Abstract
Objective:
To determine the effect of bracket type on halitosis, periodontal status, and microbial colonization.
Materials and Methods:
Forty-six patients scheduled for fixed orthodontic treatment (age 11–16 years) were selected from the orthodontic department of Suleyman Demirel University. Patients were divided into two groups with random distribution of brackets; 23 patients were treated with self-ligating brackets (group SLBs), the others with conventional brackets (group CBs). Halitosis measurements and periodontal and microbial records were obtained before the placement of brackets (T0), 1 week later (T1), and 5 weeks after bonding (T2). Periodontal parameters, including plaque index (PI), gingival index (GI), and bleeding on probing index (BOP), were obtained from all the bonded teeth. Halitosis measurements were performed at the same time. Microbial samples were obtained from the buccal surfaces of all the bonded teeth. Data were analyzed by using a repeated-measurement analysis of variance test for the comparison of parameters between groups and times.
Results:
Periodontal parameters and halitosis results were higher in the CBs group than in the SLBs group (P < .05). In the SLBs group, halitosis and BOP values revealed no pronounced changes between T1 and T2 (P > .05). Intra- and intergroup comparisons showed that there were no statistically significant differences for microbial colonization between all the time intervals (P > .05).
Conclusion:
Bracket type has an effect on halitosis and periodontal status. Therefore, self-ligating brackets may be advised in order to prevent patients from developing halitosis and to increase the likelihood of good oral hygiene during orthodontic treatment.
Keywords: Self-ligating bracket, Halitosis, Periodontal status, Microbial colonization
INTRODUCTION
Halitosis is defined as breath that is offensive to other people.1 Because bad breath can connect or disconnect an individual from the social environment, it plays an important role in self-image and social interaction.2 Halitosis is estimated to affect about 50% of the population, with varying degrees of intensity and etiology.3 If halitosis originates from the oral cavity, it is known as oral malodor.4 Oral malodor is mainly caused by volatile sulfur compounds (VSCs) that are produced by a putrefactive process that occurs within the oral cavity.5 The primary causative pathogens are Gram-negative anaerobic bacteria that produce the VSCs by metabolizing different cells/tissues located in saliva, dental plaque, and gingival crevicular fluid. Moreover, the periodontal pocket is an ideal environment for the production of VSCs with respect to the bacterial and sulfur source.6
Fixed orthodontic appliances are associated with increased risk of enamel demineralization, plaque accumulation, bacterial colonization, and gingival inflammation.7–11 Furthermore, the correlation between plaque accumulation and halitosis is clearly demonstrated in the literature.12 In a recent study, Babacan et al.13 investigated the effect of fixed appliances on oral malodor. These authors stated that oral malodor tends to increase after bracket placement and advised that halitosis be added to the list of risks in fixed appliance therapy.
Parallel to metallurgic improvements in orthodontics, manufacturers presented self-ligating brackets (SLBs) to overcome the side effects of conventional brackets (CBs). In addition to the reduced chair time and biomechanical advantages of SLBs, the possibility of better oral hygiene owing to reduced complexity and fewer retentive sites for microbial colonization is a favorable aspect of SLBs.
Previous studies have demonstrated the effect of fixed orthodontic appliances on halitosis,13 periodontal status, and microbial colonization.14–18 However, to our knowledge, no study has evaluated the effect of bracket type on halitosis.
The aim of this prospective clinical study was to compare the halitosis measurements, periodontal status, and microbial colonization parameters of patients treated with SLBs and CBs. The null hypothesis assumed that bracket type has no effect on these parameters.
MATERIALS AND METHODS
Ethical approval was obtained from the Ethical Committee of the Faculty of Medicine, Suleyman Demirel University. The study population was composed of 46 patients scheduled for fixed orthodontic treatment. Patients were included based on the following criteria: (1) permanent dentition with good general health; (2) mild to moderate crowding of teeth; (3) free of dental plaque, with absence of periodontal diseases such as gingival inflammation; (4) motivation to maintain good oral hygiene; (5) no previous orthodontic treatment; (6) no usage of antibiotics in the 3 months before the beginning of the study; and (7) absence of active caries lesions. Additionally, none of the patients had otolaryngologic or systemic problems.
All patients received oral hygiene instructions to carry on their standard oral hygiene procedure before the study. Patients were divided into two groups randomly. Twenty-three patients (11 female and 12 male; mean age, 14.48 ± 1.27 years) in the SLBs group were treated with SLBs (Damon Q; Ormco, Glendora, Calif); 23 in the CBs group (13 female and 10 male; mean age, 13.30 ± 1.61 years) were treated with CBs (Mini Taurus; Rocky Mountain Orthodontics, Denver, Colo) with elastomeric ligatures. The distribution of the average age and gender of the groups is summarized in Table 1.
Table 1.
The Distribution of the Average Age (Years) and Gender of the Groupsa
Halitosis measurements and microbial and periodontal records were obtained in each group at T0, T1, and T2 by the same periodontist. All patients were instructed to brush their teeth after dinner the evening before the first measurements were taken and then refrain from eating and drinking until the appointment next morning. They were also requested to avoid spicy foods, onions, and garlic for 48 hours prior to the appointment. After obtaining first records, the study groups were bonded immediately in one session.
Halitosis Measurements
A Halimeter (Interscan Corp, Chatsword, Calif) was used to evaluate halitosis. Halitosis values were divided into four categories and classified as normal (0–100 ppb), weak (101–150 ppb), strong (151–300 ppb), or very strong (≥301 ppb).19
Before Halimeter measurements, each patients had to keep the mouth closed for a short period of time (1–2 minutes). Then, a plastic straw connected to the Halimeter was inserted into the patient's slightly opened mouth. The straw was not supposed to touch the lips, teeth, or internal surface of the mouth. The peak value was recorded.
Periodontal Measurements
To assess oral hygiene, gingival index (GI),20 plaque index (PI),21 and bleeding on probing (BOP)22 scores were recorded. Gingival inflammation was recorded as a BOP score if bleeding occurred within 30 seconds of probing. BOP was estimated as a percentage.
Amount of Microbial Colonization
Microbial samples taken from the buccal surfaces of all bonded teeth were cultured and analyzed in the Department of Clinical Microbiology. The plaque sample was placed in 4 mL of Stuart transport medium (Merck, Darmstadt, Germany). Serial 10-fold dilutions of the transport medium with the sample of plaque were prepared as 10−4- and 0.1-mL samples and were inoculated on blood agar for numbers of total bacteria and on Mitis-Salivarius agar (Difco Laboratories Inc, Detroit, Mich) containing 0.001% of Chapman Tellurite solution (Difco), 150 g sucrose, and 3.33 mg bacitracin (Sigma Diagnostics, St. Louis, Mo) per liter agar for number of Streptococcus mutans. The agar plates were incubated for 48 hours at 37°C in anaerobic jars.
Subsequently, colonies were counted under a stereomicroscope. In addition, serial 10-fold dilutions were prepared as 10−3- and 0.1-mL samples and were inoculated on two Rogosa agar plates for number of lactobacilli. Both plates were incubated for 48 hours at 37°C, one plate in aerobic conditions and the other in an anaerobic jar. The number of colonies was then determined under a stereomicroscope. Results are expressed as colony-forming units per milliliter.
Statistical Analysis
Power analysis showed that for a power of 0.80, 20 patients would be required for each group.
The records were statistically analyzed by using SPSS (version 17.0; SPSS Inc, Chicago, Ill). The Kolmogorov-Smirnov test was applied to test for normal distribution. Results of bacteria counts were log transformed. Repeated-measurement analysis of variance was used for the comparison of parameters between groups and times. If there was any evidence of a statistically significant difference between parameters, the Bonferroni test was used to compare within times. All tests were performed with a significance level of P < .05.
RESULTS
There was no significant age difference between groups (Table 1). The mean values of halitosis, GI, PI, BOP, and microbial colonization parameters are given in Tables 2 through 6. The onset comparison of halitosis values and periodontal and microbial parameters for the two groups revealed no significant differences that verify the random assignment of brackets to the population sample.
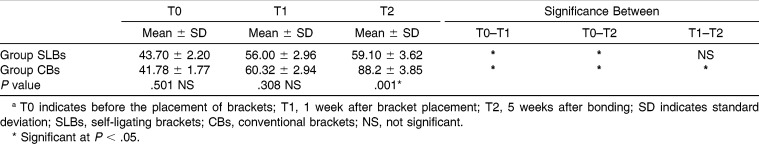
Table 2.
Comparison of Mean Halitosis Scores of Groups at Three Evaluation Times (T0, T1, and T2) and Changes Between Timesa
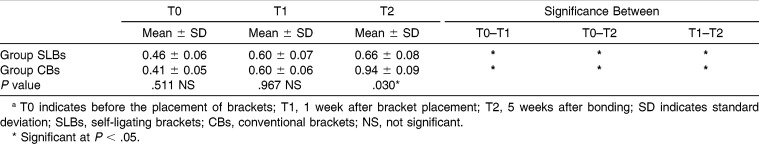
Table 3.
Comparison of Mean Plaque Index Scores of Groups at Three Evaluation Times (T0, T1, and T2) and Changes Between Timesa
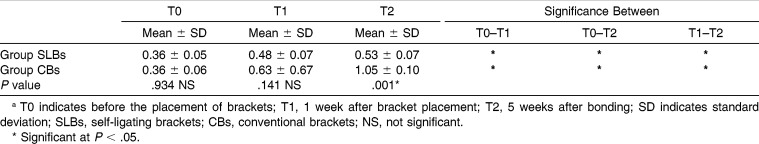
Table 4.
Comparison of Gingival Index Scores of Groups at Three Evaluation Times (T0, T1, and T2) and Changes Between Timesa
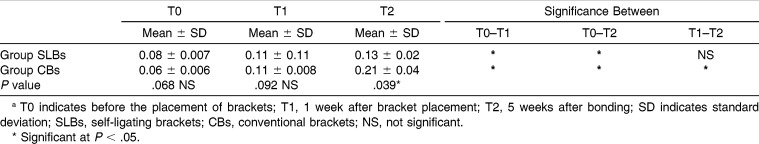
Table 5.
Comparison of Bleeding on Probing Scores of Groups at Three Evaluation Times (T0, T1, and T2) and Changes Between Timesa
Table 6.
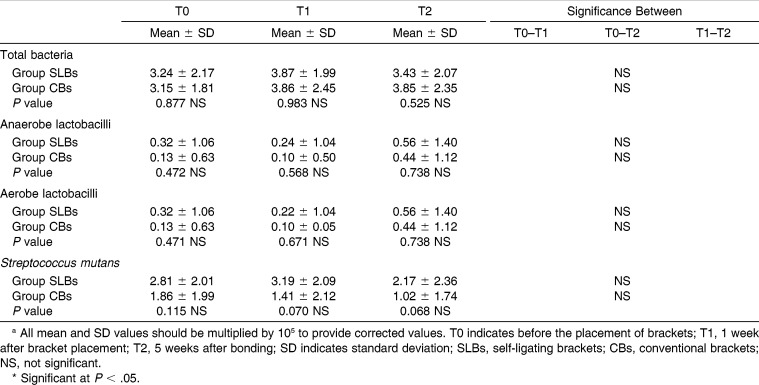
Comparison of Mean Bacterial Counts of Groups at Three Evaluation Times (T0, T1, and T2) and Changes Between Timesa
Halitosis Measurements
Intragroup evaluation showed that in the SLBs group, halitosis values significantly increased after bonding (T0–T1; P < .05). However, there were no significant differences between halitosis values 1 week and 5 weeks after bonding (T1–T2; P > .05). The halitosis values were stable after bonding in the SLBs group. In the CBs group, statistically pronounced increases between all the time intervals and halitosis values showed significant differences between T0–T1, T1–T2, and T0–T2 (P < .05). Halitosis values increased with time (Table 2).
Intergroup evaluation showed that there were no statistical differences in halitosis measurements between the SLBs and CBs groups at T0. T1 measurements of the two groups were similar, with a slight increase in the CBs group (P > .05). However, T2 halitosis values of the CBs group were significantly increased when compared with those of the SLBs group (P < .05; Table 2).
Periodontal Measurements
For GI and PI, both groups showed significant differences with time. These parameters were significantly increased in all groups (P < .05). The intergroup comparison of GI and PI measurements showed significant differences at the T2 period (P < .05; Tables 3 and 4). However, GI and PI values of the SLBs group were lower. The BOP values of both groups showed significant differences at all the time intervals, but in the SLBs group, there were no significant differences between the T1 and T2 periods (P > .05). Further, the intergroup comparison of BOP values at the T2 period showed significant differences with the lower BOP values in the SLBs group (Table 5).
Amount of Microbial Colonization
The mean and standard deviation of log-total bacteria, anaerobic and aerobic lactobacilli, and S mutans counts per milliliter are shown in Table 6. Both intra- and intergroup comparisons showed no significant differences between any time intervals for microbial colonization.
DISCUSSION
Fixed orthodontic appliances impede oral hygiene by creating new, retentive sites suitable for plaque accumulation and lead to an increase in gingival inflammation, halitosis, and enamel demineralization.11,13,23,24 Previous studies have revealed that the presence of gingivitis or periodontitis increases the risk of developing halitosis.6,25,26 However, the contribution of different bracket types to halitosis has not been evaluated yet in the literature. Additionally, only a limited number of studies have surveyed the effects of the usage of SLBs on bacterial aggregation and periodontal condition.24,27
Patient cooperation, motivation for oral hygiene procedures, and eating habits can change with time. Therefore, based on previous studies, final measurements were obtained 5 weeks after bonding.11,13,24 Similarly, elastomeric ligatures (the most common method for archwire ligation) were chosen for comparison with the SLBs.24,27
Halitosis Measurements
In both groups, halitosis values significantly increased after bonding, with lower values in the SLBs group. Halitosis values kept increasing in the CBs group after bonding, but in the SLBs group, they increased immediately after bonding then remained stable. Furthermore, T2 halitosis values of the CBs group increased significantly when compared with those of the SLBs group (P < .05; Table 1).
Only some have studied the relationship between halitosis and fixed orthodontic appliances. Babacan et al.13 evaluated the effect of fixed orthodontic treatment on oral malodor. They compared halitosis values of patients treated with conventional fixed orthodontic appliances with those of untreated controls. Measurements were taken before bonding and 1 and 4 weeks after bonding. The authors found significant increases in halitosis measurements after bonding, which is similar to our study. As in our CBs group, halitosis kept increasing with time.
McKeown2 stated that body image, self-esteem, and social interactions influence each other. Moreover, breath odor is a dynamic and interactive part of self-image that might be value-coded as bad. The study also indicated that when an individual senses a constant bad-breath problem, that person seeks defense techniques and may avoid social circumstances and relations that affect a person's well-being. Improved lifestyles, patient awareness, and methods that provide better esthetic appearance during treatment have led to an increase in the demand for adult orthodontic treatment.28 In our study, the SLBs group showed lower and more stable halitosis parameters after bonding. Since halitosis is an important part of self-image, we offer SLBs particularly to adult patients, who are in the public eye to a greater extent than are juvenile patients.
Neiders and Ramos29 mentioned that sediments of oral microorganisms of plaque on teeth or in periodontal pockets could contribute to bad breath. It has been reported13 that the production of VSCs occurs after 8 to 14 hours of maturation of plaque deposits. Protected plaque in interproximal sites produced substantial odors and was associated with overall levels of VSCs.13
Periodontal Measurements
Although all patients received routine oral hygiene education, the GI and PI parameters showed significant differences among the times in both groups (P < .05). However, in the SLBs group, the BOP values were stable after bonding. These results are consistent with the previous studies that suggest a strong relationship between fixed appliance therapy and periodontal condition.11,15,30
The increase in the values of the periodontal parameters during the treatment is probably a consequence of the plaque-retentive effect of CBs that hinders maintenance of good oral hygiene. Consistent with our results, Pellegrini et al.24 suggested a higher retention of plaque accumulation on CBs ligated with elastomeric ligature than on SLBs. To the contrary, Pejda et al.27 and Pandis et al.31 have reported that there is no difference in plaque aggregation between SLBs and CBs. In fact, our results could have been affected by the study design, such as the type of SLBs, study population, age, and different statistical analyses.
Pandis et al.31 stated that although the SLBs eliminate the need for elastics, the mechanisms of these brackets may provide additional plaque-retention spaces. In addition, the authors have suggested that the components of SLBs are not subjected to regular renewal such as in elastomeric modules. Thus, a theoretical advantage may be eliminated in reality, where calcification of the plaque leads to obstacles in the functioning of the opening-closing mechanism. In our study, the SLBs group showed better values for the periodontal parameters, probably corresponding with the SLB type used.
Amount of Microbial Colonization
Numerous studies have suggested that bonding of orthodontic appliances leads to an increase in the amount of cariogenic microorganisms.11,14–18,23,24,32–34 Only some studies compare SLBs with CBs for microbial colonization after bonding.32–34 Nonetheless, these studies have showed conflicting results. Thus, this topic remains controversial.
Pejda et al.,27 Pandis et al.,31 and Buck et al.32 have stated that bracket type does not affect microbial colonization on the dental plaque or saliva. Similarly, our results have demonstrated no significant differences in the number of total bacteria, S mutans, and lactobacilli in the dental plaque between the SLBs and CBs groups. In addition, the changes in the quantum of microbial colonization of each group at all of the time intervals were not significant.
Contrary to our results, several studies have found better microbial colonization in the SLBs group, whereas some have found better scores for the CBs group. Pellegrini et al.24 demonstrated higher total bacteria and oral S mutans colonization in the CBs group at both 1 and 5 weeks after bonding. Similarly, in a recent study, Mummola et al.33 stated that the CBs showed statistically significantly higher lactobacilli colonization when compared to that of subjects treated with SLBs. However, Pithon et al.34 reported greater bacterial accretion on the SLBs than on the CBs ligated with elastic ligature. Likewise, in an in vitro study, Garcez et al.35 indicated that there was less supragingival biofilm on the CBs ligated with stainless steel ligature than on the SLBs. All these conflicting results can be attributed to the differences in the study models, material and methods, studied bacteria, individual differences, and statistical analysis.
Pellegrini et al.24 stated that while the results reveal fewer plaque bacteria surrounding the SLBs, the mechanics of elastomeric chains or related auxiliaries with these appliances might probably cancel out the favorable effects of SLBs, probably also diminishing other claimed benefits, such as reduced friction and lower force distribution. Therefore, these aspects should be considered by the orthodontist before using elastomers over the SLBs to satisfy patient requests for colored elastics or when elastomeric chains are to be placed for extended periods, as in space-closing mechanics.
CONCLUSIONS
The null hypothesis was partially rejected; SLBs positively affected halitosis and periodontal status but did not alter microbial colonization.
CBs led to an increase in halitosis with the accretion of plaque accumulation. Therefore, these brackets with elastomeric ligatures are not recommended for use in patients with poor oral hygiene or individuals prone to bad breath.
SLBs could be considered for enabling better oral health and hygiene.
REFERENCES
- 1.Rösing CK, Loesche W. Halitosis: an overview of epidemiology, etiology and clinical management. Braz Oral Res. 2011;25:466–471. doi: 10.1590/s1806-83242011000500015. [DOI] [PubMed] [Google Scholar]
- 2.McKeown L. Social relations and breath odour. Int J Dent Hyg. 2003;1:213–217. doi: 10.1034/j.1601-5037.2003.00056.x. [DOI] [PubMed] [Google Scholar]
- 3.Meskin LH. A breath of fresh air. J Am Dent Assoc. 1996;127:1282–1286. doi: 10.14219/jada.archive.1996.0425. [DOI] [PubMed] [Google Scholar]
- 4.Delanghe G, Ghyselen J, van Steenberghe D, Feenstra L. Multidisciplinary breath-odour clinic. Lancet. 1997;19:187. doi: 10.1016/S0140-6736(05)62354-9. [DOI] [PubMed] [Google Scholar]
- 5.Tonzetich J. Direct gas chromatographic analysis of sulphur compounds in mouth air in man. Arch Oral Biol. 1971;16:587–597. doi: 10.1016/0003-9969(71)90062-8. [DOI] [PubMed] [Google Scholar]
- 6.Morita M, Wang HL. Association between oral malodor and adult periodontitis: a review. J Clin Periodontol. 2001;28:813–819. doi: 10.1034/j.1600-051x.2001.028009813.x. [DOI] [PubMed] [Google Scholar]
- 7.Zachrisson S, Zachrisson B. Gingival condition associated with orthodontic treatment. Angle Orthod. 1972;42:26–34. doi: 10.1043/0003-3219(1972)042<0026:GCAWOT>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 8.Zachrisson BU. Cause and prevention of injuries to teeth and supporting structures during orthodontic treatment. Am J Orthod. 1976;69:285–300. doi: 10.1016/0002-9416(76)90077-4. [DOI] [PubMed] [Google Scholar]
- 9.Naranjo AA, Triviño ML, Jaramillo A, Betancourth M, Botero JE. Changes in the subgingival microbiota and periodontal parameters before and 3 months after bracket placement. Am J Orthod Dentofacial Orthop. 2006;130:275.e17–e22. doi: 10.1016/j.ajodo.2005.10.022. [DOI] [PubMed] [Google Scholar]
- 10.O'Reilly MM, Featherstone Demineralization and remineralization around orthodontic appliances: an in vivo study. Am J Orthod Dentofacial Orthop. 1987;92:33–40. doi: 10.1016/0889-5406(87)90293-9. [DOI] [PubMed] [Google Scholar]
- 11.Turkkahraman H, Sayin MO, Bozkurt FY, Yetkin Z, Kaya S, Onal S. Archwire ligation techniques, microbial colonization, and periodontal status in orthodontically treated patients. Angle Orthod. 2005;75:231–236. doi: 10.1043/0003-3219(2005)075<0227:ALTMCA>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 12.Tonzetich J. Production and origin of oral malodor: a review of mechanisms and methods of analysis. J Periodontol. 1977;48:13–20. doi: 10.1902/jop.1977.48.1.13. [DOI] [PubMed] [Google Scholar]
- 13.Babacan H, Sokucu O, Marakoglu I, Ozdemir H, Nalcacı R. Effects Of fixed appliances on oral malodor. Am J Orthod Dentofacial Orthop. 2011;139:351–355. doi: 10.1016/j.ajodo.2009.03.055. [DOI] [PubMed] [Google Scholar]
- 14.Huser MC, Baehni PC, Lang R. Effects of orthodontic bands on microbiologic and clinical parameters. Am J Orthod Dentofacial Orthop. 1990;97:213–218. doi: 10.1016/S0889-5406(05)80054-X. [DOI] [PubMed] [Google Scholar]
- 15.Balenscifen JW, Madonia JV. Study of dental plaque in orthodontic patients. J Dent Res. 1970;49:320–324. doi: 10.1177/00220345700490022101. [DOI] [PubMed] [Google Scholar]
- 16.Rosenbloom RG, Tinanoff N. Salivary streptococcus mutans levels in patients before, during and after orthodontic treatment. Am J Orthod Dentofacial Orthop. 1991;100:35–37. doi: 10.1016/0889-5406(91)70046-Y. [DOI] [PubMed] [Google Scholar]
- 17.Scheie AA, Arneberg P, Krogstad O. Effect of orthodontic treatment on prevalence of streptococcus mutans in plaque and saliva. Scand J Dent Res. 1984;92:211–217. doi: 10.1111/j.1600-0722.1984.tb00881.x. [DOI] [PubMed] [Google Scholar]
- 18.Sinclair PM, Berry CW, Bennett CL, Israelson H. Changes in gingiva and gingival flora with bonding and banding. Angle Orthod. 1987;57:271–278. doi: 10.1043/0003-3219(1987)057<0271:CIGAGF>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 19.Monteiro-Amado F, Chinellato LE, Tarzia O, Rezende ML. Evaluation of oral and nasal odor patients with and without cleft lip and palate: preliminary report. Cleft Palate Craniofac J. 2004;41:661–663. doi: 10.1597/02-163.1. [DOI] [PubMed] [Google Scholar]
- 20.Sillness P, Löe H. Periodontal disease in pregnancy II. Correlation between oral hygiene and periodontal condition. Acta Odontol Scand. 1964;22:121–135. doi: 10.3109/00016356408993968. [DOI] [PubMed] [Google Scholar]
- 21.Löe H. The gingival index, the plaque index and the retention index systems. J Periodontol. 1967;38:610–616. doi: 10.1902/jop.1967.38.6.610. [DOI] [PubMed] [Google Scholar]
- 22.Greenstein G. The role of bleeding upon probing in the diagnosis of periodontal disease. A literature review. J Periodontol. 1984;55:684–688. doi: 10.1902/jop.1984.55.12.684. [DOI] [PubMed] [Google Scholar]
- 23.Forsberg CM, Brattström V, Malmberg E, Nord CE. Ligature wires and elastomeric rings: two methods of ligation, and their association with microbial colonization of Streptococcus mutans and lactobacilli. Eur J Orthod. 1991;13:416–420. doi: 10.1093/ejo/13.5.416. [DOI] [PubMed] [Google Scholar]
- 24.Pellegrini P, Sauerwein R, Finlayson T, et al. Plaque retention by self-ligating vs. elastomeric orthodontic brackets: quantitative comparison of oral bacteria and detection with adenosine triphosphate-driven bioluminescence. Am J Orthod Dentofacial Orthop. 2009;135:426.e1–e9. doi: 10.1016/j.ajodo.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 25.Lu DP. Halitosis. An etiologic classification, a treatment approach and prevention. Oral Surg Oral Med Oral Pathol. 1982;54:521–526. doi: 10.1016/0030-4220(82)90190-6. [DOI] [PubMed] [Google Scholar]
- 26.Ratcliff PA, Johnson PW. The relationship between oral malodor, gingivitis and periodontitis. A review. J Periodontol. 1999;70:485–489. doi: 10.1902/jop.1999.70.5.485. [DOI] [PubMed] [Google Scholar]
- 27.Pejda S, Varga ML, Milosevic SA, et al. Clinical and microbiological parameters in patients with self-ligating and conventional brackets during early phase of orthodontic treatment. Angle Orthod. 2013;83:133–139. doi: 10.2319/010412-8.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vanarsdall RL, Musich DR. Adult interdisciplinary therapy: diagnosis and treatment. In: Graber LW, Vanarsdall RL, Vig KWL, editors. Orthodontics Current Principles and Techniques 5th ed. St Louis, Mo: Mosby; 2012. p. 843. [Google Scholar]
- 29.Neiders M, Ramos B. Operation of bad breath clinics. Quintessence Int. 1999;30:295–301. [PubMed] [Google Scholar]
- 30.de Souza RA, de Araújo Magnani MBB, Nouer DF, da Silva CO, Klein MI, Sallum EA, Goncalves RB. Periodontal and microbiologic evaluation of 2 methods of archwire ligation: ligature wires and elastomeric rings. Am J Orthod Dentofacial Orthop. 2008;134:506–512. doi: 10.1016/j.ajodo.2006.09.067. [DOI] [PubMed] [Google Scholar]
- 31.Pandis N, Vlachopoulos K, Polychronopoulou A, Madianos P, Eliades T. Periodontal condition of the mandibular anterior dentition in patients with conventional and self-ligating brackets. Orthod Craniofac Res. 2008;11:211–215. doi: 10.1111/j.1601-6343.2008.00432.x. [DOI] [PubMed] [Google Scholar]
- 32.Buck T, Pellegrini P, Sauerwein R, et al. Elastomeric-ligated vs self-ligating appliances: a pilot study examining microbial colonization and white spot lesion formation after 1 year of orthodontic treatment. Orthodontics. 2011;12:108–121. [PubMed] [Google Scholar]
- 33.Mummolo S, Marchetti E, Giuca MR, et al. In-office bacteria test for a microbial monitoring during the conventional and self-ligating orthodontic treatment. Head Face Med. 2013;1:9:7 doi: 10.1186/1746-160X-9-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pithon MM, dos Santos RL, Nascimento LE, Osorio Ayre A, Alviano D, Bolognese AM. Do self-ligating brackets favor greater bacterial aggregation. Braz J Oral Sci. 2011;10:208–212. [Google Scholar]
- 35.Garcez AS, Suzuki SS, Ribeiro MS, Mada EY, Freitas AZ, Suzuki H. Biofilm retention by 3 methods of ligation on orthodontic brackets: a microbiologic and optical coherence tomography analysis. Am J Orthod Dentofacial Orthop. 2011;140:e193–e198. doi: 10.1016/j.ajodo.2011.04.019. [DOI] [PubMed] [Google Scholar]