Abstract
Efficient methods for the site-specific installation of structurally-defined interstrand cross-links in duplex DNA may be useful in a wide variety of fields. The work described here developed a high-yield synthesis of chemically stable interstrand cross-links resulting from a reductive amination reaction between an abasic site and the noncanonical nucleobase 2-aminopurine in duplex DNA. Results from footprinting, LC-MS, and stability studies support the formation of an N2-alkylamine attachment between the 2-aminopurine residue and the Ap site. The reaction performs best when the 2-aminopurine residue on the opposing strand is offset 1 nt to the 5’-side of the abasic site. The cross-link confers substantial resistance to thermal denaturation (melting). The cross-linking reaction is fast (complete in 4 h), employs only commercially available reagents, and can be used to generate cross-linked duplexes in sufficient quantities for biophysical, structural, and DNA repair studies.
Graphical Abstract
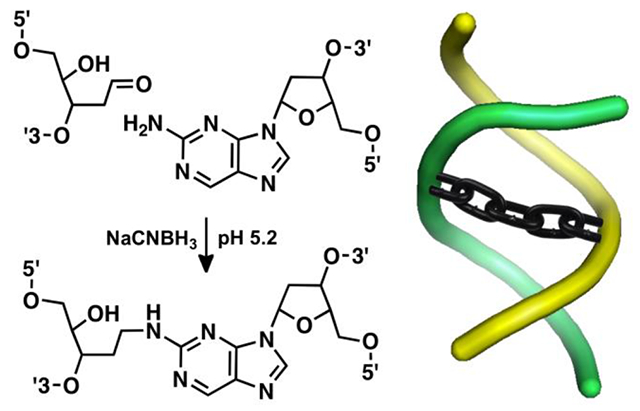
Strand separation of the double helix is required to utilize or copy the information encoded by the sequence of nucleobases in DNA. Covalent interstrand cross-links (ICLs) prevent strand separation and compromise the biological functions of DNA. Nonetheless, DNA duplexes containing site-specific interstrand cross-links (ICL) have applications in a variety of fields. For example, the duplex stabilization provided by ICLs can modulate the properties of DNA-based nanomaterials1,2 and can enable some biophysical and structural experiments.3 ICL formation also can be used for the selective detection of nucleic acid sequences.4–6 Finally, duplexes containing structurally-defined ICLs are critical reagents for the study of cellular DNA repair processes.7–10
Unfortunately, the preparation of DNA duplexes containing site-specific, chemically-defined ICLs is not a simple task. Treatment of duplexes with cross-linking agents such as formaldehyde or nitrogen mustards typically generates complex mixtures of cross-linked products in low yield.11–13 A number of creative solid-phase methods for the preparation of cross-linked duplexes have been developed, but these typically require multi-step organic syntheses.14–18 Many applications would benefit from simple methods that employ readily available commercial oligonucleotides and reagents to achieve rapid and high-yield preparation of duplexes containing site specific, chemically-defined ICLs.
Here we describe an approach that exploits the reactivity of an abasic (Ap) site for the preparation of cross-linked DNA duplexes in high yield. Ap sites are easily introduced into DNA by treatment of the corresponding 2’-deoxyuridine (dU)-containing oligonucleotide with the repair enzyme uracil DNA glycosylase (UDG).19,20 Both UDG and dU-containing oligonucleotides are commercially available from a variety of sources. Ap sites can also be generated in oligonucleotides by chemical and photochemical means.21–23
Ap sites exist in DNA as a mixture of the ring-closed hemiacetal and the ring-opened aldehyde (Scheme 1).24 We previously have characterized the formation of ICLs resulting from the reaction of the abasic aldehyde residue with the exocyclic amino groups of the nucleobases adenine and guanine.25–27 Adenine residues located one nucleotide to the 3’-side of the Ap site on the opposing strand can generate equilibrium cross-link yields of 15-85%, depending upon the sequence context.25 Guanine residues located one nucleotide to the 5’-side of the Ap site on the opposing strand generate cross-links in yields of 2-3%.26,27 Addition of sodium cyanoborohydride (NaCNBH3) to dG-Ap cross-linking reactions produced higher yields (~20%) of a reduced alkylamine cross-link via a reductive amination reaction.26,27
Scheme 1.
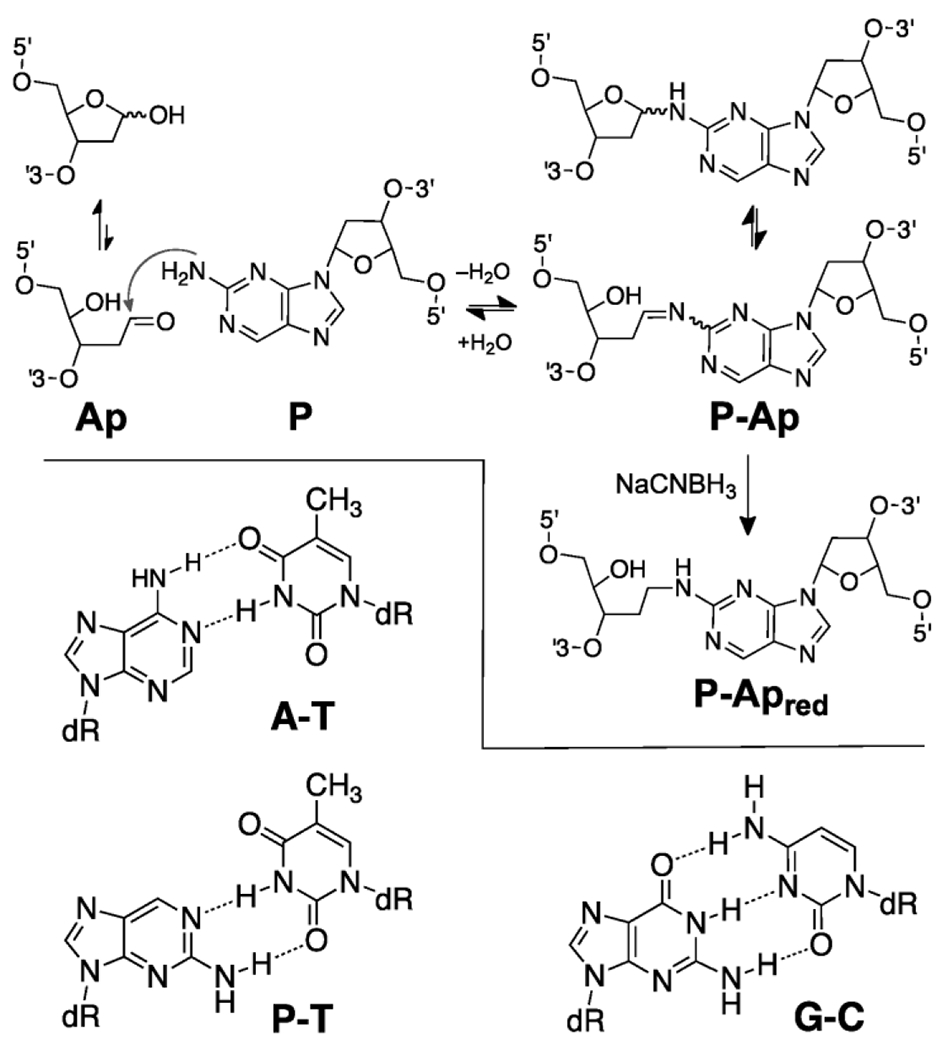
Formation and structure of the reduced and unreduced P-Ap ICLs.
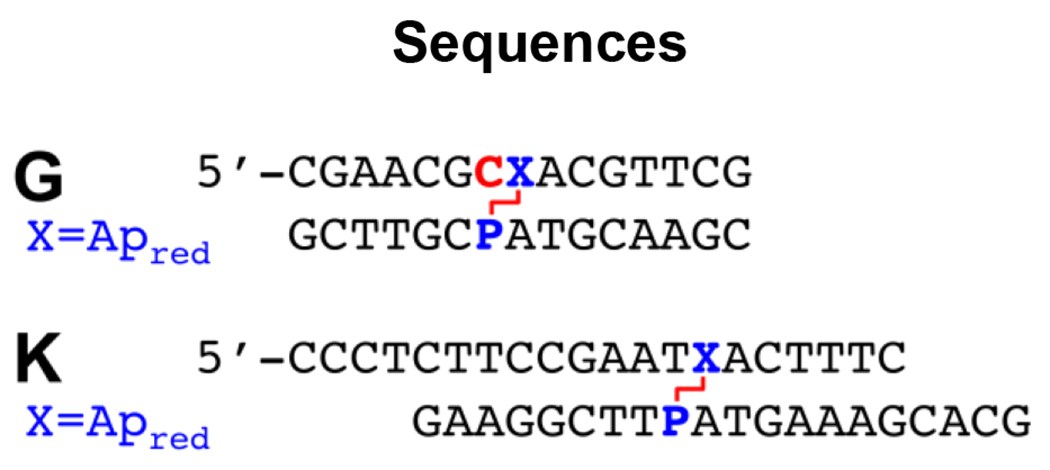
In the work described here, we characterized ICL formation arising from reaction of the non-canonical nucleobase 2-aminopurine (P) with an Ap site on the opposing strand. P is a positional isomer of adenine that pairs with thymine or cytosine residues in duplex DNA to place the exocyclic amino group in a location that is isostructural with that of guanine (Scheme 1).28–30 P is not found naturally in cellular DNA but has been widely used as a fluorescent reporter in biophysical studies.31,32 P-containing oligonucleotides are commercially available from a variety of vendors. We find that very high yields of cross-linked duplex (~85%) were obtained via a reductive amination reaction (Scheme 1) in sequences where the P residue was offset one base to the 5’-side of the Ap site on the opposing strand (Figure 1A). Several lines of experimental evidence established that this reaction generates a chemically-stable alkylamine cross-link between the Ap site and the P residue on the opposing strand. Installation of the P-Apred cross-link dramatically stabilized the DNA duplexes, as measured by thermal melting analyses. Finally, we showed that duplexes containing the P-Apred cross-link can be prepared and purified on a scale (≥ 30 nmol) suitable for use in DNA-repair experiments, crystallization trials, and biophysical studies.
Figure 1. Gel electrophoretic analysis of the P-Ap cross-link in duplex B.
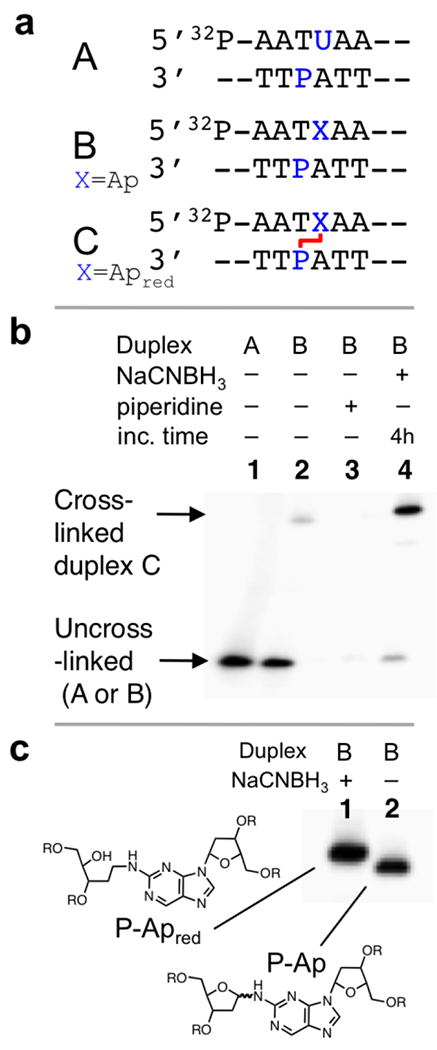
Panel a: The Ap-containing duplex B was generated by treatment of the corresponding dU-containing duplex A with UDG. The central cross-linking sequence is shown here and the complete sequence of the 35 nt duplexes are provided in the Supporting Information. Panel b: Gel electrophoretic detection of cross-link formation in duplex B. Lane 1: Size-marker consisting of 35 nt 32P-labeled dU-containing oligonucleotide; lane 2: Ap-containing duplex B, frozen immediately after mixing and stored at −20 °C prior to gel electrophoretic analysis; lane 3: Ap-containing duplex B treated subjected to piperidine workup (0.1 M, 95 °C, 15 min) to induce strand cleavage at the Ap site; lane 4: The 5’-32P-labeled duplex B was incubated in sodium acetate buffer (750 mM, pH 5.2) containing NaCNBH3 (250 mM) at 37 °C for 4 h. The 32P-labeled 2’-deoxyoligonucleotides were resolved on a 20% denaturing polyacrylamide gel and the radioactivity in each band quantitatively measured by storage phosphor autoradiography. Panel c: Gel electrophoretic separation of the reduced and unreduced P-Ap cross-links.
RESULTS AND DISCUSSION
Gel Electrophoretic Analysis of P-Ap Cross-Link Formation in Duplex B.
We first investigated the P-Ap cross-linking reaction in a sequence where the P residue on the opposing strand was offset 1 nt to the 5’-side of the Ap site (duplex B, Figure 1A). This is analogous to the dG-Ap cross-linking sequence motif.27 We prepared a 35 base pair (bp), Ap-containing DNA duplex B by treatment of the corresponding dU-containing duplex A with UDG as described previously.19,25–27,33–36 It may be important to point out that the cross-linking reactions described here require a true Ap site and cannot occur with the tetrahydrofuran (F) abasic site that is often used as a chemically-stable surrogate for the true abasic site.37 To enable analysis of the cross-linking reaction, the Ap-containing strand was labeled with a 5’-32P-phosphoryl group. The P residue was located on the unlabeled complementary strand. Labeled oligonucleotides were resolved by electrophoresis on a denaturing 20% polyacrylamide gel and visualized using storage phosphor autoradiography.38 Successful installation of the Ap site in duplex B was confirmed by piperidine workup (0.1 M, 90 °C, 15 min) to induce strand cleavage at the Ap site.20,39–41 The resulting short 32P-labeled product migrates faster than the full-length 35 nt oligomers in the 20% denaturing polyacrylamide gel (Figure 1B, lanes 1 and 2; the cleavage product is not shown in lane 3, but can be seen in the full-size image of the gel in Figure S1).
We conducted a preliminary survey of cross-link formation in several different buffers both with and without the reducing agent NaCNBH3 (Table 1). NaCNBH3 was employed with the expectation that a reductive amination reaction might generate increased yields of a chemically-stable N2-alkyl cross-link (P-Apred, Scheme 1).26,27,42 In a classical reductive amination reaction, NaCNBH3 selectively reduces the intermediate protonated imine and not the aldehyde starting material, thus drawing the aldehyde-imine equilibrium forward to provide good yields of the alkylamine product (in contrast, NaBH4 is expected to reduce both the aldehyde and the iminium ion).42,43 The yield of cross-linked duplex obtained under each condition was assessed by measurement of the characteristic slowly-migrating cross-link band on the gel (Figure 1B, lane 4).25,27 Consistent with our earlier work,27 substantial yields (up to 44%) of cross-linked duplex were generated by incubation of duplex B for 4 h in the absence of NaCNBH3 in three of the four buffers tested (Table 1). The unreduced cross-link reaches its final equilibrium yield rapidly, within of 3 h in pH 7 buffer (Figure S2). Reactions carried out in the presence of NaCNBH3 produced dramatically increased yields of the P-Ap cross-link in every buffer examined (Table 1). Differences in the concentration of NaCNBH3 across the range of 50-250 mM did not result in large changes in the cross-link yield. For subsequent experiments, we selected a set of standard conditions involving incubation of the DNA in sodium acetate buffer (750 mM, pH 5) containing NaCNBH3 (250 mM) at 24 °C.
Table 1. ICL yields generated in duplex B under various conditions.
The Ap-containing duplex B was incubated under the conditions indicated for 4 h at 37 °C. The 32P-labeled 2’-deoxyoligonucleotides were resolved on a 20% denaturing polyacrylamide gel and the yield of slowly-migrating cross-link band quantitatively measured by storage-phosphor autoradiography.
| Cross-link Yield (%) | ||||||
|---|---|---|---|---|---|---|
| Buffer╲NaCNBH3 conc. (mM) | 0 | 5 | 50 | 100 | 200 | 250 |
| NH4OAc (50 mM, pH 4.5) | 0 | 78 | 85 | 84 | 84 | 81 |
| (NH4)Pi (50 mM, pH 5.5) | 17 | 57 | 57 | 67 | 70 | 72 |
| NaOAc (750 mM, pH 5.2) | 44 | 58 | 87 | 86 | 86 | 85 |
| NaPi (10 mM, pH 7.2) | 37 | 32 | 36 | 43 | 48 | 46 |
A time course experiment showed that the reductive amination reaction reached its final, maximum yield of 85±3% within 4 h under our standard conditions (Figure 2). The reduced cross-linked duplex showed a slight decrease in gel mobility relative to the unreduced cross-link (Figure 1C). Curiously, the opposite trend in relative gel mobilities was observed previously for the reduced and unreduced dG-Ap cross-links.27
Figure 2. Time-course for the formation of the P-Apred in duplex B.
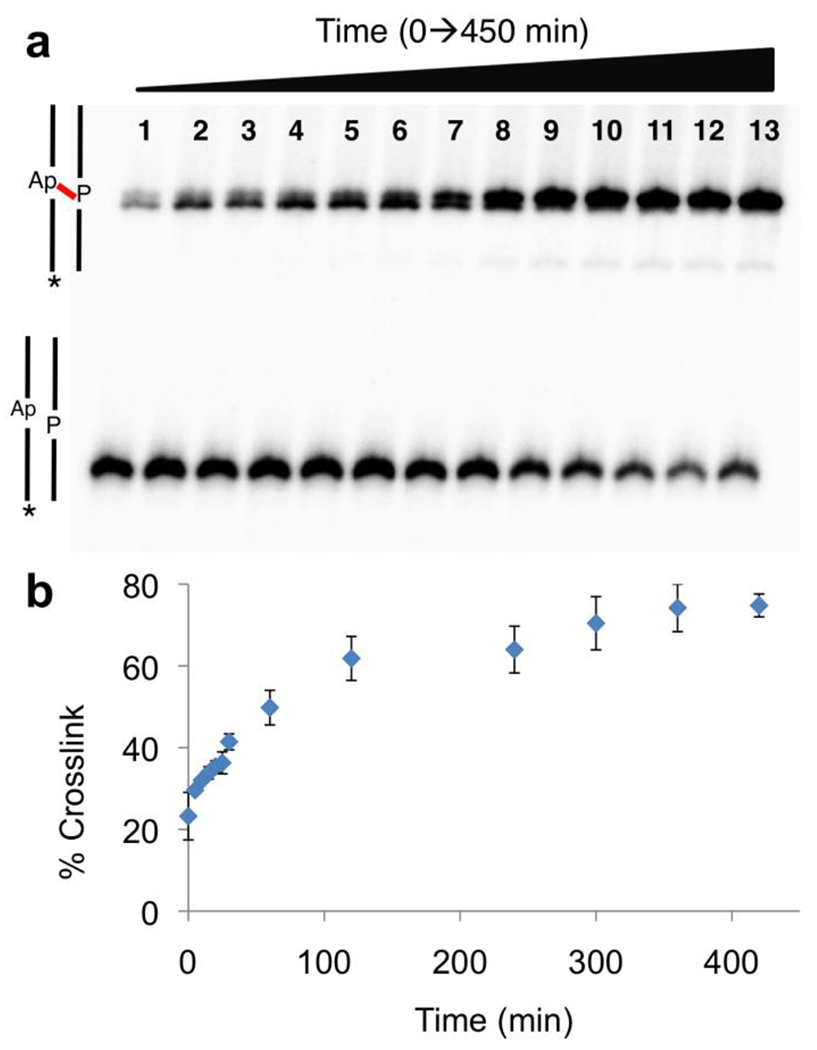
Panel a: The Ap-containing duplex was incubated in sodium acetate buffer (750 mM, pH 5.2) containing NaCNBH3 (250 mM) at 37 °C. Aliquots were removed at 0, 5, 10, 15, 20, 25, 30, 60, 120, 240, 300, 360 and 420 min and frozen prior to gel analysis. The 32P-labeled 2’-deoxyoligonucleotides were resolved on a 20% denaturing polyacrylamide gel and the radioactivity in each band quantitatively measured by storage-phosphor autoradiography. The fast-migrating band corresponds to the 32P-labeled full-length Ap-containing strand from uncross-linked duplex and the upper, slowly-migrating band corresponds to the cross-linked DNA duplexes. Panel b: Plot of cross-link yield versus time derived from the gel electrophoretic data (shown in Panel a).
It may be informative to compare our cross-linking reaction conditions and yields to those reported by Admiraal and O’Brien, who used the P-Ap cross-linking reaction in an interesting study that examined the inhibition of Ap-derived cross-link formation by base excision repair enzymes.44 Their study used a different duplex sequence (duplex D) from the one used here, a different buffer (MES, pH 6.5), and a NaBH4 workup at the end of the reaction, rather than NaCNBH3 present during the reaction.44 These conditions provided a 5% cross-link yield in duplex D.44 When we applied Admiraal and O’Brien’s reaction conditions and workup procedure to duplex C, we obtained a 23% cross-link yield. These results allow two useful conclusions: i. reductive amination conditions, employing NaCNBH3 in the reaction mixture, provide improved cross-link yields over those obtained without reducing agent or with a NaBH4 workup and, ii. sequences flanking the P-Ap motif can significantly affect cross-link yields.
Footprinting Confirms Cross-Link Attachment Is At the P Residue.
We employed iron-EDTA footprinting to rigorously pinpoint the location of cross-link generated by the reductive amination process. For this experiment, the cross-link was generated using our standard conditions except using an analog of duplex B in which the P-containing strand was 5’-32P-labeled. The isolated cross-link was subjected to strand cleavage using iron-EDTA-H2O2,45 resulting in the generation of a library of labeled fragments. The location where the “ladder” of single-stranded fragments ends in the electropherogram reveals the site of cross-link attachment.25,27,46,47 In this case, the results provided evidence that the cross-link attachment was at the P residue in duplex C (Figure S3).
Characterization of the P-Ap Using LC-MS/MS.
We employed LC-MS/MS methods to further characterize the chemical composition of the cross-links in the reduced and unreduced P-Ap ICLs. The cross-linked duplex C was prepared by incubation of an unlabeled analog of duplex B in sodium acetate buffer (pH 5.2) containing NaCl (100 mM) for 4 h at 37 °C either in the presence or absence of the reducing agent NaCNBH3 (250 mM). The resulting cross-linked duplexes were isolated from a denaturing polyacrylamide gel and subjected to digestion using a four-enzyme cocktail consisting of nuclease P1, alkaline phosphatase, and phosphodiesterases I and II.
Selected-ion chromatograms from LC-MS/MS analysis of the unreduced cross-link, obtained using previously reported experimental conditions,48–50 revealed two peaks eluting at 26.4 and 29.1 min displaying the m/z 368→252 transition anticipated for the neutral loss of 2-deoxyribose from the unreduced P-Ap cross-link (Figure 3A). Further cleavage of the m/z 252 ion produced a fragment ion at m/z 136 in MS/MS/MS, corresponding to the [M+H]+ ion of the 2-aminopurine free base (Figure S4). Together the data suggest that the initial m/z 368→252 transition arises via loss of the cross-linked 2-deoxyribose adduct from the 2-aminopurine nucleoside (Figure 3). Multiple peaks in the LC-MS/MS ion chromatogram (Figure 3A) likely reflect an equilibrating mixture consisting of the α- and β anomers of the pyranose and furanose isomers (Figure S4) in which the two major peaks correspond to the α and β forms of the pyranose isomer. Previous NMR analyses have shown that the pyranose isomers predomoninate in this type of nucleosidic cross-link remnant.35,51
Figure 3. LC-MS/MS analysis is consistent with the proposed chemical structures of the unreduced and reduced forms of the P-Ap cross-link.
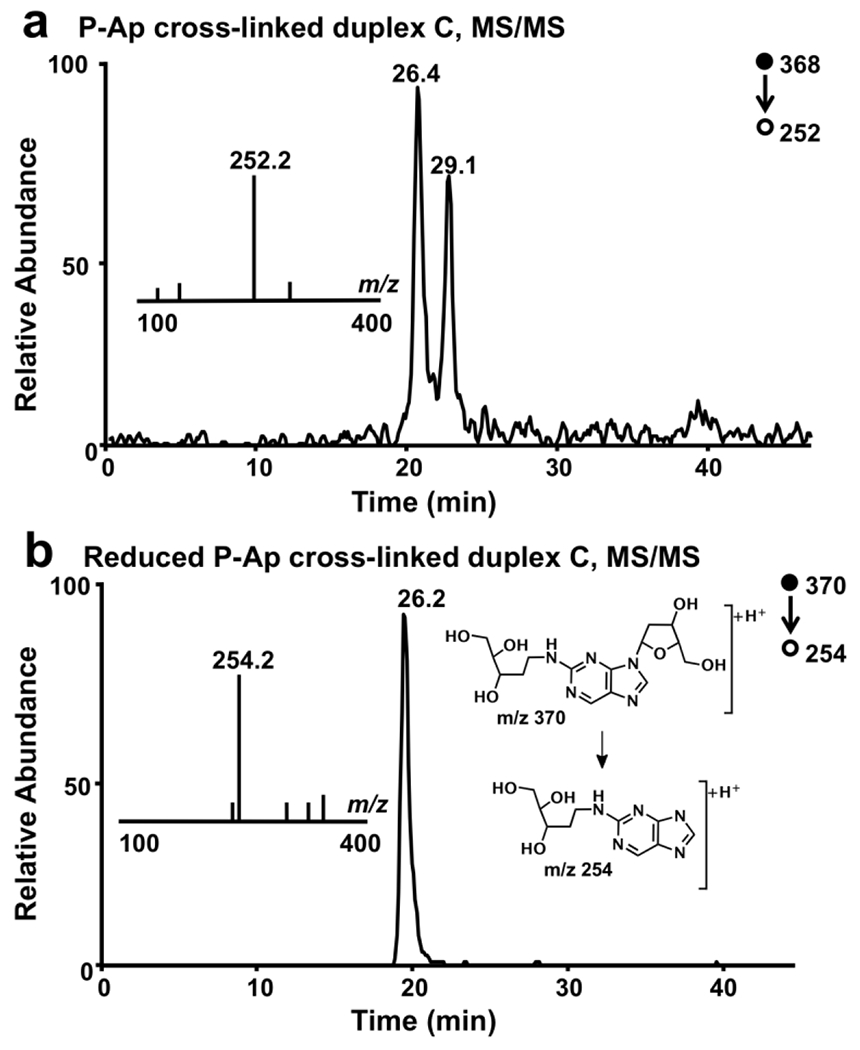
Panel a: Selected-ion chromatogram for monitoring the m/z 368→252 transition for the unreduced P-Ap cross-link. Panel b: Selected-ion chromatogram for monitoring the m/z 370→254 transition for the reduced P-Ap cross-link.
Selected-ion chromatograms from LC-MS/MS analysis of the reduced cross-link revealed a single peak eluting at 26.2 min displaying the m/z 370→254 transition expected for the neutral loss of a 2-deoxyribose from the P-Apred cross-link (Figure 3B). Further cleavage of the m/z 252 ion in MS/MS/MS produced prominent fragment ions at m/z values of 236, 218, and 136. This suggests that fragmentation of the reduced cross-link remnant proceeded via initial loss of 2-deoxyribose from the N9-position of the 2-aminopurine residue, followed by two neutral losses of water (–18 Da) and, ultimately, net loss of the reduced 2-deoxyribose unit (–118 Da) from the N2-position of the base to generate a fragment ion at m/z 136 corresponding to the [M+H]+ ion of the 2-aminopurine free base (Figure S5). Overall, the observed m/z values and fragmentations are consistent with the proposed structures of the P-Ap ICLs shown in Scheme 1 and Figure 3.
The Reduced P-Ap Cross-Linkage Is Chemically Stable.
Duplexes containing the reduced and unreduced cross-link were purified by gel electrophoresis and their stability examined under a range of conditions. We found that the isolated, unreduced cross-link in duplex C rapidly relaxed back toward an equilibrium mixture composed of approximately 25% cross-linked and 75% uncross-linked duplex over the course of about 3 h in HEPES buffer (pH 7.4, 50 mM) containing NaCl (100 mM) at 24 °C (Figure S6). This is consistent with the expected reversible nature of the unreduced, imine-derived P-Ap cross-link. The unreduced cross-link in duplex C was labile to heat (90 °C, 15 min), basic conditions (piperidine or pH 10), or acidic conditions (pH 3, Figure 4).
Figure 4. The reduced P-Ap cross-link is stable and the unreduced P-Ap cross-link is relatively unstable.
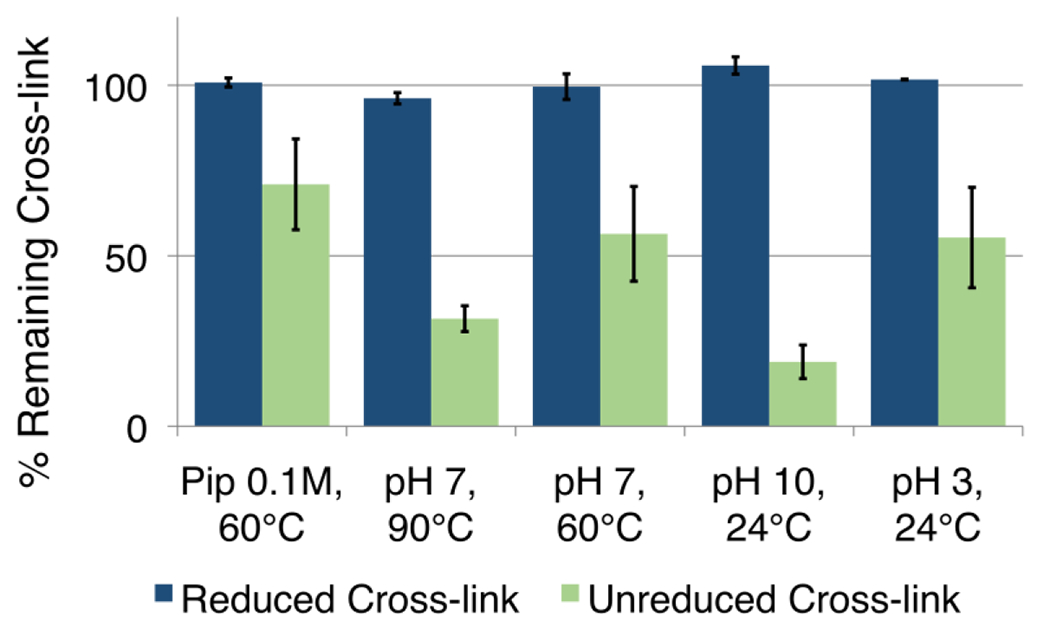
The unreduced cross-link was generated by incubation of the Ap-containing duplex B in HEPES buffer (50 mM, pH 7) containing NaCl (100 mM) at 37 °C (72 h). The reduced cross-link was generated by incubation of the Ap-containing duplex B in sodium acetate buffer (750 mM, pH 5.2) and NaCNBH3 (250 mM) at 37 °C (24 h). Following formation of the cross-links, the reaction mixtures were subjected to the following work-up conditions: pH adjusted to 3, 15 min; pH adjusted to 10, 15 min; 60 °C, 15 min; 90 °C, 15 min, 100 mM piperidine, 60° C, 15 min The amount of remaining cross-link following treatment to each condition (relative to that present at the end of the cross-link generation reaction) was measured by gel electrophoretic analysis. The yellow bars represent the relative yields of reduced cross-link and the red bars represent the relative yields of unreduced cross-link.
In contrast, duplex C containing the reduced, alkylamine cross-link (P-Apred) remained completely intact at pH 7 and 24 °C over the course of 100 h (Figure S6). In addition, the P-Apred is stable against heat (90 °C, 15 min), basic conditions (piperidine or pH 10), or acidic conditions (pH 3, Figure 4).
Covalent ICLs can stabilize DNA duplexes against thermal melting (heat-induced strand dissociation).14,15,33,52 Indeed, we found that the P-Apred cross-link provided significant duplex stabilization as indicated by thermal melting analysis. The melting temperatures for the parent dU-containing duplex A, the Ap-containing duplex B, and the cross-linked duplex C were 64 °C, 61 °C, and 75 °C, respectively (Figure 5A). Conceptually, the duplex stabilization provided by a DNA cross-link is similar to the thermal stabilization of short DNA duplexes by single-stranded linkers in DNA hairpin (stem-loop) structures (Figure 5).53 Thymine-containing tetraloops are particularly effective for stabilizing short duplexes against thermal melting.53 Therefore, we compared the thermal stabilization provided by P-Apred to that provided by the loop of a hairpin structure. For this purpose, we prepared the sequence-matched stem-loop structure E and the 16 bp cross-linked duplex F. The stem-loop displayed a melting temperature of 65 °C, consistent with literature values for similar structures (Figure 5B).53 In contrast, the corresponding 10 bp duplex without the T4 linker is predicted to melt at or below room temperature. Duplex F containing the P-Apred cross-link showed superior stability against thermal denaturation, displaying a melting temperature of 72 °C. Interestingly, the cross-linked duplex G, in which the P residue was paired with cytosine (rather than thymine) displayed two distinct melting transitions at approximately 63 °C and 73 °C (Figure S7), suggesting that the two duplex regions flanking the cross-link melt independently. Similar biphasic melting behavior has been observed previously in a different cross-linked duplex (see Figure 6 in reference 52). Overall, the results show that thermal stability of a duplex containing the P-Apred cross-link is slightly greater than that of a hairpin structure containing a thymine tetraloop.
Figure 5. The reduced P-Ap cross-link stabilizes duplexes against thermal melting.
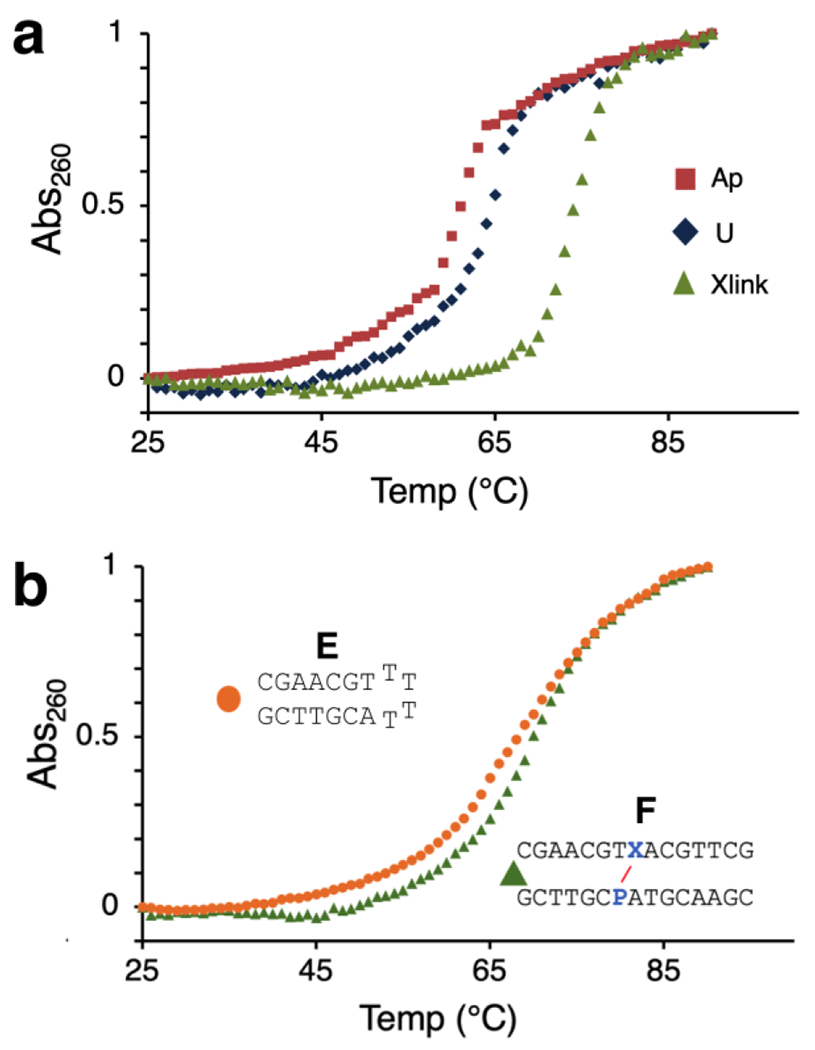
Panel a: Comparison of thermal melting profiles of the uncross-linked dU-containing duplex A, the uncross-linked Ap-containing duplex B, and duplex C containing the reduced P-Ap cross-link. Panel b: Comparison of thermal melting of the stem-loop structure E and a sequence-matched duplex F, containing the P-Apred cross-link.
Figure 6. Generation of slowly-migrating bands in DNA duplexes that vary the location of P with respect to Ap.
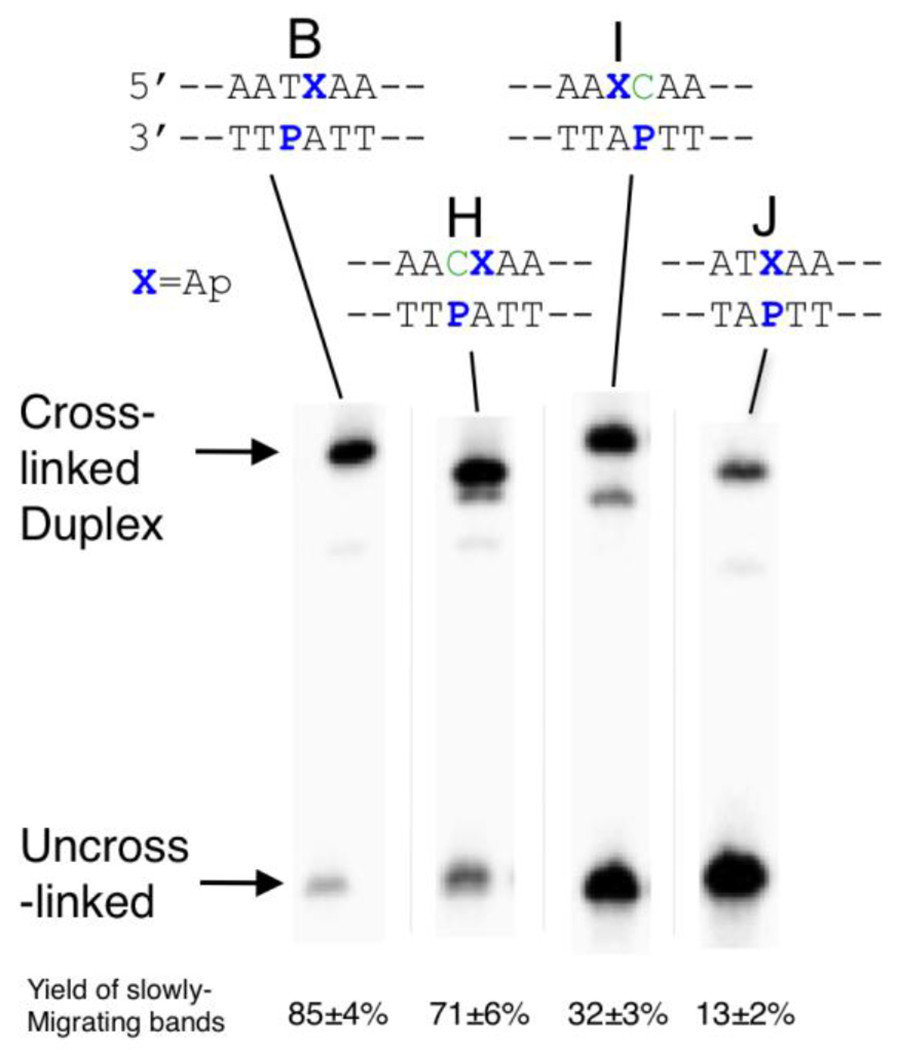
The 5’-32P-labeled, Ap-containing duplexes were incubated in sodium acetate buffer (750 mM, pH 5.2) containing NaCNBH3 (250 mM) at 37 °C for 4 h. The 32P-labeled 2’-deoxyoligonucleotides were resolved on a 20% denaturing polyacrylamide gel and the radioactivity in each band quantitatively measured by storage phosphor autoradiography.
Effects of P/Ap Juxtapositioning on Cross-Link Formation.
We prepared a series of duplexes (H-J) in which the location of the P residue with respect to the Ap site on the opposing strand was varied (Figure 6). We also varied the base-pairing partner of the P residue. Cross-linking reactions of these duplexes were carried out in the presence of NaCNBH3 using our standard conditions. We examined the effects of pairing the P residue with a C residue (duplex H). P can base pair with either C or T.29,30 The total yield of slowly-migrating, cross-linked bands (84%) in duplex H containing the P-C pair was similar to that generated in duplex B (86%) containing the P-T base pair (Figure 6); however, multiple slowly-migrating bands were generated by duplex H. In duplex I, where the P residue on the opposing strand is offset 1 nt to the 3’-side of the Ap site, the total yield of slowly-migrating bands is markedly decreased (55%) relative to duplex B and, again, two distinct bands were observed. When the P residue was placed directly opposing the Ap site (duplex J) the yield of slowly-migrating bands was dramatically decreased (13%). The P-Apred cross-link derived from duplex B has been rigorously characterized (vide supra), while the cross-linked bands generated from duplexes H-J have not. It is possible that multiple bands reflect mixtures of reduced and unreduced P-Ap cross-links or attachment of the Ap aldehyde residue at multiple sites on the opposing strand. Regardless, it seems clear that the preferred sequence motif for P-Ap cross-link formation is that in which the P residue on the opposing strand is offset 1 nt to the 5’-side of the Ap as seen in duplex B (and the corresponding cross-linked duplex C). The other cross-linking motifs shown in Figure 6 require further characterization but, ultimately, the P-Ap cross-linking reaction may afford access to a family of cross-linked duplexes with varied three-dimensional structures.
Large-Scale Preparation of a Duplex Containing the Reduced P-Ap Cross-Link.
Some applications in structural biology and biophysics require substantial amounts (≥10 nmol) of cross-linked DNA for spectroscopic measurements or crystallization. To demonstrate the preparative utility of the P-Ap cross-linking reaction, we carried out a large scale synthesis of a 16 bp cross-linked duplex with overhanging “sticky” ends suitable for ligation into a plasmid vector (duplex K).9,54 To prepare the requisite Ap-containing duplex, we treated the corresponding dU-containing duplex (5 nmol) with UDG (35 units) for 1 h. The DNA was ethanol precipitated and the Ap-containing duplex incubated in sodium acetate buffer (pH 5.2, 750 mM) containing NaCNBH3 (250 mM) for 4 h. Cross-linked DNA from six reactions was isolated by excising the band of cross-linked DNA from six lanes of a single denaturing 20% polyacrylamide gel (2 mm x 30 cm x 20 cm) to yield 38 nmol (63% yield). Thermal melting analysis provided evidence that the isolated material contained the expected reduced covalent cross-link.
CONCLUSIONS
In the work described here, we characterized the formation of ICLs derived from the reaction of a P residue with an Ap site in duplex DNA. In the absence of NaCNBH3, we observed a rapid, but reversible, formation of cross-linked DNA in good yields. When the cross-linking reaction in duplex B was carried out in the presence of the reducing agent NaCNBH3 we obtained high cross-link yields (~85%) within a 4 h reaction time. The results of LC-MS experiments, stability studies, and footprinting were consistent with formation of a chemically-stable alkylamine linkage resulting from a reductive amination reaction (Scheme 1). The P-Apred cross-link provides substantial stabilization against thermal melting of duplex DNA (Figure 5). Both the rate and yield of the P-Ap cross-link formation reported here are superior to those typically observed for the canonical nucleobases guanine and adenine.25,27 The reason(s) for this deserve further investigation, but it is worth noting that Sczepanski et al. previously observed a similar superiority of P over other nucleobases in reactions with oxidized abasic sites.55
The best cross-link yield was obtained when the P residue was located 1 nt to the 5’-side of the Ap site on the opposing strand (Figures 1A and 6), but gel electrophoretic evidence indicates that cross-links also form in other orientations (Figure 6). The preferred sequence motif for the P-Ap cross-link (seen in duplex B) may arise naturally from the helical twist of duplex DNA combined with the groove location of the amine residue on the nucleobase. This analysis begins with the recognition that Ap-containing duplexes may retain a B-like structure, especially when a purine residue directly opposes the Ap site.56,57 Importantly, the distances between the C1-atom of the Ap site and the exocyclic N2 amino group of P in the minor groove are expected to be significantly different depending upon whether the P residue is shifted to the 5’- or 3’-side of the Ap site (duplexes A and I, respectively). In a B-helix, when the P residue is offset to the 5’-side of the Ap site (reading on the Ap strand, see: duplex B), the N2-C1 distance is approximately 4 Å; whereas, when the P residue is offset to the 3’-side of the Ap site (duplex I) the N2-C1 distance is approximately 9 Å (Figure 7). An identical structural imperative can be used to rationalize the preferred sequence motif for dG-Ap cross-link formation, where the amine nucleophile similarly resides in the minor groove.27 The same logic also explains the preferred sequence motif for dA-Ap cross-link formation, in which the A-residue is offset to the 3’-side of the Ap site because the N6-amino group resides in the major groove of the double helix.25 This type of structural analysis can be used to rationalize the preferred sequence motif is preferred for the generation of Ap-derived cross-links involving N4-aminocytidine in which the hydrazine nucleophile resides in the major groove.33 Our analysis is informed by Hopkins’ incisive description of how helical twist and groove locations of the exocyclic amino groups of adenine and guanine residues combine to dictate the preferred sequence motifs for cross-link formation by agents such as mitomycin C and formaldehyde.58 Finally, we suggest that cross-link formation involving a residue directly opposing an Ap site (e.g. duplex J) is generally disfavored because the reaction requires “pinching” the double helix below its normal 20 Å width, necessitating staggering or zippering of flanking base pairs to accommodate the new covalent bond.
Figure 7. The preferred sequence for P-Ap cross-link formation may arise naturally from the helical twist of duplex DNA combined with the groove location of the amine residue on the nucleobase.
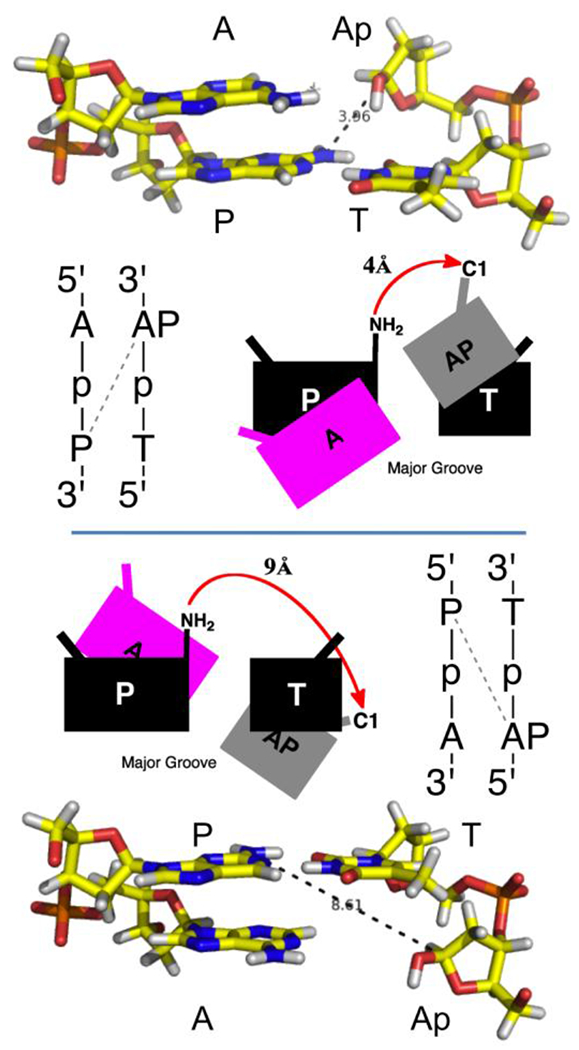
In a B-helical structure, containing the preferred sequence motif for cross-link formation (top), the C1-atom of the Ap site and the exocyclic N2-amino group of P are in close proximity, suggesting a facile reaction in which little structural reorganization is required. In the disfavored sequence motif (bottom), the two reaction centers are separated by approximately 9 Å, suggesting that significant and energetically costly structural rearrangements may be required to accommodate cross-link formation. Structures were built by modification of pdb code 2kv0.
Overall, we find that the reductive amination reaction between an appropriately positioned P residue with an Ap site in duplex DNA generates a high yield of a stable, covalent, site specific, chemically-defined ICL. The preparation of these cross-linked duplexes requires only common benchtop manipulations (e.g. pipeting, microcentrifugation, and gel electrophoresis) and commercially available oligonucleotides and chemical reagents. The method is suitable for preparation of substantial amounts (≥30 nmol) of cross-linked duplexes and, therefore, may be useful for the preparation of cross-linked duplexes for various applications including structural biology, biophysical studies, and the investigation of DNA repair mechanisms.
Supplementary Material
ACKNOWLEDGMENTS
KSG and YW thank the National Institute of Environmental Health Science of the National Institutes of Health for support of this work (ES 021007). NP was supported in part by an NRSA T32 institutional training grant (ES018827).
Footnotes
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.biochem
Methods, formation of reduced P-Ap cross-link, complete gel image; time course for formation of unreduced cross-link, footprinting to pinpoint location of cross-link attachment in duplex containing P-Ap cross-link, LC-MS/MS/MS analysis of duplex containing unreduced P-Ap cross-link; LC-MS/MS/MS analysis of duplex containing reduced P-Ap cross-link; time-course for dissociation of unreduced P-Ap cross-link in a DNA duplex; biphasic melting of cross-linked duplex containing reduced P-Ap cross-link in which P is paired with a C residue.
The authors declare no competing financial interest.
REFERENCES
- (1).Abdallah HO, Ohayon YP, Chandrasekaran AR, Sha R, Fox KR, Brown T, Rusling DA, Mao C and Seeman NC (2016) Stabilisation of self-assembled DNA crystals by triplex-directed photo-cross-linking. Chem. Comm 52, 8014–8017. [DOI] [PubMed] [Google Scholar]
- (2).Rajendran A, Endo M, Katsuda Y, Hidaka K and Sugiyama H (2011) Photo-cross-linking-assisted thermal stability of DNA origami structures and its application for higher-temperature self assembly. J. Am. Chem. Soc 133, 14488–14491. [DOI] [PubMed] [Google Scholar]
- (3).Erlanson DA, Chen L and Verdine GL (1993) DNA methylation through a locally unpaired intermediate. J. Am. Chem. Soc 115, 12583–12584. [Google Scholar]
- (4).Fujimoto K, Yamada A, Yoshimura Y, Tsukaguchi T and Sakamoto T (2013) Details of the ultrafast DNA photo-cross-linking reaction of 3-cyanovinylcarbazole nucleoside: cis-trans isomeric effect and the application for SNP-based genotyping. J. Am. Chem. Soc 135, 16161–16167. [DOI] [PubMed] [Google Scholar]
- (5).Imani-Nejad M, Shi R, Zhang X, Gu L-Q and Gates KS (2017) Sequence-specific covalent capture coupled with high-contrast nanopore detection of a disease-derived nucleic acid sequence. ChemBioChem 18, 1383–1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Shi R, Imani-Nejad M, Zhang X, Gu L-Q and Gates KS (2018) Generation and single-molecule characterization of a sequence-selective covalent cross-link mediated by mechlorethamine at a C-C mismatch in duplex DNA for discrimination of a disease-relevant single nucleotide polymorphism. Bioconjugate Chem, DOI: 10.1021/acs.bioconjchem.1028b00663. [DOI] [PubMed] [Google Scholar]
- (7).Price NE, Li L, Gates KS and Wang Y (2017) Replication and repair of a reduced 2’-deoxyguanosine-abasic site cross-link in human cells. Nucleic Acids Res. 45, 6486–6493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Roy U, Mukherjee S, Sharma A, Frank EG and Schärer OD (2016) The structure and duplex content of DNA interstrand cross-links affects the activity of DNA polymerase eta. Nucleic Acids Res. 44, 728–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Semlow DR, Zhang J, Budzowska M, Drohat AC and Walter JC (2016) Replication-dependent unhooking of DNA interstrand cross-links by the NEIL3 glycosylase. Cell 167, 498–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Kato N, Kawasoe Y, Williams HL, Coates E, Roy U, Shi Y, Beese LS, Schärer OD, Yan H, Gottesman ME, Takahashi TS and Gautier J (2017) Sensing and processing of DNA interstrand crosslinks by the mismatch repair pathway. Cell Rep. 21, 1375–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Huang H and Hopkins PB (1993) DNA interstrand cross-linking by formaldehyde: nucleotide sequence preference and covalent structure of the predominant cross-link formed in synthetic oligonucleotides. J. Am. Chem. Soc 115, 9402–9408. [Google Scholar]
- (12).Millard JT, Raucher S and Hopkins PB (1990) Mechlorethamine cross-links deoxyguanosine residues at 5’-GNC sequences in duplex DNA fragments. J. Am. Chem. Soc 112, 2459–2460. [Google Scholar]
- (13).Imani-Nejad M, Johnson KM, Price NE and Gates KS (2016) A new cross-link for an old cross-linking drug: the nitrogen mustard anticancer agent mechlorethamine generates cross-links derived from abasic sites in addition to the expected drug-bridged cross-links. Biochemistry 55, 7033–7041. [DOI] [PubMed] [Google Scholar]
- (14).Tomás-Gamasa M, Serdjukow S, Su M, Müller M and Carell T (2014) “Post-it” type connected DNA created with a reversible covalent cross-link. Angew. Chem. Int. Ed. Eng 53, 796–800. [DOI] [PubMed] [Google Scholar]
- (15).Pujari SS, Leonard P and Seela F (2014) Oligonucleotides with “clickable” sugar residues: synthesis, duplex stability, and terminal versus central interstrand cross-linking of 2′-O-propargylated 2-aminoadenosine with a bifunctional azide. J. Org. Chem 79, 4423–4437. [DOI] [PubMed] [Google Scholar]
- (16).Harwood EA, Hopkins PB and Sigurdsson ST (2000) Chemical synthesis of cross-link lesions found in nitrous acid treated DNA: A general method for the preparation of N2-substituted 2’-deoxyguanosines. J. Org. Chem 65, 2959–2964. [DOI] [PubMed] [Google Scholar]
- (17).Mukherjee S, Guainazzi A and Schärer OD (2014) Synthesis of struturally diverse major groove DNA interstrand crosslinks using three different aldehyde precursors. Nucleic Acids Res. 42, 7429–7435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Haque MM, Sun H, Liu S, Wang Y and Peng X (2014) Photoswitchable formation of a DNA interstrand cross-link by a coumarin-modified oligonucleotide. Angew. Chem. Int. Ed. Eng 53, 7001–7005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Varshney U and van de Sande JH (1991) Specificities and kinetics of uracil excision from uracil-containing DNA oligomers by Escherichia coli uracil DNA glycosylase. Biochemistry 30, 4055–4061. [DOI] [PubMed] [Google Scholar]
- (20).Stuart GR and Chambers RW (1987) Synthesis and properties of oligodeoxynucleotides with an AP site at a preselected position. Nucleic Acids Res. 15, 7451–7462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Péoc’h D, Meyer A, Imbach J-L and Rayner B (1991) Efficient chemical synthesis of oligodeoxynucleotides containing a true abasic site. Tetrahedron Lett. 32, 207–210. [Google Scholar]
- (22).Shishkina IG and Johnson F (2000) A new method for the postsynthetic generation of abasic sites in oligomeric DNA. Chem. Res. Toxicol 13, 907–912. [DOI] [PubMed] [Google Scholar]
- (23).Iocono JA, Gildrea B and McLaughlin LW (1990) Mild acid hydrolysis of 2-pyrimidinone-containing DNA fragments generates apurinic/apyrimidinic sites. Tetrahedron Lett. 31, 175–178. [Google Scholar]
- (24).Wilde JA, Bolton PH, Mazumdar A, Manoharan M and Gerlt JA (1989) Characterization of the equilibrating forms of the abasic site in duplex DNA using 17O-NMR. J. Am. Chem. Soc 111, 1894–1896. [Google Scholar]
- (25).Price NE, Johnson KM, Wang J, Fekry M,I, Wang Y and Gates KS (2014) Interstrand DNA–DNA Cross-Link Formation Between Adenine Residues and Abasic Sites in Duplex DNA. J. Am. Chem. Soc 136, 3483–3490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Dutta S, Chowdhury G and Gates KS (2007) Interstrand crosslinks generated by abasic sites in duplex DNA. J. Am. Chem. Soc 129, 1852–1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Johnson KM, Price NE, Wang J, Fekry MI, Dutta S, Seiner DR, Wang Y and Gates KS (2013) On the Formation and Properties of Interstrand DNA-DNA Cross-links Forged by Reaction of an Abasic Site With the Opposing Guanine Residue of 5’-CAp Sequences in Duplex DNA. J. Am. Chem. Soc 135, 1015–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Dallmann A, Dehmel L, Peters T, Mügge C, Griesinger C, Tuma J and Ernsting NP (2010) 2-Aminopurine incorporation perturbs the dynamics and structure of DNA. Angew. Chem. Int. Ed. Eng 49, 5989–5992. [DOI] [PubMed] [Google Scholar]
- (29).Law SM, Eritja R, Goodman MF and Breslauer KJ (1996) Spectroscopic and colorimetric characterization of DNA duplexes containing 2-aminopurine. Biochemistry 35, 12329–12337. [DOI] [PubMed] [Google Scholar]
- (30).Fagan PA, Fábrega C, Eritja R, Goodman MF and Wemmer DE (1996) NMR study of the conformation of the 2-aminopurine:cytosine mismatch in DNA. Biochemistry 35, 4026–4033. [DOI] [PubMed] [Google Scholar]
- (31).Shipova K and Gates KS (2005) A fluorimetric assay for the spontaneous release of an N7-alkylguanine residue from duplex DNA. Bioorg. Med. Chem. Lett 15, 2111–2113. [DOI] [PubMed] [Google Scholar]
- (32).Jones AC and Neely RK (2015) 2-aminopurine as a fluorescent probe of DNA conformation and the DNA–enzyme interface. Quart. Rev. Biophys 48, 244–279. [DOI] [PubMed] [Google Scholar]
- (33).Gamboa Varela J and Gates KS (2015) A Simple, High-Yield Synthesis of DNA Duplexes Containing a Covalent, Thermally-Reversible Interstrand Cross-link At a Defined Location Angew. Chem. Int. Ed. Eng 54, 7666–7669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Yang Z, Johnson KM, Price NE and Gates KS (2015) Characterization of interstrand DNA-DNA cross-links derived from abasic sites using bacteriophage φ29 DNA polymerase. Biochemistry 54, 4259–4266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Price NE, Catalano MJ, Liu S, Wang Y and Gates KS (2015) Chemical and structural characterization of interstrand cross-links formed between abasic sites and adenine residue in duplex DNA. Nucleic Acids Res. 43, 3434–3441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Gamboa Varela J and Gates KS (2016) Simple, high-yield syntheses of DNA duplexes containing interstrand DNA-DNA cross-links between an N4-aminocytidine residue and an abasic site. Curr. Protoc. Nucleic Acid Chem 65, 5.16.11–15.16.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Takashita M, Chang C-N, Johnson F, Will S and Grollman AP (1987) Oligodeoxynucleotides containing synthetic abasic sites. J. Biol. Chem 262, 10171–10179. [PubMed] [Google Scholar]
- (38).Johnson RF, Pickett SC and Barker DL (1990) Autoradiography using storage phosphor technology. Electrophoresis 11, 355–360. [DOI] [PubMed] [Google Scholar]
- (39).Maxam AM and Gilbert W (1980) Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 65, 499–560. [DOI] [PubMed] [Google Scholar]
- (40).Gates KS (2009) An overview of chemical processes that damage cellular DNA: spontaneous hydrolysis, alkylation, and reactions with radicals. Chem. Res. Toxicol 22, 1747–1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Gates KS, Nooner T and Dutta S (2004) Biologically relevant chemical reactions of N7-alkyl-2’-deoxyguanosine adducts in DNA. Chem. Res. Toxicol 17, 839–856. [DOI] [PubMed] [Google Scholar]
- (42).Borch RF, Bernstein MD and Durst HD (1971) The cyanohydridoborate anion as a selective reducing agent. J. Am. Chem. Soc 93, 2897–2904. [Google Scholar]
- (43).Kurtz AJ, Dodson ML and Lloyd RS (2002) Evidence for multiple imino intermediates and identification of reactive nucleophiles in peptide-catalyzed beta-elimination at abasic sites. Biochemistry 41, 7054–7064. [DOI] [PubMed] [Google Scholar]
- (44).Admiraal SJ and O’Brien PJ (2015) Base Excision Repair Enzymes Protect Abasic Sites in Duplex DNA from Interstrand Cross-Links. Biochemistry 54, 1849–1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Pogozelski WK, McNeese TJ and Tullius TD (1995) What species is responsible for strand scission in the reaction of [Fe(II)EDTA]2− and H2O2 with DNA? J. Am. Chem. Soc 117, 6428–6433. [Google Scholar]
- (46).Luce RA and Hopkins PB (2001) Chemical cross-linking of drugs to DNA. Methods Enzymol. 340, 396–412. [DOI] [PubMed] [Google Scholar]
- (47).Sczepanski JT, Jacobs AC, Majumdar A and Greenberg MM (2009) Scope and mechanism of interstrand crosslink formation by the C4’-oxidized abasic site. J. Am. Chem. Soc 131, 11132–11139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (48).Lai C, Cao H, Hearst JE, Corash L, Luo H and Wang Y (2008) Quantitative Analysis of DNA Interstrand Cross-Links and Monoadducts Formed in Human Cells Induced by Psoralens and UVA Irradiation. Anal. Chem 80, 8790–8798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49).Cao H, Hearst JE, Corash L and Wang Y (2008) LC-MS/MS for the Detection of DNA Interstrand Cross-Links Formed by 8-Methoxypsoralen and UVA Irradiation in Human Cells. Anal. Chem 80, 2932–2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (50).Wang Y and Wang Y (2003) Structure elucidation of DNA interstrand crosslink lesions by a combination of nuclease P1 digestion with mass spectrometry. Anal. Chem 75, 6306–6313. [DOI] [PubMed] [Google Scholar]
- (51).Catalano MJ, Liu S, Andersen N, Yang Z, Johnson KM, Price NA, Wang Y and Gates KS (2015) Chemical structure and properties of the interstrand cross-link formed by the reaction of guanine residues with abasic sites in duplex DNA. J. Am. Chem. Soc 137, 3933–3945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (52).Stevens K and Madder A (2009) Furan-modified oligonucleotides for fast, high-yielding and site-selective DNA inter-strand cross-linking with non-modified complements. Nucleic Acids Res. 37, 1555–1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (53).Senior MM, Jones RA and Breslauer KJ (1988) Influence of loop residues on the relative stabilities of DNA hairpin structures. Proc. Nat. Acad. Sci. USA 85, 6242–6246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (54).Enoiu M, Ho TV, Long DT, Walter JC and Scharer OD (2012) Construction of plasmids containing site-specific DNA interstrand cross-links for biochemical and cell biological studies. Methods Mol. Biol 920, 203–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (55).Sczepanski JT, Heimstra CN and Greenberg MM (2011) Probing DNA interstrand cross-link formation by an oxidized abasic site using nonnative nucleotides. Bioorg. Med. Chem 19, 5788–5793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (56).Chen J, Dupradeau F-Y, Case DA, Turner CJ and Stubbe J (2008) DNA oligonucleotides with A, T, G, or C opposite an abasic site: structure and dynamics. Nucleic Acids Res. 36, 253–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (57).Lhomme J, Constant J-F and Demeunynck M (2000) Abasic DNA structure, reactivity, and recognition. Biopolymers 52, 65–83. [DOI] [PubMed] [Google Scholar]
- (58).Hopkins PB, Millard JT, Woo J, Weidner MF, Kirchner JJ, Sigurdsson ST and Raucher S (1991) Sequence preferences of DNA interstrand cross-linking agents: Importance of minimal DNA structural reorganization in the cross-linking reactions of mechlorethamine, cisplatin, and mitomycin C. Tetrahedron 47, 2475–2489. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


