Important Compound Classes
Title
BRD9 Bifunctional Degraders and Their Methods of Use
Patent Publication Number
WO 2021/055295 A1
Publication Date
March 25, 2021
Priority Application
US 62/900,863; US 62/900,860; US 62/900,865; US 62/900,869
Priority Date
September 16, 2019; September 16, 2019; September 16, 2019; September 16, 2019
Inventors
Lorber, J.; Sendzik, M.; Chen, X.; Goude, M.; Harrington, E. M.; Hollingworth, G. J.; Vulpetti, A.; Zoller, T.
Assignee Company
Novartis AG, Switzerland
Disease Area
Cancer
Biological Target
BRD9
Summary
Mammalian SWI/SNF (SWItch Sucrose Non-Fermentable) (mSWI/SNF) is a family of ATP-dependent chromatin remodeling complexes, which regulate the chromatin architecture to enable DNA accessibility, ensuring timely and appropriate control of gene expression. The bromodomain-containing protein 9 (BRD9), which is a subunit of the SWI/SNF complex, has emerged as a drug target from genetic screens (CRISPR, shRNA) showing a critical functional dependency in synovial sarcoma and acute myeloid leukemia (AML) but having little or no impact on most other cells.
There is limited understanding of BRD9 function beyond acetyl-lysine recognition based on early chemical probes. To date, the majority of drug discovery efforts have focused on blocking the activity of its bromodomain based antagonizing the established role of this domain as a histone acylated lysine reader. Small molecule inhibitors of BRD9 did not reproduce cell-type selective proliferative effects until they were incorporated into molecules containing the Cereblon-binding (CRBN-binding) IMID from thalidomide.
These bifunctional molecules direct the formation of complexes containing BRD9 and CRBN and lead to the ubiquitination and degradation of BRD9 protein. These observations suggest that BRD9 has an essential scaffolding role beyond its bromodomain reader function and has revived the idea that BRD9 is druggable and is a valuable target. Therefore, BRD9-directed chemical degraders have the potential to be efficacious in treating a range of BRD9-mediated hematopoietic proliferative disorders, such as cancer.
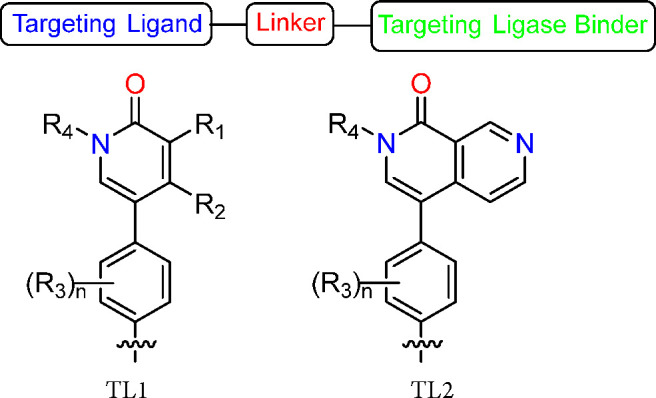
The present application describes a series of novel compounds containing Targeting Ligand-Linker-Targeting Ligase Binder, wherein Targeting Ligand is a group that is capable of binding to a bromodomain-containing protein 9 (BRD9); the Linker is a group that covalently links to the Targeting Ligand to the Targeting Ligase Binder; and Targeting Ligase Binder is a group that is capable of binding to a ligase (e.g., Cereblon E3 Ubiquitin ligase) and particularly BRD9 degraders for the treatment of cancer. Further, the application discloses compounds and their preparation, use, pharmaceutical composition, and treatment.
Definitions
R1 and R2 = H or C1–6 alkyl or R1 and R2 together with the atoms to which they are attached form an aryl or heteroaryl;
R3 = C1–6 alkyl, C1–6 alkoxyl and halogen;
R4 = H and C1–6 alkyl; and
n = 0, 1, or 2.
Key Structures
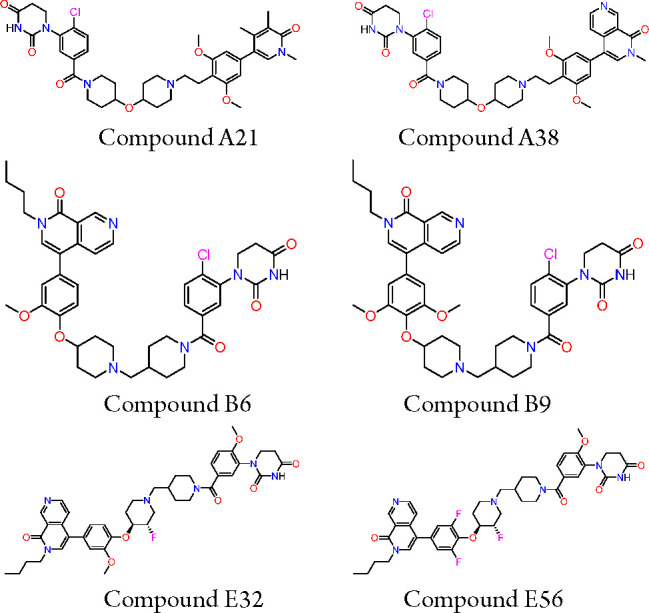
Biological Assay
The in vitro BRD9 protein degradation assay was performed. The compounds described in this application were tested for their ability to inhibit BRD9. The BRD9 DC50 (μM) are shown in the following table.
Biological Data
The table below shows representative
compounds that were tested for BRD9 inhibition. The biological data
obtained from testing representative examples are listed in the following
table.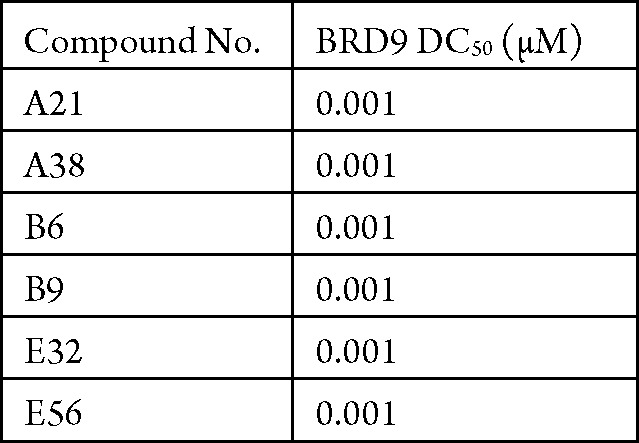
Claims
Total claims: 32
Compound claims: 28
Pharmaceutical composition claims: 1
Method for inhibition claims: 1
Method of treatment claims: 2
Recent Review Articles
-
1.
Gokani S.; Bhatt L. K.. Eur. J. Pharmacol. 2021, 911, 174523.
-
2.
D’Amico F.; Mukhopadhyay R.; Ovaa H.; Mulder M. P. C.. ChemBioChem 2021, 22, 2011.
-
3.
Coughlan A. Y.; Testa G.. Int. J. Cancer 2021, 149, 1732.
-
4.
Tsuda M.; Fukuda A.; Kawai M.; Araki O.; Seno H.. Cancer Sci. 2021, 112, 490.
-
5.
Zhu X.; Liao Y.; Tang L.. OncoTargets Ther. 2020, 13, 13191.
-
6.
Bechter O.; Schoffski P.. Pharmacol. Ther. 2020, 208, 107479.
The author declares no competing financial interest.
Special Issue
Published as part of the ACS Medicinal Chemistry Letters special issue “Epigenetics 2022”.


