Abstract
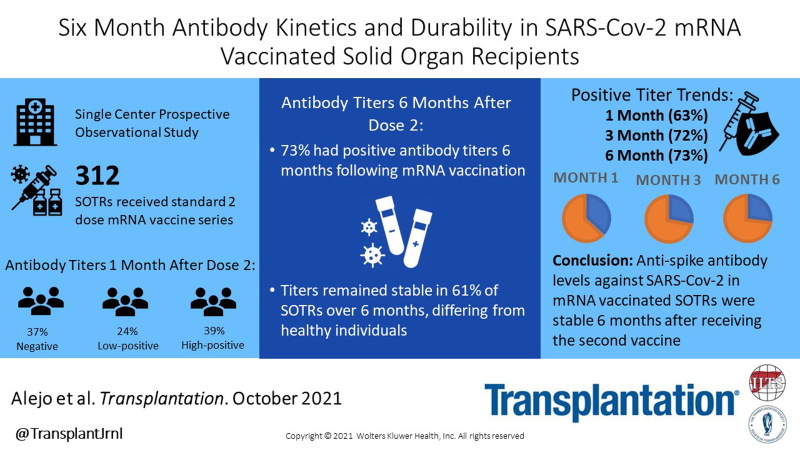
We previously reported the overall stability of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) antispike antibodies in vaccinated solid organ transplant recipients (SOTRs) 3 mo after receiving 2 doses of BNT162b2 or mRNA-1237 vaccines.1 In the interim, the US Food and Drug Administration fully authorized the BNT162b2 vaccine for people 16 y and older and expanded Emergency Use Authorization to allow for a third dose in immunocompromised individuals.2 The Centers for Disease Control and Prevention currently recommends an additional dose for mild to moderately immunocompromised patients at least 28 d following mRNA dose 2, but optimal timing for booster doses in SOTRs has not been determined.3 Declines in SARS-CoV-2 antibodies over time were observed 3 mo postvaccination in the general population,4 but antibody longevity in SOTRs is unknown. Semi-quantitative antispike antibody testing was performed using the Roche Elecsys (R) anti-SARS-CoV-2 S or the EUROIMMUN IgG enzyme immunoassays 1, 3, and 6 mo after dose 2. Participants provided informed consent, and this study was approved by the Johns Hopkins Institutional Review Board (IRB00248540).
Three hundred twelve SOTRs received a second mRNA vaccine between January 6, 2021, and April 14, 2021. None reported a third dose during the study period. Median (interquartile range) age was 62 (48–69) y, 65% were female, and 92% were White. One hundred fifty-six (50%) were kidney transplant recipients, and median time from transplant was 7.2 (3.3–14.3) y. Regarding immunosuppression, 224 (72%) were taking an antimetabolite, 250 of 312 (80%) tacrolimus, and 169 of 312 (54%) steroids. At median 29 (28–32) d after dose 2, 198 of 312 (63%) patients had positive titers; at median 91 (89–94) d after dose 2, 178 of 246 (72%) had positive titers, and at 181 (174–186) d after dose 2, 227 of 312 (72%) had positive titers. Forty-three of 114 (38%) patients with negative 1-mo titers developed positive titers by 6 mo; 12 of 43 had high-positive titer levels at 6 mo. Among 198 patients with positive titers at 1 mo, 14 (7.1%) fell below the threshold of positivity at 6 mo (Table 1). The 4 patients whose titers fell from high positive to negative experienced this by 3 mo.
TABLE 1.
Antispike antibody sero-response 6 mo after receiving second mRNA SARS-CoV-2 vaccine stratified by antibody response 1 mo after receiving second mRNA vaccine, N (%)
| Sero-response after 6 mo | |||||
|---|---|---|---|---|---|
| Negative | Low positive | High positive | Totals (1 mo) | ||
| Sero-response after 1 mo | Negative | 71 (62.3) | 31 (27.2) | 12 (10.5) | 114 (36.5) |
| Low positive | 9 (12.0) | 24 (32.0) | 42 (56.0) | 75 (24.0) | |
| High positive | 5 (4.1) | 23 (18.7) | 95 (77.2) | 123 (39.4) | |
| Totals (6 mo) | 85 (27.2) | 78 (25) | 149 (47.8) | 312 (100) | |
SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
In this study of SARS-CoV-2 antibody kinetics and durability, 73% of SOTRs had positive antibody titers 6 mo following mRNA vaccination; titers increased in 27% over 6 mo, decreased in 12%, and remained stable in 61% of patients over 6 mo. This differs from healthy individuals, who had overall stability of antibody positivity over 6 mo.5 It is unknown what degree of antibody titer decline confers increased risk of breakthrough infection.
This study is limited by the lack of an immunocompetent control group, the unknown correlation with neutralizing antibody, and the absence of antinucleocapsid testing, prohibiting analysis of asymptomatic exposure. The presence of coronavirus disease 2019 infection would likely increase antibody levels.
In conclusion, antispike antibody levels against SARS-CoV-2 in SOTRs are relatively stable 6 mo after receipt of the second vaccine. Further investigation into the effect of booster vaccination on antibody titers, as well as understanding B- and T-cell responses against emerging variants of concern, is needed to better inform timing of booster vaccination.
ACKNOWLEDGMENTS
The authors thank the Johns Hopkins transplant vaccine study team, including Mayan Teles, BS; Julia Lopez, BS; Michael T. Ou, BS; Ross S. Greenberg, BA; Jake A. Ruddy, BS; Muhammad Asad Munir, MBBS; Michelle R. Krach, MS; and Iulia Barbur, BSE. They also thank Andrew H. Karaba, MD, PhD, and Ms. Yolanda Eby for project support and guidance.
Footnotes
J.L.A., J.M., T.P.-Y.C., A.T.A., C.N.S., B.J.B., R.K.A., A.A.R.T., M.L.L., D.S.W., A.B.M., J.M.G.-W., D.L.S., and W.A.W. participated in conception or design of the work, the acquisition, analysis, or interpretation of data for the work, and drafting or revising the work critically for important intellectual content. J.L.A., J.M., T.P.-Y.C., A.T.A., B.J.B., R.K.A., A.A.R.T., M.L.L., A.B.M., J.M.G.-W., D.L.S., and W.A.W. gave final approval of the version to be published. J.L.A., J.M., T.P.-Y.C., A.T.A., C.N.S., B.J.B., R.K.A., A.A.R.T., M.L.L., D.S.W., A.B.M., J.M.G., D.L.S., and W.A.W. agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
This research was made possible with the generous support of the Ben-Dov family. This work was supported by the ASTS Fryer Resident Scientist Award (J.M.); grants T32DK007713 (J.L.A.), F32DK124941 (B.J.B), K01DK114388-03 (M.L.L.), K01DK101677 (A.B.M.), and K23DK115908 (J.M.G.-W.) from the National Institute of Diabetes and Digestive and Kidney Diseases; grant K24AI144954 (D.L.S.) from the National Institute of Allergy and Infectious Disease; and grant K23AI157893 (W.A.W.).
The analyses described here are the responsibility of the authors alone and do not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US government.
D.L.S. received consulting and speaking honoraria from Sanofi, Novartis, CLS Behring, Jazz Pharmaceuticals, Veloxis, Mallinckrodt, and Thermo Fisher Scientific. M.L.L. is the Social Media Editor for Transplantation. R.K.A. has grant/research support from Aicuris, Astellas, Chimerix, Merck, Oxford Immunotec, Qiagen, and Takeda/Shire. The other authors declare no conflicts of interest.
REFERENCES
- 1.Boyarsky BJ, Chiang TP, Teles AT, et al. Antibody kinetics and durability in SARS-CoV-2 mRNA vaccinated solid organ transplant recipients. Transplantation. 2021:105:e137–e138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.FDA Office of Media Affairs. Coronavirus (COVID-19) update: FDA authorizes additional vaccine dose for certain immunocompromised individuals. Available at https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-additional-vaccine-dose-certain-immunocompromised. Published 2021. Accessed August 12, 2021.
- 3.National Center for Immunization and Respiratory Diseases (NCIRD), Division of Diseases. COVID-19 vaccines for moderately to severely immunocompromised people. Available at https://www.cdc.gov/coronavirus/2019-ncov/vaccines/recommendations/immuno.html. Published 2021. Accessed September 10, 2021.
- 4.Widge AT, Rouphael NG, Jackson LA, et al. ; mRNA-1273 Study Group. Durability of responses after SARS-CoV-2 mRNA-1273 vaccination. N Engl J Med. 2021;384:80–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Doria-Rose N, Suthar MS, Makowski M, et al. ; mRNA-1273 Study Group. Antibody persistence through 6 months after the second dose of mRNA-1273 vaccine for COVID-19. N Engl J Med. 2021;384:2259–2261. [DOI] [PMC free article] [PubMed] [Google Scholar]