Abstract
Background:
Frailty disproportionally affects people with HIV (PWH) and increased frailty in this already vulnerable population is associated with worse neurocognitive functioning. Whether frailty interacts with current and modifiable markers of HIV disease severity to synergistically increase risk for HIV-associated neurocognitive disorders (HAND), however, is unknown and important for informing the clinical care of aging PWH.
Setting:
UC San Diego’s HIV Neurobehavioral Research Program.
Methods:
Participants were 178 PWH evaluated between 2014 and 2019. HIV disease severity was measured by current CD4 count and plasma HIV RNA. HAND diagnoses were made according to the Frascati criteria using a 7-domain neuropsychological battery, and the Fried phenotype criteria were used to assess frailty syndrome (0–5 symptoms). The independent and interactive effects of frailty and current HIV disease severity (ie, CD4 count and plasma HIV RNA) on HAND were examined using multiple logistic regressions.
Results:
There was an interaction between CD4 count and frailty on HAND. Simple slopes showed that CD4 count and the likelihood of HAND were negatively associated at >1.25 symptoms of frailty, and conversely, frailty and HAND were negatively associated at 642 or less cells/mm3. There were no significant independent or interactive effects of plasma HIV RNA and frailty on the likelihood of HAND.
Conclusions:
In addition to monitoring CD4 count, assessing for frailty may be critical in older adults with HIV to potentially mitigate poor neurobehavioral outcomes. Longitudinal follow-up studies are needed to determine the directionality of these findings.
Keywords: aging, frailty, CD4, AIDS, neuropsychology, cognition
INTRODUCTION
As of 2018, almost 50% of people with HIV (PWH) in the United States were aged 50 years or older.1 Although incredible advancements in antiretroviral treatment have allowed PWH to achieve lifespans comparable with those without HIV, PWH are still more likely to have decreased healthspan.2,3 For example, older PWH are more likely to acquire age-related comorbidities at younger ages and have more non-HIV conditions, polypharmacy, and greater overall disease burden than their seronegative counterparts.4–6 HIV-associated neurocognitive disorders (HAND) are also highly prevalent among PWH and are associated with multifactorial detriments to quality of life.7
PWH also have higher rates of frailty and at younger ages, which further increases vulnerability to poor health-related outcomes.8–12 In fact, greater frailty seems to be linearly associated with worse neurocognitive functioning among PWH.13 Whether frailty confers even greater risk of HAND in the presence of other risk factors, however, is unknown. For example, among individuals with Alzheimer disease, frailty has been shown to moderate the relationship between neuropathological disease burden and clinical manifestation of dementia.14 Among PWH, examining a possible interaction between frailty and HIV disease severity on HAND is important for informing the clinical care of aging PWH because these are modifiable risk factors that, if intervened on, could improve the quality of life and prolong healthspan.
Thus, the current study examined the interaction between frailty and markers of current HIV disease severity on likelihood of HAND. We hypothesized that worse HIV disease severity (ie, low CD4 and/or high HIV RNA viral load) would be associated with greater risk of HAND only at higher levels of frailty and vice versa. Importantly, our aims and hypotheses are only concerned with markers of current disease severity (ie, CD4 cell count and HIV RNA viral load) because they are intervenable. Notably, we did not examine antiretroviral therapy (ART) use as a predictor because our sample consisted of very few individuals who were off ART (n = 5). Although low nadir CD4 has been previously established as a predictor of HAND,15 this measure is not modifiable. In addition, we examined frailty symptoms in a continuous fashion because prefrailty (1–2 symptoms) has been shown to predict poor health outcomes among PWH; thus, considering the full range of symptoms may be more sensitive in detecting a cumulative effect of frailty on HAND. In sum, examination of the independent and interactive effects of important modifiable risk factors (eg, frailty and HIV disease severity) on the risk of HAND is needed to further elucidate possible mechanisms for the development of HAND and inform multimodal comprehensive clinical interventions.
METHODS
Participants
Participants were 178 PWH (n = 84 no HAND; n = 94 HAND) enrolled in National Institutes of Health–funded research studies at the UC San Diego’s HIV Neurobehavioral Research Program from 2014 to 2019. The exclusion criteria were as follows: (1) presence of a neurological condition known to impact neurocognitive functioning (eg, non-HIV neurological disorder such as Parkinson disease or stroke); (2) history of head injury with loss of consciousness for longer than 30 minutes, seizure disorder, or central nervous system neoplasm; (3) diagnosis of a psychotic disorder or severe learning disorder (eg, Wide Range Achievement Test-Fourth Edition reading score <70); and (4) positive urine toxicology for illicit substances (excluding marijuana) on the day of testing. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee (UCSD Human Research Protections Program IRB00000355) and with the Helsinki Declaration of 1975 and its later amendments or comparable ethical standards.
HAND Classification
All participants completed a comprehensive neurocognitive assessment that evaluated 7 domains consistent with large-scale studies of HAND.16 Raw neurocognitive test scores were converted to T scores that adjusted for demographics based on established normative standards (age, education, sex, and race/ethnicity where available). Participants also completed a modified version of an activities of daily living self-report questionnaire (Lawton & Brody, 1969) and were classified as having a functional deficit if they reported decreased ability to independently perform 2 or more instrumental activities of daily (eg, managing finances and cooking). HAND diagnoses were made using Frascati criteria,17 which require cognitive impairment (>1 SD below the mean) in at least 2 neurocognitive domains. HAND classification includes 3 subtypes, as follows, in order of most severe to least severe: HIV-associated dementia (HAD, n = 5, 5%), mild neurocognitive disorder (n = 21, 22%), and asymptomatic neurocognitive impairment (n = 68, 72%). Because of the small numbers of individuals in some of the diagnostic levels, HAND diagnoses were evaluated dichotomously (HAND vs. no HAND).
Frailty Assessment
Frailty was assessed using the Fried Frailty Index,18 which consists of 5 domains of physical functioning: (1) unintentional weight loss (self-reported ≥10 pounds lost in the past year), (2) weakness (<20th percentile of normative sample for grip strength, stratified by sex and body mass index), (3) exhaustion (self-reported through elevations on items 7 and 9 of the Center for Epidemiologic Studies—Depression scale), (4) slow walking speed (<20th percentile of normative sample for total seconds to walk 15 feet, stratified by sex and height), and (5) low physical activity (<383 kcal/wk for men and <270 kcal/wk for women, self-reported through the International Physical Activity Questionnaire). Frailty was examined as a count of the presence of deficits in each of the 5 domains (possible range = 0–5).
Psychiatric Assessment
The Beck Depression Inventory Second Edition (BDI-II) was used to assess current depressive symptoms. The WHO Composite International Diagnostic Interview was administered to diagnose participants for current and lifetime major depressive disorder, as defined by the Fourth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV).
Neuromedical Assessment
All participants underwent a standardized medical history interview, neuromedical examination, and blood and urine collection. The HIV serological status was confirmed using enzyme-linked immunosorbent assay and Western blot test, and HIV RNA levels were measured in plasma by reverse transcription polymerase chain reaction (Amplicor; Roche Diagnostics, Indianapolis, IN; lower limit of quantitation of 50 copies/mL). The current CD4 count was measured in plasma using flow cytometry. Self-reported HIV disease and treatment variables included nadir CD4, AIDS status, estimated duration of HIV disease, and current ART regimen. Comorbid medical conditions (ie, hepatitis C, diabetes, hypertension, and hyperlipidemia) were determined by self-report and/or medical chart review when available.
Statistical Analyses
Demographics, psychiatric and neuromedical characteristics, and frailty symptoms were compared by HAND status using independent t test, Wilcoxon test, or χ2 test as appropriate. Nominal logistic regressions were conducted to examine the odds of having a HAND diagnosis as a function of number of frailty symptoms, markers of current HIV disease severity (CD4 and plasma HIV RNA viral load), and their interaction. Covariates were chosen based on univariable differences by HAND status. These included age, premorbid verbal IQ estimate, hypertension, hyperlipidemia, and diabetes. Pairwise and regression analyses were performed using JMP Pro, version 14.0.0 (JMP, Version <14.0.0>; SAS Institute Inc, Cary, NC, 1989–2007). The CAHOST Microsoft Excel 2013 workbook was used to determine regions of moderation significance using the Johnson–Neyman procedure.19
RESULTS
Participant characteristics are displayed in Table 1. HAND subtypes included 5% HAD (n = 5), 22% mild neurocognitive disorder (n = 21), and 72% asymptomatic neurocognitive impairment (n = 68). Participants with HAND were older (P = 0.01) and had lower estimated premorbid verbal IQs (P < 0.001) than those without HAND. HAND groups were comparable on all other demographic, psychiatric, and HIV disease characteristics (ps > 0.10). HAND groups did not differ on the Fried frailty status or the average number of Fried frailty symptoms; however, participants with HAND were more likely to meet the criteria for slow walking speed compared to those without HAND (P = 0.001). Participants with HAND also had higher rates of diabetes (P = 0.04), hypertension (P < 0.001), and hyperlipidemia (P = 0.04) than those without HAND.
TABLE 1.
Participant Demographics and Clinical Characteristics by HAND Diagnosis (N = 178)
| No HAND (N = 84) | HAND (N = 94) | P | |
|---|---|---|---|
| Demographics | |||
| Age | 52.15 (11.15) | 56.21 (10.63) | 0.01 |
| Sex (female) | 14 (17%) | 8 (9%) | 0.10 |
| Education (yr) | 14.33 (2.48) | 14.24 (2.68) | 0.47 |
| Race/ethnicity (non-Hispanic white) | 34 (40%) | 44 (47%) | 0.40 |
| Premorbid verbal IQ estimate* | 107.20 (14.41) | 99.27 (12.69) | <0.001 |
| Unemployed | 51 (57%) | 67 (70%) | 0.08 |
| Psychiatric characteristics | |||
| BDI-II Score | 8.24 (8.73) | 8.68 (0.99) | 0.39 |
| Current MDD (yes) | 8 (10%) | 5 (6%) | 0.26 |
| Lifetime MDD (yes) | 47 (56%) | 59 (63%) | 0.31 |
| HIV disease characteristics | |||
| History of AIDS (yes) | 46 (55%) | 62 (66%) | 0.10 |
| log10 plasma HIV RNA | 1.29 (0.66) | 1.13 (0.68) | 0.26 |
| Plasma HIV RNA detectability† | 4 (5%) | 6 (6%) | 0.74 |
| Current CD4 count | 641 [508–863] | 576 [421–809] | 0.26 |
| Nadir CD4 count | 197 [53–352] | 174 [30–303] | 0.29 |
| Estimated years living with HIV | 18.59 (9.11) | 19.56 (9.15) | 0.46 |
| On ART (yes) | 82 (98%) | 91 (97%) | 0.92 |
| Fried Frailty | |||
| Average number of frailty symptoms | 1.16 (1.14) | 1.04 (0.98) | 0.79 |
| Frailty | 5 (6%) | 10 (11%) | 0.26 |
| Prefrailty | 38 (46%) | 43 (46%) | 0.99 |
| Weight loss (yes) | 11 (13%) | 14 (15%) | 0.73 |
| Weakness (yes) | 11 (13%) | 13 (14%) | 0.89 |
| Exhaustion (yes) | 31 (37%) | 26 (28%) | 0.19 |
| Slow walking speed (yes) | 3 (4%) | 18 (19%) | 0.001 |
| Low physical activity (yes) | 17 (20%) | 26 (28%) | 0.25 |
| Comorbid health conditions | |||
| Hepatitis C (yes) | 21 (26%) | 17 (18%) | 0.22 |
| Diabetes (yes) | 9 (11%) | 21 (23%) | 0.04 |
| Hypertension (yes) | 31 (38%) | 59 (64%) | <0.001 |
| Hyperlipidemia (yes) | 34 (42%) | 53 (58%) | 0.04 |
All values presented as mean (SD), median [IQR], or n (%); groups were compared using independent t test, Wilcoxon test, or χ2 test as appropriate.
Values in bold indicate P-value <0.05.
WRAT-4 = Wide Range Achievement Test-Fourth Edition.
≤50 copies/mL.
ART, antiretroviral therapy; BDI-II, Beck Depression Inventory—Second Edition; MDD, major depressive disorder.
A logistic regression model was used to examine the independent and interactive effects of plasma CD4 count and the number of frailty symptoms on the likelihood of a HAND diagnosis, covarying for factors that differed between HAND groups (ie, age, estimated premorbid verbal IQ, hypertension, hyperlipidemia, and diabetes). The interaction between CD4 and number of frailty symptoms was significant (b = −0.001, SE = 0.001, and P = 0.037). Independent main effects of CD4 (b = −0.0004, SE = 0.0006, and P = 0.510) and number of frailty symptoms (b = 0.014, SE = 0.190, and P = 0.942) were not significant. Higher estimated premorbid verbal IQ significantly related to lower likelihood of HAND (b = −0.047, SE = 0.013, and P = 0.001), but the other covariates did not significantly explain the variance in the likelihood of HAND (age: b = 0.037, SE = 0.19, and P = 0.050; hypertension: b = 0.315, SE = 0.196, and P = 0.107; hyperlipidemia: b = −0.040, SE = 0.200, and P = 0.843; and diabetes: b = 0.131, SE = 0.252, and P = 0.603).
The critical level in which the interaction between CD4 and number of frailty symptoms was significant was probed using the Johnson–Neyman technique. When examining frailty as the moderator, results revealed that the negative relationship between CD4 and the likelihood of HAND was significant only among participants with frailty greater than 1.25 symptoms (Fig. 1A). That is, among participants with more than 1.25 frailty symptoms, lower CD4 related to higher likelihood of HAND. For clinical interpretability, Figure 1B illustrates simple slopes of the interaction effect at higher (2 or more symptoms) and lower levels of frailty (0 or 1 symptoms). When examining CD4 as the moderator, results showed that the positive relationship between frailty symptoms and the likelihood of HAND was significant only among participants with less than or equal to 642 CD4 cells/mm3 (Fig. 1C). That is, among participants with ≤642 CD4 cells/mm3, a higher number of frailty symptoms related to higher likelihood of HAND (Fig. 1D).
FIGURE 1.
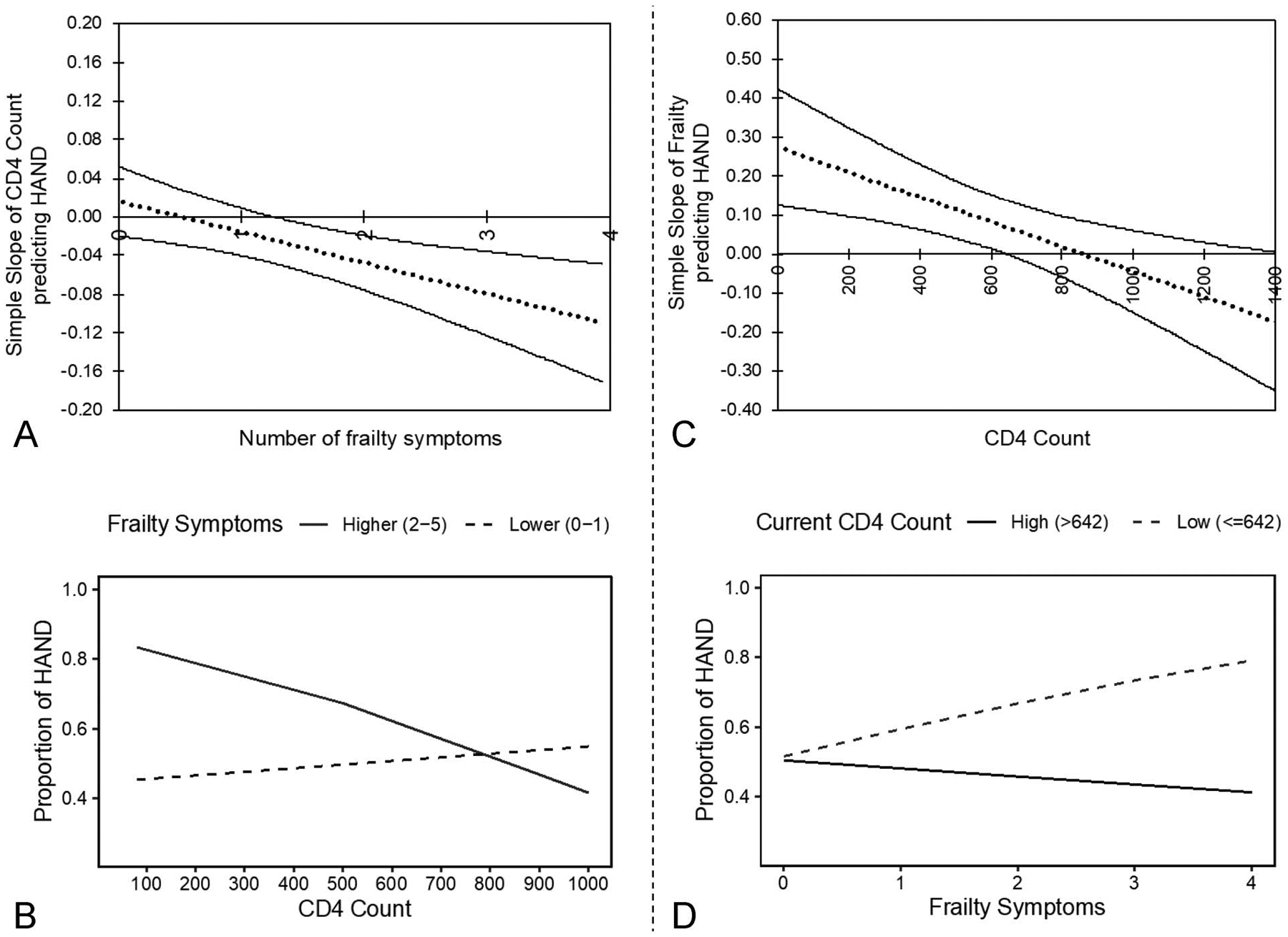
A, Using the Johnson–Neyman technique, the simple slope of CD4 count is only significant above 1.25 symptoms of frailty, such that the lower CD4 count relates to higher likelihood of HAND among participants with >1.25 frailty symptoms. B, The negative relationship between CD4 count and likelihood of HAND among participants with 2–5 frailty symptoms is depicted by the solid line. By contrast, the CD4 count is not significantly related to the likelihood of HAND among participants with 0–1 frailty symptoms, as depicted by the dashed line. C, Using the Johnson–Neyman technique, the simple slope of frailty is only significant at or below a CD4 count of 642, such that higher frailty relates to higher likelihood of HAND among participants with ≤642 CD4 cells. D, The positive relationship between frailty symptoms and the likelihood of HAND among participants with ≤642 CD4 cells, as depicted by the dashed line. By contrast, frailty symptoms are not significantly related to the likelihood of HAND among participants with >642 CD4 cells, as depicted by the solid line.
The next logistic regression revealed no significant independent or interactive effects of plasma HIV RNA viral load and number of frailty symptoms on the likelihood of HAND (ps > 0.05). Notably, the distribution of plasma HIV RNA viral load values was non-normal (right skewed) and restricted in range because most participants were virally suppressed (n = 168, 94% of the entire sample). When we removed those who were virally unsuppressed (n = 21), the moderation between CD4 and frailty still held (b = −0.002, SE = 0.001, and P = 0.015).
DISCUSSION
This study investigated the independent and interactive effects of current HIV disease severity (ie, CD4 cell count, plasma HIV RNA viral load) and the number of frailty symptoms on the likelihood of HAND. We found a significant interaction between CD4 and frailty on HAND. When frailty was examined as the moderating variable, the relationship between CD4 and HAND became significant at 1.25 or more symptoms (2 symptoms for clinical relevance), such that lower CD4 was associated with increased likelihood of HAND. Inversely, when CD4 was examined as the moderator, the relationship between frailty and HAND became significant at 642 or less cells/mm3, such that higher number of frailty symptoms was associated with increased likelihood of HAND. The plasma HIV RNA viral load did not associate with HAND or interact with frailty.
Results highlight the importance of considering both frailty and CD4 in PWH at risk of neurocognitive impairment. We found that frailty is critical even at low levels. This is consistent with previous research showing that 1 to 2 symptoms of frailty (prefrailty) is associated with neurocognitive impairments among community-dwelling adults20 and is predictive of neurocognitive decline over 2 years among PWH.21 Furthermore, rates of frailty and prefrailty in our sample were consistent with other samples of PWH.22 Our results additionally suggest that frailty, in conjunction with low CD4, can be especially problematic for cognition to the extent of a diagnosable neurocognitive disorder. Interestingly and unexpectedly, CD4 was shown to be important even up to 642 cells/mm3, which is at a level traditionally accepted as within the normal range (500–1500 cells/mm3). Although nadir CD4 has been demonstrated to predict HAND,15 this study uniquely establishes a relationship between current CD4 (interacting with frailty) and HAND, which has significant clinical implications because unlike nadir, current cell count is modifiable.
To the best of our knowledge, this is the first study to investigate the interaction of frailty and markers of current HIV disease severity on HAND. Several limitations of this study should be noted to guide future studies. First, the sample of relatively healthy individuals with few having plasma HIV RNA viral load less than 50 copies/mL. This restricted range decreases our confidence in concluding that HIV RNA viral load does not have the same interactive effect with frailty on HAND as CD4 at this time. Cerebrospinal fluid HIV RNA viral load was considered because its levels (at least as high as plasma HIV RNA levels viral load) have been shown to correlate with HAND.23 However, because of the small sample size of participants with CSF data (n = 48), analyses on CSF values were not done at this time but would be important to investigate in future studies. Another limitation is that the number of frailty symptoms in our sample only ranged from 0 to 4 (5 possible), which may have impacted the magnitude of its interactive effect with CD4. Finally, our sample consisted of few females (12%), which limits our ability to generalize our findings to these individuals.
In summary, our findings support an interaction between frailty and CD4 count on the likelihood of HAND. Clinically, providers may be impactful in mitigating HAND by screening for frailty in the context of relatively lower CD4 counts or more closely follow CD4 counts in the context of 2 or more symptoms of frailty. A CD4 count below 642 cells mm3 should prompt clinicians to assess for frailty, and identification of 2 frailty symptoms in PWH should prompt for closer monitoring of CD4 to identify or possibility mitigate neurocognitive impairment. Future studies examining the moderating effect of frailty on neurodegeneration and HAND would be an important next step to further investigate the role of frailty on HIV disease and cognition.
ACKNOWLEDGMENTS
* The San Diego HIV Neurobehavioral Research Center [HNRC] group is affiliated with the University of California, San Diego, the Naval Hospital, San Diego, and the Veterans Affairs San Diego Healthcare System and includes the following: director: Robert K. Heaton, PhD, codirector: Igor Grant, MD, associate directors: J. Hampton Atkinson, MD; Ronald J. Ellis, MD, PhD; and Scott Letendre, MD, center manager: Jennifer Iudicello, PhD; Donald Franklin, Jr.; and Melanie Sherman, neuroassessment Core: Ronald J. Ellis, MD, PhD (PI); Scott Letendre, MD; Thomas D. Marcotte, PhD; Christine Fennema-Notestine, PhD.; Debra Rosario, MPH; and Matthew Dawson, neurobiology core: Cristian Achim, MD, PhD (PI); Ana Sanchez, PhD, and Adam Fields, PhD, neurogerm core: Sara Gianella Weibel, MD (PI); David M. Smith, MD; Rob Knight, PhD; and Scott Peterson, PhD, developmental core: Scott Letendre, MD (PI) and J. Allen McCutchan, participant accrual and retention unit: J. Hampton Atkinson, MD (PI) Susan Little, MD and Jennifer Marquie-Beck, MPH, data management and information systems unit: Lucila Ohno-Machado, PhD (PI) and Clint Cushman, statistics unit: Ian Abramson, PhD (PI); Florin Vaida, PhD (Co-PI); Anya Umlauf, MS; and Bin Tang, MS.
The views expressed in this article are those of the authors and do not reflect the official policy or position of the Department of the Navy, Department of Defense, nor the US Government.
Supported by the National Institute of Mental Health [Grant number R01 MH099987, P30 MH062512], the National Institute on Drug Abuse [Grant number T32 DA031098 (stipend support to NSS)], and the National Institute on Alcohol Abuse and Alcoholism [Grant number F31 AA027198 (stipend support to EWP)]. The HIV Neurobehavioral Research Center (HNRC) is supported by Center award P30MH062512 from NIMH.
Footnotes
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Centers for Disease Control and Prevention. HIV Surveillance Report, 2018; vol 31; 2020. Available at http://www.cdc.gov/hiv/library/reports/hiv-surveillance.html. Accessed March 2020. [Google Scholar]
- 2.Deeks SG. HIV infection, inflammation, immunosenescence, and aging. Annu Rev Med. 2011;62:141–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moore RC, Fazeli PL, Jeste DV, et al. Successful cognitive aging and health-related quality of life in younger and older adults infected with HIV. AIDS Behav. 2014;18:1186–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kong AM, Pozen A, Anastos K, et al. Non-HIV comorbid conditions and polypharmacy among people living with HIV age 65 or older compared with HIV-negative individuals age 65 or older in the United States: a retrospective claims-based analysis. AIDS Patient Care STDs. 2019;33:93–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guaraldi G, Orlando G, Zona S, et al. Premature age-related comorbidities among HIV-infected persons compared with the general population. Clin Infect Dis. 2011;53:1120–1126. [DOI] [PubMed] [Google Scholar]
- 6.Rodriguez-Penney AT, Iudicello JE, Riggs PK, et al. Co-morbidities in persons infected with HIV: increased burden with older age and negative effects on health-related quality of life. AIDS Patient Care STDs. 2013;27:5–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jones JD, Kuhn T, Levine A, et al. Changes in cognition precede changes in HRQoL among HIV+ males: longitudinal analysis of the multicenter AIDS cohort study. Neuropsychology. 2019;33:370–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Desquilbet L, Jacobson LP, Fried LP, et al. HIV-1 infection is associated with an earlier occurrence of a phenotype related to frailty. J Gerontol A Biol Sci Med Sci. 2007;62:1279–1286. [DOI] [PubMed] [Google Scholar]
- 9.Erlandson KM, Schrack JA, Jankowski CM, et al. Functional impairment, disability, and frailty in adults aging with HIV-infection. Curr HIV/AIDS Rep. 2014;11:279–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Levett TJ, Cresswell FV, Malik MA, et al. Systematic review of prevalence and predictors of frailty in individuals with human immunodeficiency virus. J Am Geriatr Soc. 2016;64:1006–1014. [DOI] [PubMed] [Google Scholar]
- 11.Rubtsova AA, Sabbag S, Sundermann E, et al. Frailty and neurocognitive impairment: impacts on quality of life in HIV. J Assoc Nurses AIDS Care. 2020;31:290–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Piggott DA, Erlandson KM, Yarasheski KE. Frailty in HIV: epidemiology, biology, measurement, interventions, and research needs. Curr HIV/AIDS Rep. 2016;13:340–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oppenheim H, Paolillo EW, Moore RC, et al. Neurocognitive functioning predicts frailty index in HIV. Neurology. 2018;91:e162–e170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wallace LMK, Theou O, Godin J, et al. Investigation of frailty as a moderator of the relationship between neuropathology and dementia in Alzheimer’s disease: a cross-sectional analysis of data from the Rush Memory and Aging Project. Lancet Neurol. 2019;18:177–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ellis RJ, Badiee J, Vaida F, et al. CD4 nadir is a predictor of HIV neurocognitive impairment in the era of combination antiretroviral therapy. AIDS. 2011;25:1747–1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heaton RK, Clifford DB, Franklin DR Jr, et al. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology. 2010;75:2087–2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Antinori A, Arendt G, Becker JT, et al. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007;69: 1789–1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–M156. [DOI] [PubMed] [Google Scholar]
- 19.Carden SW, Holtzman NS, Strube MJ. CAHOST: an Excel workbook for facilitating the johnson-neyman technique for two-way interactions in multiple regression. Front Psychol. 2017;8:1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Robertson DA, Savva GM, Coen RF, et al. Cognitive function in the prefrailty and frailty syndrome. J Am Geriatr Soc. 2014;62: 2118–2124. [DOI] [PubMed] [Google Scholar]
- 21.Paolillo EW, Sun-Suslow N, Pasipanodya EC, et al. Pre-frailty predicts cognitive decline at 2-year follow-up in persons living with HIV. J Neurovirol. 2019;1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Önen N, Patel P, Baker J, et al. Frailty and pre-frailty in a contemporary cohort of HIV-infected adults. J Frailty Aging. 2014;3:158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Letendre S. Central nervous system complications in HIV disease: HIV-associated neurocognitive disorder. Top Antivir Med. 2011;19: 137–142. [PMC free article] [PubMed] [Google Scholar]


