Supplemental digital content is available in the text.
Key Words: Post-acute COVID-19; Fatigue; Cognition; Employment, Quality of life
Objective
This report describes persistent symptoms associated with post-acute COVID-19 syndrome (PACS) and the impact of these symptoms on physical function, cognitive function, health-related quality of life, and participation.
Design
This study used a cross-sectional observational study design. Patients attending Mount Sinai’s post-acute COVID-19 syndrome clinic completed surveys containing patient-reported outcomes.
Results
A total of 156 patients completed the survey, at a median (range) time of 351 days (82–457 days) after COVID-19 infection. All patients were prevaccination. The most common persistent symptoms reported were fatigue (n = 128, 82%), brain fog (n = 105, 67%), and headache (n = 94, 60%). The most common triggers of symptom exacerbation were physical exertion (n = 134, 86%), stress (n = 107, 69%), and dehydration (n = 77, 49%). Increased levels of fatigue (Fatigue Severity Scale) and dyspnea (Medical Research Council) were reported, alongside reductions in levels of regularly completed physical activity. Ninety-eight patients (63%) scored for at least mild cognitive impairment (Neuro-Qol), and the domain of the EuroQol: 5 dimension, 5 level most impacted was Self-care, Anxiety/Depression and Usual Activities.
Conclusions
Persistent symptoms associated with post-acute COVID-19 syndrome seem to impact physical and cognitive function, health-related quality of life, and participation in society. More research is needed to further clarify the relationship between COVID-19 infection and post-acute COVID-19 syndrome symptoms, the underlying mechanisms, and treatment options.
What Is Known
Post-acute COVID-19 syndrome (PACS, or long COVID) is characterized by persistent and debilitating symptoms that are still present at least 4 wks after initial infection. Symptoms often occur in the absence of severe acute infection or preexisting comorbidities. Millions of Americans are at risk of developing PACS.
What Is New
The presence of persistent PACS symptoms negatively impacts physical and cognitive function, health-related quality of life, and participation in society. Physical exertion and dehydration are the major factors causing symptom exacerbation. Sixty-three percent of patients scored for at least mild cognitive impairment.
After the dramatic influx of patients with persistent, debilitating symptoms after acute SARS-CoV-2 (COVID-19) infection, the National Institutes of Health announced an initiative to fully investigate the post-acute sequelae of COVID-19 (post-acute COVID-19 syndrome [PASC]). Post-acute COVID-19 syndrome can take many forms, from post–intensive care unit syndrome1 to pulmonary fibrosis secondary to aggressive COVID-19 pneumonia.2 However, PACS (also known as long COVID) is one of the most troubling manifestations of PASC that has been reported to date. It is characterized by persistent symptoms that are still present at least 4 wks after initial infection and often lasting for several months.3 Despite the highly debilitating nature of PACS, the long-lasting symptoms often occur in the absence of severe acute infection, medically explainable physical symptoms, or preexisting comorbidities.4–6
Several studies have documented the most common persistent symptoms after severe COVID-19 infection. These symptoms include fatigue, dyspnea, “brain fog”/various cognitive symptoms, pain, anxiety, depression, and gastrointestinal issues.3,4,6–9 In these cohorts, the symptoms arising from COVID-19 increased disability and negatively impacted physical function and quality of life7,8 and affected participation in general life activities and the ability to work.9 There is a critical need to classify the prevalence of specific persistent symptoms that follow acute COVID-19 infection and the impact of these symptoms on patient-reported outcomes that are well validated in other conditions. This will facilitate the establishment of diagnostic criteria for PACS and accurate tracking of responses to various prospective therapies.
It has been hypothesized that persistent symptoms after acute COVID-19 infection result from an immune-mediated disruption to the autonomic nervous system.10,11 Similar to other postviral autoimmune conditions (such as Guillain-Barré syndrome), COVID-19 infection seems to act as an immune trigger.12 This immune response, coupled with a lack of access to acute COVID-19 treatments offered only in a hospital setting, may explain why even those with less severe acute infection are still experiencing persistent symptoms.
It is clear that in the wake of the COVID-19 pandemic, a second, longer-term public health emergency has emerged. It is imperative to understand the burden of this novel condition with millions Americans at risk of developing PACS by the end of the pandemic. This study describes the persistent symptoms reported by a cohort of patients with PACS, the majority of whom were infected with COVID-19 in early 2020 and not hospitalized. The impact of these symptoms on physical function, cognitive function, health-related quality of life, and participation is also reported.
METHODS
This was a retrospective observational study of patients attending Mount Sinai’s PACS clinic. Approval for publication was provided and requirement for patient consent was waived by the Mount Sinai Program for Protection of Human Subjects (IRB 21-01147). This study conforms to all strengthening the reporting of observational studies in epidemiology guidelines and reports the required information accordingly (see Supplementary Checklist, Supplemental Digital Content 1, http://links.lww.com/PHM/B417).
Participants
This was a convenience sample exploring symptom characteristics of patients attending the Mount Sinai’s PACS clinic. This is an interdisciplinary clinic consisting of physicians, physical therapists, and dietitians. Patients were either referred by a physician or self-referred. All patients had confirmed (by polymerase chain reaction [PCR] and/or antibody test) or probable (diagnosed by a medical doctor in accordance with World Health Organization recommendations13) previous COVID-19 infection and diagnosis of PACS (defined as experiencing symptoms >12 wks since initial symptom onset). Inclusion criteria for the present study were attending the Mount Sinai’s PACS clinic between March 2020 and March 2021 and completion of the patient-reported outcome survey.14 There were no exclusion criteria.
Data Collection and Outcomes
Data were collected using REDCap (Research Electronic Data Capture) electronic data capture tools hosted at Mount Sinai Health System. REDCap is a secure, web-based application designed to support data capture for research studies. Participants were provided with a link to the survey via e-mail on March 14, 2021, as part of their clinical care.
Baseline demographic data included sex, age, body mass index, race, and comorbidities. COVID-19 clinical data included duration of COVID-19 symptoms (at survey completion), PCR (obtained from nasopharyngeal swab), and antibody test completion and results, need for hospitalization at time of COVID-19 infection, and vaccination status.
Patient-reported outcomes included current persistent symptoms and triggers of symptom exacerbation, and screening tools for fatigue (Fatigue Severity Scale, Fatigue Visual Analog Scale), breathlessness (Medical Research Council Breathlessness Scale), completion of regular moderate and vigorous intensity physical activity (author developed), cognitive function (Neuro-Qol), health-related quality of life (EuroQol: 5 dimension, 5 level [EQ-5D-5L]), anxiety (generalized anxiety disorder scale [GAD-7]), depression (patient health questionnaire-2 [PHQ-2]), disability (World Health Organization Disability Assessment Schedule), and pre– and post–COVID-19 employment status (author developed).
Statistical Analyses
Statistical analyses were undertaken with Stata (Stata Statistical Software Release: V.14; StataCorp). Data were analyzed using descriptive statistics and reported using number and percentage, or median and range.
RESULTS
The survey was sent to 386 patients, with 156 (48%) responding. The median (range) time to follow-up time since the onset of COVID-19 infection was 351 days (82–457 days; Table 1). The most common symptoms reported were fatigue (n = 128, 82%), brain fog (n = 105, 67%), headache (n = 94, 60%), sleep disturbance (n = 92, 59%), and dizziness (n = 85, 54%; Fig. 1). The most common triggers of symptom exacerbation reported were physical exertion (n = 134, 86%), stress (n = 107, 69%), dehydration (n = 77, 49%), weather changes (n = 58, 37%), consuming large meals (n = 44, 28%), premenstrual period (n = 34, 22%), and alcohol consumption (n = 34, 22%).
TABLE 1.
Patient (N = 156) baseline demographic and COVID-19–related data
| All Patients (N = 156) | Confirmed COVID-19 (n = 87) | Presumed COVID-19 (n = 69) | |
|---|---|---|---|
| Female | 107 (69) | 54 (62) | 53 (77) |
| Age, median (range), yr | 44 (13–79) | 45 (13–79) | 44 (14–79) |
| BMI, median (range), kg/m2 | 24 (16–52) | 24 (17–52) | 24 (16–42) |
| Race | |||
| White | 119 (76) | 65 (75) | 54 (78) |
| Asian | 8 (5) | 3 (3) | 5 (7) |
| Black or African American | 6 (4) | 4 (5) | 2 (3) |
| American Indian or American Native | 2 (1) | 1 (1) | 1 (1) |
| Native Hawaiian or Pacific Islander | 0 (0) | 0 (0) | 0 (0) |
| Other | 15 (10) | 8 (9) | 7 (10) |
| Hispanic or Latinx | 10 (7) | 3 (4) | 7 (10) |
| Duration of symptoms, median (range), d | 351 (82–457) | 350 (157–424) | 355 (82–457) |
| PCR completed | 98 (63) | 57 (66) | 41 (59) |
| PCR positive | 34 (22) | 34 (39) | 0 (0) |
| Antibody test completed | 149 (96) | 86 (99) | 63 (91) |
| Antibody positive | 80 (51) | 80 (92) | 0 (0) |
| PCR and/or antibody positive | 87 (56) | 87 (100) | 0 (0) |
| Hospitalized for COVID-19 | 17 (11) | 16 (18) | 1 (1) |
| Received COVID-19 vaccinationa | 87 (56) | 45 (52) | 42 (61) |
| Most prevalent comorbidities | |||
| Cancer (any type) | 30 (20) | 10 (11) | 20 (29) |
| Asthma | 30 (20) | 13 (15) | 17 (25) |
| Anxiety | 18 (12) | 12 (14) | 6 (9) |
| Depression | 13 (8) | 8 (9) | 5 (7) |
| Hypertension | 11 (7) | 7 (8) | 4 (6) |
Data are presented as n (%) unless otherwise indicated.
aAll COVID-19 vaccination occurred after COVID-19 infection.
BMI, body mass index.
FIGURE 1.
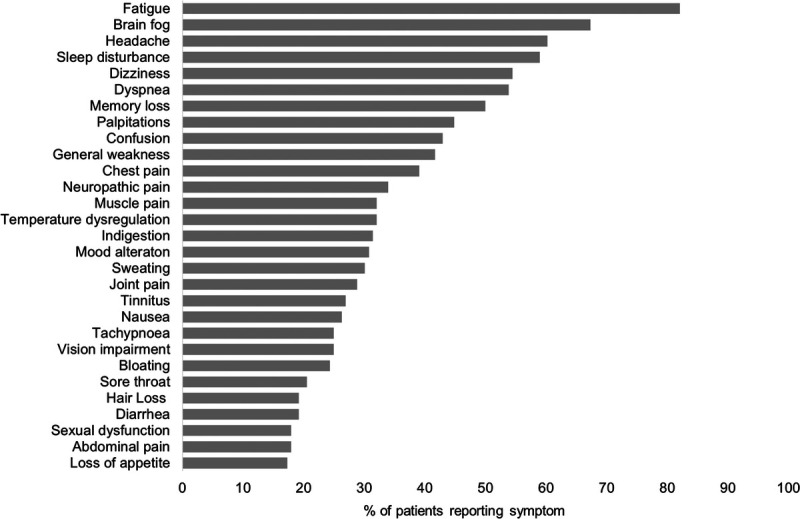
Most commonly reported persistent symptoms by all patients (N = 156).
The median (range) Fatigue Severity Scale average score was 5.6 (1–7) of 7, with 122 patients (78%) reporting an Fatigue Severity Scale average score of 4 or greater, indicating problematic fatigue. Sixty-three patients (40%) reported a score of 3 or more (of 5) on the Medical Research Council Breathlessness Scale, suggesting moderate to severe disability due to dyspnea. When compared with pre–COVID-19 infection levels, patients were completing 150 mins/wk of physical activity less frequently after COVID-19 infection, when asked separately about moderate and vigorous intensities (Fig. 2).
FIGURE 2.
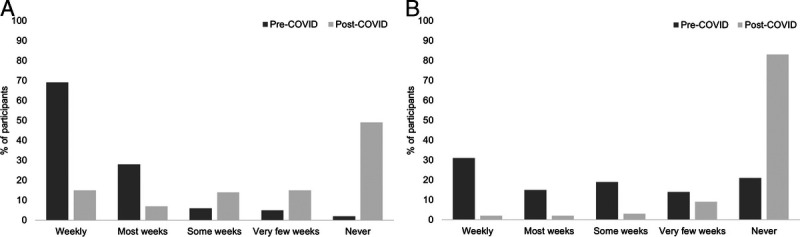
Levels of moderate (A) and vigorous (B) intensity physical activity regularly completed (150 mins/wk) before and after COVID-19 infection in all patients (N = 156).
Ninety-eight patients (63%) scored for at least mild cognitive impairment on the Neuro-Qol (Fig. 3). The domains of the EQ-5D-5L impacted the most (reported as slight problems or greater) were self-care, anxiety/depression, and usual activities (Table 2). The median (range) EQ-5D-5L Visual Analog Scale score was 64 (6–99) of 100, with a higher score indicating greater health-related quality of life. Twenty-nine patients (19%) scored 10 or greater on the GAD-7, indicating possible anxiety disorder. Forty-three patients (28%) scored 3 or greater on the PHQ-2, indicating possible major depressive disorder. The median (range) World Health Organization Disability Assessment Schedule total score was 14 (0–44) of 100. A total of 134 patients (86%) answered pre– and post–COVID-19 employment questions; the number of patients in full-time work reduced from 102 (76%) pre–COVID-19 to 55 (41%) at the time of follow-up (Fig. 4).
FIGURE 3.
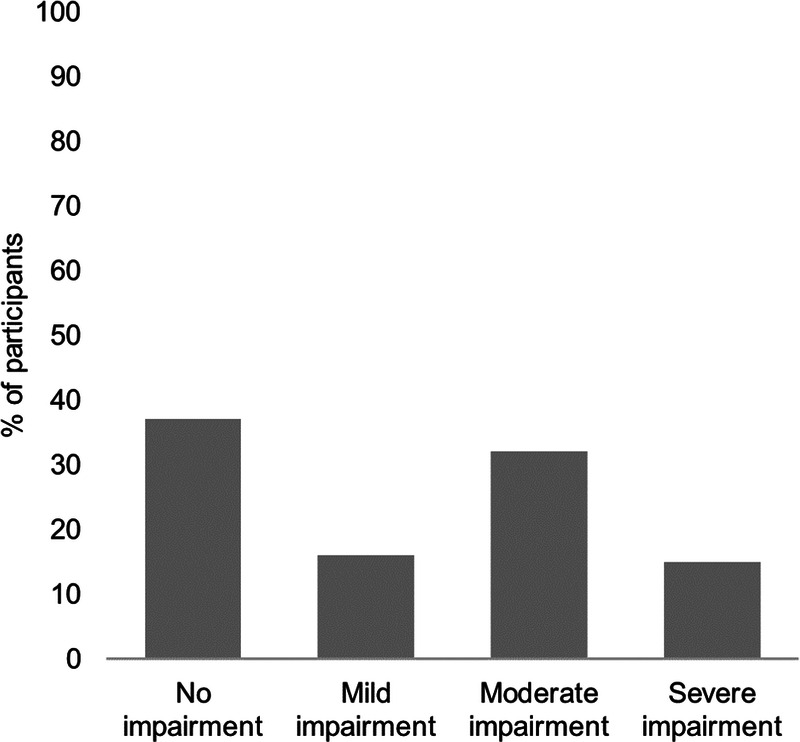
Neuro-QoL level of impairment according to t scores in patients who completed the measure (n = 155). Neuro-QoL scores have a mean of 50 and SD of 10 in a referent population. T scores 40–45 indicate mild dysfunction, t scores 30–40 indicate moderate dysfunction, and t scores less than 30 indicate severe dysfunction.
TABLE 2.
Patient (N = 156) responses to the EQ-5D-5L
| No Problems | Slight Problems | Moderate Problems | Severe Problems | Extreme Problems | |
|---|---|---|---|---|---|
| Domain | |||||
| Mobility | 68 (44) | 42 (27) | 28 (18) | 17 (11) | 1 (1) |
| Self-care | 23 (15) | 48 (31) | 39 (25) | 35 (22) | 11 (7) |
| Usual activities | 29 (19) | 64 (41) | 45 (29) | 11 (7) | 7 (4) |
| Pain discomfort | 113 (72) | 26 (17) | 12 (8) | 5 (3) | 0 (0) |
| Anxiety/depression | 26 (17) | 59 (38) | 49 (31) | 16 (10) | 6 (4) |
Data are presented as n (%).
FIGURE 4.
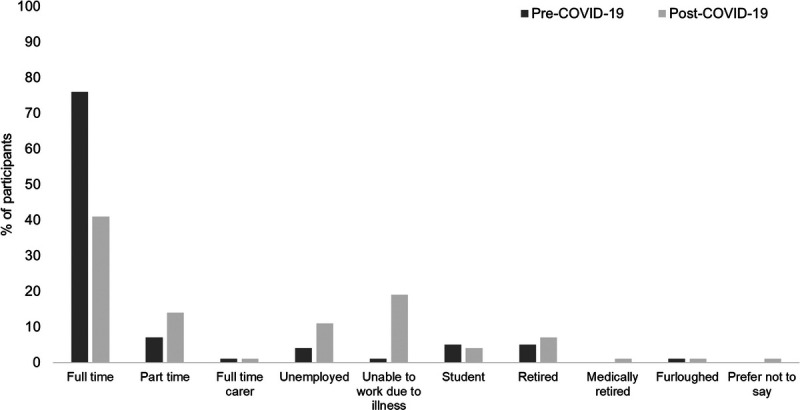
Employment status before and after COVID-19 infection in participants who answered employment questions (n = 134).
DISCUSSION
This observational study of a cohort of patients with PACS reported that COVID-19–related symptoms are persistent for at least 2 mos, and often longer than 12 mos, with fatigue, brain fog, sleep disturbance, dizziness, dyspnea, memory loss, and palpitations being identified as the most common. The most common triggers of symptom exacerbation in this cohort were physical exertion, stress, and dehydration. The negative impact of PACS on a variety of patient-reported outcomes has been demonstrated.
Just less than 50% of the patients included in this study had a negative PCR test or tested seronegative for antibodies. Issues relating to false-negative rates are well documented with PCR testing.15–17 Similarly, there is literature to support the idea that antibody levels in patients who experienced less severe acute infection tend to fade rapidly.18 In acknowledgement of the potential for health disparities to rapidly arise from an overreliance on COVID-19 tests, the Centers for Disease Control and Prevention has recently recommended against using seropositive status as the sole diagnostic criteria for any post-acute sequelae of COVID-19.19 To fully understand all of the possible presentations of PACS, studies must incorporate and report data from both seronegative and seropositive patient populations.
The most common symptoms observed in this cohort are consistent with those previously reported.3,6,20–23 The pattern of PACS symptoms resembles other postviral syndromes, including dysautonomia,24 postural orthostatic tachycardia syndrome,25 and myalgic encephalomyelitis.26 It is unsurprising that physical exertion was the most common cause of symptom exacerbation, as this is a feature shared by some of these conditions. The potential for the worsening of symptoms after physical exertion is the most important consideration when prescribing rehabilitation therapies for people with PACS.27
The presence of cognitive dysfunction in more than half (63%) of patients, in combination with reduced usual activities and self-care scores on the EQ-5D-5L, highlights that patients with PACS may have a reduced ability to participate in society. Employment was also impacted in most patients; however, it is difficult to determine whether this was specifically due to disability related to COVID-19 infection or possibly to the broader implications of the pandemic on the ability for workplaces to operate as usual. Levels of self-reported physical activity were greatly reduced, likely coinciding with the potential for symptom exacerbation; this raises concerns given the known longer-term health risks of physical inactivity.28
The proportion of participants reporting anxiety (19%) and depression (28%) on the PHQ-2 and GAD-7 was slightly higher than reported as part of their medical history; however, it was similar to what is expected in the normal population29 and lower than that observed in chronic individuals with obstructive pulmonary disease30 and cardiac disease.31 However, these levels were greater than what participants’ reported as part of their pre–COVID-19 medical history. With a lack of pre–COVID-19 PHQ-2 and GAD-7 data available, it is difficult to make conclusions about the impact of PACS on anxiety and depression.
Some limitations of the present study include the use of clinical survey data answered in retrospect, a lack of comparison group, and/or pre–COVID-19 measures. In addition, the use of noncondition-specific patient-reported outcomes can increase the risk of inaccuracy and recall bias.
CONCLUSIONS
The presence of persistent symptoms associated with PACS seems to impact physical and cognitive function, health-related quality of life, and participation in society. The data reported contribute to the recognition and research of long COVID as recommended by the World Health Organization32 and will help inform future rehabilitation strategies.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank the patients in the study, the frontline healthcare workers at Mount Sinai Health System, and the wider research team at the Abilities Research Center and the Center for Post-COVID Care at Mount Sinai. The authors also thank the RTW Charitable Foundation for supporting multiple PACS initiatives in the Abilities Research Center.
Footnotes
Financial disclosure statements have been obtained, and no conflicts of interest have been reported by the authors or by any individuals in control of the content of this article.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Web site (www.ajpmr.com).
Contributor Information
Laura Tabacof, Email: laura.tabacof@MOUNTSINAI.ORG.
Jenna Tosto-Mancuso, Email: jenna.tosto@mountsinai.org.
Jamie Wood, Email: Jamie.wood@mountsinai.org.
Mar Cortes, Email: mar.cortes@mountsinai.org.
Amy Kontorovich, Email: amy.kontorovich@mountsinai.org.
Dayna McCarthy, Email: dayna.mccarthy@mountsinai.org.
Dahlia Rizk, Email: dahlia.rizk@mountsinai.org.
Gabriela Rozanski, Email: Gabriela.rozanski@mountsinai.org.
Erica Breyman, Email: erica.breyman@mountsinai.org.
Leila Nasr, Email: leila.nasr@mountsinai.org.
Christopher Kellner, Email: christopher.kellner@mountsinai.org.
Joseph E. Herrera, Email: joseph.herrera@mountsinai.org.
David Putrino, Email: DAVID.PUTRINO@MOUNTSINAI.ORG.
REFERENCES
- 1.Martillo MA Dangayach NS Tabacof L, et al. : Postintensive care syndrome in survivors of critical illness related to coronavirus disease 2019: cohort study from a New York City Critical Care Recovery Clinic. Crit Care Med 2021;49:1427–38 [DOI] [PubMed] [Google Scholar]
- 2.McGroder CF Zhang D Choudhury MA, et al. : Pulmonary fibrosis 4 months after COVID-19 is associated with severity of illness and blood leucocyte telomere length. Thorax 2021;76:1242–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nalbandian A Sehgal K Gupta A, et al. : Post-acute COVID-19 syndrome. Nat Med 2021;27:601–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davis HE Assaf GS McCorkell L, et al. : Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. medRxiv 2020:2020.12.24.20248802. doi: 10.1101/2020.12.24.20248802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carvalho-Schneider C Laurent E Lemaignen A, et al. : Follow-up of adults with noncritical COVID-19 two months after symptom onset. Clin Microbiol Infect 2021;27:258–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bliddal S Banasik K Pedersen OB, et al. : Acute and persistent symptoms in non-hospitalized PCR-confirmed COVID-19 patients. Sci Rep 2021;11:13153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carfì A Bernabei R Landi F, Gemelli Against COVID-19 Post-Acute Care Study Group : Persistent symptoms in patients after acute COVID-19. JAMA 2020;324:603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang C Huang L Wang Y, et al. : 6-Month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet 2021;397:220–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tenforde MW Kim SS Lindsell CJ, et al. IVY Network Investigators; CDC COVID-19 Response Team; IVY Network Investigators : Symptom duration and risk factors for delayed return to usual health among outpatients with COVID-19 in a multistate health care systems network—United States, March–June 2020. Morb Mortal Wkly Rep 2020;69:993–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dani M Dirksen A Taraborrelli P, et al. : Autonomic dysfunction in ‘long COVID’: rationale, physiology and management strategies. Clin Med 2021;21:e63–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goldstein DS: The extended autonomic system, dyshomeostasis, and COVID-19. Clin Auton Res 2020;30:299–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davido B Seang S Tubiana R, et al. : Post–COVID-19 chronic symptoms: a postinfectious entity? Clin Microbiol Infect 2020;26:1448–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.World Health Organization : WHO COVID-19 case definition. Available at: https://www.who.int/publications-detail-redirect/WHO-2019-nCoV-Surveillance_Case_Definition-2020.2. Accessed February 18, 2021
- 14.National Institute for Health and Care Excellence : COVID-19 rapid guideline: managing the long-term effects of COVID-19. Available at: https://www.nice.org.uk/guidance/ng188. Accessed July 16, 2021 [PubMed]
- 15.Arevalo-Rodriguez I Buitrago-Garcia D Simancas-Racines D, et al. : False-negative results of initial RT-PCR assays for COVID-19: a systematic review. PLoS One 2020;15:e0242958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kucirka LM Lauer SA Laeyendecker O, et al. : Variation in false-negative rate of reverse transcriptase polymerase chain reaction–based SARS-CoV-2 tests by time since exposure. Ann Intern Med 2020;173:262–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zitek T: The appropriate use of testing for COVID-19. West J Emerg Med 2020;21:470–2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ibarrondo FJ Fulcher JA Goodman-Meza D, et al. : Rapid decay of anti–SARS-CoV-2 antibodies in persons with mild COVID-19. N Engl J Med 2020;383:1085–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Centers for Disease Control and Prevention Healthcare workers. Published February 11, 2020. Available at: https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-care/post-covid-conditions.html. Accessed July 16, 2021
- 20.Townsend L Dyer AH Jones K, et al. : Persistent fatigue following SARS-CoV-2 infection is common and independent of severity of initial infection. PLoS One 2020;15:e0240784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peghin M Palese A Venturini M, et al. : Post–COVID-19 symptoms 6 months after acute infection among hospitalized and non-hospitalized patients. Clin Microbiol Infect 2021;27:1507–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Logue JK Franko NM McCulloch DJ, et al. : Sequelae in adults at 6 months after COVID-19 infection. JAMA Netw Open 2021;4:e210830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moghimi N Di Napoli M Biller J, et al. : The neurological manifestations of post-acute sequelae of SARS-CoV-2 infection. Curr Neurol Neurosci Rep 2021;21:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reichgott MJ: Clinical evidence of dysautonomia, in Walker HK, Hall WD, Hurst JW. (eds): Clinical Methods: The History, Physical, and Laboratory Examinations, 3rd ed. Boston, Butterworths, 1990. Available at: http://www.ncbi.nlm.nih.gov/books/NBK400/. Accessed February 1, 2021 [PubMed] [Google Scholar]
- 25.Bryarly M Phillips LT Fu Q, et al. : Postural orthostatic tachycardia syndrome: JACC Focus Seminar. J Am Coll Cardiol 2019;73:1207–28 [DOI] [PubMed] [Google Scholar]
- 26.Carruthers BM van de Sande MI De Meirleir KL, et al. : Myalgic encephalomyelitis: International Consensus Criteria. J Intern Med 2011;270:327–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.World Physiotherapy . World Physiotherapy Response to COVID-19 Briefing Paper 9. Safe rehabilitation approaches for people living with Long COVID: physical activity and exercise. London, UK: World Physiotherapy, 2021. Available at: https://world.physio/sites/default/files/2021-06/Briefing-Paper-9-Long-Covid-FINAL.pdf. Accessed July 1, 2021
- 28.Warburton DER, Nicol CW, Bredin SSD: Health benefits of physical activity: the evidence. CMAJ 2006;174:801–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kessler RC Berglund P Demler O, et al. : Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry 2005;62:593–602 [DOI] [PubMed] [Google Scholar]
- 30.Pumar MI Gray CR Walsh JR, et al. : Anxiety and depression—important psychological comorbidities of COPD. J Thorac Dis 2014;6:1615–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Celano CM, Huffman JC: Depression and cardiac disease: a review. Cardiol Rev 2011;19:130–42 [DOI] [PubMed] [Google Scholar]
- 32.Wise J: Long COVID: WHO calls on countries to offer patients more rehabilitation. BMJ 2021;372:n405. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


