Abstract
Estrogens induce proliferation of estrogen receptor (ER)-positive MCF-7 breast cancer cells by stimulating G1/S transition associated with increased cyclin D1 expression, activation of cyclin-dependent kinases (Cdks), and phosphorylation of the retinoblastoma protein (pRb). We have utilized blockade of cyclin D1-Cdk4 complex formation through adenovirus-mediated expression of p16INK4a to demonstrate that estrogen regulates Cdk inhibitor expression and expression of the Cdk-activating phosphatase Cdc25A independent of cyclin D1-Cdk4 function and cell cycle progression. Expression of p16INK4a inhibited G1/S transition induced in MCF-7 cells by 17-β-estradiol (E2) with associated inhibition of both Cdk4- and Cdk2-associated kinase activities. Inhibition of Cdk2 activity was associated with delayed removal of Cdk-inhibitory activity in early G1 and decreased cyclin A expression. Cdk-inhibitory activity and expression of both p21Cip1 and p27Kip1 was decreased, however, in both control and p16INK4a-expressing cells 20 h after estrogen treatment. Expression of Cdc25A mRNA and protein was induced by E2 in control and p16INK4a-expressing MCF-7 cells; however, functional activity of Cdc25A was inhibited in cells expressing p16INK4a. Inhibition of Cdc25A activity in p16INK4a-expressing cells was associated with depressed Cdk2 activity and was reversed in vivo and in vitro by active Cdk2. Transfection of MCF-7 cells with a dominant-negative Cdk2 construct inhibited the E2-dependent activation of ectopic Cdc25A. Supporting a role for Cdc25A in estrogen action, antisense CDC25A oligonucleotides inhibited estrogen-induced Cdk2 activation and DNA synthesis. In addition, inactive cyclin E-Cdk2 complexes from p16INK4a-expressing, estrogen-treated cells were activated in vitro by treatment with recombinant Cdc25A and in vivo in cells overexpressing Cdc25A. The results demonstrate that functional association of cyclin D1-Cdk4 complexes is required for Cdk2 activation in MCF-7 cells and that Cdk2 activity is, in turn, required for the in vivo activation of Cdc25A. These studies establish Cdc25A as a growth-promoting target of estrogen action and further indicate that estrogens independently regulate multiple components of the cell cycle machinery, including expression of p21Cip1 and p27Kip1.
Estrogenic steroids, including 17-β-estradiol (E2), regulate cellular function in a wide variety of tissues and influence proliferation in the female reproductive tract and mammary gland (31). A role for estrogens in breast cancer etiology is well established and clearly relates to their growth-stimulatory action (35). Estrogens elicit proliferative responses in breast cancer cells in vivo (85) and in vitro (43) and are essential for initiation and progression of breast cancer in animal models (35). Studies of estrogen receptor (ER)-positive breast cancer cell lines indicate that estrogens (41) and antiestrogens (86) act on sensitive populations of cells in early to mid-G1 phase.
G1/S transition is under the control of cyclin-dependent kinases (Cdks) activated by specific complex formation with regulatory cyclins. Cdk4 and Cdk6 are activated by binding to D-type cyclins and act early in G1 phase, while Cdk2 kinase functions in conjunction with cyclins E and A and is necessary for progression through late G1 and entry into S phase (81, 83, 92, 98). A primary target of Cdk action in G1 phase is the retinoblastoma susceptibility gene product (pRb), which mediates G1 arrest through sequestration of transcriptional factors of the E2F-DP family. Phosphorylation of pRb and other members of the pocket protein family (p107 and p130) by active cyclin-Cdk complexes leads to release of E2F and DP transcription factors and transcription of requisite genes for S-phase entry (98). Recently a parallel, Cdk2-driven pathway promoting the G1/S transition independent of D cyclin-Cdk4 activation, pRb phosphorylation, and E2F release has been described in model systems utilizing cooperative Ras-Myc activation (40), and overexpression of cyclin E (45, 74).
Cdk activation depends upon removal of inhibitory Thr/Tyr phosphorylation by members of the Cdc25 phosphatase family (17, 21, 25, 77). Cdc25 phosphatases are candidate oncogenes and are overexpressed in a wide variety of tumors, including roughly 30% of breast carcinomas (20). Cdc25A expression is required for S-phase entry (17, 27, 33) and is induced in G1 (3, 27, 33) by Myc (18, 74) and E2F (7, 19, 30, 93). Cdc25A is active from mid-G1 through S phase and participates in activation of Cdk2 (3, 27, 33). Overexpression of Cdc25A is sufficient for transformation of Rb−/− fibroblasts and cooperates with Ras in causing tumors in mice (20). Coexpression of Cdc25A and cyclin E elicits G1/S transition in fibroblasts (93) and in U2-OS cells independent of pRb inactivation (74).
D-type cyclins play an essential role in recognition of extracellular growth stimuli and initiation of G1 transit (71, 80), and several lines of evidence have linked estrogen regulation of cellular proliferation to cyclin D1 expression. Estrogen-induced proliferation of normal uterine and breast epithelium in vivo is associated with increased expression of cyclin D1 mRNA and protein (2, 23, 73, 90). Cyclin D1−/− knockout mice exhibit normal development of reproductive tissues and mammary gland ductal epithelium, yet estrogen-dependent development of lobular-alveolar structures in mammary epithelium during pregnancy is disrupted (14, 84). Expression of cyclin D1 in breast tumor isolates correlates with ER-positive status (28, 52, 59). MCF-7 breast cancer cells treated with estrogen exhibit increased expression of cyclin D1 mRNA and protein, formation of active cyclin D1-Cdk4 complexes, and phosphorylation of pRb leading to G1/S transition (1, 15, 64, 69). Estrogen-induced S-phase entry in these cells is inhibited by microinjection of antibodies to cyclin D1 (44). Ectopic expression of cyclin D1 regulates exit from G0 in MCF-7 cells (102) and is sufficient for Cdk activation and S-phase entry in MCF-7 and T47D breast cancer cells (56, 68). Antiestrogen-induced growth arrest of ER-positive breast cancer cells is associated with decreased cyclin D1 expression (97). Collectively, these studies are consistent with a model of estrogen action in which receptor activation induces increased cyclin D1 expression, Cdk4 activation, and cell cycle progression. An upstream role for cyclin D1 has been suggested by recent reports describing direct physical interactions between cyclin D1 and the ER, leading to recruitment of steroid receptor coactivators and activation of ER-dependent transcription. This occurs in the absence of hormone and is independent of D cyclin association with Cdk4 (49, 57, 101, 103).
Constraint upon Cdk activity and G1 progression is provided by the universal Cdk inhibitors of the Cip-Kip family, including p21Cip1 and p27Kip1, and the specific Cdk4 and Cdk6 inhibitors of the INK4 family, typified by p16INK4a (26, 39, 65, 82, 91). The p16INK4a gene product inhibits formation of active D cyclin-Cdk complexes through specific binding interactions with Cdk4 or Cdk6 that prevent D cyclin-Cdk association (46, 50, 63). Overexpression of p16INK4a in cells with functional pRb results in inhibition of both Cdk4- and Cdk6-associated kinase activity and pRb phosphorylation, with subsequent cell cycle arrest (46, 50). In addition, inhibition of D cyclin-Cdk4 complex formation by p16INK4a prevents sequestration of p21Cip1 and p27Kip1 by these complexes in early G1, leading to suppression of cyclin E-Cdk2 activity (32, 48, 53).
Adenoviral transduction of p16INK4a into MCF-7 cells leads to G1 arrest associated with inhibited Cdk activity (8, 9). Previous studies in our laboratory indicated that cell cycle progression induced by estradiol requires action of the steroid through mid-G1, well beyond the point of cyclin D1-Cdk4 activation (15). In this study, we utilized adenovirus-mediated overexpression of p16INK4a to examine in detail the role of cyclin D1-Cdk4 complexes in cell cycle progression induced in these cells by estrogen. This approach allowed for the study of estrogen action independent of D cyclin-Cdk4 complex formation and cell cycle progression. Our results demonstrate that functional association of cyclin D1-Cdk4 is required for estrogen-induced Cdk2 activation and G1/S transition and that estrogen regulates expression of p21Cip1, p27Kip1, and Cdc25A independent of D cyclin-Cdk4 function. The results further demonstrate a requirement for in vivo activation of Cdc25A by active Cdk2.
MATERIALS AND METHODS
Reagents and antibodies.
Cell culture media and antibiotics, E2, insulin, epidermal growth factor (EGF), histones, glutathione-agarose beads, RNase A, propidium iodide, and other chemicals were from Sigma Chemical Co. (St. Louis, Mo.) unless otherwise noted. ICI 182,780 was kindly supplied by Alan Wakeling at Zeneca Pharmaceuticals (Alderly Park, Cheshire, United Kingdom). Recombinant glutathione S-transferase (GST)-pRb, protein A/G beads, GST-agarose beads, affinity-purified antibodies to Cdk2 (M2), Cdk4 (C22), pRb (C15), p27Kip1 (C19), p21Cip1 (C19), Cdc25A (N15), Raf-1 (C12), Pim-1 (C20), and cyclin D1 (H295), as well as monoclonal antibodies to cyclin E (HE12 and HE111) and cyclin A (BF683), were from Santa Cruz Biotechnology (Santa Cruz, Calif.). Monoclonal anti-Cdc25A (Ab-3) and anti-E2F-1 (Ab-7) antibodies were from Labvision (Freemont, Calif.), and both monoclonal antiactin and anti-hemagglutinin (HA) (12CA5) antibodies were from Boehringer Mannheim (Indianapolis, Ind.) [γ-32P]ATP, Tran35S label, and [methyl-3H]thymidine were from ICN (Irvine, Calif.). Fetal bovine serum (FBS) was from Summit Biotechnology (Fort Collins, Colo.). Horseradish peroxidase-conjugated protein A/G and secondary antibodies were from Jackson Immunoresearch (West Grove, Pa.). MG132, geldanamycin, PD98059, and roscovitine were from Calbiochem (La Jolla, Calif.). Flavopiridol was acquired from the National Cancer Institute (Bethesda, Md.). Antisense and control oligonucleotides for c-myc and CDC25A were based upon published sequences and were obtained from Genosys (Houston, Tex.) in partially phosphothiorated form (three linkages at 3′ and 5′ ends). Oligonucleotide sequences were as follows: antisense CDC25A, 5′-GGGCTCGGGCCCAGTTCCAT-3′; antisense c-myc, 5′-AAGCTAACGTTGAGGGGCAT-3′; and nonsense control oligonucleotide, 5′-AGATAGCTTAGTGCGGACGA-3′.
Cell culture and transfections.
MCF-7 cells were a kind gift from R. P. Shiu (13) and were maintained in Dulbecco's minimal essential medium (DMEM) supplemented with antibiotics and 5% FBS. MCF-7/tTA cells stably expressing the Tet transactivator protein were derived by transfection with pTet-off (Clontech, Palo Alto, Calif.) and selection in geneticin. For experiments requiring growth arrest, cells were plated in 60- or 100-mm-diameter dishes and grown to 50 to 60% confluency. Cells were growth arrested by 2 to 3 days of culture in phenol red-free DMEM–0.1% FBS with 10 nM ICI 182,780. This protocol results in arrest of greater than 90% of the cells in G0/G1 as described previously (15, 69). Stock solutions of E2 and ICI 182,780 were prepared in ethanol and added to growth-arrested cultures as indicated in the text. Control cultures received equal amounts of ethanol or dimethyl sulfoxide (DMSO) as vehicle controls where appropriate. MCF-7/MVLN cells (66) were maintained and treated for experiments in the same fashion as MCF-7 cells. MCF-7/MVLN cells are derived from MCF-7 cells and contain a stably integrated vitellogenin A2-luciferase reporter of estrogen-induced transcriptional activity. Plasmid vectors for p16 (pBPSTRI-p16) and cyclin E (pMTcyclin E) were provided by G. Peters (48) and J. M. Roberts (83), respectively. Transfections were carried out on the day of growth arrest with Superfect transfection reagent (Qiagen, Valencia, Calif.).
Viral vectors and infection of MCF-7 cells.
A replication-defective adenoviral vector for expression of p16INK4a (Ad.p16) was constructed in the laboratory of P.S. (8) by homologous recombination in human embryonic kidney 293 cells. Adenoviral backbone (Ad type 5, 9.24-100mu) was cotransfected by calcium phosphate precipitation with shuttle vector pCC2, which contains a p16INK4a expression cassette. Control adenovirus (Ad.Con) and adenoviral vectors expressing p27Kip1 (Ad.p27) and p21Cip1 (Ad.p21) were derived similarly (9). Adenoviral vectors for expression of cyclin E (Ad.cycE), Cdk2 (Ad.Cdk2), the constitutively active caaX mutant of Raf-1 (Ad.Raf-1caaX), and dominant-negative Ras (Ad.RasN17) were kindly provided by J. Nevins (40, 76). Adenoviruses were propagated in 293 cells (American Type Culture Collection), and titers of viral lysates for use in experiments were determined by a standard plaque assay. For experiments with MCF-7 cells, cultures undergoing growth arrest were infected with adenoviral vectors at appropriate multiplicities of infection (MOIs) in phenol red-free DMEM–0.1% FBS. Unless otherwise noted, MOIs were 50 PFU/cell. After infection, the medium was replaced with phenol red-free DMEM–0.1% FBS plus ICI 182,780, and the cultures were incubated for an additional 48 to 72 h before treatment and harvest. A plasmid retroviral vector for Cdc25A was obtained from M. Roussel (78). The retroviral vector was produced by transfection of PT67 cells (Clontech) and selection of producer pools in geneticin. MCF-7 cells were infected with retroviral supernatants in the presence of 4 μg of Polybrene per ml selected in 0.5 mg of geneticin per ml, and surviving colonies were pooled for experiments.
Flow cytometric analysis and thymidine incorporation.
MCF-7 cells were growth arrested and treated as described above. For flow cytometric analysis, cells were harvested in saline-EDTA, fixed in cold 70% ethanol, and stored at −20°C. Fixed cells were subsequently washed, treated with 100 μg of RNase A per ml, and stained with 50 μg of propidium iodide per ml. Analysis of DNA content was performed in a Becton-Dickinson FACScan with a minimum of 15,000 events collected for analysis with Becton-Dickinson Cell Quest software. For flow cytometric analysis of transfected cultures, MCF-7 cells were transfected with pEGFPN1 (Clontech) along with appropriate plasmids (1:5 mass ratio) and treated as for other experiments. Harvested cells were fixed in 0.5% formalin followed by ethanol fixation and propidium iodide staining. At least 30,000 events were collected for analysis of DNA content in the cell fraction exhibiting green fluorescence. Thymidine uptake was performed as described previously (15). Briefly, growth-arrested MCF-7 cultures at 20 to 30% confluency in 24-well plates were treated as described in the text, and 1 μCi of [methyl-3H]thymidine per well (60 Ci/mmol) was added 18 to 20 h after treatment. After allowing 8 to 12 h for uptake, the monolayer was washed twice with ice-cold 5% trichloroacetic acid, solubilized with 0.2 N NaOH, and counted in aqueous scintillant with a Packard β-scintillation counter (Packard Instrument Co., Downers Grove, Ill.).
Western blot analysis.
Cells were lysed as described previously (15). Briefly, following treatment, cell monolayers were washed in ice-cold phosphate-buffered saline and lysed by addition of ice-cold NP-40 lysis buffer (20 mM Tris [pH 7.5], 250 mM NaCl, 0.5% NP-40, 0.1 mM EDTA, 1 mM NaOV4, 10 mM NaF, 10 μg of aprotinin per ml, 10 μg of leupeptin per ml, 1 mM phenylmethyl sulfonyl fluoride). Cells were scraped off of the plates, briefly sonicated, and centrifuged at 15,000 × g for 10 min at 4°C to remove cellular debris. The supernatant was aliquoted and frozen at −80°C for later use. For Western blots, equal amounts of protein (50 to 100 μg) were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to nitrocellulose, and membranes were incubated in blocking buffer (20 mM Tris [pH 7.5], 150 mM NaCl, 0.1% Tween 20, 0.5% casein) for 10 to 15 min. Membranes were incubated with primary antibodies in blocking buffer 2 h at room temperature or overnight at 4°C. All primary antibodies were used at 0.5 μg/ml. Bound antibodies were detected with horseradish peroxidase-conjugated secondary antibodies and chemiluminescent immunodetection (ECL; Amersham). Equal protein loading was verified for all lysate blots by reprobing for actin expression. For Western blots of immunoprecipitated proteins, proteins were immunoprecipitated from 0.5 to 1 mg of cell lysate, and horseradish peroxidase-conjugated protein A/G was used for detection to minimize interference by the precipitating antibodies.
Immune complex kinase assays.
Histone kinase assays were performed essentially as described previously (15). Equal amounts of lysate proteins prepared as described above (100 to 200 μg) were precleared and immunoprecipitated with 1 μg of anti-Cdk2 (M2), anti-Pim-1 (C20), or monoclonal anti-cyclin E (HE111) antibodies, along with protein A/G agarose beads for 4 to 18 h in NP-40 lysis buffer. The immunoprecipitates were washed three times in lysis buffer, washed twice in kinase buffer (20 mM HEPES [pH 7.5], 20 mM MgCl2), and resuspended in kinase buffer supplemented with 400 μg of histones per ml (type III-SS), 10 μM ATP, 1 mM dithiothreitol, 0.5 mM EGTA, 0.2 mM NaOV4, and 5 μCi of [γ-32P]ATP (7,000 Ci/mmol). Kinase reaction mixtures were incubated for 1 h at room temperature. For pRb kinase assays, cell lysates were prepared in Tween 20 lysis buffer as described previously (47), and immunoprecipitations were carried out with anti-Cdk4 (C22) antibodies. Precipitates were washed in Tween 20 lysis buffer, and the kinase reaction mixtures contained 0.2 μg of GST-pRb fusion protein per sample (amino acids 769 to 928). All kinase assays were stopped with 25 μl of 2× SDS-PAGE sample buffer. Reaction products were separated on SDS-PAGE gels, followed by autoradiography with Kodak XAR film and quantification by video densitometry.
Northern blot analysis and luciferase assay.
Total cellular RNA was extracted from 100-mm-diameter dishes by a standard guanidinium isothiocynate procedure. Analysis of mRNA expression was performed following separation of 10 μg of total RNA per lane and transfer to nylon membranes. Membranes were hybridized at high stringency with cDNA probes labeled with [α-32P]dCTP by random primer extension (Decaprime, Ambion, Austin, Tex.). Relative mRNA expression was assessed by densitometry of autoradiograms normalized to β-actin or 18S rRNA expression. For luciferase assay of estrogen-induced transcriptional activity, MCF-7/MVLN cell lysates were prepared and assayed for enzymatic activity with a commercial assay kit (Promega, Madison, Wis.) and measured with a Packard scintillation counter in the single-photon counting mode. Results were obtained from measurements in triplicate and equalized for protein content. E2F-induced transcriptional activity was measured by cotransfection of MCF-7 cultures with a cyclin A promoter-luciferase construct (100) along with pSV-β-gal (Promega) for normalization of transfection efficiency. Cultures were infected with adenoviral vectors, growth arrested, and treated with E2. Lysates were prepared after 20 h and assayed for luciferase and β-galactosidase activity with a commercial assay (Promega).
Assay of Cdk inhibitors, Cdc25A treatment of cyclin E complexes, and Cdc25A assay.
For assay of Cdk inhibitors, active Cdk2 was immunoprecipitated from lysates of E2-treated MCF-7 cells (2 mg of cell lysate) by using 500 ng of anti-Cdk2 antibody and protein A/G agarose beads. Cdk2 precipitates were washed, divided (50 ng of bead-bound antibody per aliquot), and incubated for 1 h at room temperature with lysates (0.2 mg) of cells treated as indicated in the text. After incubation, complexes were washed and assayed for histone kinase activity as described above. Treatment of cyclin E-Cdk2 complexes with purified GST-Cdc25A was carried out by incubating cyclin E immunoprecipitates, prepared from 100 μg of cell lysate, with 2 μg of GST-Cdc25A for 30 min at 30°C in phosphatase buffer (50 mM Tris [pH 8], 150 mM NaCl, 2 mM dithiothreitol, 2.5 mM EDTA). Phosphatase-treated complexes were washed and assayed for histone kinase activity. GST-Cdc25A was purified by affinity chromatography of crude bacterial lysates on glutathione-agarose beads as described previously (17). The assay of endogenous Cdc25A activity in MCF-7 cell lysates was adapted from the method of Galaktionov and Beach (17). Cdc25A was immunoprecipitated from MCF-7 cell lysates (1 to 2 mg) with anti-Cdc25A (N15) and eluted from the washed beads with 0.1 M glycine (pH 2.5). Tyrosine-phosphorylated cyclin B1-Cdc2 complexes precipitated from lysates of hydroxyurea-treated MCF-7 cells were washed and incubated with the eluted Cdc25A for 1 h at 30°C in phosphatase buffer followed by two washes and a standard histone kinase assay. For assay of activity of exogenously expressed Cdc25A, MCF-7/tTA cells were transfected with pBI-HACdc25A (74) along with plasmid constructs for p16INK4a or dominant-negative Cdk2 (92) at a mass ratio of 1:5, followed by growth arrest and treatment. The control plasmid was pcDNA3 (Invitrogen). Cdc25A activity was assayed as described above in anti-HA immunoprecipitates from 100 μg of lysate. For pull-down assays of Cdc25A-associated kinase activity (72), cell lysates (200 μg) were precleared with GST-agarose beads followed by incubation with 8 μg of GST-Cdc25A on glutathione-agarose beads for 2 h at 4°C. The beads were washed and assayed for histone kinase activity as described above. Immunodepletion of lysates before assay was carried out by incubation with 1 μg of the specified antibodies or control immunoglobulin G (IgG) along with protein A/G beads. In vitro treatment of Cdc25A with active Cdk2 was carried out by incubating Cdc25A immunoprecipitates with active, soluble cyclin A-Cdk2 complexes in kinase buffer with 0.1 mM ATP. Cyclin A-Cdk2 was purified on glutathione-agarose beads from lysates MCF-7 cells transfected with a plasmid encoding GST-cyclin A (72) and treated for 24 h with hydroxyurea.
Metabolic labeling and immunoprecipitation.
MCF-7 cells in six-well plates were growth arrested and treated with E2 as required, and at appropriate times, proteins were labeled with [35S]Met/Cys as described previously (15). Cells were starved in phenol red-free, Met/Cys-free DMEM for 20 min at 37°C, followed by labeling in Met/Cys-free medium plus 50 μCi of Tran35S label (1,000 Ci/mmol) for 1 to 2 h at 37°C. Monolayers were washed twice in ice-cold phosphate-buffered saline, and lysates were prepared in NP-40 lysis buffer as described above for Western blots. For immunoprecipitation analysis, lysates were equalized based on protein concentration and precleared with protein A/G agarose beads followed by incubation with 1 μg of the appropriate antibodies (anti-cyclin D1 [HD11], anti-Cdk4 [C22], and anti-Cdc25A [Ab-3]) along with protein A/G agarose beads. After 18 h at 4°C, immunoprecipitates were washed in lysis buffer, suspended in SDS-PAGE sample buffer, boiled, and separated on SDS-PAGE gels. Gels were soaked in 1 M salicylate before drying and fluorography.
RESULTS
Adenoviral transduction of p16INK4a blocks E2-induced G1/S transition, formation of cyclin D1-Cdk4 complexes, and pRb phosphorylation.
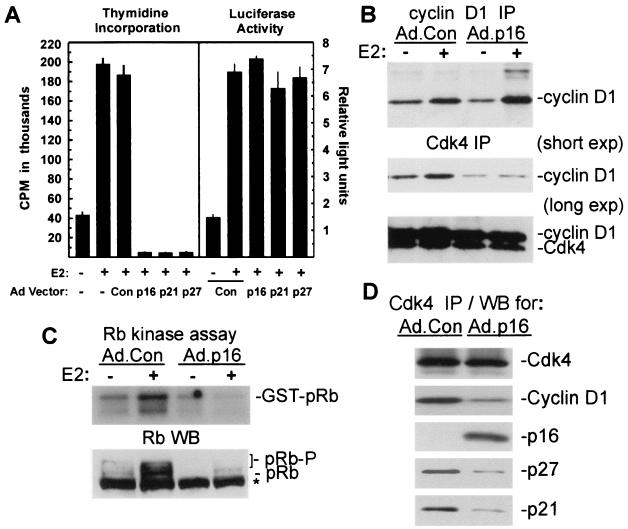
Estrogen treatment of growth-arrested MCF-7 cells elicits cyclin D1 expression, Cdk activation, pRb phosphorylation, and ultimately the onset of DNA synthesis (1, 15, 64, 69). Cell cycle progression in these cells is regulated by cyclin D1 (44, 61, 68, 102), and adenoviral transduction of the Cdk inhibitors p16INK4a, p21Cip1, and p27Kip1 into MCF-7 cells elicits G1 arrest (8, 9). To determine the effects of Cdk inhibitor transduction on E2-induced S-phase entry in MCF-7 cells, cultures were infected with control adenovirus or vectors expressing the appropriate Cdk inhibitors, and growth was arrested by serum withdrawal and antiestrogens. Assays of thymidine uptake 20 h after growth arrest and treatment with 10 nM E2 (Fig. 1A, left panel) demonstrated that DNA synthesis induced by estrogen treatment was completely inhibited in MCF-7 cells transduced with vectors for p16INK4a, p21Cip1, or p27Kip1. Flow cytometric analysis of DNA content confirmed the specific inhibition of E2-induced G1/S transition by transduction of Cdk inhibitors (data not shown). Recent studies have shown that ER-cyclin D1 interactions elicit ER-dependent transcription in ER-positive cells, independent of ligand binding and independent of interactions between cyclin D1 and Cdk4 (57, 103). Transduction of MCF-7/MVLN cells with Ad.p16, Ad.p21, or Ad.p27 had no effect on upon E2-induced transcription from an ERE-luciferase reporter (Fig. 1A, right panel). Thus, while ectopic expression of p16INK4a, p21Cip1, or p27Kip1 provided an effective blockade of G1/S transition induced by estrogen, ligand-induced transcription mediated by the ER was unaffected by Cdk inhibitor expression.
FIG. 1.
Blockade of estrogen-induced G1/S transition in MCF-7 cells by p16INK4a is associated with inhibition of cyclin D1-Cdk4 complex formation and pRb kinase activity. (A, left panel) Cdk inhibitor effects on E2-induced DNA synthesis. MCF-7 cells were infected with adenoviral vectors for p16INK4a, p21Cip1, p27Kip1, or with control adenovirus (MOI = 50), growth arrested, and treated with 10 nM E2 as indicated. Thymidine incorporation was measured from 20 to 32 h after treatment with E2 and is given as the mean ± standard deviation from four replicates. (A, right panel) Analysis of estrogen-induced transcriptional activity in MCF-7/MVLN cells. MCF-7/MVLN cells were infected with the indicated adenoviral vectors (MOI = 50), growth arrested, and treated with 10 nM E2 for 24 h. Results from the luciferase assay are given as relative light units (mean ± standard deviation from three replicates) based on an arbitrary designation. (B) Analysis of de novo-synthesized cyclin D1 and Cdk4. MCF-7 cells were infected with Ad.Con or Ad.p16 (MOI = 50) followed by growth arrest and treatment with 10 nM E2 as indicated. Cultures were labeled for 2 h with Tran35S label, 4 h after estrogen treatment. Immunoprecipitations (IP) were performed with antibodies to cyclin D1 (upper panel) and Cdk4 (lower panel). Two different exposures are given for Cdk4 precipitates. (C) Assay of Cdk4-associated kinase activity. MCF-7 cells were infected with Ad.p16 and Ad.Con (MOI = 50), growth arrested, and treated for 6 h with 10 nM E2 as indicated. Lysates were assayed for Cdk4-associated pRb kinase activity in an immunocomplex assay with GST-pRb as the substrate. (C, lower panel) Western blotting analysis of total pRb phosphorylation: MCF-7 cultures were infected as indicated and treated for 20 h with E2. Western blot analysis of pRb was performed with whole-cell lysates. Slowly migrating, phosphorylated pRb forms are indicated (pRb-P). Nonspecific bands on Western blots are indicated by an asterisk. (D) Analysis of p21Cip1-p27Kip1 association with Cdk4. MCF-7 cells were infected as indicated, followed by growth arrest and a 10-h treatment with 10 nM E2. Proteins in Cdk4 immunoprecipitates were analyzed by Western blotting (WB) analysis for p16INK4a, cyclin D1, p21Cip1, p27Kip1, and Cdk4.
Estrogen treatment elicits formation of active cyclin D1-Cdk4 complexes in MCF-7 cells (1, 15, 64, 69). Specific binding interactions of p16INK4a with Cdk4 and Cdk6 in vivo inhibit complex formation with D-type cyclins and prevent kinase activation (46, 50, 63). We analyzed cyclin D1 synthesis and cyclin D1-Cdk4 complex formation in MCF-7 cells expressing p16INK4a. MCF-7 cells were infected with Ad.Con or Ad.p16 and growth arrested, and de novo-synthesized proteins were labeled from 4 to 6 h after E2 treatment. Estrogen treatment increased cyclin D1 synthesis as described previously (15), and this increase was independent of p16INK4a expression (Fig. 1B). Complex formation between de novo-synthesized cyclin D1 and Cdk4 was increased by E2 in Ad.Con-infected cells, but was markedly inhibited in p16INK4a-expressing cells irrespective of estrogen treatment (Fig. 1B, lower panels). Cdk4 immunoprecipitates from Ad.p16-infected cells also contained detectable p16INK4a on long film exposures (data not shown). Western blotting studies confirmed the equivalent expression of cyclin D1 in lysates of E2-treated MCF-7 and MCF-7/MVLN cells, whether infected with Ad.Con or Ad.p16 (data not shown).
Immunocomplex assays of Cdk4-associated kinase activity in Ad.Con- and Ad.p16-infected MCF-7 cultures demonstrated that estrogen treatment increased Cdk4-associated pRb kinase activity in Ad.Con-infected cells measured 6 h after E2 treatment, while expression of p16INK4a blocked induction of kinase activity (Fig. 1C). Phosphorylation of pRb in vivo was assessed by Western blotting of lysates from cultures treated for 20 h with E2. Estrogen treatment elicited pRb phosphorylation, as demonstrated by the appearance of slower-migrating forms in Ad.Con-infected cells, while phosphorylated forms of pRb were not evident in E2-treated MCF-7 cells expressing p16INK4a (Fig. 1C, lower panel). Cdk4-specific phosphorylation of Ser 780 of pRb (37) was also inhibited by p16INK4a, as shown when blots were reprobed with phosphospecific antibodies to this residue (data not shown).
Binding of p21Cip1 and p27Kip1 to newly formed, intact cyclin D1-Cdk4 complexes leads to sequestration of Cdk-inhibitory activity and is a critical element of cell cycle transit in early G1 (65, 67, 82, 83). Since p16INK4a effectively inhibited formation of cyclin D1-Cdk4 complexes in the studies shown above, the association of p21Cip1 and p27Kip1 with Cdk4 was evaluated by Western blot analysis of Cdk4 immunoprecipitates from control and p16INK4a-expressing MCF-7 cells treated with estrogen. Association of both p21Cip1 and p27Kip1 with Cdk4 was markedly decreased in cells expressing p16INK4a when compared to controls (Fig. 1D), while association of p16INK4a with Cdk4 was readily evident. In these experiments, transduction of MCF-7 cells with p16INK4a again inhibited the association of cyclin D1 with Cdk4.
Expression of p16INK4a inhibits Cdk2 activation in estrogen-treated MCF-7 cells.
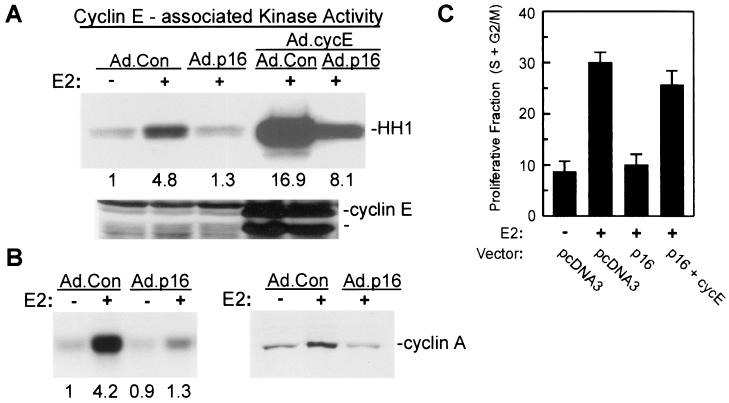
In normal cells, activation of Cdk2 in mid-to-late G1 phase depends upon prior formation of active D cyclin-Cdk4 or -Cdk6 complexes (82, 83). To evaluate cyclin E-Cdk2 activation in p16INK4a-expressing MCF-7 cells, cultures of Ad.Con- and Ad.p16-infected MCF-7 cells were treated with E2 for 10 h and cyclin E immunoprecipitates were assayed for kinase activity. Estrogen treatment activated cyclin E-Cdk2 in Ad.Con-infected cells (Fig. 2A), as has been previously demonstrated (1, 15, 64, 69), while cyclin E-Cdk2 activity was completely inhibited in MCF-7 cells expressing p16INK4a. Cyclin E expression is limiting for G1-phase Cdk2 activation in MCF-7 cells (87) and is regulated by E2F (22). Estrogen treatment does not, however, elicit increased cyclin E expression in MCF-7 cells despite pRb inactivation and E2F release (15, 69). In multiple experiments, expression of endogenous cyclin E was not affected by estrogen treatment or by p16INK4a expression (Fig. 2A) (data not shown). We further tested the extent to which p16INK4a inhibition of cyclin E-Cdk2 activity relates to limiting expression of cyclin E in these cells. Ad.Con- or Ad.p16-infected MCF-7 cultures were coinfected with adenoviral vector for cyclin E. Cyclin E expression and associated kinase activity were elevated in Ad.cycE-transduced cells treated with estrogen (Fig. 2A). Cyclin E-associated kinase activity was again suppressed by coexpression of p16INK4a, yet exceded that in E2-treated control cultures. The data demonstrate that overexpression of cyclin E facilitates formation of active cyclin E-Cdk2 complexes in the context of p16INK4a-mediated blockade of cyclin D1-Cdk4 function.
FIG. 2.
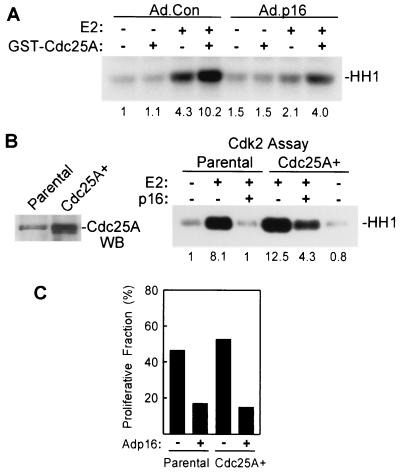
Expression of p16INK4a prevents Cdk2 activation in E2-treated MCF-7 cells. (A) For assay of E2-induced cyclin E-Cdk2 activity, MCF-7 cells were infected with Ad.Con or Ad.p16 (MOI = 50). Additional cultures were coinfected with Ad.cycE as indicated (all MOIs = 50). Cultures were growth arrested and treated with 10 nM E2 for 12 h, and cyclin E-associated histone kinase activity was determined with equal amounts of lysate. The histone band (HH1) is indicated. Relative kinase activities based on densitometry are indicated under the appropriate lanes. Western blot analysis of cyclin E expression in the same lysates is given in the lower panel with identical arrangement of lanes. (B, left panel) Effects of p16INK4a expression on Cdk2-associated kinase activity in MCF-7 cells. MCF-7 cells were infected with the indicated adenoviral vectors, growth arrested, and treated for 20 h with E2. Histone kinase activity was determined with Cdk2 immunoprecipitates. Numbers represent relative kinase activities. (B, right panel) Cyclin A expression in control and p16INK4a-expressing MCF-7 cells was determined by Western blotting of whole-cell lysates from MCF-7 cells infected as indicated and treated for 20 h with E2. (C) Reversal of p16INK4a-mediated G1 arrest by cyclin E. MCF-7/tTA cells were transfected with pEGFPN1 along with control vector (pcDNA3), pBPSTRI-p16 (p16), or pMTcyclin E (cycE). The cultures were growth arrested and treated for 24 h with E2, and the proliferative fraction (S+G2/M) was determined by DNA content analysis of the transfected population as given in Materials and Methods. The results are given as the mean ± standard deviation from three independent experiments.
Cyclin E-Cdk2 activity in MCF-7 cells peaks 10 to 12 h after release of growth arrest (15, 69), and Cdk2 activity is associated more predominantly with cyclin A as MCF-7 cells approach the G1/S border (69). Kinase activity in Cdk2 immunoprecipitates was assayed from MCF-7 cultures transduced with Ad.Con or Ad.p16 and treated for 20 h with E2, and again, Cdk2 activation was inhibited by p16INK4a expression (Fig. 2B, left panel). Cyclin A expression in late G1 is regulated by E2F (10, 75) and is increased by estrogen treatment of growth-arrested MCF-7 cells (69). Expression of cyclin A is inhibited by p16INK4a in U2-OS osteosarcoma cells (48, 53), likely through repression of the cyclin A promoter, which represents one proposed mechanism of cell cycle arrest mediated by active pRb (38). Western blot analysis indicated that cyclin A expression in response to estrogen was inhibited by Ad.p16 transduction of MCF-7 cells (Fig. 2B, right panel). The results in Fig. 2 demonstrate that Cdk2 activation elicited by estrogen treatment of MCF-7 cells requires functional association of cyclin D1-Cdk4 and further suggest that p16INK4a inhibition of Cdk2 activity relates to limiting cyclin expression, particularly that of cyclin A at the G1/S border.
Given the reversal of Cdk2 inhibition evident when p16INK4a-expressing MCF-7 cells were cotransduced with Ad.cycE, we evaluated whether G1/S transition was facilitated in these cells when cyclin E was overexpressed. Overexpression of cyclin E or cyclin A provides relief of p16INK4a-mediated G1 arrest in U2-OS cells (53). For these experiments, MCF-7 cells were transfected with plasmid vector for p16INK4a along with either control plasmid, or a plasmid vector for cyclin E. G1/S transition induced in these cells by E2 was again inhibited by p16INK4a expression, and this inhibition was almost fully reversed upon transfection of cells with vector for cyclin E (Fig. 2C). In separate experiments, transfection of MCF-7 cells with plasmid cyclin E vector fully reversed p16INK4a suppression of activity associated with cotransfected, exogenous HA-Cdk2 (data not shown).
Cdk-inhibitory activity and p21Cip1 and p27Kip1 expression are downregulated late in G1 independent of p16INK4a expression.
Activation of Cdk2, and G1 transit requires elimination of Cdk-inhibitory activity associated with p21Cip1 and p27Kip1 (39, 65, 82, 83). Previous studies indicate that Cdk-inhibitory activity in MCF-7 cells is predominantly attributable to p21Cip1 (64, 69) and that estrogen treatment leads to removal of Cdk inhibition in part through redistribution of p21Cip1 into cyclin D1-Cdk4 complexes (64). Expression of p16INK4a in U2-OS cells prevents Cdk inhibitor sequestration by D cyclin-Cdk4 complexes leading to inhibition of Cdk2 (32, 48, 53). Since the experiments described above demonstrated that cyclin D1-Cdk4 complex formation and Cdk2 activation were effectively inhibited by transduction with Ad.p16, we determined whether inhibition of Cdk2 activity in p16INK4a-expressing MCF-7 cells relates to a failure in elimination of Cdk inhibitors. We performed functional assays of Cdk-inhibitory activity in whole-cell lysates from control and Ad.p16-infected cultures treated with estrogen for a range of times (0 to 20 h). Our results indicated that Cdk-inhibitory activity in MCF-7 lysates was effectively eliminated by 20-h estrogen treatment in both p16INK4a-expressing and nonexpressing cells (Fig. 3A). Downregulation of this activity was delayed, however, in cells expressing p16INK4a, with the majority of Cdk-inhibitory activity still present at 7.5 to 10 h after treatment (Fig. 3A). At these times, Cdk-inhibitory activity was absent, and substantial cyclin E-Cdk2 activity was detected in control MCF-7 cells treated with estrogen (Fig. 3A) (data not shown). The results indicate that 20-h estrogen treatment leads to elimination of Cdk-inhibitory activity in MCF-7 cells independent of sequestration by D cyclin-Cdk4 complexes, while removal of Cdk inhibition at earlier times is effectively prevented by p16INK4a. Thus, in early G1 and during the normal time frame of cyclin E-Cdk2 activation, p16INK4a-mediated blockade of D cyclin-Cdk4 complex formation maintains Cdk-inhibitory activity and prevents Cdk2 activation.
FIG. 3.
Estradiol downregulates Cdk-inhibitory activity and decreases expression of p21Cip1 and p27Kip1 proteins in MCF-7 cells arrested in G1 by p16INK4a expression. (A) Effects of p16INK4a expression on downregulation of Cdk-inhibitory activity in MCF-7 cells. Cdk-inhibitory activity in Ad.Con- and Ad.p16-infected MCF-7 cells was assayed after treatment of growth-arrested cells with 10 nM E2 for the indicated times. Whole-cell lysates were mixed with Cdk2 immunoprecipitates followed by assay of histone kinase activity. Results are presented in graphic form based on densitometric measurements. (B, left panels) Expression of p21Cip1 and p27Kip1 proteins in MCF-7 cells. Cultures were infected with Ad.p16 and Ad.Con (MOI = 50), growth arrested, and treated with 10 nM E2 for 20 h. Lysates were assayed by Western blotting (WB) for expression of p27Kip1, p21Cip1, p16INK4a, and actin as indicated. (B, right panels) Levels of Cdk2, p21Cip1, and p27Kip1 proteins in cyclin E immunoprecipitates (IP) from cultures treated in the same fashion were analyzed by Western blotting. (C) Proteasomal regulation of Cdk inhibitor levels. Ad.p16-infected MCF-7 cells were growth arrested and treated with 10 nM E2 and the specific proteasome inhibitor MG132 (10 μM) as indicated. Lysates prepared after 20 h were analyzed by Western blotting for p21Cip1, p27Kip1, and actin (upper three panels). In the lower panel, cyclin E immunoprecipitates from cultures treated in the same fashion were analyzed for p27Kip1 and p21Cip1 content by Western blotting. (D) Estrogen regulation of ectopic p27Kip1 expression. MCF-7 cells were infected with Ad.p27, growth arrested, and treated for 20 h with E2. Expression of p27Kip1 was determined by Western blotting of whole-cell lysates. Lysates of uninfected, growth-arrested MCF-7 cells (NV) were included for comparison. (E) Western blot analysis of p27Kip1 (T187A) expression in MCF-7 cells. MCF-7 cells were transfected with the HA-p27-T187A vector, growth arrested, and treated with E2 for 20 h. Expression of the p27Kip1 mutant was determined by Western blot analysis of lysates with anti-HA antibodies.
Estrogen treatment of growth-arrested MCF-7 cells leads to decreased expression of both p21Cip1 and p27Kip1 and formation of cyclin E-Cdk2 complexes depleted in content of either Cdk inhibitor (15, 69). We determined whether regulation of p21Cip1 and p27Kip1 expression by E2 is dependent upon cyclin D1-Cdk4 complex formation. Western blotting analysis indicated that steady-state protein levels of p21Cip1 and p27Kip1 declined 50 to 60% in MCF-7 cells treated for 20 h with E2, independent of p16INK4a-induced G1 blockade (Fig. 3B, left panels). In agreement with earlier studies (69), we did not observe any decline in levels of p21Cip1 and p27Kip1 on Western blots until at least 8 h after estrogen treatment, and at 12 h after E2 treatment, 70 to 75% of p21Cip1 and p27Kip1 remained (J. Wimalasena and S. Ahamed, submitted for publication). Cyclin E immunoprecipitates from both Ad.Con- and Ad.p16-infected cultures were depleted of p21Cip1 and p27Kip1 following E2 treatment (Fig. 3B, right panels), and Cdk2 in these complexes was phosphorylated in response to estrogen, as demonstrated by the appearance of faster-migrating forms of the enzyme (25).
In mammalian cells undergoing G1 transit, p27Kip1 protein is subject to ubiquitin-targeted degradation in the proteasome, leading to a shortened half-life for the protein and decreased overall abundance (62, 82). Expression of p21Cip1 is regulated by the proteasome as well (6). Treatment of MCF-7 cells with the specific proteasome inhibitor MG132 along with E2 prevented downregulation of both p21Cip1 and p27Kip1, leading to a 15-fold increase in p21Cip1 levels and an 8-fold increase in p27Kip1 (Fig. 3C). The control protease inhibitor E64 had no effect on levels of p21Cip1 or p27Kip1 in MCF-7 cells (data not shown). Inhibition of proteasome function by MG132 also led to increased p21Cip1 in cyclin E-Cdk2 complexes (Fig. 3C, lower panels), while levels of p27Kip1 in these complexes were unchanged. As described previously (64, 69), we found that Cdk-inhibitory activity in whole-cell lysates of MCF-7 cells was eliminated by immunodepletion with p21Cip1 antibodies (data not shown). Given this apparent intracellular excess of p21Cip1 and the greater extent of p21Cip1 accumulation, a predominance of p21Cip1 in cyclin E-Cdk2 complexes might be expected when proteasome function is inhibited. In further support of proteolytic mechanisms of Cdk inhibitor downregulation, estrogen treatment also decreased expression of ectopic p21Cip1 and p27Kip1 expressed from a cytomegalovirus promoter in MCF-7 cells transduced with Ad.p21 or Ad.p27, as observed for the endogenous proteins (Fig. 3D) (data not shown).
Downregulation of p27Kip1 requires Cdk2-mediated phosphorylation of the protein on Thr 187 (55, 79, 83, 89). Transfection of MCF-7 cells with the epitope-tagged T187A mutant of p27Kip1 and Western blotting analysis of expression of the protein demonstrated that phosphorylation of this residue was required for downregulation of the protein subsequent to estrogen stimulation (Fig. 3E). Epitope-tagged wild-type p27Kip1 expressed in MCF-7 cells was downregulated by estrogen treatment, as observed above with endogenous and ectopic p27Kip1 (data not shown). The T187A mutant of p27Kip1 accumulated in cells treated with MG132, indicating that steady-state levels of the protein are nonetheless regulated to some extent by proteasomal degradation. Together the results in Fig. 2 and 3 suggest that estrogen treatment of MCF-7 cells leads to elimination of Cdk-inhibitory activity in two phases. The early phase (0 to 8 h) is dependent upon sequestration of the Cdk inhibitors by D cyclin-Cdk4 complexes and mediates rapid removal of Cdk-inhibitory activity allowing activation of cyclin E-Cdk2 in mid-G1. At later times following estrogen administration (8 to 20 h), Cdk inhibitors are subject to degradation mediated via the proteasome, a mechanism of Cdk inhibitor removal independent of cyclin D1-Cdk4 function. Furthermore, downregulation of p27Kip1 in MCF-7 cells requires phosphorylation at Thr 187, although this occurs under conditions of only minimal Cdk2 activation.
Estrogen induces Cdc25A expression in MCF-7 cells.
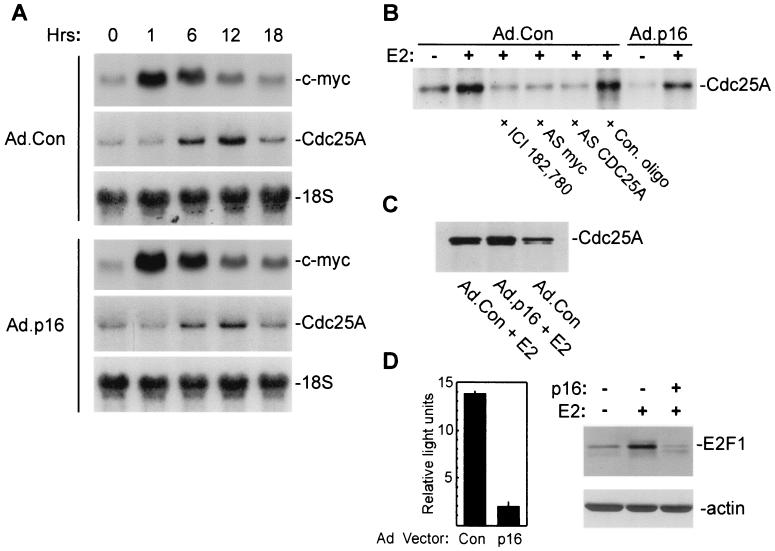
Cdc25A is required for S-phase entry and activates Cdk2 in vitro through removal of inhibitory phosphorylation (25, 27, 33). Our studies indicated that Cdk2 was phosphorylated after estrogen treatment in p16INK4a-expressing MCF-7 cells, yet remained inactive (Fig. 3). Hence, we wished to ascertain the extent of Cdc25A expression in estrogen-treated cells. Cdc25A is a downstream target of Myc-induced transactivation (18, 74). Transcription of c-myc is rapidly induced by E2 in MCF-7 cells (11, 12), and increased expression of Cdc25A protein in estrogen-treated MCF-7 cells has been reported (96). In preliminary studies, estrogen induced c-myc mRNA expression in both Ad.Con- and Ad.p16-infected cells, with peak expression 1 h after treatment (Fig. 4A). Estrogen treatment also increased the expression of Cdc25A mRNA in both control and p16INK4a-expressing MCF-7 cells, with maximal expression by 10 to 12 h (Fig. 4A). Metabolic labeling of de novo-synthesized proteins from 10 to 12 h after E2 treatment demonstrated increased synthesis of Cdc25A (Fig. 4B), which was completely inhibited by cotreatment of cultures with the steroidal antiestrogen ICI 182,780. Prior treatment of MCF-7 cultures with antisense DNA oligonucleotides directed to the translation-initiation regions of either c-myc or CDC25A also inhibited E2-induced Cdc25A protein synthesis, while control oligonucleotides and p16INK4a transduction had no effect (Fig. 4B). Western blot analysis of Cdc25A immunoprecipitates demonstrated an increase in total Cdc25A protein expression induced in MCF-7 cells by E2 irrespective of p16INK4a-expression (Fig. 4C). In addition to Myc, Cdc25A expression is also regulated by transcription factors of the E2F family through transactivation by free E2F-1 and repression or derepression mediated by E2F-pocket protein complexes (7, 19, 30, 93). Functional analysis of E2F transcriptional activity in estrogen-treated MCF-7 cells demonstrated that E2F activity generated following estrogen treatment is largely inhibited by p16INK4a expression (Fig. 4D, left panel). In addition to eliciting E2F activity through Cdk activation and pRb phosphorylation, estrogen treatment of MCF-7 cells induces E2F-1 expression (95). Western blot analysis confirmed that E2 treatment increased E2F-1 protein expression (fourfold) in control MCF-7 cells (Fig. 4D, right panels); however, transduction with p16INK4a yielded marked suppression of E2F-1 expression. The results in Fig. 4 demonstrate that Cdc25A is expressed in response to E2 in control and p16INK4a-expressing MCF-7 cells and would further indicate that Cdc25A induction in cells transduced with p16INK4a occurs in the presence of minimal E2F activity.
FIG. 4.
Estradiol induces expression of Cdc25A mRNA and protein in MCF-7 cells. (A) MCF-7 cells were infected with Ad.p16 or Ad.Con (MOI = 50), growth arrested, and treated for the indicated times with 10 nM E2. Total RNA was analyzed for expression of c-myc and Cdc25A mRNA by Northern blotting. Membranes were reprobed for 18S rRNA as a loading control. (B) Cdc25A protein synthesis. Ad.p16- and Ad.Con-infected MCF-7 cultures were growth arrested and treated with 10 nM E2 or 10 nM E2 plus 500 nM ICI 182,780 as indicated. Cultures were labeled with Tran35S-label from 10 to 12 h after treatment. Control (Con.), antisense CDC25A, or c-myc antisense (AS) oligonucleotides (oligo) (10 μM) were added to the culture medium 18 h before treatment and remained in the culture until labeling. Equal amounts of protein lysates were immunoprecipitated with monoclonal anti-Cdc25A (Ab-3) antibodies and analyzed by SDS-PAGE. (C) Western blot analysis of Cdc25A expression. MCF-7 cultures were infected with Ad.Con or Ad.p16, growth arrested, and treated for 20 h with E2 as indicated. Cdc25A was immunoprecipitated with monoclonal anti-Cdc25A (Ab-3) and analyzed by Western blotting with anti-Cdc25A (N15). (D, left panel) E2F-dependent transcriptional activity was measured in lysates of Ad.Con- and Ad.p16-infected cells 30 h after treatment with E2 as given in Materials and Methods. Values are given as relative light units (mean ± standard deviation from three replicates). Values given represent estrogen-induced transcription, with activity in untreated cells subtracted. (D, right side panels) Western blot analysis of E2F-1 and actin expression in whole-cell lysates of MCF-7 cells treated as described above in panel C.
Cdc25A regulates estrogen-induced Cdk2 activation and G1/S transit in vivo, but is inactive in p16INK4a-expressing MCF-7 cells.
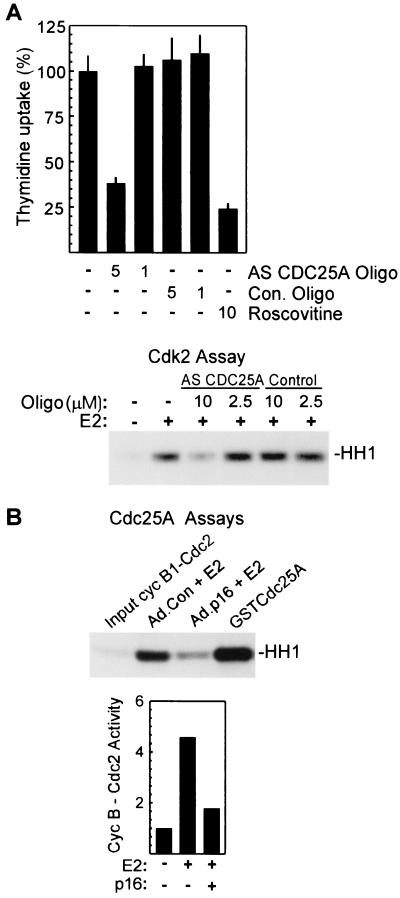
Cdc25A is rate limiting for G1 transit (3, 78), and microinjection of anti-Cdc25A antibodies inhibits S-phase entry in vivo (17, 27, 33). In experiments to clarify the in vivo role of Cdc25A in MCF-7 cells, antisense CDC25A oligonucleotides, which effectively inhibit synthesis of the protein (Fig. 4B), were found to inhibit estrogen-induced DNA synthesis, while control oligonucleotides had no inhibitory effect (Fig. 5A, upper panel). In agreement with earlier studies (68), the Cdk2 inhibitor roscovitine also inhibited DNA synthesis induced by estrogen. Cdc25A activates Cdk2 in vitro (25) and promotes G1 transit in vivo through Cdk2 activation as well (3, 27, 33). In direct support of an in vivo role for Cdc25A in the activation of Cdk2 in MCF-7 cells, cyclin E-dependent Cdk2 activity measured 12 h after estrogen treatment of growth-arrested MCF-7 cells was specifically inhibited by antisense CDC25A oligonucleotides (Fig. 5A, lower panel).
FIG. 5.
Cdc25A is required for estrogen-induced Cdk2 activation and DNA synthesis in MCF-7 cells and is inactive in cells expressing p16INK4a. (A, upper panel) Inhibition of S-phase entry by antisense (AS) CDC25A oligonucleotides (Oligo). DNA synthesis in E2-treated MCF-7 cells was assayed as given above in Fig. 1. Antisense oligonucleotides were added to the culture medium at the indicated concentrations 18 h before estrogen treatment and remained in the culture throughout the assay. Values represent percent thymidine incorporation relative to that in estrogen-treated control (Con.) cultures and are given as the mean ± standard deviation from four replicates. (A, lower panel) Inhibition of Cdk2 activation by antisense CDC25A. MCF-7 cells were treated with antisense CDC25A or control oligonucleotides as described above, and lysates prepared 12 h after E2 treatment were assayed for cyclin E-associated histone kinase activity. (B) Assay of endogenous Cdc25A activity. Cdc25A immunoprecipitates were prepared from MCF-7 cultures treated as indicated, and Cdc25A was eluted from the beads as described in Materials and Methods. The function of eluted Cdc25A was assayed as activation of cyclin B1-Cdc2 complexes measured in a standard histone kinase assay. In lane 4, maximal activation is demonstrated by treatment of complexes with purified recombinant GST-Cdc25A. Results from a separate experiment are presented graphically in the lower portion of panel B, with activity in lysates of untreated, Ad.Con-infected cells taken as 1.
Previous studies suggest that Cdc25A is phosphorylated and activated in vivo by cyclin E-Cdk2 in late G1 (27). To determine if Cdc25A expressed in estrogen-treated MCF-7 cells is fully active, Cdc25A was immunoprecipitated from lysates of estrogen-treated control and p16INK4a-expressing cells and assayed for activation of cyclin B1-Cdc2 complexes (3, 17). In multiple experiments, Cdc25A activity was markedly lower in p16INK4a-expressing cells than in Ad.Con-infected cells (Fig. 5B). Equal amounts of Cdc25A were precipitated from E2-treated cells irrespective of p16INK4a expression (Fig. 4C) (data not shown). In control experiments, Cdc2-activating activity in Cdc25A immunoprecipitates was inhibited by sodium orthovanadate and by blocking peptide included in the immunoprecipitation reaction (data not shown). As a further control, purified recombinant GST-Cdc25A was found to readily activate cyclin B1-Cdc2 complexes in the assay (Fig. 5B). In agreement with previous studies (3, 27), Cdc25A activity was largely absent in growth-arrested cultures (Fig. 5B, lower panel). These results would indicate that Cdc25A is largely inactive in MCF-7 cells transduced with p16INK4a, despite increased expression of the protein in response to estrogen, and might suggest a failure to activate Cdc25A in vivo in these cells.
Inactive cyclin E-Cdk2 complexes from p16INK4a-expressing cells are activated in vitro by Cdc25A.
Given our results in Fig. 5 indicating that Cdc25A is inactive in p16INK4a-expressing cells, we determined if cyclin E-Cdk2 complexes in p16INK4a-expressing MCF-7 cells were inactive due to inhibitory phosphorylation and, as such, could be activated in vitro by Cdc25A phosphatase (64). Cyclin E immunoprecipitates from control and p16INK4a-expressing MCF-7 cells were incubated with purified, recombinant GST-Cdc25A before assay of kinase activity. Cyclin E-Cdk2 from Ad.p16-infected, E2-treated cells was activated by treatment with Cdc25A (Fig. 6A), yielding histone kinase activity similar to that in complexes from estrogen-treated control cultures (relative activity of 4.0 versus 4.3). Active cyclin E-Cdk2 complexes from E2-treated, Ad.Con-infected cultures were further activated by treatment with Cdc25A (activity of 10.2). As reported previously (64), Cdc25A phosphatase did not activate cyclin E-Cdk2 complexes from untreated MCF-7 cultures, irrespective of p16INK4a expression, perhaps owing to the presence of Cdk inhibitors in the complexes and/or a lack of activating phosphorylation. Inclusion of sodium orthovandate in the in vitro phosphatase reaction completely inhibited activation of cyclin E-Cdk2 complexes by GST-Cdc25A (data not shown). The results indicate that a substantial fraction of cyclin E-Cdk2 complexes elicited in both control and p16INK4a-expressing MCF-7 cells remain inactive due to inhibitory phosphorylation. Following treatment in vitro with Cdc25A, the active fraction of Cdk2 is increased irrespective of in vivo p16INK4a expression, yet remains greater when derived from cells not expressing p16INK4a.
FIG. 6.
Cyclin E-Cdk2 complexes from p16INK4a-expressing MCF-7 cells are activated in vitro and in vivo by Cdc25A. (A) Activation of cyclin E-Cdk2 complexes by Cdc25A in vitro. MCF-7 cells were infected with Ad.Con or Ad.p16, growth arrested, and treated for 20 h with E2 as indicated. Cyclin E immunoprecipitates were incubated with GST-Cdc25A and assayed for histone kinase activity. Relative kinase activities from densitometric measurements are given beneath the figure. (B) Effect of in vivo overexpression of Cdc25A on Cdk2 activity in MCF-7 cells. (Left panel) Cdc25A expression was assayed in parental MCF-7 cells and in cells transduced with retroviral vector for Cdc25A. Western blot analysis of Cdc25A immunoprecipitates from proliferating parental and Cdc25A-overexpressing cells is shown. (Right panel) Cdk2-associated kinase activity was assayed in lysates of parental and Cdc25A-overexpressing MCF-7 cells infected with Ad.Con or Ad.p16. Cultures were growth arrested and treated for 20 h with 10 nM E2 as indicated. Relative activity based on densitometry is provided beneath each lane. (C) Flow cytometric analysis of estrogen-induced S-phase entry in parental and Cdc25A-overexpressing MCF-7 cells is given. Parental and Cdc25A-overexpressing MCF-7 cells were infected with Ad.Con or Ad.p16 as indicated and growth arrested, and the proliferative fraction (S+G2/M) was determined 24 h after E2 treatment by flow cytometric analysis of DNA content.
In vivo overexpression of Cdc25A leads to early activation of Cdk2 and accelerated S-phase entry in HeLa cells and NIH 3T3 fibroblasts (3, 78). MCF-7 cells transduced with retroviral Cdc25A expression vector overexpressed Cdc25A protein 8- to 10-fold (Fig. 6B). These cells exhibited elevated functional Cdc25A activity, yet did not exhibit accelerated G1/S transition or early activation of cyclin E-Cdk2 complexes, which would indicate that following estrogen stimulation, Cdc25A activity is not limiting in MCF-7 cells (data not shown). When the effects of p16INK4a expression on Cdk2 activation were assessed in these cells, transduction with Ad.p16 inhibited Cdk2 activation in parental MCF-7 cells as before, but inhibited that in Cdc25A-overexpressing cells to a lesser degree (Fig. 6B). Cdk2 activity was higher overall in Cdc25A-overexpressing cells. In flow cytometric assays of S-phase entry after estrogen treatment, however, in vivo overexpression of Cdc25A provided no relief from the G1/S blockade afforded by p16INK4a transduction (Fig. 6C). The results indicate that despite the increase in Cdk2 activity provided by Cdc25A overexpression, the degree of activation achieved is nonetheless insufficient for G1/S transition. Together the results in Fig. 5 and 6 lend support to an in vivo role for Cdc25A in Cdk2 activation and G1/S transit induced by estrogen in MCF-7 cells. The results also indicate that a population of Cdk2 in p16INK4a-expressing MCF-7 cells can be activated in vitro and in vivo by Cdc25A. Cdk2 inhibition and cell cycle arrest in these cells are, however, a result of mechanisms of which Cdc25A is only a part.
Cdc25A activity is regulated in vivo by p27Kip1 expression and Cdk2 activity.
Cdc25A interacts with cyclin E-Cdk2 complexes through a cyclin-binding domain similar to that in Cip and Kip Cdk inhibitors and competes with p21Cip1 for cyclin-Cdk2 binding (72). Activation of Cdc25A has been related to this interaction with cyclin-Cdk complexes in vitro and in vivo (17, 27). To further investigate the mechanisms of Cdc25A activation in MCF-7 cells, we sought to determine whether p27Kip1 overexpression had any effect on Cdc25A activation. As shown in Fig. 7A, Cdc25A activity was inhibited in MCF-7 cells transduced with Ad.p27, similar to effects seen in Ad.p16-infected cells. Assay of Cdk2 activity in the same lysates confirmed that p27Kip1 overexpression effectively inhibited Cdk2 in these cells, similar to the effects seen with Ad.p16 transduction (Fig. 7A). MG132, which effectively inhibited downregulation of p21Cip1 and p27Kip1 (Fig. 3), inhibited Cdc25A activation as well (data not shown). These results would indicate that deregulated expression of Cdk inhibitors inhibits Cdc25A activation in MCF-7 cells, whether occurring as a result of ectopic overexpression, through inhibition of proteasome function, or as observed in cells where cyclin D1-Cdk4 function is inhibited by overexpression of p16INK4a.
FIG. 7.
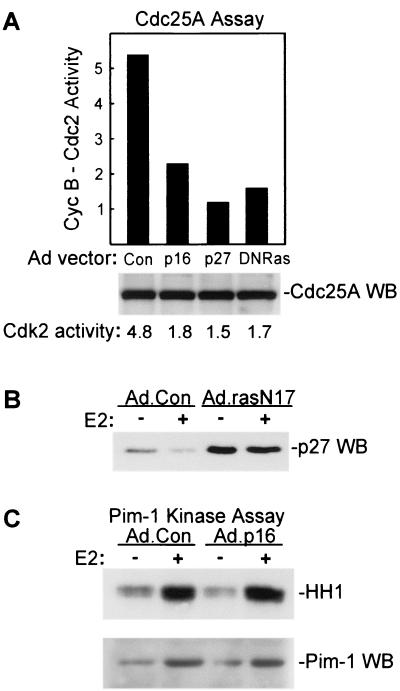
Cdc25A activation in vivo is inhibited by p27Kip1 and by dominant-negative Ras. (A, top panel) Effects of transduction with Ad.p16, Ad.p27, and Ad.RasN17 on generation of Cdc25A activity in MCF-7 cells. MCF-7 cells were infected with Ad.Con, Ad.p16, Ad.RasN17, or Ad.p27 as indicated, and Cdc25A activity was assayed following growth arrest and a 20-h estrogen treatment. Results are presented in graph form with input cyclin B1-Cdc2 activity taken as a value of 1. (A, lower panel) Western blotting (WB) analysis of Cdc25A expression in MCF-7 cells. Cdc25A was immunoprecipitated from 600 μg of the same lysates assayed above. Relative Cdk2 activities assayed in the same lysates are given below the panel based on densitometric analysis. (B) Expression of p27Kip1 was assayed by Western blotting of lysates from MCF-7 cells infected with Ad.Con and Ad.rasN17 vectors following growth arrest and 20-h treatment with estrogen as indicated. (C) Pim-1-associated histone kinase activity (upper panel) was determined as given in Materials and Methods with lysates of control and Ad.p16-infected MCF-7 cells 20 h after E2 treatment. Western blot analysis of Pim-1 protein expression in the same lysates is given in the lower panel.
Catalytic activity of Cdc25A is increased in vitro upon phosphorylation by cyclin E-Cdk2 (27), Raf-1 (19), and Pim-1 (54). Recent studies indicate that E2 activates the Ras-Raf-Erk pathway (5, 70), and our own studies have demonstrated that adenoviral transduction of a dominant-negative Ras mutant (Ad.RasN17) inhibits E2-induced Cdk2 activation and G1/S transition in MCF-7 cells (J.W., unpublished data). We examined the effects of Ad.RasN17 transduction on Cdc25A activation in vivo, and as depicted in Fig. 7A, dominant-negative Ras inhibited Cdc25A activity without influencing expression of the protein. Cdk2 activity was inhibited by Ad.RasN17 transduction as well. Ras activity is required for p27Kip1 downregulation (34, 88), and dominant-negative Ras expression led to an accumulation of p27Kip1 in both control and estrogen-treated cultures (Fig. 7B). Previous studies and our own unpublished results indicate that growth factor treatment of MCF-7 cells leads to more prolonged and pronounced activation of the Ras-Raf-Erk pathway than the transient activation observed with estrogen treatment alone (44, 70). Cdc25A activity was inhibited by p16INK4a, however, when MCF-7 cells were treated with estrogen and growth factors in combination, as was observed in cultures treated only with estrogen (data not shown). Our studies thus suggest some role for the Ras–Raf-1 pathway in estrogen-induced activation of Cdc25A. While this potentially involves direct activation of Cdc25A by interaction with active Raf-1, the results may reflect a requirement for Ras in p27Kip1 downregulation and subsequent activation of Cdk2.
Pim-1 kinase is expressed at the G1/S border in a wide variety of cells (42) and activates Cdc25A in vitro (54). We examined whether Cdc25A activity in MCF-7 cells was related to expression and/or activity of Pim-1 in control and p16INK4a-expressing MCF-7 cells. Estrogen treatment of growth-arrested cells elicited increased Pim-1-associated histone kinase activity, and this was associated with increased expression of the protein (Fig. 7C). Increased Pim-1 expression and kinase activity were evident in both control and p16INK4a-expressing cells, which would not suggest any relationship between Pim-1 activity and the relative inactivity of Cdc25A in cells transduced with p16INK4a.
Cdk2 activates Cdc25A in vivo and in vitro.
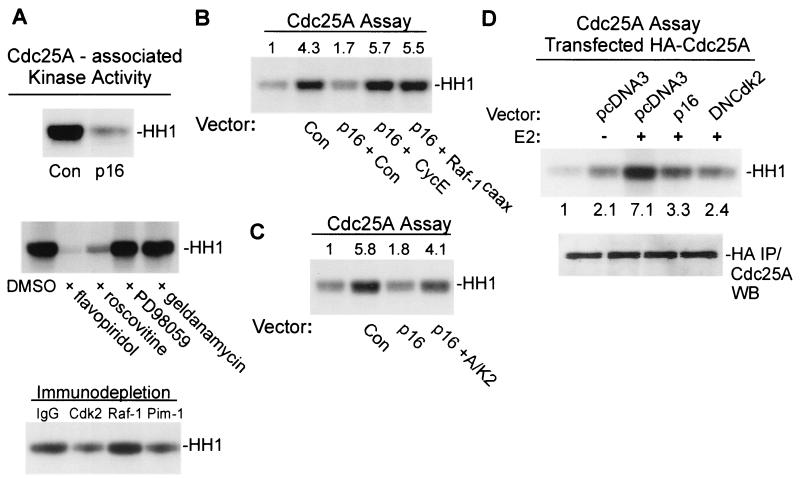
Active cyclin E-Cdk2 complexes associate with Cdc25A in vitro and with ectopic Cdc25A in vivo (72). To our knowledge, no study has, as yet, demonstrated in vivo association of endogenous Cdc25A and cyclin-Cdk complexes. To assess interactions of Cdc25A and potential in vivo activators in MCF-7 cells, kinase activity associated with Cdc25A in vitro was measured in pull-down kinase assays using beads coated with recombinant Cdc25A and lysates from control and p16INK4a-transduced MCF-7 cells treated for 12 h with E2 (mid-G1). Histone kinase activity bound to Cdc25A was readily demonstrable in lysates of estrogen-treated cells, but was greatly diminished in cells expressing p16INK4a (Fig. 8A, top panel). No kinase activity was associated with beads coated only with GST (data not shown). Kinase activity associated with Cdc25A was almost completely abolished by addition of flavopiridol and roscovitine to the in vitro assay (Fig. 8A, middle panel). As controls, PD98059, a specific MEK inhibitor, and geldanamycin, an inhibitor of tyrosine kinases, were found to have no effect on Cdc25A-associated kinase activity in vitro. Immunodepletion of MCF-7 lysates with specific antibodies to Cdk2 and Pim-1 decreased kinase activity associated with Cdc25A, while antibodies to Raf-1 had no effect (Fig. 8A, bottom panel). These results indicate that the active Cdk2 and Pim-1 elicited in estrogen-treated MCF-7 cells can interact with Cdc25A in vitro and would support a potential role for these regulators in Cdc25A activation in vivo.
FIG. 8.
Cdc25A is activated in vivo by Cdk2. (A) Analysis of Cdc25A-associated kinase activity. (A, top panel) Control-infected and Ad.p16-infected MCF-7 cells were growth arrested and treated for 12 h with E2. Lysates were precleared and incubated with GST-Cdc25A-coated beads, and histone kinase activity associating with Cdc25A was measured as given in Materials and Methods. (A, middle panel) The in vitro assay of Cdc25A-associated kinase activity was carried out with the addition of flavopiridol, roscovitine, PD9059, or geldanamycin (all 2.5 μM). DMSO was used as a solvent control. Lysates were from estrogen-treated MCF-7 cells. (A, bottom panel) Immunodepletion analysis of Cdc25A-associated kinase activity is shown. Lysates of estrogen-treated MCF-7 cells were subjected to immunodepletion with the indicated specific antibodies or control goat IgG before incubation with Cdc25A beads and assay. (B) Reversal of Cdc25A inhibition in vivo. MCF-7 cells were infected with Ad.Con and Ad.p16 vectors along with Ad.Con, Ad.cycE, or Ad.Raf-1caax as indicated. Cdc25A activity was assayed following growth arrest and a 20-h treatment with E2. (C) Reactivation of Cdc25A in vitro. Cdc25A immunoprecipitates from lysates of estrogen-treated Ad.p16-infected MCF-7 cells prepared as described above were incubated in vitro with soluble, active cyclin A-Cdk2 complexes as described in Materials and Methods and assayed for activation of cyclin B1-Cdc2 complexes. Activity in Cdc25A immunoprecipitates from Ad.Con-infected cells is given for comparison. In panels C and D, relative activity based on densitometry is given above the respective lanes. (D) Activation and inhibition of ectopic Cdc25A activity. MCF-7/tTA cells were transfected with pBI-HACdc25A along with control plasmid (pcDNA3), pBPSTRI-p16 (p16), or dominant-negative Cdk2 vector (DNCdk2). Following growth arrest and E2 treatment (20 h), Cdc25A activity was assayed in anti-HA immunoprecipitates as given in Materials and Methods. Relative activity is given under each lane. In the lower panel, equal expression of HA-Cdc25A was verified by anti-Cdc25A immunoblot analysis of anti-HA immunoprecipitates prepared from the same lysates.
Cdc25A is inactive in MCF-7 cells where Cdk2 is rendered inactive by transduction with p16INK4a, p27Kip1, or dominant-negative Ras (Fig. 5 and 7). In light of these results and since active Cdk2 from E2-treated MCF-7 cells associates with Cdc25A in vitro (Fig. 8A), we determined if Cdc25A activity in p16INK4a-expressing MCF-7 cells could be restored in vivo or in vitro by Cdk2. To examine this, MCF-7 cells were infected with Ad.p16 along with Ad.Con, Ad.cycE, or adenoviral vector expressing constitutively active Raf-1caax, and Cdc25A activity was assayed after growth arrest and E2 treatment. Ad.cycE transduction increases Cdk2 activity in p16INK4a-expressing MCF-7 cells (Fig. 2) and led to generation of Cdc25A activity in these cells comparable to that in Ad.Con-infected cells (Fig. 8B). Expression of the Raf-1 mutant also activated Cdc25A in p16INK4a-expressing cells. In vitro treatment of Cdc25A immunoprecipitates from p16INK4a-expressing cells with soluble, active cyclin A-Cdk2 also increased activity to levels similar to those in immunoprecipitates from Ad.Con-transduced cells (Fig. 8C).
To test directly whether in vivo inhibition of Cdk2 activity leads to suppression of Cdc25A activity, MCF-7 cells were transfected with HA-tagged Cdc25A along with vectors for p16INK4a or a kinase-inactive, dominant-negative Cdk2 mutant. Assays of Cdc25A activity in HA immunoprecipitates demonstrated that activity of the exogenous enzyme was increased following E2 treatment (Fig. 8D) and furthermore demonstrated that Cdc25A activation was inhibited by dominant-negative Cdk2. Transfection with p16INK4a inhibited activation of ectopic Cdc25A as well. Our results in Fig. 7 and 8 indicate that the inability of estrogen to generate full Cdc25A activity in MCF-7 cells under conditions of G1 blockade enforced by p16INK4a expression stems from low levels of Cdk2 activity. Cdk2 inhibition is, in turn, associated with inhibition of p21Cip1-p27Kip1 sequestration in p16INK4a-expressing cells, as well as a lack of cyclin A expression late in G1. Full activity of Cdc25A in vivo thus requires activation of cyclin-Cdk complexes and the availability of these complexes for interaction with Cdc25A.
DISCUSSION
Our studies demonstrate that estrogens promote cell cycle progression in MCF-7 cells at multiple points within the machinery governing G1/S transition (see the schematic in Fig. 9). Using adenoviral transduction of p16INK4a to provide G1 blockade, we have shown that estrogen regulates expression of Cdk inhibitors and induces expression of Cdc25A and that regulation at this level is independent of D cyclin-Cdk4 function. The data show that formation of ternary complexes between cyclin D1-Cdk4 and p21Cip1 and p27Kip1 is an essential aspect of estrogen action in G1, since expression of p16INK4a in MCF-7 cells completely inhibited S-phase entry induced by E2 treatment (Fig. 1). Blockade of cyclin D1-Cdk4 association by p16INK4a prevented sequestration of p21Cip1 and p27Kip1, inhibited cyclin A induction by preventing pRb inactivation and E2F release, and led to abolition of Cdk2 activity (Fig. 2). As a consequence of Cdk2 inhibition, activation of Cdc25A in vivo was inhibited as well (Fig. 5, 7, and 8).
FIG. 9.
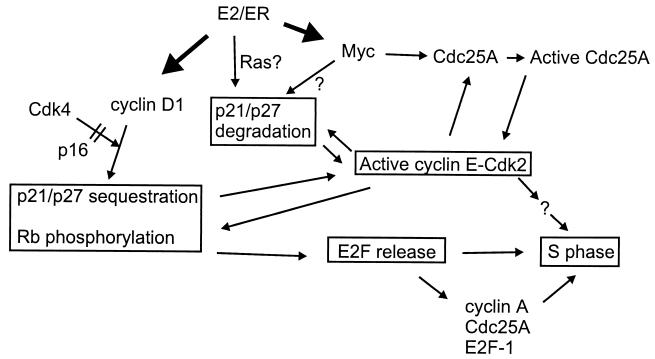
Schematic model of estrogen-mediated promotion of S-phase entry. Transcriptional activation of Myc and cyclin D1 expression in early G1 (dark arrows) facilitates cyclin E-Cdk2 activation in mid-to-late G1- and S-phase entry. Expression of cyclin D1 and complex formation with Cdk4 leads to sequestration of p21Cip1 and p27Kip1 Cdk inhibitors and initiates phosphorylation and inactivation of pocket proteins, including pRb. Conversely, expression of p16INK4a prevents cyclin D1-Cdk4 association, delays removal of Cdk-inhibitory activity, and effectively inhibits pocket protein phosphorylation and release of E2F transcription factors. Estrogen downregulates expression of both p21Cip1 and p27Kip1 independent of cyclin D1-Cdk4 function and at least in part through the proteasome. It is not yet clear to what extent this is related to estrogen-induced Myc expression, Ras activation, or induction of as-yet-unidentified mediators. Myc further participates in cyclin E-Cdk2 activation by eliciting Cdc25A expression. As suggested in earlier studies, full activation of both Cdc25A and Cdk2 hinges upon interaction and mutual activation between these two regulators. Ultimately, active cyclin E-Cdk2 likely elicits S-phase entry both through contribution to pocket protein phosphorylation and E2F release and through phosphorylation of additional, unknown mediators of S-phase entry. Upon inactivation of pocket proteins, derepression at E2F-dependent promoters and consequent induction of cyclin A, Cdc25A, and E2F-1 provides further reinforcement for G1/S transition and progression through the S phase.
Three previous studies in U2-OS osteosarcoma cells associated Cdk2 inhibition and G1 arrest by p16INK4a with inhibition of D cyclin-Cdk4 complex formation, redistribution of Cdk inhibitors from D cyclin-Cdk4 into cyclin E-Cdk2 complexes, and cyclin A repression (29, 32, 53). In our studies, ectopic p16INK4a was found in association with Cdk4, inhibited formation of complexes between Cdk4 and cyclin D1, and prevented association of p21Cip1 and p27Kip1 proteins with these complexes (Fig. 1). Our studies essentially agree with those with U2-OS cells in that p16INK4a expression in MCF-7 cells caused delayed removal of Cdk-inhibitory activity in early G1 (0 to 8 h after E2 treatment, Fig. 3), leading to inhibition of cyclin E-Cdk2 activation. In contrast, our studies show that downregulation of Cdk-inhibitory activity was evident in both control and p16INK4a-expressing cells 20 h after estrogen administration. Cdk inhibitor downregulation at this time was associated with decreased expression of p21Cip1 and p27Kip1, required proteasomal action, and was reflected in decreased p21Cip1 and p27Kip1 content of cyclin E-Cdk2 complexes (Fig. 3). This suggests a functional dissociation of Cdk inhibitor sequestration in early G1 and downregulation through protein degradation in the proteasome in mid to late G1. It is not clear at this time to what extent these particular observations are specific to MCF-7 cells. Studies with U2-OS cells have utilized asynchronous cell populations (29, 32, 53), which might not allow for discrimination of Cdk inhibitor regulation in early and late G1. The relative contributions of complex formation and sequestration and protein degradation to removal of the Cdk-inhibitory threshold associated with p21Cip1 and p27Kip1 are not known at this time. As with p16INK4a-transduced cells, MCF-7 cells treated with MG132 do not exhibit active Cdk2, nor do they enter S phase (Fig. 1 and 2) (data not shown). Thus, Cdk inhibitor regulation at the level of protein degradation would appear to be necessary, though insufficient, for G1/S transition in MCF-7 cells. Our results would suggest that sequestration and degradation of Cdk inhibitors are requisite and complementary mechanisms facilitating Cdk2 activation in MCF-7 cells.
Our observation that inactive cyclin E-Cdk2 complexes in p16INK4a-expressing MCF-7 cells were phosphorylated (Fig. 3) led us to investigate Cdc25A expression and function in MCF-7 cells. Expression of Cdc25A is transcriptionally regulated by Myc (18, 74) and E2F-1 (7, 19, 30, 93), both of which are expressed in MCF-7 cells in response to estrogen (11, 12, 95). Estrogen treatment of MCF-7 cells induced Cdc25A expression independent of p16INK4a-induced G1 blockade (i.e., where pRb remained in the hypophosphorylated state and both E2F-1 expression and functional E2F activity were minimal) (Fig. 1 and 4). Synthesis of Cdc25A protein induced by E2 was inhibited by c-myc antisense oligonucleotides (Fig. 4); thus, Cdc25A expression in these cells would appear to be a downstream effect of estrogen-induced Myc expression.
The activity of cyclin E-Cdk2 complexes from p16INK4a-expressing MCF-7 cells was increased by in vitro treatment with recombinant Cdc25A, although the levels of activity did not equal that of enzyme-treated complexes derived from control cultures (Fig. 6). This indicates that at least some portion of cyclin E-Cdk2 complexes in p16INK4a-expressing cells were inactive due to inhibitory phosphorylation. In vivo overexpression of Cdc25A partially restored Cdk2 activation in p16INK4a-expressing cells as well, although no relief of the p16INK4a-mediated blockade of G1/S transition was provided (Fig. 6B and C). Thus, both in vivo and in vitro, Cdc25A failed to restore full activation of Cdk2 derived from cells expressing p16INK4a. The data would indicate that in MCF-7 cells, the role of Cdc25A in Cdk2 activation is secondary to the requirement for D cyclin-Cdk4 complex formation and Cdk inhibitor sequestration in early G1.
Our studies do, however, support an in vivo role for Cdc25A in G1/S transition induced in MCF-7 cells by estrogen, since antisense CDC25A oligonucleotides inhibited expression of Cdc25A, inhibited Cdk2 activation, and prevented the onset of DNA synthesis (Fig. 4 and 5). In conjunction with increased Cdc25A expression, estrogen treatment increased measurable in vivo Cdc25A activity in MCF-7 cells, but this activity was largely absent when these cells were transduced with p16INK4a (Fig. 4, 5, 7, and 8). Previous studies demonstrated that in HeLa cells, expression and activity of Cdc25A increase as cells approach the G1/S border (3, 27). In these cells Cdc25A was phosphorylated in vivo at the G1/S border, and in vitro phosphorylation or activation of Cdc25A was associated with cyclin E-Cdk2 in cell lysates (27). Our studies provide a direct demonstration of the separate regulation of Cdc25A expression and activity in MCF-7 cells and thus lend in vivo support to earlier studies suggesting that full enzymatic activity of Cdc25A requires activation in vivo through phosphorylation (17, 19, 27, 54). Cdc25A synthesis in MCF-7 cells occurs in mid-G1 phase, and activation of the enzyme in vivo correlates with Cdk2 activity. The relative inactivity of Cdc25A in p16INK4a-expressing cells is associated with a lack of cyclin E-Cdk2 kinase activity and may directly relate to the disruption of p21Cip1-p27Kip1 sequestration by p16INK4a. Overexpression of p27Kip1 also led to inhibition of Cdc25A activity (Fig. 7). The evidence at this time indicates that deregulated expression of p27Kip1-p21Cip1, cyclin A repression, and the consequent inhibition of Cdk2 leads to Cdc25A inhibition in p16INK4a-expressing cells. Accordingly, in vivo overexpression of cyclin E and incubation in vitro with active Cdk2 restored activity of Cdc25A derived from p16INK4a-transduced cells (Fig. 8B and C). In addition, active Cdk2 in MCF-7 cell lysates associated with Cdc25A in vitro (Fig. 8A). Chemical Cdk inhibitors flavopiridol and roscovitine (51) also inhibited kinase activity associating with Cdc25A in vitro (Fig. 8A). To underscore the independence of Cdc25A expression and in vivo activation, ectopic Cdc25A was activated following estrogen treatment of growth-arrested MCF-7 cells (Fig. 8D). Furthermore, activation of exogenous Cdc25A was prevented by expression of p16INK4a and a dominant-negative Cdk2 mutant (Fig. 8D).
Cdc25A is phosphorylated and activated in vitro by cyclin E-Cdk2 (27), Raf-1 (19), and Pim-1 (54), although no previous study has conclusively demonstrated whether these constitute mediators of Cdc25A phosphorylation or activation in vivo. Estrogen elicits activation of cyclin E-Cdk2 (1, 15, 64, 69), Raf-1 (5, 70), and Pim-1 (Fig. 7) (this study) in MCF-7 cells, so, potentially, any of these mediators might participate in Cdc25A activation in vivo. The inhibition of Cdc25A activity we observed in MCF-7 cells transduced with dominant-negative Ras might suggest a requirement for the Ras–Raf-1 pathway in Cdc25A activation, yet this was also associated with p27Kip1 accumulation and depressed Cdk2 activity (Fig. 7). Raf-1 activation by E2 is transient (5, 70) and may not coincide with the time frame of Cdc25A activation in mid-G1. A constitutively active Raf-1 mutant activated Cdc25A in vivo in MCF-7 cells expressing p16INK4a (Fig. 8B), which would suggest that Raf-1–Cdc25A interactions are nonetheless functional in vivo. Recent studies have suggested, however, that these interactions serve to regulate phosphorylation and activity of Raf-1 (99) and thus may lie outside the realm of any direct participation in G1 transit. Our studies cannot at this time support a role for Pim-1 in Cdc25A activation, since Cdc25A remained inactive in p16INK4a-expressing cells where Pim-1 was activated (Fig. 7). Active Pim-1 kinase in lysates of E2-treated MCF-7 cells, however, was found to associate with Cdc25A in vitro (Fig. 8), as described previously (54).
In conclusion, a wide range of studies have suggested roles for cyclin D1 and p16INK4a in control of growth and senescence of normal mammary epithelial cells (4, 16, 73) and in mammary carcinogenesis (36, 59, 94). Estrogen induces expression of cyclin D1 mRNA and protein in normal uterine and mammary gland epithelium and in MCF-7 cells (1, 2, 15, 23, 69, 73, 90), and our studies and the others cited herein would suggest that cyclin D1 is a requisite downstream target of estrogen action with respect to growth promotion. Our studies cannot support recent investigations suggesting that direct cyclin D1-ER interactions serve to promote growth in a Cdk4-independent fashion (49, 57, 101, 103). Rather, the studies described herein would suggest that in MCF-7 cells, growth promotion by cyclin D1 is mediated largely through interactions with Cdk4. These studies further demonstrate that estrogen independently regulates multiple components of the cell cycle machinery in addition to cyclin D1, including expression of p21Cip1-p27Kip1 and Cdc25A (Fig. 9). Our studies thus indicate that estrogen exerts a regulatory influence upon Cdk2 activation in MCF-7 cells independent of cyclin D1-Cdk4 function and identify Cdc25A as a growth-promoting target for estrogen action. Recent investigations demonstrate that cyclin E and Cdc25A function cooperatively along with Myc to generate active Cdk2 and elicit G1/S transition independent of pRb inactivation (74). Cyclin E and Cdc25A are overexpressed in a substantive portion of breast carcinomas (20, 24, 36, 58, 59) and cyclin E expression, cyclin E-Cdk2 kinase activity, and p27Kip1 expression may hold predictive value with respect to the proliferative rate and severity of the disease (58–60). Estrogens modulate expression and function of a variety of genes across a range of target cell types, and our studies fit this paradigm of multifaceted regulation. Elucidation of the multiple sites of estrogen action may be of considerable importance to our understanding of the normal biology of estrogen target tissues and our understanding of breast cancer etiology.
ACKNOWLEDGMENTS
We thank Eva Bukovska for technical assistance and Richard Andrews for performing the flow cytometric analysis. We are also grateful to Joseph Nevins for providing adenoviral vectors for dominant-negative Ras, Raf-1caax, and cyclin E. Plasmid vectors used for Northern blotting analysis of Cdc25A, c-myc, and cyclin D1 were kindly provided by Yue Xiong and David Beach (Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.). MCF-7/MVLN cells were provided by Michel Pons (INSERM, Strasbourg, France). The retroviral expression vector for Cdc25A was kindly provided by M. Roussel (St. Jude's Children's Research Hospital, Memphis, Tenn.). The GST-cyclin A expression vector was provided by Bruce Mayer (Harvard Medical School, Boston, Mass.). Plasmid vectors for p16INK4a, cyclin E, and dominant-negative Cdk2 were kindly provided by G. Peters, J. Roberts, and E. Harlow, respectively. The p27Kip1 wild-type and T187A vectors were provided by J.-Y. Kato at the Nora Institute of Science and Technology, Japan. We also thank J. Lukas and E. Santoni-Rugiu (Institute of Cancer Biology, Danish Cancer Society) for the HA-Cdc25A expression vector.
This work was supported by National Institutes of Health grant CA-68538 and by CA-84048 (J.W.).
ADDENDUM
While this paper was in the review process, a related study was published (4a) that provides support for a role for Cdc25A in breast cancer and in proliferation of MCF-7 cells in vitro. This study demonstrates that Cdc25A is overexpressed in up to 50% of breast carcinomas and that Cdc25A overexpression was associated with poor patient survival. Cdc25A overexpression also correlated with elevated Cdk2 activity in tumor tissues. In addition, antisense CDC25A oligonucleotides were shown to specifically inhibit Cdk2 activity and proliferation in asynchronous cultures of MCF-7 cells.
REFERENCES
- 1.Altucci L, Addeo R, Cicatiello L, Dauvois S, Parker M G, Truss M, Beato M, Sica V, Bresciani F, Weisz A. 17beta-Estradiol induces cyclin D1 gene transcription, p36D1–p34cdk4 complex activation and p105Rb phosphorylation during mitogenic stimulation of G(1)-arrested human breast cancer cells. Oncogene. 1996;12:2315–2324. [PubMed] [Google Scholar]
- 2.Altucci L, Addeo R, Cicatiello L, Germano D, Pacilio C, Battista T, Cancemi M, Petrizzi V B, Bresciani F, Weisz A. Estrogen induces early and timed activation of cyclin-dependent kinases 4, 5, and 6 and increases cyclin messenger ribonucleic acid expression in rat uterus. Endocrinology. 1997;138:978–984. doi: 10.1210/endo.138.3.5002. [DOI] [PubMed] [Google Scholar]
- 3.Blomberg I, Hoffmann I. Ectopic expression of Cdc25A accelerates the G1/S transition and leads to premature activation of cyclin E- and cyclin A-dependent kinases. Mol Cell Biol. 1999;19:6183–6194. doi: 10.1128/mcb.19.9.6183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brenner A J, Stampfer M R, Aldaz C M. Increased p16 expression with first senescence arrest in human mammary epithelial cells and extended growth capacity with p16 inactivation. Oncogene. 1998;17:199–205. doi: 10.1038/sj.onc.1201919. [DOI] [PubMed] [Google Scholar]
- 4a.Cangi M G, Cukor B, Suong P, Signoretti S, Moreira G, Jr, Ranashinge M, Cady B, Pagano M, Loda M. Role of the Cdc25A phosphatase in human breast cancer. J Clin Investig. 2000;106:753–761. doi: 10.1172/JCI9174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Castoria G, Barone M V, Di Domenico M, Bilancio A, Ametrano D, Migliaccio A, Auricchio F. Non-transcriptional action of oestradiol and progestin triggers DNA synthesis. EMBO J. 1999;18:2500–2510. doi: 10.1093/emboj/18.9.2500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang Y C, Lee Y S, Tejima T, Tanaka K, Omura S, Heintz N H, Mitsui Y, Magae J. mdm2 and bax, downstream mediators of the p53 response, are degraded by the ubiquitin-proteasome pathway. Cell Growth Differ. 1998;9:79–84. [PubMed] [Google Scholar]
- 7.Chen X, Prywes R. Serum-induced expression of the cdc25A gene by relief of E2F-mediated repression. Mol Cell Biol. 1999;19:4695–4702. doi: 10.1128/mcb.19.7.4695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Craig C, Kim M, Ohri E, Wersto R, Katayose D, Li Z, Choi Y H, Mudahar B, Srivastava S, Seth P, Cowan K. Effects of adenovirus-mediated p16INK4A expression on cell cycle arrest are determined by endogenous p16 and Rb status in human cancer cells. Oncogene. 1998;16:265–272. doi: 10.1038/sj.onc.1201493. [DOI] [PubMed] [Google Scholar]
- 9.Craig C, Wersto R, Kim M, Ohri E, Li Z, Katayose D, Lee S J, Trepel J, Cowan K, Seth P. A recombinant adenovirus expressing p27Kip1 induces cell cycle arrest and loss of cyclin-Cdk activity in human breast cancer cells. Oncogene. 1997;14:2283–2289. doi: 10.1038/sj.onc.1201064. [DOI] [PubMed] [Google Scholar]
- 10.DeGregori J, Kowalik T, Nevins J R. Cellular targets for activation by the E2F1 transcription factor include DNA synthesis- and G1/S-regulatory genes. Mol Cell Biol. 1995;15:4215–4224. doi: 10.1128/mcb.15.8.4215. . (Erratum, 15:5846–5847.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dubik D, Dembinski T C, Shiu R P. Stimulation of c-myc oncogene expression associated with estrogen-induced proliferation of human breast cancer cells. Cancer Res. 1987;47:6517–6521. [PubMed] [Google Scholar]
- 12.Dubik D, Shiu R P. Transcriptional regulation of c-myc oncogene expression by estrogen in hormone-responsive human breast cancer cells. J Biol Chem. 1988;263:12705–12708. [PubMed] [Google Scholar]
- 13.Dubik D, Shiu R P. Mechanism of estrogen activation of c-myc oncogene expression. Oncogene. 1992;7:1587–1594. [PubMed] [Google Scholar]
- 14.Fantl V, Stamp G, Andrews A, Rosewell I, Dickson C. Mice lacking cyclin D1 are small and show defects in eye and mammary gland development. Genes Dev. 1995;9:2364–2372. doi: 10.1101/gad.9.19.2364. [DOI] [PubMed] [Google Scholar]
- 15.Foster J S, Wimalasena J. Estrogen regulates activity of cyclin-dependent kinases and retinoblastoma protein phosphorylation in breast cancer cells. Mol Endocrinol. 1996;10:488–498. doi: 10.1210/mend.10.5.8732680. [DOI] [PubMed] [Google Scholar]
- 16.Foster S A, Wong D J, Barrett M T, Galloway D A. Inactivation of p16 in human mammary epithelial cells by CpG island methylation. Mol Cell Biol. 1998;18:1793–1801. doi: 10.1128/mcb.18.4.1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Galaktionov K, Beach D. Specific activation of cdc25 tyrosine phosphatases by B-type cyclins: evidence for multiple roles of mitotic cyclins. Cell. 1991;67:1181–1194. doi: 10.1016/0092-8674(91)90294-9. [DOI] [PubMed] [Google Scholar]
- 18.Galaktionov K, Chen X, Beach D. Cdc25 cell-cycle phosphatase as a target of c-myc. Nature. 1996;382:511–517. doi: 10.1038/382511a0. [DOI] [PubMed] [Google Scholar]
- 19.Galaktionov K, Jessus C, Beach D. Raf1 interaction with Cdc25 phosphatase ties mitogenic signal transduction to cell cycle activation. Genes Dev. 1995;9:1046–1058. doi: 10.1101/gad.9.9.1046. [DOI] [PubMed] [Google Scholar]
- 20.Galaktionov K, Lee A K, Eckstein J, Draetta G, Meckler J, Loda M, Beach D. CDC25 phosphatases as potential human oncogenes. Science. 1995;269:1575–1577. doi: 10.1126/science.7667636. [DOI] [PubMed] [Google Scholar]
- 21.Gautier J, Solomon M J, Booher R N, Bazan J F, Kirschner M W. Cdc25 is a specific tyrosine phosphatase that directly activates p34cdc2. Cell. 1991;67:197–211. doi: 10.1016/0092-8674(91)90583-k. [DOI] [PubMed] [Google Scholar]
- 22.Geng Y, Eaton E N, Picon M, Roberts J M, Lundberg A S, Gifford A, Sardet C, Weinberg R A. Regulation of cyclin E transcription by E2Fs and retinoblastoma protein. Oncogene. 1996;12:1173–1180. [PubMed] [Google Scholar]
- 23.Geum D, Sun W, Paik S K, Lee C C, Kim K. Estrogen-induced cyclin D1 and D3 gene expressions during mouse uterine cell proliferation in vivo: differential induction mechanism of cyclin D1 and D3. Mol Reprod Dev. 1997;46:450–458. doi: 10.1002/(SICI)1098-2795(199704)46:4<450::AID-MRD2>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 24.Gray-Bablin J, Zalvide J, Fox M P, Knickerbocker C J, DeCaprio J A, Keyomarsi K. Cyclin E, a redundant cyclin in breast cancer. Proc Natl Acad Sci USA. 1996;93:15215–15220. doi: 10.1073/pnas.93.26.15215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gu Y, Rosenblatt J, Morgan D O. Cell cycle regulation of CDK2 activity by phosphorylation of Thr160 and Tyr15. EMBO J. 1992;11:3995–4005. doi: 10.1002/j.1460-2075.1992.tb05493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harper J W, Adami G R, Wei N, Keyomarsi K, Elledge S J. The p21 Cdk-interacting protein Cip1 is a potent inhibitor of G1 cyclin-dependent kinases. Cell. 1993;75:805–816. doi: 10.1016/0092-8674(93)90499-g. [DOI] [PubMed] [Google Scholar]
- 27.Hoffmann I, Draetta G, Karsenti E. Activation of the phosphatase activity of human cdc25A by a cdk2-cyclin E dependent phosphorylation at the G1/S transition. EMBO J. 1994;13:4302–4310. doi: 10.1002/j.1460-2075.1994.tb06750.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hui R, Cornish A L, McClelland R A, Robertson J F, Blamey R W, Musgrove E A, Nicholson R I, Sutherland R L. Cyclin D1 and estrogen receptor messenger RNA levels are positively correlated in primary breast cancer. Clin Cancer Res. 1996;2:923–928. [PubMed] [Google Scholar]
- 29.Iavarone A, Massague J. Repression of the CDK activator Cdc25A and cell-cycle arrest by cytokine TGF-beta in cells lacking the CDK inhibitor p15. Nature. 1997;387:417–422. doi: 10.1038/387417a0. [DOI] [PubMed] [Google Scholar]
- 30.Iavarone A, Massagué J. E2F and histone deacetylase mediate transforming growth factor β repression of cdc25A during keratinocyte cell cycle arrest. Mol Cell Biol. 1999;19:916–922. doi: 10.1128/mcb.19.1.916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Imagawa W, Yang J, Guzman R, Nandi S. Control of mammary gland development. In: Knobil E, Neill J D, editors. The physiology of reproduction. New York, N.Y: Raven Press; 1994. pp. 1033–1063. [Google Scholar]
- 32.Jiang H, Chou H S, Zhu L. Requirement of cyclin E-Cdk2 inhibition in p16INK4a-mediated growth suppression. Mol Cell Biol. 1998;18:5284–5290. doi: 10.1128/mcb.18.9.5284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jinno S, Suto K, Nagata A, Igarashi M, Kanaoka Y, Nojima H, Okayama H. Cdc25A is a novel phosphatase functioning early in the cell cycle. EMBO J. 1994;13:1549–1556. doi: 10.1002/j.1460-2075.1994.tb06417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kawada M, Yamagoe S, Murakami Y, Suzuki K, Mizuno S, Uehara Y. Induction of p27Kip1 degradation and anchorage independence by Ras through the MAP kinase signaling pathway. Oncogene. 1997;15:629–637. doi: 10.1038/sj.onc.1201228. [DOI] [PubMed] [Google Scholar]
- 35.Key T J, Pike M C. The role of oestrogens and progestagens in the epidemiology and prevention of breast cancer. Eur J Cancer Clin Oncol. 1988;24:29–43. doi: 10.1016/0277-5379(88)90173-3. [DOI] [PubMed] [Google Scholar]
- 36.Keyomarsi K, Pardee A B. Redundant cyclin overexpression and gene amplification in breast cancer cells. Proc Natl Acad Sci USA. 1993;90:1112–1116. doi: 10.1073/pnas.90.3.1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kitagawa M, Higashi H, Jung H K, Suzuki-Takahashi I, Ikeda M, Tamai K, Kato J, Segawa K, Yoshida E, Nishimura S, Taya Y. The consensus motif for phosphorylation by cyclin D1-Cdk4 is different from that for phosphorylation by cyclin A/E-Cdk2. EMBO J. 1996;15:7060–7069. [PMC free article] [PubMed] [Google Scholar]
- 38.Knudsen K E, Fribourg A F, Strobeck M W, Blanchard J M, Knudsen E S. Cyclin A is a functional target of retinoblastoma tumor suppressor protein-mediated cell cycle arrest. J Biol Chem. 1999;274:27632–27641. doi: 10.1074/jbc.274.39.27632. [DOI] [PubMed] [Google Scholar]
- 39.Koff A, Ohtsuki M, Polyak K, Roberts J M, Massague J. Negative regulation of G1 in mammalian cells: inhibition of cyclin E-dependent kinase by TGF-beta. Science. 1993;260:536–539. doi: 10.1126/science.8475385. [DOI] [PubMed] [Google Scholar]
- 40.Leone G, DeGregori J, Sears R, Jakoi L, Nevins J R. Myc and Ras collaborate in inducing accumulation of active cyclin E/Cdk2 and E2F. Nature. 1997;387:422–426. doi: 10.1038/387422a0. . (Erratum, 387:932.) [DOI] [PubMed] [Google Scholar]
- 41.Leung B S, Potter A H. Mode of estrogen action on cell proliferation in CAMA-1 cells. II. Sensitivity of G1 phase population. J Cell Biochem. 1987;34:213–225. doi: 10.1002/jcb.240340307. [DOI] [PubMed] [Google Scholar]
- 42.Liang H, Hittelman W, Nagarajan L. Ubiquitous expression and cell cycle regulation of the protein kinase PIM-1. Arch Biochem Biophys. 1996;330:259–265. doi: 10.1006/abbi.1996.0251. [DOI] [PubMed] [Google Scholar]
- 43.Lippman M E, Bolan G. Oestrogen-responsive human breast cancer in long term tissue culture. Nature. 1975;256:592–593. doi: 10.1038/256592a0. [DOI] [PubMed] [Google Scholar]
- 44.Lukas J, Bartkova J, Bartek J. Convergence of mitogenic signalling cascades from diverse classes of receptors at the cyclin D-cyclin-dependent kinase-pRb-controlled G1 checkpoint. Mol Cell Biol. 1996;16:6917–6925. doi: 10.1128/mcb.16.12.6917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lukas J, Herzinger T, Hansen K, Moroni M C, Resnitzky D, Helin K, Reed S I, Bartek J. Cyclin E-induced S phase without activation of the pRb/E2F pathway. Genes Dev. 1997;11:1479–1492. doi: 10.1101/gad.11.11.1479. [DOI] [PubMed] [Google Scholar]
- 46.Lukas J, Parry D, Aagaard L, Mann D J, Bartkova J, Strauss M, Peters G, Bartek J. Retinoblastoma-protein-dependent cell-cycle inhibition by the tumour suppressor p16. Nature. 1995;375:503–506. doi: 10.1038/375503a0. [DOI] [PubMed] [Google Scholar]
- 47.Matsushime H, Quelle D E, Shurtleff S A, Shibuya M, Sherr C J, Kato J Y. D-type cyclin-dependent kinase activity in mammalian cells. Mol Cell Biol. 1994;14:2066–2076. doi: 10.1128/mcb.14.3.2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McConnell B B, Gregory F J, Stott F J, Hara E, Peters G. Induced expression of p16INK4a inhibits both CDK4- and CDK2-associated kinase activity by reassortment of cyclin-CDK-inhibitor complexes. Mol Cell Biol. 1999;19:1981–1989. doi: 10.1128/mcb.19.3.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McMahon C, Suthiphongchai T, DiRenzo J, Ewen M E. P/CAF associates with cyclin D1 and potentiates its activation of the estrogen receptor. Proc Natl Acad Sci USA. 1999;96:5382–5387. doi: 10.1073/pnas.96.10.5382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Medema R H, Herrera R E, Lam F, Weinberg R A. Growth suppression by p16ink4 requires functional retinoblastoma protein. Proc Natl Acad Sci USA. 1995;92:6289–6293. doi: 10.1073/pnas.92.14.6289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Meijer L, Kim S H. Chemical inhibitors of cyclin-dependent kinases. Methods Enzymol. 1997;283:113–128. doi: 10.1016/s0076-6879(97)83011-x. [DOI] [PubMed] [Google Scholar]
- 52.Michalides R, Hageman P, van Tinteren H, Houben L, Wientjens E, Klompmaker R, Peterse J. A clinicopathological study on overexpression of cyclin D1 and of p53 in a series of 248 patients with operable breast cancer. Br J Cancer. 1996;73:728–734. doi: 10.1038/bjc.1996.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mitra J, Dai C Y, Somasundaram K, El-Deiry W S, Satyamoorthy K, Herlyn M, Enders G H. Induction of p21WAF1/CIP1 and inhibition of Cdk2 mediated by the tumor suppressor p16INK4a. Mol Cell Biol. 1999;19:3916–3928. doi: 10.1128/mcb.19.5.3916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mochizuki T, Kitanaka C, Noguchi K, Muramatsu T, Asai A, Kuchino Y. Physical and functional interactions between Pim-1 kinase and Cdc25A phosphatase. Implications for the Pim-1-mediated activation of the c-Myc signaling pathway. J Biol Chem. 1999;274:18659–18666. doi: 10.1074/jbc.274.26.18659. [DOI] [PubMed] [Google Scholar]
- 55.Muller D, Bouchard C, Rudolph B, Steiner P, Stuckmann I, Saffrich R, Ansorge W, Huttner W, Eilers M. Cdk2-dependent phosphorylation of p27 facilitates its Myc-induced release from cyclin E/cdk2 complexes. Oncogene. 1997;15:2561–2576. doi: 10.1038/sj.onc.1201440. [DOI] [PubMed] [Google Scholar]
- 56.Musgrove E A, Sarcevic B, Sutherland R L. Inducible expression of cyclin D1 in T-47D human breast cancer cells is sufficient for Cdk2 activation and pRB hyperphosphorylation. J Cell Biochem. 1996;60:363–378. doi: 10.1002/(SICI)1097-4644(19960301)60:3%3C363::AID-JCB8%3E3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 57.Neuman E, Ladha M H, Lin N, Upton T M, Miller S J, DiRenzo J, Pestell R G, Hinds P W, Dowdy S F, Brown M, Ewen M E. Cyclin D1 stimulation of estrogen receptor transcriptional activity independent of cdk4. Mol Cell Biol. 1997;17:5338–5347. doi: 10.1128/mcb.17.9.5338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nielsen N H, Arnerlov C, Emdin S O, Landberg G. Cyclin E overexpression, a negative prognostic factor in breast cancer with strong correlation to oestrogen receptor status. Br J Cancer. 1996;74:874–880. doi: 10.1038/bjc.1996.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nielsen N H, Emdin S O, Cajander J, Landberg G. Deregulation of cyclin E and D1 in breast cancer is associated with inactivation of the retinoblastoma protein. Oncogene. 1997;14:295–304. doi: 10.1038/sj.onc.1200833. [DOI] [PubMed] [Google Scholar]
- 60.Nielsen N H, Loden M, Cajander J, Emdin S O, Landberg G. G1-S transition defects occur in most breast cancers and predict outcome. Breast Cancer Res Treat. 1999;56:105–112. doi: 10.1023/a:1006208419350. [DOI] [PubMed] [Google Scholar]
- 61.Ohtsubo M, Theodoras A M, Schumacher J, Roberts J M, Pagano M. Human cyclin E, a nuclear protein essential for the G1-to-S phase transition. Mol Cell Biol. 1995;15:2612–2624. doi: 10.1128/mcb.15.5.2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pagano M, Tam S W, Theodoras A M, Beer-Romero P, Del Sal G, Chau V, Yew P R, Draetta G F, Rolfe M. Role of the ubiquitin-proteasome pathway in regulating abundance of the cyclin-dependent kinase inhibitor p27. Science. 1995;269:682–685. doi: 10.1126/science.7624798. [DOI] [PubMed] [Google Scholar]
- 63.Parry D, Bates S, Mann D J, Peters G. Lack of cyclin D-Cdk complexes in Rb-negative cells correlates with high levels of p16INK4/MTS1 tumour suppressor gene product. EMBO J. 1995;14:503–511. doi: 10.1002/j.1460-2075.1995.tb07026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Planas-Silva M D, Weinberg R A. Estrogen-dependent cyclin E-cdk2 activation through p21 redistribution. Mol Cell Biol. 1997;17:4059–4069. doi: 10.1128/mcb.17.7.4059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Polyak K, Kato J Y, Solomon M J, Sherr C J, Massague J, Roberts J M, Koff A. p27Kip1, a cyclin-Cdk inhibitor, links transforming growth factor-beta and contact inhibition to cell cycle arrest. Genes Dev. 1994;8:9–22. doi: 10.1101/gad.8.1.9. [DOI] [PubMed] [Google Scholar]
- 66.Pons M, Gagne D, Nicolas J C, Mehtali M. A new cellular model of response to estrogens: a bioluminescent test to characterize (anti) estrogen molecules. BioTechniques. 1990;9:450–459. [PubMed] [Google Scholar]
- 67.Poon R Y, Toyoshima H, Hunter T. Redistribution of the CDK inhibitor p27 between different cyclin · CDK complexes in the mouse fibroblast cell cycle and in cells arrested with lovastatin or ultraviolet irradiation. Mol Biol Cell. 1995;6:1197–1213. doi: 10.1091/mbc.6.9.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Prall O W J, Rogan E M, Musgrove E A, Watts C K W, Sutherland R L. c-Myc or cyclin D1 mimics estrogen effects on cyclin E-Cdk2 activation and cell cycle reentry. Mol Cell Biol. 1998;18:4499–4508. doi: 10.1128/mcb.18.8.4499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Prall O W J, Sarcevic B, Musgrove E A, Watts C K W, Sutherland R L. Estrogen-induced activation of Cdk4 and Cdk2 during G1-S phase progression is accompanied by increased cyclin D1 expression and decreased cyclin-dependent kinase inhibitor association with cyclin E-Cdk2. J Biol Chem. 1997;272:10882–10894. doi: 10.1074/jbc.272.16.10882. [DOI] [PubMed] [Google Scholar]
- 70.Pratt M A, Satkunaratnam A, Novosad D M. Estrogen activates raf-1 kinase and induces expression of Egr-1 in MCF-7 breast cancer cells. Mol Cell Biochem. 1998;189:119–125. doi: 10.1023/a:1006827015320. [DOI] [PubMed] [Google Scholar]
- 71.Roussel M F, Theodoras A M, Pagano M, Sherr C J. Rescue of defective mitogenic signaling by D-type cyclins. Proc Natl Acad Sci USA. 1995;92:6837–6841. doi: 10.1073/pnas.92.15.6837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Saha P, Eichbaum Q, Silberman E D, Mayer B J, Dutta A. p21CIP1 and Cdc25A: competition between an inhibitor and an activator of cyclin-dependent kinases. Mol Cell Biol. 1997;17:4338–4345. doi: 10.1128/mcb.17.8.4338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Said T K, Conneely O M, Medina D, O'Malley B W, Lydon J P. Progesterone, in addition to estrogen, induces cyclin D1 expression in the murine mammary epithelial cell, in vivo. Endocrinology. 1997;138:3933–3939. doi: 10.1210/endo.138.9.5436. [DOI] [PubMed] [Google Scholar]
- 74.Santoni-Rugiu E, Falck J, Mailand N, Bartek J, Lukas J. Involvement of Myc activity in a G1/S-promoting mechanism parallel to the pRb/E2F pathway. Mol Cell Biol. 2000;20:3497–3509. doi: 10.1128/mcb.20.10.3497-3509.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schulze A, Zerfass K, Spitkovsky D, Middendorp S, Berges J, Helin K, Jansen-Durr P, Henglein B. Cell cycle regulation of the cyclin A gene promoter is mediated by a variant E2F site. Proc Natl Acad Sci USA. 1995;92:11264–11268. doi: 10.1073/pnas.92.24.11264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sears R, Leone G, DeGregori J, Nevins J R. Ras enhances Myc protein stability. Mol Cell. 1999;3:169–179. doi: 10.1016/s1097-2765(00)80308-1. [DOI] [PubMed] [Google Scholar]
- 77.Sebastian B, Kakizuka A, Hunter T. Cdc25M2 activation of cyclin-dependent kinases by dephosphorylation of threonine-14 and tyrosine-15. Proc Natl Acad Sci USA. 1993;90:3521–3524. doi: 10.1073/pnas.90.8.3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sexl V, Diehl J A, Sherr C J, Ashmun R, Beach D, Roussel M F. A rate limiting function of cdc25A for S phase entry inversely correlates with tyrosine dephosphorylation of Cdk2. Oncogene. 1999;18:573–582. doi: 10.1038/sj.onc.1202362. [DOI] [PubMed] [Google Scholar]
- 79.Sheaff R J, Groudine M, Gordon M, Roberts J M, Clurman B E. Cyclin E-CDK2 is a regulator of p27Kip1. Genes Dev. 1997;11:1464–1478. doi: 10.1101/gad.11.11.1464. [DOI] [PubMed] [Google Scholar]
- 80.Sherr C J. D-type cyclins. Trends Biochem Sci. 1995;20:187–190. doi: 10.1016/s0968-0004(00)89005-2. [DOI] [PubMed] [Google Scholar]
- 81.Sherr C J. Cancer cell cycles. Science. 1996;274:1672–1677. doi: 10.1126/science.274.5293.1672. [DOI] [PubMed] [Google Scholar]
- 82.Sherr C J, Roberts J M. Inhibitors of mammalian G1 cyclin-dependent kinases. Genes Dev. 1995;9:1149–1163. doi: 10.1101/gad.9.10.1149. [DOI] [PubMed] [Google Scholar]
- 83.Sherr C J, Roberts J M. CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev. 1999;13:1501–1512. doi: 10.1101/gad.13.12.1501. [DOI] [PubMed] [Google Scholar]
- 84.Sicinski P, Donaher J L, Parker S B, Li T, Fazeli A, Gardner H, Haslam S Z, Bronson R T, Elledge S J, Weinberg R A. Cyclin D1 provides a link between development and oncogenesis in the retina and breast. Cell. 1995;82:621–630. doi: 10.1016/0092-8674(95)90034-9. [DOI] [PubMed] [Google Scholar]
- 85.Soule H D, McGrath C M. Estrogen responsive proliferation of clonal human breast carcinoma cells in athymic mice. Cancer Lett. 1980;10:177–189. doi: 10.1016/0304-3835(80)90042-7. [DOI] [PubMed] [Google Scholar]
- 86.Sutherland R L, Hall R E, Taylor I W. Cell proliferation kinetics of MCF-7 human mammary carcinoma cells in culture and effects of tamoxifen on exponentially growing and plateau-phase cells. Cancer Res. 1983;43:3998–4006. [PubMed] [Google Scholar]
- 87.Sweeney K J, Swarbrick A, Sutherland R L, Musgrove E A. Lack of relationship between CDK activity and G1 cyclin expression in breast cancer cells. Oncogene. 1998;16:2865–2878. doi: 10.1038/sj.onc.1201814. [DOI] [PubMed] [Google Scholar]
- 88.Takuwa N, Takuwa Y. Ras activity late in G1 phase required for p27kip1 downregulation, passage through the restriction point, and entry into S phase in growth factor-stimulated NIH 3T3 fibroblasts. Mol Cell Biol. 1997;17:5348–5358. doi: 10.1128/mcb.17.9.5348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tomoda K, Kubota Y, Kato J. Degradation of the cyclin-dependent-kinase inhibitor p27Kip1 is instigated by Jab1. Nature. 1999;398:160–165. doi: 10.1038/18230. [DOI] [PubMed] [Google Scholar]
- 90.Tong W, Pollard J W. Progesterone inhibits estrogen-induced cyclin D1 and cdk4 nuclear translocation, cyclin E- and cyclin A-cdk2 kinase activation, and cell proliferation in uterine epithelial cells in mice. Mol Cell Biol. 1999;19:2251–2264. doi: 10.1128/mcb.19.3.2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Toyoshima H, Hunter T. p27, a novel inhibitor of G1 cyclin-Cdk protein kinase activity, is related to p21. Cell. 1994;78:67–74. doi: 10.1016/0092-8674(94)90573-8. [DOI] [PubMed] [Google Scholar]
- 92.van den Heuvel S, Harlow E. Distinct roles for cyclin-dependent kinases in cell cycle control. Science. 1993;262:2050–2054. doi: 10.1126/science.8266103. [DOI] [PubMed] [Google Scholar]
- 93.Vigo E, Müller H, Prosperini E, Hateboer G, Cartwright P, Moroni M C, Helin K. CDC25A phosphatase is a target of E2F and is required for efficient E2F-induced S phase. Mol Cell Biol. 1999;19:6379–6395. doi: 10.1128/mcb.19.9.6379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wang T C, Cardiff R D, Zukerberg L, Lees E, Arnold A, Schmidt E V. Mammary hyperplasia and carcinoma in MMTV-cyclin D1 transgenic mice. Nature. 1994;369:669–671. doi: 10.1038/369669a0. [DOI] [PubMed] [Google Scholar]
- 95.Wang W, Dong L, Saville B, Safe S. Transcriptional activation of E2F1 gene expression by 17beta-estradiol in MCF-7 cells is regulated by NF-Y-Sp1/estrogen receptor interactions. Mol Endocrinol. 1999;13:1373–1387. doi: 10.1210/mend.13.8.0323. [DOI] [PubMed] [Google Scholar]
- 96.Wang W, Smith R, Safe S. Aryl hydrocarbon receptor-mediated antiestrogenicity in MCF-7 cells: modulation of hormone-induced cell cycle enzymes. Arch Biochem Biophys. 1998;356:239–248. doi: 10.1006/abbi.1998.0782. [DOI] [PubMed] [Google Scholar]
- 97.Watts C K, Brady A, Sarcevic B, deFazio A, Musgrove E A, Sutherland R L. Antiestrogen inhibition of cell cycle progression in breast cancer cells is associated with inhibition of cyclin-dependent kinase activity and decreased retinoblastoma protein phosphorylation. Mol Endocrinol. 1995;9:1804–1813. doi: 10.1210/mend.9.12.8614416. [DOI] [PubMed] [Google Scholar]
- 98.Weinberg R A. The retinoblastoma protein and cell cycle control. Cell. 1995;81:323–330. doi: 10.1016/0092-8674(95)90385-2. [DOI] [PubMed] [Google Scholar]
- 99.Xia K, Lee R S, Narsimhan R P, Mukhopadhyay N K, Neel B G, Roberts T M. Tyrosine phosphorylation of the proto-oncoprotein Raf-1 is regulated by Raf-1 itself and the phosphatase Cdc25A. Mol Cell Biol. 1999;19:4819–4824. doi: 10.1128/mcb.19.7.4819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yoshizumi M, Wang H, Hsieh C M, Sibinga N E, Perrella M A, Lee M E. Down-regulation of the cyclin A promoter by transforming growth factor-beta1 is associated with a reduction in phosphorylated activating transcription factor-1 and cyclic AMP-responsive element-binding protein. J Biol Chem. 1997;272:22259–22264. doi: 10.1074/jbc.272.35.22259. [DOI] [PubMed] [Google Scholar]
- 101.Zwijsen R M, Buckle R S, Hijmans E M, Loomans C J, Bernards R. Ligand-independent recruitment of steroid receptor coactivators to estrogen receptor by cyclin D1. Genes Dev. 1998;12:3488–3498. doi: 10.1101/gad.12.22.3488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zwijsen R M, Klompmaker R, Wientjens E B H G M, Kristel P M P, van der Burg B, Michalides R J A M. Cyclin D1 triggers autonomous growth of breast cancer cells by governing cell cycle exit. Mol Cell Biol. 1996;16:2554–2560. doi: 10.1128/mcb.16.6.2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zwijsen R M, Wientjens E, Klompmaker R, van der Sman J, Bernards R, Michalides R J. CDK-independent activation of estrogen receptor by cyclin D1. Cell. 1997;88:405–415. doi: 10.1016/s0092-8674(00)81879-6. [DOI] [PubMed] [Google Scholar]