Abstract
Background
Hypersensitivity reactions induced by chemotherapeutic drugs may influence the course of the oncologic disease by preventing doctors from prescribing first‐line therapy. In order to prevent another hypersensitivity reaction to the culprit chemotherapeutic agent, the physician can decide between two possibilities: premedication or desensitisation protocols. Rapid drug desensitisation showed successful results for most patients, but some of them may develop symptoms. Although omalizumab is not licensed as premedication or adjuvant therapy in chemotherapy desensitisation protocols, there have been published some case reports and small sample size studies that indicated promising results.
Methods
We reviewed all the published literature regarding the use of omalizumab during chemotherapy desensitisation protocols.
Results and conclusions
We found a great heterogeneity between the doses and the interval between omalizumab injections and chemotherapy ‐ rapid drug desensitisation, but most of the studies showed promising results. As a corollary, we propose a dose regimen of omalizumab administered before the first desensitisation protocol. Then, omalizumab should be administered one day before every chemotherapy regimen. Omalizumab might be used as an adjuvant therapy and might be a solution for a hopeless situation.
Keywords: chemotherapy hypersensitivity, drug desensitisation, omalizumab
1. INTRODUCTION
Chemotherapy (CHT) is one of the basic medical approaches for treating patients with different types of cancer. Chemotherapy agents are used either alone or in combination with targeted therapies constituted by monoclonal antibodies or other biologicals. 1
Hypersensitivity reactions (HSRs) induced by CHT can be defined as unpredicted signs and symptoms not consistent with a toxicity reaction. 2 The mechanisms responsible for HSRs are not fully understood and may vary between IgE‐mediated, non‐IgE‐mediated or unclear pathogenic events. 3
Almost all CHT drugs can induce HSRs, but they are reported only in about 5% of patients; this percentage may be substantially underestimated because of unreported mild and moderate reactions. 4 According to recent data, CHT‐induced HSRs are the third leading cause of fatal drug‐induced anaphylaxis in the United States 5 ; in Europe they are amongst top 5 inducers. 6
Based on the risk of generating HSRs, it is possible to divide the chemotherapeutic agents into three groups: drugs with high, intermediate, or low potential to cause HSRs, as summarized in Table 1. The reactions can be caused by the parent compound, its metabolites, or by the solvent. Depending on this grouping, the problem of CHT‐induced HSRs is notable for patients treated with platinum compounds, taxanes, L‐asparaginase, epipodophyllotoxins and is lower for others. 7
TABLE 1.
Classificasion of chemotherapeutic agents based on risk to induce hypersensitivity reactions 7
| Drugs with high potential | Drugs with intermediate potential | Drugs with low potential |
|---|---|---|
| Platinum compounds | Anthracyclines | Cytarabine |
| Oxaliplatin | Doxorubicin | Cyclophosphamide |
| Carboplatin | Daunorubicin | Ifosfamide |
| Cisplatin | Epirubicin | |
| Taxanes | Idarubicin | |
| Paclitaxel | 6‐Mercaptopurine | |
| Docetaxel | Azathioprine | |
| Other cytostatic drugs | Methotrexate | |
| L‐Asparaginase | ||
| Procarbazine | ||
| Epipodophyllotoxins | ||
| Teniposide | ||
| Etoposide |
Unlike other drugs that can be easily replaced when a HSR occurs, CHT drugs are often unique and essential in the treatment of neoplastic disease and thereby the management of HSRs to CHT is crucial. 8
The diagnosis of HSR is based on history, in vivo tests (skin prick, intradermal and provocation tests) and in vitro tests. The clinical manifestations are variable, unpredictable and involve the skin (e.g., rash, pruritus, urticaria, angioedema, palmar erythema, facial flushing), the respiratory tract (e.g., bronchospasm), the gastrointestinal tract (e.g., abdominal pain, nausea, diarrhea), and the cardiovascular system (alterations in blood pressure and heart rate). 9 Severe reactions usually develop during the infusion of the chemotherapy and most of them are IgE‐mediated as it has been clearly demonstrated for platinum salts, 10 , 11 whereas mild to moderate reactions can occur either during treatment or in the 24 up to 72 h after CHT administration and are apparently related to other mechanisms like mast cell and basophil activation and degranulation or complement activation. 12 , 13
In respect of skin tests usefulness, their importance has been proved in platin salts‐induced HSRs, demonstrating an IgE‐mediated mechanism particularly for carboplatin and oxaliplatin. 14 Prick and intradermal skin tests to platinum drugs are valuable diagnostic tools; their high sensitivity has been shown by several studies on carboplatin (66%–100%) and oxaliplatin (26%–100%). 15 , 16 In addition, the severity of the reaction appears to be related to the sensitivity of skin tests. 11
In clinical practice, if the first‐line treatment led to a HSR, the oncologist might switch to a second line therapy which can be less effective and can lead to significant morbidity. 17 However, if the culprit drug is associated with increased life expectancy and increased quality of life or if there is no therapeutic alternative, the physician must weigh the benefit of continuing the treatment against the risk of a potential fatal anaphylactic reaction during the following administration of chemotherapy. 7
In order to prevent another HSR to the culprit chemotherapeutic agent, the physician can decide between two possibilities: premedication or desensitisation protocols.
2. PREMEDICATION
Premedication schedules are carried out to prevent HSRs; they include administration of corticosteroids and antihistamines prior to chemotherapy infusion. Premedication is effective as well as recommended to prevent HSRs to different CHT, such as taxanes, epipodophillotoxins and pegasparaginase. 7 , 18 This procedure has dramatically decreased the incidence of HSRs to taxanes down to 2%–4% of cases. 19 Instead, for platinum salts, premedication is ineffective in preventing IgE‐mediated HSRs. 20 , 21
Occasionally, in case of premedication failure or if the procedure cannot be implemented, a drug desensitisation protocol could be recommended. Rapid drug desensitisation (RDD) is the best option for mast cell‐mediated HSRs, whether the involved mechanism is IgE‐mediated or not. 17
3. DESENSITISATION TO CHEMOTHERAPEUTIC AGENTS
RDD is a procedure that induces a temporary tolerance to a drug, by consecutive administration of small doses of the culprit drug until the total therapeutic dose is reached. 15
RDD induces transient unresponsiveness, so patients need to be re‐desensitised each time they are re‐exposed to the culprit CHT. Throughout RDD, patients with IgE and non‐IgE HSRs can safely receive needed medication while minimizing or completely inhibiting adverse reactions. Due to the extensive clinical usefulness and success of RDD, the molecular mechanisms of inducing temporary tolerance have been extensively investigated but are still incompletely understood. 17
The standardized 12‐step protocol is the most used in RDD and can help achieve a temporary tolerance of the drug; the protocol usually starts with 1/1000 from the final dose and subsequently, the doses are doubled at each step at fixed time intervals. 17 , 22
Patients who have had severe anaphylactic reactions to CHT or who have reacted at an early stage in the standard 12‐step RDD, may experience fewer symptoms when using a 16‐step protocol according to Mariana Castells' one. 17
RDD showed successful results for most patients, but some of them may develop symptoms despite intense pre‐treatment and extra antiallergic medication received during the desensitisation procedure. For these special cases, omalizumab might be used as an adjuvant therapy to induce CHT tolerance.
Omalizumab has also been used in achieving tolerance to other drugs such as insulin, elosulfase ɑ, acetylsalicylic acid. 23 , 24 , 25 The largest evidence is regarding the use of omalizumab in acetylsalicylic acid desensitisation for aspirin exacerbated respiratory disease. 25
4. OMALIZUMAB
Omalizumab is a recombinant humanized anti‐IgE monoclonal antibody that specifically binds to the C‐ε‐3 domain of free IgE and the surface IgE of IgE expressing B cells but not to IgE bound to high or low affinity IgE receptors (FcεRI, respectively FcεRII) and therefore they do not trigger effector cell degranulation. 26 The complexes formed between omalizumab and IgE result in a significant decrease in free IgE in serum. They also prevent IgE from binding to effector cells, resulting in decreased mediator release in response to allergens 27 and in a reversible downregulation of FcεRI receptors on basophils, mast cells, and dendritic cells. 28 , 29 Recent studies have shown that Omalizumab is able to detach IgE from high affinity IgE receptors, although the exact mechanism is still under investigation. 30
It was approved for the treatment of severe allergic asthma, chronic spontaneous urticaria and severe chronic rhinosinusitis with nasal polyps. In addition, it has been studied as an add‐on therapy for immunotherapy and other disorders such as food allergy, atopic dermatitis, idiopathic anaphylaxis, inducible urticaria, allergic bronchopulmonary aspergillosis and mastocytosis. 31 , 32 The studies' results suggest that anti‐IgE monoclonal antibodies may have important immunotherapeutic benefits for the management of the above‐mentioned disorders. Although the complete mechanism of action of omalizumab is not fully understood, some authors suggest that it may reduce mast cell releasability, it reverses basopenia and improves basophil IgE receptor function. Also, it may reduce the activity of IgG autoantibodies against FcεRI and IgE. 33
5. OMALIZUMAB IN CHEMOTHERAPY‐RDD: CLINICAL EVIDENCE
Although omalizumab is not licensed as premedication or adjuvant therapy in CHT desensitisation protocol, there have been published some case reports and small sample size studies that indicated promising results. We have reviewed articles, published abstracts, and conference proceedings on Pubmed, Embase, Cochrane Library and Web of Science that were published before June 10, 2021. Medical Subject Heading and keywords were used together, including omalizumab, chemotherapy, oxaliplatin, carboplatin, desensitisation. This approach was also combined with a manual search of references in all selected studies. Until now, there have been published two clinical trials and ten case reports. 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 All the results are synthesized chronologically in Table 2.
TABLE 2.
Omalizumab in chemotherapy desensitisation—current data
| Author (Ref.) | Patients | Drug involved | Omalizumab dose | Interval between omalizumab doses (days) | Interval between omalizumab and CHT (days) | Number of omalizumab doses | Number of desensitisation protocols | Tolerance |
|---|---|---|---|---|---|---|---|---|
| Cahill, 2012 34 | 1 | Oxaliplatin | 150 mg | 14 | UNK | 6 | 5 | Mild reactions |
| Ojaimi, 2014 35 | 1 | Carboplatin | 300 mg | 14 | 1 | 9 | 5 | Yes |
| Saura, 2016 36 | 3 | Carboplatin | UNK | UNK | UNK | UNK | 15 | Yes |
| Garcia, 2016 37 | 1 | Carboplatin | UNK | UNK | UNK | UNK | UNK | Mild reaction |
| Hong, 2018 38 | 5 | CHT (platins, taxanes) or monoclonal antibodies | 300 mg | 28 | UNK | 3 | 3 | Mild reactions |
| Stein, 2018 39 | 9 | Oxaliplatin | 300 mg | 14 | 7 | Median = 5 (1–10) | UK | Yes |
| Prieto‐Garcia, 2019 40 | 1 | Oxaliplatin | 300 mg | 15 | 7 | 13 | 13 | Yes |
| De Las Vaceillas Sánchez, 2019 41 | 1 | Oxaliplatin | 600/300 mg | 14 | 1 | 6 | 5 | Yes |
| Cuevas, 2019 42 | 1 | Oxaliplatin | 300 mg | UNK | 6 | 1 | 1 | Anaphylaxis |
| Sanchez‐Morillas, 2020 43 | 2 | Carboplatin | 300/150 mg | 7/14 a | 1 | 9 | 6 | Yes |
| Carboplatin | 300/150 mg | 7/14 a | 1 | 6 | 4 | Mild reaction | ||
| Oude Elberink, 2020 44 | 1 | Carboplatin | 300 mg | 14 | 11 b | 9 | 9 | Yes |
| Penella, 2020 45 | 1 | Oxaliplatin | 300 mg | 14 | 19/14 c | 5 | 5 | Yes |
Abbreviations: CHT, chemotherapy; RDD, rapid drug desensitisation; UNK, unknown.
7 days between 300 and 150 mg of Oma; 14 days between the next 150 mg doses.
Before first desensitisation.
19 days between the first dose of Oma and CHT‐RDD, then 14 days.
The first description of omalizumab use as premedication for oxaliplatin desensitisation was made by Cahill et al. 34 in a 68‐year‐old patient with colon cancer who developed anaphylaxis to oxaliplatin. Initially, the patient received two doses of 150 mg omalizumab at 2 weeks interval, before a 16‐step desensitisation protocol. He tolerated four subsequent oxaliplatin desensitisation protocols with only mild reactions.
Ojaimi et al. 35 reported the case of a 63‐year‐old non‐atopic female who failed different desensitisation protocols to carboplatin, but after receiving premedication with omalizumab, she successfully completed five RDD (modified over 4 days). The authors used a series of nine fortnightly doses of 300 mg of omalizumab given subcutaneously. The first RDD was administered 1 day after the third dose of omalizumab. This is the only report that monitored both skin tests to carboplatin and total IgE values. Intradermic skin tests to carboplatin 10 mg/ml became negative and the value of total IgE decreased at the end of the study.
A series of three patients who benefited from pre‐treatment with omalizumab before chemotherapy desensitisation was reported by Saura et al. 36 All three cases had a history of carboplatin anaphylactic reaction (proven by tryptase elevation) during classical desensitisation protocols. By using omalizumab before RDD, all three patients tolerated the total number of 15 desensitisation procedures.
Another case report of successful use of omalizumab as premedication to carboplatin desensitisation was described by Garcia et al in a 52‐year‐old female. 37
Hong's study 38 included five patients who had IgE‐mediated reaction to CHT including platins, taxanes, or monoclonal antibodies and a breakthrough reaction during desensitisation protocol. The participants received a dose of 300 mg omalizumab every four weeks, at three consecutive visits. There is no information about the time frame between omalizumab administration and CHT‐RDD, except that the two drugs were not given on the same day. Out of these five patients, only three concluded the study protocol (one dropped out due to cytokine storm‐like reaction and another due to disease progression). The investigator concluded that omalizumab did not completely abrogate breakthrough reactions in all patients, but the symptoms were milder and the three patients reported significantly less symptoms during desensitisation protocols.
Stein et al. 39 reported a small sample size study that enrolled nine patients diagnosed with stage IV colon cancer and oxaliplatin HSRs during chemotherapy protocol. Omalizumab was administered in a fixed dose of 300 mg every 2 weeks, alternating with Oxaliplatin chemotherapy, administered also at 2 weeks interval. First dose of omalizumab was administered 7 days prior to Oxaliplatin. Only one patient experienced a HSR during the first cycle of oxaliplatin. The other patients were able to tolerate the desensitisation protocols, the median number of cycles was 5 (range 1–10). The reasons for dropping out were disease progression or chemotherapy related side effects.
Omalizumab was also helpful in achieving tolerance to oxaliplatin in a patient who suffered anaphylaxis after completing the desensitisation protocol. Omalizumab 300 mg was administered every 15 days, one week before the chemotherapeutic agent and the patient was able to tolerate 13 more cycles. 40
A different dose regimen was used by De Las Vecillas Sánchez et al. 41 in a patient with severe anaphylaxis (with elevated tryptase level) during desensitisation protocol to oxaliplatin. Two doses of omalizumab (600 mg and after 2 weeks another 300 mg) were administered 15 days, respectively 1 day prior to RDD. The patient had a mild reaction with slight increase of the tryptase level; subsequently, she tolerated four more RDD with omalizumab premedication with no noticeable reactions. The high sensitisation to oxaliplatin and the elevated total IgE (>5000 kU/L) might be the reason for deciding on the high initial dose of omalizumab.
The only unsuccessful reported omalizumab‐RDD was described in a patient who received 300 mg 6 days prior to oxaliplatin desensitisation. 42 Clinical symptoms and increased tryptase levels were reported during the first step of the desensitisation protocol. No other attempt to achieve tolerance was noted.
Sanchez‐Morillas et al. 43 reported two patients with severe anaphylactic reactions during CHT (carboplatin) who were premedicated with omalizumab in RDD. The authors did not recommend a previous RDD in the absence of omalizumab premedication. Initially, both patients received 300 mg omalizumab; 7 days later they received another 150 mg. This course of action was performed twice a month, until the end of chemotherapy.
The 16 steps chemotherapy desensitisation protocols were performed 24 h after the second dose of omalizumab. The first patient tolerated six cycles of carboplatin desensitisation, while the second one was able to finish only four cycles but with mild cutaneous reactions that were resolved with antihistamines and corticosteroids. As a conclusion, for patients with a history of CHT‐induced severe anaphylactic reaction, the authors propose a bimonthly 300 mg omalizumab premedication protocol, with at least one dose administered before the first RDD. 43
This premedication plan was adapted by Oude Elberink et al. 44 in a patient with a successful carboplatin desensitisation schedule after receiving omalizumab 300 mg fortnightly, with the first dose given 11 days before the RDD. Interestingly, in this case report, omalizumab was also used as premedication when a new cycle of desensitisation protocol to carboplatin was needed because of the relapse of the ovarian cancer.
Prolonging the desensitisation protocol to oxaliplatin alongside premedication with 300 mg omalizumab have proven successful in a patient with metastatic sigmoid adenocarcinoma who experienced anaphylaxis during standard desensitisation. On the first cycle, the time frame between omalizumab administration and chemotherapy was 19 days, the interval being shortened to 14 days on four subsequent administrations. The authors highlighted the decrease of total IgE value from 4690 to 575 kU/L. 45
6. DISCUSSION
CHT‐RDD must be used for oncologic patients when no other therapeutical options with the same benefits are available. In case of RDD failure, omalizumab might be used as an adjuvant therapy and might be a solution for a hopeless situation. Although the main anti‐IgE mechanism of action of omalizumab is well defined, its complete mastermind of processes in other diseases, including in RDD, is still enigmatic.
It makes sense to try inducing tolerance by using omalizumab in patients who fail platinum salts desensitisation, because the underlying mechanism of platinum salts‐induced hypersensitivity is IgE‐mediated. All the aforementioned studies included patients diagnosed with platinum salt‐hypersensitivity. The selection of omalizumab dose was independent of the patients' weight or the total IgE values, opposed to asthma or nasal polyposis. Instead, the choice of omalizumab dose was like the one used in chronic urticaria.
We found a great heterogeneity between the doses and the interval between omalizumab injections and CHT‐RDD; some studies used the anti‐IgE therapy only before the first RDD, but most of the studies used it before all the subsequent RDD.
Most of the authors preferred to administer omalizumab fortnightly, except Hong et al. 38 The time interval between the last omalizumab injection and the desensitisation protocol varied largely, ranging from 24 h to 19 days. Some of the authors preferred using every other week regimen for omalizumab, while administering the chemotherapy as prescribed by the oncologist every 21 days. 35 , 44
As a corollary of these results, we propose a dose regimen of 300 mg omalizumab administered twice before the first CHT‐RDD, 15 days and 1 day prior to the first CHT infusion. Then, omalizumab should be administered one day before the CHT regimen, while the interval between CHT might vary depending on the drug used in the respective oncologic disease (Figure 1).
FIGURE 1.
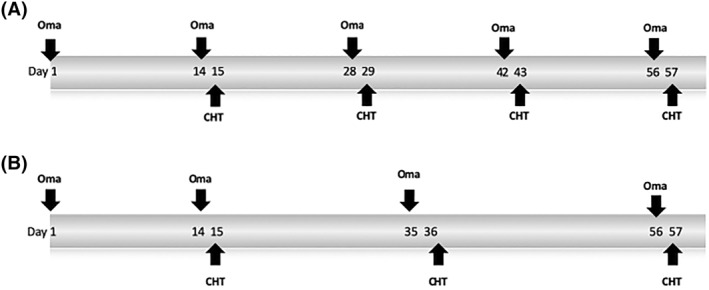
Timeframe between Omalizumab (Oma) and CHT‐RDD: (A) Patients whose CHT regimen is every 14 days and (B) Patients whose CHT regimen is every 21 days. CHT, chemotherapy; RDD, rapid drug desensitisation
The results of most of the studies were promising, the patients tolerated subsequent CHT‐RDD without HSRs or accompanied by mild reactions only. Unfortunately, a homogeneous definition of mild reactions was not given in the above‐mentioned studies.
7. FINAL REMARKS
RDD has become a key component of the management of CHT‐HSR. It is the only effective approach for overcoming the HSR to first‐line therapy, thus representing an important progression in patients' treatment and prognosis. Understanding the mechanism of action implied to RDD will allow improvement in patients' treatment.
Omalizumab, an anti‐IgE drug, may be used as an adjuvant therapy in RDD for patients with IgE‐mediated CHT‐HSRs in troublesome desensitisations despite premedication. Although the above‐mentioned case reports and small size clinical trials showed promising results, further studies with larger number of patients are necessary for setting up standard recommendations for omalizumab adjuvant‐RDD protocol.
CONFLICT OF INTEREST
All authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
Roxana Silvia Bumbacea and Dragos Bumbacea designed and supervised the study. Selda Ali, Sabina Loredana Corcea and Roxana Silvia Bumbacea performed the research. Selda Ali, Sabina Loredana Corcea, Cornelia Nitipir and Victor Strambu reviewed the references. Selda Ali, Sabina Loredana Corcea, Victor Strambu and Luiza Spiru wrote the manuscript. Luiza Spiru, Sabina Loredana Corcea and Victor Strambu contributed to tables and figure. Dragos Bumbacea and Roxana Silvia Bumbacea revised the article critically for important intellectual content. All authors read and approved the final manuscript for publication
ACKNOWLEDGEMENT
The authors would like to thank Rama Boustani and Ruxandra Udrea for their help and support with the language editing and figure design.
Bumbacea RS, Ali S, Corcea SL, et al. Omalizumab for successful chemotherapy desensitisation: What we know so far. Clin Transl Allergy. 2021;e12086. 10.1002/clt2.12086
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. De Vita Junior VT, Lawrence TS, Rosenberg, SA . Principles and Practice of Oncology. 11th ed. Wolter Kluver Health; 2018. chapter 20. [Google Scholar]
- 2. Syrigou E, Syrigos K, Saif MW. Hypersensitivity reactions to oxaliplatin and other antineoplastic agents. Curr Allergy Asthma Rep. 2008;8:56‐62. [DOI] [PubMed] [Google Scholar]
- 3. Baldo BA, Pagani M. Adverse events to nontargeted and targeted chemotherapeutic agents: emphasis on hypersensitivity responses. Immunol Allergy Clin North Am. 2014;34(3):565‐596. viii. [DOI] [PubMed] [Google Scholar]
- 4. Syrigou E, Syrigos K, Saif MW. Hypersensitivity reactions to oxaliplatin and other antineoplastic agents. Curr Allergy Asthma Rep. 2008;8(1):56‐62. 10.1007/s11882-008-0011-0. PMID: 18377776. [DOI] [PubMed] [Google Scholar]
- 5. Jerschow E, Lin RY, Scaperotti MM, McGinn AP. Fatal anaphylaxis in the United States, 1999‐ 2010: temporal patterns and demographic associations. J Allergy Clin Immunol. 2014;134:1318‐1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. RibeiroVaz I, Marques J, Demoly P, Polonia J, Gomes ER. Drug‐induced anaphylaxis: a decade review of reporting to the Portuguese Pharmacovigilance Authority. Eur J Clin Pharmacol. 2013;69:673‐681. [DOI] [PubMed] [Google Scholar]
- 7. Pagani M, Bavbek S, Alvarez‐Cuesta E, et al. Hypersensitivity reactions to chemotherapy: an EAACI position paper. Allergy. 2021. [DOI] [PubMed] [Google Scholar]
- 8. Bonamichi‐Santos R, Castells M. Desensitization for drug hypersensitivity to chemotherapy and monoclonal antibodies. Curr Pharm Des. 2016;22:6870‐6880. [DOI] [PubMed] [Google Scholar]
- 9. Pagani M. The complex clinical picture of presumably allergic side effects to cytostatic drugs: symptoms, pathomechanism, reexposure, and desensitization. Med Clin North Am. 2010;94(4):835‐852. xiii. [DOI] [PubMed] [Google Scholar]
- 10. Herrero T, Tornero P, Infante S, et al. Diagnosis and management of hypersensitivity reactions caused by oxaliplatin. J Investig Allergol Clin Immunol. 2006;16(5):327‐330. PMID: 17039675. [PubMed] [Google Scholar]
- 11. Caiado J, Castells M. Presentation and diagnosis of hypersensitivity to platinum drugs. Curr Allergy Asthma Rep. 2015 Apr;15(4):15. 10.1007/s11882-015-0515-3. PMID: 26130472. [DOI] [PubMed] [Google Scholar]
- 12. Shepherd GM. Hypersensitivity reactions to chemotherapeutic drugs. Clin Rev Allergy Immunol. 2003;24:253‐262. [DOI] [PubMed] [Google Scholar]
- 13. Picard M, Castells MC. Re‐visiting hypersensitivity reactions to taxanes: a comprehensive review. Clin Rev Allergy Immunol. 2015 Oct;49(2):177‐191. 10.1007/s12016-014-8416-0. PMID: 24740483. [DOI] [PubMed] [Google Scholar]
- 14. Otani IM, Wong J, Banerji A. Platinum chemotherapy hypersensitivity: prevalence and management. Immunol Allergy Clin North Am. 2017;37(4):663‐677. 10.1016/j.iac.2017.06.003. PMID: 28965633. [DOI] [PubMed] [Google Scholar]
- 15. Castells MC, Tennant NM, Sloane DE, et al. Hypersensitivity reactions to chemotherapy: outcomes and safety of rapid desensitization in 413 cases. J Allergy Clin Immunol. 2008;122(3):574‐580. [DOI] [PubMed] [Google Scholar]
- 16. Leguy‐Seguin V, Jolimoy G, Coudert B, et al. Diagnostic and predictive value of skin testing in platinum salt hypersensitivity. J Allergy Clin Immunol. 2007;119(3):726‐730. 10.1016/j.jaci.2006.11.640. PMID: 17258305. [DOI] [PubMed] [Google Scholar]
- 17. Castells M, Sancho‐Serra Mdel C, Simarro M. Hypersensitivity to antineoplastic agents: mechanisms and treatment with rapid desensitization. Cancer Immunol Immunother. 2012;61(9):1575‐1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. De Angelo DJ, Arellano M, Advani A, et al. Prevention and management of asparaginase/pegasparaginase‐associated toxicities in adults and older adolescents: recommendations of an expert panel. Leuk Lymphoma. 2011;52:2237‐2253. [DOI] [PubMed] [Google Scholar]
- 19. Pagani M, Bavbek S, Dursun AB, et al. Role of skin tests in the diagnosis of hypersensitivity reactions to taxanes: results of a multicentre study. J Allergy Clin Immunol Pract. 2018;7:990‐997. [DOI] [PubMed] [Google Scholar]
- 20. Polyzos A, Tsavaris N, Kosmos C, et al. Hypersensitivity reactions to carboplatin administration are common but not always severe. A 10‐year experience. Oncology. 2001;61:129‐133. [DOI] [PubMed] [Google Scholar]
- 21. Brandi G, Pantaleo MA, Galli C, et al. Hypersensitivity reactions related to oxaliplatin (OHP). Br J Cancer. 2003;89:477‐481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Morales AR, Shah N, Castells M. Antigen‐IgE desensitization in signal transducer and activator of transcription 6‐deficient mast cells by suboptimal doses of antigen. Ann Allergy Asthma Immunol. 2005;94(5):575‐580. 10.1016/S1081-1206(10)61136-2. PMID: 15945561. [DOI] [PubMed] [Google Scholar]
- 23. Matheu V, Franco A, Perez E, Hernández M, Barrios Y. Omalizumab for drug allergy. J Allergy Clin Immunol. 2007;120(6):1471‐1472. author reply 1472‐3. 10.1016/j.jaci.2007.07.037. PMID: 17854881. [DOI] [PubMed] [Google Scholar]
- 24. Arroabarren E, Aznal E, Anda M, Sanchez‐Valverde F. Anaphylaxis after Elosulfase A infusion: omalizumab as coadjuvant for enzyme replacement therapy desensitization. Pediatr Allergy Immunol. 2019;30(4):491‐494. 10.1111/pai.13049. PMID: 30817035. [DOI] [PubMed] [Google Scholar]
- 25. Waldram J, Walters K, Simon R, Woessner K, Waalen J, White A. Safety and outcomes of aspirin desensitization for aspirin‐exacerbated respiratory disease: a single‐center study. J Allergy Clin Immunol. 2018;141(1):250‐256. 10.1016/j.jaci.2017.05.006. PMID: 28550988. [DOI] [PubMed] [Google Scholar]
- 26. Stokes J. Anti‐IgE treatment for disorders other than asthma. Front Med (Lausanne). 2017;4:152. 10.3389/fmed.2017.00152. PMID: 28983485; PMCID: PMC5613080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. MacGlashan D. Loss of receptors and IgE in vivo during treatment with anti‐ IgE antibody. J Allergy Clin Immunol. 2004;114:1472‐1474. [DOI] [PubMed] [Google Scholar]
- 28. Beck LA, Marcotte GV, MacGlashan D, Togias A, Saini S. Omalizumab‐induced reductions in mast cell FceRI expression and function. J Allergy Clin Immunol. 2004;114:527‐530. [DOI] [PubMed] [Google Scholar]
- 29. Fernandez J, Ruano‐Zaragoza M, Blanca‐Lopez N. Omalizumab and other biologics in drug desensitization. Curr Opin Allergy Clin Immunol. 2020;20(4):333‐337. 10.1097/ACI.0000000000000648. PMID: 32398420. [DOI] [PubMed] [Google Scholar]
- 30. Maggi L, Rossettini B, Montaini G, et al. Omalizumab dampens type 2 inflammation in a group of long‐term treated asthma patients and detaches IgE from FcεRI. Eur J Immunol. 2018;48(12):2005‐2014. 10.1002/eji.201847668. PMID: 30252930. [DOI] [PubMed] [Google Scholar]
- 31. Dantzer JA, Wood RA. The use of omalizumab in allergen immunotherapy. Clin Exp Allergy. 2018;48(3):232‐240. 10.1111/cea.13084. PMID: 29315922. [DOI] [PubMed] [Google Scholar]
- 32. Bumbăcea RS, Deaconu CG, Berghea EC. Management problems in severe chronic inducible urticaria: two case reports. Exp Ther Med. 2019;18(2):960‐963. 10.3892/etm.2019.7651. PMID: 31384330; PMCID: PMC6640052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kaplan AP, Giménez‐Arnau AM, Saini SS. Mechanisms of action that contribute to efficacy of omalizumab in chronic spontaneous urticaria. Allergy. 2017;72(4):519‐533. 10.1111/all.13083. PMID: 27861988; PMCID: PMC5915348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cahill KN, Harrison P, de Asis M, Castells M. Use of omalizumab to achieve successful desensitization after oxaliplatin anaphylaxis [Abstract]. J Allergy Clin Immunol. 2012;129(2):AB103. 10.1016/j.jaci.2011.12.515 [DOI] [Google Scholar]
- 35. Ojaimi S, Harnett PR, Fulcher DA. Successful carboplatin desensitization by using omalizumab and paradoxical diminution of total IgE levels. J Allergy Clin Immunol Pract. 2014;2(1):105‐106. 10.1016/j.jaip.2013.08.009. PMID: 24565780. [DOI] [PubMed] [Google Scholar]
- 36. Saura Foix MP, Fernandez AP, Garcia Gonzalez F, et al. Use of omalizumab in carboplatin desensitisation, our experience. Allergy. 2016;71(Suppl 102):505‐527. 10.1111/all.12477 26687298 [DOI] [Google Scholar]
- 37. Garcia‐Campos J, Perez‐Padilla CI, Posadas‐Miranda T, Requena‐Quesada G, Campos‐Suarez G, De la Higuera Artesero R. Use of omalizumab as preventive treatment in a carboplatin’s desensitization Poster Session TPS 19‐33. Allergy. 2016;71:413‐504. 10.1111/all.12976 [DOI] [Google Scholar]
- 38. Hong DI. Omalizumab as adjuvant therapy in chemotherapy desensitization [Abstract]. J Allergy Clin Immunol. 2018;141(Suppl. 2):AB410. 10.1016/j.jaci.2017.12.968 [DOI] [Google Scholar]
- 39. Stein S, Uboha NV, Dooley K, Hochster HS. A pilot study of omalizumab to reduce the incidence of oxaliplatin‐induced hypersensitivity reaction (HSR): an interim analysis. J Clin Oncol. 2018;36(Suppl 15):e15555. 10.1200/JCO.2018.36.15_suppl.e15555 [DOI] [PubMed] [Google Scholar]
- 40. Prieto‐García A, Noguerado B, Rojas P, Torrado I, Rodríguez‐Fernández A, Tornero P. Unexpected anaphylaxis after completing a desensitization protocol to oxaliplatin: successful adjuvant use of omalizumab. J Investig Allergol Clin Immunol. 2019;29(1):53‐55. 10.18176/jiaci.0326. PMID: 30785102. [DOI] [PubMed] [Google Scholar]
- 41. De Las Vecillas Sánchez L, Lopez C, Peñasco Y, et al. Successful high risk oxaliplatin desensitization using omalizumab. Allergy. 2019;74:736. [Google Scholar]
- 42. Cuevas C, Tornero P, Garcia‐Gutierrez I, Rojas P, Torrado I, Prieto A. Failure of adjuvant omalizumab in drug desensitization. Allergy. 2019;74:736. [Google Scholar]
- 43. Sánchez‐Morillas L, Casado Herráez A, Rubio‐Perez M, et al. Usefulness of omalizumab in rapid drug desensitization in patients with severe anaphylaxis induced by carboplatin: open questions. J Investig Allergol Clin Immunol. 2020;30(4):298‐300. 10.18176/jiaci.0499. PMID: 32101175. [DOI] [PubMed] [Google Scholar]
- 44. Oude Elberink HNG, Jalving M, Dijkstra H, van de Ven AAJM. Modified protocol of omalizumab treatment to prevent carboplatin‐induced drug hypersensitivity reactions: a case study. Clin Transl Allergy. 2020;10:5. 10.1186/s13601-020-0309-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Penella J, Quan P, Carvallo A, et al. Successful desensitization to oxaliplatin after a single initial dose of omalizumab in a patient with elevated IgE levels. J Investig Allergol Clin Immunol. 2020;30(4):293‐295. 10.18176/jiaci.0496. PMID: 32101174. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


