Condensed Abstract
ARIC initiated community-based surveillance, in 1987, for myocardial infarction (MI) and coronary heart disease (CHD) incidence and mortality and created a prospective cohort of 15,792 African American and white adults aged 45–64 years. The primary aims were to improve understanding of the decline in CHD mortality and identify determinants of subclinical atherosclerosis and CHD in African American and white middle-aged adults. ARIC has examined areas including health disparities, genomics, heart failure (HF), and prevention, producing more than 2,300 publications. Results have had strong clinical impact and demonstrate the importance of population-based research in the spectrum of biomedical research to improve health.
Keywords: epidemiology, cohort study, adult, risk factors, surveillance, health disparity
Introduction
The ARIC study was funded in 1985 to conduct population-based surveillance for MI incidence and CHD mortality in four US communities (Forsyth County, NC, Jackson, MS, suburban Minneapolis, MN and Washington County, MD) and initiate a prospective cohort study of approximately 16,000 middle-aged African American and white adults recruited from the same communities (1) (Central Illustration). The study conducted community surveillance of cardiovascular disease (CVD) in geographically diverse regions from 1987 through 2014, producing annual estimates of incidence of acute MI and CHD case fatality and mortality in these four communities. ARIC also recruited a cohort of 15,792 African American and white adults aged 45–64 years to participate in what has now been seven clinic examinations and 33 years of follow-up to identify health events. The data collected over this time have generated research resulting in over 2,300 peer-reviewed publications. Data collection in the cohort employs phone interviews and clinic examinations and has evolved to use novel technology such as wearable electrocardiogram (ECG) patches and has expanded to include in depth cognitive testing to facilitate research on vascular contributions to cognitive impairment and dementia (VCID). More recently the ARIC data have contributed to large data sharing efforts such as the National Heart, Lung, and Blood Institute (NHLBI) Trans-Omics for Precision Medicine (TOPMed) program.(2)
Central Illustration. ARIC Study Design.
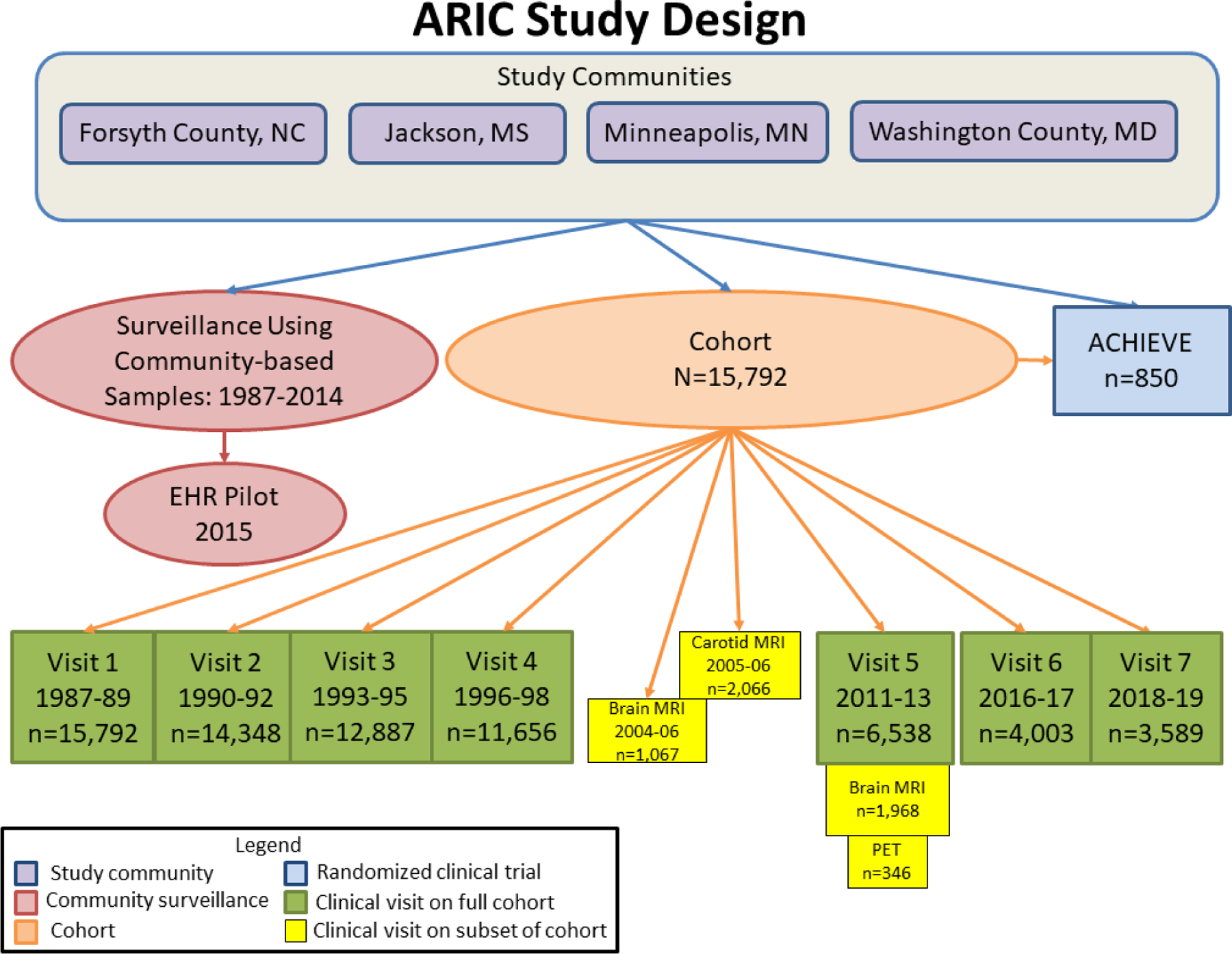
Design of prospective cohort study with clinic examinations; community surveillance for myocardial infarction, coronary heart disease and heart failure, including feasibility pilot study of surveillance using Electronic Health Record data; and Aging and Cognitive Health Evaluation in Elders (ACHIEVE) randomized clinical trial with participants recruited from cohort and de novo participants from study communities. Information about ARIC can be found on the study website: https://sites.cscc.unc.edu/aric/.
Original Goals
The impetus for the development and design of the ARIC study was the 1978 Conference on the Decline in CHD Mortality sponsored by the NHLBI.(3) The workshop sought to address the unexpected decline in heart disease mortality during the prior 10-year period and to identify potential contributors to the decline. The experts’ recommendations included conducting longitudinal investigations to better characterize and understand the observed downward trends.
1978 Conference on the Decline in CHD Mortality
The workshop goal was to review existing data to determine if the decline was real and identify contributors to the decline with emphasis on factors that could be modified to achieve greater impact. The invited experts evaluated data from vital statistics, national and regional health interview surveys, and hospital discharge surveys. They concluded that the downward trends were real and that improvements in both risk factor prevalence in the population as well as improvements in treatment probably affected the downward trend in CHD. The conference participants were unable to make conclusive statements about the relative contributions of disease incidence or survival to the observed trends or consider variation in the trends, such as across demographic traits. The conference recommendations included a call to develop a prospective surveillance system for “a limited number of discrete geographic areas in the U.S. for cardiovascular disease,” in order to examine trends and test hypotheses. (p. xxvi) The 40th anniversary of this landmark workshop was marked by a symposium sponsored by the NHLBI at the 2018 American Heart Association (AHA) Annual Scientific Sessions Conference.(4)
Following the 1978 conference the NHLBI conducted a CVD surveillance feasibility pilot study to develop and test a protocol for community surveillance of CHD death and MI.(5) Subsequently, in 1985, the NHLBI designed and initiated the ARIC study with two components: community surveillance and a cohort study. Four geographically diverse US communities were selected. The combination of community surveillance with a prospective cohort study sampled from each region resulted in the application of the rigorous validation procedures developed for community surveillance also being applied to cohort data on cardiovascular outcomes. Historically, cohort studies alone have rarely been sufficiently large and are not optimally designed to allow characterization of trends in CVD. The pairing of the cohort study with a community surveillance arm allowed for investigation of risk factors of cardiovascular morbidity and mortality identified in the community surveillance trends. It further allowed more complete and standardized characterization of a broader range of cardiovascular events in the cohort, including subclinical evidence of CVD.
Cohort Study
An initial goal of the ARIC cohort study was to identify risk factors for subclinical atherosclerosis measured by carotid intima-media thickness and for progression of subclinical atherosclerosis to clinical CVD (MI, CHD death, and stroke). (Supplemental Appendix) There also was an interest in determining whether “novel” risk factors (e.g., hemostatic factors, lipid subfractions, systemic inflammation, etc.) were causally associated with CVD or might improve CVD risk prediction. A secondary goal of having a cohort in ARIC was to allow testing of the consistency of community surveillance findings within a cohort of participants taking part in detailed clinical examinations. As cardiovascular epidemiology evolved, research on the ARIC cohort came to include risk factors for prediction of additional cardiovascular outcomes (e.g., stroke, HF, atrial fibrillation or AF, peripheral artery disease, venous thromboembolism) conditions with a major vascular component (retinal disease, chronic kidney disease or CKD, cognitive decline and dementia) and most other outcomes at older age (e.g. hospitalizations, cancer, infections, fractures). As laboratory measurements improved, ARIC has studied genomic, metabolomic, proteomic, and other mechanistic pathways for CVD. Ancillary studies have provided an increasing proportion of ARIC research funding, including expansion beyond CVD to CKD, diabetes, chronic lung disease, cancer, aging, cognitive decline/dementia, and other chronic conditions.
Ten Key Findings
Community Surveillance of MI and CHD
ARIC data are some of the most rigorously-validated population-based data on incidence and case-fatality of CHD in US populations and were regularly cited to document the burden of CHD in the US, including the AHA Heart Disease and Stroke Statistical Update.(Supplemental Appendix) (6–8) ARIC observed a relatively stable incidence of hospitalization for MI in the first decade of surveillance data (1987–96) with significant decreases in CHD mortality, along with a significant increase in survival after MI hospitalization among residents 35–74 years of the four ARIC communities (9). These findings garnered attention as an apparent paradox and generated consideration of potential explanatory hypotheses (10–12). Trends in CHD mortality from the World Health Organization Monitoring Trends and Determinants in Cardiovascular Disease (WHO MONICA) Project were published shortly afterward with a conclusion that decreased disease occurrence was the primary driver of trends rather than rather than improvements in case fatality rates.(13,14) Other surveillance studies subsequently reported similar trends as was found in ARIC for this 10-year period and further possible explanations (15,16). The improvements accelerated through 2008, after accounting for the introduction of increasingly sensitive troponin assays, that would have masked declines in incidence (Figure 1)(17). More recently ARIC investigators reported that the proportion of MI hospitalizations attributable to young patients increased from 1995 to 2014 and was especially pronounced among women, suggesting emerging need to refocus prevention strategies among the younger age demographic (18).
Figure 1. Age- and biomarker-adjusted rate in hospitalized MI or CHD death.
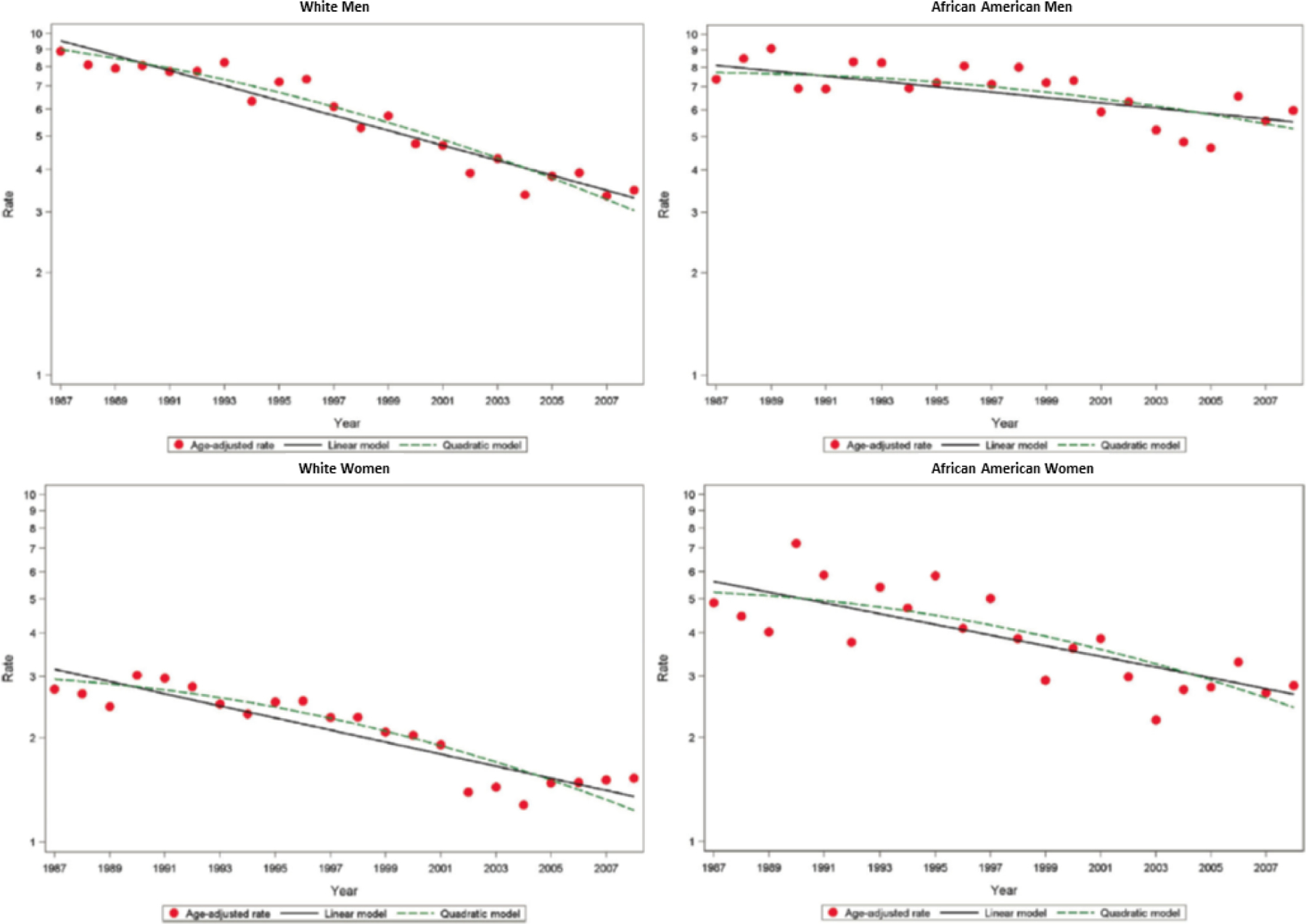
Age- and biomarker-adjusted rate in first hospitalized MI or CHD death without prior MI, estimated per 1000 persons and age-adjusted trends estimated by linear and quadratic Poisson regression, for men and women aged 35 to 74 years, ARIC 1987–2008. Republished with permission from Wayne D. Rosamond, Lloyd E. Chambless, Gerardo Heiss, et al. Twenty-Two–Year Trends in Incidence of Myocardial Infarction, Coronary Heart Disease Mortality, and Case Fatality in 4 US Communities, 1987–2008. Circulation. 2012;125: 1848–1857.
HF and Stroke in Community-Based Populations, including African Americans
Given the age and inclusion of both African Americans and white participants, the ARIC study has become an important source of data on HF in a community-based study.(19–26) Research using ARIC has provided important information on novel risk factors for HF (including Troponin T) and created a useful HF prediction model.(27,28) In subsequent research a stroke risk prediction model was created, one of the few that included African Americans.(29) ARIC community surveillance for HF added specificity to prior community surveillance efforts because it differentiated acute decompensated HF (ADHF) from chronic stable HF.(30,31) ARIC documented a disparity in incidence of ADHF with higher incidence among African American men than other sex and race-specific groups.(30) Racial disparities in HF incidence were investigated in the cohort as well as in the community surveillance data, with the higher incidence in African Americans largely explained by higher levels of risk factors.(19) ARIC reported that incidence of ADHF was higher in African American men and women, than in white men and women (Figure 2). The investigators documented trends of increasing ADHF hospitalizations between 2005 and 2014, with increases in cases of HF with preserved ejection fraction.(32) Echocardiographic data from the 2011–13 examination was used to investigate age-associated changes in left ventricular diastolic function and incident HF or death in ARIC.(26)
Figure 2. Age-adjusted rates of hospitalized ADHF events: 2005 to 2009.
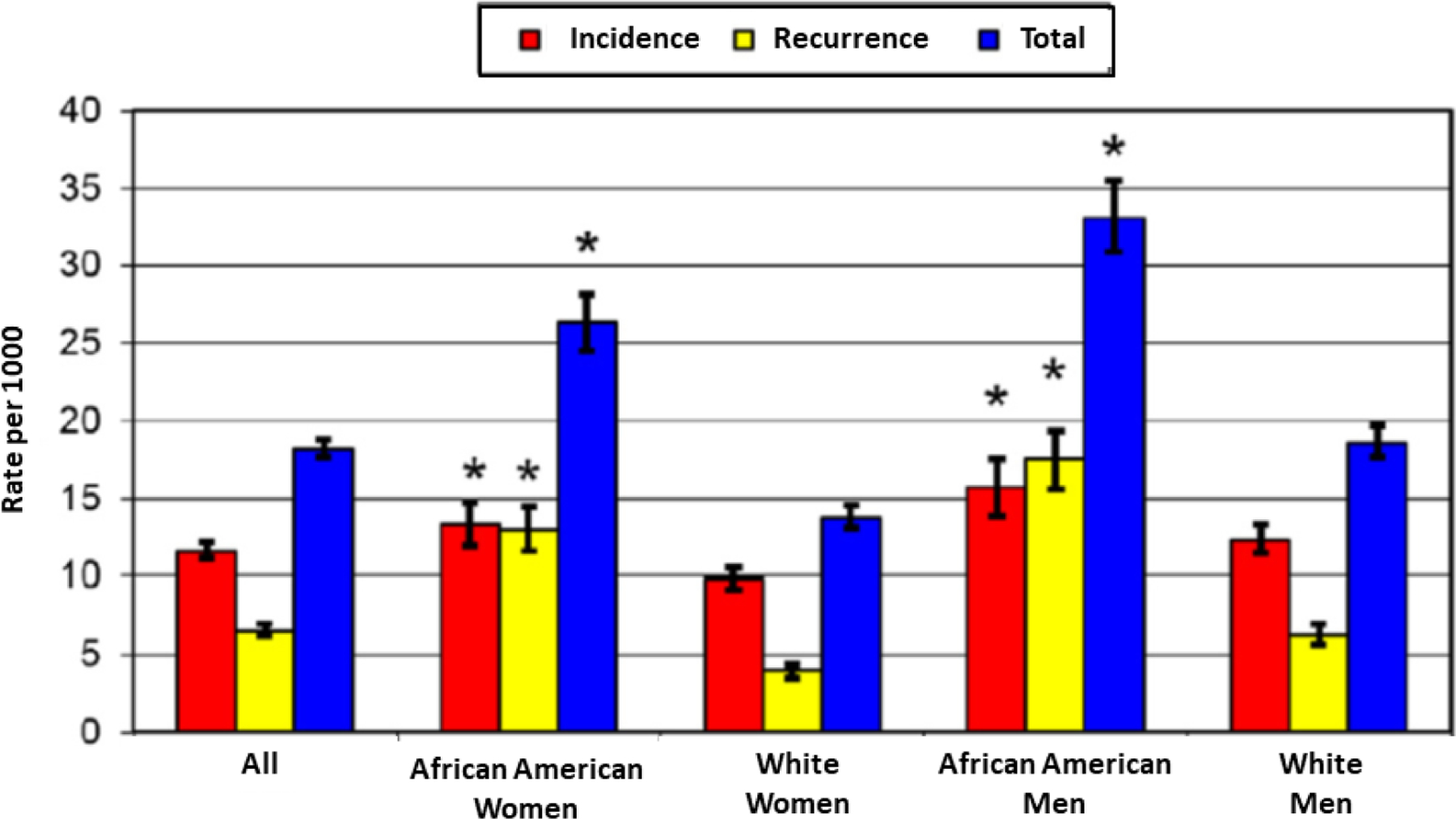
Rates of first, recurrent, and all ADHF hospitalizations are adjusted for age by the direct method, according to the US 2000 population distribution. * p value < 0.05 for the gender-specific comparison between African American and white groups. Republished with permission from Chang PP, Chambless LE, Shahar E, et al. Incidence and survival of hospitalized acute decompensated heart failure in four US communities (from the Atherosclerosis Risk in Communities Study). Am J Cardiol. 2014 Feb 1;113(3):504–10.
Health Disparities
The number of African American participants and the length of follow-up have facilitated extensive research on health disparities in ARIC, with early demonstrations of higher CVD risk in African Americans.(33–39) With 10 years of follow-up, ARIC showed that hypertension was a somewhat stronger risk factor for CHD in African American women, but was statistically significant for all race-sex groups.(39) Despite higher prevalence of hypertension and diabetes in African Americans, the magnitude of the associations with CVD were similar in African Americans and whites, but in African Americans the higher prevalence of these two risk factors leads to higher attributable risks for CVD. With 20 years of follow-up ARIC used data on seven risk factors and health behaviors included in AHA’s definition of ideal cardiovascular health to document a low prevalence of ideal cardiovascular health in ARIC participants, with notably lower prevalence of ideal cardiovascular health in African Americans than whites.(40)
With variability across the four communities of ARIC it has also been possible to study the detrimental effect of disadvantaged neighborhoods on CHD. ARIC investigators used information from the 1990 US Census on neighborhood characteristics, including demographic characteristics, median housing value, and median household income, education, and occupation for a random sample of housing units to define levels of neighborhood deprivation.(37) The ARIC publications were foundational in underscoring the importance of neighborhood factors in influencing risk factors as well as risk after adjusting for individual levels of risk factors. ARIC demonstrated that residing in deprived neighborhoods was associated with increased prevalence of CHD and increased prevalence of risk factors, with the neighborhood associations persisting after adjusting for individual levels of risk factors (Figure 3).
Figure 3. CHD Incidence Rates for Race and Neighborhood Groups.
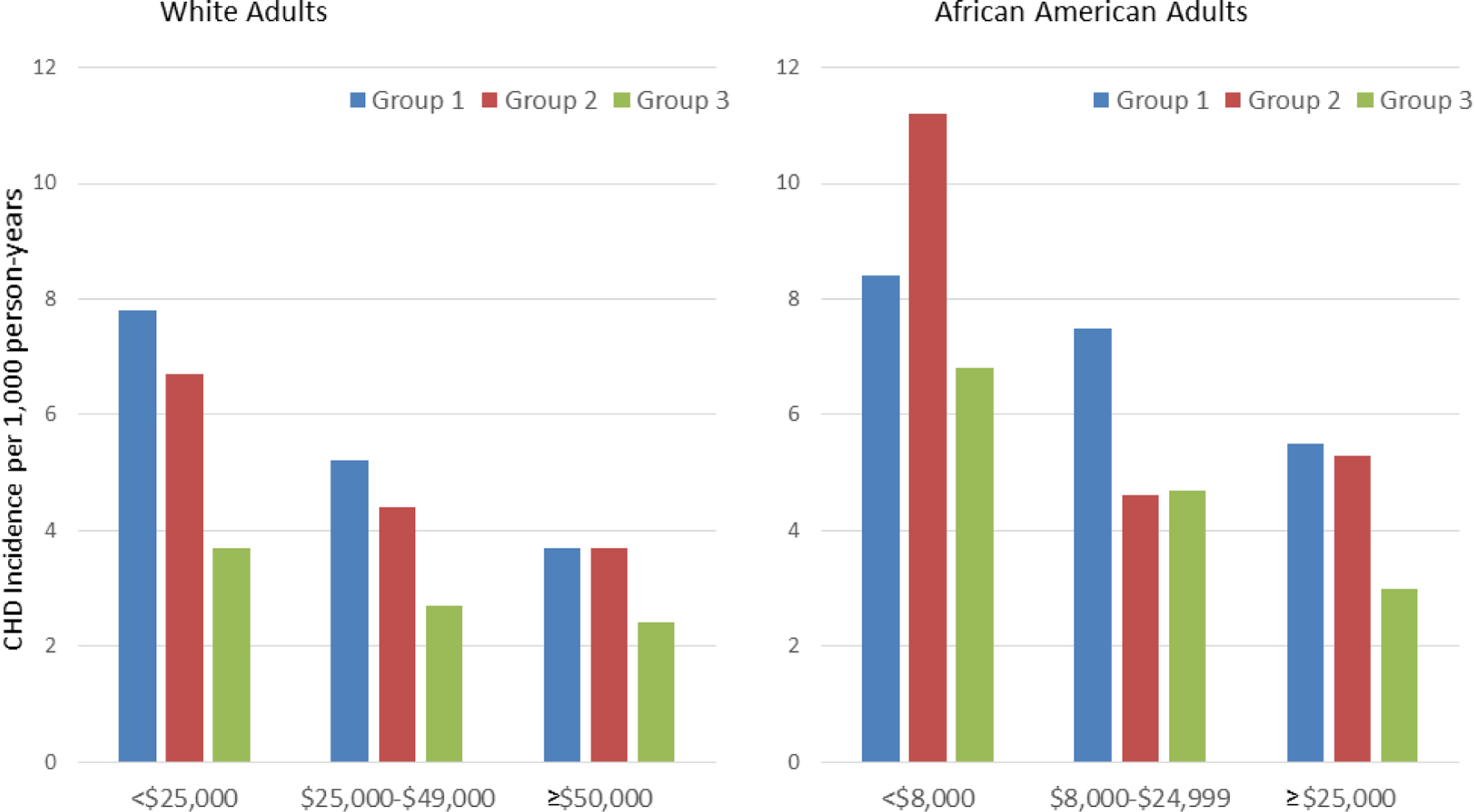
CHD incidence rates were adjusted for age, study site, and sex and are shown according to neighborhood group and personal income level. In whites and African Americans, neighborhood groups were defined according to summary socioeconomic scores. Group 1 (scores in the lowest third) corresponds to the most disadvantaged neighborhoods, and group 3 (scores in the highest third) corresponds to the most advantaged neighborhoods. Neighborhood scores were created using six neighborhood characteristics of wealth and income: 1) median household income, 2) median value of housing units, 3) percent of households receiving interest, dividend, or rental income, 4) percent of households with adults 25 years and older who completed high school, 5) percent of adults 25 years and older who completed college, and 6) percent of employed persons 16 years and older who were occupied in executive, managerial, or professional occupations. Republished with permission from Diez Roux AV, Merkin SS, Arnett D, e tal. Neighborhood of residence and incidence of coronary heart disease. N Engl J Med. 2001 Jul 12;345(2):99–106.
The community surveillance data documented disparities in rates of MI and CHD mortality between African Americans and whites, with an increasing or unchanged rate of MI among African American men and women over time, in contrast with declining rates in whites from these four communities.(9,17)
Epidemiology of Subclinical Atherosclerosis
The baseline ARIC cohort examination included some of the first B-mode ultrasound measurements of carotid artery with reading of intima-media thickness (IMT) and adjudicated plaque presence or absence in a population-based study to measure subclinical atherosclerosis and its risk factors (41–46). ARIC showed that carotid IMT and plaque presence were associated with CHD incidence (Figure 4) and improved CHD risk prediction over prediction using traditional risk factors alone.(44) This finding elicited comments on clinical utility of carotid ultrasound and an editorial calling for a prospective randomized trial on ultrasound imaging.(47–49) Subsequent meta-analyses on carotid IMT and progression of IMT, both of which included ARIC data, found small improvement in risk prediction with limited clinical utility.(50,51) The 2013 ACC/AHA Guideline on the Assessment of Cardiovascular Risk recommended against including carotid IMT in clinical practice. Advances in carotid imaging techniques have improved risk prediction and though this recommendation was repeated by the recent U.S. Preventive Services Task Force, an accompanying editorial commented on the enduring controversy in this area.(52–55) Recent research in ARIC using data from the Carotid MRI ancillary study demonstrated an association of carotid artery plaques with a lipid core with CVD events independent of traditional risk factors and maximum carotid IMT. (56)
Figure 4. CHD Incidence Rate per 1,000 Person-Years by CIMT and Plaque.
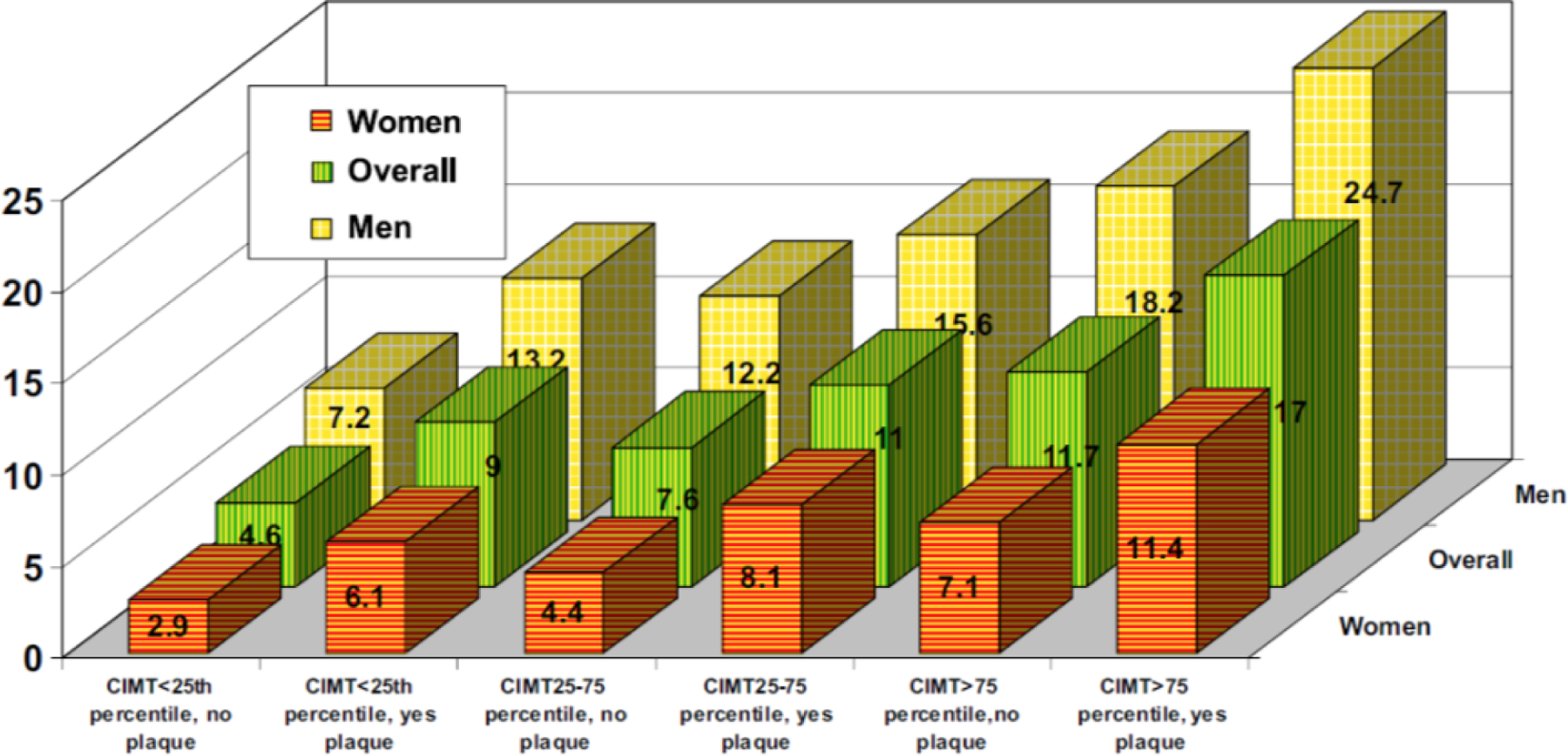
Adjusted CHD incidence rate for women, men, and overall, by categories of carotid intima-media thickness (CIMT) and presence or absence of plaque. For every CIMT category (i.e., <25th percentile, 25th to 75th percentile, and >75th percentile), for the overall group (green bars), men (yellow bars), or women (orange bars), having carotid artery plaque is associated with a higher incidence of coronary heart disease. Source: Nambi et al. J Am Coll Cardiol 2010(44)
Novel Cardiovascular Biomarkers
One of the valuable aspects of longitudinal cohort studies with repeated clinic examinations at which blood specimens are collected and carefully preserved is the great potential for future research on stored specimens to discover new biomarkers associated with cardiovascular events accumulated over follow up. ARIC data have provided vanguard information from the general population supporting or refuting many novel risk markers for future CHD (e.g., hemostatic factors, lipid and lipoproteins, markers of inflammation, estimated kidney function, albuminuria, homocysteine, troponin T, troponin I, and NT-pro BNP).(27,57–66) ARIC provided early corroboration of the association of high sensitivity Troponin T (Roche Diagnostics, Indianapolis, IN) with CHD and HF hospitalizations.(62). This finding improved the understanding of the cardiovascular risk associated with low concentrations of this biomarker measured with new troponin assays.(67) Although some of these risk markers have somewhat improved prediction of CVD (e.g., troponin T and I), ARIC has dispelled arguments for many novel risk factors (e.g., hemostatic factors and homocysteine) being added to risk equations for predicting CHD.(68)
Genomics Discoveries
Genetics research conducted in ARIC has contributed to advances through novel study questions, size of the cohort, and especially the number of African American participants. ARIC data were key in research identifying proprotein convertase subtilisin/kexin type 9 (PCSK9) mutations that determine low cholesterol levels and decreased CHD risk, including the finding that PCSK9 carriers did not have obvious phenotypic manifestations that would bode poorly for the outcome of pharmacologic therapy. Anti-PCSK9 lipid lowering therapies with monoclonal antibodies were approved in record time and have been shown to reduce both LDL-C and CVD events.(69) This PCSK9 example and other work in ARIC underscores the importance of diversity in genetics studies, especially for studies including low frequency and rare variants.(70) Genetics research emphasizes combining data from multiple studies to strengthen statistical rigor and the inclusion of African American and white participants in ARIC make it an important contributor. ARIC participated in a study on resequencing of ANGPTL4 to identify variants associated with reduced triglycerides and increased HDL, and to two genome-wide association studies, one on uric acid concentration and risk of gout and one on renal function and CKD.(71–73) Genetic research has also been conducted using ARIC data exclusively. ARIC used Mendelian randomization principles to investigate five single nucleotide polymorphisms known to be strongly associated with fasting glucose and subclinical atherosclerosis.(74) ARIC showed an association of the fasting glucose genetic risk score with mean carotid IMT, and though not a clinically meaningful difference did suggest a possible causal association of fasting glucose with atherosclerosis. ARIC investigated association of genetic variants with diabetic nephropathy and showed association of glucose transporter 1 (GLUT1) polymorphisms (e.g., Enh2 and XbaI) with albuminuria in whites with diabetes but not African Americans (where variants are more rare).(75)
Cardiovascular Risk and Cognitive Decline, Mild Cognitive Impairment, and Dementia
The comprehensive clinic examinations conducted in the first 10 years of ARIC included a short battery of cognitive tests, chosen for their broad but efficient coverage of three major cognitive domains (episodic memory, processing speed, and language). These assessments provided invaluable baseline data for subsequent research in the 2010s on the vascular contribution to brain aging, including cognitive decline and progression to dementia. As there are currently no effective treatments for dementia, ARIC’s focus on the vascular contribution meets widespread interest in factors which might be intervened on to delay or prevent cognitive decline and dementia. ARIC reported the association of midlife higher systolic blood pressure with decline in cognitive test scores in whites but not African American participants. ARIC described the importance of hypertension and diabetes in mid-life as contributing factors to later cognitive decline, particularly if it is followed by lower blood pressure at older ages.(76,77) Other mid-life predictors of cognitive decline, dementia and imaging-defined brain damage that were documented in ARIC include orthostatic hypotension, prediabetes, smoking, and physical inactivity. Broadly, these findings suggest that managing vascular risk factors may provide an effective strategy to lower dementia risk with age. These findings resulted from work of the ARIC NCS Study, funded by multiple NIH Institutes interested in the intersection of CVD and dementia, which initiated detailed cognitive testing and brain imaging with participants in the 2011–13 examination. (Supplemental Appendix) Data generated from this large ancillary study have facilitated ongoing in-depth research on cardiovascular risk factors and cognitive decline and dementia. The ARIC PET Study, an independently funded ancillary study of ARIC conducted brain amyloid PET imaging in conjunction with the 2011–13 clinic examination in a subsample of 346 participants from among the nearly 2,000 who received a brain MRI in ARIC NCS with some additional exclusion criteria (heavy current alcohol use, renal dysfunction, prolonged (>450 milliseconds) QTc interval, or neuropsychological results consistent with dementia).(78) Midlife (45–64 years), but not late life (67–88 years), cardiovascular risk factors were associated with brain amyloid deposition, including body mass index ≥30 kg/m2, current smoking, hypertension, serum cholesterol ≥200 mg/dL, and diabetes (Figure 5). ARIC data inform the recommendations to prevent and treat vascular risk factors as one of the more promising strategies to prevent dementia.(79,80)
Figure 5. Odds Ratios for Elevated SUVR by Vascular Risk Factors and Visit.
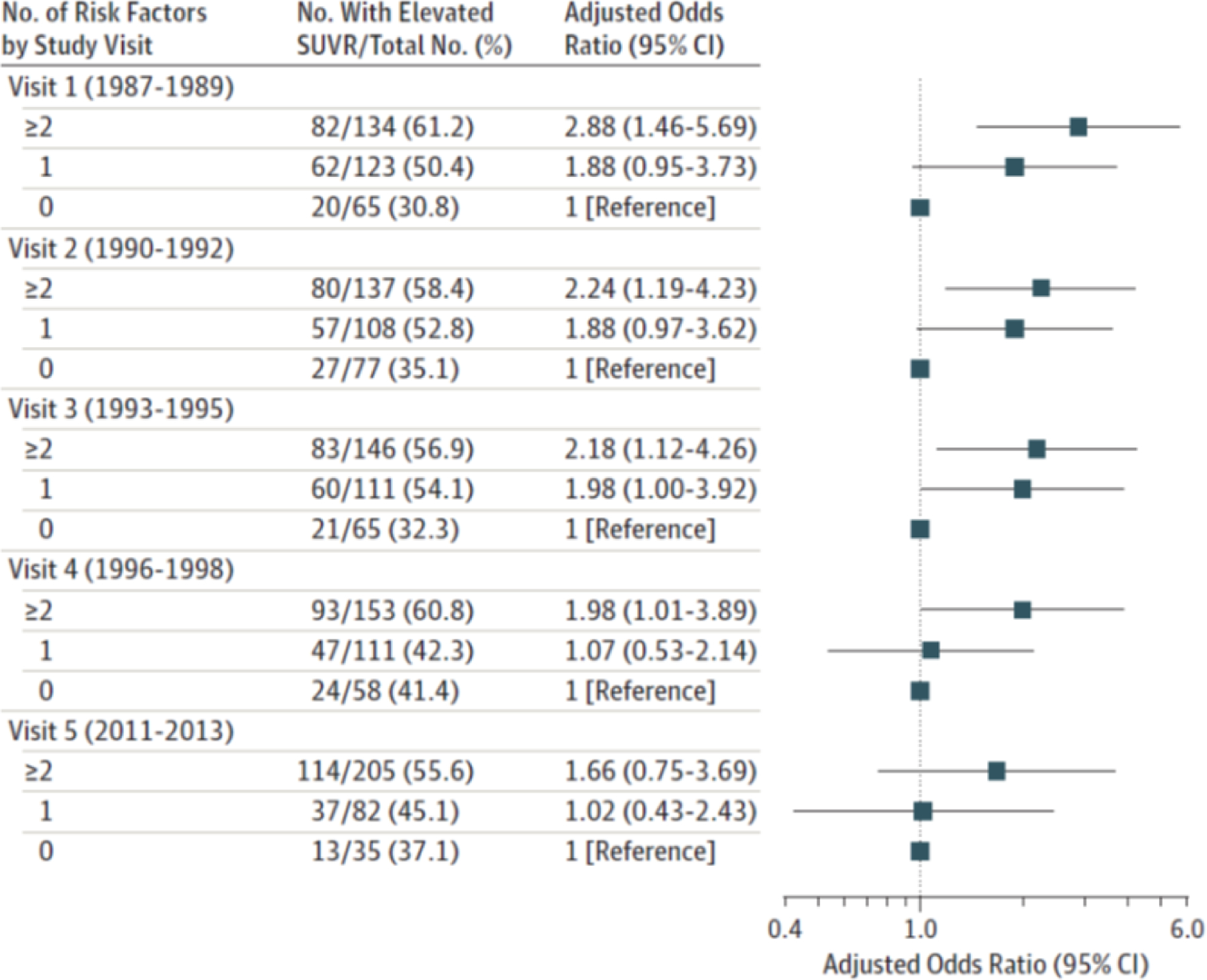
Adjusted Odds Ratios for Global Cortex Florbetapir SUVRs > 1.2 by Number of Vascular Risk Factors, Midlife Through Late Life Adjusted odds ratios (with 95% confidence intervals as error bars) are shown for number of vascular risk factors for visits 1 (at midlife) through 5 (at late life) for standardized uptake value ratios (SUVRs) > 1.2. Models are adjusted for age (at Visit 5, 2011–13), sex, race, education level, and apolipoprotein E ε4 genotype. Vascular risk factors include body mass index ≥30 kg/m2, current smoking, hypertension, diabetes, and total serum cholesterol ≥200 mg/dL. Republished with permission from Gottesman RF, Schneider AL, Zhou Y, et al. Association Between Midlife Vascular Risk Factors and Estimated Brain Amyloid Deposition. JAMA. 2017 Apr 11;317(14):1443–1450.
Diabetes, Kidney Disease and Cardiovascular Risk
ARIC reported diabetes incidence in African American women of 25.1 per 1,000 person-years compared with 10.4 in white women, and 23.4 in African American men compared with 15.9 in white men.(81) Differences in potentially modifiable risk factors such as body mass index accounted for almost half of the excess risk in African American women. ARIC published evidence linking glycated hemoglobin among individuals with and without diabetes (where other data were very limited) to incident diabetes and CVD, thus bolstering the use of glycated hemoglobin in updated clinical diabetes diagnostic guidelines.(82,83) Glycated hemoglobin was significantly associated with risk of diabetes and CHD even after adjustment for fasting glucose level.
ARIC data were used to demonstrate the burden and risk of moderate kidney dysfunction in the general population.(84–88) This research helped foster an emphasis on the broader epidemiology of CKD and the guidelines that followed.(89,90) ARIC data demonstrated racial disparities in risk factors and socioeconomic factors explained most of the excess risk of CKD in diabetics but only approximately half of the excess risk among individuals without diabetes.(91) This research contributed to efforts to discover genetic variants in the Apolipoprotein L1 (APOL1) present in approximately one quarter of African-Americans increase risk of CKD and dialysis. ARIC data showed that in contrast to odds ratios of approximately 10 in case-control studies, the APOL1 risk variants have a relative risks of approximately two in the general population informing population-wide policies.(92) ARIC data and investigators are also central to the influential, global CKD Prognosis Consortium.(93,94)
AF and Venous Thromboembolism
Investigations in ARIC helped to establish that risk of AF is lower in African Americans than whites and that there is a link between obesity and AF. (95–98) ARIC found that overall 56.5% of AF cases could be explained by having borderline or elevated risk factor levels (blood pressure, body mass index, fasting serum glucose, and smoking status) however incidence rates were lower in African Americans than in whites at each risk profile (optimal, borderline, or high) (Figure 6) (98). Recent findings on subclinical AF assessed using an ambulatory ECG monitor indicate that prevalence of subclinical AF is 2.5%, of which 75% was classified as intermittent. Observational data from ARIC suggested that clinical trials of weight loss interventions to prevent AF may warrant consideration given the growing epidemic of AF. Recently funded NIH grants are investigating contributions of left atrial abnormalities to AF.(99)
Figure 6. Time free from AF by sex, race, and risk factors.
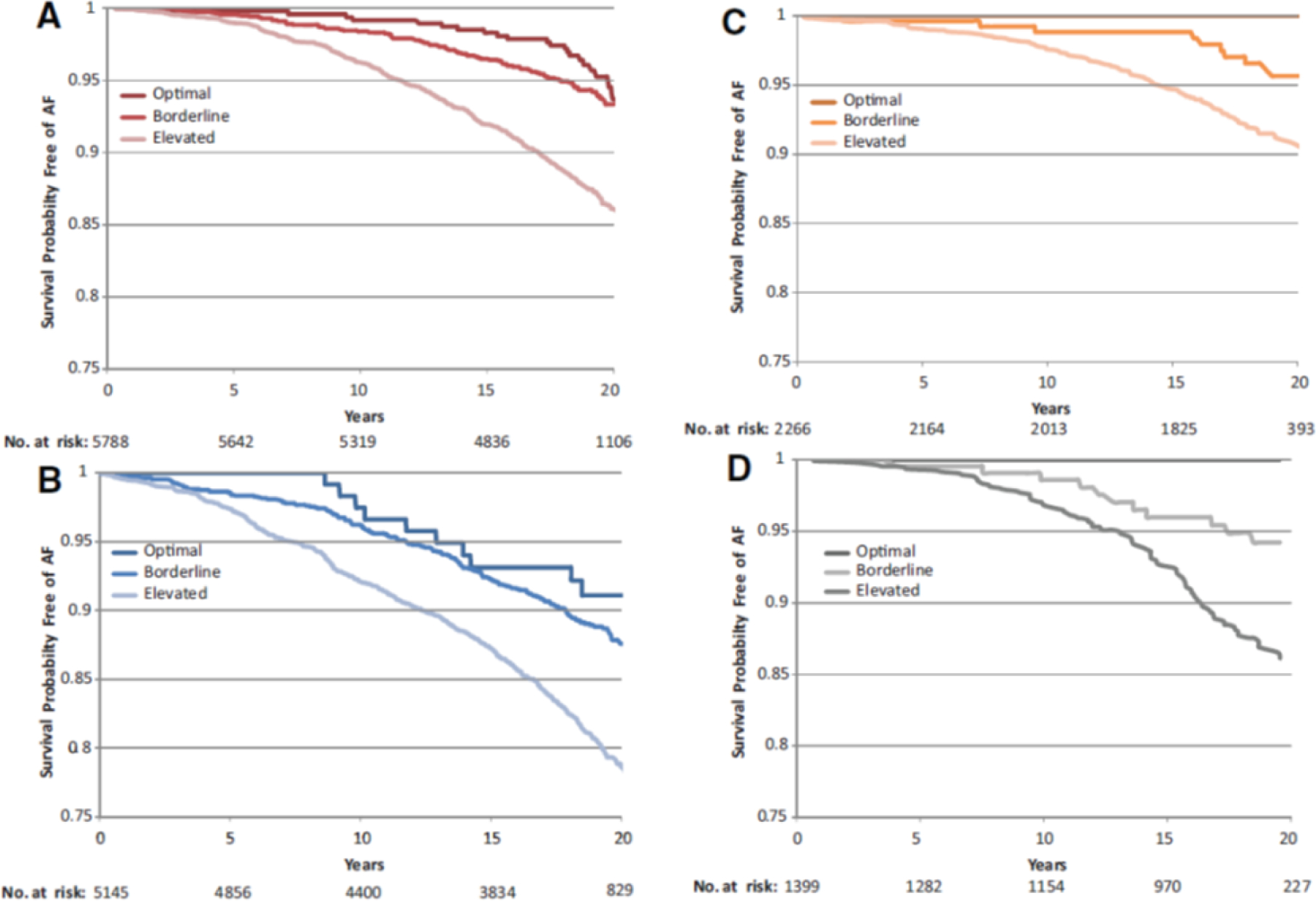
Survival curves adjusted for age, study center, education, and height showing time free from AF according to risk factor group (optimal, borderline, or elevated) in white women (A), white men (B), African American women (C), and African American men (D). The numbers of subjects at risk throughout the duration of study follow-up are shown on the x axis. An optimal risk factor profile was defined as no history of cardiac disease (HF or CHD); systolic BP <120 mm Hg, diastolic BP <80 mm Hg, and no use of antihypertensive medication; body mass index (BMI) <25 kg/m2; fasting serum glucose <100 mg/dL, no use of antidiabetic medication, and no history of physician-diagnosed diabetes mellitus; and never a smoker. A borderline risk factor profile was defined as having any of the following criteria and no elevated risk factor profile characteristics: systolic BP of 120 to 139 mm Hg and/or diastolic BP of 80 to 89 mm Hg and no use of antihypertensive medication; BMI of 25 to <30 kg/m2; fasting serum glucose 100 to 125 mg/dL, no use of antidiabetic medication, and no history of physician-diagnosed diabetes mellitus; and a former smoker. An elevated risk factor profile was defined as having any of the following criteria: history of cardiac disease (HF or CHD); systolic BP ≥140 mm Hg, diastolic BP ≥90 mm Hg, or use of antihypertensive medication; BMI ≥30 kg/m2; fasting serum glucose ≥126 mg/dL, use of antidiabetic medication, or history of physician diagnosed diabetes mellitus; or a current smoker. Republished with permission from Huxley RR, Lopez FL, Folsom AR, et al. Absolute and attributable risks of atrial fibrillation in relation to optimal and borderline risk factors: the Atherosclerosis Risk in Communities (ARIC) study. Circulation. 2011 Apr 12;123(14):1501–8.
ARIC documented that at age 45 years, the remaining lifetime risk of venous thromboembolism was 8.1% (95% confidence interval, 7.1–8.7). High-risk groups were African Americans (11.5% lifetime risk), those with obesity (10.9%), heterozygous for the factor V Leiden (17.1%), or with sickle cell trait or disease (18.2%). ARIC also provided other evidence for lifestyle factors contributing to risk of venous thromboembolism, offering possible keys to prevention.(100–102)
Contributions to Prevention
Cardiovascular epidemiology has been a foundational research component of cardiovascular prevention science through the past decades.(103) Given the overall size of the ARIC cohort and the number of African American participants, the study has contributed results to virtually every AHA Scientific Statement related to prevention guidelines and policy published in the last two decades (104–109). ARIC provided data for the ubiquitous ACC/AHA CVD risk equation, including most of the data for African Americans’ risk estimation. (52) Every AHA Heart Disease and Stroke Statistical Update published in Circulation in the past 10 years has included ARIC data.(8) This publication is cited over 1000 times per year in the medical literature. ARIC provided the first report documenting the prevalence of AHA’s “ideal CVD health metrics (AHA’s Life’s Simple Seven) and evidence that the number of health metrics is related to CVD rates in a dose-response fashion (Figure 7).(40) ARIC investigators extended this research to consider the ideal CVD health metric and risk of HF, venous thrombosis, and other vascular conditions.(102,110–112) The ARIC study also helped to demonstrate the importance of a healthy lifestyle those with a high underlying genetic risk (Figure 8).(113)
Figure 7. CVD Incidence Rate According to Ideal Health Behaviors and Factors.
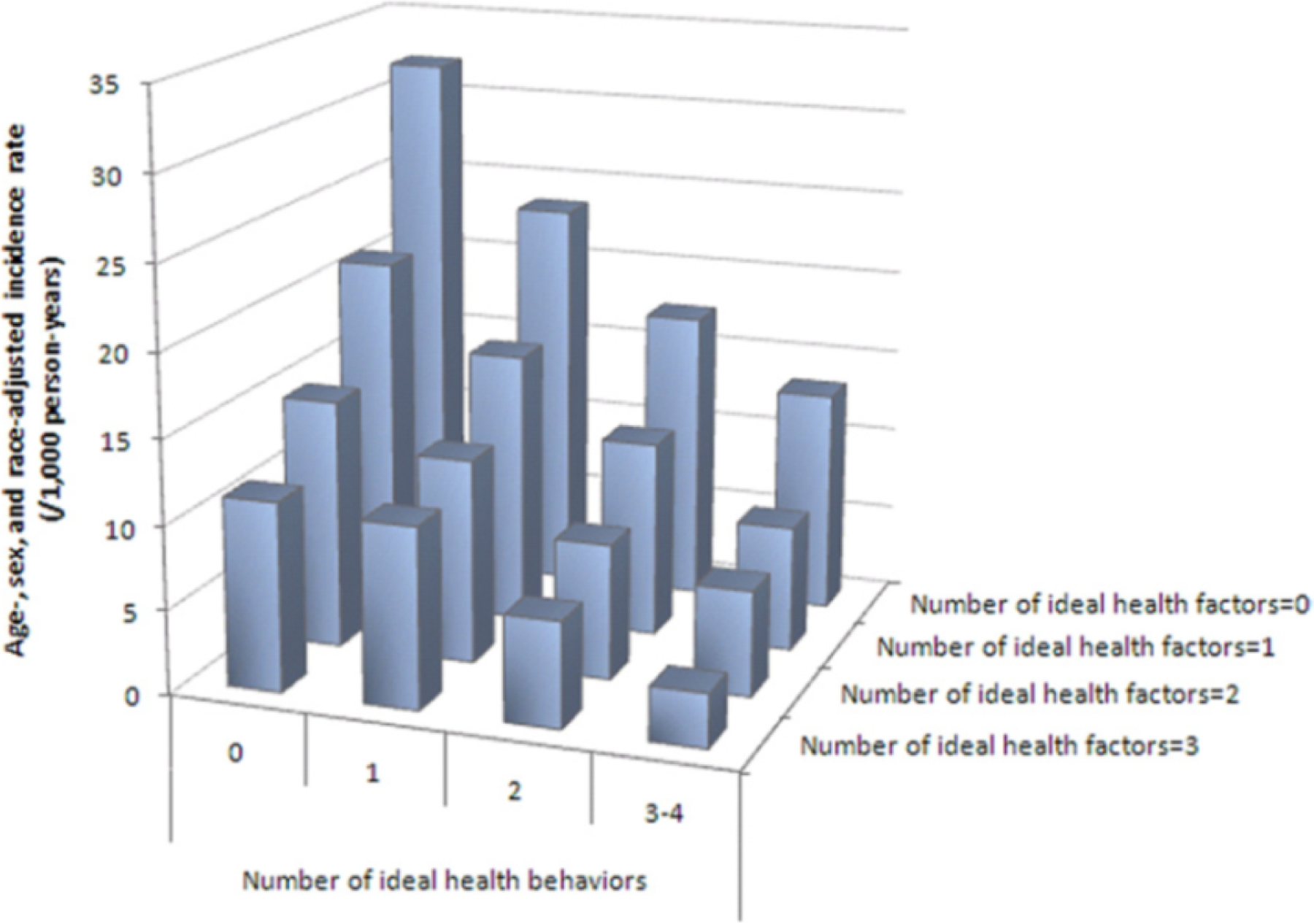
Age-, sex-, and race-adjusted incidence rate of CVD according to the number of ideal cardiovascular health behaviors (nonsmoking, body mass index, physical activity, healthy diet score) and health factors (total cholesterol, blood pressure, and glucose), ARIC (Atherosclerosis Risk in Communities), 1987 to 2007. Source: Folsom et al. J Am Coll Cardiol 2011(40)
Figure 8. Risk of Coronary Events, According to Genetic and Lifestyle Risk.
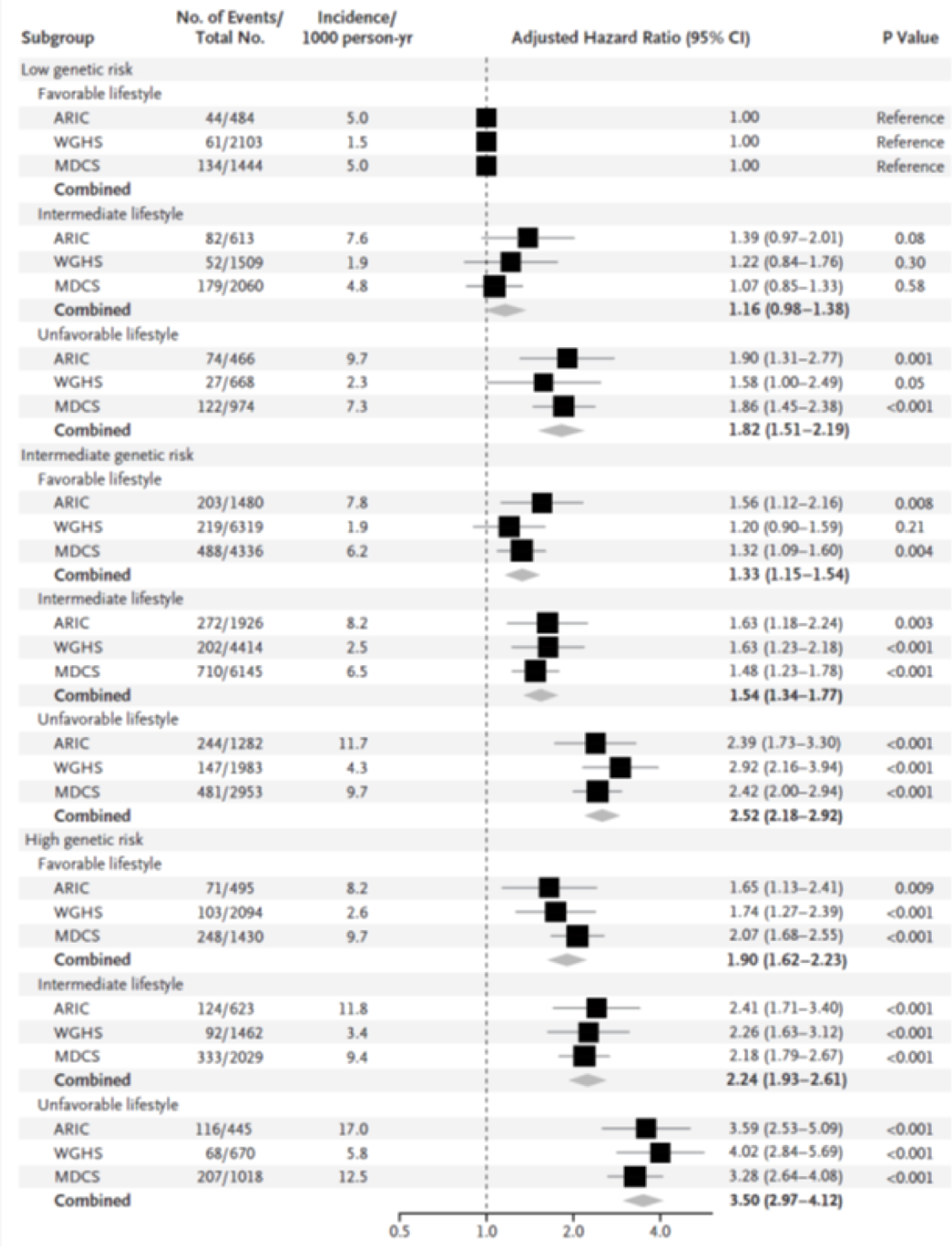
Shown are adjusted hazard ratios for coronary events in each of the three prospective cohorts, according to genetic risk and lifestyle risk: the Atherosclerosis Risk in Communities (ARIC) cohort, the Women’s Genome Health Study (WGHS) cohort, and the Malmö Diet and Cancer Study (MDCS) cohort. In these comparisons, participants at low genetic risk with a favorable lifestyle served as the reference group. There was no evidence of a significant interaction between genetic and lifestyle risk factors (P = 0.38 for interaction in the ARIC cohort, P = 0.31 in the WGHS cohort, and P = 0.24 in the MDCS cohort). Unadjusted incidence rates are reported per 1000 person-years of follow-up. A random-effects meta-analysis was used to combine cohort-specific results. Republished with permission from Khera AV, Emdin CA, Drake I, et al. Genetic Risk, Adherence to a Healthy Lifestyle, and Coronary Disease. N Engl J Med. 2016 Dec 15;375(24):2349–2358.
Future Research Opportunities
In the last decade, ARIC has strengthened engagement of outside investigators through ancillary studies following changes in the NHLBI’s approach to supporting cohort studies.(114,115) A review of approved ancillary study proposals over time indicates increases in the number of proposals just before clinical examinations in 2011–13 and 2018–19, suggesting a response by investigators to the “platform model” of support for clinic examination components through independently funded NIH grants. The most recent contract supported clinic examination, Visit 7, included components supported by 12 independently funded ancillary studies. ARIC uses Medicare data on cohort participant hospitalizations and out-patient visits in combination with medical discharge records obtained by the study with consideration of the limitations of these administrative data.(116) Engagement of new investigators in ARIC led to the development of new areas of research, enhanced existing research areas and produced new research projects.
Vascular Contributions to Cognitive Impairment and Dementia
As the ARIC cohort has aged, the ARIC NCS Study, now in its third funding cycle, is focusing on cognition in late-life and understanding factors that enhance resilience to detrimental aspects of aging. Given the depth of characterization of CVD in mid-life from multiple prior clinic examinations, there continue to be research questions on cognitive function and decline that may be addressed in ARIC. Investigation in the role of systemic inflammation on cognitive decline is possible using blood biomarkers measured in early clinic examinations. ARIC used blood biomarkers of inflammation to create an inflammation composite score that was significantly associated with cognitive decline over 20 years.(117) Ongoing work focuses on discovering plasma proteomic markers and pathways related to dementia risk, the connections between cognitive and physical function and resilience with aging whereby some individuals retain high function at advanced age, sometimes despite having imaging signs of brain pathology. Increased emphasis is placed on detailed physiologic measures (e.g. hearing and gait) and outpatient monitoring of health (e.g. physical activity, ambulatory ECG and blood pressure). As the cohort becomes very old (currently aged 80–100 years) the interplay of late life events with mid-life risk factors and trajectories in the modern, highly medicalized, era will be important to study.
Progression of HF in a Community-Based Cohort
The ARIC cohort experience of HF burden is a growing research area due to the increasing burden of HF in the US. ARIC recently used the 2011–13 examination data, with subsequent follow-up for HF hospitalizations or death, to assess scoring algorithms for HF with preserved ejection fraction.(118) Echocardiographic data from the 2011–13 examination was used in combination with time-averaged cumulative systolic blood pressure (from 5 visits) and subsequent follow-up for HF hospitalization to investigate association of cumulative systolic blood pressure with cardiac structure and function and risk of HF.(119) Investigators found worse cardiac function and increased risk of HF with preserved ejection fraction was association with time-averaged systolic blood pressure in mid- to late-life. Recently funded NIH grants will be investigating different avenues of HF research including the role of adipokines, detection of transthyretin cardiac amyloidosis, and metabolomic signatures associated with HF.(99)
Multi-omic Studies
Multi-omic studies have the ability to better define mechanisms of disease susceptibility and identify novel biomarkers of disease risk and protection. Leveraging its stored biorepository, the ARIC study has pioneered the use of DNA methylation, metabolomics and proteomics in large diverse longitudinal population studies.(120–122) Quality assurance and laboratory reproducibility using biospecimens from multiple clinic visits has been a key focus at every step. (123) Partnerships with industry were developed with careful attention to data sharing policies of and input from NHLBI.(124) Research based on recently funded NIH grants on metabolomics and proteomics will be investigating questions in many areas, including cognitive decline, dementia, CKD, and venous thromboembolism.(99)
Cardiovascular Surveillance Using Electronic Health Record Data
The ARIC CVD surveillance program included computer-based efficiencies from the outset to facilitate the community-based surveillance such as computer-based linking of all hospitalization records for an individual within a 28-day period and use of computer algorithms to streamline the classification process. The most recent contract renewal of the study ended the community surveillance and launched a feasibility study of cardiovascular surveillance using digitally transmitted electronic health record (EHR) data (Central Illustration). The goal of this feasibility study was to assess the practicability, accuracy and costs of EHR‐ based population surveillance of hospitalized MI and HF following ARIC protocol specifications. Research findings have been presented on MI classification using EHR-based abstraction data compared with manually abstracted data, and evaluation of the effectiveness of algorithm extraction protocols for hospitalization laboratory data.(125,126)
Beyond the ARIC Cohort
Future investigations may involve expansion beyond the original study cohort. Engagement of proxies for cohort participants has become important as the participants aged, with more than 95% of cohort participants identifying proxies who may be contacted on their behalf. These proxies are an important source of information on the participant but are themselves potential research participants in areas like health effects on caregivers of persons living with dementia. During the COVID-19 pandemic, closest family members were questioned about social support and isolation. Another example of expansion beyond the original ARIC cohort is the embedded clinical trial underway on the effect of hearing loss intervention on slowing declines in cognition, physical and social functioning among adults with hearing loss. This trial, Aging and Cognitive Health Evaluation in Elders (ACHIEVE), is ongoing with final results expected in 2022.(127) The ARIC cohort participants recruited for the trial are more deeply characterized than de novo participants and the cohort study infrastructure supports the recruitment of new participants from among the community.
Limitations and Lessons Learned
One limitation in ARIC is the confounding of race and field center because at the Jackson field center African Americans were exclusively recruited, and the Forsyth County field center recruited whites and African Americans. This design was implemented to ensure the sample size was sufficient for statistically reliable analysis but does limit generalizability. Although the sample of African Americans in ARIC is substantial, it has not always been sufficient for the study of more rare outcomes. Another limitation is attrition in the study over time and this was a particular concern for analyses conducted using data that spanned the gap between clinic visits in 1999–2011. ARIC analyses on cognitive change have evaluated the potential for differential attrition.(128) Improvements were made in ultrasound methods developed after the carotid IMT ultrasound examinations performed in early clinic visits and a study was conducted in 2005–06 in a subset of ARIC participants to perform MRI imaging of the carotid arteries. In ARIC, and all longitudinal studies, investigators weight the benefits of using newly developed improved technologies and maintaining comparability with previous data collection methods.
In spite of these limitations, several lessons can be identified as key to successful epidemiology and as strengthening ARIC’s ability to move science forward and produce findings with lasting clinical impact: a distributed data model encouraging analysis and publication at the field centers and laboratories as well as the coordinating center, a team science approach across multiple disciplines, openness to ancillary study collaboration with the scientific community, participation in consortia publications to broaden clinical impact, wide data sharing, and fostering training of the next generation of investigators through publications and ancillary studies. ARIC has approved over 400 ancillary study proposals and more than half of these have successfully secured funding. In addition to distributing ARIC data to the NIH database of Genotypes and Phenotypes (dbGaP) and NHLBI’s Biologic Specimen and Data Repository Information Coordinating Center (BioLINCC), the ARIC Coordinating Center distributes ARIC data to the scientific community on request via data distribution agreements for individual research projects, with almost 250 data distribution agreements executed to date.(129,130) ARIC partnered in the development of the Cohorts for Heart and Aging Research in Genomic Epidemiology (CHARGE) Consortium that was formed by investigators from five prospective cohort studies in the US and Europe to investigate genetic determinants of risk factors and measures of subclinical disease as well as clinical events.(131) The CHARGE consortium facilitated genome wide association study (GWAS) meta-analyses and replication studies in its large, well characterized cohort studies. The ARIC investigators also collaborated to create an Analysis Commons to bring together multi-omics data from diverse population-based studies. The Commons provides the computational tools needed in a cloud-computing environment and the administrative support necessary to protect sensitive data that adhere to data sharing policies and management of data access, harmonization efforts, and analytic tool development.(132)
From the Seven Countries Study to MONICA, cardiovascular epidemiology has a history of collaborating across countries to broaden the generalizability of findings and strengthen the statistical reliability of results (13,133). The CHARGE consortium continued this tradition of international collaborations and ARIC has also partnered in the Emerging Risk Factors Collaboration, which includes data from over 100 population-based studies from predominantly Western populations of Europe, Australia, and North America.(134) ARIC is part of the Chronic Kidney Disease Prognosis Consortium including cohorts from around the world and evaluated the improvement in CVD risk prediction with inclusion of kidney function measures.(93) ARIC is a data partner in the Global Alzheimer’s Association Interactive Network, which has a primary goal to advance research into the causes and preventions and treatment of Alzheimer’s and other neurodegenerative diseases, and enables queries of a study’s data attributes to facilitate collaborations.(135) As a large biracial cohort, ARIC contributes important diversity to these international research collaborations.
The ARIC study was begun with the intention to improve the understanding of trends of decline in CHD mortality and to identify determinants of subclinical atherosclerosis in African American and white middle-aged adults. The study has met this goal and as the cohort has aged and the research base has broadened, the study developed an inclusive team-based approach to research on the broad impact of vascular disease including, but also beyond, clinical CVD and its sequelae. The early investment in comprehensive examinations developed by thoughtful and broad perspectives allowed the development of a unique data source for investigation of the vascular contributions to cognitive impairment and dementia and a clinical trial to reduce their risk. The ARIC study, and other NHLBI-supported cardiovascular cohort studies included in this review series, demonstrate the importance of population-based research in the spectrum of biomedical research to improve health.
Conclusions
ARIC and other NHLBI-supported cohort studies have amassed a wealth of data from multiple standardized examinations over decades and rigorously validated clinical outcomes. These studies form the bedrock of single-cohort and consortial observational research.(94,131,132,134) ARIC fits within a diverse landscape of cohort studies supported by NHLBI, representing African American and white adults born in 1923–42.(136) NHLBI launched other biracial cohort studies of younger and older adults and cohort studies including other race-ethnic groups, namely participants of Hispanic/Latino origin and Asian origin, predominantly of Chinese descent. The community surveillance arm of ARIC provided the unique opportunity to evaluate how well cohort findings generalized to the source populations for the cardiovascular events included in surveillance.
Supplementary Material
Highlights.
ARIC generated findings used in developing patient care guidelines, improved treatment, and preventive care policy
ARIC deeply characterized a biracial cohort and followed it for multiple outcomes over 33 years
ARIC documented 27-year community-based trends in MI, CHD mortality, and 10-year trends in HF
Future efforts focus on cardiovascular risk and late-life outcomes including cognitive decline and functional aging
ACKNOWLEDGMENTS:
The authors thank the staff and participants of the ARIC study for their important contributions. The views expressed in this manuscript are those of the authors and do not necessarily represent the views of the National Heart, Lung, and Blood Institute; the National Institutes of Health; or the U.S. Department of Health and Human Services. Information about ARIC can be found on the study website: https://sites.cscc.unc.edu/aric/.
Josef Coresh: Dr. Coresh has investigator initiated grant support from the NIH and National Kidney Foundation (NKF received research support from industry). Scientific advisor to Healthy.io and Alike.
Christie M. Ballantyne: Grant/Research Support- All significant. (All paid to institution, not individual): Abbott Diagnostic, Akcea, Amgen, Esperion, Novartis, Regeneron, Roche Diagnostic, NIH, AHA, ADA. Consultant- Abbott Diagnostics, Akcea, Althera, Amarin*, Amgen, Arrowhead, Astra Zeneca, Corvidia, Denka Seiken*, Esperion, Gilead, Janssen, Matinas BioPharma Inc, New Amsterdam*, Novartis, Novo Nordisk, Pfizer, Regeneron, Roche Diagnostic, Sanofi-Synthelabo*
Funding:
The Atherosclerosis Risk in Communities study has been funded in whole or in part with Federal funds from the National Heart, Lung, and Blood Institute, National Institutes of Health, Department of Health and Human Services, under Contract nos. (HHSN268201700001I, HHSN268201700002I, HHSN268201700003I, HHSN268201700004I, and HHSN268201700005I). Neurocognitive data are collected by U01 2U01HL096812, 2U01HL096814, 2U01HL096899, 2U01HL096902, 2U01HL096917 from the NIH (NHLBI, NINDS, NIA and NIDCD), and with previous brain MRI examinations funded by R01-HL70825 and biomarkers by R01-HL134320 from the NHLBI.
Abbreviations:
- AF
atrial fibrillation
- ARIC
Atherosclerosis Risk in Communities
- CHD
coronary heart disease
- CVD
cardiovascular disease
- ECG
electrocardiogram
- HF
heart failure
- MI
myocardial infarction
- MRI
magnetic resonance imaging
- NCS
Neurocognitive Study
- NHLBI
National Heart, Lung, and Blood Institute
Footnotes
Disclosures:
Jacqueline D. Wright: None
Aaron R. Folsom: None
A. Richey Sharrett: None
David Couper: None
Lynne E. Wagenknecht: None
Thomas H. Mosley, Jr.: None
Eric A. Boerwinkle: None
Wayne D. Rosamond: None
Gerardo Heiss: None
Significant where noted (>$10,000); remainder modest (<$10,000).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.The ARIC Investigators. The Atherosclerosis Risk in Communities (ARIC) study: design and objectives. Am J Epidemiol 1989;129:687–702. [PubMed] [Google Scholar]
- 2.Taliun D, Harris DN, Kessler MD, et al. Sequencing of 53,831 diverse genomes from the NHLBI TOPMed Program. Nature 2021;590: 290–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Havlik RJ, Feinleib M, editors. Proceedings of the 1978 Conference on the Decline in Coronary Heart Disease Mortality. Bethesda, MD: U.S. Department of Health, Education, and Welfare, Public Health Service, National Institutes of Health, 1979. [Google Scholar]
- 4.Goff DC Jr., Khan SS, Lloyd-Jones D, et al. Bending the curve in cardiovascular disease mortality: Bethesda + 40 and beyond. Circulation 2021;143:837–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.CCSP Coordinating Center. Community Cardiovascular Surveillance Program: Final Report to the National Heart, Lung, and Blood Institute. University of Maryland; Baltimore MD: 1984. [Google Scholar]
- 6.Benjamin EJ, Virani SS, Callaway CW, et al. Heart disease and stroke statistics—2018 update: a report from the American Heart Association. Circulation 2018;137:e67–492. [DOI] [PubMed] [Google Scholar]
- 7.Benjamin EJ, Muntner P, Alonso A, et al. Heart disease and stroke statistics—2019 update: a report from the American Heart Association. Circulation 2019;139:e56–528. [DOI] [PubMed] [Google Scholar]
- 8.Virani SS, Alonso A, Benjamin EJ, et al. Heart disease and stroke statistics—2020 update: a report from the American Heart Association. Circulation 2020;141:e139–596. [DOI] [PubMed] [Google Scholar]
- 9.Rosamond WD, Chambless LE, Folsom AR, et al. Trends in the incidence of myocardial infarction and in mortality due to coronary heart disease, 1987 to 1994. N Engl J Med 1998;339:861–7. [DOI] [PubMed] [Google Scholar]
- 10.Levy D, Thom TJ. Death rates from coronary disease—progress and a puzzling paradox. N Engl J Med 1998;339:915–7. [DOI] [PubMed] [Google Scholar]
- 11.Gornel DL. Rates of death from coronary heart disease. N Engl J Med 1999;340:730. [DOI] [PubMed] [Google Scholar]
- 12.Gray DP, Steele R, Sweeney K. Rates of death from coronary heart disease. N Engl J Med 1999; 340:730–2. [PubMed] [Google Scholar]
- 13.Tunstall-Pedoe H, Kuulasmaa K, Mähönen M, Tolonen H, Ruokokoski E, Amouyel P. Contribution of trends in survival and coronary-event rates to changes in coronary heart disease mortality: 10-year results from 37 WHO MONICA project populations. Monitoring trends and determinants in cardiovascular disease. Lancet 1999;353:1547–57. [DOI] [PubMed] [Google Scholar]
- 14.Rosamond WD, Chambless LE, Folsom AR. Survival trends, coronary event rates, and the MONICA project. Monitoring trends and determinants in cardiovascular disease. Lancet 1999; 354:864–5. [DOI] [PubMed] [Google Scholar]
- 15.Goldberg RJ, Yarzebski J, Lessard D, Gore JM. A two-decades (1975 to 1995) long experience in the incidence, in-hospital and long-term case-fatality rates of acute myocardial infarction: a community-wide perspective. J Am Coll Cardiol 1999;33:1533–9. [DOI] [PubMed] [Google Scholar]
- 16.Roger VL, Jacobsen SJ, Weston SA, et al. Trends in the incidence and survival of patients with hospitalized myocardial infarction, Olmsted County, Minnesota, 1979 to 1994. Ann Intern Med 2002;136:341–8. [DOI] [PubMed] [Google Scholar]
- 17.Rosamond WD, Chambless LE, Heiss G, et al. Twenty-two-year trends in incidence of myocardial infarction, coronary heart disease mortality, and case fatality in 4 US communities, 1987–2008. Circulation 2012;125:1848–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arora S, Stouffer GA, Kucharska-Newton AM, et al. Twenty-year trends and sex differences in young adults hospitalized with acute myocardial infarction. Circulation 2019;139:1047–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Loehr LR, Rosamond WD, Chang PP, Folsom AR, Chambless LE. Heart failure incidence and survival (from the Atherosclerosis Risk in Communities study). Am J Cardiol 2008;101: 1016–22. [DOI] [PubMed] [Google Scholar]
- 20.Folsom AR, Yamagishi K, Hozawa A, Chambless LE. Absolute and attributable risks of heart failure incidence in relation to optimal risk factors. Circ Heart Fail 2009;2:11–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Loehr LR, Rosamond WD, Poole C, et al. The potentially modifiable burden of incident heart failure due to obesity: the atherosclerosis risk in communities study. Am J Epidemiol 2010;172: 781–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Agarwal SK, Simpson RJ Jr., Rautaharju P, et al. Relation of ventricular premature complexes to heart failure (from the Atherosclerosis Risk in Communities [ARIC] study). Am J Cardiol 2012; 109:105–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Avery CL, Loehr LR, Baggett C, et al. The population burden of heart failure attributable to modifiable risk factors: the ARIC (Atherosclerosis Risk in Communities) study. J Am Coll Cardiol 2012;60:1640–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gupta DK, Shah AM, Castagno D, et al. Heart failure with preserved ejection fraction in African Americans: the ARIC (Atherosclerosis Risk in Communities) study. J Am Coll Cardiol HF 2013;1: 156–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shah AM, Cheng S, Skali H, et al. Rationale and design of a multicenter echocardiographic study to assess the relationship between cardiac structure and function and heart failure risk in a biracial cohort of community-dwelling elderly persons: the Atherosclerosis Risk in Communities study. Circ Cardiovasc Imaging 2014;7:173–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shah AM, Claggett B, Loehr LR, et al. Heart failure stages among older adults in the community: the Atherosclerosis Risk in Communities study. Circulation 2017;135:224–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nambi V, Liu X, Chambless LE, et al. Troponin T and N-terminal pro-B-type natriuretic peptide: a biomarker approach to predict heart failure risk—the Atherosclerosis Risk in Communities study. Clin Chem 2013;59:1802–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Agarwal SK, Chambless LE, Ballantyne CM, et al. Prediction of incident heart failure in general practice: the Atherosclerosis Risk in Communities (ARIC) study. Circ Heart Fail 2012;5:422–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chambless LE, Heiss G, Shahar E, Earp MJ, Toole J. Prediction of ischemic stroke risk in the Atherosclerosis Risk in Communities study. Am J Epidemiol 2004;160:259–69. [DOI] [PubMed] [Google Scholar]
- 30.Chang PP, Chambless LE, Shahar E, et al. Incidence and survival of hospitalized acute decompensated heart failure in four US communities (from the Atherosclerosis Risk in Communities study). Am J Cardiol 2014;113:504–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rosamond WD, Chang PP, Baggett C, et al. Classification of heart failure in the Atherosclerosis Risk in Communities (ARIC) study: a comparison of diagnostic criteria. Circ Heart Fail 2012;5:152–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chang PP, Wruck LM, Shahar E, et al. Trends in hospitalizations and survival of acute decompensated heart failure in four US communities (2005–2014): ARIC study community surveillance. Circulation 2018;138:12–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Arora S, Stouffer GA, Kucharska-Newton A, et al. Fifteen-year trends in management and outcomes of non-ST-segment-elevation myocardial infarction among Black and White patients: the ARIC Community Surveillance study, 2000–2014. J Am Heart Assoc 2018;7:e010203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Folsom AR, Stevens J, Schreiner PJ, McGovern PG. Body mass index, waist/hip ratio, and coronary heart disease incidence in African Americans and whites. Atherosclerosis Risk in Communities study investigators. Am J Epidemiol 1998;148:1187–94. [DOI] [PubMed] [Google Scholar]
- 35.Sorlie PD, Sharrett AR, Patsch W, et al. The relationship between lipids/lipoproteins and atherosclerosis in African Americans and whites: the Atherosclerosis Risk in Communities study. Ann Epidemiol 1999;9:149–58. [DOI] [PubMed] [Google Scholar]
- 36.Fox ER, Alnabhan N, Penman AD, et al. Echocardiographic left ventricular mass index predicts incident stroke in African Americans: Atherosclerosis Risk in Communities (ARIC) study. Stroke 2007;38:2686–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Diez Roux AV, Merkin SS, Arnett D, et al. Neighborhood of residence and incidence of coronary heart disease. N Engl J Med 2001;345: 99–106. [DOI] [PubMed] [Google Scholar]
- 38.Diez-Roux AV, Nieto FJ, Muntaner C, et al. Neighborhood environments and coronary heart disease: a multilevel analysis. Am J Epidemiol 1997;146:48–63. [DOI] [PubMed] [Google Scholar]
- 39.Jones DW, Chambless LE, Folsom AR, et al. Risk factors for coronary heart disease in African Americans: the Atherosclerosis Risk in Communities study, 1987–1997. Arch Intern Med 2002; 162:2565–71. [DOI] [PubMed] [Google Scholar]
- 40.Folsom AR, Yatsuya H, Nettleton JA, Lutsey PL, Cushman M, Rosamond WD. Community prevalence of ideal cardiovascular health, by the American Heart Association definition, and relationship with cardiovascular disease incidence. J Am Coll Cardiol 2011;57:1690–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.The ARIC Study Group. High-resolution B-mode ultrasound scanning methods in the Atherosclerosis Risk in Communities study (ARIC). J Neuroimaging 1991;1:68–73. [PubMed] [Google Scholar]
- 42.Li R, Duncan BB, Metcalf PA, et al. B-mode-detected carotid artery plaque in a general population. Atherosclerosis Risk in Communities (ARIC) study investigators. Stroke 1994;25:2377–83. [DOI] [PubMed] [Google Scholar]
- 43.Li R, Cai J, Tegeler C, Sorlie P, Metcalf PA, Heiss G. Reproducibility of extracranial carotid atherosclerotic lesions assessed by B-mode ultrasound: the Atherosclerosis Risk in Communities study. Ultrasound Med Biol 1996;22:791–9. [DOI] [PubMed] [Google Scholar]
- 44.Nambi V, Chambless L, Folsom AR, et al. Carotid intima-media thickness and presence or absence of plaque improves prediction of coronary heart disease risk: the ARIC (Atherosclerosis Risk in Communities) study. J Am Coll Cardiol 2010;55: 1600–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sharrett AR, Sorlie PD, Chambless LE, et al. Relative importance of various risk factors for asymptomatic carotid atherosclerosis versus coronary heart disease incidence: the Atherosclerosis Risk in Communities study. Am J Epidemiol 1999; 149:843–52. [DOI] [PubMed] [Google Scholar]
- 46.Wagenknecht L, Wasserman B, Chambless L, et al. Correlates of carotid plaque presence and composition as measured by MRI: the Atherosclerosis Risk in Communities study. Circ Cardiovasc Imaging 2009;2:314–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rohatgi A, Berry JD. Assessing clinical utility of carotid intima-media thickness on the basis of reclassification. J Am Coll Cardiol 2010;56:1068–9. author reply 1069–70. [DOI] [PubMed] [Google Scholar]
- 48.Rundek T, Salameh MJ. Carotid plaque assessment: a bumpy road to improved risk prediction. J Am Coll Cardiol 2010;56:1069. author reply 1069–70. [DOI] [PubMed] [Google Scholar]
- 49.Stein JH, Johnson HM. Carotid intima-media thickness, plaques, and cardiovascular disease risk: implications for preventive cardiology guidelines. J Am Coll Cardiol 2010;55:1608–10. [DOI] [PubMed] [Google Scholar]
- 50.Den Ruijter HM, Peters SA, Anderson TJ, et al. Common carotid intima-media thickness measurements in cardiovascular risk prediction: a meta-analysis. JAMA 2012;308:796–803. [DOI] [PubMed] [Google Scholar]
- 51.Lorenz MW, Polak JF, Kavousi M, et al. Carotid intima-media thickness progression to predict cardiovascular events in the general population (the PROG-IMT collaborative project): a meta-analysis of individual participant data. Lancet 2012;379:2053–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Goff DC Jr., Lloyd-Jones DM, Bennett G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014;63:2935–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Brinjikji W, Huston J 3rd., Rabinstein AA, Kim GM, Lerman A, Lanzino G. Contemporary carotid imaging: from degree of stenosis to plaque vulnerability. J Neurosurg 2016;124:27–42. [DOI] [PubMed] [Google Scholar]
- 54.Krist AH, Davidson KW, Mangione CM, et al. Screening for asymptomatic carotid artery stenosis: US Preventive Services Task Force Recommendation Statement. JAMA 2021;325:476–81. [DOI] [PubMed] [Google Scholar]
- 55.Chaturvedi S To screen or not to screen for carotid stenosis: is that the question? JAMA Neurol 2021;78:383–4. [DOI] [PubMed] [Google Scholar]
- 56.Brunner G, Virani SS, Sun W, et al. Associations between carotid artery plaque burden, plaque characteristics, and cardiovascular events: the ARIC Carotid Magnetic Resonance Imaging Study. JAMA Cardiol 2021;6:79–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hwang SJ, Ballantyne CM, Sharrett AR, et al. Circulating adhesion molecules VCAM-1, ICAM-1, and E-selectin in carotid atherosclerosis and incident coronary heart disease cases: the Atherosclerosis Risk in Communities (ARIC) study. Circulation 1997;96:4219–25. [DOI] [PubMed] [Google Scholar]
- 58.Folsom AR, Nieto FJ, McGovern PG, et al. Prospective study of coronary heart disease incidence in relation to fasting total homocysteine, related genetic polymorphisms, and B vitamins: the Atherosclerosis Risk in Communities (ARIC) study. Circulation 1998;98:204–10. [DOI] [PubMed] [Google Scholar]
- 59.Folsom AR, Desvarieux M, Nieto FJ, Boland LL, Ballantyne CM, Chambless LE. B vitamin status and inflammatory markers. Atherosclerosis 2003; 169:169–74. [DOI] [PubMed] [Google Scholar]
- 60.Matsushita K, Selvin E, Bash LD, Astor BC, Coresh J. Risk implications of the new CKD Epidemiology Collaboration (CKD-EPI) equation compared with the MDRD study equation for estimated GFR: the Atherosclerosis Risk in Communities (ARIC) study. Am J Kidney Dis 2010;55: 648–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Grams ME, Astor BC, Bash LD, Matsushita K, Wang Y, Coresh J. Albuminuria and estimated glomerular filtration rate independently associate with acute kidney injury. J Am Soc Nephrol 2010;21:1757–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Saunders JT, Nambi V, de Lemos JA, et al. Cardiac troponin T measured by a highly sensitive assay predicts coronary heart disease, heart failure, and mortality in the Atherosclerosis Risk in Communities study. Circulation 2011;123: 1367–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Oluleye OW, Folsom AR, Nambi V, Lutsey PL, Ballantyne CM, Troponin T. B-type natriuretic peptide, C-reactive protein, and cause-specific mortality. Ann Epidemiol 2013;23:66–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Folsom AR, Lutsey PL, Nambi V, et al. Troponin T, NT-proBNP, and venous thromboembolism: the Longitudinal Investigation of Thromboembolism Etiology (LITE). Vasc Med 2014;19: 33–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.McEvoy JW, Chen Y, Nambi V, et al. High-sensitivity cardiac troponin T and risk of hypertension. Circulation 2015;132:825–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jia X, Sun W, Hoogeveen RC, et al. High-sensitivity troponin I and incident coronary events, stroke, heart failure hospitalization, and mortality in the ARIC study. Circulation 2019;139:2642–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Keller T, Münzel T, Blankenberg S. Making it more sensitive: the new era of troponin use. Circulation 2011;123:1361–3. [DOI] [PubMed] [Google Scholar]
- 68.Folsom AR, Chambless LE, Ballantyne CM, et al. An assessment of incremental coronary risk prediction using C-reactive protein and other novel risk markers: the Atherosclerosis Risk in Communities study. Arch Intern Med 2006;166: 1368–73. [DOI] [PubMed] [Google Scholar]
- 69.Cohen JC, Boerwinkle E, Mosley TH Jr., Hobbs HH. Sequence variations in PCSK9, low LDL, and protection against coronary heart disease. N Engl J Med 2006;354:1264–72. [DOI] [PubMed] [Google Scholar]
- 70.Coventry A, Bull-Otterson LM, Liu X, et al. Deep resequencing reveals excess rare recent variants consistent with explosive population growth. Nat Commun 2010;1:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Romeo S, Pennacchio LA, Fu Y, et al. Population-based resequencing of ANGPTL4 uncovers variations that reduce triglycerides and increase HDL. Nat Genet 2007;39:513–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dehghan A, Köttgen A, Yang Q, et al. Association of three genetic loci with uric acid concentration and risk of gout: a genome-wide association study. Lancet 2008;372:1953–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Köttgen A, Glazer NL, Dehghan A, et al. Multiple loci associated with indices of renal function and chronic kidney disease. Nat Genet 2009;41: 712–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rasmussen-Torvik LJ, Li M, Kao WH, et al. Association of a fasting glucose genetic risk score with subclinical atherosclerosis: the Atherosclerosis Risk in Communities (ARIC) study. Diabetes 2011;60:331–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hsu CC, Kao WL, Steffes MW, et al. Genetic variation of glucose transporter-1 (GLUT1) and albuminuria in 10,278 European Americans and African Americans: a case-control study in the Atherosclerosis Risk in Communities (ARIC) study. BMC Med Genet 2011;12:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gottesman RF, Schneider AL, Albert M, et al. Midlife hypertension and 20-year cognitive change: the Atherosclerosis Risk in Communities Neurocognitive Study. JAMA Neurol 2014;71: 1218–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gottesman RF, Albert MS, Alonso A, et al. Associations between midlife vascular risk factors and 25-year incident dementia in the Atherosclerosis Risk in Communities (ARIC) Cohort. JAMA Neurol 2017;74:1246–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gottesman RF, Schneider AL, Zhou Y, et al. Association between midlife vascular risk factors and estimated brain amyloid deposition. JAMA 2017;317:1443–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ding J, Davis-Plourde KL, Sedaghat S, et al. Antihypertensive medications and risk for incident dementia and Alzheimer’s disease: a meta-analysis of individual participant data from prospective cohort studies. Lancet Neurol 2020;19:61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lazar RM, Howard VJ, Kernan WN, et al. A primary care agenda for brain health: a scientific statement from the American Heart Association. Stroke 2021. March 15 [E-pub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Brancati FL, Kao WH, Folsom AR, Watson RL, Szklo M. Incident type 2 diabetes mellitus in African American and white adults: the Atherosclerosis Risk in Communities study. JAMA 2000;283: 2253–9. [DOI] [PubMed] [Google Scholar]
- 82.Selvin E, Steffes MW, Zhu H, et al. Glycated hemoglobin, diabetes, and cardiovascular risk in nondiabetic adults. N Engl J Med 2010;362: 800–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.2. Classification and diagnosis of diabetes: Standards of Medical Care in Diabetes—2018. Diabetes Care 2018;41:S13–27. [DOI] [PubMed] [Google Scholar]
- 84.Muntner P, He J, Astor BC, Folsom AR, Coresh J. Traditional and nontraditional risk factors predict coronary heart disease in chronic kidney disease: results from the Atherosclerosis Risk in Communities study. J Am Soc Nephrol 2005;16:529–38. [DOI] [PubMed] [Google Scholar]
- 85.Bash LD, Coresh J, Köttgen A, et al. Defining incident chronic kidney disease in the research setting: the ARIC study. Am J Epidemiol 2009;170:414–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lamprea-Montealegre JA, Sharrett AR, Matsushita K, Selvin E, Szklo M, Astor BC. Chronic kidney disease, lipids and apolipoproteins, and coronary heart disease: the ARIC study. Atherosclerosis 2014;234:42–6. [DOI] [PubMed] [Google Scholar]
- 87.Matsushita K, Sang Y, Ballew SH, et al. Cardiac and kidney markers for cardiovascular prediction in individuals with chronic kidney disease: the Atherosclerosis Risk in Communities study. Arterioscler Thromb Vasc Biol 2014;34:1770–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lazarus B, Chen Y, Wilson FP, et al. Proton pump inhibitor use and the risk of chronic kidney disease. JAMA Intern Med 2016;176:238–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis 2002;39:S1–266. [PubMed] [Google Scholar]
- 90.Sarnak MJ, Levey AS, Schoolwerth AC, et al. Kidney disease as a risk factor for development of cardiovascular disease: a statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Circulation 2003;108:2154–69. [DOI] [PubMed] [Google Scholar]
- 91.Krop JS, Coresh J, Chambless LE, et al. A community-based study of explanatory factors for the excess risk for early renal function decline in blacks vs whites with diabetes: the Atherosclerosis Risk in Communities study. Arch Intern Med 1999;159:1777–83. [DOI] [PubMed] [Google Scholar]
- 92.Foster MC, Coresh J, Fornage M, et al. APOL1 variants associate with increased risk of CKD among African Americans. J Am Soc Nephrol 2013; 24:1484–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Matsushita K, Coresh J, Sang Y, et al. Estimated glomerular filtration rate and albuminuria for prediction of cardiovascular outcomes: a collaborative meta-analysis of individual participant data. Lancet Diabetes Endocrinol 2015;3: 514–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Matsushita K, van der Velde M, Astor BC, et al. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet 2010;375:2073–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Alonso A, Agarwal SK, Soliman EZ, et al. Incidence of atrial fibrillation in whites and African-Americans: the Atherosclerosis Risk in Communities (ARIC) study. Am Heart J 2009;158:111–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Rooney MR, Soliman EZ, Lutsey PL, et al. Prevalence and characteristics of subclinical atrial fibrillation in a community-dwelling elderly population: the ARIC study. Circ Arrhythm Electrophysiol 2019;12:e007390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Loehr LR, Soliman EZ, Poon AK, et al. The prevalence of atrial fibrillation on 48-hour ambulatory electrocardiography in African Americans compared to Whites: the Atherosclerosis Risk in Communities (ARIC) study. Am Heart J 2019; 216:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Huxley RR, Lopez FL, Folsom AR, et al. Absolute and attributable risks of atrial fibrillation in relation to optimal and borderline risk factors: the Atherosclerosis Risk in Communities (ARIC) study. Circulation 2011;123:1501–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Office of Extramural Research. Research Portfolio Online Reporting Tools: Expenditures and Results (RePORTER), version 2020.9. National Institutes of Health. 2020. Available at: https://reporter.nih.gov/. Accessed April 28, 2021. [Google Scholar]
- 100.Bell EJ, Lutsey PL, Basu S, et al. Lifetime risk of venous thromboembolism in two cohort studies. Am J Med 2016;129:339.e19–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Steffen LM, Folsom AR, Cushman M, Jacobs DR Jr., Rosamond WD. Greater fish, fruit, and vegetable intakes are related to lower incidence of venous thromboembolism: the Longitudinal Investigation of Thromboembolism Etiology. Circulation 2007;115:188–95. [DOI] [PubMed] [Google Scholar]
- 102.Folsom AR, Olson NC, Lutsey PL, Roetker NS, Cushman M. American Heart Association’s Life’s Simple 7 and incidence of venous thromboembolism. Am J Hematol 2015;90:E92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Blackburn H, Epstein FH. History of the Council on Epidemiology and Prevention, American Heart Association. The pursuit of epidemiology within the American Heart Association: prehistory and early organization. Circulation 1995;91:1253–62. [DOI] [PubMed] [Google Scholar]
- 104.Angell SY, McConnell MV, Anderson CAM, et al. The American Heart Association 2030 impact goal: a presidential advisory from the American Heart Association. Circulation 2020; 141:e120–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2019;74: 1376–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Carnethon MR, Pu J, Howard G, et al. Cardiovascular health in African Americans: a scientific statement from the American Heart Association. Circulation 2017;136:e393–423. [DOI] [PubMed] [Google Scholar]
- 107.Meschia JF, Bushnell C, Boden-Albala B, et al. Guidelines for the primary prevention of stroke. Stroke 2014;45:3754–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ganesh SK, Arnett DK, Assimes TL, et al. Genetics and genomics for the prevention and treatment of cardiovascular disease: update. Circulation 2013;128:2813–51. [DOI] [PubMed] [Google Scholar]
- 109.Pearson TA, Palaniappan LP, Artinian NT, et al. American Heart Association guide for improving cardiovascular health at the community level, 2013 update. Circulation 2013;127: 1730–53. [DOI] [PubMed] [Google Scholar]
- 110.Garg PK, O’Neal WT, Chen LY, et al. American Heart Association’s Life Simple 7 and risk of atrial fibrillation in a population without known cardiovascular disease: the ARIC (Atherosclerosis Risk in Communities) study. J Am Heart Assoc 2018;7:e008424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Shah AM, Claggett B, Folsom AR, et al. ideal cardiovascular health during adult life and cardiovascular structure and function among the elderly. Circulation 2015;132:1979–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sengeløv M, Cheng S, Biering-Sørensen T, et al. Ideal cardiovascular health and the prevalence and severity of aortic stenosis in elderly patients. J Am Heart Assoc 2018;7:e007234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Khera AV, Emdin CA, Drake I, et al. Genetic risk, adherence to a healthy lifestyle, and coronary disease. N Engl J Med 2016;375:2349–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sorlie PD, Bild DE, Lauer MS. Cardiovascular epidemiology in a changing world—challenges to investigators and the National Heart, Lung, and Blood Institute. Am J Epidemiol 2012;175: 597–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Roger VL, Boerwinkle E, Crapo JD, et al. Strategic transformation of population studies: recommendations of the working group on epidemiology and population sciences from the National Heart, Lung, and Blood Advisory Council and Board of External Experts. Am J Epidemiol 2015;181:363–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kucharska-Newton AM, Heiss G, Ni H, et al. Identification of heart failure events in Medicare claims: the Atherosclerosis Risk in Communities (ARIC) study. J Card Fail 2016;22:48–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Walker KA, Gottesman RF, Wu A, et al. Systemic inflammation during midlife and cognitive change over 20 years: the ARIC study. Neurology 2019;92:e1256–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Selvaraj S, Myhre PL, Vaduganathan M, et al. Application of diagnostic algorithms for heart failure with preserved ejection fraction to the community. J Am Coll Cardiol HF 2020;8:640–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Teramoto K, Nadruz W Jr., Matsushita K, et al. Mid-to late-life time-averaged cumulative blood pressure and late-life cardiac structure, function, and heart failure. Hypertension 2020;76:808–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Yu B, Zheng Y, Nettleton JA, Alexander D, Coresh J, Boerwinkle E. Serum metabolomic profiling and incident CKD among African Americans. Clin J Am Soc Nephrol 2014;9:1410–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Roetker NS, Pankow JS, Bressler J, Morrison AC, Boerwinkle E. Prospective study of epigenetic age acceleration and incidence of cardiovascular disease outcomes in the ARIC study (Atherosclerosis Risk in Communities). Circ Genom Precis Med 2018;11:e001937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Tin A, Yu B, Ma J, et al. Reproducibility and variability of protein analytes measured using a multiplexed modified aptamer assay. J Appl Lab Med 2019;4:30–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Bose M, Wu C, Pankow JS, et al. Evaluation of microarray-based DNA methylation measurement using technical replicates: the Atherosclerosis Risk in Communities (ARIC) study. BMC Bioinformatics 2014;15:312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.National Heart, Lung, and Blood Institute. Third party involvement in NHLBI-supported clinical trials and other population-based studies: awardee/contractor third party related issues. Available at: https://www.nhlbi.nih.gov/grants-and-training/policies-and-guidelines/third-party-involvement-in-nhlbi-supported-clinical-trials-and-other-population-based-studies-awardee-contractor-third-party-related-issues. Accessed April 28, 2021.
- 125.Kucharska-Newton A, Bullo M, Loop M, et al. Completeness in the abstraction of cardiac biomarkers and cardiac pain data from electronic health records (EHR). Findings from the Atherosclerosis Risk in Communities (ARIC) study. Circulation 2018;137:AMP23. [Google Scholar]
- 126.Bogle BM, Rosamond WD, Folsom AR, et al. Feasibility of electronic health records-based community surveillance of cardiovascular disease: findings from the Atherosclerosis Risk in Communities study. Circulation 2018;137: AMP21. [Google Scholar]
- 127.ClinicalTrials.gov. Aging and cognitive health evaluation in elders (ACHIEVE). Available at: https://clinicaltrials.gov/ct2/show/NCT03243422?term=NCT03243422&draw=2&rank=1. Accessed April 28, 2021.
- 128.Gottesman RF, Rawlings AM, Sharrett AR, et al. Impact of differential attrition on the association of education with cognitive change over 20 years of follow-up: the ARIC neurocognitive study. Am J Epidemiol 2014;179:956–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.National Center for Biotechnology Information. Database of Genotypes and Phenotypes (dbGaP). National Library of Medicine. Available at: https://www.ncbi.nlm.nih.gov/gap/. Accessed April 28, 2021. [Google Scholar]
- 130.Coady SA, Mensah GA, Wagner EL, Goldfarb ME, Hitchcock DM, Giffen CA. Use of the National Heart, Lung, and Blood Institute Data Repository. N Engl J Med 2017;376:1849–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Psaty BM, O’Donnell CJ, Gudnason V, et al. Cohorts for Heart and Aging Research in Genomic Epidemiology (CHARGE) consortium: design of prospective meta-analyses of genome-wide association studies from 5 cohorts. Circ Cardiovasc Genet 2009;2:73–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Brody JA, Morrison AC, Bis JC, et al. Analysis commons, a team approach to discovery in a big-data environment for genetic epidemiology. Nat Genet 2017;49:1560–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Farchi G, Capocaccia R, Verdecchia A, Menotti A, Keys A. Risk factors changes and coronary heart disease in an observational study. Int J Epidemiol 1981;10:31–40. [DOI] [PubMed] [Google Scholar]
- 134.Danesh J, Erqou S, Walker M, et al. The Emerging Risk Factors Collaboration: analysis of individual data on lipid, inflammatory and other markers in over 1.1 million participants in 104 prospective studies of cardiovascular diseases. Eur J Epidemiol 2007;22:839–69. [DOI] [PubMed] [Google Scholar]
- 135.Toga AW, Neu SC, Bhatt P, Crawford KL, Ashish N. The Global Alzheimer’s Association Interactive Network. Alzheimers Dement 2016;12: 49–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.National Heart, Lung, and Blood Institute. Population and Epidemiology Studies. Available at: https://www.nhlbi.nih.gov/science/population-and-epidemiology-studies. Accessed April 28, 2021.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


