Abstract
Objectives
To report clinical outcome, development of humoral and T-cell mediated immunity in convalescent COVID-19 people with multiple sclerosis (pwMS) treated with ofatumumab in the ALITHIOS study from a single center.
Methods
Testing for SARS-Cov2 IgG antibodies was performed on two occasions with at least three months apart between the two testing. During the second antibody testing, interferon-γ ELISpot was used to assess cellular immunity.
Results
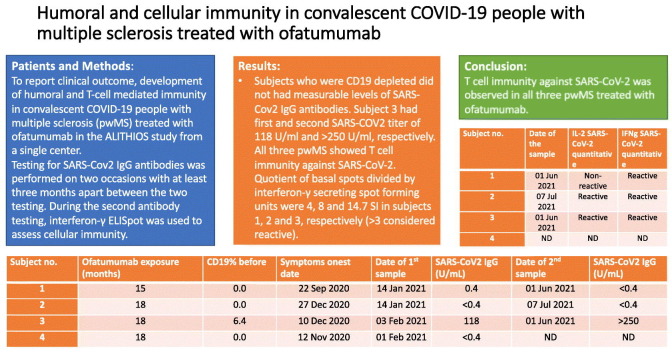
All four subjects had mild COVID-19 infection without any sequelae. In all subjects except subject 2, COVID-19 was confirmed with PCR. Subjects 1, 2 and 4 had normal levels of IgM and IgG without measurable counts of CD19 cells prior to COVID-19. Subject 3 administered the last dose of ofatumumab 24 days prior to COVID-19 symptoms, but had a gap of 28 weeks of ofatumumab application beforehand due to low IgM levels. Subject 4 received COVID-19 vaccinations before second testing, so second testing and T-cell immunity testing were not performed. Subjects who were CD19 depleted did not had measurable levels of SARS-Cov2 IgG antibodies. Subject 3 had first and second SARS-COV2 titer of 118 U/ml and > 250 U/ml, respectively. All three pwMS showed T cell immunity against SARS-CoV-2. Quotient of basal spots divided by interferon-γ secreting spot forming units were 4, 8 and 14.7 SI in subjects 1, 2 and 3, respectively (>3 considered reactive).
Conclusion
While no antibody response was observed in pwMS who were CD19+ lymphocyte depleted, T cell immunity against SARS-CoV-2 was observed in all three pwMS treated with ofatumumab.
Keywords: Multiple sclerosis, Ofatumumab, COVID-19, Antibodies, T-cell immunity
Graphical abstract
1. Introduction
Ofatumumab is a fully human anti-CD20 monoclonal antibody approved for the treatment of active relapsing multiple sclerosis (MS). It is administered as a monthly subcutaneous injection and its efficacy and safety have been demonstrated in two phase III randomized controlled trials (Hauser et al., 2020). The current global pandemic has caused great concern regarding the use of lymphocyte depleting agents in MS and the risk of COVID-19. Reports regarding the use of other anti-CD20 therapies in persons with multiple sclerosis (pwMS), such as ocrelizumab and rituximab are conflicting. While three studies did not show association between B-cell depleting DMTs and higher probability of a more serious clinical course of COVID-19 (Hughes et al., 2020; Louapre et al., 2020; Salter et al., 2021), two studies indicated that treatment with ocrelizumab or rituximab was associated with increased risk of severe COVID-19 (Sormani et al., 2021a; Stastna et al., 2021). Whether this is also true for ofatumumab treated pwMS is not known. Another important question is how do the B-cell depleting agents affect the development of humoral and cellular immunity after the infection and whether there is an impact on the vaccine response.
The aim of the present study is to report clinical outcome, development of anti-SARS-Cov2 antibodies and development of T-cell mediated immunity in convalescent COVID-19 pwMS treated with ofatumumab in ALITHIOS study from a single center.
2. Materials and methods
Four pwMS with COVID-19 who were treated with 20 mg of ofatumumab subcutaneously every four weeks were identified. The subjects were previously enrolled into the phase III ASCLEPIOS trial and continued with the open-label extension study (Hauser et al., 2020).
Testing for humoral immunity was performed in the Clinical Institute for Laboratory Diagnostics, University Hospital Center Zagreb, Zagreb, Croatia. Blood samples were drawn during the next two study visits after the recovery of COVID-19, with at least three months apart between two tests. Testing for SARS-CoV2 antibodies was performed per the manufacturer's instructions, using Cobas e 801 analytical unit for immunoassay tests (F. Hoffmann-La Roche Ltd.) (https://diagnostics.roche.com/global/en/products/params/elecsys-anti-sars-cov-2.html, n.d.). Antibody titer ≥0.8 U/mL was considered positive, as recommended by the manufacturer.
During the second antibody testing ELISpot, was used to assess cellular immunity. This testing was performed in SGS Analytics Laboratory, Germany GmbH, München, Germany. For ELISpot analysis, the CoV-iSpot Interferon-g + Interleukin 2 kit (AID Autoimmun Diagnostika GmbH, Straßberg, Germany) was used according to the manufacturer's instructions.
Shortly, isolated peripheral blood mononuclear cells (PBMC) were stimulated with two peptide mixes (Pan Corona peptide mix (covers multiple different corona subtypes) and SARS-CoV-2 specific peptide mix) and a negative control. 200.000 cells (freshly isolated PBMCs) per well were seeded and incubated with SARS-CoV-2 peptide mix for 20 h. Pokeweed mitogen was used as positive control. SARS-CoV-2 peptide mix consists of two antigen-specific peptide pools. The first peptide pool consists of 26 peptides with a length of 15 to 22 amino acids from the spike protein (Seq ID YP_009724390.1) and is referred to as the SARS-CoV-2 S peptide pool. The second pool consists of 10 highly specific sequences of the nucleocapsid protein (Seq ID YP_009724397.2), the matrix glycoprotein (Seq ID YP_009724393.1) and the coat protein (Seq ID YP_009724392.1) with a length of 10 to 20 amino acids, and is referred to as the SARS-CoV-2 NME peptide pool. The two pools were combined and are available as a SARS-CoV-2 peptide mix. Most of the SARS-CoV-2 specific peptides contained in the AID SARS-CoV-2 peptide mix is located in the N-terminal area of the spike protein, while that in the AID PAN-Corona conserved regions contained in the peptide mix represent the C-terminal area.
Section of two cytokines was measured: interferon γ (IFNg) and interleukin-2 (IL-2). Number of spots/spot forming units was counted and depending on the number of spots after stimulation compared to the basal spots, the response was classified as reactive, nonreactive or equivocal. The thresholds for classification are as follows: if basal spots 0–1 SI: qualitative ≤5 – nonreactive, qualitative between 5 and 7 – equivocal and qualitative ≥7 – reactive; if basal spots 2–20: qualitative ≤2 – nonreactive, qualitative between 2 and ≤ 3 – equivocal and qualitative >3 – reactive.
In order to determine background levels in the assays utilized, historical data from the laboratory on cellular immunity from healthy controls and non-MS convalescent patients was used.
3. Results
Demographic and clinical characteristics of the subjects along with laboratory findings are summarized in Table 1 . Subject 1 developed fever, muscle and joint pain lasting for three days. Pharyngeal swab testing for the SARS-CoV-2 viral RNA was positive. Subject 2 had fever, anosmia, ageusia, headache and fatigue lasting for twelve days. Testing for SARS-CoV-2 was not performed, but two members of her household tested positive for SARS-CoV-2 viral RNA. Subject 3 experienced fever and muscle pain for twelve days and her pharyngeal swab testing for the SARS-CoV-2 viral RNA was positive. Subject 4 experienced fever and muscle pain that lasted for five days with positive pharyngeal swab testing for the SARS-CoV-2 viral RNA. None of the subjects were hospitalized due to COVID-19. They received antipyretics and analgesics as needed, including acetaminophen, ibuprofen and diclofenac. Subject 3 received three days of 500 mg azithromycin daily. All of the patients recovered without sequelae.
Table 1.
Characteristics of patients on ofatumumab and COVID-19. Levels of CD19, IgG, and IgM levels before the infection are presented as well as PCR findings of nasopharyngeal swabs for the presence of SARS-Cov2 and finding of SARS-Cov2 antibodies after the infection.
| Subject no. | Age, Sex | Ofatumumab exposure (months) | CD19% before | IgM before (g/L) | IgG before (g/L) | Sars-Cov2 PCR | Symptoms onset date | Symptom duration (days) | Date of 1st sample | SARS-CoV2 IgG (U/mL) | Date of 2nd sample | SARS-CoV2 IgG (U/mL) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M, 35 | 15 | 0.0 | 0.59 | 10.60 | + | 22 Sep 2020 | 3 | 14 Jan 2021 | 0.4 | 01 Jun 2021 | <0.4 |
| 2 | F, 31 | 18 | 0.0 | 0.82 | 8.45 | ND | 27 Dec 2020 | 12 | 14 Jan 2021 | <0.4 | 07 Jul 2021 | <0.4 |
| 3 | F, 34 | 18 | 6.4 | 0.38 | 7.95 | + | 10 Dec 2020 | 12 | 03 Feb 2021 | 118 | 01 Jun 2021 | >250 |
| 4 | F, 32 | 18 | 0.0 | 1.91 | 11.0 | + | 12 Nov 2020 | 5 | 01 Feb 2021 | <0.4 | ND | ND |
M-male. F-female. IgM-immunoglobulin M. IgG-immunoglobulin G. PCR-polymerase chain reaction. ND-not done.
Reference range: CD19 (6.5–27.0%), IgM (0.40–2.30 g/L), IgG (5.65–17.65 g/L), ACOV2 S (<0.8 U/mL)
All subjects had normal levels of IgM and IgG before the infection. In subjects 1,2 and 4, CD19+ B cells were depleted, and these were the patients that were adherent with their monthly ofatumumab injections. As well, these three subjects tested negative for SARS-Cov2 antibodies following the infection on first testing. Subject 4 received COVID-19 vaccinations before second testing, so both second testing and T-cell immunity testing were not performed. Subject 3 administered the last dose of ofatumumab 24 days prior to COVID-19 symptoms but had a gap of 28 weeks of ofatumumab application beforehand due to low IgM levels. That subject tested positive for SARS-COV-2 IgG antibodies on both measurements, with increasing titers with time.
Using interferon-γ ELISpot, we observed that subjects 1, 2 and 3 showed T cell immunity against SARS-CoV-2 (Table 2 ). Quotient of basal spots divided by interferon-γ secreting spot forming units were 4, 8 and 14.7 SI in subjects 1, 2 and 3, respectively. ELISpot data from healthy controls and non-MS convalescent patients are presented in Table 2.
Table 2.
Results of the cellular immunity using interleukin-2 (IL-2) and interferon-γ (IFNg) ELISpot in pwMS on ofatumumab, healthy controls, and non-MS convalescent patients.
| Subject no. | Age, Sex | Date of the sample | ELISpot: IL-2 basal spots (SI) | ELISpot: IL-2 SARS-CoV-2 spots (SI) | IL-2 SARS-CoV-2 quantitative | ELISpot: IFNg basal spots (SI) | ELISpot: IFNg SARS-CoV-2 spots (SI) | IFNg SARS-CoV-2 quantitative |
|---|---|---|---|---|---|---|---|---|
| 1 | M, 35 | 01 Jun 2021 | 4 | 8 | Non-reactive | 2 | 8 | Reactive |
| 2 | F, 31 | 07 Jul 2021 | 2 | 12 | Reactive | 0 | 8 | Reactive |
| 3 | F, 34 | 01 Jun 2021 | 9 | 52 | Reactive | 3 | 44 | Reactive |
| 4 | F, 32 | ND | ND | ND | ND | ND | ND | ND |
| Healthy control – 1 | F, 47 | 19 Nov 2020 | 0 | 0 | Non-reactive | 0 | 0 | Non-reactive |
| Healthy control – 2 | F, 38 | 19 Nov 2020 | 5 | 4 | Non-reactive | 2 | 1 | Non-reactive |
| non-MS convalescent patient – 1 | F, 48 | 18 Nov 2020 | 1 | 25 | Reactive | 0 | 27 | Reactive |
| non-MS convalescent patient – 2 | F, 38 | 18 Nov 2020 | 1 | 23 | Reactive | 1 | 19 | Reactive |
ND-not done, MS- multiple sclerosis.
4. Discussion
This report provides clinical and laboratory data on COVID-19 in pwMS treated with ofatumumab. To the best of our knowledge, there is only one prior report on COVID-19 and ofatumumab. Flores-Gonzalez et al. presented a patient treated with ofatumumab who had complete B-cell depletion with normal serum IgM and IgG values (Flores-Gonzalez et al., 2021). The patient had an asymptomatic COVID-19 with an adequate humoral response and presence of anti-SARS-CoV-2 IgG three months after the infection. In contrast, three patients presented in this study who had complete CD19+ B-cell depletion did not mount an antibody response. On the other hand, patient 3, whose B-cells recovered due to ofatumumab dosing interruption, had anti-SARS-Cov2 antibodies.
These finding raise several important points. Three of the reported subjects who were depleted of CD19+ lymphocytes did not develop an antibody response, which corroborates findings from ocrelizumab treated patients in whom therapy with anti-CD20 monoclonal antibodies was significantly associated with a reduced probability of developing antibodies after COVID-19 (Sormani et al., 2021b; Bigaut et al., 2021). This raises the question whether patients who recovered from COVID-19 and did not develop an antibody response will in fact have adequate immunity from subsequent SARS-Cov-2 infections. Studies on healthy people and subjects taking rituximab for rheumatoid arthritis have shown that T-cell mediated responses may contribute to protection against SARS-CoV-2 (Sekine et al., 2020; DiPiazza et al., 2021; Bonelli et al., 2021; Benucci et al., 2021). SARS-CoV-2-specific memory lymphocytes exhibit characteristics associated with potent antiviral function: memory T cells secret cytokines and expand upon antigen re-encounter (Rodda et al., 2021). Maybe the best example of the importance of T cells in human immunity to SARS-CoV-2 are case studies of COVID-19 patients with agammaglobulinemia. COVID-19 patients with X-linked or autosomal recessive agammaglobulinemia were able to recover from infection without oxygen ventilation or intensive care, suggesting that while B cells and antibodies are critical for preventing infection or reducing inoculum size, T cell responses may be sufficient to clear infection with minimal disease (Soresina et al., 2020). Data on T-cell immunity in people with MS taking another B-cell depleting therapy, Ocrelizumab are emerging, emphasizing T-cell immunity as an important factor in the protection against SARS-CoV-2. The first study compared B cell and T cell responses longitudinally in 20 pwMS on anti-CD20 antibody monotherapy with 10 HC after BNT162b2 or mRNA-1273 mRNA vaccination. In this study all pwMS treated with aCD20 therapy generated antigen-specific CD4 and CD8 T cell responses after vaccination (Apostolidis et al., 2021). Moreover, several subsequent studies, some of which have still not been peer-reviewed, confirmed that pwMS who were treated with ocrelizumab generated comparable SARS-CoV-2-specific T-cell responses with healthy controls and/or pwMS on other MS therapies (Brill et al., 2021; Sabatino et al., 2021; Gadani et al., 2021). However, it is not yet clear to which extent of T cell response and for how long it is adequate to protect patients against virus infection after recovery from COVID-19 or vaccination.
Finally, the presented subject whose levels of CD19+ lymphocytes recovered had an adequate antibody response to SARS-Cov-2. This has implications not only for long-term COVID-19 immunity but as well as for COVID-19 vaccine readiness. It has been demonstrated that pwMS treated with ocrelizumab have an attenuated response to vaccines (Bar-Or et al., 2020). Ofatumumab, unlike ocrelizumab, has a relative rapid repopulation of CD20+ lymphocytes after treatment interruption (Baker et al., 2020). In that way, a treatment gap could be made before COVID-19 vaccination in order to enable a proper antibody response. The data for ocrelizumab suggest that delaying the dose up to nine months since the previous one is associated with B-cell repopulation but without clinical signs of MS activity (Barun et al., 2021). However, how long ofatumumab should be withheld and what effect that would leave on possible disease reactivation is not yet known.
In conclusion, we report on four pwMS treated with ofatumumab with mild COVID-19. While no antibody response was observed in pwMS who were CD19+ lymphocyte depleted, T cell immunity against SARS-CoV-2 was observed in all three pwMS treated with ofatumumab. A COVID-19 substudy in the ALITHIOS trial is currently ongoing to further investigate and verify these findings.
Authors' contributions
Study concept and design: Adamec, Habek. Acquisition of data: Adamec, Rogić, Penz, Braun, Habek. Analysis and interpretation of data: Adamec, Rogić, Penz, Braun, Habek. Drafting of the manuscript: Habek. Critical revision of the manuscript for important intellectual content: Adamec, Rogić, Penz, Braun, Habek. Administrative, technical, and material support: Adamec, Rogić, Penz, Braun, Habek.
Funding
This study was funded by Novartis Pharma AG, Basel, Switzerland.
Financial & competing interest disclosure
IA: Participated as a clinical investigator and/or received consultation and/or speaker fees from: Biogen, Sanofi Genzyme, Merck, Bayer, Novartis, Pliva/Teva, Roche, Alvogen, Actelion, Alexion Pharmaceuticals, TG Pharmaceuticals.
DR: Reports no conflict of interest.
MH: Participated as a clinical investigator and/or received consultation and/or speaker fees from: Biogen, Sanofi Genzyme, Merck, Bayer, Novartis, Pliva/Teva, Roche, Alvogen, Actelion, Alexion Pharmaceuticals, TG Pharmaceuticals.
References
- Apostolidis S.A., Kakara M., Painter M.M., Goel R.R., Mathew D., Lenzi K., Rezk A., Patterson K.R., Espinoza D.A., Kadri J.C., Markowitz D.M., Markowitz C., Mexhitaj I., Jacobs D., Babb A., Betts M.R., ETL Prak, Weiskopf D., Grifoni A., Lundgreen K.A., Gouma S., Sette A., Bates P., Hensley S.E., Greenplate A.R., Wherry E.J., Li R., Bar-Or A. Cellular and humoral immune responses following SARS-CoV-2 mRNA vaccination in patients with multiple sclerosis on anti-CD20 therapy. Nat. Med. 2021 Sep;14 doi: 10.1038/s41591-021-01507-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker D., Roberts C.A.K., Pryce G., Kang A.S., Marta M., Reyes S., Schmierer K., Giovannoni G., Amor S. COVID-19 vaccine-readiness for anti-CD20-depleting therapy in autoimmune diseases. Clin. Exp. Immunol. 2020;202:149–161. doi: 10.1111/cei.13495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Or A., Calkwood J.C., Chognot C., Evershed J., Fox E.J., Herman A., Manfrini M., McNamara J., Robertson D.S., Stokmaier D., Wendt J.K., Winthrop K.L., Traboulsee A. Effect of ocrelizumab on vaccine responses in patients with multiple sclerosis: the VELOCE study. Neurology. 2020;95:e1999–e2008. doi: 10.1212/WNL.0000000000010380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barun B., Gabelić T., Adamec I., Babić A., Lalić H., Batinić D., Krbot Skorić M., Habek M. Influence of delaying ocrelizumab dosing in multiple sclerosis due to COVID-19 pandemics on clinical and laboratory effectiveness. Mult. Scler. Relat. Disord. 2021;48 doi: 10.1016/j.msard.2020.102704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benucci M., Damiani A., Infantino M., Manfredi M., Grossi V., Lari B., Gobbi F.L., Sarzi-Puttini P. Presence of specific T cell response after SARS-CoV-2 vaccination in rheumatoid arthritis patients receiving rituximab. Immunol. Res. 2021:1–3. doi: 10.1007/s12026-021-09212-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigaut K., Kremer L., Fabacher T., Lanotte L., Fleury M.C., Collongues N., de Seze J. Impact of disease-modifying treatments of multiple sclerosis on anti-SARS-CoV-2 antibodies: an observational study. Neurol. Neuroimmunol. Neuroinflamm. 2021;8 doi: 10.1212/NXI.0000000000001055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonelli M.M., Mrak D., Perkmann T., Haslacher H., Aletaha D. SARS-CoV-2 vaccination in rituximab-treated patients: evidence for impaired humoral but inducible cellular immune response. Ann. Rheum. Dis. 2021 doi: 10.1136/annrheumdis-2021-220408. annrheumdis-2021-220408. [DOI] [PubMed] [Google Scholar]
- Brill L., Rechtman A., Zveik O., Haham N., Oiknine-Djian E., Wolf D.G., Levin N., Raposo C., Vaknin-Dembinsky A. Humoral and T-cell response to SARS-CoV-2 vaccination in patients with multiple sclerosis treated with ocrelizumab. JAMA Neurol. 2021 Dec 1;78(12):1510–1514. doi: 10.1001/jamaneurol.2021.3599. e213599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiPiazza A.T., Graham B.S., Ruckwardt T.J. T cell immunity to SARS-CoV-2 following natural infection and vaccination. Biochem. Biophys. Res. Commun. 2021;538:211–217. doi: 10.1016/j.bbrc.2020.10.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores-Gonzalez R.E., Hernandez J., Tornes L., Rammohan K., Delgado S. Development of SARS-CoV-2 IgM and IgG antibodies in a relapsing multiple sclerosis patient on ofatumumab. Mult. Scler. Relat. Disord. 2021;49 doi: 10.1016/j.msard.2021.102777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadani S.P., Reyes-Mantilla M., Jank L., Harris S., Douglas M., Smith M.D., Calabresi P.A., Mowry E.M., Fitzgerald K.C., Bhargava P. Discordant humoral and T cell immune responses to SARS-CoV-2 vaccination in people with multiple sclerosis on anti-CD20 therapy. EBioMedicine. 2021 Oct 16;73:103636. doi: 10.1016/j.ebiom.2021.103636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser S.L., Bar-Or A., Cohen J.A., Comi G., Correale J., Coyle P.K., Cross A.H., de Seze J., Leppert D., Montalban X., Selmaj K., Wiendl H., Kerloeguen C., Willi R., Li B., Kakarieka A., Tomic D., Goodyear A., Pingili R., Häring D.A., Ramanathan K., Merschhemke M., Kappos L., ASCLEPIOS I and ASCLEPIOS II Trial Groups Ofatumumab versus teriflunomide in multiple sclerosis. N. Engl. J. Med. 2020;383:546–557. doi: 10.1056/NEJMoa1917246. [DOI] [PubMed] [Google Scholar]
- https://diagnostics.roche.com/global/en/products/params/elecsys-anti-sars-cov-2.html accessed May 27, 2020.
- Hughes R., Pedotti R., Koendgen H. COVID-19 in persons with multiple sclerosis treated with ocrelizumab - A pharmacovigilance case series. Mult. Scler. Relat. Disord. 2020;42 doi: 10.1016/j.msard.2020.102192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louapre C., Collongues N., Stankoff B., Giannesini C., Papeix C., Bensa C., Deschamps R., Créange A., Wahab A., Pelletier J., Heinzlef O., Labauge P., Guilloton L., Ahle G., Goudot M., Bigaut K., Laplaud D.A., Vukusic S., Lubetzki C., De Sèze J., Covisep investigators, Derouiche F., Tourbah A., Mathey G., Théaudin M., Sellal F., Dugay M.H., Zéphir H., Vermersch P., Durand-Dubief F., Françoise R., Androdias-Condemine G., Pique J., Codjia P., Tilikete C., Marcaud V., Lebrun-Frenay C., Cohen M., Ungureanu A., Maillart E., Beigneux Y., Roux T., Corvol J.C., Bordet A., Mathieu Y., Le Breton F., Boulos D.D., Gout O., Guéguen A., Moulignier A., Boudot M., Chardain A., Coulette S., Manchon E., Ayache S.S., Moreau T., Garcia P.Y., Kumaran D., Castelnovo G., Thouvenot E., Taithe F., Poupart J., Kwiatkowski A., Defer G., Derache N., Branger P., Biotti D., Ciron J., Clerc C., Vaillant M., Magy L., Montcuquet A., Kerschen P., Coustans M., Guennoc A.M., Brochet B., Ouallet J.C., Ruet A., Dulau C., Wiertlewski S., Berger E., Buch D., Bourre B., Pallix-Guiot M., Maurousset A., Audoin B., Rico A., Maarouf A., Edan G., Papassin J., Videt D. Clinical characteristics and outcomes in patients with coronavirus disease 2019 and multiple sclerosis. JAMA Neurol. 2020;77:1079–1088. doi: 10.1001/jamaneurol.2020.2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodda L.B., Netland J., Shehata L., Pruner K.B., Morawski P.A., Thouvenel C.D., Takehara K.K., Eggenberger J., Hemann E.A., Waterman H.R., Fahning M.L., Chen Y., Hale M., Rathe J., Stokes C., Wrenn S., Fiala B., Carter L., Hamerman J.A., King N.P., Gale M., Jr., Campbell D.J., Rawlings D.J., Pepper M. Functional SARS-CoV-2-specific immune memory persists after mild COVID-19. Cell. 2021;184:169–183.e17. doi: 10.1016/j.cell.2020.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatino J.J., Mittl K., Rowles W., Mcpolin K., Rajan J.V., Zamecnik C.R., Dandekar R., Alvarenga B.D., Loudermilk R.P., Gerungan C., Spencer C.M., Sagan S.A., Augusto D.G., Alexander J., Hollenbach J.A., Wilson M.R., Zamvil S.S., Bove R. Impact of multiple sclerosis disease-modifying therapies on SARS-CoV-2 vaccine-induced antibody and T cell immunity. medRxiv [Preprint]. 2021 Sep 20 doi: 10.1101/2021.09.10.21262933. 2021.09.10.21262933. [DOI] [Google Scholar]
- Salter A., Fox R.J., Newsome S.D., Halper J., Li D.K.B., Kanellis P., Costello K., Bebo B., Rammohan K., Cutter G.R., Cross A.H. Outcomes and risk factors associated with SARS-CoV-2 infection in a North American registry of patients with multiple sclerosis. JAMA Neurol. 2021 doi: 10.1001/jamaneurol.2021.0688. https://pubmed.ncbi.nlm.nih.gov/33739362/#:~:text=JAMA%20Neurol,10.1001/jamaneurol.2021.0688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekine T., Perez-Potti A., Rivera-Ballesteros O., Strålin K., Gorin J.B., Olsson A., Llewellyn-Lacey S., Kamal H., Bogdanovic G., Muschiol S., Wullimann D.J., Kammann T., Emgård J., Parrot T., Folkesson E., Karolinska COVID-19 Study Group, Rooyackers O., Eriksson L.I., Henter J.I., Sönnerborg A., Allander T., Albert J., Nielsen M., Klingström J., Gredmark-Russ S., Björkström N.K., Sandberg J.K., Price D.A., Ljunggren H.G., Aleman S., Buggert M. Robust T cell immunity in convalescent individuals with asymptomatic or mild COVID-19. Cell. 2020;183:158–168.e14. doi: 10.1016/j.cell.2020.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soresina A., Moratto D., Chiarini M., Paolillo C., Baresi G., Focà E., Bezzi M., Baronio B., Giacomelli M., Badolato R. Two X-linked agammaglobulinemia patients develop pneumonia as COVID-19 manifestation but recover. Pediatr. Allergy Immunol. 2020;31:565–569. doi: 10.1111/pai.13263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sormani M.P., De Rossi N., Schiavetti I., Carmisciano L., Cordioli C., Moiola L., Radaelli M., Immovilli P., Capobianco M., Trojano M., Zaratin P., Tedeschi G., Comi G., Battaglia M.A., Patti F., Salvetti M., Musc-19 Study Group Disease-modifying therapies and coronavirus disease 2019 severity in multiple sclerosis. Ann. Neurol. 2021 Apr;89(4):780–789. doi: 10.1002/ana.26028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sormani M.P., Schiavetti I., Landi D., Carmisciano L., De Rossi N., Cordioli C., Moiola L., Radaelli M., Immovilli P., Capobianco M., Brescia Morra V., Trojano M., Tedeschi G., Comi G., Battaglia M.A., Patti F., Fragoso Y.D., Sen S., Siva A., Furlan R., Salvetti M. SARS-CoV-2 serology after COVID-19 in multiple sclerosis: an international cohort study. Mult. Scler. 2021;13524585211035318 doi: 10.1177/13524585211035318. [DOI] [PubMed] [Google Scholar]
- Stastna D., Menkyova I., Drahota J., Mazouchova A., Adamkova J., Ampapa R., Grunermelova M., Peterka M., Recmanova E., Rockova P., Rous M., Stetkarova I., Valis M., Vachova M., Woznicova I., Horakova D. Multiple sclerosis, neuromyelitis optica spectrum disorder and COVID-19: A pandemic year in Czechia. Mult. Scler. Relat. Disord. 2021;54 doi: 10.1016/j.msard.2021.103104. [DOI] [PMC free article] [PubMed] [Google Scholar]