We and others previously reported persistent immunogenicity of anti-severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccines in the first month after kidney transplantation (KT) with, nevertheless, a significant drop in antibody (Ab) titer.1-3 However, the mid-term impact of starting immunosuppression for KT on Ab titers has not been described yet.
We report the evolution of anti-SARS-CoV-2 Ab titer 100 d after KT in 14 KT recipients (KTRs) transplanted between January 2021 and June 2021 who received at least 1 vaccine dose before KT.
We assessed the antireceptor-binding domain Ab titers (Elecsys anti-SARS-CoV-2, Roche Diagnostics GmbH, Mannheim, Germany; positive threshold ≥0.8 U/mL) on the day of transplantation (day 0), 28 d after transplantation (day 28), and 100 d after transplantation (day 100). This work received institutional review board approval.
Ten patients had received their second dose of either the mRNA BNT162b2 (Pfizer-BioNTech, n = 8) or ChAdOx1 nCoV-19 vaccine (AstraZeneca, n = 2) at a mean time of 57 (range, 25–78) d before KT.1 Four patients had received a single dose of vaccine (Pfizer-BioNTech n = 2, AstraZeneca n = 2) at a mean time of 29 (range, 13–50) d before KT, of which 2 patients received a second dose of the same vaccine 2 mo after KT. The mean age was 58 (range, 32–77) y; 57% were male. Twelve patients were on dialysis before transplantation (mean duration, 54 [range, 20–122] mo). One patient had prior history of coronavirus disease 2019 (COVID-19).
Sensitized patients (n = 3) and KTRs who received a graft from a living donor (n = 2) were inducted with thymoglobulin and basiliximab, respectively. All patients received an association of tacrolimus, mycophenolate, and steroids. None developed COVID-19 infection to date.
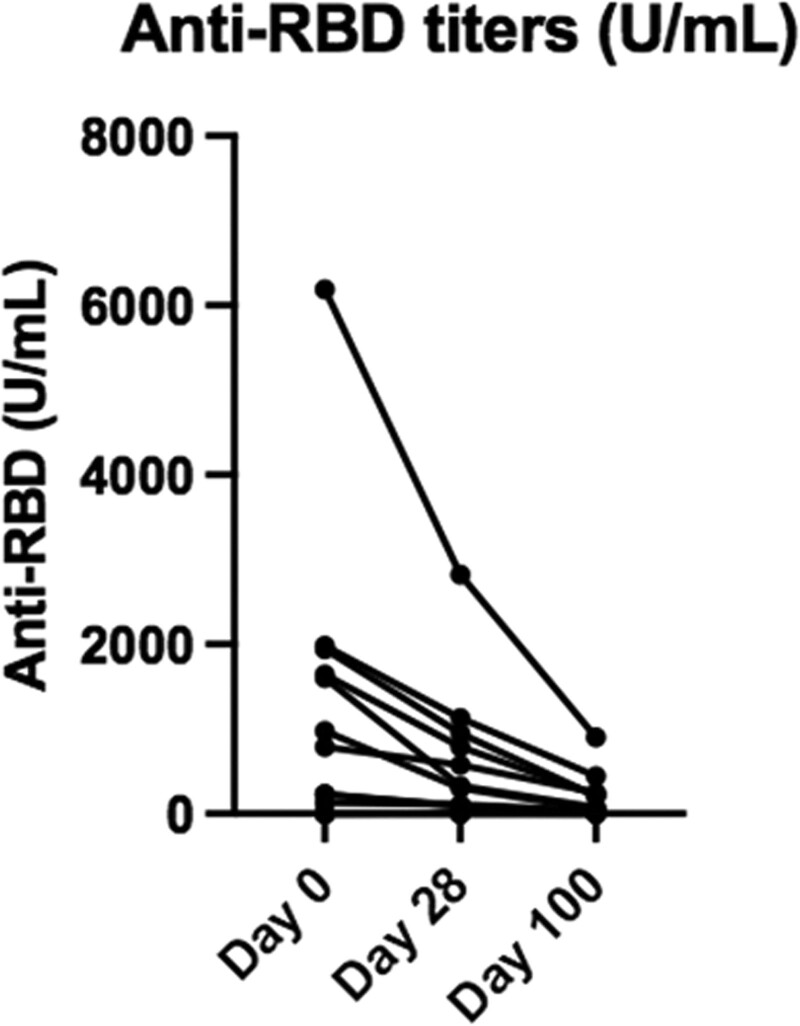
Anti-SARS-CoV-2–receptor-binding domain (RBD) Ab levels significantly decreased from day 0 to 100, with mean (±SEM) titers at 1124 ± 441, 514 ± 205, and 146 ± 39 U/mL at day 0, 28, and 100, respectively (Figure 1). Compared with day 0, it represents a 54% (P = 0.0034) and 87% (P = 0.0015) drop in Ab titers at day 28 and 100, respectively. The patient with a history of COVID-19 before KT showed a similar decline (54% and 85% drop at day 28 and 100, respectively). Of the 4 patients who had received a single dose of vaccine, 2 had no detectable Ab on day 100 (despite one of them receiving a second dose in the meantime) and 2 had very low Ab titers (<1.5 U/mL). Similar to our previous report,1 the Ab titer drop was not significantly different in patients inducted with thymoglobulin (n = 3) or basiliximab (n = 2) compared with the others (90% and 82% drop, respectively, versus 86% at day 100).
FIGURE 1.
Evolution of anti-RBD antibody titer. Mean (±SEM) antibody titer on d 0, 28, and 100: 1124 (±441), 514 (±205), and 146 (±39) U/mL (P = 0.003, 0.002; Wilcoxon test). RBD, receptor-binding domain; SEM, standard error of the mean.
Our results contrast with the long-term persistent humoral response in immunocompetent hosts.4 Doria-Rose et al showed that anti-SARS-CoV-2-RBD Ab activity remained high 6 mo after the second dose of mRNA-1273 vaccine. This suggests that immunosuppressive therapy hinders the immunization rate in KTRs5 and reduces the mid-term immunogenicity of mRNA vaccines administered before KT, which highlights the clinical relevance of a booster dose of vaccine early after KT. The optimal timing to administrate this additional dose needs further investigation.
Footnotes
G.F., A.D., and A.S. participated equally in this work.
The authors declare no funding or conflicts of interest.
G.F., A.D., A.S., and N.K. participated in conceptualization of the research idea, study design, and data analysis. G.F. participated in data acquisition. A.S. and B.K. performed serologic analysis. A.S. performed statistical analysis. G.F., A.D., A.S., B.K., M.D.M., M.M., T.D., A.B., J.D.G., L.B., J.C.Y., E.G., and N.K. took care of the patients. All authors discussed and reviewed the article.
REFERENCES
- 1.Fernandes G, Devresse A, Scohy A, et al. Impact of kidney transplantation on humoral immunity against SARS-CoV-2: a case-series from Belgium. Transplantation. 2021;105:e257–e258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yi SG, Eager T, Moore L, et al. Persistent immunogenicity of the mRNA COVID-19 vaccine in patients vaccinated before kidney transplant. Transplantation. 2021;105:e133–e134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mohamadou I, Nkok J, Galichon P, et al. Immediate impact of induction treatment on post vaccination SARS-Cov-2 serology in kidney transplant recipients. Transplantation. 2021;105:e135–e136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Doria-Rose N, Suthar MS, Makowski M, et al. ; mRNA-1273 Study Group. Antibody persistence through 6 months after the second dose of mRNA-1273 vaccine for Covid-19. N Engl J Med. 2021;384:2259–2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Georgery H, Devresse A, Yombi J-C, et al. Disappointing immunization rate after 2 doses of the BNT162b2 vaccine in a Belgian cohort of kidney transplant recipients. Transplantation. 2021;105:e283–e284. [DOI] [PMC free article] [PubMed] [Google Scholar]