Abstract
Objective:
To determine if the shape of the oropharyngeal airway is related to the vertical morphology of the skeletofacial complex, including the hyoid bone.
Materials and Methods:
Cone beam computed tomography scans from 50 pretreatment adult orthodontic records were used to obtain skeletal and airway measurements. Linear regression statistics were used to compare soft tissue variables to hard tissue predictor variables.
Results:
Transverse airway widening was significantly increased when the distance between the hyoid and vertebrae was reduced; when the three-dimensional (3D) facial axis angle decreased (became more vertical); when the 3D mandibular plane angle increased; when the width of the hyoid increased, or when the calculated length of the geniohyoid decreased.
Conclusions:
A laterally elliptical airway—found when the face is more vertical and when the hyoid is closer to the cervical vertebrae—is hypothetically more resistant to collapse. Patients with a retrognathic, skeletal deep bite and a rounded oropharynx should be identified and corrected early to prevent potential airway problems.
Keywords: Oropharynx shape, CBCT, Facial skeleton, Hyoid, OSA
INTRODUCTION
Upper airway dimension is a contributing factor to airway collapsibility in oxygen-limiting disorders such as obstructive sleep apnea (OSA).1–15 However, the findings related to patients with OSA are ambiguous. There is a lack of correlation between pharyngeal size and the apneic index of OSA patients.
Pharyngeal resistance to airway collapse on inspiration is decreased in OSA patients, but the overlap with normal individuals confuses the use of this parameter. Waking measurements of airway resistance in OSA individuals often display normal values.16
The suprahyoid muscle group lifts the hyoid to improve airway patency. Electromyography (EMG) of these muscles may be elevated in both sleeping and awake OSA and non-OSA individuals. It is believed that OSA individuals activate airway musculature to compensate for increased airway loads while sleeping. EMG activity might well be activated fully while awake but is unable to meet the required level of activation necessary for patency when the airway is loaded during sleep.17
The etiology of OSA is thus unclear: EMG activity, pharyngeal size, and pharyngeal resistance may be normal in OSA sufferers. Leiter's model8 introduced the physics of airway shape in OSA etiology. An airway that has a cross section that is longer anterior-posteriorly (A–P) than laterally is considered more at risk for collapse during sleep.8
The muscles associated with the anterior border of the pharynx influence the shape and volume of the airway. A laterally elliptical hypopharynx displays increased volume when pulled anteriorly by muscle action, compared to an airway that is originally rounded or elongated in the A–P direction (Figure 1).8 The lack of muscles on the lateral walls of the pharynx requires the internal pressure of the airway to maintain patency. It is the posterior and lateral walls that collapse in apneic individuals.18–20 Turbulence in the pharynx causes an increase in the velocity of air, with a lowering of surrounding pressure (the Bernoulli effect), which elevates the soft tissue structure into the narrowed space and closes the airway.21 It is the inspiratory activation of the muscles in the A–P plane, including the genioglossus, that stabilizes and dilates the airway anteriorly.21 The shape of the upper airway can also be constrained laterally by fat pads. It follows that the OSA patients with the smallest airway lumens, oriented in the A–P direction, require the greatest amount of EMG activity to maintain airway patency. Thus, there is a balance between airway lumen, EMG activity, and shape.
Biomechanical efficiency factors such as the position of the hyoid impact the relative muscle activity displayed and may determine whether or not a threshold effect is reached, resulting in OSA. Additionally, the physics of a transverse elliptical pharynx allow for less pharyngeal resistance, with less airway turbulence.8
A two-dimensional cephalometric study by Otsuka et al.22 compared variables between responders and nonresponders with regard to a titratable oral appliance. Surprisingly, middle and inferior airway space and oropharyngeal airway cross-sectional area were significantly larger in the group that did not respond to a titratable mandibular advancing appliance. However, only the sagittal component can be seen in cephalometric radiographs. Ogawa et al.23 demonstrated the utility of three-dimensional (3D) airway diagnosis with cone beam computed tomography (CBCT) imaging. Two-dimensional images are not capable of measuring cross-sectional areas of the oropharynx.
A CBCT evaluation by Schendel et al.24 showed that as the airway grows, the total volume, length, area, and volume-to-length index all increase until age 20, then remain relatively stable until age 50, when they begin to decrease profoundly. They found that 45-year-olds had only slightly larger airways than did 15-year-olds. Other anatomical factors exist that have an influence upon airway patency, but they are not fully understood.25–28
The present study was designed to clarify the relationship between airway shape, craniofacial morphology, and the hyoid using i-CAT® (Imaging Sciences International, Hatfield, Pa) CBCT images of adults that presented for orthodontic treatment. The specific aims were to (1) determine if the airway is laterally elliptical when the hyoid is lower, further back, angled, or smaller in width and (2) determine the correlation with vertical and horizontal facial morphology. Understanding the anatomical factors in the development of airway patency issues may improve diagnosis for risk factors of airway obstructions and possibly contribute to averting them with various treatment modalities.29–34
MATERIALS AND METHODS
This retrospective study was approved by the local Research Subjects Review Board. Fifty pretreatment CBCT records were collected from a private orthodontics practice. Inclusion criteria included the following: patient age of 18 years or older (nongrowing individuals); the hyoid had to be visible in its entirety; the subject must not have undergone previous orthognathic surgery; and the subject had to be dentate.
DICOM files were imported into Dolphin v.11 and oriented with the axial plane equal to the occlusal plane—defined by the mesiobuccal cusp tips of both maxillary first molars and the incisal embrasure between the maxillary central incisors. The occlusal plane is parallel to the planes of interest—the cross sections of the oropharynx (Figure 2). The midsagittal plane was subjectively determined.
Measurements of the hyoid, the craniofacial complex, and the oropharyngeal airway were made by digitizing 48 landmarks (see Table 1) using Dolphin 3D Imaging 11 software.
Table 1.
Landmark Definitions
The software was created to calculate specified measurements from the XYZ coordinates of the landmarks. It was necessary to modify conventional cephalometric measurements for 3D analysis. The angle of interest for all craniofacial measurements (two angles are always created at the intersection of two planes) was parallel to the sagittal plane. The following lists (Tables 2–4) show each measurement made and the definition of each according to the landmarks listed in Table 1.
Table 2.
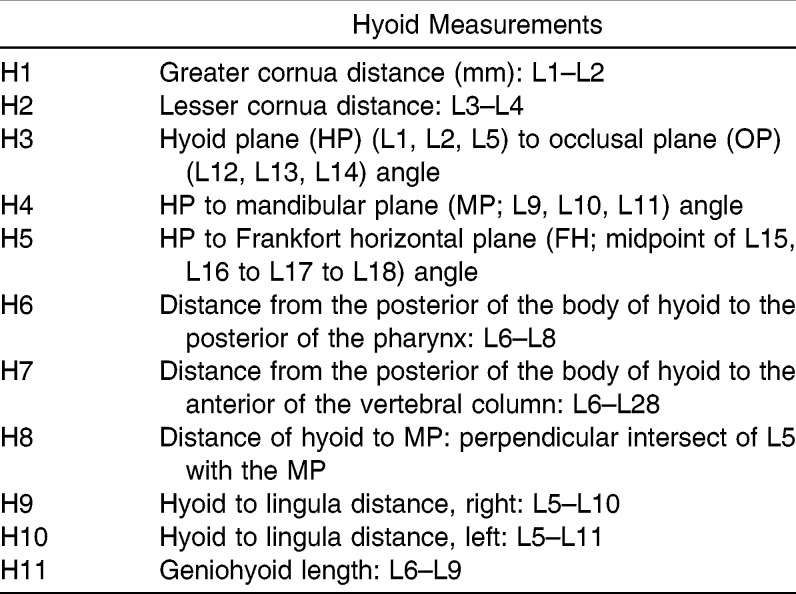
Hyoid Measurements
Table 3.
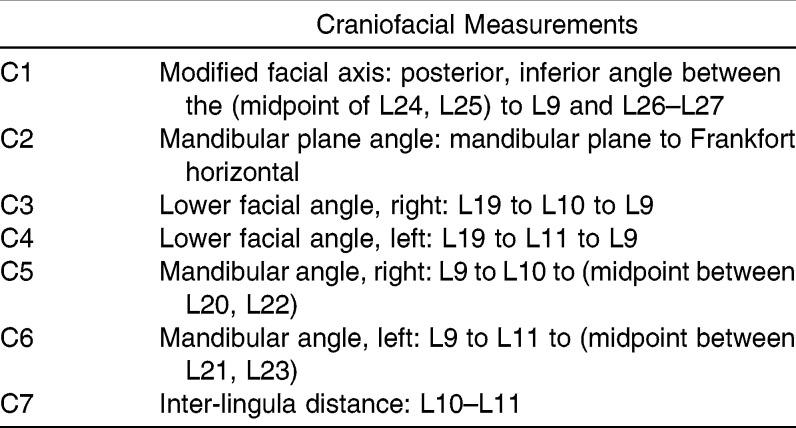
Craniofacial Measurements
Table 4.
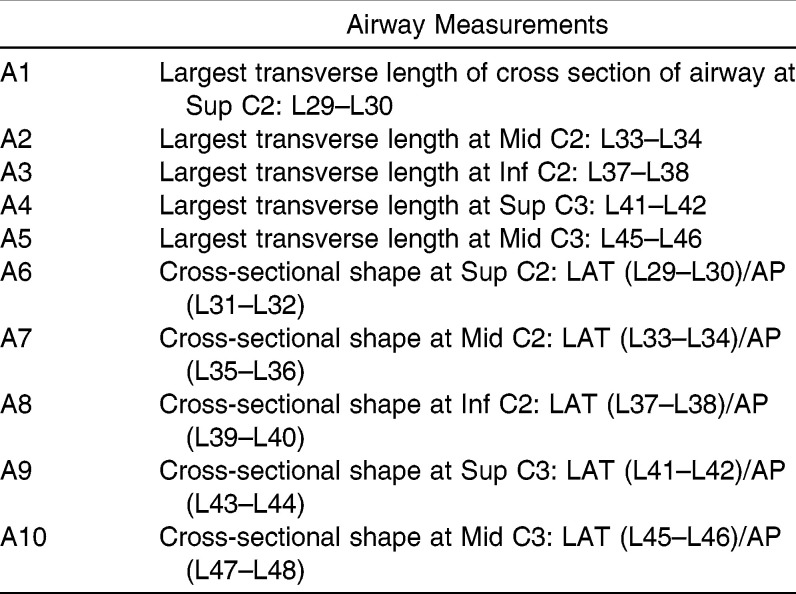
Airway Measurements
The SAS statistical software package (SAS Institute Inc, Cary, NC) was used to calculate linear regression using the hard tissue hyoid and craniofacial measurements (H1–H11 and C1–C7) as the independent variables and the airway measurements (A1–A10) as the dependent variables. Descriptive statistics were performed for each variable, including mean, standard deviation, and range. Pearson correlations compared each of the airway variables to each other. The normality of the outcome variables was tested with a Shapiro-Wilks test, which found that six of the 10 airway variables needed to be transformed via Box-Cox to obtain normality. A4 was squared, and the log of A6 through A10 was taken. Only the significant variables (p ≤ .05) were included in the linear regression equation.
RESULTS
Linear regression analysis showed that there are significant predictor hard tissue variables for all of the outcome airway variables. The significant hyoid predictor variables were the width between the greater cornua (H1); the width between the lesser cornua (H2); the distance of the anterior body of the hyoid from the nearest vertebra along the hyoid plane (H7); the angle of the hyoid plane to the occlusal plane (H3); the angle of the hyoid plane to Frankfort horizontal (H5); and the calculated length of geniohyoid muscle (H11). The most significant craniofacial predictor variables were modified facial axis (C1) and modified mandibular plane angle (C2).
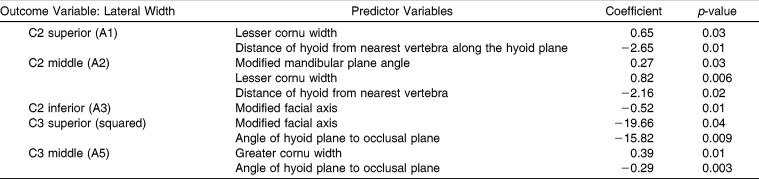
Table 5 shows the significant predictor variables for the lateral width of each level of the airway. It also shows the coefficient and p-value for each significant predictor variable. A negative coefficient represents an inverse relationship between the predictor and outcome variables. For example, the first two lines of the table can be interpreted in the following ways: the width between the lesser cornua of the hyoid increases by 0.65 mm or the distance between the hyoid body and the anterior surface of the vertebral column along the plane of the hyoid decreases by 2.65 mm; the width of the airway at the superior aspect of C2 increases by 1 mm.
Table 5.
Predictor Variables for the Lateral Width of the Airway
Table 6 shows the shape data as the outcome variables, in which each is represented as the log of the ratio of lateral to AP width of the airway at each of the five levels. Because each outcome variable in Table 6 has had its log taken, the coefficients of the predictor variables appear very small, but all are highly significant.
Table 6.
Predictor Variables for the Lateral/Anterior-Posterior (A-P) Ratio of the Airway
The relationship of hard tissue anatomy to the shape of the airway can be summarized as follows:
The more vertical the craniofacial morphology, according to the modified facial axis and modified mandibular plane angle, the more laterally wide and transversely elliptical the airway.
The closer the hyoid body and the vertebrae, the more laterally wide and transversely elliptical the airway.
The closer the hyoid is to the chin, the more transversely elliptical the airway. (For example, in a retrognathic, vertically developed individual, the hyoid is closer to the chin and the vertebrae.)
The wider the hyoid in the greater cornu or lesser cornu areas, the more laterally wide and transversely elliptical the airway.
The steeper the pitch of the hyoid relative to the Frankfort horizontal, the more elliptical the airway.
DISCUSSION
The position of the hyoid descends over time in vertical patients with a steep facial axis,11 apparently worsening the biomechanics necessary for a proper EMG level of the genioglossus and the suprahyoid muscles in the modality explained by Leiter.8 An increased need for excessive nighttime EMG activity and tonus is required for patency, which may not be achieved in this milieu. Collapsibility is a function of lumen size, not only lumen shape. Grauer et al.35 indicated a qualitatively narrower and smaller airway in vertical, as compared with normal, facial types. It is suggested that vertical facial types might well have a higher rate of OSA were it not for a compensated elliptical airway shape creating a transverse resistance to compression in the elliptical pharynxes.
Iwasaki et al.28 showed that while the size of the upper airway did not differ statistically among facial types, the simulated maximal pressure and velocity of the dolichofacial type were significantly higher than those of the brachyfacial type. The higher velocities and lower pressures found in the vertical skeletal types indicate a constriction of flow consistent with a narrowing of the airway.
A simple analogy is drawn in obtaining a photographic exposure. A combination of factors, with the potential for compensatory balances, yields a proper exposure. For example, a longer exposure can make up for a sensor with low light sensitivity. A similar interaction of anatomic (oropharyngeal shape and size) and physiologic factors (muscle activation) is necessary to avoid OSA.
Confounding factors in this study are that the images were captured in upright, alert patients with no attempt to control respiration or swallowing (ie, to mimic a nongrowing, orthodontic patient population). The head is tipped up during CBCT capture, until the occlusal plane is horizontal, so postural change of the airway is inevitable. The sample of 50 random patients offers a wide distribution of possible attributes. Dental classifications and sexual dimorphism were avoided at the current level of investigation because the sample was intended to be statistically random for morphologic traits and OSA status.
OSA is a multifactorial problem, of which shape is a major component that has been addressed in biophysical and physiologic research. Investigating 3D shape as it relates to skeletal morphology is proposed using a simple measurement made possible by CBCT imaging. This study was not intended to prove that a positive relationship exists between certain airway shapes and OSA subjects. It was designed to serve as a descriptive population study of how horizontal and vertical craniofacial measurements relate to 3D airway shape and as a precursor to additional research. Previous studies describing the cross-sectional relationship to the vertebrae and the hyoid involved two dimensions only.
CONCLUSIONS
Vertical aspects of skeletal morphology in relationship to the hyoid bone are linked to the shape of the airway, as the subjects in this study were not known to have an airway obstruction. No study has yet fully integrated the interplay among airway morphology, the physiology of airway dynamics (including suprahyoid muscle activity), and biomechanics and the size and shape of the pharyngeal airway.
OSA has a multifactorial etiology, and shape seems to be an important factor for airway patency. It is not known whether persons with a vertical facial pattern are more “resistant” to airway collapse because of the correlation with a transverse elliptical airway. It also does not mean that they are “immune” from airway obstructive disease. Other factors, such as obesity, decreased volume, inadequate muscle activity, aging, etc, may have an overarching effect.
Indeed, it may be more important than previously realized to take the vertical dimension into consideration in comprehensive treatment planning for airway patency. Brachyfacial patients with a flat mandibular plane angle that are retrognathic or bimaxillary skeletally retrusive and with a low hyoid may be at greater risk for sleep apnea or other obstructive sleep disorders later in life if an associated airway morphology is determined to be more “rounded” in configuration. This remains to be proven with confirmed OSA patients, instead of through inference through biophysical and morphologic data. Treatment plans that decrease the vertical and A–P skeletal dimensions in patients who are retrognathic and brachyfacial should be avoided.
By inference, this study proposes that it may be prudent to have a CBCT scan to assess airway shape in selected orthodontic patients with planned procedures that might compromise or negatively influence the airway.
Figure 1.
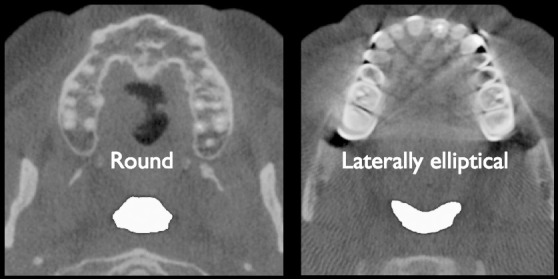
Cross section of the oropharynx. Based on shape, the left image is more at risk for collapse. The right image is a more desirable, less collapsible shape.
Figure 2.
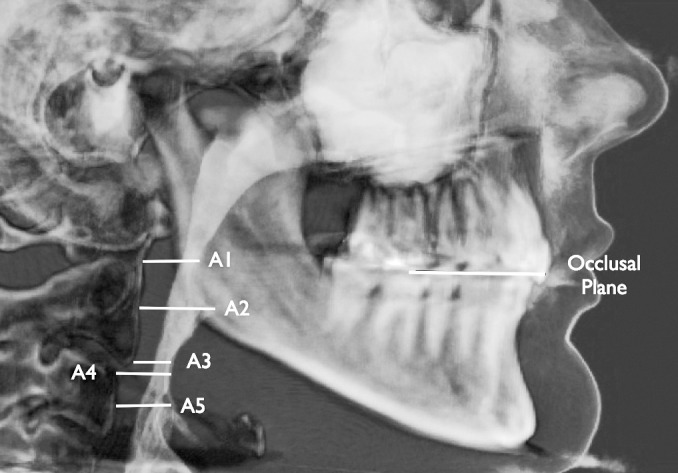
The five levels of the airway cross sections measured.
ACKNOWLEDGMENTS
Many thanks go to Joel Brodsky, DDS, MS, who provided the CBCT scans and made the study possible; David Rivshin, MS, for creating the software used to calculate the measurements; and J. Daniel Subtelny, DDS, MS, DSc (Hon) and Leonard S. Fishman, DDS, for their intellectual guidance and endless support.
REFERENCES
- 1.Clark GT, Arand D, Chung E, Tong D. Effect of anterior mandibular positioning on obstructive sleep apnea. Am Rev Respir Dis. 1993;147:624–629. doi: 10.1164/ajrccm/147.3.624. [DOI] [PubMed] [Google Scholar]
- 2.Choi JK, Hur YK, Lee JM, Clark GT. Effects of mandibular advancement on upper airway dimension and collapsibility in patients with obstructive sleep apnea using dynamic upper airway imaging during sleep. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;109:712–719. doi: 10.1016/j.tripleo.2009.11.034. [DOI] [PubMed] [Google Scholar]
- 3.deBerry-Borowiecki B, Kukwa A, Blanks RH. Cephalometric analysis for diagnosis and treatment of obstructive sleep apnea. Laryngoscope. 1988;98:226–234. doi: 10.1288/00005537-198802000-00021. [DOI] [PubMed] [Google Scholar]
- 4.Cuccia AM, Caradonna C. Mandibular advancement devices: indications and predictors of treatment outcome. A review. Minerva Stomatol. 2007;56:427–443. [PubMed] [Google Scholar]
- 5.Eikermann M, Jordan AS, Chamberlin NL, et al. The influence of aging on pharyngeal collapsibility during sleep. Chest. 2007;131:1702–1709. doi: 10.1378/chest.06-2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haskell JA, McCrillis JM, Haskell BS, Scheetz JP, Scarfe WC, Farman AG. Effects of mandibular advancement device (MAD) on airway dimensions assessed with cone-beam computed tomography. Semin Orthod. 2009;15:132–158. [Google Scholar]
- 7.Johal A, Patel SI, Battagel JM. The relationship between craniofacial anatomy and obstructive sleep apnoea: a case-controlled study. J Sleep Res. 2007;16:319–326. doi: 10.1111/j.1365-2869.2007.00599.x. [DOI] [PubMed] [Google Scholar]
- 8.Leiter JC. Upper airway shape: is it important in the pathogenesis of obstructive sleep apnea. Am J Respir Crit Care Med. 1996;153:894–898. doi: 10.1164/ajrccm.153.3.8630569. [DOI] [PubMed] [Google Scholar]
- 9.Malhotra A, Pillar G, Fogel R, Beauregard J, Edwards J, White DP. Upper-airway collapsibility: measurements and sleep effects. Chest. 2001;120:156–161. doi: 10.1378/chest.120.1.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oliven A, Aspandiarov E, Gankin I, Gaitini L, Tov N. Collapsibility of the relaxed pharynx and risk of sleep apnoea. Eur Respir J. 2008;32:1309–1315. doi: 10.1183/09031936.00139407. [DOI] [PubMed] [Google Scholar]
- 11.Pae EK, Quas C, Quas J, Garret N. Can facial type be used to predict changes in hyoid bone position with age? A perspective based on longitudinal data. Am J Orthod Dentofacial Orthop. 2008;134:792–797. doi: 10.1016/j.ajodo.2006.10.039. [DOI] [PubMed] [Google Scholar]
- 12.Schwab RJ. Pro: sleep apnea is an anatomic disorder. Am J Respir Crit Care Med. 2003;168:270–271; discussion 273. doi: 10.1164/rccm.2305014. [DOI] [PubMed] [Google Scholar]
- 13.Pierce R, White D, Malhotra A, et al. Upper airway collapsibility, dilator muscle activation and resistance in sleep apnoea. Eur Respir J. 2007;30:345–353. doi: 10.1183/09031936.00063406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stuck BA, Neff W, Hörmann K, et al. Anatomic changes after hyoid suspension for obstructive sleep apnea: an MRI study. Otolaryngol Head Neck Surg. 2005;133:397–402. doi: 10.1016/j.otohns.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 15.Choi JK, Goldman M, Koyal S, Clark G. Effect of jaw and head position on airway resistance in obstructive sleep apnea. Sleep Breath. 2000;4:163–168. doi: 10.1007/s11325-000-0163-1. [DOI] [PubMed] [Google Scholar]
- 16.Haponik EF, Smith PL, Bohlman ME, Allen RP, Goldman SM, Bleecker ER. Computerized tomography in obstructive sleep apnea. Correlation of airway size with physiology during sleep and wakefulness. Am Rev Respir Dis. 1983;127:221–226. doi: 10.1164/arrd.1983.127.2.221. [DOI] [PubMed] [Google Scholar]
- 17.White DP, Ballard RD. Pharyngeal muscle activity and upper airway resistance in obstructive apnea patients versus controls. In: Ipsa FG, Suratt PM, Remmers JE, editors. Sleep and Respiration. New York, NY: Wiley-Liss; 1990. pp. 243–251. [PubMed] [Google Scholar]
- 18.Sher AE, Thorpy MJ, Shprintzen RJ, Spielman AJ, Burack B, McGregor PA. Predictive value of Müller maneuver in selection of patients for uvulopharyngoplasty. Laryngoscope. 1985;95:1483–1487. doi: 10.1288/00005537-198512000-00009. [DOI] [PubMed] [Google Scholar]
- 19.Guilleminault CM, Hill MW, Simmons FB, Dement WC. Obstructive sleep apnea: electromyographic and fiber optic studies. Exp Neurol. 1978;62:48–67. doi: 10.1016/0014-4886(78)90040-7. [DOI] [PubMed] [Google Scholar]
- 20.Weitzman ED, Pollak CP, Borowiecki BB. The hypersomnia sleep-apnea syndrome: site and mechanisms of upper airway obstruction. In: Guilleminault C, Dement WC, editors. Sleep Apnea Syndromes. New York, NY: Alan R. Liss Inc; 1978. pp. 235–238. [Google Scholar]
- 21.Leiter JC. Analysis of pharyngeal resistance and genioglossal EMG activity using a model of orifice flow. J Appl Physiol. 1992;73:576–583. doi: 10.1152/jappl.1992.73.2.576. [DOI] [PubMed] [Google Scholar]
- 22.Otsuka R, Almeida FR, Lowe AA, Ryan F. A comparison of responders and nonresponders to oral appliance therapy for the treatment of obstructive sleep apnea. Am J Orthod Dentofacial Orthop. 2006;129:222–229. doi: 10.1016/j.ajodo.2004.06.035. [DOI] [PubMed] [Google Scholar]
- 23.Ogawa T, Enciso R, Memon A, Mah JK, Clark GT. Evaluation of 3D airway imaging of obstructive sleep apnea with cone-beam computed tomography. Stud Health Technol Inform. 2005;111:365–368. [PubMed] [Google Scholar]
- 24.Schendel S, Jacobson R, Khalessi S. Airway growth and development: a computerized 3-dimensional analysis. J Oral Maxillofac Surg. 2012;70:2174–2183. doi: 10.1016/j.joms.2011.10.013. [DOI] [PubMed] [Google Scholar]
- 25.Lowe AA, Gionhaku N, Takeuchi K, Fleetham JA. Three-dimensional CT reconstructions of tongue and airway in adult subjects with obstructive sleep apnea. Am J Orthod Dentofacial Orthop. 1986;90:364–374. doi: 10.1016/0889-5406(86)90002-8. [DOI] [PubMed] [Google Scholar]
- 26.Abramson Z, Susarla S, Troulis M, Kaban L. Age-related changes of the upper airway assessed by 3-dimensional computed tomography. J Craniofac Surg. 2009;20(suppl 1):657–663. doi: 10.1097/SCS.0b013e318193d521. [DOI] [PubMed] [Google Scholar]
- 27.Iwasaki T, Hayasaki H, Takemoto Y, Kanomi R, Yamasaki Y. Oropharyngeal airway in children with Class III malocclusion evaluated by cone-beam computed tomography. Am J Orthod Dentofacial Orthop. 2009;136:318.e1–318.e9. doi: 10.1016/j.ajodo.2009.02.017. [DOI] [PubMed] [Google Scholar]
- 28.Iwasaki T, Saitoh I, Takemoto Y, et al. Evaluation of upper airway obstruction in Class II children with fluid-mechanical simulation. Am J Orthod Dentofacial Orthop. 2011;139:e135–e145. doi: 10.1016/j.ajodo.2010.08.014. [DOI] [PubMed] [Google Scholar]
- 29.Battagel JM, L'Estrange PR, Nolan P, Harkness B. The role of lateral cephalometric radiography and fluoroscopy in assessing mandibular advancement in sleep-related disorders. Eur J Orthod. 1998;20:121–132. doi: 10.1093/ejo/20.2.121. [DOI] [PubMed] [Google Scholar]
- 30.Hilgers ML, Scarfe WC, Scheetz JP, Farman AG. Accuracy of linear temporomandibular joint measurements with cone beam computed tomography and digital cephalometric radiography. Am J Orthod Dentofacial Orthop. 2005;128:803–811. doi: 10.1016/j.ajodo.2005.08.034. [DOI] [PubMed] [Google Scholar]
- 31.Moshiri M, Scarfe WC, Hilgers ML, Scheetz JP, Silveira AM, Farman AG. Accuracy of linear measurements from imaging plate and lateral cephalometric images derived from cone-beam computed tomography. Am J Orthod Dentofacial Orthop. 2007;132:550–560. doi: 10.1016/j.ajodo.2006.09.046. [DOI] [PubMed] [Google Scholar]
- 32.Honey OB, Scarfe WC, Hilgers MJ, et al. Accuracy of cone-beam computed tomography imaging of the temporomandibular joint: comparisons with panoramic radiology and linear tomography. Am J Orthod Dentofacial Orthop. 2007;132:429–438. doi: 10.1016/j.ajodo.2005.10.032. [DOI] [PubMed] [Google Scholar]
- 33.Cho HJ. A three-dimensional cephalometric analysis. J Clin Orthod. 2009;43:235–252; discussion 235; quiz 273. [PubMed] [Google Scholar]
- 34.Conley RS, Legan HL. Correction of severe obstructive sleep apnea with bimaxillary transverse distraction osteogenesis and maxillomandibular advancement. Am J Orthod Dentofacial Orthop. 2006;129:283–292. doi: 10.1016/j.ajodo.2005.11.029. [DOI] [PubMed] [Google Scholar]
- 35.Grauer D, Cevidanes LS, Styner M, Ackerman JL, Proffit W. Pharyngeal airway volume and shape from cone-beam computed tomography: relationship to facial morphology. Am J Orthod Dentofacial Orthop. 2009;136:805–814. doi: 10.1016/j.ajodo.2008.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]