Abstract
Background
Psoriasis is an immune-mediated inflammatory skin disease that causes significant physical and psychological burden to the patient. While there is currently no curative treatment, recent breakthroughs involving stem cell therapy, in particular, adipose tissue-derived from mesenchymal stem cells (AD-MSCs), have been promising. This single-arm study evaluated the feasibility, safety, and efficacy of AD-MSC infusions for the treatment of moderate to severe psoriasis.
Methods
A single-center, open-label pilot study was conducted involving seven subjects with moderate to severe psoriasis. Patients received intravenous injections of AD-MSCs (0.5×106 cells/kg) monthly for 12 weeks. The primary outcome was patient safety evaluated by the incidence of adverse events (AEs). Secondary parameters included changes in the Psoriasis Area and Severity Index (PASI), Dermatology Life Quality Index (DLQI), Body Surface Area (BSA), and Pruritus Scores on the Visual Analogue Scale (VAS).
Results
A total of 7 patients, including 6 males and 1 female, with an average age of 50.71 years (range, 35–65 years) were enrolled in this study. Four patients completed the trial and two participants completed the one-year follow-up. There were 16 AEs (including 1 grade 2 event and 15 grade 1 events) recorded during the treatment period and 1 serious adverse event (SAE) documented during the follow-up period. The most common AEs were transient fevers (5/16) which were likely to be related to the infusions, followed by pharyngitis (3/16), and headaches (2/16). Both of them were unlikely to be related to the infusions. The procedure was determined to be safe, and no SAEs relating to AD-MSCs were observed. Two patients reached and maintained a PASI-50, indicating a 50% improvement in the PASI score, after one year without any treatment.
Conclusions
These results suggested that intravenous injection of AD-MSCs is safe and may be a therapeutic option for the treatment of patients with psoriasis. Future studies involving larger test cohorts and a control group are warranted.
Keywords: Mesenchymal stem cells (MSCs), clinical trials, cellular therapy, stem cell transplantation, psoriasis
Introduction
Psoriasis is an immune-mediated chronic systemic inflammatory skin disorder that is associated with an increased risk of comorbidities, such as hypertension, hyperlipidemia, major adverse cardiovascular events, inflammatory arthritis, and malignancies (1). The condition affects 0.51–11.43% of the adult population (2) and causes significant physical and psychological burden (3) which may lead to mental disorders such as depression (4), anxiety (5), and even suicide (6). Although the introduction of biological therapies has made some progress in recent years, there is currently no satisfactory therapy for the management of psoriasis (7) and no curative treatment. Many patients remain untreated, have inadequate responses or have treatment-related toxic effects (8). Therefore, the development of novel therapeutic strategies for the management and treatment of psoriasis is urgently needed.
The pathogenesis of psoriasis is characterized by T cell-mediated immune responses with complex cellular networks, including macrophages, keratinocytes, and T and B cells (9). Interleukin (IL)-23 and helper T cells type 17 (Th17) responses are considered central mechanisms of the disease (10). Recent investigations have suggested that the effects of mesenchymal stem cells (MSCs) on T cells contribute to the pathogenesis of psoriasis, and thus, MSCs may be a promising therapeutic target (11-14). MSCs modulate both innate and adaptive responses by changing the profile of cytokines and chemokines secreted by dendritic cells and macrophages. Moreover, they inhibit the proliferation of activated T and B cells and impede the cytotoxic activity of natural killer (NK) cells (15,16). Under psoriatic conditions, MSCs inhibit the activity of the Thl7 cells, reducing the expression of IL-17 and tumor necrosis factor (TNF)-α, and increasing the production of IL-10 (17,18). Understanding the immunological mechanisms underlying psoriasis may contribute to the development of novel therapeutic strategies.
Recent studies have suggested that adipose-derived mesenchymal stem cells (AD-MSCs) could be potential candidates for psoriasis cell-replacement therapy (19). AD-MSCs are usually obtained from adipose tissues which are abundant and convenient materials to get. According to the criteria of the International Society for Cellular Therapy position statement, AD-MSCs must be plastic-adherent when maintained in standard culture conditions with expression of CD105, CD73 and CD90, and lack expression of CD45, CD34, CD14 or CD11b, CD79alpha or CD19 and HLA-DR surface molecules. Thirdly, AD-MSCs must differentiate to osteoblasts, adipocytes and chondroblasts in vitro (20). AD-MSCs can act on a variety of immune cells and regulate immune molecules through inter-cell contacts, which play a complex role in immunomodulatory function (21). Compared with the MSCs derived from bone marrow (BM) or umbilical cord matrix (UCM), AD-MSCs can minimize immunological rejection (22) and blocked the T cell activation process in an earlier phase than BM-MSCs or UCM-MSCs, yielding a greater proportion of T cells in the non-activated state (23). Studies have successfully used cell-based therapy in the imiquimod (IMQ)-induced psoriasis mouse model and demonstrated that AD-MSCs significantly prevented the development of psoriasis (18,24). However, there has been a paucity of case reports documenting the safety and efficacy of AD-MSC infusions in psoriasis patients (25,26). While these case reports suggest that AD-MSCs may be a new feasible treatment option for psoriasis, to date, there have been no clinical trials of AD-MSCs for this indication. We supposed that AD-MSCs could be delivered to the targeted areas in vivo where T cells had been activated and proliferated with the increased anti-inflammatory paracrine activity to inhibit inflammation and had a therapeutical effect on psoriasis. Therefore, in this study, we developed a protocol and conducted a pilot trial to investigate the safety of AD-MSC infusions in patients with moderate to severe psoriasis. We present the following article in accordance with the TREND reporting checklist (available at https://dx.doi.org/10.21037/atm-21-5028).
Methods
Preparation of AD-MSCs
Clinical-grade AD-MSCs were isolated and cultured in a Good Manufacturing Practice (GMP) environment in total online: https://cdn.amegroups.cn/static/public/atm-21-5028-1.pdf. A total of 3×107 AD-MSCs (within 5 passage) were washed 3 times with saline, centrifuged and collected. Cells were resuspended in 20 mL saline (Baxter Medical Supplies Company, Shanghai, China) and 2 mL 20% human blood albumin. The cell mixture was filtered through a 100 µm cell strainer (BD FALCON), and placed in a 100-mL saline bag for transplantation. Each batch of AD-MSCs was tested for microbiological contamination. Biological efficacy was evaluated by phenotypic analysis and differentiation function tests in total online: https://cdn.amegroups.cn/static/public/atm-21-5028-1.pdf. The quality of the AD-MSCs was accredited by the National Institutes of Food and Drug Control (NIFDC).
Participants
Patients with moderate to severe psoriasis vulgaris who received outpatient services in the Dermatology Department of Guangdong Provincial Hospital of Chinese Medicine (GPHCM) or were recruited by advertisement in Guangzhou, China from November 29th, 2017 to December 5th, 2018, were consecutively enrolled into this pilot study. Patients were included in the study if they presented with moderate to severe psoriasis vulgaris [Psoriasis Area and Severity Index (PASI) >10 or Body Surface Area (BSA) >10%]; were aged between 18 to 65 years old; and were willing to sign the informed consent form. Patients were excluded from the study if they presented with any of the following: (I) guttate psoriasis, inverse psoriasis, or psoriatic rash exclusively associated with the face; (II) acute progressive psoriasis and erythroderma tendency; (III) pregnancy, lactational or plans to be pregnant within 1 year; (IV) significant anxiety or depression with Self-rating Anxiety Scale (SAS) >50 or the Self-rating Depression Scale (SDS) >53, or other psychiatric disorders; (V) a history of primary cardiovascular, respiratory, digestive, urinary, endocrinologic or hematologic diseases, which cannot be controlled through standard treatments; (VI) malignancy, infections, electrolyte imbalance, acid-base disturbance, allergic constitutions, abnormal tumor markers or other organ dysfunctions; (VII) patients receiving topical treatments (i.e., corticosteroids or retinoic acid or Vitamin D analogs) within 2 weeks, systemic therapy or phototherapy [ultraviolet radiation B (UVB) and psoralen combined with ultraviolet A (PUVA)] within 4 weeks, or biological therapy within 12 weeks; and (VIII) patients involved in other clinical trials or have completed a clinical trial within one month. Patients with the following abnormal clinical test results were also excluded: abnormal serum calcium levels (Ca2+ >2.9 or <2 mmol/L); aspartate aminotransferase (AST) or alanine aminotransferase (ALT) levels 2 times beyond the normal upper limit; creatinine and cystatin C greater than the normal upper limit; hemoglobin elevated 20 g/L more than the normal upper limit or anemia; platelet count less than 75.0×109/L; white blood cell count less than 3.0×109/L; or any other abnormal laboratory test results. Furthermore, patients whose medical conditions were assessed as not suitable for this study were also excluded.
Study design
This was a single-center, single-arm pilot study. Each subject signed a written informed consent form prior to the commencement of the study. All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013). The study received ethical approval from the Guangdong Provincial Hospital of Chinese Medicine (GPHCM) Ethics Committee (No. S2017-01-01). This study has been registered in the Clinicaltrials.gov (Identifier: NCT03265613).
During the run-in and follow-up periods, urea ointment was administered by the investigators as the basic concomitant treatment if it was deemed necessary. Patients were administered intravenous infusions of AD-MSCs at weeks 0, 4, and 8, at 0.5×106 cells/kg body weight and an infusion rate of generally 2–3 mL/min. To ensure the safety of the patients, hospitalized observations were mandated for 48 hours after the procedure. Electrocardiography (ECG), heart rate, blood pressure, respiration, and oxygen saturation were monitored and recorded every 30 minutes for 5 hours after the infusion. All patients were followed up regularly by weekly telephone consults. The PASI and BSA assessments, as well as routine laboratory tests were performed monthly. Intervention was discontinued for patients with abnormal clinically meaningful laboratory results in accordance with the study protocol in total online: https://cdn.amegroups.cn/static/public/atm-21-5028-2.pdf.
Measurement of outcomes
The primary outcome was to evaluate the safety and tolerability of the intravenous infusion of AD-MSCs through the identification of adverse events (AEs) and serious adverse events (SAEs). Clinically relevant AEs (i.e., grades 3–5) related to AD-MSCs administration were considered dose-limiting toxicity (DLT) (27). Intensity of AEs was assessed according to the Common Terminology Criteria for AEs, V. 5.0, of the National Cancer Institute which ranges from 1 to 5 (https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_5.0/).
Secondary outcomes were assessed by measuring changes in Psoriasis Area and Severity Index (PASI), BSA, pruritus score on the Visual Analogue Scale (VAS), and DLQI (Dermatology Life Quality Index) from baseline to week 12 after infusion. The proportion of patients who achieve at least 50% or 75% improvement in PASI score (PASI-50 and PASI-75, respectively) from baseline to week 12 was also assessed. All PASI and BSA scores were evaluated by the same investigators.
Data management
Data was entered using the double-entry method through Epidata version 3.1 database. SPSS version 18 software (IBM Corp., Armonk, NY, USA) was used for all statistical analyses. Missing data were addressed with multiple imputation. The number and percentages of patients who experienced AEs, SAEs, treatment-related AEs, treatment-related SAEs, and grade 3–5 AEs were examined.
Statistical analysis
Percentages were used for dichotomous variables while means and standard deviations were used for continuous variables. A Chi-square test, Fisher’s exact test, or t-test was used to analyze differences before and after treatment. Analyses were performed at the individual level. A P value <0.05 was considered statistically significant.
Results
Clinical-grade human AD-MSCs
Clinical-grade AD-MSCs were isolated via a series of bioprocesses including cell isolation, expansion, characterization, certification, and safety assessment. Cultured AD-MSCs were spindle-shaped with a fibroblast-like morphology in total online: https://cdn.amegroups.cn/static/public/atm-21-5028-1.pdf. Phenotypic analysis and differentiation experiments were performed to characterize the AD-MSCs. Flow cytometry analysis showed that the purity of the AD-MSCs was greater than 96%, which was confirmed by the high positive rates of CD105, CD73, CD90, and CD44, and negative expression of the hematopoietic markers CD34, CD45, CD11b, CD19, CD45, and HLA-DR in total online: https://cdn.amegroups.cn/static/public/atm-21-5028-1.pdf. Oil-red O staining was performed to detect the presence of lipids, thereby confirming adipogenic differentiation. Likewise, osteogenic and chondrogenic differentiation were confirmed by the presence of mineralized plaques and cartilaginous substrates, respectively, as shown by Alizarin Red and Alcian Blue staining, respectively in total online: https://cdn.amegroups.cn/static/public/atm-21-5028-1.pdf.
Baseline characteristics of the patients
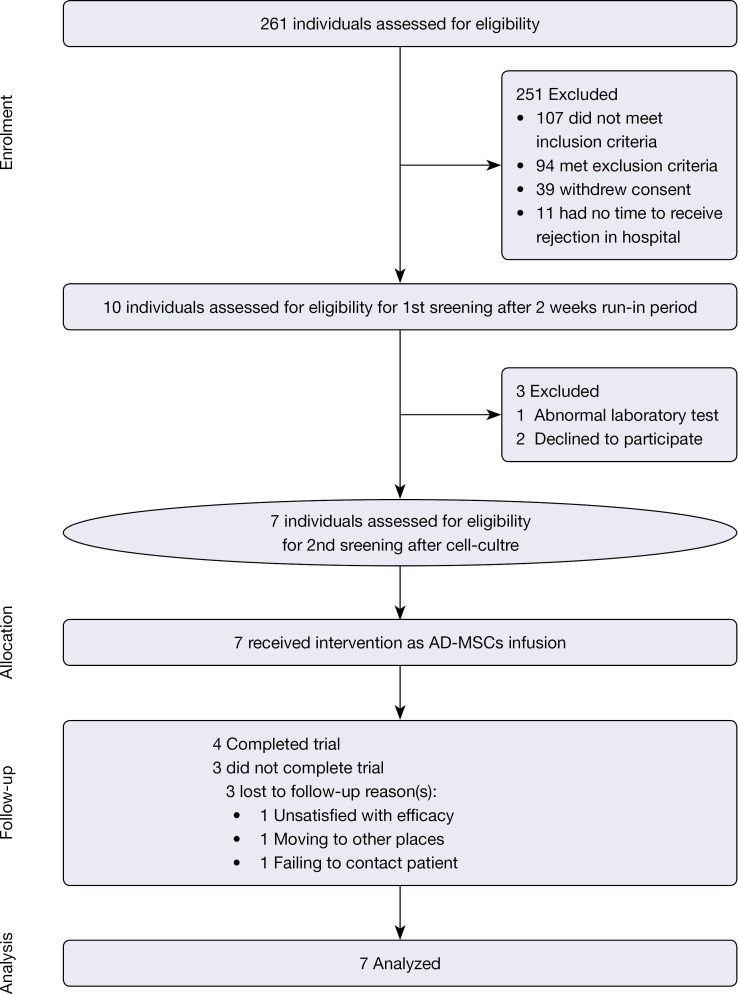
A total of 261 patients were recruited for this study. In accordance with the inclusion and exclusion criteria, 7 patients, including 1 female and 6 males, were finally enrolled (Figure 1). Ultimately, 4 patients completed the study and 3 patients withdrew. Table 1 summarizes the baseline characteristics of the 7 patients. The severity of psoriasis at baseline was moderate to severe with a mean PASI score of 9.8 (Table 1).
Figure 1.
A flow diagram depicting the patient selection process.
Table 1. Clinical characteristics of the participants.
| Characteristic | Value (N=7) |
|---|---|
| Gender, n (%) | |
| Male | 6 (85.7) |
| Female | 1 (14.3) |
| Age (years), mean ± SD | 50.71±10.45 |
| Disease duration (months), mean ± SD | 176±94.47 |
| Outcome measure, mean ± SD | |
| BSA (%) | 14.91±4.43 |
| PASI | 9.80±1.83 |
| VAS | 5.57±0.73 |
| DLQI | 7.71±3.99 |
SD, standard deviation; BSA, body surface area; PASI, Psoriasis Area and Severity Index; VAS, Pruritus Scores on the Visual Analogue Scale; DLQI, Dermatology Life Quality Index.
Outcome measures
Primary outcome measures
All subjects tolerated the procedure well. A total of 16 AEs were reported during the treatment period, all of which were of mild (grade 1, 94%) or moderate (grade 2, 6%) intensity (see Table 2).
Table 2. The incidence of adverse events during the trial period.
| Patient | Adverse event | Date of onset | Date of resolution | Any therapy after adverse event occurred? | Grade of AEs according to Common Terminology Criteria for AEs1 | Were the AEs related to AD-MSCs infusion? | Did the adverse event resolve or persist? |
|---|---|---|---|---|---|---|---|
| P1 | Transient fever for 16 hours (<38.5 °C) | 2017.11.29 | 2017.11.30 | No | 1 | Related | Relieved and resolved |
| Headache for 4 days | 2017.12.8 | 2017.12.12 | No | 1 | Unrelated | Relieved and resolved | |
| ALT abnormal for several days (<3.0× ULN, baseline was normal) | 2017.12.20 | 2017.12.22 | No | 1 | Unrelated | Relieved and resolved | |
| P2 | Transient fever for 12 hours (<39 °C) | 2018.3.14 | 2018.3.14 | No | 1 | Unrelated | Relieved and resolved |
| Pharyngitis for 1 week | 2018.3.20 | 2018.3.27 | No | 1 | Unrelated | Relieved and resolved | |
| Pharyngitis for 4 days | 2018.4.12 | 2018.4.16 | Yes, antiviral drug | 2 | Unrelated | Relieved and resolved | |
| P3 | Feeling hot for 3 days | 2018.5.24 | 2018.5.27 | No | 1 | Unrelated | Relieved and resolved |
| Transient fever for 22 hours (<39 °C) | 2018.6.21 | 2018.6.22 | Yes, Dexamethasone 5 mg iv; Phenergan 25 mg im; fluid infusion |
1 | Related | Relieved and resolved | |
| Headache for 4 days | 2018.6.28 | 2018.7.31 | No | 1 | Unrelated | Relieved and resolved | |
| Flu like symptoms (pharyngitis, cough, dizziness) | 2018.7.20 | 2018.8.7 | Yes, throat tablet consisting of Chinese herbs | 1 | Unrelated | Relieved and resolved | |
| P5 | Dizziness for 1 hour during the infusion of AD-MSCs | 2018.6.19 | 2018.6.19 | No | 1 | Related | Relieved and resolved |
| Transient fever for 8 hours | 2018.6.20 | 2018.6.20 | No | 1 | Related | Relieved and resolved | |
| P6 | Transient fever for 20 hours | 2018.8.30 | 2018.8.31 | No | 1 | Related | Relieved and resolved |
| Erythema of skin after being bitten by mosquitoes | 2018.9.19 | 2018.9.26 | No | 1 | Unrelated | Relieved and resolved | |
| P7 | Mild abdominal pain | 2018.10.20 | 2018.10.27 | No | 1 | Unrelated | Relieved and resolved |
| Pharyngitis for 2 days | 2018.12.1 | 2018.12.3 | No | 1 | Unrelated | Relieved and resolved |
US Department of Health and Human Services. Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0. Published 27 Nov. 2017 (v5.0: Nov 27 2017). https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_5.0/. 1, the CTCAE defines adverse events all grades on a scale from 1 to 5: with 1, a mild or asymptomatic adverse event; 2, a moderate adverse event; 3, a severe or medically significant but not life-threatening adverse event; 4, a life-threatening adverse event with consequences; and 5, death. ALT, alanine transaminase; ULN, upper limits of normal; AD-MSC, adipose tissue derived mesenchymal stem cell.
The most common AE was transient fever (5, 31.2%), followed by pharyngitis (3, 18.6%), headaches (2, 12.5%), flu like symptoms (1, 6.25%), dizziness (1, 6.25%), mild abdominal pain (1, 6.25%), erythema of the skin after being bitten by mosquitoes (1, 6.25%), and transient ALT abnormality for several days (1, 6.25%). Except for fever, none of the AEs were deemed to be attributable to the administration of the AD-MSCs by the Data Monitoring Committee (DMC).
No laboratory test abnormalities occurred (see Table 3) other than those related to the reported AEs (see Table 2). No relevant vital sign abnormalities and no expected AEs, such as acute infusional toxicity, pulmonary embolism, and infections, were observed.
Table 3. Safety assessment of laboratory tests before and after three infusions in each patient.
| Patient | Leukocyte count (109/L) | Neutrophil percentage (%) | ALT (U/L) | AST (U/L) | II polymer (mg/mL) | Cr (μmol/L) |
AFP (ng/mL) |
CEA (ng/mL) |
CA199 (ng/mL) |
CA724 (ng/mL) |
|||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 12 | 0 | 12 | 0 | 12 | 0 | 12 | 0 | 12 | 0 | 12 | 0 | 12 | 0 | 12 | 0 | 12 | 0 | 12 | ||||||||||
| P1 | 4.66 | 5.02 | 54.3 | 57.7 | 21 | 17 | 23 | 19 | 0.41 | 0.40 | 65 | 69 | 4.03 | 3.42 | 0.8 | 0.88 | 7.06 | 5.6 | 0.8 | 0.8 | |||||||||
| P2 | 4.41 | – | 68.1 | – | 36 | – | 35 | – | 0.31 | – | 74 | – | 1.97 | – | 3.31 | – | 3.41 | – | 2.2 | – | |||||||||
| P3 | 5.38 | 6.14 | 54.2 | 48.2 | 26 | 24 | 21 | 20 | 0.24 | 0.32 | 62 | 67 | 6.15 | 5.19 | 1.83 | 1.75 | 6.29 | 5.12 | 1 | 1 | |||||||||
| P4 | 6.22 | – | 66.6 | – | 13 | – | 19 | – | 0.22 | – | 69 | – | 2.96 | – | 2.3 | – | 7.86 | – | 3.6 | – | |||||||||
| P5 | 6.24 | – | 54.5 | – | 23 | – | 24 | – | 0.26 | – | 84 | – | 3.6 | – | 2.57 | – | 17.99 | – | 1.6 | – | |||||||||
| P6 | 6.69 | 6.08 | 63.1 | 62.1 | 38 | 105 | 23 | 42 | 0.40 | 0.35 | 97 | 90 | 1.59 | 1.48 | 2.61 | 2.66 | 10.96 | 11.39 | 3.6 | 6 | |||||||||
| P7 | 7.56 | 6.81 | 53.2 | 57.4 | 35 | 32 | 27 | 25 | 0.34 | 0.33 | 77 | 79 | – | – | 2.11 | – | 6.66 | 7.31 | 1.1 | 1.36 | |||||||||
ALT, alanine aminotransferase; AST, aspartate aminotransferase; Cr, creatinine; AFP, alpha fetoprotein; CEA, carcinoembryonic antigen; CA, carbohydrate antigen.
One SAE occurred during the follow-up period. Patient 3 was diagnosed with gastric cancer by gastroscopy in a routine physical examination without any epigastric discomfort almost 6 months after the last infusion. She was hospitalized on November 19th, 2018. The patient underwent laparoscopic subtotal gastrectomy with gastrojejunostomy and perigastric lymph node dissection under general anesthesia on November 26th, 2018. She recovered well and was discharged from the hospital one week after surgical treatment. The pathological result of the gastric cancer was poorly differentiated adenocarcinoma (partly signet ring cell carcinoma) without any lymphatic metastasis. The short tandem repeat (STR) analysis showed that the cancerous tissue was different from that of the MSC donor in total online: https://cdn.amegroups.cn/static/public/atm-21-5028-3.pdf. Therefore, the gastric cancer was considered a primary disease and the SAE was considered unlikely to be related to AD-MSC infusion. At the time of publication, no tumor recurrence had been detected in this patient.
Aside from the above-mentioned SAE, no malignancies or deaths were reported in the whole trial.
Secondary outcome measures
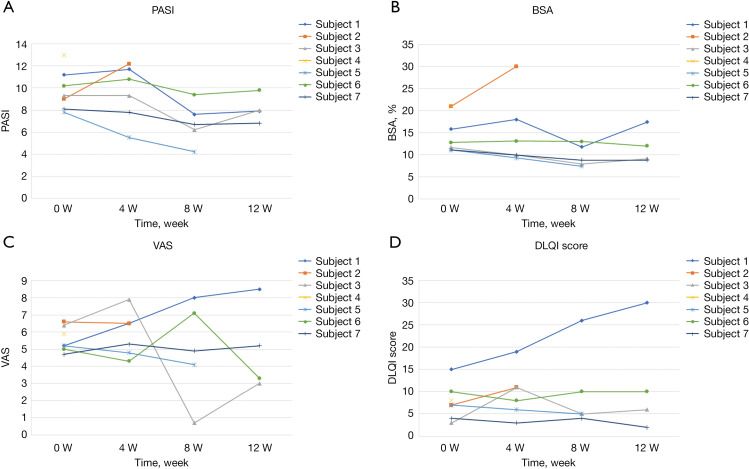
The improvement rate of PASI score from baseline to week 12 ranged from -0.36% to 46%. Some of the outcome measures of the 7 participants were showed in Figure 2. The PASI scores gradually decreased from baseline to week 12 (Figure 2A). However, there were no statistically significant differences in PASI, BSA, VAS, nor DLQI scores between baseline and week 12 (see Figure 2B-2D). None of the participants reached PASI-50 or PASI-75 during the course of the trial.
Figure 2.
The figure showed four outcome measures of the 7 participants including PASI score, BSA, VAS and DLQI. (A) PASI score; (B) BSA for psoriasis; (C) VAS; (D) DLQI. PASI, Psoriasis Areas Severity Index; BSA, body surface area; VAS, Visual Analog Score; DLQI, Dermatology Life Quality Index.
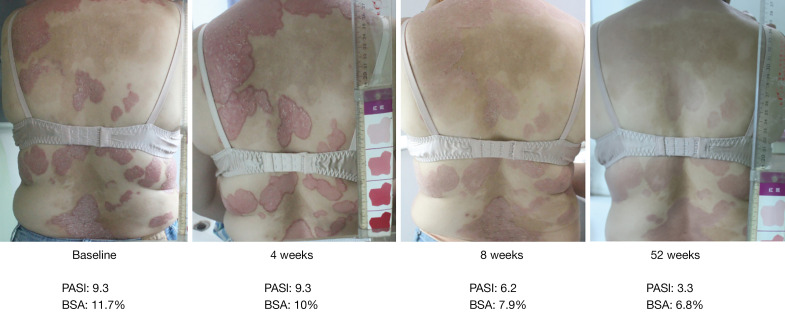
During the follow-up period, 2 patients found that the lesions continued to decrease without any treatment and even reached PASI-50 6 months after the treatment period. One of those patients maintained PASI-50 at the one-year follow-up (see Figure 3).
Figure 3.
Representative skin images of participants before and after treatment. The four images were skin images of one of the participant before and after treatment. From the images, the erythema, scaling and infiltration of the lesion had improved significantly after treatment. The PASI score declined gradually from baseline (before treatment 9.3) to 52 weeks (3.3) after treatment. And the BSA also decreased (from 11.7% to 6.8%). The participant reached PASI-50 at week 52. BSA, body surface area; PASI, Psoriasis Area and Severity Index.
Discussion
To the best of our knowledge, this is the first clinical trial to report the outcomes of AD-MSC infusions in patients with moderate to severe psoriasis. The results of this trial indicated that an intravenous infusion of 0.5×106 cells/kg of body weight of allogeneic MSCs was safe and well tolerated in our patients. No AD-MSC related SAEs were reported. There were no clinical complications related to the cell infusion, no changes in vital signs, no loss of hepatic or renal function, nor changes to blood cell counts. No major toxicity was observed up to one year after the last infusion of AD-MSCs. Transient fever, which is common after infusions, was considered to be related to the intervention. A study involving endobronchial administration of autologous AD-MSCs-SVF (Stromal Vascular Fraction) in patients with idiopathic pulmonary fibrosis reported transient fevers in 7 out of 14 patients (28). The mechanisms for the fevers are unclear but may be related to acute inflammatory reactions by a subset of patients to particular preparations of MSCs (29).
Previous preclinical studies have demonstrated an increased tumor burden in vivo, raising concerns of tumorigenicity related to MSCs (30). However, recent studies have suggested a low probability of malignant transformation and tumor formation with MSCs (31). In randomized controlled trials (RCTs) of participants without malignancies, there were no differences between patients who received AD-MSCs and control participants (32). In this trial, a SAE of gastric cancer was reported six months after the last infusion. Previous reports have suggested that the shortest time required for the transformation of normal gastric mucosa to gastric cancer is 14 months (33). The patient had a family history of tumors due to her mother’s nasopharyngeal carcinoma. In addition, the patient’s history of moderate to severe psoriasis for over 20 years also increased the risk of tumors (34). According to the STR analysis, the cancer was considered a primary malignancy. Contrary to induced pluripotent stem cells (iPSC) (35) or embryonic stem cells, MSCs are not associated with a risk of teratoma formation, because they are adult stem cells with restricted differentiation potential. A meta-analysis examining more than 1,000 patients treated with MSCs also reported no association with acute toxicity, organ system complications, infections, malignancies, or death (36). Therefore, besides the AE of transient fevers, this current pilot trial suggested that the administration of AD-MSCs is safe for patients with psoriasis.
The study demonstrated that AD-MSCs may have a long-lasting efficacy in psoriasis patients. Two of the patients reported that their lesions continued to decrease during the follow-up period. One patient maintained PASI-50 for almost three years. Similarly, previous studies have reported that patients treated with MSCs could maintain PASI-50 for 9.7 months (25), or even 4–5 years (37). This is an encouraging and attractive feature of MSC treatment, even though the underlying immunomodulatory mechanisms are still not fully understood. Investigations have shown that AD-MSCs can inhibit the secretion of IL-17 by peripheral blood mononuclear cells (PBMCs) (38) and restore the physiological phenotypical profile of psoriatic MSCs (39). In IMQ-induced mouse models, MSCs were reported to inhibit Th17 cytokines (IL-17, IL-23) and exert immune modulation in psoriasis, which may be a key factor for improving psoriasis (40-42). Another research provide an explanation for the therapeutic effects of MSC infusion by first suppressing neutrophil function and then downregulating the production of type I interferon (IFN-I) by plasmacytoid dendritic cells (pDCs) (43). It is possible that the limited short-term efficacy of the pilot study was due to insufficient dose. The low dose and the infusion interval used in our study was based on the safety observations from prior case reports (25). Compared to other clinical trials (44,45), the infusion interval was too long and the number of infusions was inadequate, as the AD-MSCs were likely cleared up by the human body after infusion. Therefore, a dose- and frequency-finding design would be desirable in the next phase for AD-MSC infusions.
There were several limitations to this trial. The sample size and follow-up periods were limited and there was no control group and no blinding in this trial. Future randomized controlled trials comparing certain basic treatments and AD-MSCs infusions should be conducted. To enable blinding in the experiments, the AD-MSCs and the placebo cells should be delivered in some type of packaging to enable blinding. This current trial, in agreement with previous reports, demonstrated good safety of AD-MSC infusions. Thus, in future trial, the patient exclusion criteria could be appropriately relaxed with the exception of patients with a family history of tumors or who exhibit significant risk factors for cancer. These experiments were also limited by the treatment method of intravenous infusion. Novel modes of drug administration, including subcutaneous infusions and external applications, may be developed and studied in the future.
Conclusions
This pilot study demonstrated that intravenous injection of AD-MSCs is safe and well tolerated in patients with moderate to severe psoriasis. Future controlled long-term studies with larger samples are needed to confirm the efficacy of this innovative cell therapy for the treatment of patients with psoriasis.
Supplementary
The article’s supplementary files as
Acknowledgments
We thank Professors Jian-Wen Guo, Xun Zhang, Yuqi Yang (Guangdong Provincial Hospital of Chinese Medicine, Guangzhou, China), and Jing Wang (Center of Excellence in Tissue Engineering, Institute of Basic Medical Sciences, School of Basic Medicine, Peking Union Medical College Hospital, Peking Union Medical College, Chinese Academy of Medical Sciences, Beijing, China) for their comments, suggestions, and conducting the study in their centers.
Funding: This research was supported by grants from the Special Funding for TCM Science and Technology Research of Guangdong Provincial Hospital of Chinese Medicine (Project number: YN2016QJ02), the Traditional Chinese Medicine Bureau Foundation of Guangdong Province (Project number: No. 20183005), National Natural Science Foundation of China (U20A20397, 81873302) and the Guangdong Provincial Clinical Research Center for Chinese Medicine Dermatology (No. 2020B1111170012).
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013). The study received ethical approval from the Guangdong Provincial Hospital of Chinese Medicine (GPHCM) Ethics Committee (No. S2017-01-01). Written informed consent was obtained from all participants and confidentiality was ensured.
Reporting Checklist: The authors have completed the TREND reporting checklist. Available at https://dx.doi.org/10.21037/atm-21-5028
Data Sharing Statement: Available at https://dx.doi.org/10.21037/atm-21-5028
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/atm-21-5028). The authors have no conflicts of interest to declare.
(English Language Editor: J. Toeh)
References
- 1.Takeshita J, Grewal S, Langan SM, et al. Psoriasis and comorbid diseases: Epidemiology. J Am Acad Dermatol 2017;76:377-90. 10.1016/j.jaad.2016.07.064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Michalek IM, Loring B, John SM. A systematic review of worldwide epidemiology of psoriasis. J Eur Acad Dermatol Venereol 2017;31:205-12. 10.1111/jdv.13854 [DOI] [PubMed] [Google Scholar]
- 3.Luo Y, Ru Y, Sun X, et al. Characteristics of psoriasis vulgaris in China: a prospective cohort study protocol. Ann Transl Med 2019;7:694. 10.21037/atm.2019.10.46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dominguez PL, Han J, Li T, et al. Depression and the risk of psoriasis in US women. J Eur Acad Dermatol Venereol 2013;27:1163-7. 10.1111/j.1468-3083.2012.04703.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fleming P, Bai JW, Pratt M, et al. The prevalence of anxiety in patients with psoriasis: a systematic review of observational studies and clinical trials. J Eur Acad Dermatol Venereol 2017;31:798-807. 10.1111/jdv.13891 [DOI] [PubMed] [Google Scholar]
- 6.Singh S, Taylor C, Kornmehl H, et al. Psoriasis and suicidality: A systematic review and meta-analysis. J Am Acad Dermatol 2017;77:425-440.e2. 10.1016/j.jaad.2017.05.019 [DOI] [PubMed] [Google Scholar]
- 7.Lebwohl MG, Kavanaugh A, Armstrong AW, et al. US Perspectives in the Management of Psoriasis and Psoriatic Arthritis: Patient and Physician Results from the Population-Based Multinational Assessment of Psoriasis and Psoriatic Arthritis (MAPP) Survey. Am J Clin Dermatol 2016;17:87-97. 10.1007/s40257-015-0169-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lebwohl MG, Bachelez H, Barker J, et al. Patient perspectives in the management of psoriasis: results from the population-based Multinational Assessment of Psoriasis and Psoriatic Arthritis Survey. J Am Acad Dermatol 2014;70:871-81.e1-30. [DOI] [PubMed]
- 9.Lowes MA, Suárez-Fariñas M, Krueger JG. Immunology of psoriasis. Annu Rev Immunol 2014;32:227-55. 10.1146/annurev-immunol-032713-120225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Griffiths CEM, Armstrong AW, Gudjonsson JE, et al. Psoriasis. Lancet 2021;397:1301-15. 10.1016/S0140-6736(20)32549-6 [DOI] [PubMed] [Google Scholar]
- 11.Liu R, Yang Y, Yan X, et al. Abnormalities in cytokine secretion from mesenchymal stem cells in psoriatic skin lesions. Eur J Dermatol 2013;23:600-7. 10.1684/ejd.2013.2149 [DOI] [PubMed] [Google Scholar]
- 12.Liu R, Wang Y, Zhao X, et al. Lymphocyte inhibition is compromised in mesenchymal stem cells from psoriatic skin. Eur J Dermatol 2014;24:560-7. 10.1684/ejd.2014.2394 [DOI] [PubMed] [Google Scholar]
- 13.Liu RF, Wang F, Wang Q, et al. Research Note Mesenchymal stem cells from skin lesions of psoriasis patients promote proliferation and inhibit apoptosis of HaCaT cells. Genet Mol Res 2015;14:17758-67. 10.4238/2015.December.21.49 [DOI] [PubMed] [Google Scholar]
- 14.Niu X, Chang W, Liu R, et al. mRNA and protein expression of the angiogenesis-related genes EDIL3, AMOT and ECM1 in mesenchymal stem cells in psoriatic dermis. Clin Exp Dermatol 2016;41:533-40. 10.1111/ced.12783 [DOI] [PubMed] [Google Scholar]
- 15.English K. Mechanisms of mesenchymal stromal cell immunomodulation. Immunol Cell Biol 2013;91:19-26. 10.1038/icb.2012.56 [DOI] [PubMed] [Google Scholar]
- 16.Hou R, Li J, Niu X, et al. Stem cells in psoriasis. J Dermatol Sci 2017;86:181-6. 10.1016/j.jdermsci.2016.11.006 [DOI] [PubMed] [Google Scholar]
- 17.Ghannam S, Pène J, Moquet-Torcy G, et al. Mesenchymal stem cells inhibit human Th17 cell differentiation and function and induce a T regulatory cell phenotype. J Immunol 2010;185:302-12. 10.4049/jimmunol.0902007 [DOI] [PubMed] [Google Scholar]
- 18.Rokunohe A, Matsuzaki Y, Rokunohe D, et al. Immunosuppressive effect of adipose-derived stromal cells on imiquimod-induced psoriasis in mice. J Dermatol Sci 2016;82:50-3. 10.1016/j.jdermsci.2015.12.007 [DOI] [PubMed] [Google Scholar]
- 19.Owczarczyk-Saczonek A, Krajewska-Włodarczyk M, Kruszewska A, et al. Stem Cells as Potential Candidates for Psoriasis Cell-Replacement Therapy. Int J Mol Sci 2017;18:2182. 10.3390/ijms18102182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006;8:315-7. 10.1080/14653240600855905 [DOI] [PubMed] [Google Scholar]
- 21.Razmkhah M, Abedi N, Hosseini A, et al. Induction of T regulatory subsets from naïve CD4+ T cells after exposure to breast cancer adipose derived stem cells. Iran J Immunol 2015;12:1-15. [PubMed] [Google Scholar]
- 22.Tan K, Zheng K, Li D, et al. Impact of adipose tissue or umbilical cord derived mesenchymal stem cells on the immunogenicity of human cord blood derived endothelial progenitor cells. PLoS One 2017;12:e0178624. 10.1371/journal.pone.0178624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ribeiro A, Laranjeira P, Mendes S, et al. Mesenchymal stem cells from umbilical cord matrix, adipose tissue and bone marrow exhibit different capability to suppress peripheral blood B, natural killer and T cells. Stem Cell Res Ther 2013;4:125. 10.1186/scrt336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sah SK, Park KH, Yun CO, et al. Effects of Human Mesenchymal Stem Cells Transduced with Superoxide Dismutase on Imiquimod-Induced Psoriasis-Like Skin Inflammation in Mice. Antioxid Redox Signal 2016;24:233-48. 10.1089/ars.2015.6368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.De Jesus MM, Santiago JS, Trinidad CV, et al. Autologous Adipose-Derived Mesenchymal Stromal Cells for the Treatment of Psoriasis Vulgaris and Psoriatic Arthritis: A Case Report. Cell Transplant 2016;25:2063-9. 10.3727/096368916X691998 [DOI] [PubMed] [Google Scholar]
- 26.Comella K, Parlo M, Daly R, et al. First-in-man intravenous implantation of stromal vascular fraction in psoriasis: a case study. Int Med Case Rep J 2018;11:59-64. 10.2147/IMCRJ.S163612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Paoletti X, Le Tourneau C, Verweij J, et al. Defining dose-limiting toxicity for phase 1 trials of molecularly targeted agents: results of a DLT-TARGETT international survey. Eur J Cancer 2014;50:2050-6. 10.1016/j.ejca.2014.04.030 [DOI] [PubMed] [Google Scholar]
- 28.Tzouvelekis A, Paspaliaris V, Koliakos G, et al. A prospective, non-randomized, no placebo-controlled, phase Ib clinical trial to study the safety of the adipose derived stromal cells-stromal vascular fraction in idiopathic pulmonary fibrosis. J Transl Med 2013;11:171. 10.1186/1479-5876-11-171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hendrickson JE, Hillyer CD. Noninfectious serious hazards of transfusion. Anesth Analg 2009;108:759-69. 10.1213/ane.0b013e3181930a6e [DOI] [PubMed] [Google Scholar]
- 30.Djouad F, Plence P, Bony C, et al. Immunosuppressive effect of mesenchymal stem cells favors tumor growth in allogeneic animals. Blood 2003;102:3837-44. 10.1182/blood-2003-04-1193 [DOI] [PubMed] [Google Scholar]
- 31.Li J, Xu SQ, Zhao YM, et al. Comparison of the biological characteristics of human mesenchymal stem cells derived from exfoliated deciduous teeth, bone marrow, gingival tissue, and umbilical cord. Mol Med Rep 2018;18:4969-77. 10.3892/mmr.2018.9501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Toyserkani NM, Jørgensen MG, Tabatabaeifar S, et al. Concise Review: A Safety Assessment of Adipose-Derived Cell Therapy in Clinical Trials: A Systematic Review of Reported Adverse Events. Stem Cells Transl Med 2017;6:1786-94. 10.1002/sctm.17-0031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oh YS, Massey BT. Rapid development of intestinal type gastric adenocarcinoma. Case Rep Gastroenterol 2011;5:192-5. 10.1159/000326960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chiesa Fuxench ZC, Shin DB, Ogdie Beatty A, et al. The Risk of Cancer in Patients With Psoriasis: A Population-Based Cohort Study in the Health Improvement Network. JAMA Dermatol 2016;152:282-90. 10.1001/jamadermatol.2015.4847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yasuda S, Kusakawa S, Kuroda T, et al. Tumorigenicity-associated characteristics of human iPS cell lines. PLoS One 2018;13:e0205022. 10.1371/journal.pone.0205022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lalu MM, McIntyre L, Pugliese C, et al. Safety of cell therapy with mesenchymal stromal cells (SafeCell): a systematic review and meta-analysis of clinical trials. PLoS One 2012;7:e47559. 10.1371/journal.pone.0047559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen H, Niu JW, Ning HM, et al. Treatment of Psoriasis with Mesenchymal Stem Cells. Am J Med 2016;129:e13-4. 10.1016/j.amjmed.2015.11.001 [DOI] [PubMed] [Google Scholar]
- 38.Baharlou R, Ahmadi-Vasmehjani A, Faraji F, et al. Human adipose tissue-derived mesenchymal stem cells in rheumatoid arthritis: Regulatory effects on peripheral blood mononuclear cells activation. Int Immunopharmacol 2017;47:59-69. 10.1016/j.intimp.2017.03.016 [DOI] [PubMed] [Google Scholar]
- 39.Campanati A, Orciani M, Sorgentoni G, et al. Indirect co-cultures of healthy mesenchymal stem cells restore the physiological phenotypical profile of psoriatic mesenchymal stem cells. Clin Exp Immunol 2018;193:234-40. 10.1111/cei.13141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim CH, Lim CY, Lee JH, et al. Human Embryonic Stem Cells-Derived Mesenchymal Stem Cells Reduce the Symptom of Psoriasis in Imiquimod-Induced Skin Model. Tissue Eng Regen Med 2019;16:93-102. 10.1007/s13770-018-0165-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee YS, Sah SK, Lee JH, et al. Human umbilical cord blood-derived mesenchymal stem cells ameliorate psoriasis-like skin inflammation in mice. Biochem Biophys Rep 2017;9:281-8. 10.1016/j.bbrep.2016.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Imai Y, Yamahara K, Hamada A, et al. Human amnion-derived mesenchymal stem cells ameliorate imiquimod-induced psoriasiform dermatitis in mice. J Dermatol 2019;46:276-8. 10.1111/1346-8138.14768 [DOI] [PubMed] [Google Scholar]
- 43.Chen M, Peng J, Xie Q, et al. Mesenchymal Stem Cells Alleviate Moderate-to-Severe Psoriasis by Reducing the Production of Type I Interferon (IFN-I) by Plasmacytoid Dendritic Cells (pDCs). Stem Cells Int 2019;2019:6961052. 10.1155/2019/6961052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Álvaro-Gracia JM, Jover JA, García-Vicuña R, et al. Intravenous administration of expanded allogeneic adipose-derived mesenchymal stem cells in refractory rheumatoid arthritis (Cx611): results of a multicentre, dose escalation, randomised, single-blind, placebo-controlled phase Ib/IIa clinical trial. Ann Rheum Dis 2017;76:196-202. 10.1136/annrheumdis-2015-208918 [DOI] [PubMed] [Google Scholar]
- 45.Freitag J, Ford J, Bates D, et al. Adipose derived mesenchymal stem cell therapy in the treatment of isolated knee chondral lesions: design of a randomised controlled pilot study comparing arthroscopic microfracture versus arthroscopic microfracture combined with postoperative mesenchymal stem cell injections. BMJ Open 2015;5:e009332. 10.1136/bmjopen-2015-009332 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as