Abstract
Case series
Patients: Femal, 39-year-old • Femal, 64-year-old • Femal, 52-year-old • Femal, 34-year-old • Femal, 90-year-old
Final Diagnosis: Lipedema
Symptoms: Edema • pain
Medication: —
Clinical Procedure: Medications
Specialty: Endocrinology and Metabolic
Objective:
Unusual clinical course
Background:
Lipedema is a chronic progressive disease characterized by the abnormal accumulation of fat in the subcutaneous region. Both medical and surgical treatments have been described in international guidelines; however, the current literature is biased toward promoting liposuction as the primary treatment of lipedema, and this can lead to the misapprehension that liposuction is the only form of definitive treatment.
Case Reports:
In the present study, we report 5 cases at various stages of the evolution of lipedema, all with different therapeutic objectives. Case 1 reported having persistent bruising and pain, case 2 reported pain and fat deposition, case 3 reported night cramps and discomfort, case 4 reported leg thickening, and case 5 reported redness in the legs. All of were diagnosed with lipedema in different evolution stages. Our purpose was to demonstrate the possibility of non-surgical therapy, as well as to improve signs and symptoms of lipedema, using the QuASiL questionnaire and measuring changes in volumes and proportions. Good aesthetic outcomes improve both social and psychological status.
Conclusions:
Currently, there are many described therapies available for lipedema. Liposuction surgery for lipedema should be considered one possible tool. Treatment objectives can be different for each patient. It is imperative to understand each patient’s needs in order to offer the best therapy attainable that meets patient requirements and induces a better quality of life. Non-surgical treatment of lipedema is feasible in selected cases, and it can meet the criteria for achieving selected clinical objectives.
Keywords: Lipedema, Lymphedema, Obesity, Obesity Management
Background
Lipedema is an inherited, chronic, progressive disease characterized by the abnormal accumulation of fat in subcutaneous tissue, mainly in the lower and upper limbs [1]. It is a potentially aggressive disease because it limits mobility and damages the lymphatic vascular system [2], leading to deformities and loss of quality of life. Its prevalence in females is believed to be 11–39% [3–6]. The intensity of presentation is greatest in the inflammatory phase, manifesting as pain [7], sensitivity to touch, edema [8], chronic fatigue, and unprovoked ecchymosis [5,9].
Lipedema is often misdiagnosed as obesity, lymphedema, or chronic venous disease, although these diagnoses can often occur concomitantly [1,10,11]. Lipedema is not influenced substantially by diet or exercise [12]. In the absence of targeted treatment, there is no significant improvement in body dis-proportions or in signs and symptoms. Signs of lipedema are disproportionately increased limb volume, adipose tissue on the limbs, symmetric tissue, palpable tissue nodules, painful tissue (not always), and limb swelling (pitting or non-pitting), and the hands and feet not affected [13].
Clinical and surgical treatments for lipedema have been described extensively in international guidelines. The aim of all of these is to improve the signs and symptoms, to reduce volumes and disproportions of the affected limbs, and to prevent progression [14–18]. Manual lymphatic drainage and elastic compression have been reported to alleviate symptoms [5].
Diuretics are not indicated, as there is no accumulation of liquid. However, there is thickening of adipose tissue and there is no standard pharmacological protocol to treat this [19]. Diet has been presented as an adjuvant therapy [3]; however, like physical exercise, it has been little studied [19]. Most often the most commonly used diet strategies are anti-inflammatory, low-carbohydrate, and ketogenic diets [20]. The current literature is biased in the sense that it presents liposuction as the main treatment for lipedema [3], and this can lead to the misapprehension that it is its only definitive treatment.
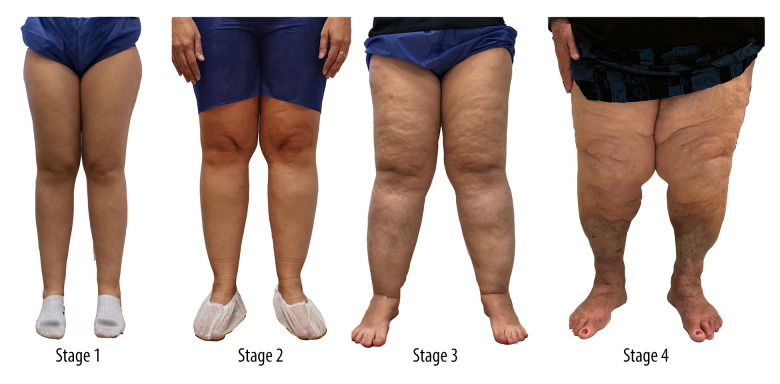
The treatment of lipedema must be individualized and must consider the technical limitations and clinical aspects of each patient. In the present study, we describe a series of 5 cases of lipedema in various stages (Figure 1 [21]), all of which were successfully treated clinically.
Figure 1.
Classification of lipedema by evolutionary stages [21]. Stage 1: normal skin with an enlarged hypodermis; Stage 2: unevenness of the skin texture with pleats of fat and large piles of tissue growing like unencapsulated masses; Stage 3: hardening and thickening of the subcutaneous with large nodules and protrusion of cushions/accumulations of fat, especially in the thighs and around the knees; Stage 4: lipedema with lymphedema, so-called lipolymphedema.
Stage I lipedema is characterized by normal skin with enlarged hypodermis. Stage II is defined as uneven skin texture with pleats of fat and large piles of tissue growing like un-encapsulated masses. Stage III is characterized by hardening and thickening of the subcutaneous tissue with large nodules and protrusion of cushions/accumulations of fat, especially in the thighs and around the knees. Finally, stage IV is associated with lymphedema, and is also called lipolymphedema.
Case Reports
Case 1, a Patient in Stage 1
The patient was a 39-year-old woman with persistent ‘bruising’ and pain that worsened with standing. Edema of her lower limbs worsened in the afternoon. She also reported ‘cellulite’ and fat on her legs that started 5 years prior to presentation, when she started taking contraceptives, in addition to finding her legs ‘disproportionately thick’. On the Lipedema Symptom Assessment Questionnaire (QuASiL: minimum 0, maximum 150 [22]) she presented 89 points. Superficial and deep-color Doppler ultrasound examinations of the lower limbs (EDCV-MMII) revealed only sparse reticular structures. Lymphoscintigraphy was within normal limits.
On past surgical history, she reported having undergone ‘hydrolipo’ 3 years prior and varicose vein surgery 5 years prior.
Physical examination revealed sparse reticular veins, absence of Godet and Stemmer signs, and pain on palpation of areas with greater fat deposition. The bioimpedance test (Tanita®, BC-601, Illinois, United States) showed a body mass index (BMI) of 21.2 kg/cm2 with 29.8% body fat and lower-limb volume of 19 668.55 mL.
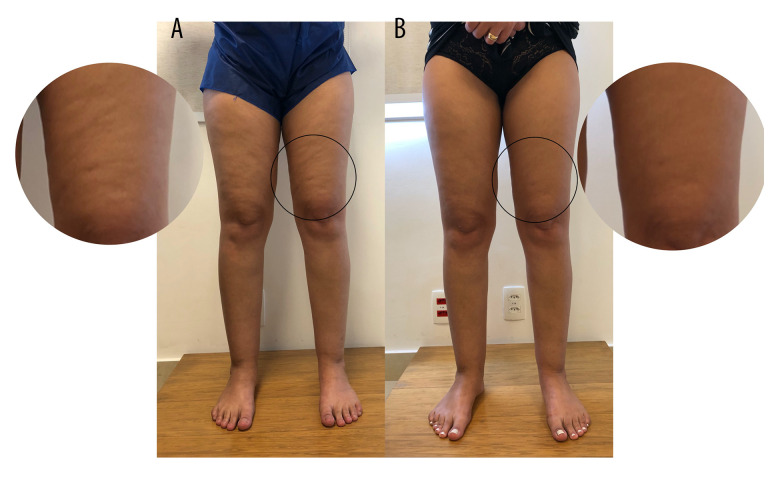
A diagnosis of grade I lipedema was made and clinical treatment was suggested based on the aforementioned guidelines. These included anti-inflammatory dietary measures, regular aquatic physical exercise, manual lymphatic drainage, and antioxidant phytotherapeutics in regular-usage doses. The patient returned with symptom improvement, with a score of 81 in QuASiL (improvement of 8.9%) and volumetric decrease of 491.62 mL. After 8 months of clinical treatment, she achieved 58 points on the QuASiL (improvement of 34.83%) and total volume loss of 1230.54 mL. There was significant improvement in her aesthetic concerns of gynecoid lipodystrophy (Figure 2). Her final BMI was 21.4 kg/cm2.
Figure 2.
Patient with stage 1 lipedema before (A) and after (B) treatment, showing significant aesthetic improvement of gynecoid lipodystrophy (cellulitis), although it was not the main objective of the treatment.
Case 2, a Patient in Stage 1
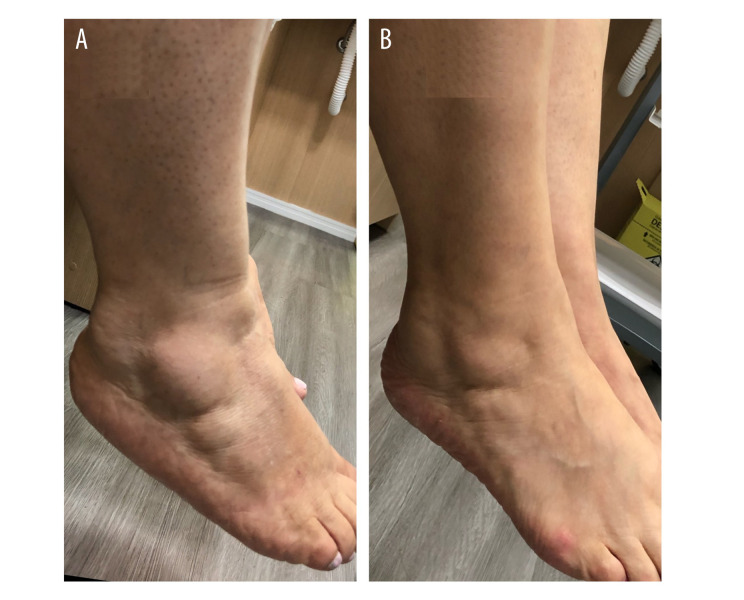
The patient was a 64-year-old woman who presented with pain and fat in her legs since adolescence. On physical examination, there were fatty nodules in both lateral malleoli that were painful to palpation; her QuASiL score was 44. Stage 1 lipedema was diagnosed, and clinical treatment was provided with anti-inflammatory dietary measures, aquatic physical exercise, manual lymphatic drainage, and antioxidant herbal medicines. After 1 month of treatment, she reported significant improvement in the nodules (Figure 3) and symptoms, scoring 11 points on the QuASiL.
Figure 3.
Decrease in painful fat nodules in the lateral malleolus in a patient with stage 1 lipedema. (A) Before. (B) After.
Case 3, a Patient in Stage 2
A 52-year-old woman presented with a 7-month history of ankle edema. She reported discomfort in her calf, with a sensation that she could feel her veins, as well as sporadic night cramps and the appearance of spider veins in her right thigh. She had been taking chlortalidone and phlebotonics. On physical examination, she had telangiectasias, severe pain on palpation of the medial pre-tibial and genicular fat, absence of Godet or Stemmer signs, and 103 points on the QuASiL. EDCV-MMII revealed bilateral varicose tributaries, without reflux in saphenous veins or the deep venous system. The bioimpedance exam showed a BMI of 29.5 kg/cm2, 38.6% body fat, and lower-limb volume of 23 737.71 mL. Stage 2 lipedema was diagnosed.
Testing for IgG hypersensitivity to food was requested, which identified yogurt, cow’s milk, cashews, poppy seeds, and mushrooms as the strongest triggers of hypersensitivity.
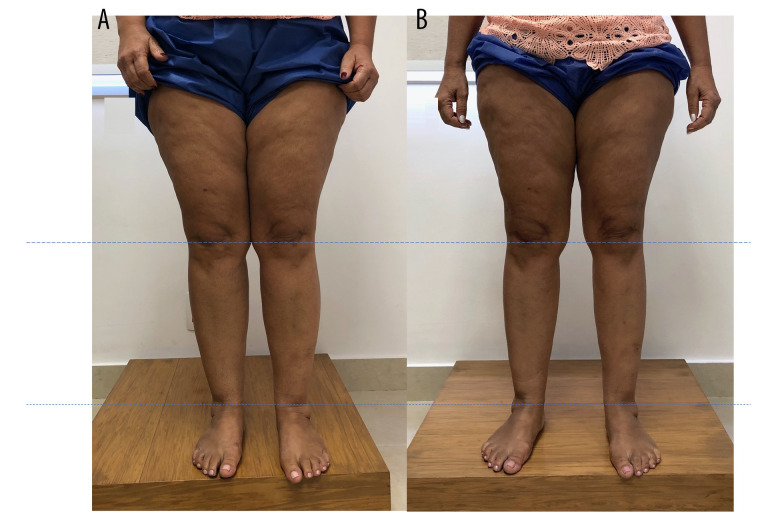
Clinical treatment was initiated, including changes in lifestyle habits, combined with an anti-inflammatory diet and progression to a low-carbohydrate diet associated with aquatic exercise, manual lymphatic drainage, and antioxidant herbal medicines. After 1 month, she returned with 69 points on the QuASiL (33% improvement) and volume loss of 968.96 mL. After 5 months of clinical treatment, she scored 22 points on the QuASiL (78.64% improvement), with total limb volume of 21 817.51 mL (a difference of 1920.2 mL) (Figure 4).
Figure 4.
Patient with stage 2 lipedema (A), showing a slight improvement and decrease in fat deposition in the lower limbs (B).
Case 4, a Patient in Stage 3
The patient was a 34-year-old woman with longstanding ‘stubborn constitution’ and weight gain after menarche. She reported that, during adolescence, her legs thickened despite her being very active. Dieting caused her to lose truncal fat, without changes in the lower limbs.
She reported a maximum weight of 95 kg, which reduced with exercise to a minimum of 65 kg, but without decreasing the volume of the legs, which remained the same.
Physical examination revealed absence of Godet and Stemmer signs, deposition of fat that was tender to palpation in the lower limbs, and 115 points on the QuASiL. EDCV-MMII revealed small varicosities in the thighs and legs, without significant reflux, with a measured dermal thickness of 22.4 mm and 21.2 mm in the right and left pre-tibial regions, respectively.
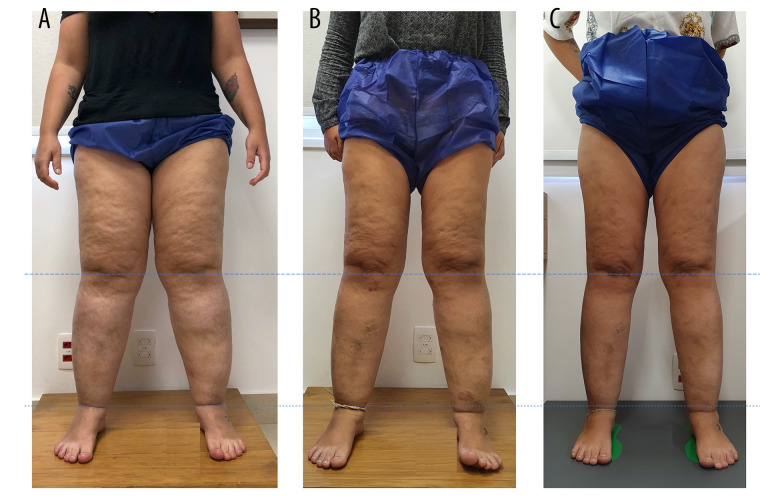
The bioimpedance exam showed a BMI of 34.2 kg/cm2, 41.6% body fat, and lower-limb volume calculated by bioimpedance of 32 572.21 mL. She was diagnosed with grade III lipedema. Although surgical treatment with tumescent liposuction was indicated, the patient initially opted for clinical treatment that included an anti-inflammatory diet followed by a ketogenic diet associated with aquatic physical exercise, manual lymphatic drainage, and antioxidant herbal medicines. After 1 month of treatment, she returned with 107 points on the QuASiL (6.9% improvement), and a volume loss of 3972.44 mL. After 3 months, she scored 86 points on the QuASiL (25.21% improvement), with a volumetric loss of 6472.47 mL. After 6 months, she achieved 76 points on the QuASiL (33.91% improvement), with volumetric loss of 6,346.34 mL. After 11 months, she achieved 59 points in QuASiL (48.6% improvement) with final volume of 21 832.31 mL (volumetric loss 10 739.90 mL) (Figures 5, 6).
Figure 5.
Patient with stage 3 lipedema (A), with significant improvement in fat deposition and volume in the lower limbs in 6 months (B) and 11 months (C).
Figure 6.
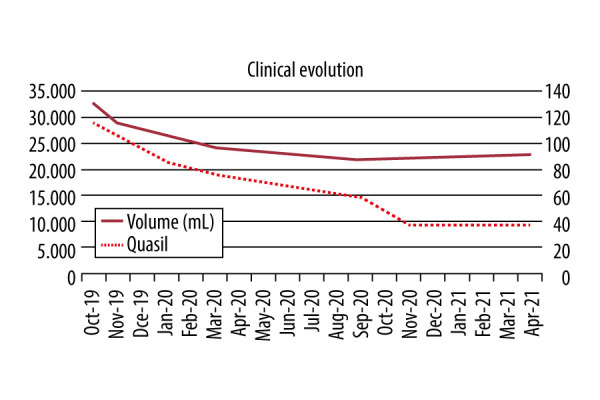
Clinical evolution of case 4.
Case 5, a patient in stage 4
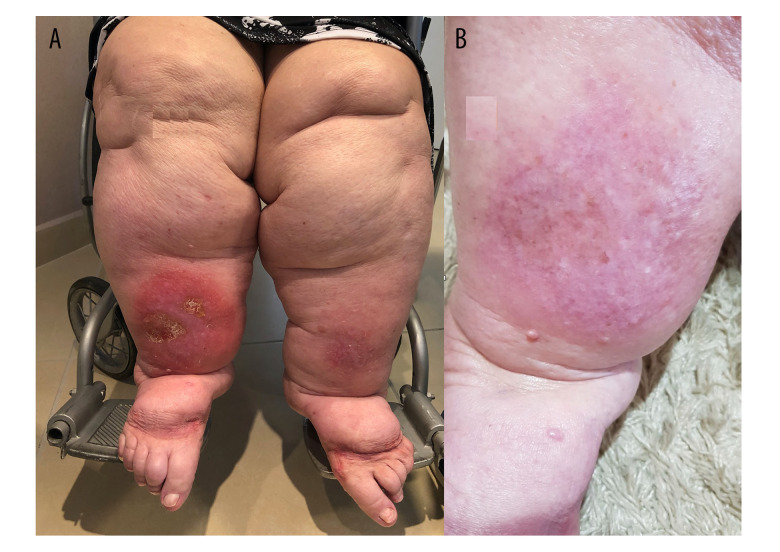
A 90-year-old woman presented with ‘redness in the leg’ of 1-year duration, with no improvement following unspecified treatments. She reported that, since adolescence, her legs had been slowly growing in volume and she had never managed to prevent progression. Two years prior to presentation, orthopedic surgical treatment of the knees was indicated after significant weight loss. She reported loss of mobility, which progressed to inability to walk. She was unable to lose weight despite changes in eating habits. She scored 75 points on the QuASiL. On physical exam she presented with redness, lymphangitis, erysipelas, severe columnar form, and painful symmetric fat tissue. Pitting edema over her feet suggested associated lymphedema, and the bilateral edema also suggested that the right lymphangitis was not the only cause of lymph-edema (Figure 7). EDCV-MMII revealed only small superficial varicosities, while pre-tibial skin and subcutaneous thickness of 57.27 mm left and 55.9 mm right confirmed lipedema [23]. Lymphoscintigraphy was not requested because of infection. After starting clinical measures to treat lipedema (anti-inflammatory diet, moderate exercise, antioxidants and lymphatic drainage) and lymphedema (lymphatic drainage) associated with the treatment of erysipelas (amoxicillin-clavulanate and enoxaparin), there was quick resolution of erysipelas and stasis dermatitis.
Figure 7.
Patient with lipolymphedema, stage 4 lipedema, with erysipelas that was difficult to treat before (A) and after (B) non-surgical treatment for lipedema.
Discussion
There is a need to define the objective of the treatment for lipedema in cooperation with the patient. These objectives can vary widely: improvement of signs and symptoms, weight loss, fat, reduction of limb volume, and improvement of mobility and aesthetics. Often, the patient’s aesthetic desire unduly overrides the objective need to improve mobility and symptoms, and to prevent progression. Using objective clinical tools for analysis and monitoring of lipedema, such as the QuASiL [22], symptom monitoring questionnaires that are validated and culturally adapted, and volumetric measurements of limbs and bioimpedance, it is possible to quantify clinical progression. BMI does not differentiate obesity from lipedema. Nevertheless, we have no tool do measure the aesthetic aspects of the disease.
The top priority in the treatment of lipedema, regardless of type, is the recovery and preservation of mobility [3]. Medial contact of the thighs when walking, which already occurs in the early stages of the disease, is difficult to quantify; however, it can be considered the beginning of mobility limitation.
Secondarily, the objective is to improve symptoms, as assessed by the QuASiL questionnaire, in addition to volumetric and proportional improvements. Finally, the aesthetic improvement that is eventually achieved improves psychological status.
In the first case, it can be argued that lipedema had been treated surgically by previous liposuction; however, the return of lipedema symptoms demonstrated the need for clinical treatment even after surgical treatment. The prior varicose vein surgery highlights comorbidities and overlap of symptoms. She experienced volumetric, symptomatic, and aesthetic improvements. In the second case, symptoms and fat nodules showed significant improvement. In the third case, in addition to the symptomatic improvement, there was significant volume loss. In the fourth patient, who had grade I obesity on the basis of BMI, there was disproportion and substantial fat deposition in the lower limbs. In this case, lipedema mimicked obesity; nevertheless, there was significant volumetric decrease in addition to symptomatic improvement. In the fifth case, the initial objective was the treatment of refractory erysipelas; nevertheless, clinical treatment facilitated the resolution of the persistent infection.
The clinical treatment of lipedema involves, in most aspects, the search for a healthy lifestyle, including changes in attitudes. These measures are mostly harmless to those who do not have lipedema and can be suggested to all patients who do not have contraindications. Although compression bandages or garments are widely recognized treatment, they were not used for the present patients because of high pain patients reported before lowering inflammation. We opted for lymphatic drainage as a strategy the patients would find more acceptable. Hesperidin and diosmin are antioxidant treatments currently suggested for lipedema treatment [13], and use of quercetin, pycnogenol [24], flavonoids [25], rutosides [26], and butcher’s broom [27] was previously reported in treatment of lipedema and/or lymphedema. It was suggested that a combined sequence of natural complex antioxidants sets up a reductant cascade to match the progressive oxidative stresses of the free-radical cascade [28].
In patients with lipedema with comorbid diseases such as varicose veins of the lower limbs (53% of cases) or obesity (50% of cases) [29], failure in the clinical treatment of lipedema may cause failure in the treatment of the associated disease [30]. The high prevalence of the disease and co-presentation with other common diseases, as well as its numerous clinical variations, justify the consideration of clinical treatment as initial and supporting treatments for patients with lipedema.
Surgical treatment should not be performed in the inflammatory phase of the disease [10], and the best time for decision-making is when the patient has reached the best symptomatic state. Undoubtedly, the advent of liposuction that protects the lymphatic system brought new perspectives and hope in the treatment of this disease [31]; nevertheless, liposuction does not exclude non-surgical therapy.
This series of cases exemplifies and contextualizes lipedema, which until recently had few therapeutic options in the daily practice of vascular surgeons. Our study has several limitations inherent to a series of cases: not all aspects of lipedema were addressed, and patients were selected to exemplify chosen aspects.
Conclusions
Currently, there are many described therapies available for lipedema. Liposuction surgery for lipedema should be considered a possible tool to be used and not the only available treatment. Treatment objectives should be individualized for each patient. It is imperative to understand each patient’s needs to offer the best therapy attainable that meets patient requirements and induces a better quality of life. Some patients may demand better aesthetics, which in some cases is reachable without surgery. Most patients report pain and discomfort, symptoms that can improve without surgery. Other patients may be concerned about leg volume. Volume and disproportion reduction may seem challenging without surgery, but we have shown it is possible. Complications of lipedema, like ulcers, lymphangitis, and erysipelas, can be the main patient concern and can be treated without liposuction. Because of all available therapies, lipedema requires an integrated multispecialty and multidisciplinary teamwork patient-centered approach.
Non-surgical treatment of lipedema is feasible in selected cases, and it can meet the criteria for achieving selected clinical objectives.
Footnotes
Department and Institution Where Work Was Done
Cirurgia Vascular, Amato – Instituto de Medicina Avançada, São Paulo, SP, Brazil.
Declaration of Figures’ Authenticity
All figures submitted have been created by the authors who confirm that the images are original with no duplication and have not been previously published in whole or in part.
References:
- 1.Amato ACM, Markus DV, dos Santos RV. [Lipedema associated with obesity, lymphedema and venous insufficiency: A case report.] Diagnóstico e Tratamento. 2020;25(11):4–8. [in Portuguese] [Google Scholar]
- 2.Ma W, Gil HJ, Escobedo N, et al. Platelet factor 4 is a biomarker for lymphatic-promoted disorders. JCI Insight. 2020;5(13):e135109. doi: 10.1172/jci.insight.135109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sandhofer M, Hanke CW, Habbema L, et al. Prevention of progression of lipedema with liposuction using tumescent local anesthesia: Results of an International Consensus Conference. Dermatologic Surgery. 2020;46(2):220–28. doi: 10.1097/DSS.0000000000002019. [DOI] [PubMed] [Google Scholar]
- 4.Shavit E, Wollina U, Alavi A. Lipoedema is not lymphoedema: A review of current literature. Int Wound J. 2018;15(6):921–18. doi: 10.1111/iwj.12949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fife CE, Maus EA, Carter MJ. Lipedema: A frequently misdiagnosed and misunderstood fatty deposition syndrome. Adv Skin Wound Care. 2010;23(2):81–84. doi: 10.1097/01.ASW.0000363503.92360.91. [DOI] [PubMed] [Google Scholar]
- 6.Marshall M, Schwahn-Schreiber C. [Prevalence of lipedema among working women in Germany.] Phlebologie. 2011;40(03):127–34. [in German] [Google Scholar]
- 7.Allen EV, Hines EA, Hines EA. Lipedema of the legs: A syndrome characterized by fat legs and orthostatic edema. Proc Staff Meet Mayo Clinic. 1940;15:184–87. [Google Scholar]
- 8.Okhovat JP, Alavi A. Lipedema: A review of the literature. Int J Low Extrem Wounds. 2015;14(3):262–67. doi: 10.1177/1534734614554284. [DOI] [PubMed] [Google Scholar]
- 9.Forner-Cordero I, Szolnoky G, Forner-Cordero A, Kemény L. Lipedema: An overview of its clinical manifestations, diagnosis and treatment of the dis-proportional fatty deposition syndrome – systematic review. Clin Obes. 2012;2(3–4):86–95. doi: 10.1111/j.1758-8111.2012.00045.x. [DOI] [PubMed] [Google Scholar]
- 10.Amato ACM. Is lipedema a unique entity? EC Clinical and Medical Cases Reports. 2020;2(3):1–7. [Google Scholar]
- 11.La Torre YSD, Wadeea R, Rosas V, Herbst KL. Lipedema: Friend and foe. Horm Mol Biol Clin Invest. 2018;33(1):1–10. doi: 10.1515/hmbci-2017-0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crescenzi R, Marton A, Donahue PMC, et al. Tissue sodium content is elevated in the skin and subcutaneous adipose tissue in women with lipedema. Obesity. 2018;26(2):310–17. doi: 10.1002/oby.22090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Herbst KL, Kahn LA, Iker E, et al. Standard of care for lipedema in the United States. Phlebology. :2021. doi: 10.1177/02683555211015887. [Online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Langendoen SI, Habbema L, Nijsten TEC, Neumann HAM. Lipoedema: From clinical presentation to therapy. A review of the literature. Br J Dermatol. 2009;161(5):980–86. doi: 10.1111/j.1365-2133.2009.09413.x. [DOI] [PubMed] [Google Scholar]
- 15.Pedersen BK, Saltin B. Evidence for prescribing exercise as therapy in chronic disease. Scand J Med Sci Sports. 2006;16(S1):3–63. doi: 10.1111/j.1600-0838.2006.00520.x. [DOI] [PubMed] [Google Scholar]
- 16.Wounds UK . Best Practice Guidelines: The management of lipoedema. London: Wounds UK; 2017. Available at: www.wounds-uk.com. [Google Scholar]
- 17.Damstra R, Habbema L, Hendrickx A, et al. Lipedema guidelines in the Netherlands. 2014. Available at: https://www.gdlymph.eu/assets/pdf/Dutch-lipoedema-guideline-2014.pdf.
- 18.Reich-Schupke S, Schmeller W, Brauer WJ, et al. S1 guidelines: Lipedema. J Dtsch Dermatol Ges. 2017;15(7):758–67. doi: 10.1111/ddg.13036. [DOI] [PubMed] [Google Scholar]
- 19.Alcolea J, Alonso Álvarez B, Arroyo Bielsa A, et al. [Consensus Document Lipedema 2018. Includes current status of lipedema 2019.] 2019. Available at: https://aelinfedema.org/wpcontent/uploads/2019/11/Consenso-Lipedema-v.Sep-2019pdf [in Spanish]
- 20.Keith L, Seo CA, Rowsemitt C, et al. Ketogenic diet as a potential intervention for lipedema. Med Hypotheses. 2021;146(11):110435. doi: 10.1016/j.mehy.2020.110435. [DOI] [PubMed] [Google Scholar]
- 21.Dayan E, Kim JN, Smith ML, et al. Lipedema – the disease they call FAT: An overview for clinicians. 1st edn. New york: Lipedema Simplified Publications; 2017. [Google Scholar]
- 22.Amato ACM, Amato FCM, Benitti DA, Dos Santos RV. Translation, cultural adaptation, and validation of a lipedema symptoms questionnaire. J Vasc Bras. 2020;19:e20200049. doi: 10.1590/1677-5449.200049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Amato ACM, Saucedo DZ, Santos K da S, Benitti DA. Ultrasound criteria for lipedema diagnosis. Phlebology. 2021;36(8):651–58. doi: 10.1177/02683555211002340. [DOI] [PubMed] [Google Scholar]
- 24.Herbst KL. Subcutaneous adipose tissue diseases: Dercum disease, lipedema, familial multiple lipomatosis, and madelung disease. In: Feingold KR, Anawalt B, Boyce A, et al., editors. South Dartmouth (MA): 2000. [PubMed] [Google Scholar]
- 25.Buck DW, Herbst KL. Lipedema: A relatively common disease with extremely common misconceptions. Plast Reconstr Surg Glob Open. 2016;4(9):e1043. doi: 10.1097/GOX.0000000000001043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Faga A. A double-blind, cross-over trial of O-(β-hydroxyethyl) rutosides (benzopyrones) in the treatment of lymphoedema of the arms and legs. Br J Plast Surg. 1988;41(4):444–45. doi: 10.1016/0007-1226(88)90092-6. [DOI] [PubMed] [Google Scholar]
- 27.Mondry TE NS. Bucher’s broom and selenium improve lipedema: A retrospective case study. Alternative & Integrative Medicine. 2013;02(04):1000119. [Google Scholar]
- 28.Fishman RB. Antioxidants and phytotherapy. Lancet. 1994;344(8933):1356. [Google Scholar]
- 29.Child AH, Gordon KD, Sharpe P, et al. Lipedema: An inherited condition. Am J Med Genet A. 2010;152A(4):970–76. doi: 10.1002/ajmg.a.33313. [DOI] [PubMed] [Google Scholar]
- 30.Földi M, Idiazabal G. The role of operative management of varicose veins in patients with lymphedema and/or lipedema of the legs. Lymphology. 2000;33(4):167–71. [PubMed] [Google Scholar]
- 31.Rapprich S, Dingler A, Podda M. [Liposuction is an effective treatment for lipedema – results of a study with 25 patients.] Journal of the German Society of Dermatology. 2011;9(1):33–41. doi: 10.1111/j.1610-0387.2010.07504.x. [in German] [DOI] [PubMed] [Google Scholar]