Abstract
Background:
Childhood adversity is reliably associated with immune alterations in adulthood, including increases in inflammatory processes. However, relatively few studies have investigated these associations in clinical populations such as cancer patients who are at risk for negative immune-related health outcomes. The current study tested the hypothesis that childhood maltreatment would be associated with alterations in immune-related gene expression in monocytes from women with breast cancer.
Methods:
Women (n=86) were recruited after diagnosis with early-stage breast cancer but before onset of adjuvant therapy with radiation, chemotherapy, and/or endocrine therapy. Participants completed questionnaires to assess childhood maltreatment (Childhood Trauma Questionnaire; CTQ) and depressive symptoms (CESD) and provided blood samples for immune assessment. CD14+ monocytes were isolated for RNA extraction and gene expression analyses.
Results:
Based on responses to the CTQ, 28% of participants were classified as experiencing physical and/or emotional abuse or neglect and 7% as experiencing sexual abuse. Genome-wide transcriptional profiling of isolated monocytes identified 202 gene transcripts that differed in average expression level by >25% over the range of maltreatment exposure. Bioinformatics analyses of those gene transcripts identified a significantly greater prevalence of NF-κB-binding motifs within the promoters of up-regulated vs. down-regulated genes (p = .028) in women exposed to childhood maltreatment, indicating greater inflammatory signaling. Parallel analyses of Type I interferon signaling also indicated greater prevalence of Interferon Response Factor (IRF)-related binding sites in women with a childhood maltreatment history (p = .020). Results remained significant in analyses controlling for current depression; however, NF-κB and IRF-related gene expression was higher in women with both maltreatment exposure and current depression.
Conclusions:
In women recently diagnosed with early-stage breast cancer, childhood maltreatment was associated with increases in the classical NF-kB-related proinflammatory signaling pathway and with increases in the Type I interferon system. These results suggest a broad pattern of chronic immunologic activation in breast cancer patients with a history of childhood maltreatment, particularly those who are currently experiencing clinically significant depressive symptoms. These findings have implications for the long-term health and well-being of maltreatment exposed breast cancer patients.
INTRODUCTION
Childhood adversity predicts a variety of mental and physical health problems in adulthood, including depression, cardiovascular disease, diabetes, autoimmune disease, and cancer (Chapman et al., 2004; Dube et al., 2009; Felitti et al., 1998; Wegman & Stetler, 2009). One of the biological processes that is thought to mediate the effects of childhood adversity on poor adult health is alterations in immune function (Kuhlman, Chiang, Horn, & Bower, 2017; Miller, Chen, & Parker, 2011). There is now substantial evidence that exposure to a variety of stressors in childhood is associated with elevated inflammatory activity and other immune alterations in later life. In particular, childhood adversity is associated with elevated levels of circulating inflammatory markers, including CRP, IL-6, and TNF-α, in adulthood (Baumeister, Akhtar, Ciufolini, Pariante, & Mondelli, 2016) and with alterations in inflammation-related gene expression (Levine, Cole, Weir, & Crimmins, 2015). Exposure to childhood adversity is also associated with dysregulation in the adaptive immune system, including alterations in T cells and elevated antibody titers to latent viruses (indicating poor immune control) (Elwenspoek, Kuehn, Muller, & Turner, 2017). However, most of this work has focused on non-clinical samples, with fewer studies conducted among individuals with inflammation- or immune related medical conditions who are arguably most at risk for the negative effects of inflammatory activity.
Inflammation is particularly relevant in the context of cancer given its role in tumor growth and progression (Hanahan & Weinberg, 2011). Preclinical models have elegantly demonstrated the role of monocytes/macrophages in breast cancer where they are recruited into the tumor microenvironment to promote angiogenesis, invasion, and metastasis (Sloan et al., 2010; Williams, Yeh, & Soloff, 2016). In studies with breast cancer survivors, systemic markers of inflammation (e.g., CRP) predict breast cancer recurrence and mortality, supporting the relevance of systemic inflammation for breast cancer prognosis (Pierce et al., 2009; Villasenor et al., 2014).
Despite the importance of immune function in this population, only a handful of studies have examined links between childhood adversity and markers of inflammation and other indices of immune dysregulation in cancer patients and survivors. We have previously reported that childhood adversity is associated with elevated circulating levels of IL-6 in breast cancer survivors (Crosswell, Bower, & Ganz, 2014). Further, Fagundes and colleagues found that childhood adversity was associated with higher antibody titers to latent herpesviruses (EBV and CMV) in breast cancer survivors, suggesting poor cellular immune control over viral latency (Fagundes, Glaser, Malarkey, & Kiecolt-Glaser, 2013). To our knowledge, only one small study (n=20) of newly diagnosed breast cancer patients has examined links between childhood adversity and alterations in immune-related gene expression, which may be a more sensitive indicator of cellular inflammatory activity (Han et al., 2016). This study found that peripheral blood mononuclear cells (PBMC) from patients with a history of childhood trauma showed altered expression of gene transcripts related to inflammatory signaling as well as other aspects of immunologic activation, including IL-22, IL-17, and C-C chemokine receptor 5 signaling. This study also suggested increased activity of genes regulated by the NF-κB family of transcription factors, which play a key role in both classical inflammatory responses in monocytes as well as activation of adaptive immune responses by T and B lymphocytes. The observed alterations suggest that childhood adversity may affect a broad range of immunoregulatory processes beyond the classical monocyte pro-inflammatory program involving IL-1β, IL-6, IL-8/CXCL8, and TNF-α, with these results also suggesting potential alterations B cell and Th17 helper T cell activation.
The current study was designed to examine the association between childhood maltreatment and alterations in inflammatory and immune-related gene expression in monocytes from women recently diagnosed with early-stage breast cancer. We have previously shown that childhood maltreatment is associated with elevated depressive symptoms and fatigue in this sample (Bower et al., 2019; K. R. Kuhlman et al., 2017). The present analyses examined whether childhood maltreatment might also be associated with alterations in monocyte gene regulation, including activation of pro-inflammatory and antiviral transcription pathways as well as differentiation toward CD16- classical monocyte transcriptomes vs. CD16+ “non-classical” monocyte transcriptome.
Monocyte gene regulation and cell differentiation is particularly relevant for the health of breast cancer patients due the monocyte’s role as a systemically circulating precursor to tumor-associated macrophages (TAMs). TAMs mediate innate immune, inflammatory, and tissue regenerative responses in the tumor microenvironment that can either promote tumor progression and metastasis (e.g., via angiogenesis, tumor invasion, and other manifestations of the “M2” gene expression profile) or promote anti-tumor immune responses (e.g., via “M1” gene expression profiles and antigen-presenting cell support for cellular immune responses by cytotoxic T lymphocytes) depending on the particular gene expression profile they express (Lamkin et al., 2016; Lamkin et al., 2019). Previous studies have shown that chronic stress can upregulate monocyte production and alter monocyte gene expression and differentiation profiles via beta-adrenergic signaling from the sympathetic nervous system (Heidt et al., 2014; Lamkin et al., 2016; Lamkin et al., 2019; McKim et al., 2018; Miller et al., 2014; Powell et al., 2013), resulting in increased tumor progression and metastasis in mouse models of human cancer (Sloan et al., 2010; Williams et al., 2016). Monocyte/macrophage differentiation toward tumor-promoting or tumor-suppressive gene expression profiles is mediated in part by the effects of beta-adrenergic signaling on the key pro-inflammatory transcription factor, NF-κB, as well as the family of Interferon Response Factor (IRF) transcription factors involved in mediating Type I interferon innate antiviral responses (Heidt et al., 2014; Irwin & Cole, 2011; McKim et al., 2018; Powell et al., 2013). However, stress-related differences in circulating monocyte gene expression have not previously been examined in human cancer patients or survivors.
Based on research linking childhood adversity with inflammatory activity (Baumeister et al., 2016; Levine et al., 2015), we hypothesized that exposure to childhood maltreatment would be associated with elevated proinflammatory gene expression in circulating monocytes from breast cancer patients. In addition, we examined depressive symptoms as a potential modifier of this association, based on research showing that adult depression current depression may exacerbate immune effects of childhood adversity (Danese et al., 2008).
METHODS
Patients and Procedures:
Patients were recruited from oncology practices in Los Angeles to participate in a longitudinal, observational study of cancer-related fatigue (RISE study)(Bower et al., 2019). Women were eligible if they had been recently diagnosed with Stage 0-IIIA breast cancer and had not yet started adjuvant or neoadjuvant therapy with radiation, chemotherapy, or endocrine therapy. Primary recruitment sites were UCLA and Cedars Sinai Medical Center (CSMC).
Participants completed a baseline assessment after diagnosis but before onset of adjuvant therapy (with radiation, chemotherapy, and/or endocrine therapy) as well as four post-treatment follow-ups. The focus here is on the baseline assessment, when monocytes were collected. Monocyte isolation was conducted for the first 88 participants, based on funding availability, and 86 of those participants also provided questionnaire data. The Institutional Review Boards at UCLA and CSMC approved the study, and all participants provided written informed consent.
Measures:
Data were collected from self-report questionnaires, blood collection, and medical chart review.
Demographic characteristics were obtained from self-report at baseline and included age, race/ethnicity, marital status, income, and education. In addition, height and weight were measured for determination of body mass index (BMI).
Disease and treatment-related information was obtained from medical record abstraction and included cancer stage, type of surgery received (if any), and time from diagnosis to baseline assessment. The Charlson Comorbidity Index was used to assess other medical comorbidities (Katz, Chang, Sangha, Fossel, & Bates, 1996). Patients were also asked about previous history of breast or other cancers.
History of childhood maltreatment was assessed with the Childhood Trauma Questionnaire (Bernstein, 1998), a 28-item measure that includes questions about physical, emotional, and sexual abuse, as well as physical and emotional neglect that occurred during childhood. Women were categorized into one of three groups based on type and severity of maltreatment using an algorithm with established sensitivity and specificity (Walker et al., 1999). These groups were: no maltreatment (scored 0), physical and/or emotional abuse or neglect but no sexual abuse (scored .5), and sexual abuse with or without physical and/or emotional abuse or neglect (scored 1).
Depressive symptoms were assessed with the Center for Epidemiologic Studies - Depression Scale (CES-D; Radloff, 1977). The CES-D is a 20-item scale assessing frequency of depressive symptoms over the past week. This scale was developed for use in the general population and has been found to be valid and reliable in samples of breast cancer survivors (Hann, Winter, & Jacobsen, 1999). Participants rate each symptom with response options ranging from 0 (rarely or none of the time) to 3 (most or all of the time). Responses are summed to create an overall score reflecting depressive symptoms, with scores of 16 or above suggesting clinically significant depressive symptoms (Radloff, 1977).
Monocyte gene expression: Blood samples were collected by venipuncture, peripheral blood mononuclear cells (PBMC) were isolated by standard ficoll gradient centrifugation, and CD14+ monocytes were isolated from PBMC by immunomagnetic positive selection (MACS, Miltenyi Biotec). CD14+ cells were selected because they are a primary source of proinflammatory cytokines and proinflammatory gene expression in the context of chronic stress (Miller et al., 2008; Miller et al., 2014; Powell et al., 2013). RNA was extracted (Qiagen RNeasy), tested for suitable mass (Nanodrop ND1000) and integrity (Agilent TapeStation), converted to fluorescent cRNA (Ambion TotalPrep) and hybridized to Illumina Human HT-12 v4 BeadArrays following the manufacturer’s standard protocol in the UCLA Neuroscience Genomics Core Laboratory. All samples were assayed in a single batch, quantile normalized, and log2-transformed for statistical analysis as described below. All samples yielded technically valid results according to study-specific probe signal distribution metrics (median intensity > 100 units).
Analyses
Our primary analysis quantified the relationship between CTQ maltreatment and inflammatory gene expression using genome-wide transcriptional profiles as input into the TELiS promoter-based bioinformatics analysis quantifying activity of the proinflammatory transcription factor, NF-κB, using methods described in our previous research (Bower et al., 2015; Cole, Yan, Galic, Arevalo, & Zack, 2005). Briefly, normalized gene expression values were log2 transformed for standard linear statistical model analyses estimating the magnitude of differential gene expression as a function of CTQ classification while controlling for age, race (White vs. Non), BMI, and timing of questionnaire completion relative to surgery (before vs. after). All genes showing a point estimate of >25% differential expression over the range of CTQ maltreatment scores (0/.5/1) served as input into TELiS analyses testing differential prevalence of NF-κB-binding motifs (quantified by TRANSFAC V$NFKAPPAB_01 position-specific weight matrix) in core promoter sequences of up- vs. down-regulated genes. Motif prevalence was quantified by the average (log-) ratio of transcription factor-binding motifs in up- vs. down-regulated promoters across 9 combinations of 3 core promoter sequence lengths (−300, −600, and −1000 to +200 nucleotides relative to the RefSeq transcription start site) and 3 motif detection stringencies (TRANSFAC mat_sim values of .80, .90, and .95).
To determine whether maltreatment exposure was associated with activation of the other major axis of monocyte gene regulation involving the Type I interferon system, we also tested for differential prevalence of Interferon Response Factor-binding sites / interferon-stimulated response element (ISRE) motifs using TRANSFAC V$ISRE_01. In all analyses, statistical testing of the average log-ratio’s difference from the null hypothesis value of 0 was based on standard errors derived from bootstrap resampling of linear model residual vectors, which provides a non-parametric assessment of statistical significance while appropriately controlling for correlation among genes.
To cross-validate results from the primary analysis of transcription factor activation, we also conducted secondary Transcript Origin Analyses (TOA) (Cole, Hawkley, Arevalo, & Cacioppo, 2011) to address the same general question through the lens of monocyte cellular differentiation programs - specifically testing whether the same set of genes showing >25% differential expression across the maltreatment severity groups derived from classical CD16- pro-inflammatory monocytes or from the CD16+ non-classical monocyte phenotype associated with chronic immunologic activation. TOA mapped the empirically observed differences in gene expression onto the transcriptomic differences in isolated classical vs. non-classical monocytes (Wong et al., 2011), with statistical testing of deviation from the null hypothesis value of 0 based on standard errors derived from bootstrap resampling of linear model residual vectors as above.
To determine whether the effects of childhood maltreatment might be mediated at least in part by current depressive symptoms we conducted an ancillary follow-up analysis that added to the basic analytic model an additional variable indicating whether participants showed clinically relevant levels of depressive symptoms (CESD ≥ 16 = 1, 0 otherwise). To ensure estimated maltreatment effects were not confounded by any correlated differences in cancer stage, other comorbid conditions, or history of breast cancer or other types of cancer, ancillary follow-up analyses controlled for those variables. To determine whether depressive symptoms might moderate the effect of maltreatment history, we conducted a second ancillary follow-up analysis that additionally included a Depressive Symptoms x Maltreatment Score interaction term. Throughout these ancillary analyses, genes showing > 25% differential expression as a function of each substantive target parameter served as input into TELiS and TOA analyses as described above.
RESULTS
Characteristics of the 86 study participants are provided in Table 1. Participants were predominantly White and college-educated and had been diagnosed with Stage 0, I, or II breast cancer, comparable to the parent sample of 270 women (Bower et al., 2019). The majority had their tumors surgically resected via lumpectomy or mastectomy prior to baseline assessment, though some women were scheduled to receive neoadjuvant chemotherapy and thus completed their baseline assessments before surgery. On average, women scheduled to receive neoadjuvant therapy were younger than the rest of the sample, though no differences in other demographic characteristics (i.e., income, education) or in childhood maltreatment exposure were observed. Note that cancer stage could not be determined for these women given that their tumors were not available for pathological evaluation.
Table 1.
Demographic and medical characteristics
| Full sample (n=86) | No trauma exposure (n=56) | Physical, emotional, and/or sexual trauma (n=30) | |
|---|---|---|---|
|
| |||
| Age (mean, SD) | 55.95 (11.8) | 56.5 (12.6) | 54.9 (10.3) |
|
| |||
| BMI (mean, SD) | 24.2 (5.3) | 24.1 (5.4) | 24.3 (5.2) |
|
| |||
| Race | |||
| White | 65 | 45 (69%) | 20 (31%) |
| Non-white | 21 | 11 (52%) | 10 (48%) |
|
| |||
| Education | |||
| Less than college | 19 | 13 (68%) | 6 (32%) |
| College graduate | 38 | 26 (68%) | 12 (32%) |
| Post-graduate degree | 29 | 17 (59%) | 12 (41%) |
|
| |||
| Income | |||
| Under 60K | 21 | 11 (52%) | 10 (48%) |
| 60–100K | 12 | 8 (67%) | 4 (33%) |
| 100K+ | 53 | 37 (70%) | 16 (30%) |
|
| |||
| Comorbidities (Charlson Comorbidity Index)* | |||
| 0 | 62 | 41 (66%) | 21 (34%) |
| 1 | 17 | 8 (47%) | 9 (53%) |
| 2+ | 7 | 7 (100%) | 0 (0%) |
|
| |||
| Stage at diagnosis | |||
| 0 | 11 | 6 (54%) | 5 (46%) |
| 1 | 37 | 24 (65%) | 13 (35%) |
| 2 | 18 | 13 (72%) | 5 (28%) |
| 3 | 5 | 4 (80%) | 1 (20%) |
| Indeterminate (neoadjuvant)/missing | 15 | ||
|
| |||
| ER/PR status | |||
| ER+ | 67 | 43 (64%) | 24 (36%) |
| ER− | 5 | 4 (80%) | 1 (20%) |
| PR+ | 58 | 36 (62%) | 22 (38%) |
| PR− | 14 | 11 (79%) | 3 (21%) |
| Indeterminate/missing | 14 | ||
|
| |||
| Her2 status | |||
| Her2+ | 6 | 4 (67%) | 2 (33%) |
| Her2− | 57 | 39 (68%) | 18 (32%) |
| Indeterminate/missing | 23 | ||
|
| |||
| Surgery at enrollment | |||
| None (neoadjuvant) | 12 | 7 (58%) | 5 (42%) |
| Lumpectomy | 46 | 29 (63%) | 17 (37%) |
| Mastectomy | 28 | 20 (71%) | 8 (29%) |
|
| |||
| History of previous cancer (breast or other) | 17 | 11 (65%) | 6 (35%) |
|
| |||
| Depressive symptoms (CESD)* | 11.93 (10.1) | 9.93 (9.6) | 15.67 (10.0) |
p<.05
With respect to childhood maltreatment exposure, 28% of participants were classified as experiencing physical and/or emotional abuse or neglect and 7% as experiencing sexual abuse, with a total of 35% of the sample (n=30) classified as maltreatment exposed. These rates are similar to those observed in the parent sample (Bower et al., 2019) and in demographically comparable samples of women (Walker et al., 1999). For descriptive purposes, we combined the two maltreatment groups when evaluating potential differences in demographic and disease-related characteristics (see Table 1). There were no statistically significant group differences on most characteristics, though there was a non-significant trend for higher rates of maltreatment exposure among non-White women (p =.16) and a higher level of medical comorbidities in the non-maltreated group (p = .045). Depressive symptoms were elevated in the sample as a whole, with 31% of women endorsing clinically significant depressive symptomatology (CESD ≥ 16). Women with a history of childhood maltreatment reported significantly higher levels of depressive symptoms (p = .01) and had higher rates of clinically significant depression (p = .009).
Monocyte gene regulation
To test this study’s primary hypothesis that monocytes from women with a history of childhood maltreatment would show increased proinflammatory signaling activity, we conducted genome-wide transcriptional profiling of isolated monocytes and identified 202 gene transcripts that differed in average expression level by >25% over the range of maltreatment exposure (119 up-regulated and 83 down-regulated), after controlling for any correlated differences in age, race (White vs. Non), body mass index, and timing of questionnaire response relative to surgery (before vs. after). TELiS analyses of those gene transcripts identified a significantly greater prevalence of NF-κB-binding motifs within the promoters of up-regulated vs. down-regulated genes (mean 1.69-fold asymmetry; mean log2 prevalence ratio = 0.76 ± standard error 0.34, p = .028) in women exposed to maltreatment (Figure 1A). Parallel analyses of Type I interferon signaling also indicated increased activity in monocytes from women with a childhood maltreatment history (mean ISRE asymmetry = 2.94-fold; 1.55 ± 0.67, p = .020). Similar results emerged in ancillary analyses that additionally controlled for cancer stage (NF-κB: 1.68-fold; 0.75 ± 0.43, p = .079; ISRE: 4.40-fold; 2.14 ± 0.77, p = .006), for previous history of breast cancer and previous history of other cancers (NF-κB: 1.59-fold; 0.67 ± 0.31, p = .031; ISRE: 3.30-fold; 1.72 ± 0.47, p = .003), and for comorbid medical conditions (NF-κB: 1.56-fold; 0.65 ± 0.31, p = .036; ISRE: 2.91-fold; 1.54 ± 0.62, p = .013).
Figure 1.
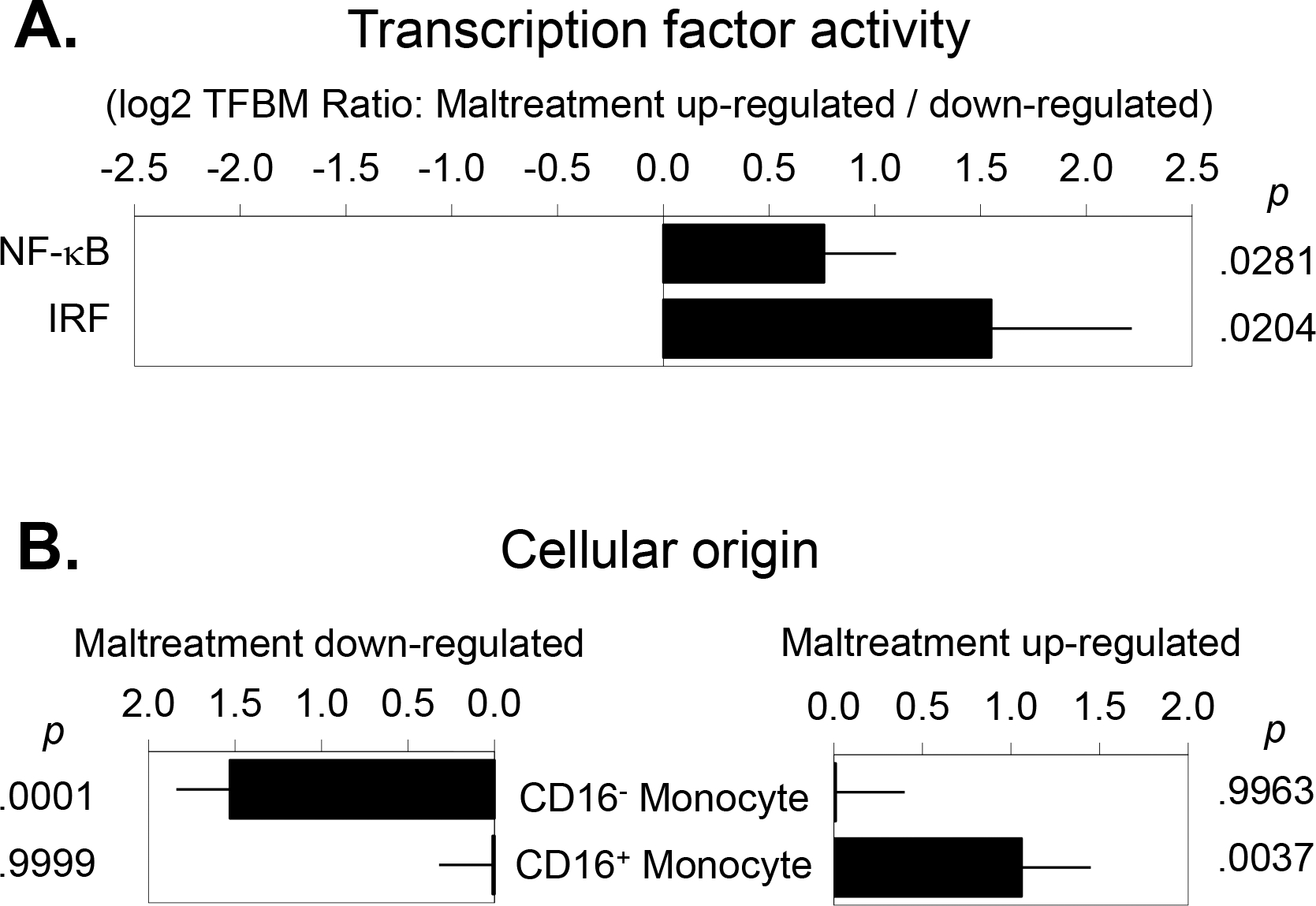
(A) Bioinformatics analysis of transcription factor activity indicated increased activity of the proinflammatory transcription factor NF-κB and increased activity of interferon-related transcription factors (IRF) in breast cancer patients exposed to childhood maltreatment. (B) Transcript origin analyses identified genes down-regulated in maltreatment-exposed women as originating primarily from classical CD16- monocytes, and up-regulated genes as originating predominately from non-classical CD16+ monocytes.
Monocyte subset differentiation
To cross-validate results from the primary analysis of transcription factor activation, we conducted secondary Transcript Origin Analyses (TOA) to determine whether the genes differentially expressed over the range of maltreatment exposure derived from classical CD16- monocytes vs. non-classical CD16+ monocytes (Figure 1B). Results indicated a significant bias toward non-classical monocyte origin for the 119 genes up-regulated in association with maltreatment history (mean TOA diagnosticity score = 1.06 ± 0.39, p = .0037) and a significant bias toward classical monocyte origin for the 83 genes down-regulated in association with maltreatment history (1.53 ± 0.31, p < .001).
Role of depressive symptoms
To determine whether the effects of childhood maltreatment were mediated by current depressive symptoms, we conducted an ancillary follow-up analysis that added to the basic analytic model a covariate identifying participants with clinically significant depressive symptoms (CESD ≥ 16). This analysis identified 54 gene transcripts that were differentially expressed by > 25% as a function of depressive symptoms (23 up-regulated and 31 down-regulated). TELiS analyses of those gene transcripts showed no significant indication of differential NF-κB or IRF activity (both p > .80) and TOA analyses showed no differential derivation from classical vs. non-classical monocytes (all p > .40) based on CESD scores. However, in these analyses controlling for current depressive symptomatology, results continued to link maltreatment to increased activity of NF-κB (2.24-fold; 1.16 ± 0.33, p = .001) and IRFs (2.57-fold; 1.36 ± 0.64, p = .037), and to implicate CD16+ non-classical monocytes in up-regulated gene expression (0.83 ± 0.40, p = .020) and CD16- classical monocytes in down-regulated gene expression (1.83 ± 0.36, p < .001)
To determine whether the effects of childhood maltreatment might be most pronounced in participants who currently experience clinically significant levels of depressive symptoms (i.e., moderation of maltreatment effects by depression), a second set of ancillary analyses identified all gene transcripts showing >25% difference in the quantitative magnitude of maltreatment effects among women showing CESD scores ≥ 16 relative vs. those with CESD scores < 16 (i.e., as a function of a CESD category x CTQ score interaction term). Consistent with previous findings (Danese et al., 2008), the relationship between maltreatment history and biological outcomes was more pronounced in those who also reported current depressive symptoms. Analysis of a maltreatment history x depressive symptom interaction term indicated higher levels of NF-kB activity (1.47-fold; 0.55 ± 0.28, p = .047) and IRF activity (3.53-fold; 1.82 ± 0.66, p = .006). TOA analyses failed to indicate any selective origin of genes up-regulated in association with this interaction (0.18 ± 0.37, p = .345) but did identify classical monocyte subset as a preferential source of genes down-regulated in this interaction (0.83 ± 0.31, p = .004).
DISCUSSION
The present data demonstrate significant alterations in immune-related gene expression among breast cancer patients with a history of childhood maltreatment. We focused specifically on monocyte gene expression and found that maltreatment was associated with increases in the classical NF-κB-related proinflammatory signaling pathway, consistent with previous work on childhood adversity and elevated inflammatory processes (Baumeister et al., 2016; Levine et al., 2015). In addition, we observed marked up-regulation of the other major axis of myeloid cell immune response involving the Type I interferon system and IRF transcription factors. Consistent with that observation, monocyte differentiation analyses found that childhood maltreatment was associated with increased activity of the non-classical CD16+ monocyte subset that is typically associated with broader chronic immunologic activation involving T and B cell biology, rather than involving the more classical CD16- monocyte subset typically involved in focal inflammatory reactions generated by the innate immune system.
The profile of monocyte transcriptional alteration observed here in association with childhood adversity is not consistent with the Conserved Transcriptional Response to Adversity (CTRA) profile that has previously been linked to more contemporaneous forms of adversity (Cole, 2019). The CTRA is mediated in part by sympathetic nervous system-induced up-regulation of classical monocyte production by the bone marrow (Heidt et al., 2014; McKim et al., 2018; Powell et al., 2013) and increased NF-κB activity accompanied by decreased IRF activity. In contrast, the simultaneous up-regulation of NF-κB and IRF activity and increased non-classical monocyte activation observed here appears to reflect a distinct profile of immunologic dysregulation. Non-classical monocytes are often up-regulated in association with chronic activation of the adaptive immune system (e.g., T and B cell activation associated with chronic infections or autoimmune diseases) (Kapellos et al., 2019). This monocyte-derived indication of broader immunoregulatory alteration would be consistent with previous data linking childhood trauma exposure with alterations in EBV and CMV antibody titers, T lymphocyte alterations, IL-17 signaling, and other forms of adaptive immune dysregulation in women recently diagnosed with breast cancer (Han et al., 2016) and in breast cancer survivors (Fagundes et al., 2013) .
Of note, the presence of Type I IFN activation in monocytes from maltreatment exposed women does not mean that the proinflammatory system is not activated. Non-classical monocytes have strong inflammatory potential (Wong et al., 2011) and we observed upregulation of the proinflammatory transcription factor NF-κB associated with childhood maltreatment. Consistent with this hypothesis, we observed incidentally that the NF-κB family member showing the strongest indications of activation in promoter binding-site bioinformatic analyses was the atypical p50 subcomponent (which showed a 2.64-fold activity signal, p = .002, vs. the 1.69-fold, p = .041 signal for our a priori-targeted pan-NF-κB pattern). NF-κB p50 plays a key role in non-classical myeloid cell differentiation and development of M2 macrophages (Porta et al., 2009), and can thereby facilitate tumor growth (Saccani et al., 2006). Non-classical monocytes are biased to differentiate into M2 macrophages that can promote tumor progression and metastasis in the context of breast cancer (Qian & Pollard, 2010). In addition, non-classical monocytes play a role in wound healing (Olingy et al., 2017; Schmidl et al., 2014), and their ability to support vascular remodeling could potentially facilitate tumor angiogenesis and other pro-metastatic processes (Hanahan & Weinberg, 2011). These effects may be particularly relevant in the perioperative period (Horowitz, Neeman, Sharon, & Ben-Eliyahu, 2015), which is when immune assessment for the majority of study participants occurred.
Childhood maltreatment is a strong predictor of adult depression, which is also associated with immune dysregulation (Irwin & Miller, 2007). Indeed, levels of depressive symptoms were significantly higher among women with a history of childhood maltreatment in the current sample. However, results showed that the association between childhood maltreatment and monocyte gene expression was not driven by current depressive symptoms (i.e., effects of maltreatment remained significant despite control for current depressive symptoms). However, moderation analyses examining the synergistic effects of depression and childhood maltreatment suggest that the molecular correlates of maltreatment may be particularly pronounced among those with clinically significant depressive symptoms. More specifically, we observed greater NF-κB, IRF, and monocyte subset polarization effects of childhood maltreatment among women who scored above the clinical cut-point on the CESD. These results are consistent with previous research demonstrating heightened inflammatory activity among individuals with a clustering of childhood adversity and current depression (Danese et al., 2008). If confirmed in future studies involving larger samples, these results may identify a subgroup of individuals who are particularly sensitive to the biological effects of childhood adversity.
Despite the now robust literature documenting links between childhood adversity and poor health across the lifespan, remarkably few studies have investigated these relationships among patients with chronic illnesses, including cancer. Results from the current study suggest that childhood maltreatment exposure is associated with elevated inflammatory signaling as well as with broader indices of immune dysregulation among women with early-stage breast cancer, suggesting a biological pathway through which childhood adversity may increase risk for poor outcomes in this disease population. Limitations of this study include the relatively homogeneous sample, which was comprised primarily of White women with ER+/PR+/Her2- breast tumors and thus may not be generalizable to other samples or tumors with other characteristics (e.g., triple negative tumors). Another limitation is the absence of a non-cancer comparison group, which would inform whether the maltreatment-related alterations in monocyte gene expression are specific to women with breast cancer. The correlational nature of this study also precludes any conclusion that maltreatment directly causes the immunoregulatory alterations observed here. Future research should explore potential correlated alterations in adaptive immune activation and define in more detail the mechanistic pathways that may underlie the observed skewing toward non-classical myeloid differentiation. In addition, future work should consider whether the pathways leading to inflammation are different for women who are in the acute phase of breast cancer diagnosis and treatment as compared to breast cancer survivors, and whether maltreatment has distinct immune correlates among women with breast cancer relative to women with no cancer history. Finally, longitudinal studies with larger, more diverse samples of patients are required to determine whether the observed alterations in immune-related genes among women with childhood trauma are relevant for longer-term outcomes, including cancer recurrence or development of other immune-related co-morbidities.
Financial support:
This work was supported by National Institutes of Health/National Cancer Institute R01 CA160427 and by the Breast Cancer Research Foundation. Dr. Crespi is funded by the National Institutes of Health/National Cancer Institute P30 CA016042, and Dr. Kuhlman is funded by the National Institutes of Health/National Institute of Mental Health K08 MH112773.
REFERENCES
- Baumeister D, Akhtar R, Ciufolini S, Pariante CM, & Mondelli V (2016). Childhood trauma and adulthood inflammation: a meta-analysis of peripheral C-reactive protein, interleukin-6 and tumour necrosis factor-alpha. Mol Psychiatry, 21(5), 642–649. doi: 10.1038/mp.2015.67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein DPF, A. L . (1998). Childhood Trauma Questionnaire Manual. San Antonio: The Psychological Corporation. [Google Scholar]
- Bower JE, Asher A, Garet D, Petersen L, Ganz PA, Irwin MR, . . . Crespi CM (2019). Testing a biobehavioral model of fatigue before adjuvant therapy in women with breast cancer. Cancer, 125(4), 633–641. doi: 10.1002/cncr.31827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bower JE, Crosswell AD, Stanton AL, Crespi CM, Winston D, Arevalo J, . . . Ganz PA (2015). Mindfulness meditation for younger breast cancer survivors: a randomized controlled trial. Cancer, 121(8), 1231–1240. doi: 10.1002/cncr.29194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman DP, Whitfield CL, Felitti VJ, Dube SR, Edwards VJ, & Anda RF (2004). Adverse childhood experiences and the risk of depressive disorders in adulthood. J Affect.Disord, 82(2), 217–225. [DOI] [PubMed] [Google Scholar]
- Cole SW (2019). The Conserved Transcriptional Response to Adversity. Curr Opin Behav Sci, 28, 31–37. doi: 10.1016/j.cobeha.2019.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole SW, Hawkley LC, Arevalo JM, & Cacioppo JT (2011). Transcript origin analysis identifies antigen-presenting cells as primary targets of socially regulated gene expression in leukocytes. Proc.Natl.Acad.Sci.U.S.A, 108(7), 3080–3085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole SW, Yan W, Galic Z, Arevalo J, & Zack JA (2005). Expression-based monitoring of transcription factor activity: the TELiS database. Bioinformatics, 21(6), 803–810. [DOI] [PubMed] [Google Scholar]
- Crosswell AD, Bower JE, & Ganz PA (2014). Childhood adversity and inflammation in breast cancer survivors. Psychosom Med, 76(3), 208–214. doi: 10.1097/PSY.0000000000000041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danese A, Moffitt TE, Pariante CM, Ambler A, Poulton R, & Caspi A (2008). Elevated inflammation levels in depressed adults with a history of childhood maltreatment. Arch Gen Psychiatry, 65(4), 409–415. doi: 10.1001/archpsyc.65.4.409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dube SR, Fairweather D, Pearson WS, Felitti VJ, Anda RF, & Croft JB (2009). Cumulative childhood stress and autoimmune diseases in adults. Psychosomatic Medicine, 71(2), 243–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elwenspoek MMC, Kuehn A, Muller CP, & Turner JD (2017). The effects of early life adversity on the immune system. Psychoneuroendocrinology, 82, 140–154. doi: 10.1016/j.psyneuen.2017.05.012 [DOI] [PubMed] [Google Scholar]
- Fagundes CP, Glaser R, Malarkey WB, & Kiecolt-Glaser JK (2013). Childhood adversity and herpesvirus latency in breast cancer survivors. Health Psychol, 32(3), 337–344. doi: 10.1037/a0028595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felitti VJ, Anda RF, Nordenberg D, Williamson DF, Spitz AM, Edwards V, . . . Marks JS (1998). Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The Adverse Childhood Experiences (ACE) Study. Am.J Prev.Med, 14(4), 245–258. [DOI] [PubMed] [Google Scholar]
- Han TJ, Felger JC, Lee A, Mister D, Miller AH, & Torres MA (2016). Association of childhood trauma with fatigue, depression, stress, and inflammation in breast cancer patients undergoing radiotherapy. Psychooncology, 25(2), 187–193. doi: 10.1002/pon.3831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D, & Weinberg RA (2011). Hallmarks of cancer: the next generation. Cell, 144(5), 646–674. [DOI] [PubMed] [Google Scholar]
- Heidt T, Sager HB, Courties G, Dutta P, Iwamoto Y, Zaltsman A, . . . Nahrendorf M (2014). Chronic variable stress activates hematopoietic stem cells. Nat Med, 20(7), 754–758. doi: 10.1038/nm.3589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowitz M, Neeman E, Sharon E, & Ben-Eliyahu S (2015). Exploiting the critical perioperative period to improve long-term cancer outcomes. Nat Rev Clin Oncol, 12(4), 213–226. doi: 10.1038/nrclinonc.2014.224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin MR, & Cole SW (2011). Reciprocal regulation of the neural and innate immune systems. Nat Rev.Immunol, 11(9), 625–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin MR, & Miller AH (2007). Depressive disorders and immunity: 20 years of progress and discovery. Brain Behav.Immun, 21(4), 374–383. [DOI] [PubMed] [Google Scholar]
- Kapellos TS, Bonaguro L, Gemund I, Reusch N, Saglam A, Hinkley ER, & Schultze JL (2019). Human Monocyte Subsets and Phenotypes in Major Chronic Inflammatory Diseases. Front Immunol, 10, 2035. doi: 10.3389/fimmu.2019.02035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz JN, Chang LC, Sangha O, Fossel AH, & Bates DW (1996). Can comorbidity be measured by questionnaire rather than medical record review? Med.Care, 34(1), 73–84. [DOI] [PubMed] [Google Scholar]
- Kuhlman KR, Boyle CC, Irwin MR, Ganz PA, Crespi CM, Asher A, . . . Bower JE (2017). Childhood maltreatment, psychological resources, and depressive symptoms in women with breast cancer. Child Abuse Negl, 72, 360–369. doi: 10.1016/j.chiabu.2017.08.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhlman KR, Chiang JJ, Horn S, & Bower JE (2017). Developmental psychoneuroendocrine and psychoneuroimmune pathways from childhood adversity to disease. Neuroscience & Biobehavioral Reviews, 80, 166–184. doi: 10.1016/j.neubiorev.2017.05.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamkin DM, Ho HY, Ong TH, Kawanishi CK, Stoffers VL, Ahlawat N, . . . Sloan EK (2016). beta-Adrenergic-stimulated macrophages: Comprehensive localization in the M1-M2 spectrum. Brain Behav Immun, 57, 338–346. doi: 10.1016/j.bbi.2016.07.162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamkin DM, Srivastava S, Bradshaw KP, Betz JE, Muy KB, Wiese AM, . . . Cole SW (2019). C/EBPbeta regulates the M2 transcriptome in beta-adrenergic-stimulated macrophages. Brain Behav Immun, 80, 839–848. doi: 10.1016/j.bbi.2019.05.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine ME, Cole SW, Weir DR, & Crimmins EM (2015). Childhood and later life stressors and increased inflammatory gene expression at older ages. Soc Sci Med, 130, 16–22. doi: 10.1016/j.socscimed.2015.01.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKim DB, Yin W, Wang Y, Cole SW, Godbout JP, & Sheridan JF (2018). Social Stress Mobilizes Hematopoietic Stem Cells to Establish Persistent Splenic Myelopoiesis. Cell Rep, 25(9), 2552–2562 e2553. doi: 10.1016/j.celrep.2018.10.102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Chen E, & Parker KJ (2011). Psychological stress in childhood and susceptibility to the chronic diseases of aging: moving toward a model of behavioral and biological mechanisms. Psychol.Bull, 137(6), 959–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Chen E, Sze J, Marin T, Arevalo JM, Doll R, . . . Cole SW (2008). A functional genomic fingerprint of chronic stress in humans: blunted glucocorticoid and increased NF-kappaB signaling. Biol.Psychiatry, 64(4), 266–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Murphy ML, Cashman R, Ma R, Ma J, Arevalo JM, . . . Cole SW (2014). Greater inflammatory activity and blunted glucocorticoid signaling in monocytes of chronically stressed caregivers. Brain Behav Immun, 41, 191–199. doi: 10.1016/j.bbi.2014.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olingy CE, San Emeterio CL, Ogle ME, Krieger JR, Bruce AC, Pfau DD, . . . Botchwey EA (2017). Non-classical monocytes are biased progenitors of wound healing macrophages during soft tissue injury. Sci Rep, 7(1), 447. doi: 10.1038/s41598-017-00477-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce BL, Ballard-Barbash R, Bernstein L, Baumgartner RN, Neuhouser ML, Wener MH, . . . Ulrich CM (2009). Elevated biomarkers of inflammation are associated with reduced survival among breast cancer patients. J Clin Oncol, 27(21), 3437–3444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porta C, Rimoldi M, Raes G, Brys L, Ghezzi P, Di Liberto D, . . . Sica A (2009). Tolerance and M2 (alternative) macrophage polarization are related processes orchestrated by p50 nuclear factor kappaB. Proc Natl Acad Sci U S A, 106(35), 14978–14983. doi: 10.1073/pnas.0809784106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell ND, Sloan EK, Bailey MT, Arevalo JM, Miller GE, Chen E, . . . Cole SW (2013). Social stress up-regulates inflammatory gene expression in the leukocyte transcriptome via beta-adrenergic induction of myelopoiesis. Proc Natl Acad Sci U S A, 110(41), 16574–16579. doi: 10.1073/pnas.1310655110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian BZ, & Pollard JW (2010). Macrophage diversity enhances tumor progression and metastasis. Cell, 141(1), 39–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff LS (1977). The CES-D Scale: A self-report depression scale for research in the general population. Appl Psychol Measur, 1, 385–401. [Google Scholar]
- Saccani A, Schioppa T, Porta C, Biswas SK, Nebuloni M, Vago L, . . . Sica A (2006). p50 nuclear factor-kappaB overexpression in tumor-associated macrophages inhibits M1 inflammatory responses and antitumor resistance. Cancer Res, 66(23), 11432–11440. doi: 10.1158/0008-5472.CAN-06-1867 [DOI] [PubMed] [Google Scholar]
- Schmidl C, Renner K, Peter K, Eder R, Lassmann T, Balwierz PJ, . . . consortium, F. (2014). Transcription and enhancer profiling in human monocyte subsets. Blood, 123(17), e90–99. doi: 10.1182/blood-2013-02-484188 [DOI] [PubMed] [Google Scholar]
- Sloan EK, Priceman SJ, Cox BF, Yu S, Pimentel MA, Tangkanangnukul V, . . . Cole SW (2010). The sympathetic nervous system induces a metastatic switch in primary breast cancer. Cancer Res, 70(18), 7042–7052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villasenor A, Flatt SW, Marinac C, Natarajan L, Pierce JP, & Patterson RE (2014). Postdiagnosis C-reactive protein and breast cancer survivorship: findings from the WHEL study. Cancer Epidemiol Biomarkers Prev, 23(1), 189–199. doi: 10.1158/1055-9965.EPI-13-0852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker EA, Unutzer J, Rutter C, Gelfand A, Saunders K, VonKorff M, . . . Katon W (1999). Costs of health care use by women HMO members with a history of childhood abuse and neglect. Arch Gen Psychiatry, 56(7), 609–613. [DOI] [PubMed] [Google Scholar]
- Wegman HL, & Stetler C (2009). A meta-analytic review of the effects of childhood abuse on medical outcomes in adulthood. Psychosom Med, 71(8), 805–812. [DOI] [PubMed] [Google Scholar]
- Williams CB, Yeh ES, & Soloff AC (2016). Tumor-associated macrophages: unwitting accomplices in breast cancer malignancy. NPJ Breast Cancer, 2. doi: 10.1038/npjbcancer.2015.25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong KL, Tai JJ, Wong WC, Han H, Sem X, Yeap WH, . . . Wong SC (2011). Gene expression profiling reveals the defining features of the classical, intermediate, and nonclassical human monocyte subsets. Blood, 118(5), e16–31. doi: 10.1182/blood-2010-12-326355 [DOI] [PubMed] [Google Scholar]


