Abstract
Background:
Testicular cancer constitutes 1.0% of male cancer and typically carries a good prognosis. As far as we are aware, the role for hydrogen sulfide in testicular cancer and the level of hydrogen sulfide-synthesizing enzyme have never been addressed. Here we examined cystathionine gamma-lyase (CSE) expression in several germ-cell testicular tumors.
Materials and Methods:
Tissue microarrays were employed to examine CSE expression in 32 benign testicular samples, 88 testicular seminomas, 34 embryonal carcinomas, 4 mature teratomas, and 16 yolk sac tumors, and CSE expression was compared to that seen in benign testicular tissue.
Results:
Compared to benign testicular tissue, CSE expression was increased in all three types of testicular neoplasm but not in mature teratomas. Highest CSE expression was identified in embryonal carcinomas, which often show a relatively aggressive clinical course.
Conclusion:
For the first time, we show that CSE is increased in several common testicular germ-cell tumor types.
Keywords: Cystathionine lyase, testicular cancer, seminoma, embryonal carcinoma, yolk sac tumor, teratoma
Testicular cancer accounts for approximately 1.0% of male cancer and is the most common solid tumor among 18- to 34-year-old males. Interestingly, the incidence of testicular cancer has increased over the past several decades for unclear reasons (1). With effective treatment, the overall 5-year survival for patients with these malignancies is 97% (2-4). The risk factors of testicular cancer include family or personal histories of testicular cancer, cryptorchidism, Klinefelter’s syndrome, congenital abnormalities, infertility, age, and ethnicity, with White and Hispanic males having higher incidences of these malignancies (1-5). Testicular cancer typically presents as a painless, solid testicular mass, although in some cases they present as scrotal swelling and heaviness, accompanied by testicular or scrotal pain (5). Testicular cancer is treated by radical inguinal orchiectomy, with further treatment based on tumor staging, which includes active surveillance and possible chemotherapy (5).
Ninety-five percent of testicular malignancies are germ-cell tumors, with the remaining composed of sex cord-gonadal stromal tumors and other rarer tumor subtypes (6, 7). Common testicular germ-cell tumors include seminomas, embryonal carcinoma, yolk sac tumors (YST), choriocarcinomas, and teratomas (6). Seminomas account for ~50% of testicular germ-cell tumors and consist of large polygonal cells with abundant pale glycogen-rich cytoplasm, with distinct cell membranes, coarse chromatin, and prominent nucleoli. Seminomas often show a nested architecture within fibrous septae with a prominent lymphocytic infiltrate. Placental alkaline phosphatase, c-KIT, and octamer-binding transcription factor 4 immunoreactivity is generally present and the patient’s serum typically shows elevated lactate dehydrogenase. The majority of seminomas have isochromosome 12p and many carry c-KIT mutations (6). YSTs, or endodermal sinus tumors, account for 2.4% of adult testicular tumors but are found in 42% of mixed germ-cell tumors. There are at least 10 different histological subtypes, with vesicular or microcystic patterns being common and consisting of small cuboidal to flattened cells with frequent mitoses, nuclei of varying size, and often having refractile eosinophilic hyaline globules. Ninety-two percent are immunoreactive for α-fetoprotein and the patients often have elevated serum α-fetoprotein level (6).
Embryonal carcinomas comprise approximately 3% of germ-cell tumors but are present as a component of ~40% of mixed germ-cell tumors (6). Histologically, embryonal carcinomas show large embryonic-appearing cells with poorly defined cell borders and amphophilic or eosinophilic cytoplasm. Mitotic figures are often numerous and vascular/lymphatic invasion with extension into the epididymis and paratesticular tissues is common (6). Octamer-binding transcription factor 4, tumor necrosis factor receptor superfamily member 8 (CD30), and cytokeratin immunopositivity are frequent. Many patients with embryonal carcinomas present at late stages with vascular/lymphatic invasion (6). Teratomas constitute 35% of testicular tumors in infants and prepubertal children, and ~5% of adult testicular tumors, although they are found in ~50% of mixed germ-cell tumors. Teratomas are often composed of the three germ-cell layers, endoderm, ectoderm, and mesoderm, and are histologically complex. The tissue may appear mature or immature, and contain haphazardly arranged elements including enteric or salivary glands, smooth and skeletal muscle, fat, bone, cartilage, and respiratory epithelium. These tumors are usually benign, although malignant transformation may occur (6).
Cystathionine gamma-lyase (CSE) catalyzes hydrogen sulfide (H2S) synthesis by the conversion of cystathionine to L-cysteine, yielding pyruvate, ammonia, and H2S (8). CSE is increased in several human malignancies (9-12). Interestingly, CSE promotes tumor metastases in breast and prostatic cancer (9, 10). To our knowledge, CSE expression has not been examined in testicular germ-cell tumors. Here we employed tissue microarray (TMA) to examine CSE expression in testicular seminomas, embryonal carcinomas, mature teratomas, and YSTs, and compared CSE expression to that seen in benign testicular tissue.
Materials and Methods
TMA.
The TMA TE2081 was purchased from US Biomax, Inc. (Rockville, MD, USA). The TMA contained 96 seminoma cases, 16 YSTs, 34 embryonal tumors, 4 mature teratomas and 52 benign testes. The TMA was interrogated using a CSE antibody. All tissue samples in the TMAs were 1.0 mm in diameter.
CSE immunohistochemistry (IHC).
The concentration of primary CSE antibody was optimized to normal kidney as positive control tissue. The negative control was the same tissue interrogated without the use of primary CSE antibody. The staining of the TMA was performed at the Tissue Core Histology Laboratory Facility at the Moffitt Cancer Center. The microarray slides were stained using a Ventana Discovery XT automated system (Ventana Medical Systems, Tucson, AZ, USA) as per the manufacturer’s protocol with proprietary reagents. Briefly, slides were deparaffinized on the automated system with EZ Prep solution (Ventana Medical Systems). The heat-induced antigen retrieval method was used in Cell Conditioning (Ventana Medical Systems). Mouse monoclonal antibody to human CSE (catalog number sc-374249; Santa Cruz Biotechnology, Dallas, TX, USA) was used at a 1:1,000 concentration in Dako antibody diluent (Dako, Carpentaria, CA, USA) and slides were incubated for 60 min. Ventana anti-mouse secondary antibodies were then used for 16 min. The detection system used was the Ventana OmniMap kit. Slides were then dehydrated and cover-slipped per standard protocol.
Evaluation of staining.
Relative CSE protein expression was determined as immunostain intensity scored on a 0 to 3 scale as follows: No staining: 0, light staining: 1, moderate staining: 2, and heavy staining: 3. The percentage of cells stained was measured, with no detectable staining scored as 0, 1-33% as 1, 34-66% as 2, and 67-100% as 3. The final IHC score was the product of the score for the percentage of cells stained multiplied by the intensity score, allowing for a minimal score of 0 and a maximal score of 9. Cytoplasmic CSE staining was seen in all tissue samples examined, although at moderate levels in benign testes. Therefore, CSE staining was measured and quantified in the cytoplasmic compartment.
Statistical analysis of TMA results.
The standard error of the mean IHC score was calculated by using the standard deviation for the staining scores of each tumor type and dividing this number by the square root of the sample size (13, 14).
Statement of ethics.
All ethical guidelines concerning the use of patient tissue were carefully observed in this study.
Results
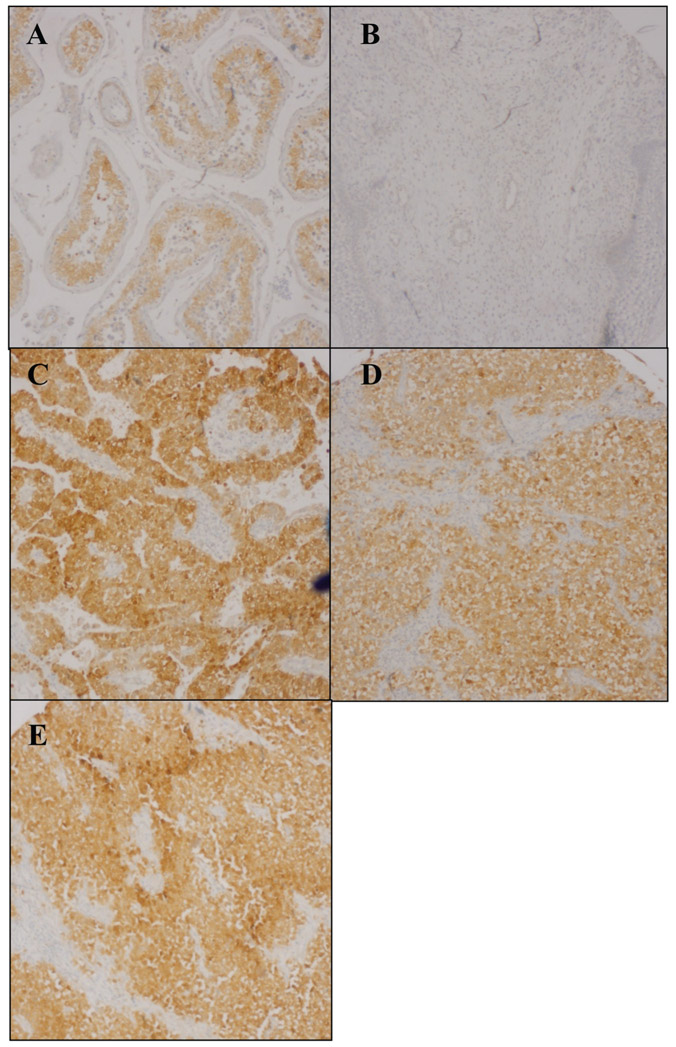
Following IHC processing, eight seminomas and 20 benign testicular tissue samples were lost in processing. The number of cases examined for CSE IHC, the quantified results for each data set are given in Table I. Examples of CSE IHC of sample tissues are shown in Figure 1. Compared to benign testicular tissue, CSE expression was increased in seminomas, embryonal, and YST tumors but not in benign teratomas. Highest CSE expression was seen in the embryonal carcinomas.
Table I.
Relative cystathionine γ-lyase (CSE) immunohistochemical (IHC) staining in different testicular tumors examined in this study compared to benign testicular tissue.
| Tumor type | Number of samples | Average CSE IHC score | SD | SEM |
|---|---|---|---|---|
| Benign testes | 32 | 1.68 | 0.52 | 0.092 |
| Seminoma | 88 | 5.76 | 2.69 | 0.29 |
| Yolk sac tumor | 16 | 3.81 | 2.97 | 0.74 |
| Embryonal carcinoma | 34 | 7.26 | 2.54 | 0.42 |
| Mature teratoma | 4 | 0.25 | 0.38 | 0.19 |
SD: Standard deviation; SEM: standard error of the mean.
Figure 1.
Representative cystathionine γ-lyase immunostaining of benign testicular tissue (A), mature teratoma (B), seminoma (C), yolk sac tumor (D), and embryonal carcinoma (E). Original magnification, ×100.
Discussion
H2S was recently identified as a gasotransmitter that plays a central role in many physiologic and pathophysiologic processes (15). H2S is synthesized by three enzymes, cystathione β-synthase, CSE, and 3-mercaptopyruvate sulfurtransferase , (8). H2S and one or more of the enzymes that synthesize it are increased in several common human malignancies, including prostate, breast, colon, oral squamous cell, and renal cell carcinomas (9-12, 15-17). A role for H2S or the enzymes that synthesize it in testicular cancer has not been previously examined. Here we used TMA to examine CSE expression in benign testicular tissue, seminomas, embryonal carcinomas, YSTs and benign testicular teratomas. Compared to benign testicular tissue, CSE expression was elevated in all three types of testicular germ-cell neoplasm but not in the mature teratomas. Highest CSE expression was seen in the embryonal carcinomas (Table I, Figure 1). A high CSE level correlated with breast cancer aggressivity and metastatic potential in animal models (10). Of the three testicular germ-cell tumor types we examined, embryonal carcinoma is the most aggressive, often first presenting with invasion/metastasis (6). Thus, the higher CSE expression we see in this tumor type, may in part, explain its increased clinical aggressivity. Taken together, our data show that increased CSE expression is part of testicular germ-cell neoplasia, and also confirms that increased H2S-synthesizing enzyme expression is a common feature of many human malignancies.
Acknowledgements
The Authors thank Jennifer Burton for help in the assembling and proofreading of this article.
Footnotes
Conflicts of Interest
The Authors report no conflicts of interest in regard to this study.
References
- 1.Smith ZL, Werntz RP and Eggener SE: Testicular cancer: Epidemiology, diagnosis, and management. Med Clin North Am 102(2): 251–264, 2018. PMID: 29406056. DOI: 10.1016/j.mcna.2017.10.003 [DOI] [PubMed] [Google Scholar]
- 2.Huyghe E, Matsuda T and Thonneau P: Increasing incidence of testicular cancer worldwide: a review. J Urol 170(1): 5–11, 2003. PMID: 12796635. DOI: 10.1097/01.ju.0000053866.68623.da [DOI] [PubMed] [Google Scholar]
- 3.Sagalowsky AI: Current considerations in the diagnosis and initial treatment of testicular cancer. Compr Ther 20(12): 688–694, 1994. PMID: 7882652. [PubMed] [Google Scholar]
- 4.Daniels J, Stutzman R and Mcleod D: A comparison of testicular tumors in black and white patients. Journal of Urology 125(3): 341–342, 2021. DOI: 10.1016/s0022-5347(17)55030-2 [DOI] [PubMed] [Google Scholar]
- 5.Baird DC, Meyers GJ and Hu JS: Testicular cancer: Diagnosis and treatment. Am Fam Physician 97(4): 261–268, 2018. PMID: 29671528. [PubMed] [Google Scholar]
- 6.Sesterhenn IA and Davis CJ Jr: Pathology of germ cell tumors of the testis. Cancer Control 11(6): 374–387, 2004. PMID: 15625525. DOI: 10.1177/107327480401100605 [DOI] [PubMed] [Google Scholar]
- 7.Young RH: Sex cord-stromal tumors of the ovary and testis: their similarities and differences with consideration of selected problems. Mod Pathol 18(Suppl 2): S81–S98, 2005. PMID: 15502809. DOI: 10.1038/modpathol.3800311 [DOI] [PubMed] [Google Scholar]
- 8.Powell CR, Dillon KM and Matson JB: A review of hydrogen sulfide (H2S) donors: Chemistry and potential therapeutic applications. Biochem Pharmacol 149: 110–123, 2018. PMID: 29175421. DOI: 10.1016/j.bcp.2017.11.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang YH, Huang JT, Chen WL, Wang RH, Kao MC, Pan YR, Chan SH, Tsai KW, Kung HJ, Lin KT and Wang LH: Dysregulation of cystathionine γ-lyase promotes prostate cancer progression and metastasis. EMBO Rep 20(10): e45986, 2019. PMID: 31468690. DOI: 10.15252/embr.201845986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang L, Shi H, Liu Y, Zhang W, Duan X, Li M, Shi X and Wang T: Cystathionine γ lyase promotes the metastasis of breast cancer via the VEGF signaling pathway. Int J Oncol 55(2): 473–487, 2019. PMID: 31173185. DOI: 10.3892/ijo.2019.4823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meram AT, Chen J, Patel S, Kim DD, Shirley B, Covello P, Coppola D, Wei EX, Ghali G, Kevil CG and Shackelford RE: Hydrogen sulfide is increased in oral squamous cell carcinoma compared to adjacent benign oral mucosae. Anticancer Res 38(7): 3843–3852, 2018. PMID: 29970504. DOI: 10.21873/anticanres.12668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sogutdelen E, Pacoli K, Juriasingani S, Akbari M, Gabril M and Sener A: Patterns of expression of H2S-producing enzyme in human renal cell carcinoma specimens: Potential avenue for future therapeutics. In Vivo 34(5): 2775–2781, 2020. PMID: 32871814. DOI: 10.21873/invivo.12102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vora M, Alattia LA, Ansari J, Ong M, Cotelingam J, Coppola D and Shackelford R: Nicotinamide phosphoribosyl transferase a reliable marker of progression in cervical dysplasia. Anticancer Res 37(9): 4821–4825, 2017. PMID: 28870901. DOI: 10.21873/anticanres.11889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vora M, Ansari J, Shanti RM, Veillon D, Cotelingam J, Coppola D and Shackelford RE: Increased nicotinamide phosphoribo-syltransferase in rhabdomyosarcomas and leiomyosarcomas compared to skeletal and smooth muscle tissue. Anticancer Res 36(2): 503–507, 2016. PMID: 26851003 [PMC free article] [PubMed] [Google Scholar]
- 15.Nagy P: Mechanistic chemical perspective of hydrogen sulfide signaling. Methods Enzymol 554: 3–29, 2015. PMID: 25725513. DOI: 10.1016/bs.mie.2014.11.036 [DOI] [PubMed] [Google Scholar]
- 16.Hellmich MR and Szabo C: Hydrogen sulfide and cancer. Handb Exp Pharmacol 230: 233–241, 2015. PMID: 26162838. DOI: 10.1007/978-3-319-18144-8_12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Szabo C, Coletta C, Chao C, Modis K, Szczesny B, Papapetropoulos A and Hellmich MR: Tumor-derived hydrogen sulfide, produced by cystathionin-β-synthase, stimulates bioenergetics, cell proliferation, and angiogenesis in colon cancer. Proc Natl Acad Sci USA 110(30): 12474–12479, 2013. PMID: 23836652. DOI: 10.1073/pnas.1306241110 [DOI] [PMC free article] [PubMed] [Google Scholar]