Abstract
Vaccination remains the most effective mechanism to reduce the impact of COVID‐19. Induction of neutralizing antibodies is a strong correlate of protection from infection and severe disease. An understanding of the cellular events that underpin the generation of effective neutralizing antibodies is therefore key to the development of efficacious vaccines that target emerging variants of concern. Analysis of the immune response to Severe Acute Respiratory Syndrome Coronavirus 2 (SARS‐CoV‐2) infection and vaccination has identified circulating T follicular helper cells (cTFH) as a robust correlate of the neutralizing antibody response. Here, we discuss the analysis of cTFH cells and their lymphoid counterparts in human humoral immune responses during COVID‐19, and in response to vaccination with SARS‐CoV‐2 spike. We discuss the phenotypic heterogeneity of cTFH cells and the utility of cTFH subsets as informative biomarkers for development of humoral immunity. We posit that the analysis of the most effective cTFH will be critical to inducing durable immunity to new variants of SARS‐CoV‐2.
Keywords: CD4+ T cells, COVID‐19, SARS‐CoV‐2, T follicular helper cells
Graphical Abstract
Review on the analysis of TFH cells during COVID‐19 infection and vaccination and the role of these cells as biomarkers of neutralising antibody development.
Abbreviations
- ACE2
angiotensin‐converting enzyme 2
- AIM
activation‐induced marker
- ASC
antibody‐secreting cells
- E
nucleoprotein
- GC
germinal center
- HCV
hepatitis C virus
- HPV
human papillomavirus
- M
membrane protein
- MBC
memory B cell
- N
envelope protein
- RBD
Receptor Binding Domain
- S
spike protein
- SARS‐CoV‐2
Severe Acute Respiratory Syndrome Coronavirus 2
- TFH cell
T follicular helper cell
- VOC
variant of concern.
1. INTRODUCTION
Severe Acute Respiratory Syndrome Coronavirus 2 (SARS‐CoV‐2) has caused more than 200 million infections and at least 4.3 million deaths since it emerged in late 2019 (https://covid19.who.int). Concerted global efforts have mapped the immune response to SARS‐CoV‐2 infection in great detail and facilitated the development of highly effective vaccines to control the spread of SARS‐CoV‐2 and the COVID‐19 pandemic.
SARS‐CoV‐2 is a single‐stranded positive‐sense RNA virus belonging to the Coronaviridae family. 1 This family encompasses six other coronaviruses known to infect humans: the endemic human coronaviruses (hCoV) 229E and NL63 (alphacoronaviruses) as well as HKU1 and OC43 (betacoronaviruses), and the highly pathogenic SARS‐CoV and MERS‐CoV (betacoronaviruses like SARS‐CoV‐2). Coronaviruses express 4 structural proteins: the surface glycoprotein spike (S), the membrane protein (M), the envelope protein (E), and the nucleoprotein (N). 1 The spike protein facilitates viral attachment and entry by engaging its cognate receptor, which in the case of SARS‐CoV‐2, SARS‐CoV, and NL63 is angiotensin‐converting enzyme 2 (ACE2). This interaction is specifically mediated by the Receptor Binding Domain (RBD) of S, located within the S1 subunit. The M and E proteins mediate virion assembly and structure, while N binds the genomic RNA to form a nucleocapsid. During infection, a plethora of additional nonstructural and accessory proteins are expressed that facilitate viral replication and immune evasion. 1
2. OVERVIEW OF HUMORAL IMMUNITY TO SARS‐COV‐2 INFECTION
Neutralizing antibodies can block the entry of SARS‐CoV‐2 into host cells, primarily by preventing spike from engaging ACE2. 2 There is increasing evidence that neutralizing antibodies are a strong correlate of protection from acquiring SARS‐CoV‐2 infection, with studies showing that plasma neutralization titers correlate strongly with vaccine efficacy. 3 , 4 Numerous studies have also shown that passive transfer of neutralizing monoclonal antibodies into animal models provides sterilizing protection against experimental SARS‐CoV‐2 challenge. 5 , 6 Most neutralizing antibodies target the RBD, 7 though neutralizing antibodies against N terminal domain of S1, and the fusion machinery within S2 have been reported. 8 , 9
The development of humoral immunity is underpinned by the activation of SARS‐CoV‐2‐specific B cells, which enter a germinal center (GC) reaction and differentiate into long‐lived, antibody‐secreting plasma cells or memory B cells (MBCs). While the majority of this response is derived from de novo priming of naïve B cells, there is some evidence that cross‐reactive MBCs that recognize epitopes conserved in endemic hCoV can be recalled upon infection with SARS‐CoV‐2. 10 , 11 However, such responses typically do not exhibit neutralizing activity. 10 , 11 In addition to neutralization, antibodies against SARS‐CoV‐2 can exert antiviral activity by clearing free virions or infected cells via Fc effector functions including antibody‐dependent phagocytosis (ADP) and antibody‐dependent cellular cytotoxicity (ADCC). 12 , 13 While most studies have focused on the Fc effector functions of S‐specific antibodies, the M, N, and ORF3a proteins can all be detected on the surface of infected cells and be targeted by antibodies that mediate NK cell activation. 14 While it remains unclear the degree to which ADCC and ADP contribute to protection against SARS‐CoV‐2 in humans, several viral challenge studies in mice have shown the protective value of many RBD‐ and NTD‐specific mAbs relies upon engagement with cellular Fc receptors in vivo. 15 , 16 , 17
During primary SARS‐CoV‐2 infection, neutralizing antibodies are generally detectable between 7 and 14 days following symptom onset, 18 peak around 23 days, and start to decline thereafter. 19 S‐specific antibodies that mediate Fc effector functions persist longer than neutralizing antibodies and could potentially contribute to protection upon reexposure. 12 The initial increase and subsequent decline of neutralizing antibodies have been linked to short‐lived antibody‐secreting cells (ASCs) that secrete high levels of antibodies in the early stages of infection and the rapidly decline afterward. 20 Despite this, S‐ and RBD‐specific IgG antibodies are maintained for at least 11 months postinfection, 21 , 22 with serologic neutralizing activity similarly maintained for at least 12 months. 22 , 23 Critically, in individuals recovered from COVID‐19, the bone marrow is populated by SARS‐CoV‐2 S‐specific ASCs (plasma cells) that are stable for at least 11 months. 21
Contrary to antibody titers, S‐specific MBCs in peripheral blood increase in frequency during convalescence 22 , 23 , 24 , 25 , 26 , 27 ), plateau at 8 months and are maintained for at least 12 months. 22 During that time, the MBC pool undergoes continued clonal evolution, with the emergence of new clones between 6 and 12 months and a gradual increase in VH gene mutational load. 22 , 26 , 27 , 28 Notably, monoclonal antibodies isolated from convalescent COVID‐19 individuals can exhibit increased binding affinity for S or RBD, and increased neutralizing potency over time. This evolution of the MBC compartment is strongly indicative of persistent GC activity and ongoing affinity maturation. 22 , 26 , 27 , 28 Interestingly, N protein and SARS‐CoV‐2 viral RNA have been detected in intestinal biopsies of convalescent COVID‐19 individuals taken ∼4 months after initial diagnosis, which suggest residual viral replication in the gut could be a potential source of antigen to support ongoing GC activity. Alternatively, antigen may be retained in the form of immune complexes on follicular dendritic cells within classical lymphoid tissues for prolonged support of ongoing GC activity. Overall, primary SARS‐CoV‐2 infection elicits a humoral immune response with hallmarks of prolonged GC activity, the generation of long‐lived plasma cells, an evolving population of MBCs and serologic maintenance of potent neutralizing antibodies.
3. THE ROLE OF TFH CELLS IN HUMORAL IMMUNITY TO SARS‐COV‐2
3.1. Overview of lymph node and circulating TFH subsets
The generation of effective humoral immune responses requires a well‐orchestrated series of cellular interactions between B cells and T follicular helper cell (TFH cells). TFH cells are a specialized subset of CD4+ T cells that provide critical “help” signaling to B cells, promoting B cell survival and differentiation into MBCs and long‐lived plasma cells. The repeated interactions of antigen‐specific TFH and B cells that take place in the GC of B cell follicles is a critical step in the generation of high‐affinity and long‐lived antibody responses following infection or vaccination. Phenotypically, human TFH cells in lymphoid tissues are characterized by a lack of CCR7, coupled with expression of the chemokine receptor CXCR5, PD‐1, markers of antigen experience (CD45RO), 29 , 30 and in some cases CD57. 31 , 32 Expression of the transcriptional repressor Bcl‐6 is a defining feature of TFH cells in lymphoid tissues. 31 , 33 , 34 TFH cells provide B cell cognate help via costimulatory molecules such as CD40L and indirectly by ICOS, as well as local secretion of cytokines including IL‐21. The importance of IL‐21 is highlighted by correlations between serum IL‐21 levels and antibody responses to influenza vaccination. 35
The anatomic localization of GC TFH within lymphoid tissues hinders their sampling in many human cohort studies. However, a subset of circulating CD4+ T cells exists in blood with significant similarities to lymph‐tissue TFH. These cells, known as circulating TFH (cTFH), share surface expression of CXCR5 with lymphoid TFH and constitute ∼10% of the CD4+ T cell compartment in peripheral blood. 36 Despite their shared expression of CXCR5 and ability to support B cell activation, there are several key transcriptional and phenotypic differences between circulating and GC TFH. While cTFH development depends on the key TFH lineage‐defining transcription factor Bcl‐6, 37 cTFH cells exhibit low or no expression of Bcl‐6, in stark contrast to GC TFH cells. 36 , 38 Circulating TFH cells do, however, express c‐Maf, 39 which can drive expression of CXCR5 and IL‐21 in the absence of Bcl‐6. 40 Additionally, cTFH typically have a quiescent phenotype and lack expression of activation markers such as PD‐1, ICO,S or CD38, while retaining expression of CCR7. Nonetheless, transcriptomic, epigenetic, and TCR repertoire analyses have provided evidence to support a clonal and developmental overlap between GC TFH and cTFH. 41 Indeed, analysis of CXCR5brightPD‐1bright CD4+ T cells from human thoracic duct lymph suggests this population is a trafficking intermediate between GC TFH and cTFH cells, which continually emigrate from lymphoid tissues. 41
Functionally, cTFH exhibit greater capacity for IL‐21 and IL‐10 secretion compared with CXCR5– CD4+ T cells, thereby allowing them to provide superior B cell help in vitro compared with conventional memory CD4+ T cells, as determined by B cell survival and differentiation in ASCs. 36 Within the cTFH population, however, studies have described significant phenotypic and functional heterogeneity. Similar to conventional memory CD4+ T cells, cTFH cells express chemokine receptors associated with distinct T helper subsets (CXCR3 and CCR6), lineage‐defining transcription factors (T‐bet, GATA3, ROR‐γt), and low levels of cytokines (IFNγ, IL‐4, IL‐17) upon activation. Consequently, cTFH can be defined as CXCR3+CCR6– T‐bet+ IFNγ‐producing cTFH1 cells (group 1 cTFH cells based on a recently proposed unifying nomenclature, 42 ), CXCR3–CCR6– GATA3+ IL‐4‐producing cTFH2 cells (group 2) and CXCR3–CCR6+ ROR‐γt+ IL‐17A‐producing cTFH17 cells (group 3). While the developmental pathways and functional implications of this phenotypic polarization within the TFH lineage are not fully understood, these distinct subsets are reported to exhibit differential capacity to provide B cell help in vitro, with CXCR3– cTFH2/17 cells exhibiting superior capacity to secrete IL‐21 and provide help to naïve B cells, as determined by B cell survival and differentiation in ASCs. 36 , 43 Although CXCR3+ cTFH1 cells do not provide significant help to naïve B cells, they can support the activation of MBCs and their differentiation into ASCs in vitro. 36 , 43
3.2. Overview of human cTFH responses following infection and vaccination
Consistent with linkage between lymph node resident TFH and cTFH populations, an activated subpopulation of PD‐1+ICOS+CD38+Ki67+ cTFH transiently emerges into the blood following viral infection or vaccination. 33 , 38 , 43 , 44 , 45 , 46 Both peptide/MHC‐II tetramers and antigen restimulation assays have demonstrated that this population contains CD4+ T cells specific for vaccine or infection‐associated antigens. 38 , 43 , 46 The appearance of this cTFH population is temporally linked to the emergence of ASCs and correlates with the magnitude and qualitative aspects of the serologic response. This phenomenon has been widely observed following vaccination of human subjects with the inactivated influenza vaccine, 38 , 43 , 44 , 47 Human Papillomavirus vaccine, 48 YF‐17D vaccine, 46 rVSV‐ZEBOV Ebola vaccine, 49 hepatitis B vaccine, 50 experimental malaria vaccination, 51 , 52 oral‐inactivated Escherichia coli vaccine, 53 as well as during acute infection with influenza virus, 45 human immunodeficiency virus, 39 , 54 hepatitis C virus, 55 Epstein–Barr virus, 56 and malaria. 57 , 58 It is clear that the analysis of cTFH cells in peripheral blood following vaccination or infection can provide important insights into humoral immunity.
While activation of the overall cTFH population is a consistent biomarker of robust serologic responses to infection or vaccination, the relationship between the 3 distinct cTFH subsets and antibody production appears to be context dependent (Figure 1). Activation of cTFH1 cells has been associated with multiple aspects of humoral immunity (antibody titers and/or ASCs and/or antigen‐specific B cells) for a number of viral infections and vaccines, including influenza, 43 , 44 , 45 HPV, 48 Yellow fever virus, 46 HIV, 54 and HCV. 55 On the other hand, vaccination with rVZV‐ZEBOV, oral‐inactivated E. coli, 53 or Plasmodium falciparum parasite protein antigens with AS01B adjuvant 52 results in activation of CXCR3– cTFH2/17 cells, which positively correlate with antibody responses to cognate antigens. Of note, although acute malaria infection drives activation of cTFH1, cTFH2, and cTFH17 cells, this does not correlate with antibody responses in children 57 and higher activation of cTFH1 cells is associated with an increased likelihood of symptomatic Plasmodium vivax infection. 58 Overall, the population of cTFH cells that emerges in peripheral blood after vaccination or infection is strongly associated with the development of protective humoral immunity. It has become evident that dividing this population into CXCR3/CCR6 subsets provides additional information and that these subsets maybe more reliable biomarkers than the total population. However, their ontogeny, their functional differences, and the context‐specific correlations between cTFH subsets and humoral immunity warrant further investigation. Comparative analysis of the TCR repertoire analysis as well as singe‐cell transcriptomic and epigenomic analyses could provide novel insights into cTFH polarization and help resolve discrepancies surrounding cTFH subsets.
FIGURE 1.
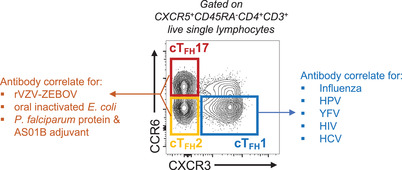
Circulating TFH subsets as correlates of antibody responses. Flow cytometry plot of cTFH subsets based on CXCR3 and CCR6 expression and their association with antibody responses in different contexts
3.3. Human cTFH responses in COVID‐19
Many recent studies have analyzed the magnitude and phenotype of cTFH cell responses during the acute and convalescent phases of SARS‐CoV‐2 infection (Table 1). 59 In the blood, activated cTFH (PD‐1+ICOS+) 60 , 61 , 62 , 63 , 64 with increased expression of CD38 60 and reduced expression of CCR7 63 are evident during acute infection. These activated PD‐1+ICOS+ cTFH cells emerge transiently during the acute phase of infection and generally wane after approximately 14 days postsymptom onset. Consequently, the use of antigen‐specific T cell assays (either activation‐induced marker (AIM) or intracellular cytokine staining) are critical for studying SARS‐CoV‐2‐specific cTFH responses during convalescence. Such assays have demonstrated that S‐specific cTFH cells that emerge during acute infection 65 persist in convalescent individuals for at least 6 months, 23 with a half‐life of ∼129 days. 24
TABLE 1.
Key studies of human cTFH responses following SARS‐CoV‐2 infection and vaccination
| Cohort | Detection method | Dominant phenotype/cytokine profile of cTFH cells | Key cTFH findings | Reference |
|---|---|---|---|---|
| Acute and convalescent cohorts | ||||
| Acute and convalescent COVID‐19 | Bulk ICOS+PD‐1+ cTFH cells | Activation of CXCR3+CCR6−cTFH cells |
|
60 |
| Acute and convalescent COVID‐19 | Antigen‐specific cTFH cells by AIM |
Greater frequency of CXCR3+CCR6− than CXCR3−CCR6+ S‐specific cTFH cells in acute samples Greater frequency of CXCR3−CCR6+ than CXCR3+CCR6− S‐specific cTFH cells in convalescent samples IFNγ producing cTFH cells IL‐17A assessed but not detected |
|
65 |
| Acute and convalescent COVID‐19 | Bulk ICOS+PD‐1+ cTFH cells | CXCR3+ cTFH cells |
|
64 |
| Convalescent cohorts | ||||
| Convalescent COVID‐19 | Antigen‐specific cTFH cells by AIM | Not reported |
|
69 |
| Convalescent COVID‐19 (up to 8 months) | Antigen‐specific cTFH cells by AIM | Increase in CCR6+ spike‐specific cTFH cells over time |
|
23 |
| Convalescent COVID‐19 | Bulk cTFH cells | CXCR3+ or CXCR3− |
|
63 |
| Convalescent COVID‐19 | Antigen‐specific cTFH cells by AIM | CXCR3+ or CXCR3− |
|
66 |
| Convalescent COVID‐19 | Antigen‐specific cTFH cells by AIM | IFNγ producing cTFH cells |
|
67 |
| Convalescent COVID‐19 | Antigen‐specific cTFH cells by AIM |
Spike‐specific cTFH 1 and cTFH 2 Membrane‐specific cTFH 1 and cTFH 1/17 Nucleocapsid‐specific cTFH 1 |
|
68 |
| Convalescent COVID‐19 (up to 150 days) | Antigen‐specific cTFH cells by AIM | Not reported |
|
24 |
| Convalescent COVID‐19 | Bulk PD‐1+CXCR5+ cTFH cells & antigen‐specific cTFH cells by AIM |
CXCR3+ or CXCR3− IFNγ and IL‐21 producing cTFH cells other cytokines not reported |
|
62 |
| Vaccination cohorts | ||||
| Healthy and MS patients on a‐CD20 therapy receiving 2 doses of mRNA vaccine | Antigen‐specific cTFH cells by AIM | CXCR3+ |
|
75 |
| Naïve and COVID‐19 recovered individuals receiving 2 doses of BNT162b or mRNA‐1273 vaccine | Antigen‐specific cTFH cells by AIM | Not reported |
|
76 |
| Naïve and COVID‐19 recovered individuals receiving 1 dose of BNT162b vaccine | Antigen‐specific cTFH cells by AIM |
IFNγ producing cTFH cells IL‐17A assessed but not detected |
|
78 |
| Naïve and COVID‐19 recovered individuals receiving 2 doses of BNT162b or mRNA‐1273 vaccine | Antigen‐specific cTFH cells by AIM | Not reported |
|
77 |
The relationship between cTFH frequency, phenotypic and functional polarization, and serologic responses to SARS‐CoV‐2 has also been assessed. The bulk PD1+ICOS+ cTFH population that emerges in the acute phase of COVID‐19 is predominantly composed of CXCR3+ cTFH1 cells, 60 , 64 similar to influenza infection. 45 However, analysis of S‐specific cTFH by AIM has demonstrated a dominant population of CXCR3–CCR6+ cells. 23 , 65 , 66 Interestingly, the proportion of CXCR3–CCR6+ S‐specific cTFH cells is higher in late convalescence (6 months) than in early convalescence (1–2 months) or during the acute phase. 23 , 65 Despite the detection of CXCR3−CCR6+ cTFH17 cells, antigen‐specific cTFH cells in recovered COVID‐19 patients consistently produce IFNγ and IL‐21, but not IL‐17, among multiple independent studies. 62 , 65 , 66 , 67
Phenotypic polarization of cTFH has been linked to the development of robust neutralizing antibody responses. Indeed, CXCR3+ cTFH1 cells are consistently associated with high titers of spike binding or neutralizing antibodies. This is observed for both the total ICOS+PD‐1+ cTFH1 population (which also correlates with the ASC response and plasma CXCL13), 60 , 62 , 63 , 64 as well as S‐specific cTFH1. 66 Activation of cTFH1 cells in acute COVID‐19 also positively correlates with the titers and avidity of RBD‐specific IgM antibodies. 60 Conversely, the relationship between cTFH2, cTFH17, and antibody responses is more variable across cohorts and assays. Identification of S‐specific cTFH has suggested that the frequency of cTFH2 responses positively correlate with neutralizing antibody titers, while S‐specific cTFH17 were a strong negative correlate of neutralizing activity. 66 In contrast, total ICOS+PD‐1+ cTFH2/17 cells have been variably correlated with the antibody response within different cohorts. 60 , 62 , 63 , 64 Altogether, the current data indicate that CXCR3+ cTFH1 cells are a strong correlate of neutralizing and total antibodies against SARS‐CoV‐2, while the role of CXCR3– cTFH2 and cTFH17 cells requires further investigation, as do the differences between total ICOS+PD‐1+ and AIM+ cTFH cells.
Although most studies have focused on S‐specific cTFH cells, cTFH specific for N and M have also been studied. 65 , 68 , 69 Frequencies of cTFH cells specific for S, N, and M positively correlated with plasma neutralization activity as well as N‐specific IgG antibodies. 69 Interestingly, polarization between cTFH1, cTFH2, and cTFH17 subsets has been reported to differ for cTFH cells specific for different SARS‐CoV‐2 antigens, 68 although the significance of this observation is currently unclear.
A potential impairment of TFH cells in some cases of severe COVID‐19 has also been reported. Specifically, GC B cells and TFH cells were diminished in lymphoid tissues in a subset of deceased COVID‐19 patients. 70 Additionally, a population of cTFH cells expressing cytotoxicity‐associated transcripts like PRF1 and GZMB (encoding perforin and granzyme B respectively) and termed cytotoxic cTFH cells was increased in hospitalized versus nonhospitalized individuals and was correlated with lower antibody titers to S. 71 These observations are however contrary to the higher antibody titers observed in severe COVID‐19, 60 since impaired TFH activity would be expected to result in lower antibody titers. These observations therefore warrant further investigation to understand the role and function of TFH cells in severe COVID‐19 and whether they relate a specific subset of patients.
In summary, the overall activation of cTFH cells during COVID‐19 as well as their phenotypic polarization are correlates of neutralizing activity and B cell responses (Figure 2). What remains unclear, however, is the ontogeny of distinct cTFH subsets and their relationship to GC TFH activity. It is pertinent to further understand the potential of cTFH cells as biomarkers of the establishment and recall of humoral immunity to SARS‐CoV‐2, especially in the context of emerging variants of concern (VOCs) with increased potential for escape from humoral immunity.
FIGURE 2.
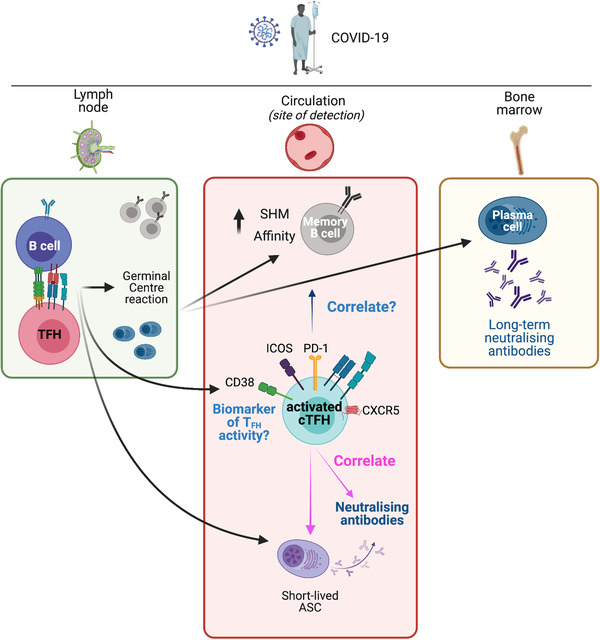
Lymphoid and circulating TFH responses in COVID‐19. SARS‐CoV‐2 antigen in the lymph nodes results in activation of antigen‐specific B cells and TFH cells. Their interaction leads to the initiation of the germinal center reaction. This results in the development of memory B cells with increased somatic hypermutation (SHM) and increased affinity, as well as long‐lived plasma cells that traffic to the bone marrow and provide a long‐term source of neutralizing antibodies. A population of short‐lived antibody‐secreting cells (ASCs) appears in the circulation and provides a raid source of neutralizing antibodies. Concurrently, a population of activated (CD38+, PD‐1+, ICOS+) cTFH cells appears in the circulation. This population contains antigen‐specific cTFH cells (not depicted). Although memory B cells and ASCs are primarily located in lymphoid tissues, they are typically measured in blood samples, where they correlate with activated cTFH cells. Activated cTFH cells also correlate with the development of neutralizing antibodies. These cTFH cells are a potential biomarker of TFH activity in lymphoid tissues but it remains to be determined if this population of cTFH cells are predictive of long‐term neutralizing antibodies, or of the development of long‐lived plasma cells and the prolonged evolution of the MBC pool. The figure was created with BioRender.com
3.4. Human cTFH cell responses after SARS‐CoV‐2 vaccination
Vaccination with approved COVID‐19 vaccines induces neutralizing antibodies that have been associated with protection from infection. Analysis of axillary draining lymph nodes after mRNA vaccination demonstrated potent GC reactions that persist for at least 12 weeks after booster immunisation. 72 Importantly, S‐specific TFH cells are induced at those sites and correlate with S‐specific GC B cells. 73 , 74 Analysis of paired lymph node and blood samples has demonstrated that while S‐specific cTFH cells with an activated phenotype (CD38+HLA‐DR+ICOS+) peak within the first month before returning to a resting phenotype and declining in frequency, S‐specific TFH cells in lymph nodes exhibit relatively constant frequencies for at least 60 days. 73 Although limited to a small number of donors, these analyses suggest that mRNA vaccines induce robust GC reactions that may underscore the strong immunogenicity profile of this vaccine.
Vaccination of naïve (previously uninfected) individuals induces S‐specific cTFH cells 75 , 76 , 77 with a CXCR3+ phenotype 75 and the ability to produce IFNγ but not IL‐17A. 78 The frequency of S‐specific cTFH cells peaks within ∼1 month after vaccination and then wanes, contrary to S‐specific TH1 cells, the frequency of which is stable for at least 6 months. 77 The frequency of S‐specific cTFH cells, as well as S‐specific conventional CD4+ TH1 cells, at 2 weeks postvaccination correlates with neutralizing antibodies to spike as well as VOCs and with S and RBD‐specific MBC responses at 1 month after vaccination. 77 This indicates a role of cTFH cells as biomarkers for the development of neutralizing antibodies and MBCs following vaccination with spike. Consistently, vaccination of individuals after recovery from COVID‐19 induces greater S‐specific cTFH responses than in naive individuals. 78 Importantly, the pre‐vaccination frequency of S‐specific cTFH cells in recovered individuals positively correlates with postvaccination neutralizing titers to ancestral viruses as well as VOCs. 76 , 78 Overall, it is becoming evident that cTFH cells have an important role as biomarkers of humoral immunity following SARS‐CoV‐2 vaccination. It will be important to further characterize and understand lymphoid and circulating TFH cell responses following the administration of different recently developed vaccine platforms and if/how they differentially induce such responses, especially in the context of heterologous prime‐boost vaccination.
4. CONCLUSIONS
There is increasing evidence that analysis of cTFH responses can provide critical insights into magnitude and qualitative aspects of the humoral immune response to SARS‐CoV‐2 infection and vaccination (Figure 1). The current data strongly support the notion that cTFH cells, and particularly the cTFH1 subset, are informative biomarkers of the development of neutralizing antibodies and MBCs targeting the wildtype Spike as well as VOCs. Nonetheless, important questions remain regarding the differential role of CXCR3/CCR6 cTFH subsets in the development of neutralizing antibodies and the persistence of spike‐specific cTFH memory. In order to effectively harness the capacity of TFH to drive strong neutralizing antibody responses to vaccination, it will be critical to understand why only some cTFH subsets positively correlate with antibody titers, and how TFH quality can be manipulated through novel vaccine platforms. Additionally, while cTFH activation and the frequency of antigen‐specific cTFH cells are biomarkers of neutralizing antibodies in the acute and early convalescent phase of COVID‐19 infection and vaccination, it is not known whether cTFH cells are predictive of long‐term neutralizing antibodies, or of the development of long‐lived plasma cells. As studies seek to understand the immunologic mechanisms underlying the prolonged evolution of the MBC pool, we will gain more information about the duration of GC TFH responses and their relationship to cTFH frequency and phenotype. Addressing these questions would be pivotal in harnessing their potential for the development of effective vaccination strategies.
AUTHORSHIPS
M. K., W. S. L., and J. A. J. prepared the manuscript. A. K. W. and S. J. K. revised the manuscript.
DISCLOSURES
The authors declare no conflicts of interest.
ACKNOWLEDGMENTS
M. K., J. A. J., A. K. W., and S. J. K. are supported by National Health and Medical Research Council (NHMRC) fellowships. The figure and graphical abstract were created with BioRender.com.
Koutsakos M, Lee WS1, Wheatley AK1, Kent SJ, Juno JA. T follicular helper cells in the humoral immune response to SARS‐CoV‐2 infection and vaccination. J Leukoc Biol. 2022;111:355–365. 10.1002/JLB.5MR0821-464R
Summary sentence: Review on the analysis of TFH cells during COVID‐19 infection and vaccination and the role of these cells as biomarkers of neutralizing antibody development.
Contributor Information
Marios Koutsakos, Email: marios.koutsakos@unimelb.edu.au.
Jennifer A Juno, Email: jennifer.juno@unimelb.edu.au.
REFERENCES
- 1. V'Kovski P, Kratzel A, Steiner S, Stalder H, Thiel V. Coronavirus biology and replication: implications for SARS‐CoV‐2. Nat Rev Microbiol. 2021;19:155‐170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cromer D, Juno JA, Khoury D, et al. Prospects for durable immune control of SARS‐CoV‐2 and prevention of reinfection. Nat Rev Immunol. 2021;21:395‐404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Khoury DS, Cromer D, Reynaldi A, et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS‐CoV‐2 infection. Nature medicine. 2021;27:1205‐1211. [DOI] [PubMed] [Google Scholar]
- 4. Earle KA, Ambrosino DM, Fiore‐Gartland A, et al. Evidence for antibody as a protective correlate for COVID‐19 vaccines. Vaccine. 2021;39:4423‐4428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rogers TF, Zhao F, Huang D, et al. Isolation of potent SARS‐CoV‐2 neutralizing antibodies and protection from disease in a small animal model. Science. 2020;369:956‐963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Baum A, Ajithdoss D, Copin R, et al. REGN‐COV2 antibodies prevent and treat SARS‐CoV‐2 infection in rhesus macaques and hamsters. Science. 2020;370:1110‐1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Piccoli L, Park YJ, Tortorici MA, et al. Mapping neutralizing and immunodominant sites on the SARS‐CoV‐2 spike receptor‐binding domain by structure‐guided high‐resolution serology. Cell. 2020;183:1024‐1042 e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chi X, Yan R, Zhang J, et al. A neutralizing human antibody binds to the N‐terminal domain of the spike protein of SARS‐CoV‐2. Science. 2020;369:650‐655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ng KW, Faulkner N, Cornish GH, et al. Preexisting and de novo humoral immunity to SARS‐CoV‐2 in humans. Science. 2020;370:1339‐1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wec AZ, Wrapp D, Herbert AS, et al. Broad neutralization of SARS‐related viruses by human monoclonal antibodies. Science. 2020;369:731‐736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dugan HL, Stamper CT, Li L, et al. Profiling B cell immunodominance after SARS‐CoV‐2 infection reveals antibody evolution to non‐neutralizing viral targets. Immunity. 2021;54:1290‐1303 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lee WS, Selva KJ, Davis SK, et al. Decay of Fc‐dependent antibody functions after mild to moderate COVID‐19. Cell Rep Med. 2021;2:100296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dufloo J, Grzelak L, Staropoli I, et al. Asymptomatic and symptomatic SARS‐CoV‐2 infections elicit polyfunctional antibodies. Cell Rep Med. 2021;2:100275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fielding C, Sabberwal P, Williamson J, et al. (2021) ADNKA overcomes SARS‐CoV2‐mediated NK cell inhibition through non‐spike antibodies. bioRxiv, 2021.04.06.438630.
- 15. Schafer A, Muecksch F, Lorenzi JCC, et al. Antibody potency, effector function, and combinations in protection and therapy for SARS‐CoV‐2 infection in vivo. J Exp Med. 2021;218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chan CEZ, Seah SGK, Chye H, et al. The Fc‐mediated effector functions of a potent SARS‐CoV‐2 neutralizing antibody, SC31, isolated from an early convalescent COVID‐19 patient, are essential for the optimal therapeutic efficacy of the antibody. PLoS One. 2021;16:e0253487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Suryadevara N, Shrihari S, Gilchuk P, et al. Neutralizing and protective human monoclonal antibodies recognizing the N‐terminal domain of the SARS‐CoV‐2 spike protein. Cell. 2021;184:2316‐2331 e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dispinseri S, Secchi M, Pirillo MF, et al. Neutralizing antibody responses to SARS‐CoV‐2 in symptomatic COVID‐19 is persistent and critical for survival. Nat Commun. 2021;12:2670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Seow J, Graham C, Merrick B, et al. Longitudinal observation and decline of neutralizing antibody responses in the three months following SARS‐CoV‐2 infection in humans. Nat Microbiol. 2020;5:1598‐1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Laing AG, Lorenc A, Del Molino Del Barrio I, et al. A dynamic COVID‐19 immune signature includes associations with poor prognosis. Nat Med. 2020. [DOI] [PubMed] [Google Scholar]
- 21. Turner JS, Kim W, Kalaidina E, et al. SARS‐CoV‐2 infection induces long‐lived bone marrow plasma cells in humans. Nature. 2021;595:421‐425. [DOI] [PubMed] [Google Scholar]
- 22. Wang Z, Muecksch F, Schaefer‐Babajew D, et al. Naturally enhanced neutralizing breadth against SARS‐CoV‐2 one year after infection. Nature. 2021;595:426‐431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dan JM, Mateus J, Kato Y, et al. Immunological memory to SARS‐CoV‐2 assessed for up to 8 months after infection. Science. 2021;371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wheatley AK, Juno JA, Wang JJ, et al. Evolution of immune responses to SARS‐CoV‐2 in mild‐moderate COVID‐19. Nat Commun. 2021;12:1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hartley GE, Edwards ESJ, Aui PM, et al. Rapid generation of durable B cell memory to SARS‐CoV‐2 spike and nucleocapsid proteins in COVID‐19 and convalescence. Sci Immunol. 2020;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sokal A, Chappert P, Barba‐Spaeth G, et al. Maturation and persistence of the anti‐SARS‐CoV‐2 memory B cell response. Cell. 2021;184:1201‐1213 e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gaebler C, Wang Z, Lorenzi JCC, et al. Evolution of antibody immunity to SARS‐CoV‐2. Nature. 2021;591:639‐644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sakharkar M, Rappazzo CG, Wieland‐Alter WF, et al. Prolonged evolution of the human B cell response to SARS‐CoV‐2 infection. Sci Immunol. 2021;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Breitfeld D, Ohl L, Kremmer E, et al. Follicular B helper T cells express CXC chemokine receptor 5, localize to B cell follicles, and support immunoglobulin production. J Exp Med. 2000;192:1545‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Schaerli P, Willimann K, Lang AB, Lipp M, Loetscher P, Moser B. CXC chemokine receptor 5 expression defines follicular homing T cells with B cell helper function. J Exp Med. 2000;192:1553‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ma CS, Deenick EK, Batten M, Tangye SG. The origins, function, and regulation of T follicular helper cells. J Exp Med. 2012;209:1241‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kim JR, Lim HW, Kang SG, Hillsamer P, Kim CH. Human CD57+ germinal center‐T cells are the major helpers for GC‐B cells and induce class switch recombination. BMC Immunol. 2005;6:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Koutsakos M, Nguyen THO, Kedzierska K. With a little help from T follicular helper friends: humoral immunity to influenza vaccination. J Immunol. 2019;202:360‐367. [DOI] [PubMed] [Google Scholar]
- 34. Lindqvist M, van Lunzen J, Soghoian DZ, et al. Expansion of HIV‐specific T follicular helper cells in chronic HIV infection. J Clin Invest. 2012;122:3271‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Pallikkuth S, Pilakka Kanthikeel S, Silva SY, Fischl M, Pahwa R, Pahwa S. Upregulation of IL‐21 receptor on B cells and IL‐21 secretion distinguishes novel 2009 H1N1 vaccine responders from nonresponders among HIV‐infected persons on combination antiretroviral therapy. J Immunol. 2011;186:6173‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Morita R, Schmitt N, Bentebibel SE, et al. Human blood CXCR5(+)CD4(+) T cells are counterparts of T follicular cells and contain specific subsets that differentially support antibody secretion. Immunity. 2011;34:108‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. He J, Tsai LM, Leong YA, et al. Circulating precursor CCR7(lo)PD‐1(hi) CXCR5(+) CD4(+) T cells indicate Tfh cell activity and promote antibody responses upon antigen reexposure. Immunity. 2013;39:770‐81. [DOI] [PubMed] [Google Scholar]
- 38. Herati RS, Muselman A, Vella L, et al. Successive annual influenza vaccination induces a recurrent oligoclonotypic memory response in circulating T follicular helper cells. Sci Immunol. 2017;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Locci M, Havenar‐Daughton C, Landais E, et al. Human circulating PD‐1+CXCR3‐CXCR5+ memory Tfh cells are highly functional and correlate with broadly neutralizing HIV antibody responses. Immunity. 2013;39:758‐69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kroenke MA, Eto D, Locci M, et al. Bcl6 and Maf cooperate to instruct human follicular helper CD4 T cell differentiation. J Immunol. 2012;188:3734‐44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Vella LA, Buggert M, Manne S, et al. T follicular helper cells in human efferent lymph retain lymphoid characteristics. J Clin Invest. 2019;129:3185‐3200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Eisenbarth SC, Baumjohann D, Craft J, et al. CD4(+) T cells that help B cells ‐ a proposal for uniform nomenclature. Trends Immunol. 2021;42:658‐669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bentebibel SE, Lopez S, Obermoser G, et al. Induction of ICOS+CXCR3+CXCR5+ TH cells correlates with antibody responses to influenza vaccination. Sci Transl Med. 2013;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Koutsakos M, Wheatley AK, Loh L, et al. Circulating TFH cells, serological memory, and tissue compartmentalization shape human influenza‐specific B cell immunity. Sci Transl Med. 2018;10. [DOI] [PubMed] [Google Scholar]
- 45. Nguyen THO, Koutsakos M, van de Sandt CE, et al. Immune cellular networks underlying recovery from influenza virus infection in acute hospitalized patients. Nat Commun. 2021;12:2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Huber JE, Ahlfeld J, Scheck MK, et al. Dynamic changes in circulating T follicular helper cell composition predict neutralising antibody responses after yellow fever vaccination. Clin Transl Immunol. 2020;9:e1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Bentebibel SE, Khurana S, Schmitt N, et al. ICOS(+)PD‐1(+)CXCR3(+) T follicular helper cells contribute to the generation of high‐avidity antibodies following influenza vaccination. Sci Rep. 2016;6:26494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Matsui K, Adelsberger JW, Kemp TJ, et al. Circulating CXCR5(+)CD4(+) T follicular‐like helper cell and memory B cell responses to human papillomavirus vaccines. PLoS One. 2015;10:e0137195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Farooq F, Beck K, Paolino KM, et al. Circulating follicular T helper cells and cytokine profile in humans following vaccination with the rVSV‐ZEBOV Ebola vaccine. Sci Rep. 2016;6:27944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Yin M, Xiong Y, Huang L, et al. Circulating follicular helper T cells and subsets are associated with immune response to hepatitis B vaccination. Hum Vaccin Immunother. 2021;17:566‐574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hill DL, Pierson W, Bolland DJ, et al. The adjuvant GLA‐SE promotes human Tfh cell expansion and emergence of public TCRbeta clonotypes. J Exp Med. 2019;216:1857‐1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Nielsen CM, Ogbe A, Pedroza‐Pacheco I, et al. Protein/AS01B vaccination elicits stronger, more Th2‐skewed antigen‐specific human T follicular helper cell responses than heterologous viral vectors. Cell Rep Med. 2021;2:100207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Cardeno A, Magnusson MK, Quiding‐Jarbrink M, Lundgren A. Activated T follicular helper‐like cells are released into blood after oral vaccination and correlate with vaccine specific mucosal B‐cell memory. Sci Rep. 2018;8:2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Niessl J, Baxter AE, Morou A, et al. Persistent expansion and Th1‐like skewing of HIV‐specific circulating T follicular helper cells during antiretroviral therapy. EBioMedicine. 2020;54:102727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Zhang J, Liu W, Wen B, et al. Circulating CXCR3(+) Tfh cells positively correlate with neutralizing antibody responses in HCV‐infected patients. Sci Rep. 2019;9:10090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Liu J, Zhou Y, Yu Q, et al. Higher frequency of CD4+CXCR5+ICOS+PD1+ T follicular helper cells in patients with infectious mononucleosis. Medicine (Baltimore). 2015;94:e2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Obeng‐Adjei N, Portugal S, Tran TM, et al. Circulating Th1‐Cell‐type Tfh cells that exhibit impaired B cell help are preferentially activated during acute Malaria in Children. Cell Rep. 2015;13:425‐39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Ioannidis LJ, Pietrzak HM, Ly A, et al. High‐dimensional mass cytometry identifies T cell and B cell signatures predicting reduced risk of Plasmodium vivax malaria. JCI Insight. 2021;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Baumjohann D, Fazilleau N. Antigen‐dependent multistep differentiation of T follicular helper cells and its role in SARS‐CoV‐2 infection and vaccination. Eur J Immunol. 2021;51:1325‐1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Koutsakos M, Rowntree LC, Hensen L, et al. Integrated immune dynamics define correlates of COVID‐19 severity and antibody responses. Cell Rep Med. 2021;2:100208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Thevarajan I, Nguyen THO, Koutsakos M, et al. Breadth of concomitant immune responses prior to patient recovery: a case report of non‐severe COVID‐19. Nat Med. 2020;26:453‐455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Zhang J, Wu Q, Liu Z, et al. Spike‐specific circulating T follicular helper cell and cross‐neutralizing antibody responses in COVID‐19‐convalescent individuals. Nat Microbiol. 2021;6:51‐58. [DOI] [PubMed] [Google Scholar]
- 63. Gong F, Dai Y, Zheng T, et al. Peripheral CD4+ T cell subsets and antibody response in COVID‐19 convalescent individuals. J Clin Invest. 2020;130:6588‐6599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Sandberg JT, Varnaite R, Christ W, et al. SARS‐CoV‐2‐specific humoral and cellular immunity persists through 9 months irrespective of COVID‐19 severity at hospitalisation. Clin Transl Immunol. 2021;10:e1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Rydyznski Moderbacher C, Ramirez SI, Dan JM, et al. Antigen‐Specific adaptive immunity to SARS‐CoV‐2 in acute COVID‐19 and associations with age and disease severity. Cell. 2020;183:996‐1012 e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Juno JA, Tan HX, Lee WS, et al. Humoral and circulating follicular helper T cell responses in recovered patients with COVID‐19. Nat Med. 2020. [DOI] [PubMed] [Google Scholar]
- 67. Rodda LB, Netland J, Shehata L, et al. Functional SARS‐CoV‐2‐specific immune memory persists after mild COVID‐19. Cell. 2021;184:169‐183 e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Sekine T, Perez‐Potti A, Rivera‐Ballesteros O, et al. Robust T cell immunity in convalescent individuals with asymptomatic or mild COVID‐19. Cell. 2020;183:158‐168 e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Boppana S, Qin K, Files JK, et al. SARS‐CoV‐2‐specific circulating T follicular helper cells correlate with neutralizing antibodies and increase during early convalescence. PLoS Pathog. 2021;17:e1009761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Kaneko N, Kuo HH, Boucau J, et al. Massachusetts consortium on pathogen readiness specimen working, G. (2020) loss of Bcl‐6‐Expressing T follicular helper cells and germinal centers in COVID‐19. Cell;183:143‐157 e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Meckiff BJ, Ramirez‐Suastegui C, Fajardo V, et al. Imbalance of regulatory and cytotoxic SARS‐CoV‐2‐Reactive CD4(+) T cells in COVID‐19. Cell. 2020;183:1340‐1353 e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Turner JS, O'Halloran JA, Kalaidina E, et al. SARS‐CoV‐2 mRNA vaccines induce persistent human germinal centre responses. Nature. 2021;596:109‐113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Mudd PA, Minervina AA, Pogorelyy MV, et al. (2021) SARS‐CoV‐2 mRNA vaccination elicits robust and persistent T follicular helper cell response in humans. bioRxiv, 2021.09.08.459485. [DOI] [PMC free article] [PubMed]
- 74. Lederer K, Parvathaneni K, Painter MM, et al. (2021) Germinal center responses to SARS‐CoV‐2 mRNA vaccines in healthy and immunocompromised individuals. medRxiv, 2021.09.16.21263686. [DOI] [PMC free article] [PubMed]
- 75. Apostolidis SA, Kakara M, Painter MM, et al. (2021) Altered cellular and humoral immune responses following SARS‐CoV‐2 mRNA vaccination in patients with multiple sclerosis on anti‐CD20 therapy. medRxiv, 2021.06.23.21259389. [DOI] [PMC free article] [PubMed]
- 76. Painter MM, Mathew D, Goel RR, et al. Rapid induction of antigen‐specific CD4(+) T cells is associated with coordinated humoral and cellular immunity to SARS‐CoV‐2 mRNA vaccination. Immunity. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Goel RR, Painter MM, Apostolidis SA, et al. (2021) mRNA vaccination induces durable immune memory to SARS‐CoV‐2 with continued evolution to variants of concern. bioRxiv, 2021.08.23.457229.
- 78. Tauzin A, Nayrac M, Benlarbi M, et al. A single dose of the SARS‐CoV‐2 vaccine BNT162b2 elicits Fc‐mediated antibody effector functions and T cell responses. Cell Host Microbe. 2021;29:1137‐1150 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]


