Abstract
Introduction:
Early recognized manifestations of GSD III include hypoglycemia, hepatomegaly, and elevated liver enzymes. Motor symptoms such as fatigue, muscle weakness, functional impairments, and muscle wasting are typically reported in the 3rd to 4th decade of life.
Objective:
In this study, we investigated the early musculoskeletal findings in children with GSD IIIa, compared to a cohort of adults with GSD IIIa.
Methods:
We utilized a comprehensive number of physical therapy outcome measures to cross-sectionally assess strength and gross motor function including the modified Medical Research Council (mMRC) scale, grip and lateral/key pinch, Gross Motor Function Measure (GMFM), Gait, Stairs, Gowers, Chair (GSGC) test, 6 Minute Walk Test (6MWT), and Bruininks-Oseretsky Test of Motor Proficiency Ed. 2 (BOT-2). We also assessed laboratory biomarkers (AST, ALT, CK and urine Glc4) and conducted whole-body magnetic resonance imaging (WBMRI) to evaluate for proton density fat fraction (PDFF) in children with GSD IIIa. Nerve Conduction Studies and Electromyography results were analyzed where available and a thorough literature review was conducted.
Results:
There were a total of 22 individuals with GSD IIIa evaluated in our study, 17 pediatric patients and 5 adult patients. These pediatric patients demonstrated weakness on manual muscle testing, decreased grip and lateral/key pinch strength, and decreased functional ability compared to non-disease peers on the GSGC, 6MWT, BOT-2, and GSGC. Additionally, all laboratory biomarkers analyzed and PDFF obtained from WBMRI were increased in comparison to non-diseased peers. In comparison to the pediatric cohort, adults demonstrated worse overall performance on functional assessments demonstrating the expected progression of disease phenotype with age.
Conclusion:
These results demonstrate the presence of early musculoskeletal involvement in children with GSD IIIa, most evident on physical therapy assessments, in addition to the more commonly reported hepatic symptoms. Muscular weakness in both children and adults was most significant in proximal and trunk musculature, and intrinsic musculature of the hands. These findings indicate the importance of early assessment of patients with GSD IIIa for detection of muscular weakness and development of treatment approaches that target both the liver and muscle.
Keywords: Glycogen storage disease type III, GSD III, whole-body magnetic resonance imaging, WBMRI, muscle strength, muscle function, physical therapy, PT, GSGC, GMFM, 6-Minute Walk Test, BOT-2, hand grip strength, lateral key pinch
1. INTRODUCTION
Glycogen storage disease type III (GSD III, MIM #232400) is a rare, autosomal recessive disorder caused by pathogenic variants in the AGL gene (MIM# 610860), resulting in a deficiency of glycogen debranching enzyme (GDE). Together with the enzyme glycogen phosphorylase, GDE plays a vital role in the degradation of glycogen. A deficiency of GDE results in abnormally structured glycogen enriched in α−1,6 branch points and an accumulation of limit dextrin-like molecules in tissues1. Nutritional management of GSD III consists of a high-protein low-carbohydrate diet and supplementation with uncooked cornstarch and medium chain fats for the prevention of hypoglycemia. A high protein diet is beneficial in that it provides an alternative source of glucose and reduces excess glycogen storage in muscle and liver, but also assists with muscle protein synthesis, possibly improving muscle function3.
Clinical manifestations of GSD III are variable and classified into two primary subtypes; GSD IIIa, which is present in 85% of all patients, is characterized by liver, heart, and muscle involvement, or GSD IIIb in which the liver is primarily affected2. Beginning in early childhood, children may exhibit hypoglycemia, hepatomegaly and elevated liver enzymes2,3. The extent of skeletal muscle involvement in GSD IIIa is reported as variable, may present as motor delays and muscle fatigue in childhood, and may be subtle and/or underestimated4. As we have previously reported, initial reports of the use of more standardized gross motor testing in children showed that 80% of children with GSD IIIa demonstrated average gross motor function below the 25th percentile for age5. Musculoskeletal features previously reported have included hypermobility at individual joints, including hyperextension at elbows and knees, and altered alignment in standing and walking characterized by an anterior pelvic tilt and lumbar lordosis, slightly increased width of base of support, genu valgum and recurvatum, hindfoot valgus, and forefoot varus3. Muscular weakness and wasting progress with age and become severe by the third or fourth decade of life; the resulting myopathy may be both proximal and distal, resulting in wheelchair dependence, difficulty with fine motor tasks, and exercise intolerance6–7. However, the musculoskeletal findings in patients with GSD III are overshadowed initially by prominent liver symptoms8. Clinically, there is a need for greater use of tools to quantify the extent of myopathy involvement early in the clinical course and follow the progression over time.
Whole-body muscle magnetic resonance imaging (WBMRI) has clinical utility in diagnosis and disease monitoring in several progressive neuromuscular disorders9–10. WBMRI can visualize the amount of intramuscular fat infiltration which develops secondary to myopathy, measured as proton-density fat-fraction (PDFF). PDFF is an accurate, quantitative value, ascribed with the help of WBMRI, and it can be used to appreciate the extent of disease and follow disease progression longitudinally10.
However, WBMRI is costly, requires expertise to interpret, and may not be readily available at all centers. Furthermore, the process of intramuscular fat accumulation can take time to develop. Muscle strength and functional testing via physical therapy (PT) assessments are alternative, non-invasive means of evaluating muscle disease and have been shown to correlate well with the degree of fatty infiltration seen on imaging10.
The purpose of this paper was to cross-sectionally evaluate the musculoskeletal findings in a cohort of individuals with GSD IIIa. To achieve this aim, a comprehensive set of PT assessments including the Gait, Stairs, Gowers, Chair (GSGC) test, Gross Motor Function Measure-88 (GMFM), 6 Minute Walk Test (6MWT), Bruininks-Oseretsky Test of Motor Proficiency Ed. 2 (BOT-2), hand grip strength, and lateral/key pinch strength were reviewed and compared to WBMRI results, a panel of biomarkers, and electromyography (EMG) and nerve conduction study (NCS) results. This study is the first to evaluate muscular weakness of individuals with GSD IIIa with a comprehensive array of physical therapy assessments in conjunction with comparison of intramuscular fat infiltration as measured by Whole Body MRI (WBMRI).
2. METHODS
2.1. Patients
Children and adults with a confirmed diagnosis of GSD III qualified to participate in this study. Informed consent was obtained from each participant (participants ≥18 years) or legal guardian (participants <18 years) to participate in the Duke GSD III Natural History study (Pro#00047556). Diagnosis was confirmed using molecular testing and/or enzyme analysis. In conjunction with metabolic clinic visits and nutritional assessments, physical therapists performed detailed muscle strength and functional testing when feasible. WBMRI scans were obtained, and routine labs were collected including urinary Glc4, and serum CK, AST, and ALT.
2.2. Muscle strength and functional testing
Manual muscle testing was performed by physical therapists experienced in neuromuscular disorders. Muscle strength was measured using a modified Medical Research Council (mMRC) scale which ranges from 0 (no contraction) to 5 (full strength); the scale was converted to a 0 to 12-point scale as described previously10. Strength testing was performed for the following movements: spinal extension, hip flexion, hip extension (with flexed and extended knee), hip abduction, hip adduction, knee extension, ankle dorsiflexion and ankle plantarflexion.
Functional tests performed included: GSGC, GMFM-88, BOT-2, and 6MWT. We have previously provided a detailed description of these functional measures10–11. For the BOT-2, the scores provided represent the Strength and Agility Composite. In addition, hand grip and lateral/key pinch strength were measured as follows: participants were seated in a chair without back or arm support, and feet fully supported with knees flexed 90 degrees. Arms were positioned at the participants side with zero degrees shoulder flexion, 90 degrees elbow flexion, neutral forearm (thumb pointed up), and 15 degrees wrist extension. Jamar hydraulic dynamometers (Sammons Preston, Bolingbrook. IL, USA) for grip and pinch were utilized. For grip measurements, the handle was adjusted to achieve a comfortable grip based on hand size (since this could change as the children grow). Instructions for lateral/key pinch were to hold the instrument between the thumb and lateral side of the index finger “as you would hold a key”. Acquisition time was for 5 seconds, with verbal encouragement provided for maximum effort, followed by a 15 second rest break between trials. A mean of 3 trials was used for calculations.
2.3. Whole-body magnetic resonance imaging
All WBMRI scans were performed on the same research 3T MRI system. A single, highly experienced radiology technologist carried out delineation of muscles for all scans, and the results were verified by a radiologist. A region of interest (ROI) was drawn around the contour of the muscles being evaluated, and a PDFF value was generated as described previously10.
Imaged muscles included in the analysis were: flexor digitorum profundus, adductor pollicis, thoracic and lumbar spinal extensors, gluteus maximus, gluteus medius, iliopsoas, rectus femoris, adductors (magnus, longus and brevis), hamstrings (semimembranosus, semitendinosus, biceps femoris), anterior tibialis and gastrocnemius. These muscles were selected as their involvement has been documented previously and is recognized clinically10.
PDFF values in muscles of healthy pediatric controls were published previously and concluded the average overall PDFF to be around 2–5%12. Our group previously published PDFF in muscles of healthy adult individuals13. These data were used to compare to the PDFF values in our GSD III cohort.
2.4. Electromyography (EMG), Nerve Conduction Study (NCS), and Neuromuscular Ultrasound
When available, results from patient electromyography (EMG) and nerve conduction studies (NCS), and/or neuromuscular ultrasound were analyzed for evidence of neuropathy and/or myopathy. The results from our patient cohort were further compared to prior EMG/NCS findings reported for GSD III patients collected as part of the comprehensive literature review.
2.5. Statistical Analysis
Patients included in the study were divided by age into two groups: children (0–18 years) and adults (18+ years) for analysis. Descriptive statistics (median and interquartile range (IQR)) were performed for each PT measure, biomarker, and PDFF. To minimize bias, only a single time point per patient was analyzed in statistical comparisons. As the focus of the study was on early musculoskeletal involvement in children with GSD IIIa, the earliest timepoint available for each test was utilized in analysis for each patient. The same timepoint was additionally used for laboratory values. For those patients with WBMRI data available, the physical therapy timepoint assessed was the visit closest to the time of the WMBRI. Due to the rare nature of the disease, and thus limited sample size, all statistical analyses were performed using non-parametric methods and reported using median and IQR. For comparisons between children and adult data a Mann-Whitney test was utilized. The Wilcoxon matched-pairs signed rank test was used for paired comparisons of grip and lateral key pinch strength between the right and left hand. The Friedman test with multiple comparisons was used to analyze performance across subdimensions or subdomains of the GMFM and GSGC, respectively. Additionally, Spearman correlations were performed where relevant to analyze the relationship between PT assessments, lab values, and PDFF. Statistical analyses were performed using GraphPad Prism 914.
2.5. Literature review
A thorough literature review was conducted using PubMed utilizing phrases such as “glycogen storage disease type 3”, “glycogen storage disease type III”, “debrancher enzyme deficiency”, “Cori’s Disease”, “muscle”, “musculoskeletal”, “physical therapy”, and “magnetic resonance imaging” in the search field.
3. RESULTS
3.1. Patients
Twenty-six patients (19 females, 7 males) with a confirmed diagnosis of GSD III enrolled in the study and were evaluated between 2007 and 2019 through prospective data collection and retrospective chart review. Four patients in our study have GSD IIIb and were excluded from analyses given the lack of muscular involvement common amongst the disease phenotype. The median age of PT assessment for children was 11.52 years (range: 3.7–17.7 years), while adults was 40.86 years (range: 19.4–58.2 years).
Eleven patients had a total of 21 WBMRI scans. The earliest scan or the scan with the closest physical therapy evaluation was utilized for the purpose of the study. Time between physical therapy evaluation and the WBMRI used for comparison is provided in Table 1. The majority of patients had PT and WBMRI evaluations performed within the same week, with the longest interval being 1 year.
Table 1:
Cohort characteristics
| ID | Sex/Age at diagnosis | Age at PT (yrs) | # of WBMRI Scans | Time (months) between PT and WBMRI | Variant 1 | Variant 2 | Cornstarch dose/dietary therapy |
|---|---|---|---|---|---|---|---|
| 1 | F/9mo | 14.6 | 1 | 0 | c.2309–1G > A | c.4260–12A > G | 1.23 g/kg/day |
| 2 | F/6mo | 13.9 | 0 | NA | Results not available | Results not available | 2.55 g/kg/day Protein supplementation. |
| 3 | F/ 6.7 mo | 15.1 | 3 | 11 | c.2309–1G > A | c.4260–12A > G | 0.7 g/kg/day |
| 4 | F/ 2.5 yo | 17.7 | 1 | 0 | c.293 + 1del | c.413G > A p.Gly138Glu |
0.5 g/kg/day |
| 5 | F/ 18 mo | 12.5 | 0 | NA | c.1276del p.Val426fs |
Not detectable | 6 g/kg/day Protein supplementation |
| 6 | M/ 15 mo | 11.3 | 0 | NA | Results not available | Results not available | 1.2 g/kg/day Protein supplementation |
| 7 | F/ 17 mo | 10.1 | 0 | NA | c.3259 + 3A > T | c.3259 + 3A > T | 0.3 g/kg/day Protein supplementation |
| 8 | F/9mo | 40.9 | 1 | 2 | c.966dup68 | c.2590C > T p.Arg864Ter c.4529dup p.Tyr1510Ter |
0.2 g/kg/day Protein supplementation |
| 9 | F/ 15 mo | 8.4 | 0 | NA | c.3299del p.Gly1100fs |
c.3299del p.Gy1100fs |
1.9 g/kg/day Protein supplementation |
| 10 | F/4yo | 8.2 | 1 | 0 | c.2929C > T p.Arg977Ter |
c.3866 T > C p.Leu1289Pro |
2.32 g/kg/day Protein supplementation |
| 12 | F/1yo | 3.7 | 0 | NA | c.4221dup p.Leu1408fs |
c.4260–12A > G | 6.1 g/kg/day Protein supplementation |
| 13 | M/12yo | 52.2 | 4 | 0 | c.100C > T p.Arg34Ter |
c.2590C > T p.Arg864Ter |
Not on cornstarch therapy Protein supplementation |
| 14 | F/ 12 mo | 14.6 | 1 | 0 | c.1384del p.Trp461_Val462insTer |
c.1384del p.Trp461_Val462insTer |
0.64 g/kg/day Protein supplementation |
| 15 | M/ 16 mo | 9.8 | 4 | 12 | c.3980G > A p.Trp1327Ter |
c.2023C > T p.Arg675Trp |
3.8 g/kg/day Protein supplementation |
| 18 | F(sibling)/4mo | 13.5 | 2 | 0 | c.2681 + 1G > T | c.2681 + 1G > T | 0.73 g/kg/day Protein supplementation |
| 19 | F(sibling)/4mo | 17.2 | 2 | 0 | c.2681 + 1G > T | c.2681 + 1G >T | 0.63 g/kg/day Protein supplementation |
| 21 | F/6mo | 19.4 | 1 | 1 | c.4529dup p.Tyr1510Ter |
c.2309–1G > A | Not on cornstarch therapy |
| 28 | F/ 8 mo | 6.9 | 0 | NA | c.1199 T > C p.Leu400Pro |
c.1199 T > C p.Leu400Pro |
Not on cornstarch therapy |
| 29 | F/18 mo | 58.2 | 0 | NA | Not available | Not available | Not on cornstarch therapy Protein supplementation |
| 32 | F/6mo | 33.6 | 0 | NA | c.410_413del p.Leu137fs |
c.2039G > T | Not on cornstarch therapy |
| 34 | M/8mo | 5.2 | 0 | NA | c.3682C > T p.Arg1228Ter |
c.3235C > T p.Gln1079Ter |
Not on cornstarch therapy |
| 35 | M/2yo | 13.1 | 0 | NA | Exon 3 del | Exon 3 del | Not on cornstarch therapy |
Table 1 abbreviations: NA = Not applicable, PT = Physical Therapy, WBMRI = Whole-body MR1.
A clinical summary of the cohort is presented in Table 1, including dietary therapy. All patients received a high protein (25–30% of daily caloric requirement) and low carbohydrate diet (<50% of total calories) and were followed closely by a metabolic dietician. All patients who fell in the “children” age group (17/22) have been described previously with the same study IDs in a publication detailing their clinical, dietary, biochemical, and hepatic imaging and histopathological findings8.
3.2. Muscle strength and function testing
The average mMRC score, henceforth referred to as strength, of all examined movements in children and adults was 8.00 (IQR Child: 2.3; IQR Adult: 1.45) indicating weakness in both groups (a score of 12 represents full strength) (Table 2). The average score for each movement is detailed in Figure 1. Ankle plantarflexion (ANK PFLX) strength was impaired in children (6.50; IQR: 5.38) and moderately lower than in adults (9.00; IQR: 6). Plantarflexion strength was assessed in a standing position in all children and half the adult patients, but the position was not specified for the remaining adults. However, while ankle dorsiflexion (ANK DFLX) was also mildly impaired in children (11.00; IQR:4.00), it was greater in children than in adults (9.00; IQR: 6). Children had decreased strength for hip abduction and flexion, and spinal extension (SPN EXT), performing similar to the adult cohort (HIP ABD: 8.00 (IQR: 3.75) vs. 7.00 (IQR: 5.25); HIP FLX: 8.00 (IQR:2) vs. 7.5 (IQR: 4); SPN EXT: 8.0 (IQR: 7) vs. 8.0 (IQR:4)). Both children and adults demonstrated weakness in hip adduction (HIP ADD), with a median score of 5.00 (IQR child: 2.5; IQR adult: 3.5)).
Table 2:
Descriptive statistics for PT tests and assessments in children and adults with GSD IIIa
| Children | Adults | |||||
|---|---|---|---|---|---|---|
| n | Median | 25th-75th Percentile | n | Median | 25th-75th Percentile | |
| mMRC Total Score | 16 | 8.00 | 6.45–8.75 | 5 | 8.00 | 6.75–8.20 |
| Grip Strength (lbs) | 12 | 37.32 | 23.56–41.87 | 5 | 37.16 | 25.92–49.88 |
| Grip Percentile | 9.09 | 3.46–16.48 | 1.77 | 0.15–13.92 | ||
| Lateral/Key Pinch (lbs) | 12 | 7.30 | 4.55–9.66 | 5 | 4.83 | 1.24–9.76 |
|
Lateral/Key Pinch
Percentile |
1.27 | 0.12–5.67 | 0.00 | 0.00–0.50 | ||
| GMFM Total Score % | 16 | 98.75 | 97.245–99.72 | 3 | 95.26 | 71.95–100.00 |
| GSGC Total Score | 17 | 5.94 | 2.21 | 3 | 15.67 | 7.51 |
| 6MWT Distance (m) | 16 | 490.5 | 60.57 | 3 | 421.5 | 92.82 |
|
6MWT Percent
Predicted |
76.67 | 17.35 | 68.09 | 6.09 | ||
|
BOT-2 Strength and
Agility Composite Standard Score |
15 | 39.00 | 31.00–42.00 | NA | NA | NA |
|
BOT-2 Strength and
Agility Composite Percentile Rank |
8.00 | 3.00–21.00 | NA | NA | ||
With regards to mMRC the normal value for the scale utilized in this study is 12.
Figure 1: Mean mMRC score for selected movements, divided by age group.
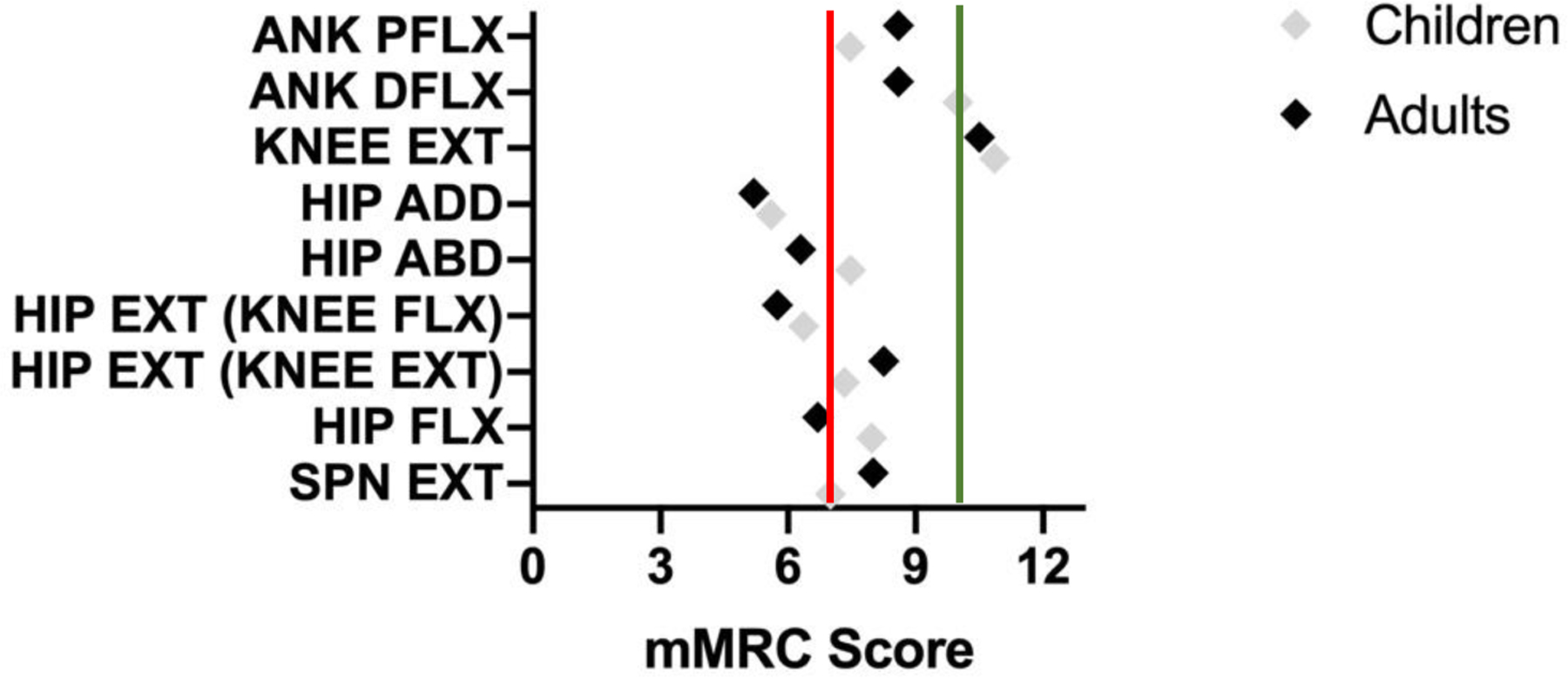
Utilizing the modified scale of 0–12, with 12 indicating full strength, it is notable that full strength was not found for any of the selected movements in children or adults with GSD IIIa. As noted in Table 3, a score of 7 (red line) indicates the movement is completed in full range against gravity, but the muscle group is unable to tolerate any additional resistance. A score of 10 (green line) indicates the muscle group can tolerate moderate, but not full, resistance.
Outcome measures to assess gross motor function included GMFM-88, GSGC, BOT-2, and the 6MWT (Table 2). While both children and adults with GSD IIIa performed well on the GMFM, median GMFM total score in children (98.75; IQR: 2.48) was greater and less variable than in adults (95.26; IQR: 28.05) (Table 2, Figure 2a). The GMFM is divided into five dimensions that analyze performance on specific motor tasks. Dimension A measures ability in lying and rolling, Dimension B measures sitting tasks, Dimension C measures crawling and kneeling tasks, Dimension D measures standing tasks, and Dimension E measures walking, running, and jumping tasks. There was a significant difference in performance of children with GSD IIIa between the dimensions of the GMFM (p<0.05), with further multiple comparisons demonstrating a significant difference between Dimensions A-C compared to Dimension E (Figure 3a). While all children scored above 95% on Dimensions A-C, 38% and 50% of children scored below 95% on Dimension D and Dimension E, respectively.
Figure 2: GMFM and GSGC Scores of Children and Adults with GSD IIIa.
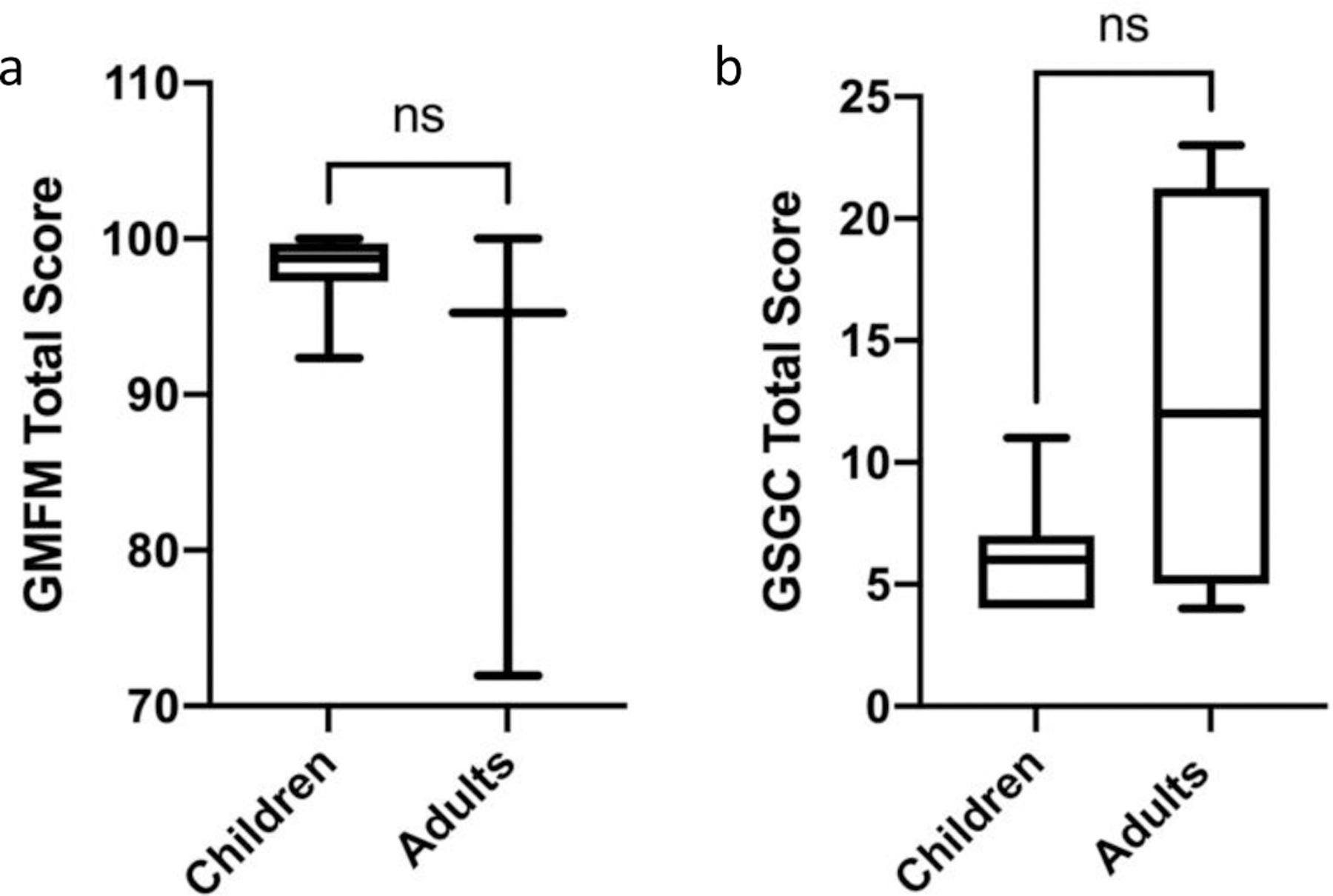
a. GMFM performance ranges from a 0–100 percent, with 100 percent representing normal function. There was no significant difference between performance on the GMFM between children and adults with GSD IIIa, as measured using a Mann Whitney test. b. GSGC performance ranges from 4–27, with 4 representing normal function and 27 representing inability to perform any of the tasks. There was no significant difference between performance on the GSGC between children and adults with GSD IIIa; however, the majority of children demonstrated at least some reduction in performance. Performance of children and adults was analyzed using a Mann Whitney test. For both graphs, ns = not significant.
Figure 3: GMFM Dimensional Scores and GSGC Subdomain Performance of Children with GSD IIIa.
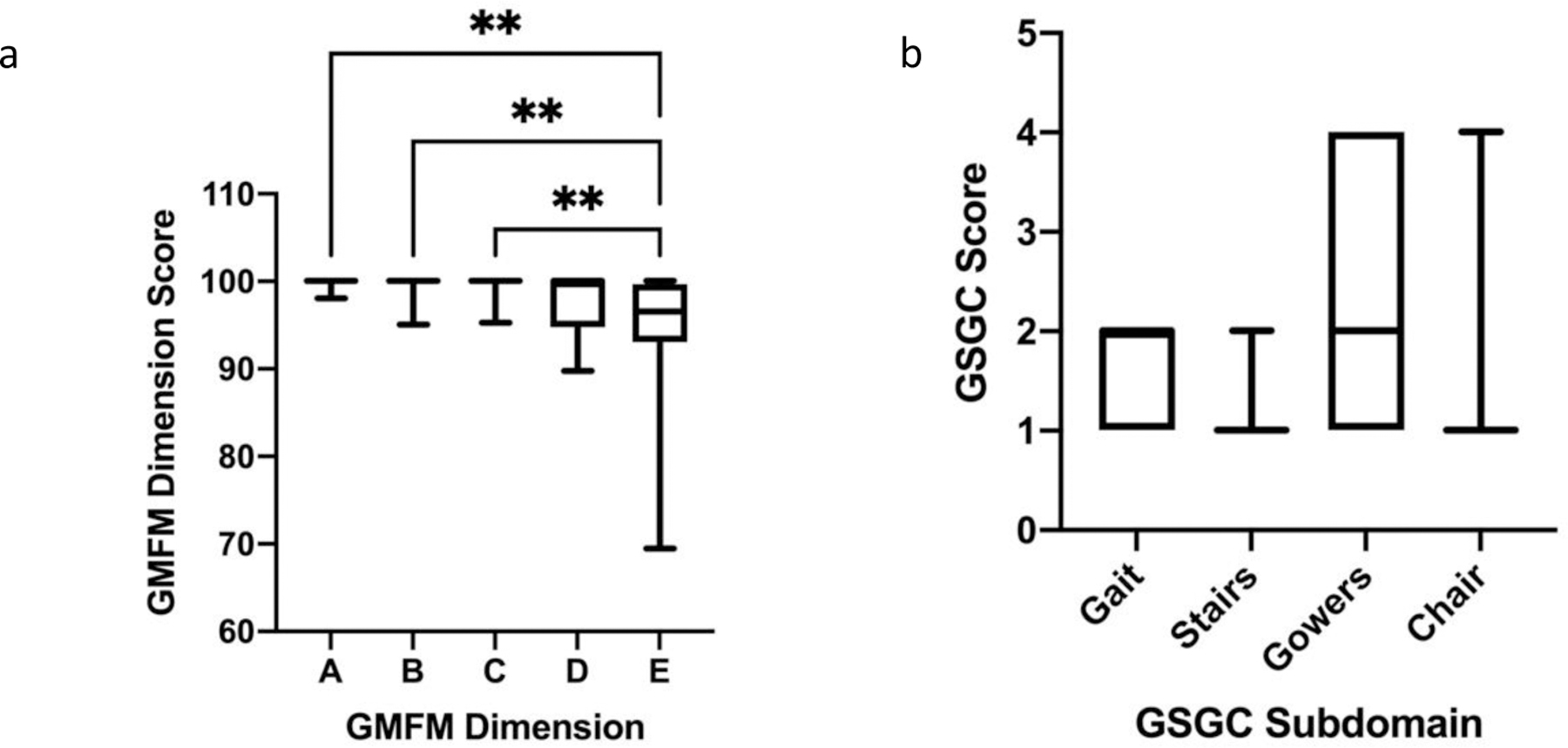
a. With respect to the GMFM Dimensional performance, a significant difference was appreciated amongst the pediatric cohort, using a Friedman test with multiple comparisons. Children with GSD IIIa demonstrated poorer performance on Dimension D, standing tasks, and Dimension E, walking, running, and jumping tasks. Only significant results from the multiple comparisons are indicated. **= p<0.01. b. On the GSGC, a significant difference was found between overall performance on the GSGC subdomains using a Friedman test; however, no significant differences were present with review of the multiple comparisons. In general a higher proportion of individuals demonstrated a poorer performance, represented by a greater score, on the Gait and Gowers subdomains. Gait, Gowers, and Stairs are graded on a 1–7 range, with 7 indicating the inability to perform the maneuver. The chair maneuver is graded on 1 −6 range, with 6 meaning inability to arise from a chair15. No individual scored the maximum for any of the four maneuvers.
On the GSGC, a score of 4 represents normal function and a score of 27 represents the inability to perform any of the tasks. With regards to the GSGC, children performed measurably better than adults with median (IQR) values of 6.00 (3) and 12 (16.25), respectively; although, this finding was not statistically significant (p=0.14) (Table 2 and Fig 2b). However, the median score for the pediatric cohort was still greater than expected for non-diseased peers. The GSGC is comprised of four functional tasks (walk 10 m, climb 4 stairs, supine to stand, and sit to stand) which are each timed and also given a qualitative score. Similar to GMFM dimensions, a statistically significant difference (p<0.05) was present among subdomain performance for children with GSD IIIa. Children experienced the greatest difficulty with the Gower and Chair tasks. While all children scored either a 1 or 2 on the Gait and Stairs portions of the assessment, 18% of children scored a 4 on the Gower maneuver, indicating they had to push up with a hand on a knee to achieve standing from supine (Figure 3b)15. Additionally, 12% of children scored a 4 on the Chair tasks indicating the need to push up with 2 hands on thighs to achieve standing from sitting (Figure 3b)15. All patients, both pediatric and adult, were able to perform all measures. For the GSGC timed measures, for supine to stand, the average time for children was 3.2 seconds (s) compared to 5.74 s for adults. On average, it took children 2.54 s to climb 4 stairs compared to 2.41 s in the adult cohort. With respect to gait speed and time to walk 10 m, the average time for children to walk 10 m was 6.41 s with an average gait speed of 1.44 m/s. The average time for the adult cohort to walk 10 m was 7.62 s with an average gait speed of 1.78 m/s. Lastly, on average it took the pediatric cohort 0.99 s to go from sitting to standing compared to an average of 1.97 s for the adult cohort. There was no significant difference in the 6MWT distance (Figure S1a) and percent predicted (Figure S1b) between children and adults. However, both children and adults performed below age-adjusted predicted score for non-diseased peers (children 67.57 (IQR: 14.97), adults 72 (IQR: 6.88)) (Figure S1b). For the Speed and Agility Composite of the BOT-2, the children examined had a median (IQR) Standard Score of 39.00 (11), with a median percentile rank of 8th (18) percentile (Figure 4). A Standard Score of 39 would be considered in the “below average” range, and a percentile rank of 18th percentile is the lower limit of “average”. By numerical score, 41% of children in our cohort placed in the below average range.
Figure 4: BOT-2 Strength and Agility Performance of Children with GSD IIIa.
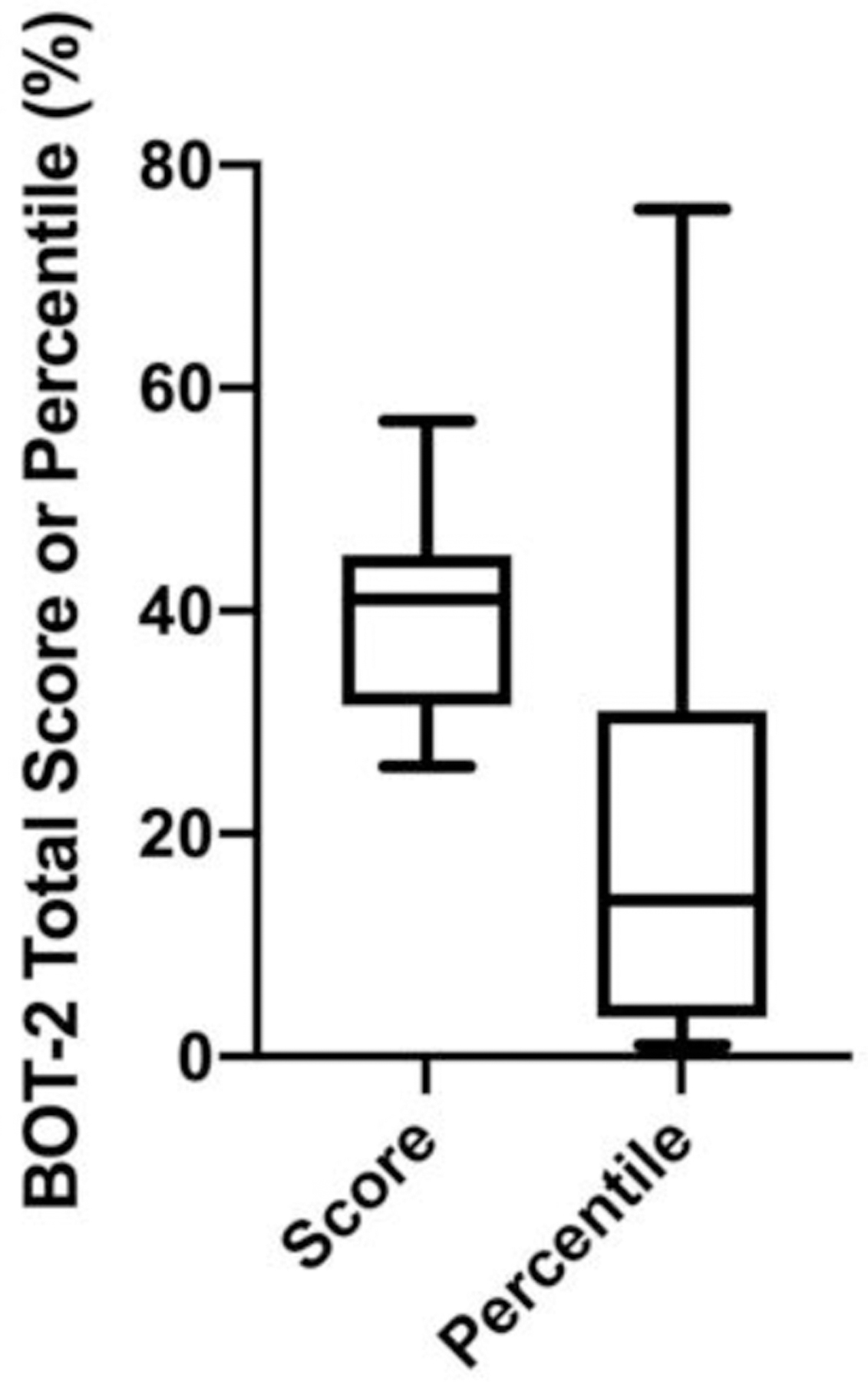
BOT-2 score and percentile performance of children with GSD IIIa demonstrates reduced performance on the measure, with the majority of individuals scoring well below the 50th percentile.
As GSD III is known to affect hand strength, we assessed both grip strength and lateral/key pinch strength. The results of both assessments for each patient were controlled for age by determining their performance as a percentile rank based on age.
Grip Strength
Grip strength in pounds (Figure 5a) was converted to percentile grip strength (Figure 5b) for each age16. Interestingly, while across all patients the right and left grip strengths (lbs) were similar (Figure 5c), the right grip percentile was significantly lower than the left (p<0.05) (Figure 5d). Overall, while the median percentile grip strength based on age was not statistically significantly lower in adults than children, both groups demonstrated significant weakness with children having a median (IQR) percentile of 9.09 (13.02) compared to that of adults (1.77; IQR:13.76) (Figure 5b).
Figure 5: Grip Strength Comparison of Children and Adults with GSD IIIa.
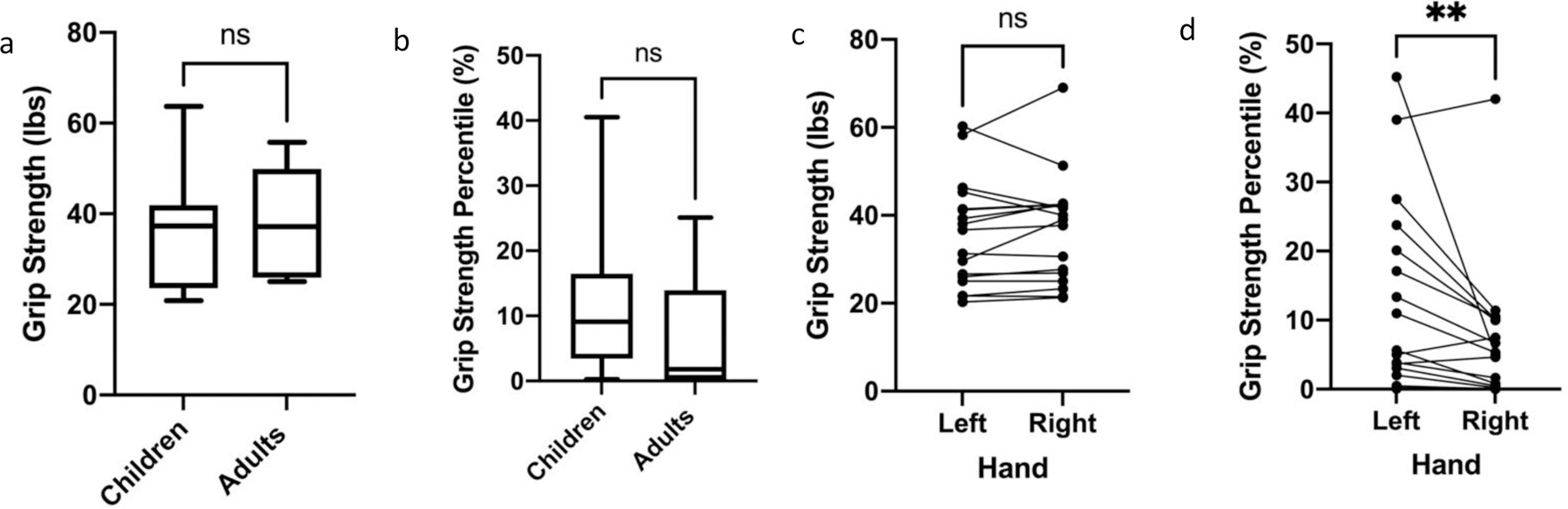
a, b. Children and adults with GSD IIIa performed similarly with respect to grip strength, measured in pounds (a) and percentile (b). c. Comparison of grip strength in pounds between the right and left hands in children and adults with GSD IIIa with a Wilcoxon Rank Sum test demonstrated no statistically significant difference between hands. d. Comparison of children and adults with GSD IIIa grip strength in percentiles demonstrated statistically significant poorer performance of the right hand compared to the left using a Wilcoxon Rank Sum test. For all graphs, ns = not significant, **p<0.01.
Lateral/Key Pinch
Lateral/key pinch strength in pounds (lbs.) (Figure 6a) was converted to percentile lateral/key pinch strength for the age group (Figure 6b). Both children and adults with GSD IIIa demonstrated profound weakness on lateral/key pinch strength measures. Among all patients tested, the median lateral/key pinch strength was similar between the right and left hands, with no significant difference between the right and left hands measured in pounds or represented by percentile (Figures 6c and d). Median key pinch strength and percentile in children (7.30 (IQR: 4.12), 1.27 (IQR: 5.56) was greater than adults (4.83 (IQR: 8.54), 0.00 (IQR: 0.495), however, the difference between children and adults was not significant (Table 2 and Figure 6a and b).
Figure 6: Lateral/Key Pinch Performance of Children and Adults with GSD IIIa.
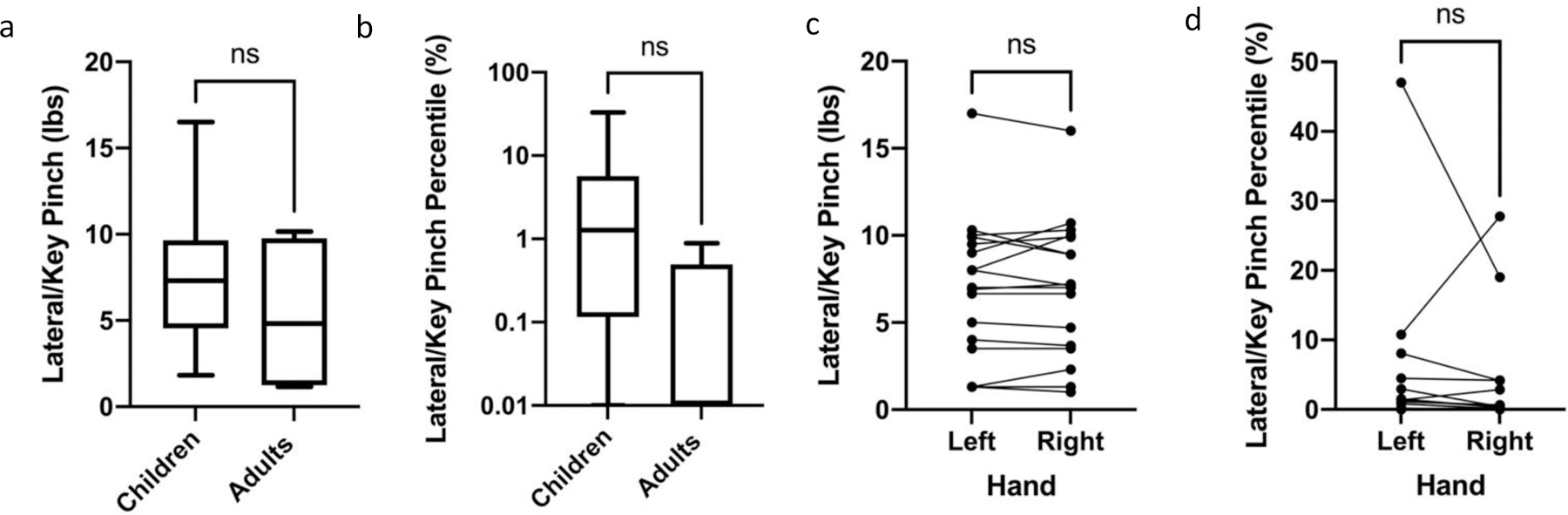
a. Comparison of children and adults with GSDIII a performed similarly with respect to lateral/key pinch, measured in pounds. Comparison via Mann Whitney test. b. Comparison of lateral/key pinch percentile performance of children and adults with GSD IIIa demonstrates similar performance of children and adults as plotted on a logarithmic scale. Comparison via Mann Whitney test. c. Comparison of lateral/key pinch strength in pounds between the right and left hands in children and adults with GSD IIIa with a Wilcoxon Rank Sum test demonstrated no statistically significant difference between hands. d. Comparison of children and adults with GSD IIIa lateral/key pinch in percentiles demonstrated no statistically significant difference between the hands using a Wilcoxon Rank Sum test, as plotted on a logarithmic scale. For all graphs, ns = not significant.
3.3. Labs
Four biochemical tests were conducted during nearly all patient visits: plasma CK, AST, ALT, and urine Glc4. The mean, standard deviation, median, and 25th-75th percentile for each age group are reported in Table 3. Of note, mean and median CK were approximately equivalent between children (1341, 753) and adults (1255, 788) with GSD IIIa. However, median AST and ALT were both greater in children (121 and 124.50) than in adults (82 and 68), respectively. Median Glc4 levels were elevated in children (10.70; IQR: 23.94) and adults (8; IQR: 32.05).
Table 3:
Comparison of Traditional mMRC Scale vs Utilized Modified Scale (0–12)
| MRC | mMRC | Our scale | Description of strength |
|---|---|---|---|
| 5 | 5 | 12 | Normal – Full strength – able to maintain test position against max resistance of examiner |
| 4+ | 11 | ||
| 4 | 4 | 10 | Good- able to maintain test position against moderate resistance |
| 4− | 9 | ||
| 3+ | 8 | ||
| 3 | 3 | 7 | Fair – able move through full range against gravity (no resistance) |
| 3− | 6 | ||
| 2+ | 5 | ||
| 2 | 2 | 4 | Poor – Moves full range in gravity eliminated position |
| 2− | 3 | ||
| 1+ | 2 | ||
| 1 | 1 | 1 | Trace – able to palpate or observe contraction but no movement possible |
| 0 | 0 | 0 | No muscle contraction present |
3.4. Whole-body Magnetic Resonance Imaging and PDFF values
Median PDFF values were greater in adults compared to children in most muscle groups tested, with the exception of flexor digitorum profundus, rectus femoris, adductor (magnus, longus, brevis), and proximal measure of the hamstring. In children, the highest percentage of intramuscular fat infiltration was observed in the rectus femoris, compared to the thoracic spinal extensors amongst the adult population. Median PDFF in all muscles in both children and adults with GSD IIIa was greater than the expected upper limit of normal of 5%, except for flexor digitorum profundus in the adult population. In children, the adductors (magnus, longus, brevis), anterior tibialis, gastrocnemius, and adductor pollicis demonstrated similar mean PDFF values compared to adults in the cohort (Figure 7a). Mean PDFF values for the other muscles scanned are shown according to age group in Figure 7a, and across all age groups in Figure 7b.
Figure 7: Mean PDFF for all muscles imaged divided by age group (a) and Mean PDFF for whole cohort (b).
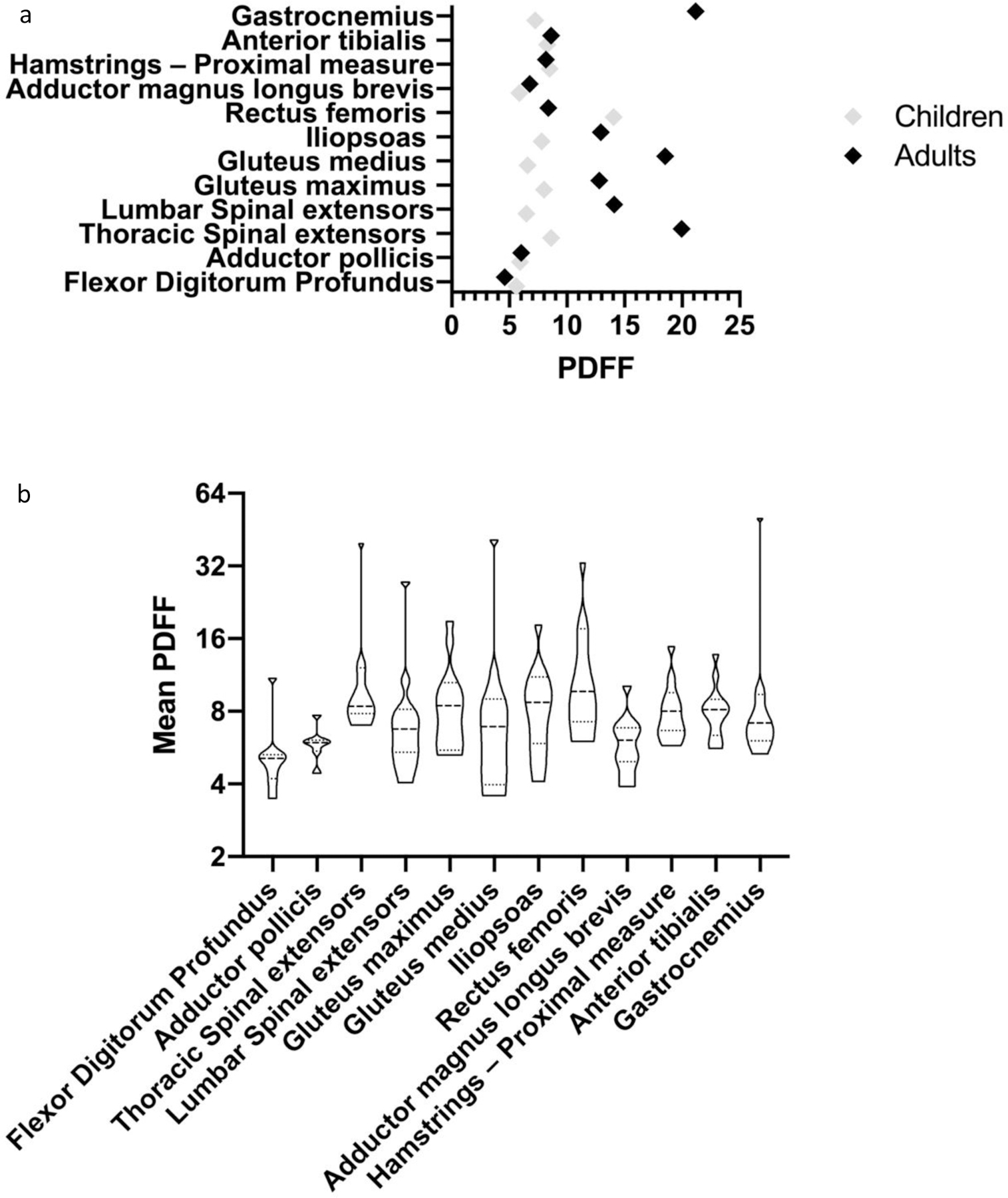
Normal values for PDFF of muscles imaged falls between 2–5% for each muscle12–13. As demonstrated in the figures above, all muscles examined in both children and adults, except for flexor digitorum profundus in adults demonstrated an increased PDFF value indicating increased glycogen and fat deposition within the muscle. As demonstrated in the figure, adults with GSD IIIa demonstrated marked elevations in PDFF in the majority of muscles examined, most notably in the gastrocnemius, gluteus medius, and thoracic spinal extensors. In children, the most notably elevation in PDFF was observed in the rectus femoris, with a median value of 10.05 (IQR: 10.3).
3.5. PDFF correlation with Strength, Functional, and Laboratory Assessments
Spearman correlation was performed comparing the physical therapy assessment total scores to laboratory values and strength (mean mMRC score) of each patient. Significant correlations (p<0.05) were found for the following relationships for the combined cohort: average MMRC and grip strength (percentile) (rs =0.76), average MMRC and GMFM total score (rs =0.90), CK, ALT, and Glc4 with grip strength (percentile) (rs =−0.81, −0.74, and-0.74, respectively), and ALT and 6MWT (% predicted) (rs =−0.79). When the pediatric cohort was analyzed separately, only the correlations between MMRC and grip strength (percentile) (rs =0.82) and GMFM total score (rs =0.954) remained significant. Furthermore, when PDFF values of each muscle group were compared to physical therapy assessment total scores and strength, the only significant correlations (p<0.05) were between the lumbar spinal extensors and GSGC total score (rs=0.732) and the proximal measure of the hamstrings and GSGC total score (rs=0.708). When the pediatric cohort was analyzed separately, two additional correlations reached significance (p<0.05), thoracic spinal extensors PDFF and BOT-2 percentile (rs= −0.82) and 6MWT (% predicted) and lumbar spinal extensors PDFF (rs = −0.786).
3.6. EMG/NCS and Neuromuscular Ultrasound
A total of 15 patients, 11 pediatric and 4 adult, were evaluated with EMG/NCS and/or neuromuscular ultrasound. Nine patients, all pediatric, were only evaluated with neuromuscular ultrasound. Two patients were only evaluated with NCS/EMG, 1 pediatric and 1 adult. Four patients were evaluated with both neuromuscular ultrasound and NCS/EMG, 1 pediatric and 3 adult.
Of the 13 total patients (10 pediatric and 3 adult) evaluated with neuromuscular ultrasound, 8 patients had normal studies. Of the remaining 5 patients (3 pediatric, 2 adult), 4 demonstrated mild enlargement of the median nerve at the wrist indicating a propensity for or diagnosis of carpal tunnel syndrome. One adult patient was reported to have mild focal enlargement of the left median nerve at the mid-humerus level of uncertain significance.
Six patients were evaluated with EMG/NCS, 3 pediatric and 3 adult. The pediatric patients evaluated included patient IDs 5, 32, and 35. Patient ID 5 exhibited mild conduction velocity slowing of the right peroneal motor response, but without evidence of widespread neuropathy or myopathy. Patient ID 32 demonstrated complex motor unit potentials and a myotonic discharge, indicating a mixture of myopathic and neuropathic findings in distal limb muscles. Patient ID 35 demonstrated mild slowing in the median nerve conduction velocity, possibly indicating early neuropathy without any evidence for myopathy. All adult patients evaluated demonstrated abnormal findings, these patients included IDs 8, 13, and 29. Patient ID 8 demonstrated increased insertional activity with widespread simple repetitive discharges and myopathic motor unit potentials, consistent with mild right carpal tunnel syndrome and diffuse myopathy. Patient ID 13 demonstrated myopathic motor unit potentials and decreased amplitude and prolonged F-wave latency of motor responses, consistent with myopathy without evidence for peripheral neuropathy. Patient ID 29 demonstrated widespread prolonged latency, decreased amplitude, and decreased conduction velocity of mixed sensory and motor responses, widespread myopathic motor unit potentials, mild denervation, and frequent myotonic discharges, consistent with sensorimotor polyneuropathy and chronic myopathy with mild features of myonecrosis.
Overall, in comparison to the prior reported GSD III EMG/NCS literature, Table 5, our patients similarly demonstrated predominantly myopathic changes, with several patients demonstrating evidence of a mixed myopathic-neuropathic process. The reported myopathic and neuropathic changes were more prominent in our adult patients than in our pediatric cohort; however, slowing of conduction velocity and motor unit potentials were present in several of our pediatric patients. Consistent with prior reports, the majority of our patients evaluated with EMG/NCS demonstrated myopathic discharges and/or myopathic motor unit potentials. Moreover, these myopathic changes were present in both proximal and distal musculature, with no evident pattern emerging, possibly due to the limited number of reports available. With the exception of findings of carpal tunnel syndrome and mild non-specific median nerve enlargement, neuromuscular ultrasound findings were unremarkable.
Table 5:
Joint Motions and Associated Muscles Evaluated on WBMRI
| PT test | Associated Muscles |
|---|---|
| Spinal Extension | Thoracic Spinal extensors, Lumbar Spinal extensors |
| Hip Flexion | Iliopsoas, Rectus femoris |
| Hip Extension (knee extended) | Gluteus maximus, Hamstrings |
| Hip Extension (knee flexed) | Gluteus maximus |
| Hip Abduction | Gluteus medius |
| Hip Adduction | Adductor magnus longus brevis |
| Knee Extension | Rectus femoris |
| Ankle Dorsiflexion | Anterior tibialis |
| Ankle Plantarflexion | Gastrocnemius |
| Hand Grip Strength | Flexor Digitorum Profundus |
| Lateral/Key Pinch | Adductor pollicis |
3.7. Literature Review
Results of the comprehensive literature review are presented in a concise manner in Table 5.
4. DISCUSSION
Early manifestations of glycogen storage disease type IIIa (GSD IIIa) have been considered to primarily be hepatic: variable hypoglycemia with or without ketosis, hepatomegaly, and elevated transaminases. Musculoskeletal involvement has been reported to present in the 3rd to 4th decade. However, there is now increasing evidence of early muscle involvement that can be missed or overlooked at younger ages17–18. To elucidate these early manifestations, our report focuses on muscle strength and functional testing. The evidence presented here suggests that it is important to identify these early musculoskeletal manifestations as it allows us to recognize the systemic involvement of GSD IIIa beyond the expected hepatic phenotype in younger patients. Recognition of early musculoskeletal involvement also informs clinicians on the importance of early physical therapy assessments to determine appropriate interventions; anticipatory, preventative management of posture and alignment for protection of the musculoskeletal system; and establishment of appropriate exercise and activity guidelines.
We assessed a comprehensive number of muscle strength and functional measures such as the GSGC, GMFM, BOT2, 6MWT, grip strength and lateral pinch strength in children with GSD IIIa. Additionally, this is the first report to look at PDFF in children with GSD IIIa. Our findings demonstrate that even at a median age of 11.6 years (Table 2) children exhibit some musculoskeletal involvement. This is corroborated in the literature: for example, Laforet et al., reported evidence of fibrosis on muscle biopsy as early as 3 years age19. Similarly, Mogahed et al., demonstrated myopathic changes on EMG in 60% of 28 children with GSD III (mean age 6.6±3.1 years) even though two-thirds of these patients were reported to exhibit no overt clinical muscle weakness17. In this study, we observed compromised muscle performance, with the children demonstrating reduced strength for all muscles tested and functional deficits identified by detailed PT assessment. The most significant weaknesses in these children were found in plantarflexion, spinal extension, and the hip musculature (weakness in flexion, extension, abduction, and adduction). Similarly, the adults demonstrated the most significant weakness in hip musculature (flexion, extension, abduction, and adduction) and spinal extension, but additionally demonstrated significant weakness with dorsiflexion (Figure 1). Due to dorsiflexion weakness, 3 of the 4 adults required bilateral ankle-foot orthosis (AFOs) to provide tow lift while walking. Furthermore, 3 of 4 adults were noted to have a Trendelenburg gait (pelvic drop of swing limb) indicative of hip abductor weakness. The observed greater weakness in plantarflexion in children with GSD IIIa compared to adults may be due to half of the adult patients likely being assessed in the less rigorous sitting position, which can lead to inflated strength scores. Additionally, both children and adults demonstrated greater hip extension weakness when the knee was flexed as opposed to extended. Flexing the knee while testing hip extension puts the hamstrings at a mechanical disadvantage, and thus isolates the gluteus maximus, making it easier to note weakness in these gluteal muscles. Overall, this suggests that the hamstrings might be masking some of the weakness of the gluteal muscles functionally. While not every participant had comments on their GMFM performance, comments were available for 11 of the patients, or half of our cohort. Upon review of these comments, it was noted that 10/11 of these patients only experienced difficulty with the hopping and jumping tasks and standing tasks such as balancing on one foot or transitioning between sitting and standing. These described difficulties in jumping, hopping, and standing may reflect the observed weaknesses in plantarflexion and hip musculature. This is further supported by the more affected performance on the Gower and Gait subdomains of the GSGC. In particular, the Gower task is very demanding as it is assessing the ability to transition from lying on the floor to standing fully upright. While GSGC scores were higher in adults (suggesting more impaired functional mobility), the scores were also compromised in children (Figure 2b). Reduced functional performance was also demonstrated on the BOT-2, with children in this cohort performing at approximately the lower bound of normal and ranking on average in the 20th percentile (Figure 4). Furthermore, both children and adults demonstrated a reduced percent predicted on the 6-MWT, with children on average performing at 76.67% predicted and adults performing at 68.09% predicted (Figure S1). The 6-MWT is a measure of endurance, thus these results suggest patient have reduced capacity for walking in the community. This combined with the observed decreased ability to hop, jump, and get off the ground can negatively impact their ability to participate in recreational/occupational activities. Future studies could also assess exercise tolerance as reported by Preisler et al to assess for early functional impairment6.
With regards to the overall pattern of muscular weakness, both the pediatric and adults cohorts demonstrated more proximal than distal weakness in the lower limbs, and profound weakness in the hands. One limitation of this study is that the proximal upper extremity was not assessed. Our adult cohort additionally exhibited significant weakness of the spinal extensors. Comparing this distribution to the findings reported in the literature review, a total of 8 studies indicated subjects had both proximal and distal involvement 18,22, 29,31–33. Three of these studies indicated greater proximal than distal involvement (references 22, 29, 33) and two studies indicated distal greater than proximal involvement (references 20, 26). A total of 5 studies additionally specifically mentioned weakness in handgrip, pinch, and intrinsic musculature of the hands 6, 20, 22, 29, 33.
GSD IIIa is also known to significantly affect smaller muscles of the hands20,25–26. We observed significant weakness in grip and lateral/key pinch in children. While the grip strength was reduced in both hands, the right grip strength measured significantly lower than the left in our cohort. However, not a single individual performed above the 45th percentile with either hand for the measure. In our cohort, children with GSD IIIa performed on average in the 9th percentile (Table 2 and Figure 5) and 1st percentile (Table 2 and Figure 6) in grip strength and lateral/key pinch, respectively, compared to non-diseased peers of their age and sex. Corroborating our findings, Decostre et al., previously identified poor handgrip and key pinch performance in the pediatric population25. Interestingly, while performance was reduced in both hands, we observed that the right grip was weaker than the left (Figure 5). Unfortunately, our data did not include dominant hand for the patients examined, which should be included in future studies. Assuming our population is reflective of the general population with approximately 90% of individuals being right hand dominant, the greater weakness in the right grip strength may indicate that overuse of the dominant hand is resulting in greater than expected weakness. These may be reliable early indicators of small muscle involvement in pediatric patients.
Although the majority of reports of electromyography (EMG) in GSD III report myopathic changes17,20, there may also be a component of neuropathic changes21–22. These prior reports are consistent with the findings reported in our patient cohort.
In this study, we also examined proton density fat fraction (PDFF), measured via whole body MRI. Corroborating the functional deficits, children exhibited elevated PDFF. Although the PDFF was less than in adults (Figure 7A), it was higher than non-diseased peers in that age group15. As previously mentioned, statistically significant correlations were found between PDFF values for the lumbar spinal extensors and proximal measure of the hamstring compared to GSGC total score. Moreover, additional correlations were found between thoracic spinal extensor PDFF and BOT-2 and 6MWT performance, when only the pediatric cohort was analyzed. Comparing our results to a prior study correlating WMBRI to functional measures in Pompe Disease (GSD II), our study similarly found a significant correlation with GSGC; however, correlation with mMRC (strength evaluation) did not reach significance for our cohort unlike the Pompe cohort10. Overall, while no clear pattern of muscular involvement based on PDFF was evident in this study, similar to prior reports in late onset Pompe Disease, the most significant involvement and highest PDFF values were predominantly found within proximal and trunk musculature10.
Future studies into the musculoskeletal involvement in pediatric patients with GSD III would be strengthened by increased sample sizes, completion of WBMRI scans in all patients evaluated, and by focusing on techniques to better evaluate accurate PDFF measurements in small muscles. Furthermore, future studies could be enhanced with more detailed item analyses for each physical therapy assessment to look for possible correlations between specific areas of muscle weakness and individual test items on which decreased functional performance was noted. Regardless, to the best of our knowledge, while studies have not quantified PDFF in this context, Muscle Ultrasound Density (MUD) and Nuclear Magnetic Resonance (NMR) spectroscopy have been reported18,26. Like PDFF, both MUD and NMR can detect muscle pathology including glycogen and fat deposition. Verbeek et al., reported higher MUD scores in children with GSD III compared to healthy controls18. They also demonstrated that proximal muscles were more affected than distal muscles. Wary et al., provided NMR evidence of fat replacement and muscle destruction in the pediatric age group within the lower limb, with no preferential involvement of proximal or distal musculature26. These findings are especially concerning as we know from Pompe disease that elevated PDFF is typically a late finding – it increases after glycogen accumulation, lysosomal rupture, and tissue fibrosis10. Thus, we suggest that even the mild increase in PDFF we have observed is a sign of significant muscle involvement and in some cases can be debilitating.
To further assess whether early muscle involvement was present, we studied clinical biomarkers of muscle and liver function. All observed biomarkers (AST, ALT, Glc4, and CK) were more elevated in our pediatric patients than adult patients, although median values for both children and adults were above normal ranges. As mentioned previously by Halaby et al, it is challenging to determine the source (liver versus muscle) of biomarkers such as Glc4 and AST 8. Young et al recently concluded that Glc4 excretion in childhood may be largely from glycogen accumulation in liver and also to some extent muscle, whereas in adults it may be largely from glycogen accumulation in the muscle27. However, the elevation of CK in childhood points to early muscle disease. Thus, these findings are consistent with prior observations that while liver involvement, suggested by elevated transaminases, is the most prominent recognized manifestation of the disease during childhood, muscular involvement cannot be overlooked as a contributor to elevated laboratory evaluations or as a component of disease phenotype. Evidence of early myopathy on laboratory exams and in clinical assessments demonstrates the importance of early assessment and intervention and has implications for current and future treatment approaches for the disease.
In conclusion, our study found that there are clinical signs demonstrating early musculoskeletal involvement in children with GSD IIIa as evidenced by weakness and decreased functional performance on physical therapy outcome measures, elevated PDFF on WBMRI imaging, and elevated laboratory biomarkers. Early muscle involvement is reflected both by isolated testing of strength in individual muscles and by detailed assessment of gross motor functional performance. This early muscular involvement in children with GSD IIIa is critical to recognize to allow for early detection and the development of targeted therapies which can address both the hepatic and muscular manifestations of the disease Compound Muscle Action Potential (CMAP), motor unit potential (MUP), Tibialis anterior (TA), First Dorsal Interosseous (FDI), Vastus Lateralis (VL), Vastus medialis (VM), Deep Tendon Reflexes (DTR), Nerve Conduction Studies (NCS)
Supplementary Material
Figure S1: 6-Minute Walk Test Performance of Children and Adults with GSD IIIa
a. 6-Minute Walk Test distance in meters was not significantly difference for children and adults with GSD IIIa as analyzed by a Mann-Whitney test. b. While no significant difference was detected between the percent predicted performance of children and adults with GSD IIIa, only two children performed at or above the expected 100%. Furthermore, 75% or greater of all children and adults performed below 75 % predicted. For both graphs, ns = not significant.
Table 4:
Descriptive statistics of biochemical values: CK, AST, ALT and Glc4, in our cohort, divided by age group
| Children | Adults | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Normal | Mean | SD | Median | 25th-75th Percentile | Mean | SD | Median | 25th-75th Percentile | |
| CK | 20–200 U/L | 1,341 | 1,506 | 753 | 93–2879 | 1,255 | 1,283 | 788 | 412.50–2331 |
| AST | 10–60 U/L | 197.60 | 214.10 | 121 | 60–242.30 | 94.80 | 38.31 | 82 | 63.50–132.5 |
| ALT | 10–60 U/L | 213.40 | 243 | 124.50 | 55.25–241 | 72.40 | 18.78 | 68 | 56–91 |
| Glc4 | <4 mmol/mol creatinine | 19.82 | 22.78 | 10.70 | 5.82–29.75 | 18.46 | 17.28 | 8 | 5.05–37.10 |
Table 6:
Literature review
| Reference | No. Cases | Age | CK (IU/L) | AST | ALT | Radiologic findings | Physical examination | EMG/NCS | Neurological Complaints | Development | Muscle Biopsy |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Moses et al (1986)28 | 16 | age range 2–27 | Elevated in all subjects | Little or no weakness (n=11), weakness limiting from physical exercise (n=3, improved with age). | N=9. Mixed pattern (n=3), myopathy (n=6). All patients had numerous short, small amplitude MUPs, most prominent in proximal muscles. | Numerous fibers with subsarcolemmal deposits of PAS + material. | |||||
| Coleman (1992)29 | 13 (7 GSD IIIa, 3 IIIb, 3 IIId) | Mean 24.8 (4–57, median 17) | GSD IIIa: Patients <15 y (n=6) CK activity less than 500. Oldest men (n-2) CK > 1000. | Mild weakness on physical exam (n=4), moderate (n=4), severe (n=2). Three older patients: proximal and distal weakness with atrophy of intrinsic hand muscles and calf. | NCS: myopathic (n=7), decreased motor nerve conduction (n=2) | Walking delayed (n=3, 18 mo-3 years) | |||||
| Kiechl et al (1999)30 | 1 | 47-year-old female | Began at 401, declined to 95 | Muscle strength was 4+/5 (MRC scale) in deltoid and biceps, otherwise normal | Reduction in CMAPs in median and peroneal nerves, low-amplitude, polyphasic potentials in biceps, deltoid, and intercostal muscles. | Diffuse mild wasting of muscles. DTRs decreased bilaterally. | Moderate variation in fiber size without obvious fibrosis. | ||||
| Kiechl et al (1999)31 | 4 | Median 52.5, range (8 months-62 years) | Mean: 470.75 (239–810), median: 417 | Slow, progressive muscle wasting of calves, proximal limb, and trunk muscles | myopathic and neurogenic features with reduced nerve conduction velocity in n=2. | DTRs decreased in arms and absent in feet. | Walking delayed to 2 years | PAS positive vacuolar myopathy, cytoplasmic glycogen pools. | |||
| Hobson-Webb et al (2010)22 | GSD IIIa (n=11); GSDIIIb (n=1)) | Mean age 31.42 (range 5–55) | Mean max 1961 (n=11, range 216–9264) | Weakness in proximal lower extremities (n=1), proximal limbs and intrinsic hand muscles (n=2), proximal and distal limbs and intrinsic hand muscles (n=1). | EMG (n=8, abnormal n=6) Myopathic MUPs n=3, neuropathic MUPs in median n=1. |
Paresthesias in fingertips, feet, distal legs. Mild distal lower extremity numbness, forearm cramping. | Mild delay in developmental milestones. Decreased endurance. | ||||
| Wary et al (2010)26 | 18 | Mean age 30.44 (11–67 yrs) | Fatty replacement in muscles (n=15). Calf muscles most frequently impacted. | Weakness (n=3), proximal >distal weakness (n=9), distal > proximal weakness (n=5), no weakness (n=1; GSD IIIb). Exercise intolerance with fatiguability. |
Glycogen vacuolation slightly higher for type 1 fibers. | ||||||
| Gershen et al (2015)32 | 1 | 25 yo male | 3261 | 225 | 290 | Normal muscle bulk and tone, mild proximal limb muscle weakness worse in legs than arms, reflexes 1/4 throughout. | Moderate variation in muscle fiber size, focal increased endomysial fibrosis, chronic endomysial inflammation. | ||||
| Mogahed et al (2015)17 | 28 | Mean Age 6.6 (SD 3.1 yrs) | Elevated (n=21), | Elevated (n=24) | Elevated (n=23) | Normal muscle power (n=20), mild weakness (n=6). Exercise intolerance, difficulty climbing stairs (n=18). | Myopathic changes (n=17), 3 with associated axonopathy | Normal muscle tone and reflexes. | Delayed sitting, standing, and walking | ||
| Decostre (2016)25 | 18 GSDIIIa | Mean (SD) 32 (13)m median 32, range 13–56 | Hip flexion weakest Pinch strength (annual loss of 1.10%), Purdue Pegboard (1.49% annual decline), handgrip (loss of 1.84% annually) |
Plantar flexion contracture (n=14) | |||||||
| Herlin et al (2016)20 | 16 GSDIIIa | Mean Age 33 (17–52 yo) | Mean 4187 (346–9104) | Muscle fatty degeneration in posterior compartments of legs | Exercise Intolerance (n=4). Weakness proximal (n=3), distal (n=8, legs n=1, arms n=5, both n=2). Distal hand muscles wasting (n=3) | Myopathic MUPs (15/16), primarily in TA and FDI. | Distal paresthesias (n=1), Normal reflexes in all patients. |
||||
| Verbeek (2016)18 | 11 (GSD IIa children, GSDIII adults) | Children (n=5, median age 4; adults (n=6, a=4, b=2), median age 31y | Increased in 3/5 GSD IIIa children (median: 420, range: 128–981); 4/4 GSD IIIa adults (median 1249, range 1093–1823) | Children: high MUD prox>distal. Adult MUD higher than children, prox>distal. | Adults with GSDIII had muscle weakness of the shoulder abductors, elbow extensors, finger flexors, knee extensors, summed-arm and summed-proximal muscles | Normal EMG results with respect to sensory and motor conductance velocities and amplitudes. | 2/5 GSDIII children and 5/6 GSDIII adults experienced neuromuscular complains | ||||
| Decostre (2017)6 | 13 GSDIIIa | Last prospective visit mean 37 (SD12) | N= 12, mean at first visit 2456 (57–9170, SD 2539, median 1981), mean at last visit 2258 (SD 2518, median 855, range 263–7716) | N=13, mean at last visit 144 (42–298, SD 85, median 126) | Progressive impairment of finger dexterity, handgrip strength, and transfer mobility after age 30. | All but 1 patient had plantar flexion contracture | |||||
| Nazari et al (2018)33 | 5 | Mean age 36.6(18–51), median age 40 | Mean: 2367.2 (1033–3367) | Mean: 137.6 (70–221) | Symmetric weakness of proximal and distal muscles; mild hand and hip muscle weakness. | Low-amplitude, short-duration, polyphasic MUPs. NCS: axonal sensory motor polyneuropathy (n=2). | Chronic axonal sensorimotor polyneuropathy (n=2). | Developmental delay (n=3), two with delayed walking. | Multiple subsarcolemmal and cytoplasmic PAS + vacuoles. | ||
| Zhang (2018)34 | 4 (GSD IIIa) | Mean 21 y(range 14–32), median 19 | Mean: 2275 (range 1126–4000) | Mean: 138.5 (range 75–202). | Mean: 122 (range 78–199) | Mild T1 signal intensity in the long head of femoris and peroneus longus muscles (increased adipose content) | All patients had MUP in proximal limbs with negative NCS | ||||
| Chehida et al (2019)21 | 50 (GSD III) | last evaluation median age: 9.87 y (16 mo-41 y) | CK elevation noticed at median age of 2.6 years (0.33–35 years) | AST elevated, decreased with age. | Muscle weakness of limbs (66%), LE (31%) >UE (19%). Weakness distribution (proximal n=1, distal n=2, both n=17). | ENMG anomalies, in 62% of patients (myogenic pattern 42%, neurogenic 12%, mixed 18%). | DTR abnormalities of lower limbs (n=14, Achilles reflex), upper limbs (n=5). | Median age walking: 18 mo (12–30 mo), late walking 43%. | |||
| Tobaly et al (2019)9 | 15 (GSDIII) | Mean age 36 yrs (range 16–59) | T1-weighted MRI sequences: symmetrical fat replacement of muscle without severe atrophy. | Total MFM scores sensitive to age, specifically in patients > 30 yrs |
Compound Muscle Action Potential (CMAP), motor unit potential (MUP), Tibialis anterior (TA), First Dorsal Interosseous (FDI), Vastus Lateralis (VL), Vastus medialis (VM), Deep Tendon Reflexes (DTR), Nerve Conduction Studies (NCS).
Funding Acknowledgement:
Research reported in this publication was supported by the National Center For Advancing Translational Sciences of the National Institutes of Health under Award Number TL1 TR002555. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.Kishnani PS, Sun B, & Koeberl DD (2019). Gene therapy for glycogen storage diseases. Human molecular genetics, 28(R1), R31–R41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Van Hoof F, Hers HG. The Subgroups of Type III Glycogenosis. Eur J Biochem. 1967;2(3):265–270. doi: 10.1111/j.1432-1033.1967.tb00134.x [DOI] [PubMed] [Google Scholar]
- 3.Kishnani PS, Austin SL, Arn P, et al. Glycogen Storage Disease Type III diagnosis and management guidelines. Genet Med. 2010;12(7):446–463. doi: 10.1097/GIM.0b013e3181e655b6 [DOI] [PubMed] [Google Scholar]
- 4.Dagli A, Sentner C, Weinstein DA. Glycogen Storage Disease Type III. In: Pagon RA, Bird TD, Dolan CR, et al. , editors. GeneReviews™ [Internet]. Seattle (WA): University of Washington, Seattle; 1993-. GeneReviews™ [Internet]. Seattle (WA): University of Washington, Seattle; 1993-. http://www.ncbi.nlm.nih.gov/books/NBK26372/. Published December 29, 2012. Accessed November 8, 2020. [Google Scholar]
- 5.Tolun AA, Boyd KF, Austin SL, et al. Utility of a urinary tetrasaccharideas a biomarker for glycogen storage disease type III. In: 11th InternationalCongress of Inborn Errors of Metabolism (ICIEM), San Diego, California;2009. [Google Scholar]
- 6.Decostre V, Laforêt P, De Antonio M, et al. Long term longitudinal study of muscle function in patients with glycogen storage disease type IIIa. Mol Genet Metab. 2017;122(3):108–116. doi: 10.1016/j.ymgme.2017.08.010 [DOI] [PubMed] [Google Scholar]
- 7.Preisler N, Pradel A, Husu E, et al. Exercise intolerance in Glycogen Storage Disease Type III: Weakness or energy deficiency? Mol Genet Metab. 2013;109(1):14–20. doi: 10.1016/j.ymgme.2013.02.008 [DOI] [PubMed] [Google Scholar]
- 8.Halaby CA, Young SP, Austin S, et al. Liver fibrosis during clinical ascertainment of glycogen storage disease type III: a need for improved and systematic monitoring. Genet Med. 2019;21(12):2686–2694. doi: 10.1038/s41436-019-0561-7 [DOI] [PubMed] [Google Scholar]
- 9.Tobaly D, Laforêt P, Perry A, et al. Whole-Body Muscle Magnetic Resonance Imaging in Glycogen-Storage Disease Type III. Muscle Nerve. 2019;60(1):mus.26483. doi: 10.1002/mus.26483 [DOI] [PubMed] [Google Scholar]
- 10.Khan AA, Boggs T, Bowling M, et al. Whole-body magnetic resonance imaging in late-onset Pompe disease: Clinical utility and correlation with functional measures. J Inherit Metab Dis. 2019. doi: 10.1002/jimd.12190 [DOI] [PubMed]
- 11.Deitz JC, Kartin D, & Kopp K (2007). Review of the Bruininks-Oseretsky test of motor proficiency, (BOT-2). Physical & occupational therapy in pediatrics, 27(4), 87–102. [PubMed] [Google Scholar]
- 12.Ortega X, Araneda D, Asahi T, et al. Variability of muscle fat fraction quantification in MRI using the Dixon technique. Radiol. 2016;22(4):149–155. doi: 10.1016/j.rchira.2016.11.005 [DOI] [Google Scholar]
- 13.Horvath JJ, Austin SL, Case LE, et al. Correlation between quantitative whole-body muscle magnetic resonance imaging and clinical muscle weakness in pompe disease. Muscle Nerve. 2015;51(5):722–730. doi: 10.1002/mus.24437 [DOI] [PubMed] [Google Scholar]
- 14.GraphPad Prism version 9.0.1 for macOS, GraphPad Software, San Diego, California USA, www.graphpad.com” [Google Scholar]
- 15.Angelini C, Semplicini C, Tonin P, Filosto M, Pegoraro E, Sorarù G, Fanin M. Progress in Enzyme Replacement Therapy in Glycogen Storage Disease Type II. Ther Adv Neurol Disord. 2009. May;2(3):143–53. doi: 10.1177/1756285609103324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McQuiddy VA, Scheerer CR, Lavalley R, McGrath T, & Lin L (2015). Normative values for grip and pinch strength for 6-to 19-year-olds. Archives of physical medicine and rehabilitation, 96(9), 1627–1633. [DOI] [PubMed] [Google Scholar]
- 17.Mogahed EA, Girgis MY, Sobhy R, Elhabashy H, Abdelaziz OM, El-Karaksy H. Skeletal and cardiac muscle involvement in children with glycogen storage disease type III. Eur J Pediatr. 2015;174(11):1545–1548. doi: 10.1007/s00431-015-2546-0 [DOI] [PubMed] [Google Scholar]
- 18.Verbeek RJ, Sentner CP, Smit GPA, et al. Muscle Ultrasound in Patients with Glycogen Storage Disease Types I and III. Ultrasound Med Biol. 2016;42(1):133–142. doi: 10.1016/j.ultrasmedbio.2015.08.013 [DOI] [PubMed] [Google Scholar]
- 19.Laforêt P, Inoue M, Goillot E, et al. Deep morphological analysis of muscle biopsies from type III glycogenesis (GSDIII), debranching enzyme deficiency, revealed stereotyped vacuolar myopathy and autophagy impairment. Acta Neuropathol Commun. 2019;7:167. doi: 10.1186/s40478-019-0815-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Herlin B, Laforět P, Labrune P, Fournier E, Stojkovic T. Peripheral neuropathy in glycogen storage disease type III: Fact or myth? Muscle and Nerve. 2016;53(2):310–312. doi: 10.1002/mus.24977 [DOI] [PubMed] [Google Scholar]
- 21.Ben Chehida A, Ben Messaoud S, Ben Abdelaziz R, et al. Neuromuscular Involvement in Glycogen Storage Disease Type III in Fifty Tunisian Patients: Phenotype and Natural History in Young Patients. Neuropediatrics. 2019;50(1):22–30. doi: 10.1055/s-0038-1669786 [DOI] [PubMed] [Google Scholar]
- 22.Hobson-Webb LD, Austin SL, Bali DS, Kishnani PS. The electrodiagnostic characteristics of Glycogen Storage Disease Type III. Genet Med. 2010;12(7):440–445. doi: 10.1097/GIM.0b013e3181cd735b [DOI] [PubMed] [Google Scholar]
- 23.Miller CG, Alleyne GA, Brooks SEH. Gross cardiac involvement in glycogen storage diseasetype III. Heart. 1972;34(8):862–864. doi: 10.1136/hrt.34.8.862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moses SW, Wanderman KL, Myroz A, Frydman M. Cardiac involvement in glycogen storage disease type III. Eur J Pediatr. 1989;148(8):764–766. doi: 10.1007/BF00443106 [DOI] [PubMed] [Google Scholar]
- 25.Decostre V, Laforêt P, Nadaj-Pakleza A, et al. Cross-sectional retrospective study of muscle function in patients with glycogen storage disease type III. Neuromuscul Disord. 2016;26(9):584–592. doi: 10.1016/j.nmd.2016.06.460 [DOI] [PubMed] [Google Scholar]
- 26.Wary C, Nadaj-Pakleza A, Laforêt P, et al. Investigating glycogenosis type III patients with multi-parametric functional NMR imaging and spectroscopy. Neuromuscul Disord. 2010;20(8):548–558. doi: 10.1016/j.nmd.2010.06.011 [DOI] [PubMed] [Google Scholar]
- 27.Young SP, Khan A, Stefanescu E, et al. Diurnal variability of glucose tetrasaccharide (Glc 4 ) excretion in patients with glycogen storage disease type III. JIMD Rep. November 2020:jmd2.12181. doi: 10.1002/jmd2.12181 [DOI] [PMC free article] [PubMed]
- 28.Moses SW, Gadoth N, Bashan N, Ben-David E, Slonim A, Wanderman KL. Neuromuscular involvement in glycogen storage disease type III. Acta Paediatr Scand. 1986;75(2):289–296. doi: 10.1111/j.1651-2227.1986.tb10201.x [DOI] [PubMed] [Google Scholar]
- 29.Coleman RA, Winter HS, Wolf B, Chen YT. Glycogen debranching enzyme deficiency: Long-term study of serum enzyme activities and clinical features. J Inherit Metab Dis. 1992;15(6):869–881. doi: 10.1007/BF01800225 [DOI] [PubMed] [Google Scholar]
- 30.Kiechl S, Willeit J, Vogel W, Kohlendorfer U, Poewe W. Reversible severe myopathy of respiratory muscles due to adult-onset type III glycogenosis. Neuromuscul Disord. 1999;9(6–7):408–410. doi: 10.1016/S0960-8966(99)00038-3 [DOI] [PubMed] [Google Scholar]
- 31.Kiechl S, Kohlendorfer U, Thaler C, et al. Different clinical aspects of debrancher deficiency myopathy. J Neurol Neurosurg Psychiatry. 1999;67(3):364–368. doi: 10.1136/jnnp.67.3.364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gershen LD, Prayson BE, Prayson RA. Pathological characteristics of glycogen storage disease III in skeletal muscle. J Clin Neurosci. 2015;22(10):1674–1675. doi: 10.1016/j.jocn.2015.03.041 [DOI] [PubMed] [Google Scholar]
- 33.Nazari F, Sinaei F, Nilipour Y, et al. Distinct clinical and genetic findings in Iranian patients with glycogen storage disease type 3. J Clin Neuromuscul Dis. 2018;19(4):203–210. doi: 10.1097/CND.0000000000000212 [DOI] [PubMed] [Google Scholar]
- 34.Zhang Y, Xu M, Chen X, et al. Genetic analysis and clinical assessment of four patients with Glycogen Storage Disease Type IIIa in China. BMC Med Genet. 2018;19(1). doi: 10.1186/s12881-018-0560-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: 6-Minute Walk Test Performance of Children and Adults with GSD IIIa
a. 6-Minute Walk Test distance in meters was not significantly difference for children and adults with GSD IIIa as analyzed by a Mann-Whitney test. b. While no significant difference was detected between the percent predicted performance of children and adults with GSD IIIa, only two children performed at or above the expected 100%. Furthermore, 75% or greater of all children and adults performed below 75 % predicted. For both graphs, ns = not significant.


