Abstract
A significant number of patients infected with the new coronavirus suffer from chronic fatigue syndrome after COVID-19, and their symptoms may persist for months after the infection. Nevertheless, no particular treatment for post-disease fatigue has been found. At the same time, many clinical trials have shown the effectiveness of l-carnitine in relieving fatigue caused by the treatment of diseases such as cancer, MS, and many other diseases. Therefore, it can be considered as a potential option to eliminate the effects of fatigue caused by COVID-19, and its consumption is recommended in future clinical trials to evaluate its effectiveness and safety.
Keywords: CFS, Chronic fatigue syndrome; PVN, paraventricular nucleus; ACE2, angiotensin-converting enzyme 2; RAS, renin-angiotensin system; IL, interleukin
Keywords: COVID-19, Fatigue, l-carnitine, Coronavirus
Highlights
-
•
The coronavirus disease is a viral infection that could induce different respiratory.
-
•
A significant number of patients infected with the new coronavirus suffer from chronic fatigue.
-
•
Clinical trials have shown the effectiveness of L-carnitine in relieving fatigue.
1. Introduction
The spread of SARS-CoV-2 in China occurred in December 2019 and the widespread of this virus around the world has so far killed hundreds of thousands of people and this mortality rate has affected the world's population [1,2]. The infection first targets the respiratory tract, and its early symptoms are shortness of breath and fever [2]. It is estimated that about 10–30% of the 147 million people, who are affected by the disease worldwide, suffer from the persistent and long-term effects of the disease [3]. Many patients complain of shortness of breath and tiredness long after the onset of this disease, which is called post-COVID or COVID-long syndrome [4,5]. Fatigue is often a sign of a serious disease such as cancer or autoimmune diseases. Chronic fatigue syndrome (CFS) is defined as a particular case in which most patients report other signs such as pain and cognitive impairment [6]. On the other hand, after viral infections, cases often experience inabilities in functions for a long-time following discharge from hospitals. In various patients, physical, cognitive, and mental disorders remain for several years [7,8]. Consistently, with advances in the study of coronavirus disease, it has been found that a high percentage of cases have continuous symptoms and one of them is fatigue [7]. As stated by the World Health Organization, cases with chronic fatigue may need rehabilitation for the management of the subsequent efficacies of the coronavirus disease; whereas, regretfully, there is no particular drug for the treatment of post-COVID-19 fatigue [9]. Considering that the treatment of fatigue is associated with difficulties, there is an urgency for efficient therapeutic options [10].
l-Carnitine is a trimethylated amino acid that is structurally similar to choline and is needed as a cofactor to convert long-chain free fatty acids to acylcarnitine and transfer them to the mitochondrial matrix [11]. Therefore, this compound plays a major role in the metabolism of fatty acids and its inadequacy will induce feelings of tiredness or general fatigue [6]. This amino acid can prevent muscle wasting or reduce the rate of muscle breakdown. l-Carnitine also has other properties such as modulating the composition and decomposition of proteins, as well as anti-apoptotic, antioxidant, and anti-inflammatory properties. Thus, fatigue can possibly be relieved by restoring serum carnitine levels through carnitine supplementation [12]. In the present review study, it is hypothesized that l-carnitine can also be effective in relieving fatigue in cases with COVID-19.
2. l-Carnitine and fatigue
l-Carnitine (3-hydroxy-4-N-trimethyl-aminobutyrate) is a micronutrient composed of essential amino acids such as lysine and methionine, which can be seen in nearly every cell of the body and has a major role in energy metabolism and production of cellular energy, bringing long-chain fatty acid to mitochondrial matrix meant for beta oxidation [6,13]. This hydrophilic amino acid is existent all through the CNS and PNS and is found mainly in the heart and skeletal muscles. l-Carnitine is biosynthesized from the amino acids lysine and methionine in the kidneys, liver, and brain and in a process that requires vitamins B6, B3, C, niacin, and iron [14]. More than 95% of the body's total carnitine is located in muscles, and it is essential for the transportation of long-chain fatty acids through the mitochondrial membrane for beta-oxidation and energy production in the form of ATP [[15], [16], [17]]. Micronutrient deficiencies can lead to fatigue by causing nutritional and metabolic disorders. Carnitine is a micronutrient that is elaborated in the generation of energy at the cellular level and is usually inadequate in cases with chronic diseases [18]. Regularly, the need for carnitine is met by consuming meat, but endogenous synthesis and incremented efficiency of renal absorbency can help to the homeostasis of this compound throughout the body. Impaired synthesis, transport, or metabolism of l-carnitine can lead to early or secondary deficiencies; this can lead to increased intracellular lipid levels in muscles. Carnitine deficiency can lead to muscle weakness and fatigue [15]. This compound is thought to alleviate fatigue by interfering with various mechanisms in the cells, such as operating as a necessary component of producing mitochondrial energy and interfering with acetylcholine synthesis in the brain, and by its anti-inflammatory and antioxidant properties. Carnitine supplementation is actively used for the treatment of CFS, fatigue caused by chemotherapy, fatigue in the elderly, and fatigue caused by different neurological diseases [19]. Supplementation of carnitine is reported to reduce cancer-related fatigue [11,20]. Patients with chronic hepatitis, when treated with l-carnitine along with interferon-gamma and ribavirin also show improvement in fatigue, absenteeism, presenteeism and work-related productivity [21]. It also reduces drug-associated hemolytic anemia in these patients [22]. Kępka, Janas [23] showed a significant reduction in l-carnitine levels of patients presented with tick-borne encephalitis virus infection. Other studies have also significantly highlighted reduction in carnitine levels among chronic fatigue syndrome patients.
Table 1 lists some of the studies performed for evaluation of the effect and safety of l-carnitine supplementation in different cases with fatigue.
Table 1.
Some clinical studies conducted on the use of l-carnitine in relieving fatigue.
| Authors(s) | Number of patients | Dosage | Results |
|---|---|---|---|
| Gramignano et al. 2005 [11] |
12 cancer patients with fatigue Average age: 60 years |
Oral use of 6 g per day for 4 weeks | Considerable increase in nutritional variables (lean body mass and appetite) after taking l-carnitine supplement Decreased reactive oxygen levels Increased glutathione peroxidase No significant change in proinflammatory cytokines |
| Crucianie et al. (2006) [17] | 38 cancer patients with fatigue | Doses of 250, 750, 1250, 1750, 2250, 2750, and 3000 mg were used twice daily for 7 days | Improvement of fatigue, mood, and sleep in most patients Improvement of dose-dependent fatigue |
| Matsui et al. 2017 [6] |
11 cancer patients with fatigue Average age: 67 years |
Daily consumption of 1500 mg for 8 weeks | Reduction of fatigue in all patients Retained plasma levels of albumin and lymphocytes throughout chemotherapy |
| Vasiljevski et al. 2021 [14] |
6 children with neurofibromatosis type 1 (NF1) and fatigue Average age: 10 years |
Daily use of 1000 mg oral l-carnitine supplement for 12 weeks | Safety of the use of l-carnitine supplement for 12 weeks in children with NF1 |
| AbuMoh'd et al. 2021 [13] |
20 athletes In two groups of l-carnitine and placebo Average age: 67 years |
2 × 1.5 g per day for 3 weeks | There was a better physiological respond to l-carnitine supplement compared with the placebo group |
3. Mechanisms associated with fatigue and l-carnitine
Carnitine is required as a nutrient by enzymes that cause translocation of long chain fatty acids, so they can enter mitochondrion membrane. These long chain fatty acids are responsible for the production of massive amount of adenosine triphosphate residues, compared to medium and small chain fatty acids [24].
Fatigue may be induced by various causes including physical and mental stress, circadian rhythm disorders, and different diseases. Sometimes the feeling of tiredness caused by influenza or other types of viral infections may remain for days or weeks. Fatigue is probably one of the body's signals to inhibit physical activities for regaining health; however, the exact mechanism that causes fatigue after infections is not well established [25]. Suggested mechanisms for fatigue include imbalances in energy production caused by increased energy requirement (e.g., caused by tumor growth, infection, fever, or surgical procedures), reduced availability of substrates (e.g., due to anorexia, nausea, or vomiting), atypical generation of compounds that disrupt metabolic homeostasis or regular functions of muscles (e.g., cytokines and proteolysis inducers), anemia, and hypoxemia. Further proposed processes associate fatigue with the pathophysiology of sleep disturbance and depression [11]. An animal-model based study showed that treatment of rats with l-carnitine against muscular fatigue reduces free-radical mediated oxidative stress by reducing the production of thiobarbituric acid reactive substances, protein carbonyl, creatine kinase and lipid hydroperoxide [26].
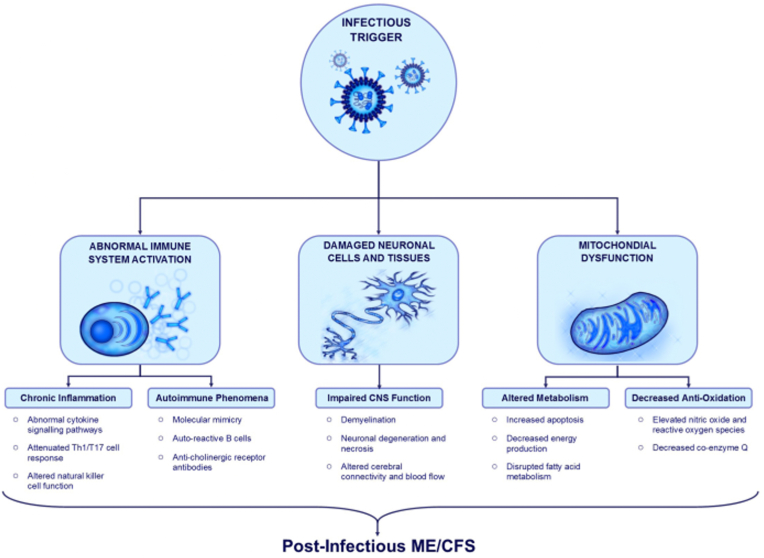
Viral infections such as the flu cause acute inflammation, and proinflammatory cytokines such as interleukin (IL) -1β or antiviral cytokines including interferons (IFNs) are made in the environment by activating Toll-like receptors. Also, during an environmental infection, in addition to fever, other atypical psychological and physical feelings are experienced, which include fatigue, depression, cognitive impairment, anorexia, and muscle or joint pains [25,27]. In addition, the most likely candidates as fatigue-inducing or transmitting agents are cytokines, including interferons [28]. Proinflammatory cytokines such as IL-1b, IL-6, and TNF-a induce fever, fatigue, anorexia, and cognitive dysfunction. Also, anti-inflammatory cytokines such as IL-10 inhibit the symptoms of acute disease [29]. Also, post-infection outcomes are affected by polymorphisms in IFN-γ +874 T/A and IL-10 92592C/A. The mentioned changes probably affect the severity of the disease and the generation of cytokines. Studies have found a link among SNPs in cytokinin genes and post-infection complications, which include fatigue, pain, neurocognitive problems, and mood disorders. Particularly, they found this association between these complications and IL-6, TNF-α, IFN-γ, and IL-10. In addition, the studies demonstrated that incremented fatigue after infection was related to the IFN-γ +874 T/A SNP T allele [2]. Fig. 1 summarizes the mechanisms associated with post-infection fatigue.
Fig. 1.
Infectious agents affect the immune system function, which leads long-term inflammation, incremented signaling of proinflammatory cytokines, and lack of normal functions of various cell types, such Th1, Th17, regulatory T cells, and NTCs. Autoimmune processes including molecular mimicry and activation of auto-reactive cells can also occur during acute infection Infectious agents with neural invasion potential can cause inflammatory and ischemic damage of the central nervous system, leading to nerve damage, demyelination, and consequent dysfunctions. Infections may also cause structural damage to the mitochondria, reducing energy generation, altering metabolism, and reducing antioxidant function [49].
Viral infection can cause chronic fatigue syndrome (CFS) by causing physiological changes in host cells [30]. Decreased serum acetyl-l-carnitine, polymorphism of serotonin genes, and autoantibodies against muscarinic cholinergic receptors were seen in cases experiencing CFS. The use of l-carnitine, a precursor of acetyl-l-carnitine, improves the clinical condition of CFS patients [31]. Dietary l-carnitine supplementation has beneficial efficacies on energy metabolism of cells and related mechanisms in regeneration of skeletal muscles. In addition, the use of l-carnitine supplement leads to an increase in the level of l-carnitine, and a considerable positive relationship has been reported among an increase in serum l-carnitine concentration and a decrease in the biochemical disorders caused by hypoxia. An increase in concentration of serum l-carnitine can increment l-carnitine transportation through skeletal muscles and neuromuscular junctions; this efficacy may reduce hypoxia and induce acetylcholine synthesis. In addition, l-carnitine may reduce the oxidative stress of skeletal muscles caused by intermittent hypoxia, thereby improving the strength, regeneration, and recovery of fatigue of muscles [32]. In fact, l-carnitine supplementation improves nitrogen balance or prevents oxidative stress by increasing protein synthesis or eliminating inflammatory processes in pathological conditions, and improves the functions of mitochondria [6,33].
4. Coronavirus disease and fatigue
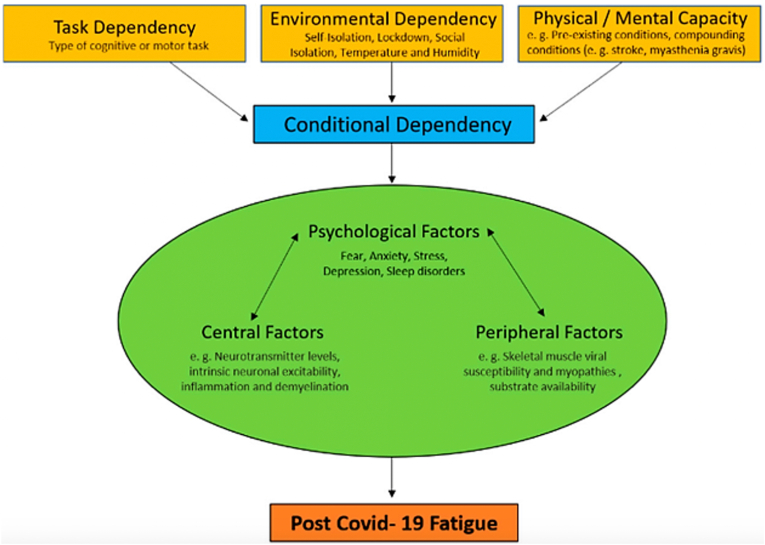
Post-infection fatigue is often seen in various conditions in viral and non-viral diseases [34]. As mentioned earlier, many patients with COVID-19 also suffer from post-disease fatigue. According to Rudroff et al. fatigue caused by the coronavirus disease is characterized by a reduction in physical or mental function due to effects on central, psychological, or environmental factors, and these factors depend on the task a person performs, his/her environmental conditions, and the physical and mental capacity of the individual (conditional dependence). Most importantly, fatigue is affected by conditional dependence factors and the interaction of central, psychological, or environmental factors (physiological factors) (Fig. 2) [7].
Fig. 2.
Factors affecting post-COVID-19 fatigue [7].
Observational studies show that the signs of the coronavirus disease persist for about 21 days after the onset of the disease, and in some patients, these symptoms last for more than 4 months. Studies show that shortness of breath and anosmia are the most regular signs reported for more than 3 weeks. The etiology and pathophysiology of fatigue after COVID19 are not well established, however, it is proposed that central and peripheral processes play a role in its occurrence. This fatigue can be caused by the cytokinin storm, which leads to inflammations and anorexia, followed by muscle loss, weakness, and fatigue. In addition, infection increases basal energy expenditure while the immune system is activated [35]. The T-lymphocyte reaction appears to occur in connection with the high release of cytokines in various cases of CFS, particularly in the initial stages. The immune response that is induced by the coronavirus disease is similar to the classical model [36]. SARS-CoV-2 also increases the release of cytokines and proinflammatory chemokines such as IL-6, TNF, and 1β-IL [3]. It has been suggested that the new corona virus, in combination with ME/CFS stimuli, acts as a physiological stressor. A major object of the virus could be the stress center of the brain, a collection of neural cells in the paraventricular nucleus (PVN) of the hypothalamus. The PVN is a set of nuclei and neural circuits that acts as a stressor, absorbing, and processing factor, which responds to a wide range of physiological stressors and plays a major role in neural regulation of endocrine and autonomic stress responses. Incoming stress signals to the PVN of the hypothalamus through a wide range of humoral and neural pathways are caused by different factors including infections, pain, emotional distress, and cardiovascular changes due to physical activity [3]. There is also growing evidence that widely different autoantibodies may cause severe corona infection. These autoantibodies may also have a major role in the symptoms of long-term fatigue in patients with COVID-19. Despite that oxidative stress cab help to this syndrome, the replacement of antioxidants and vitamins is not enough to improve the clinical symptoms of the disease. Comparably, cognitive-behavioral treatments are not enough to help with this condition. Also, hypothalamic-pituitary-adrenal insufficiency in patients with CFS has been described as a result of activation of the immune and inflammatory pathways [4].
5. l-Carnitine and coronavirus disease
Researchers are currently studying drugs such as hydroxychloroquine, AT1R blockers such as losartan and olmesartan, and indomethacin nonsteroidal anti-inflammatory drug in hopes of finding suitable treatment options for the new coronavirus disease. It has been observed that these drugs relieve the symptoms of the disease [37]. COVID-19 medications have been partly effective in inhibiting the disease and some drugs have been shown to be more effective, but have not been successful in reducing major side effects of this disease such as fatigue and lethargy [[38], [39], [40], [41]]. Further studies should be conducted on the etiology of fatigue, its duration, and related problems in patients with the coronavirus disease [35]. So far, no study has been performed on the efficacy of l-carnitine on the treatment of post-Covid-19 fatigue, but due to the favorable efficacies of this drug in the therapy of fatigue, it can be considered as an effective option in the treatment of this complication. On the other hand, it has been observed that carnitine can be efficient in counteracting pre-inflammatory conditions and decreasing oxidative stress in animal studies; thus, it is hypothesized that it can be an efficient therapy for pneumonia and Covid19 infection [42]. In COVID19, the renin-angiotensin system (RAS) is regulated and the NF-κB pathway is overexpressed. In addition, a progressive cytokine storm occurs. In all of these pathogenic processes, l-carnitine can play a modifying role in improving condition. l-Carnitine can be beneficial against the antioxidant effects of angiotensin II by inhibiting NF-κB and down-regulating NOX1 and NOX2. The medication also has anti-apoptotic and genome-stabilizing functions by inhibiting caspases and activating PARP-1. l-Carnitine is an immune system regulator that regulates proinflammatory cytokines including TNF-α, IL-6, and IL-1 and can neutralize cytokinin storms. l-Carnitine can also act as a protective agent against COVID19-induced cardiotoxicity due to disruption of the ACE2 signaling pathway, cytokinin storm, pulmonary dysfunction, and drug side effects [37]. The spike protein of SARS-CoV-2, which is the primary target of vaccines and other medications, binds to cells by binding to the angiotensin-converting enzyme 2 (ACE2) receptor. The physiological role of ACE2 is to lower blood pressure and counteract inflammation by converting proinflammatory angiotensin II to anti-inflammatory angiotensin [43]. On the other hand, l-carnitine reduces ACE1 levels and hepatitis C infection through an anti-lipogenic effect [44]. Kazmi et al. also reported the protective effect of carnitine and acetylcarnitine against Covid-19. They reported that some off-target effects may be present following the use of these drugs, thus it is necessary to examine the safety of this treatment for people with Covid-19. In addition, these researchers stated that further study should be conducted on the role of carnitine and acetylcarnitine in disease prevention [42]. Post-infection fatigue after Epstein Barr virus infection has been evidently supported by alteration in the expression of genes associated with mitochondrial function such as fatty acid metabolism and apoptosis [45]. Additionally, impairment of immune response and prolonged release of cytokine also contribute to post-infection fatigue [46]. These findings can be correlated with post-infection fatigue among covid19 patients, as covid-associated immune response also leads to the systemic production of cytokine thereby, leading to fatigue and fibromyalgia [47].
Given the importance of cytokine response in the development of chronic fatigue syndrome and its relationship with the intensity of Covid-19, attention to control and reduction of cytokine response is an important issue [48] As mentioned, l-carnitine has an effective role in production of cellular energy and tissue regeneration, which helps reduce tissue inflammation. As a result of investigating the relationship between l-carnitine consumption and cytokine response and immune system cells in patients with Covid-19 in future can be effective in recognizing the role of l-carnitine in development of chronic fatigue syndrome.
6. Conclusion
The coronavirus disease is a viral infection that could induce different respiratory, gastrointestinal, and vascular problems. Evidence shows that many patients with the disease experience a long recovery period, and symptoms such as fatigue persist for several months after infection. Fatigue depends on a variety of conditional and physiological factors, and its exact molecular mechanism has not yet been elucidated; however, it is probably caused by cytokine storm, which can cause inflammation and anorexia, followed by muscle loss, weakness, and tiredness. l-Carnitine is a trimethylated amino acid that is structurally similar to choline and is needed as a cofactor to convert long-chain free fatty acids to acylcarnitine and transfer them to the mitochondrial matrix. Dietary l-carnitine supplementation successfully has beneficial effects on the energy metabolism of the cells and related mechanisms in regeneration of muscles. Since the beneficial effects of this medication in relieving fatigue caused by diseases such as cancer, MS, etc. have been demonstrated, it can also be considered as a potential option for relieving the fatigue caused by COVID-19. Therefore, in order for evaluating the effect and safety of l-carnitine, its administration is recommended in future randomized clinical trials.
Ethical approval
All procedures performed in this study involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Sources of funding
No funding was secured for this study.
Author contribution
Dr. Parisa Delkash: conceptualized and designed the study, drafted the initial manuscript, and reviewed and revised the manuscript.
Dr. Roya Vaziri-harami: Designed the data collection instruments, collected data, carried out the initial analyses, and reviewed and revised the manuscript. Coordinated and supervised data collection, and critically reviewed the manuscript for important intellectual content.
Registration of research studies
1. Name of the registry: N/a.
2. Unique Identifying number or registration ID: N/A.
3. Hyperlink to the registration (must be publicly accessible): N/A.
Guarantor
Dr. Roya Vaziri-harami.
Consent
Not applicable.
Consent to participate
From the under 16 years old was given by a parent or legal guardian.
Availability of data and material
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Provenance and peer review
Not commissioned, externally peer-reviewed.
Declaration of competing interest
The authors deny any conflict of interest in any terms or by any means during the study.
References
- 1.Silveira D., et al. COVID-19: is there evidence for the use of herbal medicines as adjuvant symptomatic therapy? Front. Pharmacol. 2020;11:581840. doi: 10.3389/fphar.2020.581840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Islam M.F., Cotler J., Jason L.A. Post-viral fatigue and COVID-19: lessons from past epidemics. Fatig.: Biomed. Health Behav. 2020;8(2):61–69. [Google Scholar]
- 3.Mackay A. A paradigm for post-covid-19 fatigue syndrome analogous to ME/CFS. Front. Neurol. 2021;12:701419. doi: 10.3389/fneur.2021.701419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bornstein S.R., et al. Chronic post-COVID-19 syndrome and chronic fatigue syndrome: is there a role for extracorporeal apheresis? Mol. Psychiatr. 2021:1–4. doi: 10.1038/s41380-021-01148-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Townsend L., et al. Persistent fatigue following SARS-CoV-2 infection is common and independent of severity of initial infection. PLoS One. 2020;15(11):e0240784. doi: 10.1371/journal.pone.0240784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vollbracht C., Kraft K. Feasibility of vitamin C in the treatment of post viral fatigue with focus on long COVID, based on a systematic review of IV vitamin C on fatigue. Nutrients. 2021;13(4) doi: 10.3390/nu13041154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rudroff T., et al. Post-COVID-19 fatigue: potential contributing factors. Brain Sci. 2020;10(12) doi: 10.3390/brainsci10121012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stormorken E., Jason L.A., Kirkevold M. Factors impacting the illness trajectory of post-infectious fatigue syndrome: a qualitative study of adults' experiences. BMC Publ. Health. 2017;17(1):952. doi: 10.1186/s12889-017-4968-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rathi A., Jadhav S.B., Shah N. A randomized controlled trial of the efficacy of systemic enzymes and probiotics in the resolution of post-COVID fatigue. Medicines. 2021;8(9):47. doi: 10.3390/medicines8090047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gramignano G., et al. Efficacy of l-carnitine administration on fatigue, nutritional status, oxidative stress, and related quality of life in 12 advanced cancer patients undergoing anticancer therapy. Nutrition. 2006;22(2):136–145. doi: 10.1016/j.nut.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 11.Matsui H., et al. L-Carnitine supplementation reduces the general fatigue of cancer patients during chemotherapy. Mol. Clin. Oncol. 2018;8(3):413–416. doi: 10.3892/mco.2018.1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marx W., et al. Efficacy and effectiveness of carnitine supplementation for cancer-related fatigue: a systematic literature review and meta-analysis. Nutrients. 2017;9(11) doi: 10.3390/nu9111224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Malaguarnera M. Carnitine derivatives: clinical usefulness. Curr. Opin. Gastroenterol. 2012;28(2):166–176. doi: 10.1097/MOG.0b013e3283505a3b. [DOI] [PubMed] [Google Scholar]
- 14.AbuMoh'd M.F., Obeidat G., Alsababha W. Effect of oral supplementation with L-carnitine on performance time in a 5000 m race and responses of free fatty acid and carnitine concentrations in trained-endurance athletes. Montenegrin J. Sports Sci. Med. 2021;10(2):5–11. [Google Scholar]
- 15.Vasiljevski E.R., et al. L-carnitine supplementation for muscle weakness and fatigue in children with neurofibromatosis type 1: a Phase 2a clinical trial. Am. J. Med. Genet. 2021;185(10):2976–2985. doi: 10.1002/ajmg.a.62392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gnoni A., et al. Carnitine in human muscle bioenergetics: can carnitine supplementation improve physical exercise? Molecules. 2020;25(1) doi: 10.3390/molecules25010182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Malaguarnera M., et al. L-Carnitine treatment reduces severity of physical and mental fatigue and increases cognitive functions in centenarians: a randomized and controlled clinical trial. Am. J. Clin. Nutr. 2007;86(6):1738–1744. doi: 10.1093/ajcn/86.5.1738. [DOI] [PubMed] [Google Scholar]
- 18.Cruciani R.A., et al. Safety, tolerability and symptom outcomes associated with L-carnitine supplementation in patients with cancer, fatigue, and carnitine deficiency: a phase I/II study. J. Pain Symptom Manag. 2006;32(6):551–559. doi: 10.1016/j.jpainsymman.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 19.Tejani A.M., et al. Carnitine for fatigue in multiple sclerosis. Cochrane Database Syst. Rev. 2010;(2):Cd007280. doi: 10.1002/14651858.CD007280.pub2. [DOI] [PubMed] [Google Scholar]
- 20.Iwase S., et al. Efficacy and safety of an amino acid jelly containing coenzyme Q10 and l-carnitine in controlling fatigue in breast cancer patients receiving chemotherapy: a multi-institutional, randomized, exploratory trial (JORTC-CAM01) Support. Care Cancer. 2016;24(2):637–646. doi: 10.1007/s00520-015-2824-4. [DOI] [PubMed] [Google Scholar]
- 21.Malaguarnera G., et al. Acetyl-L-carnitine supplementation during HCV therapy with pegylated interferon-α 2b plus ribavirin: effect on work performance; A randomized clinical trial. Hepat. Mon. 2014;14(5) doi: 10.5812/hepatmon.11608. e11608-e11608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sato S., et al. Efficacy of L-carnitine on ribavirin-induced hemolytic anemia in patients with hepatitis C virus infection. Clin. Mol. Hepatol. 2019;25(1):65–73. doi: 10.3350/cmh.2018.0070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kępka A., et al. Serum carnitine and acyl-carnitine in patients with meningitis due to tick-borne encephalitis virus infection. Adv. Clin. Exp. Med. 2017;26(2):277–280. doi: 10.17219/acem/63006. [DOI] [PubMed] [Google Scholar]
- 24.Cruciani R.A., et al. L-carnitine supplementation for the management of fatigue in patients with cancer: an eastern cooperative oncology group phase III, randomized, double-blind, placebo-controlled trial. J. Clin. Oncol. : Off. J. Am. Soc. Clin. Oncol. 2012;30(31):3864–3869. doi: 10.1200/JCO.2011.40.2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yamato M., Kataoka Y. Fatigue sensation following peripheral viral infection is triggered by neuroinflammation: who will answer these questions? Neural Regen. Res. 2015;10(2):203–204. doi: 10.4103/1673-5374.152369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dutta A., et al. l-carnitine supplementation attenuates intermittent hypoxia-induced oxidative stress and delays muscle fatigue in rats. 2008;93(10):1139–1146. doi: 10.1113/expphysiol.2008.042465. [DOI] [PubMed] [Google Scholar]
- 27.Vollmer-Conna U., et al. Cytokine polymorphisms have a synergistic effect on severity of the acute sickness response to infection. Clin. Infect. Dis. 2008;47(11):1418–1425. doi: 10.1086/592967. [DOI] [PubMed] [Google Scholar]
- 28.Walling B.L. University of Rochester; 2018. Chemokine Insensitive CD8+ T Cell Migration. [Google Scholar]
- 29.Shendy, W., et al., Prevalence of fatigue in patients post covid. Eur. J. Mol. Clin. Med. 8(03): p. 2021.
- 30.Rasa S., et al. Chronic viral infections in myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) J. Transl. Med. 2018;16(1):268. doi: 10.1186/s12967-018-1644-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Okada T., et al. Mechanisms underlying fatigue: a voxel-based morphometric study of chronic fatigue syndrome. BMC Neurol. 2004;4(1):14. doi: 10.1186/1471-2377-4-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shen L., et al. L-carnitine's role in KAATSU training- induced neuromuscular fatigue. Biomed. Pharmacother. 2020;125:109899. doi: 10.1016/j.biopha.2020.109899. [DOI] [PubMed] [Google Scholar]
- 33.Ringseis R., Keller J., Eder K. Mechanisms underlying the anti-wasting effect of L-carnitine supplementation under pathologic conditions: evidence from experimental and clinical studies. Eur. J. Nutr. 2013;52(5):1421–1442. doi: 10.1007/s00394-013-0511-0. [DOI] [PubMed] [Google Scholar]
- 34.Poenaru S., et al. COVID-19 and post-infectious myalgic encephalomyelitis/chronic fatigue syndrome: a narrative review. Ther. Adv. Infect Dis. 2021;8 doi: 10.1177/20499361211009385. 20499361211009385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Virgens I.P.A., et al. Can COVID-19 be a risk for cachexia for patients during intensive care? Narrative review and nutritional recommendations. Br. J. Nutr. 2021;126(4):552–560. doi: 10.1017/S0007114520004420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gaber T. Assessment and management of post‐COVID fatigue. Prog. Neurol. Psychiatr. 2021;25(1):36–39. [Google Scholar]
- 37.Fakhrolmobasheri M., et al. Selenium supplementation can relieve the clinical complications of COVID-19 and other similar viral infections. Int. J. Vitam. Nutr. Res. 2020;10 doi: 10.1024/0300-9831/a000663. 0300-9831. [DOI] [PubMed] [Google Scholar]
- 38.Khan A.R., et al. Montelukast in hospitalized patients diagnosed with COVID-19. J. Asthma. 2021:1–7. doi: 10.1080/02770903.2021.1881967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Spuch C., et al. Does lithium deserve a place in the treatment against COVID-19? A preliminary observational study in six patients, case report. Front. Pharmacol. 2020;11:557629. doi: 10.3389/fphar.2020.557629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Campochiaro C., et al. Efficacy and safety of tocilizumab in severe COVID-19 patients: a single-centre retrospective cohort study. Eur. J. Intern. Med. 2020;76:43–49. doi: 10.1016/j.ejim.2020.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Beigel J.H., et al. Remdesivir for the treatment of covid-19. N. Engl. J. Med. 2020;383(19):1813–1826. doi: 10.1056/NEJMoa2007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kazmi N., Davey Smith G., Lewis S. 2021. Mendelian Randomization Analyses Show that Higher Acetyl-Carnitine and Carnitine Levels in Blood Protect against Severe Covid19. Available at: SSRN 3857663. [Google Scholar]
- 43.Aghsaeifard Z., Alizadeh R. Endocr Metab Immune Disord Drug Targets; 2021. The Role of Angiotensin Converting Enzyme in Immunity: Shedding Light on Experimental Findings. [DOI] [PubMed] [Google Scholar]
- 44.Bellamine A., et al. L-carnitine tartrate downregulates the ACE2 receptor and limits SARS-CoV-2 infection. Nutrients. 2021;13(4) doi: 10.3390/nu13041297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vernon S.D., et al. Preliminary evidence of mitochondrial dysfunction associated with post-infective fatigue after acute infection with Epstein Barr Virus. BMC Infect. Dis. 2006;6(1):15. doi: 10.1186/1471-2334-6-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Garcia M.N., et al. Evaluation of prolonged fatigue post–west nile virus infection and association of fatigue with elevated antiviral and proinflammatory cytokines. Viral Immunol. 2014;27(7):327–333. doi: 10.1089/vim.2014.0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Alizadeh R., Aghsaeifard Z. Does COVID19 activates previous chronic pain? A case series. Ann. Med. Surg. (Lond) 2021;61:169–171. doi: 10.1016/j.amsu.2020.12.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Delkash P., Vaziri-Harami R. Causes of chronic fatigue syndrome in covid-19 patients and treatment recommendations. Ann. Rom. Soc. Cell. Biol. 2021:8460–8466. [Google Scholar]
- 49.Piraino B., Vollmer-Conna U., Lloyd A.R. Genetic associations of fatigue and other symptom domains of the acute sickness response to infection. Brain Behav. Immun. 2012;26(4):552–558. doi: 10.1016/j.bbi.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.