Abstract
Objective:
To determine the levels of two sensory neuropeptides (substance P [SP] and calcitonin gene-related peptide [CGRP]) and two endogenous opioids (methionine-enkephalin [Met-Enk] and β-endorphin [β-End]) in dental pulp tissue samples subjected to controlled orthodontic intrusive forces.
Materials and Methods:
Sixteen healthy premolars were selected from eight patients who were undergoing extraction for orthodontic purposes. Eight were randomly used as controls, and the other eight were assigned to an experimental group (controlled orthodontic intrusive forces applied for 24 hours). After this period, teeth were extracted, and pulp samples were obtained. All samples were processed to quantify the expression levels of SP, CGRP, Met-Enk, and β-End using commercial radioimmunoassay kits.
Results:
All samples exhibited basal levels of both neuropeptides and endogenous opioids. After 24 hours of the intrusive stimulus, all patients reported a tolerable discomfort localized at the involved premolar. Only SP was significantly increased (P < .05). For the other molecules, no statistically significant differences were observed (P > .05); however, they expressed important increasing trends.
Conclusions:
The expression levels of SP and CGRP in dental pulp samples from the experimental group support the positive correlation between the symptomatic clinical scenario and increased expression levels of neuropeptides, clarifying the role of neurogenic inflammation in early injury response.
Keywords: Neurogenic inflammation, Endogenous opioid system, Substance P, Calcitonin gene-related peptide, Methionine-enkephalin, β-endorphin
INTRODUCTION
Orthodontic forces are capable of producing neurovascular changes within dental pulp tissue, partially mediated by the local release of neuropeptides.1,2 Not only do these influence vascular and nervous structures, but they can also target immune cells.3 Substance P (SP) is considered to be the main mediator of neurogenic inflammation.4 It presents a dose-dependent effect and causes vasodilatation by direct action over endothelial cells and indirect mast-cell stimulation for histamine release, a dual effect shared with calcitonin gene-related peptide (CGRP).5,6 CGRP is recognized as the most potent vasodilatory peptide7 and is found in nearly 50% of the neurons of the trigeminal system.6 Frequently collocated and released with SP,8 it is capable of extending this neuropeptide activity by inhibiting enzyme degradation.9 Both are well-known key factors related to painful clinical scenarios.3,8
Pain perception also activates pain-inhibition systems.10 The most relevant analgesic mechanism is the opioid endogenous system, which was identified in the late 1980s in both central and peripheral sensory neurons.11 This system consists of different opioid peptides, for instance, β-endorphin (β-End) and methionine-enkephalin (Met-Enk).12,13 Met-Enk and β-End are capable of stimulating μ and δ opioid receptors and δ opioid receptors, respectively, causing direct analgesia14,15 by attenuation of neuronal excitability and neuropeptidergic release.16
Until now, only a few reports have explored the relationship between the development of neurogenic inflammation caused by neuropeptides and the local pain modulation mechanisms mediated by the presence of endogenous opioids in the dental pulp environment. The aim of this study was to determine the levels of two sensory neuropeptides (SP and CGRP) and two endogenous opioids (Met-Enk and β-End) in dental pulp tissue samples subjected to controlled orthodontic intrusive forces.
MATERIALS AND METHODS
Subjects
A descriptive comparative pilot study was conducted in accordance with the Declaration of Helsinki. The Ethics Committee of the Faculty of Dentistry at San Luis Potosi University, San Luis Potosi, Mexico approved this study, whose objective was explained to the subjects' parents or legal guardians and for which written informed consent was obtained. Study subjects were eight patients of both sexes between 12 and 16 years old. The inclusion criteria were systemically healthy; undergoing extraction of the four first premolars for orthodontic purposes; teeth without caries, fractures, or periodontal disease tested to establish pulpal/periradicular health and with radiographically evident complete radicular formation. Patients who received recent anti-inflammatory, analgesic, or antibiotic treatment; who had root resorption (from any cause), permanent or provisional restorations in the first premolars, or radicular dilacerations; who were smokers; who were pregnant; or who had occlusal disorders were excluded. Each patient was assigned sequential numbers in the order in which they were enrolled, and they received their allocated treatment according to a computer-generated randomization schedule.
Experimental Design
Initially, a pilot study was performed to design in vitro the intrusive device to be used. Standard 0.018″ orthodontic brackets and double tubes (Sybron/Ormco, Orange, Calif) were selected for the first premolars and the permanent first molars, respectively. Both brackets were placed in plastic dentoforms using the adhesive system Prime & Bond (3M Unitek, Monrovia, Calif) and composite Filtek 350 (3M Unitek), standardizing their positions with an Anderson calibrator (Dentaurum GmbH, Ispringen, Germany). Using No.139 orthodontic pliers (Dentaurum GmbH), a 1-mm loop was formed with a retentive fold on the opposite side of a stainless steel wire (0.018″ × 0.025″). The customized wire was placed in the tube slot and adjusted to allow the loop to be bent until an acute angle was achieved. Using a dynamometer (Corex, Haag-Streit, Kowniz, Switzerland), the angulation was standardized at 40° to allow for a 150–200 g intrusive force over the plastic tooth.
Two premolars from each patient were randomly selected and assigned to two different groups: the experimental group, which would undergo the application of the controlled intrusive force for 24 hours (EXPg), and the control group (CTRg), which was used to determine the basal levels of each substance. Each patient was scheduled so as to obtain the samples from the control tooth before any clinical intervention took place. Each control tooth was anesthetized via local blockage with 4% prilocaine (Pricanest, Ropsohn Therapeutics, Bogotá, Colombia). From that moment, the entire sampling procedure was performed within a 10-minute period. After extraction, a second assistant created a 1-mm longitudinal groove over the vestibular tooth surface using a high-speed diamond bur with copious irrigation to facilitate the mechanical fracture of the tooth and the acquisition of pulpal tissue, which was immediately stored in a tagged cryovial containing 1.8 mL of 4% paraformaldehyde, according to previous studies.17 The cryovial was transported and stored at −70°C. The orthodontic device assigned to the EXPg was then placed on the first premolar. After its activation, each patient was instructed to call if any problem or severe discomfort was experienced and, if necessary, to take 400 mg of ibuprofen as a rescue analgesic. These patients were excluded. Pain was scored using a Heft-Parker visual analog scale (VAS) based on a 170-mm line determining the pain level; accordingly, no pain corresponded with 0 mm, mild pain with 1 to 54 mm, moderate pain with 55 to 113 mm, and severe pain as greater than 113 mm.
After 24 hours of controlled intrusive force application, the patient was asked to return to the university facilities, where the dental pulp from the experimental tooth was obtained as described earlier.
Radioimmunoassay
A radioimmunoassay (RIA) was performed to quantify the amount of each substance obtained from each sample. SP, CGRP, β-End, and Met-Enk release were determined by competition RIA binding assays using a human SP RIA kit (reference RK-061-05), a human CGRP RIA kit (reference RK-015-02), a human β-End RIA kit (reference RK-022-14) (Phoenix Peptide Pharmaceuticals, Burlingame, Calif), and a human Met-Enk RIA kit (reference S-2119) (Peninsula Laboratories LLC, Bachem Group, San Carlos, Calif). For each kit, 100 µL of antiserum and 100 µL of various neuropeptide/opioid concentrations (1–128 pg µL-1) or 100 µL of dental pulp tissue extracts were incubated in polypropylene tubes at room temperature for 20 hours. Then, 100 µL of radioactive 125I tracer was added and left to incubate for another 24 hours. Bound fractions were precipitated by the addition of 100 µL of a secondary antibody (goat anti-rabbit immunoglobulin G serum), 100 µL normal rabbit serum, and 500 µL RIA buffer containing 1% polyethylene glycol 4000. After 2 hours of incubation at room temperature, the suspensions were spun at 3,500 rpm (4,000 g) for 40 minutes at 4°C to precipitate the bound fractions. The supernatants were carefully aspirated, and pellet radioactivity was read on a gamma counter (Model B5002, Packard Instrument Intl, Zurich, Switzerland). All samples were assayed in duplicate, and the mean values were calculated. Finally, Scatchard analysis of the binding data was used to assess the amount of neuropeptide/opioid present in each sample.
Statistical Analysis
Results were analyzed by the Mann-Whitney U test to compare the differences among groups for continuous variables. A difference was considered to be significant if the probability of its occurring by chance alone was <5% (P < .05) in a two-tailed test.
RESULTS
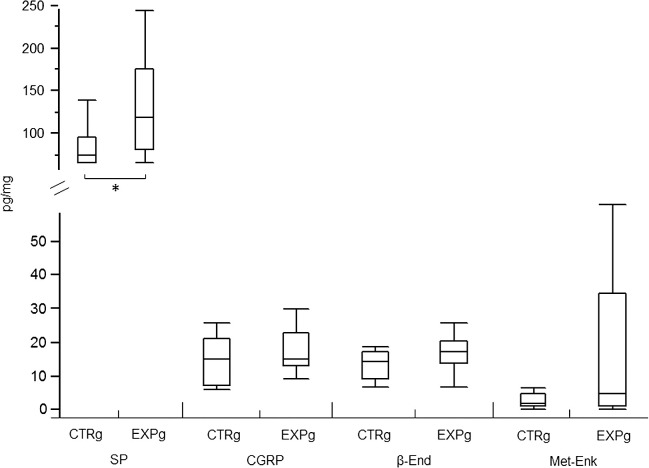
The results are shown in Figure 1. All samples from the CTRg exhibited basal levels of neuropeptides and endogenous opioids. A higher basal level was observed for SP, measuring 83.51 pg/mg of dental pulp tissue (±11.35 pg), followed by CGRP (13.73 ± 2.84 pg/mg), β-End (12.44 ± 1.74 pg/mg), and finally Met-Enk (2.29 ± 0.84 pg/mg), the least-expressed molecule at basal levels. In the EXPg, after 24 hours of the intrusive stimulus, none of the patients needed to use the rescue analgesic for severe pain, but they all reported a tolerable discomfort localized at the involved premolar. After orthodontic intrusion, SP was the only substance that exhibited statistically significantly different levels in dental pulp samples, in which a level of 131.91 (±26.32 pg) was measured (P < .05). Although none of the other samples showed statistically significant differences for the other molecules (P > 0.05), they all expressed important increasing trends in the EXPg: 16.03 (±2.74 pg), 15.64 (±2.33 pg), and 15.02 (± 9.14 pg) for CGRP, β-End, and Met-Enk, respectively.
Figure 1.
Basal and experimental levels for both neuropeptides and endogenous opioids. *P < .05. Data are presented in picograms of each substance per milligram of dental pulp sample; CTRg indicates control group; EXPg, experimental group; SP, substance P; CGRP, calcitonin gene-related peptide; β-END, β-endorphin; MET-ENK, methionine-enkephalin.
DISCUSSION
This pilot study simultaneously determined the expression levels of two proalgesic neuropeptides and two analgesic endogenous opioid peptides during an induced acute inflammation environment in eight healthy human patients. No previous reports have analyzed both types of substances in dental pulp at the same time.
An orthodontic movement methodology was selected as a well-standardized available model for human clinical trials focused on inflammation.18 The amount of intrusive force needed for each premolar assigned to the EXPg was considered. Different reports have used a large variety of forces, ranging from 35 g to 600 g.19–21 In this study, it was decided to implement 150–250 g of intrusive force. Even now, defining an orthodontic force as normal remains a paradigm.22 The use of intrusive forces avoids the creation of new interferences that may be seen with a different kind of movement, which ultimately will increase the bias or pain incidence in the study.
All basal levels for neuropeptides and endogenous opioids were determined. The presence of these two substances in dental pulp tissue concurs with the findings of previous reports.5,19,23–25 Although the origin of sensory neuropeptides lies in the trigeminal central tissue, optimal axonal transportation allows for such local storage in nerve terminals.26 For endogenous opioids, the local source is mainly located in different cellular populations, such as immunocytes.11
After the activation of orthodontic intrusive devices, patients reported a tolerable discomfort. Within the molecules capable of provoking this response, SP and CGRP are well recognized. Nevertheless, none of the subjects required analgesics or reported a degree of pain that caused elimination from the study.
The SP levels increased by 57.97%; this was a significant difference from the basal levels (P < .05). The increase of SP coincided with reports of a higher expression in symptomatic cases of irreversible pulpitis17,21,26 and cases in which inflammation was induced, such as deep restorative cavities, the use of certain whitening systems, and the placement of adhesives for cervical preparations.27 Specifically, a greater immunoreactive presence of SP on cats' teeth was reported when orthodontic movement was applied.28 CGRP expression levels were also higher but were not significantly different (P > 0.05). Such an increase was noted when irreversible pulpitis was diagnosed17,26 after 10 minutes of induced pulp inflammation27 and after orthodontic stimulation.29 When considered together, it must be remembered that both are known to be collocated within the same sensory nonmyelinated fiber, a fact corroborated by evidence that demonstrated that an increased density of both nerve-containing fibers is found in the dental pulp of mechanically stressed teeth.30 The presence of these two factors in pulp tissue may not be surprising, as 87% of the axons that enter through the apical region of a premolar are C nonmyelinated fibres.31 Our results and current evidence support the finding that orthodontic movement is not limited to the periodontal tissue but also involves biochemical and symptomatic changes that may affect the dental pulp.
None of the endogenous opioids exhibited statistically significant differences compared with control levels; however, a considerable increase was appreciable for both. Even peripheral opioid mechanisms are more evident in the function of the duration and severity of the stimulus,11 but for ethical reasons, our model intensity could not provoke any harm in healthy human patients. Opioid increase results from the recruitment of immune cells to the damaged tissue13 after the influence of chemokines that favor its migration. Orthodontic stimulus causes cellular chemotaxis, which is different in the function of elapsed time. Granulocytes are the main population of cells observed during the first day of movement,32 and this cell type is also known as the major source of opioid peptide production.33 Other studies have confirmed an increase of granulocytes that are positively marked for β-End after 6 hours of induced inflammation.34 In specific patients with lower pain thresholds due to inflammation, the increase of β-End may not be sufficient to suppress pain in which the balance between analgesia and sensitization is shifted toward pain experience,10 a similar behavior as that observed in our study.
Controversy surrounds the data obtained for Met-Enk, in which, contrary to our results, a decrease of this opioid has been reported in dental pulp after orthodontic exposure.19 Another study35 analyzed the dental pulp expression levels for SP and Met-Enk, also reporting different results from those obtained in this work. In this case, variables such as the anesthetic solution and the sampling sequence used may explain why control levels are even higher than experimental ones. Until now, the previous two reports have been the only attempts to study the simultaneous behavior of neurogenic inflammation and endogenous opioid systems during acute inflammation due to orthodontic movement in human teeth.
Available evidence offers an interesting theoretical paradox. Neuropeptides capable of causing pain favor the recruitment of some of the leukocytes responsible for releasing the endogenous opioids;36 thus, neurogenic painful responses may also indirectly activate peripheral pain modulation mechanisms, attenuating its severity. Additionally, the presence of opioids can inhibit the sensory neurons' excitability and thus the neuropeptide release. Accordingly, each substance may not exert individual effects but rather may produce cross-control mechanisms, especially as the source of both neuropeptides also expresses μ opioid and δ opioid receptors for Met-Enk and β-End.16,37 This hypothetical correlation and its relevance must be evaluated in further research. Also, further studies with more patients are needed to confirm these findings.
CONCLUSIONS
SP and CGRP levels in dental pulp samples from the EXPg support the positive correlation between the symptomatic clinical scenario and increased expression levels of neuropeptides, clarifying the role of neurogenic inflammation in the early injury response.
In the same environment, the low increasing tendency observed in the endogenous opioid levels illustrates a poorly established pain-modulating system during the first 24 hours of orthodontic intrusion.
ACKNOWLEDGMENTS
This work was supported partially by PIFI 2012 and C12-FAI-03-93.93 grants. We would like to thank American Journal Experts for assistance in editing this manuscript.
REFERENCES
- 1.Derringer KA, Jaggers DC, Linden RW. Angiogenesis in human dental pulp following orthodontic tooth movement. J Dent Res. 1996;75:1761–1766. doi: 10.1177/00220345960750100901. [DOI] [PubMed] [Google Scholar]
- 2.Vandevska-Radunovic V. Neural modulation of inflammatory reactions in dental tissues incident to orthodontic tooth movement. A review of the literature. Eur J Orthod. 1999;21:231–247. doi: 10.1093/ejo/21.3.231. [DOI] [PubMed] [Google Scholar]
- 3.Richardson JD, Vasko MR. Cellular mechanisms of neurogenic inflammation. J Pharmacol Exp Ther. 2002;302:839–845. doi: 10.1124/jpet.102.032797. [DOI] [PubMed] [Google Scholar]
- 4.Fewtrell CM, Foreman JC, Jordan CC, Oehme P, Renner H, Stewart JM. The effects of substance P on histamine and 5-hydroxytryptamine release in the rat. J Physiol. 1982;330:393–411. doi: 10.1113/jphysiol.1982.sp014347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kabashima H, Nagata K, Maeda K, Iijima T. Involvement of substance P, mast cells, TNF-alpha and ICAM-1 in the infiltration of inflammatory cells in human periapical granulomas. J Oral Pathol Med. 2002;31:175–180. doi: 10.1034/j.1600-0714.2002.310309.x. [DOI] [PubMed] [Google Scholar]
- 6.Raddant AC, Russo AF. Calcitonin gene-related peptide in migraine: intersection of peripheral inflammation and central modulation. Expert Rev Mol Med. 2011;13:e36. doi: 10.1017/S1462399411002067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cady RJ, Glenn JR, Smith KM, Durham PL. Calcitonin gene-related peptide promotes cellular changes in trigeminal neurons and glia implicated in peripheral and central sensitization. Mol Pain. 2011;7:94. doi: 10.1186/1744-8069-7-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lundy FT, Linden GJ. Neuropeptides and neurogenic mechanisms in oral and periodontal inflammation. Crit Rev Oral Biol Med. 2004;15:82–98. doi: 10.1177/154411130401500203. [DOI] [PubMed] [Google Scholar]
- 9.Vandevska-Radunovic V, Kvinnsland IH, Kvinnsland S, Jonsson R. Immunocompetent cells in rat periodontal ligament and their recruitment incident to experimental orthodontic tooth movement. Eur J Oral Sci. 1997;105:36–44. doi: 10.1111/j.1600-0722.1997.tb00178.x. [DOI] [PubMed] [Google Scholar]
- 10.Feldreich A, Ernberg M, Lund B, Rosen A. Increased beta-endorphin levels and generalized decreased pain thresholds in patients with limited jaw opening and movement-evoked pain from the temporomandibular joint. J Oral Maxillofac Surg. 2012;70:547–556. doi: 10.1016/j.joms.2011.09.013. [DOI] [PubMed] [Google Scholar]
- 11.Stein C, Lang LJ. Peripheral mechanisms of opioid analgesia. Curr Opin Pharmacol. 2009;9:3–8. doi: 10.1016/j.coph.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 12.Benarroch EE. Endogenous opioid systems: current concepts and clinical correlations. Neurology. 2012;79:807–814. doi: 10.1212/WNL.0b013e3182662098. [DOI] [PubMed] [Google Scholar]
- 13.Lesniak A, Lipkowski AW. Opioid peptides in peripheral pain control. Acta Neurobiol Exp (Wars) 2011;71:129–138. doi: 10.55782/ane-2011-1829. [DOI] [PubMed] [Google Scholar]
- 14.Przewlocki R, Przewlocka B. Opioids in chronic pain. Eur J Pharmacol. 2001;429:79–91. doi: 10.1016/s0014-2999(01)01308-5. [DOI] [PubMed] [Google Scholar]
- 15.Bodnar RJ. Endogenous opiates and behavior: 2011. Peptides. 2012;38:463–522. doi: 10.1016/j.peptides.2012.09.027. [DOI] [PubMed] [Google Scholar]
- 16.Beaudry H, Dubois D, Gendron L. Activation of spinal mu- and delta-opioid receptors potently inhibits substance P release induced by peripheral noxious stimuli. J Neurosci. 2011;31:13068–13077. doi: 10.1523/JNEUROSCI.1817-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Caviedes-Bucheli J, Lombana N, Azuero-Holguin MM, Munoz HR. Quantification of neuropeptides (calcitonin gene-related peptide, substance P, neurokinin A, neuropeptide Y and vasoactive intestinal polypeptide) expressed in healthy and inflamed human dental pulp. Int Endod J. 2006;39:394–400. doi: 10.1111/j.1365-2591.2006.01093.x. [DOI] [PubMed] [Google Scholar]
- 18.Byers MR, Narhi MV. Dental injury models: experimental tools for understanding neuroinflammatory interactions and polymodal nociceptor functions. Crit Rev Oral Biol Med. 1999;10:4–39. doi: 10.1177/10454411990100010101. [DOI] [PubMed] [Google Scholar]
- 19.Walker JA, Jr, Tanzer FS, Harris EF, Wakelyn C, Desiderio DM. The enkephalin response in human tooth pulp to orthodontic force. Am J Orthod Dentofacial Orthop. 1987;92:9–16. doi: 10.1016/0889-5406(87)90290-3. [DOI] [PubMed] [Google Scholar]
- 20.Barwick PJ, Ramsay DS. Effect of brief intrusive force on human pulpal blood flow. Am J Orthod Dentofacial Orthop. 1996;110:273–279. doi: 10.1016/s0889-5406(96)80011-4. [DOI] [PubMed] [Google Scholar]
- 21.Bowles WR, Withrow JC, Lepinski AM, Hargreaves KM. Tissue levels of immunoreactive substance P are increased in patients with irreversible pulpitis. J Endod. 2003;29:265–267. doi: 10.1097/00004770-200304000-00009. [DOI] [PubMed] [Google Scholar]
- 22.Ren Y, Maltha JC, Kuijpers-Jagtman AM. Optimum force magnitude for orthodontic tooth movement: a systematic literature review. Angle Orthod. 2003;73:86–92. doi: 10.1043/0003-3219(2003)073<0086:OFMFOT>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 23.Robinson QC, Killmar JT, Desiderio DM, Harris EF, Fridland G. Immunoreactive evidence of beta-endorphin and methionine-enkephalin-Arg-Gly-Leu in human tooth pulp. Life Sci. 1989;45:987–992. doi: 10.1016/0024-3205(89)90152-5. [DOI] [PubMed] [Google Scholar]
- 24.Casasco A, Calligaro A, Casasco M, et al. Peptidergic nerves in human dental pulp. An immunocytochemical study. Histochemistry. 1990;95:115–121. doi: 10.1007/BF00266583. [DOI] [PubMed] [Google Scholar]
- 25.Rodd HD, Boissonade FM. Immunocytochemical investigation of neurovascular relationships in human tooth pulp. J Anat. 2003;202:195–203. doi: 10.1046/j.1469-7580.2003.00153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Awawdeh L, Lundy FT, Shaw C, Lamey PJ, Linden GJ, Kennedy JG. Quantitative analysis of substance P, neurokinin A and calcitonin gene-related peptide in pulp tissue from painful and healthy human teeth. Int Endod J. 2002;35:30–36. doi: 10.1046/j.1365-2591.2002.00451.x. [DOI] [PubMed] [Google Scholar]
- 27.Caviedes-Bucheli J, Munoz HR, Azuero-Holguin MM, Ulate E. Neuropeptides in dental pulp: the silent protagonists. J Endod. 2008;34:773–788. doi: 10.1016/j.joen.2008.03.010. [DOI] [PubMed] [Google Scholar]
- 28.Nicolay OF, Davidovitch Z, Shanfeld JL, Alley K. Substance P immunoreactivity in periodontal tissues during orthodontic tooth movement. Bone Miner. 1990;11:19–29. doi: 10.1016/0169-6009(90)90012-5. [DOI] [PubMed] [Google Scholar]
- 29.Caviedes-Bucheli J, Moreno JO, Ardila-Pinto J, et al. The effect of orthodontic forces on calcitonin gene-related peptide expression in human dental pulp. J Endod. 2011;37:934–937. doi: 10.1016/j.joen.2011.03.035. [DOI] [PubMed] [Google Scholar]
- 30.Davila JE, Miller JR, Hodges JS, Beyer JP, Larson BE. Effect of neonatal capsaicin treatment on orthodontic tooth movement in male Sprague-Dawley rats. Am J Orthod Dentofacial Orthop. 2011;139:e345–e352. doi: 10.1016/j.ajodo.2009.07.019. [DOI] [PubMed] [Google Scholar]
- 31.Nair PN. Neural elements in dental pulp and dentin. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1995;80:710–719. doi: 10.1016/s1079-2104(05)80256-2. [DOI] [PubMed] [Google Scholar]
- 32.Vandevska-Radunovic V, Kvinnsland IH, Kvinnsland S, Jonsson R. Immunocompetent cells in rat periodontal ligament and their recruitment incident to experimental orthodontic tooth movement. Eur J Oral Sci. 1997;105:36–44. doi: 10.1111/j.1600-0722.1997.tb00178.x. [DOI] [PubMed] [Google Scholar]
- 33.Rittner HL, Brack A, Stein C. Pro-algesic versus analgesic actions of immune cells. Curr Opin Anaesthesiol. 2003;16:527–533. doi: 10.1097/00001503-200310000-00014. [DOI] [PubMed] [Google Scholar]
- 34.Machelska H, Schopohl JK, Mousa SA, Labuz D, Schafer M, Stein C. Different mechanisms of intrinsic pain inhibition in early and late inflammation. J Neuroimmunol. 2003;141:30–39. doi: 10.1016/s0165-5728(03)00213-3. [DOI] [PubMed] [Google Scholar]
- 35.Parris WG, Tanzer FS, Fridland GH, Harris EF, Killmar J, Desiderio DM. Effects of orthodontic force on methionine enkephalin and substance P concentrations in human pulpal tissue. Am J Orthod Dentofacial Orthop. 1989;95:479–489. doi: 10.1016/0889-5406(89)90411-3. [DOI] [PubMed] [Google Scholar]
- 36.Sehgal N, Smith HS, Manchikanti L. Peripherally acting opioids and clinical implications for pain control. Pain Physician. 2011;14:249–258. [PubMed] [Google Scholar]
- 37.Stein C, Machelska H. Modulation of peripheral sensory neurons by the immune system: implications for pain therapy. Pharmacol Rev. 2011;63:860–881. doi: 10.1124/pr.110.003145. [DOI] [PubMed] [Google Scholar]