Abstract
Systemic lupus erythematosus (SLE) is a multisystem, chronic autoimmune disease where treatment varies by patient and disease activity. Strong preclinical results and clinical correlates have motivated development of many drugs, but many of these have failed to achieve efficacy in clinical trials. FDA approval of belimumab in 2011 was the first successful SLE drug in nearly six decades. In this article, we review insights into the molecular and clinical heterogeneity of SLE from transcriptomics studies and detail their potential impact on drug development and clinical practices. We critically examine the pipeline of SLE drugs, including past failures and their associated lessons and current promising approaches. Finally, we identify opportunities for integrating these findings and drug development with new multidisciplinary advances to enhance future SLE treatment.
Keywords: systemic lupus erythematosus, therapies, patient heterogeneity, personalized medicine, systems immunology, transcriptomics
SLE is a Prototypic Autoimmune Disease with a Challenging Road to Targeted Therapies `
Systemic lupus erythematosus (SLE) is a complex autoimmune disorder that can impact any organ system. The clinical presentation of SLE is varied, unpredictable, and sometimes includes transient, evolving symptoms that mimic other diseases, leading to its classification as one of medicine’s “great imitators.” This contributes to delayed diagnosis, up to six years from initial symptoms [1], and difficulty in treatment. SLE can affect men and women across age, ethnic, and racial groups. However, there is a strong female bias with women comprising of 90% of patients [2, 3]. The disease predominately presents in women of childbearing age [4], which raises specific challenges regarding disease management during pregnancy (Box 1). The most common manifestations among SLE patients [5] are hematological [6]; musculoskeletal (i.e. arthritis); cutaneous rash; photosensitivity; constitutional symptoms (i.e. fever, fatigue, weight loss, oral or nasal ulcers); renal [7]; neuropsychiatric [8]; pleurisy [9]; pericarditis [10]; and Raynaud’s phenomenon. Patients receive induction drug regimens tailored for treatment of flares based on disease severity (Box 2) followed by drug regimens aimed at maintaining remission and preventing flares (Box 3).
Box 1. Management of SLE During Pregnancy.
SLE predominantly impacts women during reproductive age. Pregnant women with SLE are at high risk for maternal and fetal morbidities. Management of high disease activity and prevention of flares prior to conception are imperative. With proper care, women can maintain a healthy pregnancy with symptom monitoring and regular examinations by a rheumatologist and a maternal-fetal obstetrics team. Sixty years ago, women with SLE were advised against pregnancy due to high fetal death rates and severe flares. Today management of symptoms and pregnancy is possible, resulting in a decrease in pregnancy loss from 43% in the 1960s to 17% in the early 2000s [161]. Adverse outcomes include miscarriage, preterm birth, intrauterine growth retardation, pre-eclampsia, congenital heart block, and neonatal SLE [162]. Risk of preterm birth increases to approximately 60% of SLE pregnancies with increasing SLEDAI disease activity [163]. Women with active lupus nephritis, renal insufficiency, pulmonary arterial hypertension, and antiphospholipid syndrome are at increased risk for these pregnancy complications [164]. As a result, laboratory screening of antiphospholipid antibodies, testing for anti-Ro antibodies that are associated with fetal congenital heart block and neonatal lupus, and monitoring for renal involvement are recommended. Measurement of complement proteins, C3 and C4, as biomarkers for disease activity during SLE pregnancy is complicated by the fact that these proteins are generally elevated in normal pregnancies, and thus, less reliable as a marker of flares [165]. However, a study found that increased levels of Bb and sC5b-9 early in pregnancy were significantly associated with adverse outcomes in patients with SLE and/or antiphospholipid antibodies [166].
Medications that are generally acceptable during pregnancy and breastfeeding include hydroxychloroquine, azathioprine, cyclosporine, tacrolimus, and steroids and intravenous immunoglobulins [167]. Choice of drugs used during pregnancy and breastfeeding considers physician input, prevention of disease activity, reduction of harm to the fetus, and limited adverse side effects in comparison to untreated disease. Adherence to hydroxychloroquine during pregnancy has multiple benefits including a favorable risk to benefit ratio [168, 169], lowering risk of pre-eclampsia [170], lowering disease activity, decreasing steroid doses, and limiting risk of neonatal cardiac manifestations [171]. Risk assessments of biologics, such as belimumab and rituximab, are limited and require more analysis. Future studies exploring the compatibility of novel therapies with pregnancy will be important for broadening treatment options in this patient group.
Box 2. Clinician’s Corner.
SLE is the prototypical autoimmune, systemic, immune complex-mediated disease. The Lupus Foundation of America estimates 1.5 million people in the United States are affected by the disease. There is a well-documented female bias in SLE. This results in women being nine times more likely to develop the disease than men. Women of African, Asian, Hispanic/Latina and Native American ancestry or ethnicity are 2–3 times more likely to develop the disease than women of European ancestry.
SLE is thought to be caused by environmental exposure influencing genetically susceptible individuals over their lifetime. It is the most heterogeneous of autoimmune diseases with a variety of clinical manifestations, organ involvement, disease severity, and laboratory abnormalities. The disease is characterized by dysregulation of the innate and adaptive immune system leading to loss of tolerance to nuclear antigens. Antinuclear antibodies recognize a variety of antigens and are present in 95% of patients at diagnosis. Some autoantibodies are detected years before diagnosis, when patients are still asymptomatic, while others appear months before onset of clinical manifestations.
Treatment of SLE aims to control disease activity, prevent organ damage, reduce morbidity, improve patient survival and health-related quality of life. Current standard of care is dictated by the type and severity of the organ involvement. For constitutional symptoms and mild disease, antimalarials, nonsteroidal anti-inflammatory drugs, and low-dose corticosteroids are used. Broad immunosuppression with methotrexate, azathioprine, cyclophosphamide, cyclosporine, mycophenolate mofetil, leflunomide, and tacrolimus is reserved for patients with persistent manifestations and patients with moderate-severe organ involvement. All immunosuppressive drugs are associated with a significant toxicity and a wide range of morbidities.
Advances in diagnosis and treatment have improved overall survival of lupus patients, but mortality remains 3–5 times greater than in the general population. Moreover, lupus patients have significantly worse health-related quality of life compared to healthy controls or patients with other chronic diseases. There is an urgent need for new therapeutic agents with more selective mode of action targeting pathways relevant to SLE, increasing efficacy and decreasing side effects.
Many challenges including heterogeneity of the disease; lack of diagnostic, predictive and prognostic biomarkers; and flawed clinical trial design have prevented numerous agents in SLE clinical trials from meeting their primary endpoints. Multiple efforts are underway to more comprehensively map the heterogeneity and complexity of the disease at the molecular, single-cell, and tissue-specific level. Several drugs in late-stage development are focused on dysregulated pathwaysin SLE, which may provide a new generation of more effective, less toxic therapies.
Box 3. Symptomatic and Asymptomatic Flares.
An important aspect of clinical management is the treatment and prevention of disease flares. Variability in clinical trial design has resulted in different definitions of SLE flare with no universal consensus. An international meeting convened by the Lupus Foundation of America proposed defining a SLE flare as an episode associated with organ damage, significantly worse patient outcomes as evaluated by an assessor, and consideration of an increase or modification of treatment [172]. SLE flares are intermittent and may occur without any clear warning. Flares can be symptomatic, with clinical manifestations, such as joint pain, skin rash, or oral ulcers, or silent and only detected through laboratory testing of hematologic and renal parameters. Triggers such as stress, infection, injury, hormones, drugs, and UV light may exacerbate inflammation and cause immune system hyperactivity [173].Non-renal disease flares are classified as mild, moderate, or severe [174]. Mild flares are managed by a combination of hydroxychloroquine, glucocorticoids, methotrexate, and azathioprine. Treatment of non-renal severe flares adds mycophenolate mofetil and cyclophosphamide to mild flare drug regimens [174]. In all SLE patients, hydroxychloroquine is a cornerstone treatment due to multiple advantages including cardioprotective benefits [175], improved patient survival [175], reduction of disease flares and disease activity [176], and decreased thrombotic events [177]. Hydroxychloroquine is a lifelong treatment unless contraindicated due to retinal toxicity, a rare side effect of treatment where 10% of patients experience retinopathy after 20 years of use [178, 179]. Moderate-to-severe flares resulting in kidney damage from lupus nephritis are treated based on the histological class of the renal biopsy. Lupus nephritis is a serious manifestation and one of the leading causes of morbidity and mortality affecting approximately 50% of patients [18]. Glomerular lesions in immune complex-mediated lupus nephritis are classified according to the 2003 International Society of Nephrology/Renal Society nomenclature in six classes from class I with mesangial involvement to class VI with advanced sclerosing lesions in >90% of glomeruli [180, 181]. Lupus nephritis patients with class III, IV, and V lesions receive antimalarials along with immunosuppressive agents (i.e. mycophenolate acid derivatives, cyclophosphamide or calcineurin inhibitors) and corticosteroids, but management should be individualized for each patient.
Early detection and aggressive medication regimens have substantially improved SLE survival outcomes from 50% in the 1960s to 90% in the 1990s [11]. Despite this progress, SLE remains one of the leading causes of death among young women aged 15–24 [12, 13]. Drug development has been difficult with a 50+ year gap separating FDA approval of SLE therapies. Recent molecular advances are helping unravel the heterogeneity of SLE presentation and disease pathogenesis (Figure 1). These novel insights may reduce the gap between discovery and FDA-approved therapies, as demonstrated in ongoing and recently completed clinical trials (Table 1). An improved understanding of SLE heterogeneity at the clinical and mechanistic level can drive significant improvements across the care spectrum, enhancing how we diagnose, manage, and treat its manifestations. In this review, we highlight how technologies such as transcriptomics provide a novel framework for personalizing SLE treatment with current and emerging therapies. We envision using transcriptomics analysis of each patient’s immunopathology to guide selection of therapies by mechanism of action potentially enabling more targeted clinical trials and enhanced patient outcomes.
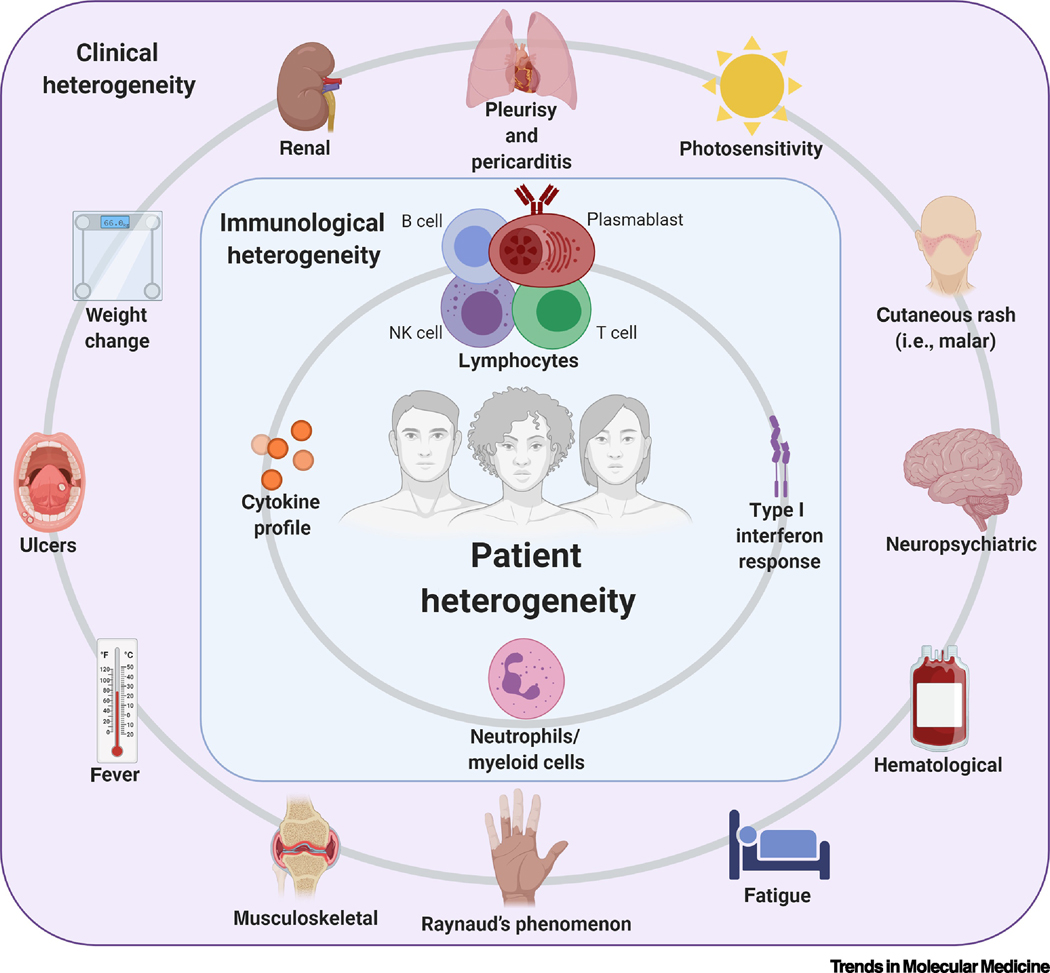
Figure 1. Heterogeneity in SLE [182].
A broad range of clinical manifestations and immunological abnormalities in SLE underlies patient heterogeneity. Patients may present any combination of characteristics and these may change over the course of disease and treatment.
Table 1.
Clinical Trials of SLE Drugs by Targeted Immune Cell or Mechanism of Action from 2005 to 2020
| Drug | ClinicalTrials.gov Identifier a | Mechanism of Action | Study Design b | Status | Primary Outcome Measure(s) |
|---|---|---|---|---|---|
| Immune Complexes | |||||
| RSLV-132 | NCT02194400 | Fc fused to RNase | RC, PC, DB | Completed phase 1 | Number of participants with treatment-related adverse events |
| RSLV-132 | NCT02660944 | Fc fused to RNase | RC, PC, DB | Recruitment status unknown, phase 2a | Proportion of participants with CLASI improvement compared to placebo at week 24 |
| Plasmacytoid Dendritic Cells and Type I Interferon | |||||
| BIIB059 | NCT02847598 (LILAC) | Anti-BDCA2 | PC, RC, DB | Completed phase 2 | Change in baseline active joint count at week 24; Change from baseline CLASI-A score at week 16 |
| BIIB059 | NCT02106897 | Anti-BDCA2 | PC, RC | Completed phase 1 | Adverse Events and Serious Adverse Events at week 32 |
| Anifrolumab | NCT01438489 (MUSE) | Anti-IFNAR subunit 1 | PC, RC, DB, MC | Completed phase 2 | SRI at week 24 with sustained oral corticosteroid reduction |
| Anifrolumab | NCT02446899 (TULIP-2) | Anti-IFNAR subunit 1 | PC, RC, DB | Completed phase 3 | BICLA response at week 52 |
| Anifrolumab | NCT02446912 (TULIP-1) | Anti-IFNAR subunit 1 | PC, RC, DB, MC | Completed phase 3 | SRI ≥4 at week 52 |
| IFN-α kinoid | NCT02665364 | Inactivated IFNα2b coupled to a carrier protein | PC, RC, DB | Active, not recruiting phase 2b | Change in IFN gene signature at week 36; BICLA with corticosteroid taping at week 36 |
| B Cells | |||||
| Rituximab | NCT00137969 (EXPLORER) | Anti-CD20 | PC, RC, DB, MC | Completed phase 2/3 | Major, partial, or no clinical response based on BILAG scores from baseline to week 52 |
| Rituximab | NCT00282347 (LUNAR) | Anti-CD20 | PC, RC, DB, MC | Completed phase 3 | Complete, partial or no renal response at week 52 |
| Obinutuzumab | NCT02550652 (NOBILITY) | Anti-CD20 | PC, RC, DB, MC | Active, not recruiting phase 2 | Complete renal response at week 52 |
| Abatacept | NCT00430677 | Recombinant CTLA4-Ig fusion protein | MC, RC, DB, PC | Terminated phase 2/3 | Time to confirmed complete renal response from day 1 to 12 months |
| Cytokines | |||||
| Belimumab | NCT00410384 (BLISS-76) | Anti-BLyS | MC, RC, DB, PC | Completed phase 3 | SRI-4 response rate at week 52 |
| Belimumab | NCT00424476 (BLISS-52) | Anti-BLyS | MC, RC, DB, PC | Completed phase 3 | SRI response rate at week 52 |
| Belimumab | NCT01639339 (BLISS-LN) | Anti-BLyS | RC, DB, PC | Completed phase 3 | Primary Efficacy Renal Response at week 104 |
| Telitacicept (RC18) | NCT02885610 | Recombinant fusion protein targeting APRIL and BLyS | PC, MC, RC, DB | Completed phase 2b | SRI-4 response rate at week 48 |
| Ustekinumab | NCT02349061 | Monoclonal antibody targeting IL-12 and IL-23 | MC, RC, DB, PC | Completed phase 2 | SRI-4 composite response at week 24 |
| Ustekinumab | NCT03517722 (LOTUS) | Monoclonal antibody targeting IL-12 and IL-23 | RC, DB, PC | Discontinued phase 3 | SRI-4 composite response at week 52 |
| hrIL-2 active | NCT02465580 | Low-dose human recombinant IL-2 | DB, RC, PC | Recruitment status unknown, phase 2 | SLEDAI-4 responders at week 24 |
| Voclosporin | NCT03021499 (AURORA) | Calcineurin inhibitor | RC, DB, PC | Completed phase 3 | Renal response at week 52 |
| Cell Signaling | |||||
| Baricitinib | NCT02708095 | JAK 1/2 inhibitor | RC, DB, PC | Completed phase 2 | Remission of arthritis and/or rash defined by SLEDAI-2K at week 24 |
| Baricitinib | NCT03616912 (BRAVE I) | JAK 1/2 inhibitor | RC, BD, PC | Recruiting phase 3 | SRI-4 response at week 52 |
| Baricitinib | NCT03616964 (BRAVE II) | JAK 1/2 inhibitor | RC, BD, PC | Recruiting phase 3 | SRI-4 response at week 52 |
| Tofacitinib | NCT02535689 | JAK 1/2/3 inhibitor | DB, RC, PC | Completed phase 1b | Safety in SLE participants after 5 years |
| BMS-986165 | NCT03252587 (PAISLEY) | Anti-TYK2 | RC, DB, PC | Recruiting phase 2 | Response criteria of SRI-4 at week 32 |
| Metformin | NCT02741960 | AMPK activation | MC, RC, DB, PC | Completed phase 4 | SELENA-SLEDAI Flare Index at 12 months |
| Combination Therapies | |||||
| Rituximab followed by belimumab | NCT03312907 (BLISS-BELIEVE) | Anti-BLyS and anti-CD20 | MC, RC, DB, PC | Active, not recruiting phase 3 | SLEDAI-2K score <=22 at week 52 |
| Rituximab followed by belimumab | NCT02260934 (CALIBRATE) | Anti-BLyS and anti-CD20 | RC, MC, OL | Completed phase 2 | Infectious adverse event at week 24, 48, and 96 |
Studies are registered at https://clinicaltrials.gov/ct2/
Abbreviations: PC, placebo controlled; RC, randomized; DB, double blind; MC, multi-center; OL, open label; CLASI-A, Cutaneous Lupus Erythematosus Disease Area and Severity Index Activity; BICLA, British Isles Lupus Assessment Group Based Composite Lupus Assessment; SRI, Systemic Lupus Erythematosus (SLE) Responder Index; BILAG, British Isles Lupus Assessment Group; SLEDAI-2K, Systemic Lupus Erythematosus Disease Activity Index 2000; SELENA, Safety of Estrogens in Lupus Erythematosus – National Assessment
Transcriptomic Analyses Reveal SLE Patient Classifications That Guide Future Treatment Strategies
Patient heterogeneity (see Glossary) is a major challenge for SLE management and drug development. A consequence of varied clinical and molecular manifestations, it leads to variability in response to treatment regimens (Figure 1). The scope of this review focuses on how transcriptomic studies are increasing our knowledge and resolution of patient heterogeneity. Other -omics are equally important in understanding the diversity of SLE patients and disease mechanisms, and we refer readers to in-depth reviews on the roles of metabolomics [14, 15], proteomics [16], epigenomics [17], and genomics [18–20]. Transcriptomic profiling across patient demographics can define more robust, data-driven classifications and better identify the mechanisms driving disease. These studies aim to better inform treatment regimens, guide clinical trials design, and develop personalized therapies based on molecular and genetic signatures of disease pathogenesis (Figure 2). Previous transcriptomic studies highlighted the importance of type I interferon (IFN) gene expression signatures [21], immature granulocytes [22], chemokine profiles [23], and CD8+ T cells and T cell exhaustion [24, 25] in distinguishing SLE patient subsets based on disease susceptibility, activity, and severity. For example, granulopoiesis-related genes were upregulated in pediatric SLE patients’ blood versus healthy controls [22]; CD14hi monocytes from newly diagnosed, untreated SLE patients showed statistically significant increases in MCP1, a proinflammatory chemokine involved in lupus nephritis [26], in comparison to healthy controls [23]; and CD4+ and CD8+ T cell expression levels from active, untreated SLE patients correlated with clinical outcomes (i.e. malar rash, arthritis, and oral ulcers) [25]. Analyses of patient tissues and fluids by single-cell sequencing of skin, kidneys, blood, and urine [27–29] have further defined the immune landscape of tissue-specific manifestations likelupus nephritis.
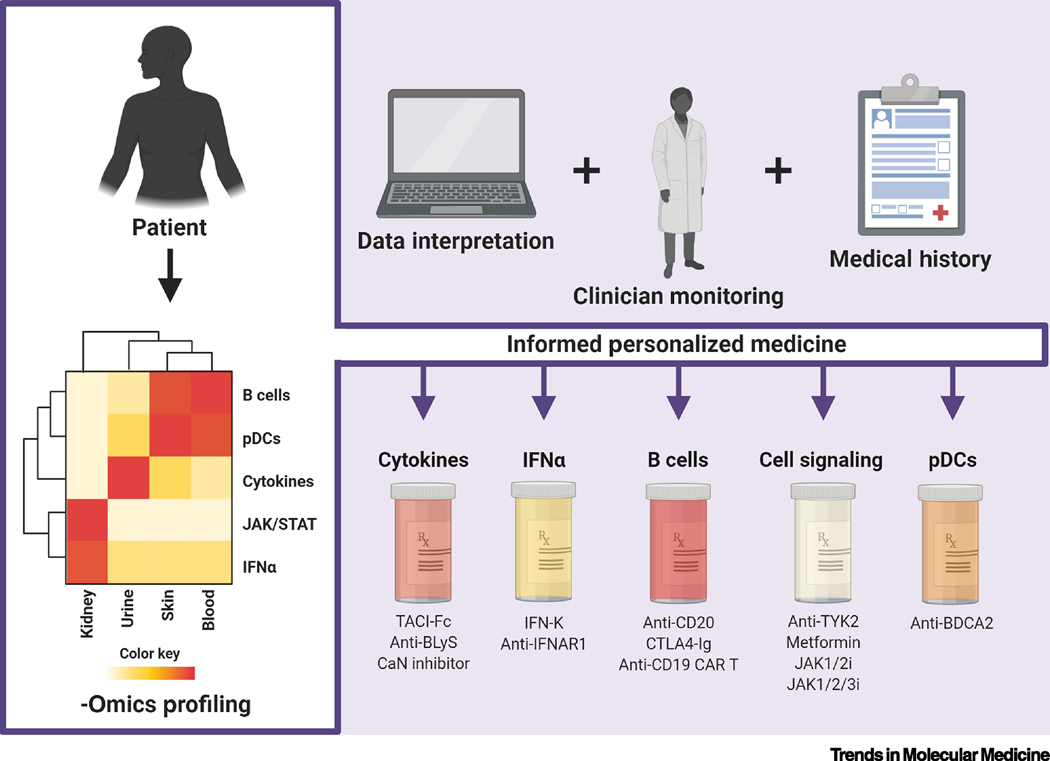
Figure 2. Patient Profiling for Personalized SLE Medicine [182].
Constructing profiles from multiple -omics approaches (i.e. transcriptomics, metabolomics, proteomics, epigenomics, and genomics) can highlight key players in each individual patient’s clinical and molecular manifestations. Interpretation of these profiles within the context of patient medical history and clinician monitoring can inform personalized medicine regimens. Specific drug targets and mechanisms can be matched to patient characteristics to optimize the likelihood of treatment success. Abbreviations used: BDCA2, blood DC antigen 2; BLyS, B lymphocyte stimulator; CaN, calcineurin, CAR, chimeric antigen receptor; IFN, interferon; IFNAR, interferon alpha and beta receptor; IFN-K, IFN-α kinoid; JAK/STAT, Janus kinase/signal transducer and activator of transcription; pDC, plasmacytoid dendritic cell; TACI, transmembrane activator and calcium modulator and cyclophylin ligand interactor; TYK2, tyrosine kinase 2
Longitudinal analyses of blood transcriptome profiles from 158 pediatric SLE patients identified seven groups, each with a distinct combination of five immune signatures: plasmablasts, type I IFN response, neutrophils/myeloid cells, and lymphocytes [30]. The plasmablast signature was the most robust biomarker of disease activity and was enriched in African American patients. Given the increased prevalence, severity, and mortality [31, 32] of SLE in African American patients, there is a major unmet need for effective therapies in this patient population. Plasmablast-targeted therapies may thus be more effective in African American patients and must be further investigated. A neutrophil signature is most associated with progression and differential treatment response of active nephritis, a leading cause of morbidity and mortality in adult and pediatric SLE patients [30]. Future studies to determine how the gene signatures associated with lupus nephritis can be exploited to develop new therapies and design better clinical trials are needed. A caveat of this study design was the observational nature, which did not allow the identification of predictors of flares. It also used the Systemic Lupus Erythematosus Disease Activity Index (SLEDAI), which is less sensitive to change compared to other indices [33]. However, machine learning approaches applied to transcriptomic data can estimate disease activity with 70% accuracy [34, 35].
A meta-analysis of 40 publicly available whole transcriptome datasets containing >7000 samples determined gene expression changes in both adult and pediatric SLE patients [36]. A 93-gene signature was differentially expressed in the blood of SLE patients versus healthy controls and correlated with disease activity. Underexplored genes associated with SLE pathogenesis were identified independent of known IFN-regulated transcriptional profiles. These genes included metallothionein family members (MT1E, MT1FI, and MT1HL1), which are upregulated in kidneys of nephritis patients [37]; and azurophilic granule gene (ELANE), a neutrophil elastase that regulates neutrophil extracellular trap (NET) formation [38]. This study used publicly available data, which restricted tissues and organ sites analyzed. However, the identification of novel disease-correlated genes may provide biomarkers or targets for future therapies and underscores the importance of fully understanding which genes and pathways impact SLE pathogenesis.
Emerging and Approved Therapies for SLE: Past Failures and Future Perspectives
The pathogenesis of SLE involves many types of immune cells and proinflammatory proteins that are potential therapeutic targets (Figure 3, Key Figure). While a detailed discussion of SLE pathogenesis is beyond the scope of this review (previously reviewed in [39]), we briefly overview the rationale for therapeutic targeting of key players in SLE disease progression below. Recent and ongoing approaches in development are detailed, along with perspectives on key knowledge gaps and unmet needs.
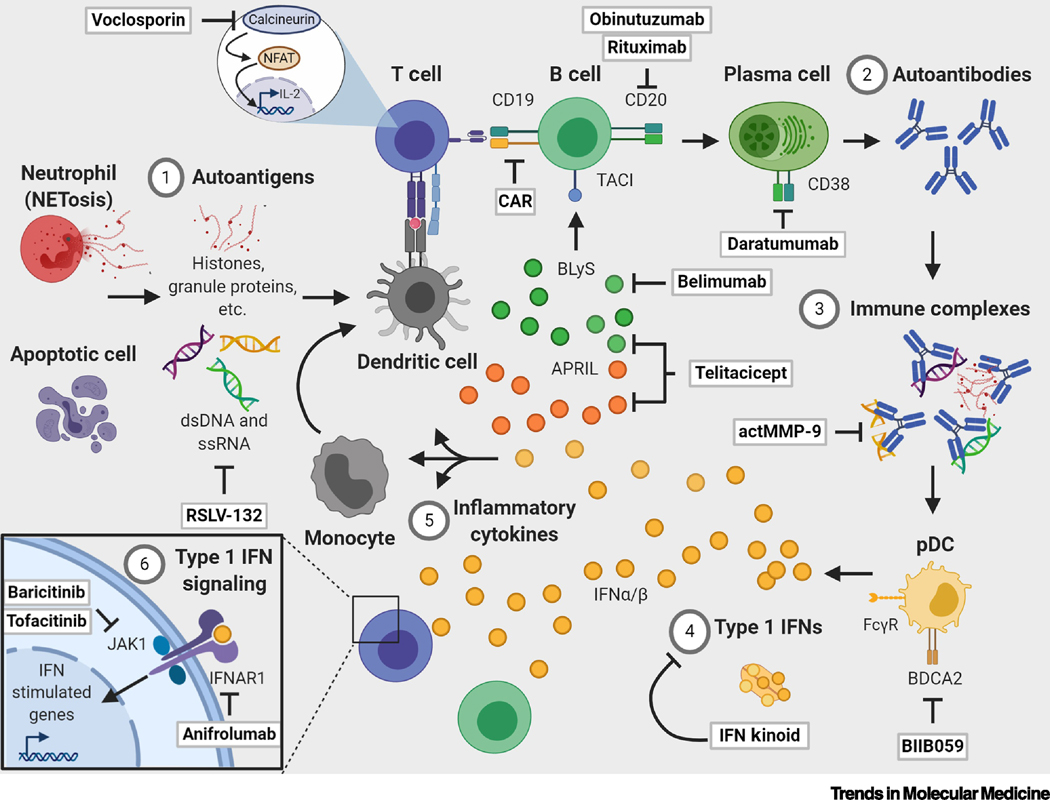
Figure 3. Pathologic mechanisms andemerging therapies in SLE [182].
1) Autoimmune responses are initiated by immune recognition of autoantigens derived from apoptotic material from dead and dying cells and neutrophil extracellular trap debris. These autoantigens include dsDNA, histones, snRNP, Ro/SSA, and La/SSB.2) Autoantigens are presented to self-reactive T cells which provide help to B cells, resulting in cellular activation and the production of pathogenic autoantibodies by plasma cells. 3) Autoantibodies bind to circulating autoantigens to form immune complexes that engage Fc gamma receptors (FcγRs) on plasmacytoid dendritic cells (pDCs), leading to internalization via endocytosis. 4) Intracellular toll-like receptors (TLRs) 7 and 9 are activated by nucleic acids in immune complexes, resulting in type I interferon (IFN) production. 5) Immune complexes and chronic immune activation contribute to excessive production of proinflammatory cytokines. IFNα is one major cytokine in this milieu, which promotes production of pro-B cell survival cytokines (e.g., APRIL, BLyS) via the innate immune system to support B cell maturation. IFNα can also contribute to pathogenic activation of T cells and differentiation of monocytes into dendritic cells. 6) Many cytokine receptors, including type I and II IFNs, transduce signals using the Janus kinase (JAK)/signal transducer and activator of transcription (STAT) pathway. Type I IFN receptors activate JAK/STAT to drive transcription of IFN-stimulated genes to create a positive feedback loop for inflammation. Immunological networks are shown with arrows. Preclinical and clinical therapeutics are highlighted in boxes and are expanded upon in the text. Abbreviations used: APRIL, a proliferation-inducing ligand; BLyS, B lymphocyte stimulator; IFNAR, interferon alpha and beta receptor; NFAT, nuclear factor of activated T cells; TACI, transmembrane activator and calcium modulator and cyclophilin ligand interactor
Immune Complexes
SLE is an immune complex-mediated disease. Deposition of circulating immune complexes composed of endogenous antigens and autoantibodies leads to tissue and organ damage. The major sources of self-antigen are derived from apoptotic material from dead and dying cells [40] and NET debris [41], while autoantibodies are produced by self-reactive B cells. Autoantibodies may predate symptoms for years in asymptomatic individuals and are associated with specific clinical manifestations in symptomatic patients, such as RNA-protein autoantibodies (anti-Ro/SSA and anti-La/SSB) for cutaneous symptoms [42] and anti-double stranded DNA (dsDNA) for lupus nephritis [43]. Specifically, IgG-linked dsDNA immune complexes are found in approximately 80% of lupus nephritis patients [44]. Deficiencies in the classical complement cascade, a system of serum proteins used to clear pathogens from the body, contribute to improper clearance and persistence of immune complexes [45, 46]. ~90% of C1q deficient patients develop SLE or SLE-like symptoms. Immune complexes are therefore a high-priority therapeutic target in SLE.
Enzymes have been used to eliminate immune complexes by degrading DNA, RNA, and protein autoantigens [47–49]. A phase 1b clinical trial infused recombinant human DNase (rhDNase) to degrade extracellular DNA in lupus nephritis patients [47]. This approach was partially motivated by the observation that low serum DNase levels are inversely correlated with nuclear autoantibodies levels in patients [50, 51]. rhDNase failed due to its short half-life and insufficient bioreactive serum concentrations [47]. RSLV-132, a human IgG1 RNase-Fc, digests extracellular RNA and inhibits toll-like receptor (TLR) activation. This therapy is in phase 2 clinical trials for cutaneous manifestations of SLE following a phase 1 trial where the therapy was well-tolerated and showed a 19-day serum half-life [48].
While nucleases are important for the degradation of extracellular DNA and RNA, other enzymes are needed to degrade other pathogenic autoantigens. A preclinical study found that a protease cleaves protein autoantigens incorporated into immune complexes. In this study active matrix metalloproteinase-9 (actMMP-9) degraded immune complexes from plasma of SLE patients and lupus-prone LPR−/− mice in vitro [49]. MMP-9 substrates include known SLE autoantigens such as C4, fibronectin, and C1q [49, 52]. In SLE patient serum, MMP-9 inversely correlates with dsDNA autoantibody levels [53], while MMP-9 inhibitors, such as D-penicillamine [54], can cause drug-induced lupus [55]. Larger studies are needed to define the toxicity, pharmacokinetics, and pharmacodynamics of actMMP-9. A unique advantage of enzymatic treatments for SLE is the potential to stop inflammatory responses by reducing excess circulating or immune complex-bound protein, RNA, and DNA. Future strategies may focus on the potential for matching enzymatic strategies to prevalent autoantigens associated with clinical manifestations, such as DNAse I to degrade extracellular DNA linked to the progression of lupus nephritis [56]. A combination of enzymatic strategies may be needed to address the diversity of pathogenic autoantibodies found in SLE patients. Enzyme-based approaches raise several questions regarding the durability of autoantigen depletion after treatment and the potential effects of cleaved products from autoantibodies and autoantigens (see Outstanding Questions). Additionally, the frequency of treatments needed to continuously degrade these autoantigens for long-term clinical benefit remains undefined since the type and persistence of autoantigens varies from patient to patient. Alternatively, nucleic acid scavenging polymers (NASs) have been used to neutralize circulating nucleic acids in NZB/W F1 and MRL/lpr lupus-prone mice [57]. Treatment with NASs reduced glomerulonephritis, ameliorated cutaneous lupus, and decreased circulating nucleic acids, but polymer toxicity remains a concern for clinical translation.
Outstanding Questions.
What frequency of immune profiling will be needed to monitor patient progress and tailor recommendations for personalized medicine?
How many and what combination therapies are needed to sufficiently address significant aspects of patient heterogeneity based on -omics profiling, sex, age, race, and medical history?
What next-generation approaches can improve safety and toxicity profiles for existing therapies?
How can plasmacytoid dendritic cells and type I interferon-targeted therapies be used to reduce disease activity without compromising antiviral immunity?
How can depletion of B cells and plasma cells be combined to therapeutically reduce pathogenic autoantibody levels?
What risk do cleaved autoantigen pose to inflammatory responses or further binding to autoantibodies?
How should novel therapies be combined with standard treatment to improve patients’ quality of life and clinical outcomes?
What is the best framework to use molecular heterogeneity/classification to match treatments with disease stage and increase efficacy?
Other strategies to block immune complexes have focused on depleting B cells to reduce autoantibodies [58, 59] or inhibiting FcγR signaling (i.e. VIB9600) to suppress proinflammatory responses [60]. Some strategies aim to enhance the clearance of nucleic acids or intracellular proteins enriched in immune complexes [47–49, 57]. Enzyme-based treatments are the most clinically advanced approach to reducing circulating or immune complex-bound autoantigens. The clinical development of NAS polymers is currently limited by concerns on dose-dependent toxicity, which is compounded by functional surface group- and structure-dependent toxicity [61]. These strategies alone only disrupt the assembly and persistence of immune complexes, and the overall feasibility and durability of this approach remains unknown. It remains to be seen whether there is potential synergy between therapies that reduce autoantibody production (e.g., B cell or plasma cell depletion) and enzymatic strategies that reduce autoantigen burden. Currently, plasmapheresis combined with intravenous cyclophosphamide, a potent alkylating agent that kills immune cells by inhibiting protein synthesis, is used to decrease pathogenic autoantibody titers [62–64], while antimalarials are used to dampen proinflammatory responses via inhibiting immune complex-activated TLRs [65]. Additional studies are still needed to define the safety profiles and efficacy of emerging treatments with existing regimens(Box 2).
Plasmacytoid Dendritic Cells and Type I Interferon
Plasmacytoid dendritic cells (pDCs) take up immune complexes via FcγRs into endosomes where they activate TLR-7 and −9, triggering type I IFN production by transcription factors such as IFN regulatory factor (IRF)-7. pDCs are professional IFN-producing cells found in <1% of blood, producing 1,000X more IFN-alpha (IFN-α) than other immune cells [66]. Significant evidence indicates IFN-α is a central mediator of SLE [67, 68]. Among adult SLE patients, 50–75% have high type I IFN signature [69]. IFN-α drives SLE by diverse mechanisms, including suppressing regulatory T cell development [70], activating autoreactive T cells [71], and supporting autoantibody production in B cells [72].
Multiple strategies are under development to inhibit type I IFNs, with the majority focused on antibodies that neutralize IFN-α [73], block its receptor [74], or induce endogenous IFN-α antibodies [75]. Anifrolumab is a monoclonal antibody that blocks the type I IFN receptor subunit 1 (IFNAR1). A phase 2 clinical trial measured the SRI-4 responses as a primary endpoint and a reduction in oral corticosteroid use at week 24 in SLE patients receiving 300 mg or 1000 mg of anifrolumab [76]. Other endpoints, such as BICLA, were assessed at week 52. The primary endpoint was met by 36.0% of SLE patients with high IFN signature treated with 300 mg of anifrolumab versus 28.2% of patients treated with 1000 mg [76]. Likewise, using the BICLA assessment, 53.5% treated at 300 mg versus 41.2% treated at 1000 mg showed a clinical response. Regardless of assessment tools used, SLE patients received better clinical responses and lower incidences of herpes zoster infection at 300 mg (5.1%) versus 1000 mg (9.5%) of anifrolumab. Phase 3 clinical trials balanced anifrolumab efficacy with risk of infection. In the phase 3 TULIP-2 trial, adults with moderate-to-severe SLE received monthly 300 mg anifrolumab or placebo intravenous infusions. The anifrolumab arm successfully reached the primary endpoint of improvement in the British Isles Lupus Assessment Group (BILAG)-based Composite Lupus Assessment (BICLA) score [77]. These results came after the highly anticipated phase 3 TULIP-1 clinical trial failed its primary endpoint [78]. The key difference between TULIP-1 and TULIP-2 was the disease activity measure used as primary endpoint: TULIP-1 used Systemic Lupus Responder Index (SRI)-4 while TULIP-2 used BICLA. SRI-4 requires complete improvement in one severely affected organ or of multiple moderate manifestations. BICLA has increased sensitivity as it allows partial improvement but retains stringency as it requires improvement in all active domains [33]. The design of TULIP-2 was inspired by the successful BICLA responses from TULIP-1’s secondary endpoint analyses. These results suggest the need to evaluate past SLE clinical trial failures to inform and improve future trial designs. Statistically significant benefits of anifrolumab reached in the TULIP-2 clinical trial include a reduction in corticosteroid use and clinically meaningful reductions in disease activity [77]. A significant potential side effect of antibodies against IFNAR1 is increased infection risk, with anifrolumab-treated patients reporting more than twice the frequency of herpes zoster (7.2%) and upper respiratory infections (21.7%) compared to placebo [77].
IFN-α kinoid (IFN-K) is a fusion of inactivated IFN-α to a carrier protein that generates endogenous polyclonal antibodies to block IFN. IFN-K received FDA fast-track designation in 2016. A subsequent phase 2b trial showed promising results including induction of neutralizing antibodies against IFN-α2b, decreased IFN signature, improved fatigue, and reduced corticosteroid dose [75]. However, IFN-K did not meet its primary endpoint (Table 1). Long-term follow-up for this trial was terminated early due to pending financial reorganization of the sponsor, leaving the status of phase 3 trials uncertain.
pDC inhibition is also being investigated to blunt type I IFN production. Human pDCs express the cell surface receptor blood DC antigen 2 (BDCA2 or CD303) [79]. BIIB059 is a humanized IgG1 monoclonal antibody that crosslinks BDCA2 [80, 81], leading to inhibition of TLR7/9 induced IFNα/β by both BDCA2 and FcγRIIa receptor internalization [79] and signaling that shares many components with the B cell receptor [80, 81]. In the phase 2 LILAC trial and a small phase I trial (Table 1), BIIB059 ameliorated cutaneous lupus symptoms, reduced pDC skin infiltration, and normalized type I IFN responses including MxA gene expression, showing benefit in both cutaneous lupus erythematosus patients and SLE patients with active joint or skin manifestations [82]. The trial design measured skin disease activity while monitoring IFN responses in whole blood and skin. The primary type I IFN producers, pDCs, preferentially accumulate in active skin lesions as well as kidneys of SLE patients [83, 84]. Larger studies are needed to determine efficacy and may also evaluate the potential for BIIB059 to ameliorate other pDC-driven organ manifestations, such as lupus nephritis. Overall, BIIB059 was well-tolerated. The main reported side effect was elevated risk of infection due to dampening of pDC-mediated antiviral responses initiated by dose-dependent internalization of BDCA-2. Prolonged (112 days) BDCA-2 internalization occurred at high-doses (20 mg/kg) of BIIB059 [82]. It remains unknown how prolonged BDCA-2 internalization reduces surface BDCA-2, whether receptor internalization limits the effects of subsequent BIIB059 doses, and how this shifts thinking on long-term dosing regimens.
Type I IFNs remain an attractive target in SLE with both cytokine and pDC inhibition demonstrating efficacy in SLE patients and preclinical models. Response rates of anifrolumab-treated patients compared to placebo were similar in patients with high- and low-IFN gene signatures [77]. These results suggest that type I IFN blockade may suppress a broad spectrum of immune activation mechanisms. One major drawback is the importance of IFN-α in antiviral responses, with any therapeutic potentially increasing risk of infection. This must be closely monitored and addressed before IFN-targeted therapies become a therapeutic option for SLE.
B Cells
B cell survival and maintenance are regulated by type I IFN [72]. SLE pathogenesis is fueled by B cells producing autoantibodies that form immune complexes. B cell depletion via rituximab, a chimeric monoclonal antibody targeting CD20, is effective in treating B cell malignancies and rheumatoid arthritis [85, 86]. However, rituximab has shown mixed efficacy in SLE patients and lupus-prone mice. In mouse models of lupus, B cell resistance [87] and poor antibody persistence [88] has led to incomplete B cell depletion. Rituximab did not improve clinical outcomes in the phase 2/3 EXPLORER study of non-renal active SLE [58] or in the LUNAR study of proliferative lupus nephritis [59, 89]. Weaknesses in the LUNAR clinical trial design obscured potential benefits of rituximab. These include a relatively small sample size that precluded a statistical assessment of differences in partial renal remission, brief duration, and use of highly effective background therapies. Regardless, rituximab is widely used by clinicians for lupus nephritis and other clinical manifestations refractory to conventional treatments, such as mycophenolate mofetil and corticosteroids [90].
Despite the failure of rituximab in clinical trials, CD20 is still considered a critical candidate to target pan B cells. Repurposing of obinutuzumab, a type II monoclonal antibody against CD20 approved for adults with follicular lymphoma and chronic lymphocytic leukemia [91], shows promise as a next-generation B cell depletion therapy for SLE. Obinutuzumab was fast tracked by the FDA for adults with proliferative lupus nephritis. B cell cytotoxicity of obinutuzumab is two-fold greater than rituximab in vitro [92]. The phase 2 NOBILITY trial showed that 40% of patients receiving obinutuzumab with standard of care achieved complete renal response compared to 18% of patients receiving placebo with standard of care (Table 1). A major limitation of B cell depletion as a therapeutic approach is the subsequent increase in B cell survival cytokines that can promote expansion of autoreactive B cells, ultimately leading to relapse [93].
B cell depletion using chimeric antigen receptor (CAR) T cells, such as Kymriah and Yescarta, is FDA-approved for the treatment of hematologic malignancies. These have revolutionized cancer care and a preclinical study shows promise in SLE. Kansal et al. demonstrated that CD8+ CAR T cells targeting CD19 are effective in NZB/W F1 and MRLfas/fas lupus mouse models [94]. CAR T cells improved survival, eliminated proteinuria, and suppressed autoantibody production. While exciting, future studies must proceed cautiously as CAR T cells have severe side effects, logistical difficulties, and are complex and expensive to manufacture. Other outstanding questions are the level of B cell depletion required for SLE treatment and whether reinfusion will be necessary.
Another lesson from successful cancer immunotherapies is the use of anti-CTLA4 antibodies to treat solid tumors. Preclinical studies demonstrate that engagement of CTLA4 disrupts T cells activation and prevents T cell help required for B cell maturation [95], thus providing a therapeutic strategy for SLE. Infusion of recombinant CTLA4 protein mitigated autoantibody production in NZB/W F1 mice [96]. Abatacept, a recombinant CTLA4-Ig fusion protein, is FDA approved to treat rheumatoid arthritis. However, multiple clinical trials testing abatacept in SLE patients failed to show improved systemic response or achieve primary endpoints of complete renal response [97–99]. The difference in efficacy of abatacept for rheumatoid arthritis versus SLE patients may be influenced by the different disease models. Rheumatoid arthritis primarily impacts the joints whereas SLE is a multi-organ disease. The lack of efficacy may also be compounded by clinical trial design such as strict definitions of complete renal response, as well as, strong glucocorticoid use which obscured partial responses in a phase 2/3 trial of abatacept for treatment of lupus nephritis [100]. In a post-hoc analysis, positive response rates with abatacept was observed using a different definition of complete renal response [101], thus highlighting the need to reimagine SLE clinical trial design.
Development of SLE treatments has largely been B cell-centric due to the importance of autoantibodies and loss of immune tolerance in B cells. Understanding the hematologic risks, adverse side effects, and dosing schedule for sustained efficacy will be critical for translation of B cell depletion strategies. Additionally, the emergence of CAR T cells for SLE may revolutionize treatment as it has for cancer immunotherapies.
Cytokines
There are many cytokines other than IFN that are critical inflammatory mediators in SLE. The maturation and survival of germinal center B cells are dependent upon signals from tumor necrosis factor (TNF) family cytokines such as B lymphocyte stimulator (BLyS) [102], a B cell growth factor upregulated in SLE patients [103, 104]. Preclinical studies show BLyS overexpression drives lupus-like disease in mice by providing key survival signals for autoreactive B cells and triggering proinflammatory T cells [105–107]. The monoclonal antibody belimumab inhibits BLyS and was the first new FDA approval for SLE in >50 years. In two phase 3 trials, belimumab reduced the numbers of plasma and B cells while significantly reducing SLE disease activity and severe flare risk [108]. Belimumab is indicated for adults and children with active, autoantibody positive SLE receiving standard treatment (Box 2) [109, 110]. In a promising phase 3 clinical trial, 43.0% of lupus nephritis patients receiving belimumab plus standard therapy met primary endpoints versus 32.3% of lupus nephritis patients given placebo (Table 1). Studies are also exploring rituximab followed by belimumab for treatment of lupus nephritis (Table 1) and SLE [111] in an attempt to neutralize BLyS after B cell depletion [93].
A proliferative inducing ligand (APRIL) is another TNF family cytokine that contributes to active nephritis [112] and B-cell mediated SLE pathology. Inhibition of BLyS and APRIL receptor with the fusion protein, TACI-Ig, improved survival and reduced proteinuria in lupus-prone NZB/W F1 mice [113]. A phase 2b trial of telitacicept (RC18), a human TACI-Fc that binds BLyS and APRIL, met primary endpoints, reduced disease activity and was well tolerated (Table 1). Telitacicept received FDA fast track designation in April 2020.
IL-17 and IL-12 family cytokines can exacerbate SLE through T and B cell mediated inflammation. Studies mechanistically linked IL-17 to SLE pathogenesis or correlated IL-17 levels with disease progression or severity [114–117]. IL-17-producing T cells can increase tissue damage in lupus-prone MRL/lpr mice [118] and enhance germinal center reactions [119]. IL-17A and IL-17-producing T cells are elevated in MRL/lpr mice, sustained by IL-12 family member IL-23, and induce nephritis when adoptively transferred into immunodeficient mice [120]. Deletion of IL-23 receptor can also prevent nephritis in lupus-prone mice [121]. In humans, IL-12 levels are higher in SLE patient sera during active disease and lower in patients receiving steroids [122, 123]. A phase 2 trial showed promising clinical responses to ustekinumab, a neutralizing antibody against the p40 protein subunit used by IL-12 and IL-23 [124, 125]. Recently, the phase 3 trial of ustekinumab was discontinued (Table 1) based on futility analysis showing no difference between treatment and standard of care (Box 2).
IL-2 is a cytokine that supports T cell activation and proliferation yet is decreased in SLE patients [126]. In lupus mouse models, a similar decrease in IL-2 reduces the number of regulatory CD4+ T cells (Tregs) leading to compromised immune tolerance [127, 128]. A phase 2 trial showed that SLE patients on standard treatment receiving low-dose IL-2 had expanded Tregs and natural killer cells and better remission rates compared to the placebo [129]. Complete remission was observed in a subset of lupus nephritis patients receiving low dose IL-2, but the sample size was small. Future larger studies are required to demonstrate efficacy of low-dose IL-2 explicitly in lupus nephritis patients. IL-2 expression is partially controlled by calcium signaling and the upstream phosphatase calcineurin. Active calcineurin dephosphorylates the transcription factor NFAT, allowing its nuclear translocation and transcription of genes including IL-2. A phase 3 trial of the calcineurin inhibitor voclosporin in patients with active lupus nephritis showed that treatment with voclosporin and standard of care doubled the proportion of complete kidney response compared to placebo and standard of care (Table 1, Box 2). This study also showed that voclosporin can be used with mycophenolate mofetil and low-dose corticosteroids for lupus nephritis treatment without increasing the rate of serious adverse events. Voclosporin is pending priority review at the FDA.
Cytokines play diverse, critical roles in causing the damaging inflammatory responses in SLE. They form soluble networks that shape the function of other cytokines and immune cells. Targeting single cytokines has been successful in other autoimmune diseases such as rheumatoid arthritis. In SLE, belimumab is a landmark first approval of a biologic and anti-cytokine therapy. As other cytokine therapies become available, it will be important to identify which patients respond to each specific cytokine therapy.
Cell Signaling
Drug targets upstream of cytokine production are appealing for their potential to inhibit multiple cytokine-mediated mechanisms with a single drug. As one example, mTOR activation is critical during T cell activation to provide appropriate metabolic support for proliferation and effector function, to prevent T cell anergy, and to transduce signals from the IL-2 receptor. Inhibition of mTOR can dampen T cell activation processes, such as reducing secretion of inflammatory cytokines including IL-17 and blunting IL-2 signaling, synergizing the effects of blocking both cytokines. Sirolimus is an inhibitor of mTORC1 with multiple effects, including suppressing IL-17 production and promoting Tregs. A phase 1/2 trial of sirolimus in patients with active SLE resistant to conventional treatment demonstrated decreased SLEDAI and BILAG disease activity scores, increased Tregs, and inhibited IL-4 and IL-17 production [130]. Metformin is a hypoglycemic agent widely used for type II diabetes mellitus [131] that inhibits mitochondrial metabolism and mTORC1 by activating AMPK. In the Roauinsan/san lupus mouse model, enhanced AMPK expression and inhibition of the mTOR pathway via metformin was associated with decreased Th17 cells and inhibition of B cell differentiation into plasma cells [132]. In a phase 4 clinical trial (Table 1), SLE patients were treated with metformin and were evaluated for major or mild-to-moderate disease flares. The trial was underpowered, but there was evidence of significantly lower frequency of infection in patients on metformin versus placebo. This suggests potential benefits of combining metformin and other targeted therapies in an attempt to decrease risk of infection.
Cytokine signaling occurs via surface receptor binding and intracellular signal transduction. Many cytokine signaling cascades converge on the downstream Janus kinase (JAK)-signal transducer and activator of transcription (STAT) node to transcribe key inflammatory genes. For example, type I IFN engage IFN-α and -β receptor subunits 1 and 2, subsequently activating JAK1 and tyrosine kinase 2 (TYK2). Receptor phosphorylation leads to activation and nuclear translocation of STAT proteins, which induces the formation of the interferon-stimulated gene factor 3 (ISGF3) complexed with IRF9. ISGF3 then translocates into the nucleus where it enhances transcription of type I IFN and pro-inflammatory cytokines, such as IL-12p40, thus creating a positive feedback loop for inflammation [133]. In 2012, the non-selective JAK1/2/3 inhibitor tofacitinib received approval for the treatment of adults with moderate-to-severe rheumatoid arthritis [134]. Preclinically, tofacitinib reduced nephritis, skin inflammation, and autoantibody production in MRL/lpr lupus-prone mice [135]. Among JAK inhibitors, baricitinib [136], anti-TYK2, and tofacitinib are currently being investigated in clinical trials for systemic and cutaneous lupus (Table 1). A phase 2 trial of the oral JAK 1/2 inhibitor baricitinib met its endpoints: patients receiving a high-dose (4 mg) showed resolution of rash or arthritis symptoms [136], but 6% of patients also experienced severe infections compared to 2% and 1% in low-dose (2 mg) and placebo groups. Phase 3 trials are recruiting patients to assess the efficacy of baricitinib in SLE (Table 1).
The benefits of JAK and mTOR inhibitors include the potential inhibition of multiple cytokines implicated in SLE pathogenesis at a common downstream or upstream signaling node. This may prove more effective than inhibition of multiple soluble proteins or extracellular receptors, replacing them with a monotherapy with distinct safety profiles and side effects and simplifying treatment regimens. Future research is needed to determine if the increased risk of infections outweighs benefits, and whether broader cytokine blockade through cell signaling versus single cytokine inhibition is beneficial or detrimental for drug efficacy and toxicity.
Key Unmet Needs in Treatment and Future Directions
Recent clinical trials in SLE have shown promise in targeting type I IFN or IFN-regulated genes via the JAK/STAT pathway, anti-IFN-α receptor, and IFN-α kinoid. Additionally, blockade of pDCs, the leading producer of IFN-α, via anti-BDCA2 met endpoints in ameliorating cutaneous lupus symptoms. These results are encouraging, but there remains a paucity of drugs to inhibit IFN-independent disease mechanisms.
In addition to the type I IFN signature, the plasmablast signature is the most distinguishing biomarker for SLE disease activity [30]. CD20 expression on B cells varies depending on stage of differentiation and location. Consequently, B cell depletion therapies, such as rituximab, fail to eliminate long-lived plasma cells responsible for autoantibody production. Alternative strategies are needed for plasma cell targeting and elimination. CD38 expressing plasma cells and plasmablasts are present at higher frequencies in SLE patients [137]. Repurposing daratumumab, an FDA-approved anti-CD38 antibody for multiple myeloma, depletes plasmablasts ex vivo in peripheral blood mononuclear cells (PBMCs) from SLE patients [138]. Follow-up in vivo studies are needed to confirm these observations and evaluate efficacy in disease. A caveat of targeted plasmablast treatment is that depletion may not decrease pathogenic autoantibodies without impacting protective antimicrobial antibodies. Additionally, the instability and changing kinetics of plasmablasts in the peripheral blood may make depletion challenging.
Neutrophils are the most abundant leukocyte, comprising between 35–80% of all circulating leukocytes. They represent a high abundance target in comparison to pDCs (0.2–0.6% of leukocytes) or plasma cells (0.2–2.0% of leukocytes in bone marrow) and are an underexplored cell type in SLE treatment. Neutrophil signatures are associated with active lupus nephritis [30, 139] and vascular disease [140]. There is also an established relationship between NETs and SLE pathogenesis [141]. The spontaneous activation and release of NETs through a process called NETosis is enhanced in SLE patients [142]. NETs are a source of autoantigens for SLE autoantibodies and impaired NET degradation due to low serum DNase I levels has been associated with greater risk of nephritis and high titers of anti-dsDNA [143]. Controlled degradation of NETs via reactive oxygen species (ROS) inhibitors, nucleases, or hydrolases are a novel class of drugs for SLE. For instance, mitochondrial NETosis is dependent on ROS and ROS scavengers, such as N-acetyl cysteine, may reduce NET formation in vitro [144]. These effects may contribute to T cell-mediated effects noted above, and improved disease outcomes in a randomized, double-blind, placebo-controlled trial with SLE patients [145, 146]. In vitro studies with patient PBMCs showed metformin reduces NET formation and pDC-induced IFNα production, while a proof-of-concept clinical trial demonstrated metformin decreased flares, prednisone use, and body weight in mild-to-moderate SLE patients treated with corticosteroids and standard immunosuppressive drugs [147]. These results suggest metformin may reduce NETs and pDC-mediated type I IFN responses, suggesting the opportunity for synergy of metformin with complementary therapies that target mechanisms other than neutrophils or pDCs. Neutrophil-mediated mechanisms of pathogenesis are an exciting new frontier for drug development in SLE and provide a crucial complement to other more established drug mechanisms.
Drug delivery and toxicity are persistent limitations for every drug candidate previously mentioned. Novel technologies are being used to develop next-generation therapies for SLE with improved persistence and efficacy combined with reduced toxicity. Among these are bispecific molecules such as the nanobody ALX-0061 [148], which binds IL-6R and human serum albumin to increase circulating half-life, and AMG 570 [149], which inhibits inducible T cell costimulator ligand and BLyS. Nanoparticles that target or enhance drug delivery in preclinical SLE models have been designed. These include dendritic cell-targeted and non-targeted nanoparticles containing mycophenolic acid [150, 151] and nanoparticles delivering mitogen-activated protein kinase siRNAs to glomeruli [152]. The safety and efficacy of novel drugs in SLE should continue to be evaluated; however, advances in drug delivery technologies may further improve odds of success for both existing and novel therapies.
Aside from therapeutic research and development, clinical trial design is an overarching problem that can block successful testing of novel SLE therapies. Improvements are stalled by restrictive patient eligibility based on disease classification criteria [153] compounded by geographically restricted trial sites that make access challenging to diverse populations. Failure to recruit adequate patients of African, Asian, and Native American descent [154] results in a lack of representation of patients with an increased risk of SLE and worse outcomes than their European descent counterparts [155]. Aggressive background immunosuppressive medications, such as those used in the LUNAR trial, may convolute outcomes of the proposed drug [156]. Because immunosuppressants and corticosteroids are not FDA approved specifically for SLE, they cannot be used as the comparator arm. Primary endpoints focused on treatment of only multi-system disease may mask treatment options beneficial to patients with organ-specific disease. These primary measures may also miss drugs that improve but not completely control SLE patient outcomes.
Towards Personalized Medicine for Systemic Lupus Erythematosus Patients
Immune profiling SLE patients using transcriptomic analysis, will allow for explicit pairing of molecular networks, disease severity, medical history, and clinical manifestations to create an integrated treatment plan (Figure 2). Outside of the approval of belimumab, most SLE treatments are non-specific with serious side effects and toxicities. Advances in personalized medicine can enable the evaluation and selection of more specific drug targets complementary to a patient’s clinical identity. Other disease models, such as cancer, have revolutionized treatment in this way. For example, trastuzumab, a monoclonal antibody against human epidermal receptor-2 (HER-2) is used for the treatment of HER-2+ breast cancers [157] and tisagenlecleucel, a CAR T cell therapy, is employed for acute lymphoblastic leukemia [158, 159]. While transcriptomics helps us better understand SLE patients, it represents a small snapshot of SLE patient heterogeneity. Other -omics approaches such as metabolomics, proteomics, epigenomics, and genomics are equally important to discovering the networks that contribute to disease and clinical manifestations. There are multiple barriers to implementing a vision of future clinical research and care integrating an -omics approach to personalization. Major logistical hurdles include the need for more trained researchers and clinicians with competency in both computational biology and SLE pathogenesis, and the lack of standardized methods for data analysis and integration of - omics with other clinical features in SLE. Another barrier for personalized medicine is the high cost, even with insurance coverage, of genetic testing and screening prior to selection of therapy. For example, genomic testing of tumor tissue can range from $300 to >$10,000 per test [160]. SLE will likely require testing of multiple samples of distinct origin (i.e. skin, kidney, blood, urine) repetitively over the life of the patient to capture the intrapatient heterogeneity of the disease. Many SLE patients would need multi -omics testing to build our understanding of which drugs best match patient clinical identities. Only a fraction may be able to use the knowledge of these tests to receive personalized treatment due to the limited toolbox of diverse, specific treatment options available to SLE patients. Re-imagining clinical trial design may increase chances of SLE drug approval through changes such as inclusion of genetic testing and updated clinical assessment tools that can stratify patients by capturing clinical and molecular heterogeneity.
Concluding Remarks
In 2011, belimumab was the first FDA-approved therapy in 55 years to expand the limited list of SLE medications. As of 2020, there are 476 interventional studies for SLE with 65 studies currently recruiting patients on ClinicalTrials.gov. Experimental and computational advances interrogating transcriptomic profiles of patients have helped validate biomarkers, stratify patient subsets, and prioritize targets for precision medicine. The goal of future therapies (Figure 3) should be to effectively manage flares, reduce corticosteroid use, improve quality of life, eliminate hospitalizations, and prevent organ damage (see Outstanding Questions). Clinical trial design should be revisited to better reflect the heterogeneity and complexity of SLE. Endpoints must ensure that failures are the result of true lack of efficacy and not poor trial design that fails to account for disease complexity. Future research to understand how novel therapies interact with each other and standard of care (Box 1–3) will be important for risk-to-benefit analysis and to determine possible synergistic treatment strategies. Combining novel therapies with personalized medicine (Figure 2) may address the challenges of patient heterogeneity, leading to future improvements in both SLE standard of care and clinical outcomes.
Highlights.
Advances in transcriptomic analyses have revealed systemic lupus erythematosus (SLE) patient classifications and underscored the importance of type I interferon-dependent and -independent gene signatures in disease pathogenesis. Results from these studies offer new insights into treatment design to target different immune cell types, proteins, and signaling pathways.
Biologic drug development broke a five-decade gap with belimumab, the first U.S. Food & Drug Administration (FDA) approved drug for adults and children with moderate-to-severe SLE. The FDA also granted fast-track designation to telitacicept for SLE and obinutuzumab for proliferative lupus nephritis.
In 2020, ClinicalTrials.gov shows >400 trials registered for SLE and >100 for lupus nephritis. Refinement of clinical trial design may improve the success of therapies in development.
Acknowledgments
Thank you to Samantha Allen and Brianda Beverly for providing useful feedback and input on this review. MEA was supported by the NIH IMSD Meyerhoff Graduate Fellows Program (5R25GM055036-24).
Glossary.
- C1q
a plasma protein of the classical complement cascade, a system used to clear pathogens via antibodies and phagocytic cells
- Chimeric antigen receptor (CAR)
engineered T cell receptors used to target a specific protein
- Cytokine
a secreted protein that affects the behavior of immune cells
- Immune signature
a gene or groups of genes in a cell type associated with a biological process or disease pathology
- Immune tolerance
prevention of an immune response to a self-antigen; an important feature of the immune system to prevent autoimmunity
- Lupus nephritis
inflammation of the kidneys and a major risk factor for mortality and morbidity in systemic lupus erythematosus patients
- Machine learning
an application of artificial intelligence where a computer is programmed to perform tasks without explicit instructions
- Nanoparticle
a particle on the scale of 1 to 100 nanometers which may be used for therapeutic purposes
- Neutrophil extracellular traps (NETs)
a network of nuclear chromatin decorated with antimicrobial effectors released from neutrophils upon apoptosis
- Patient heterogeneity
variability in a population resulting in differences in clinical and molecular manifestations
- Personalized medicine
an approach that customizes treatment based on a patient’s genetic and molecular profile
- Proteinuria
abnormal protein levels in the urine
- Small interfering RNA (siRNA)
an artificially synthesized <30 base long non-coding, double-stranded RNA used to silence a gene of interest
- Transcriptional profiling
a tool to expose a group of genes within a cell or tissue of interest at the RNA level
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Al Sawah S, et al. , SAT0423 Understanding Delay in Diagnosis, Access to Care and Satisfaction with Care in Lupus: Findings from a Cross-Sectional Online Survey in the United States. Ann. Rheum. Dis, 2015. 74(Suppl 2): p. 812–812. [Google Scholar]
- 2.McDonald G, et al. , Female Bias in Systemic Lupus Erythematosus is Associated with the Differential Expression of X-Linked Toll-Like Receptor 8. Front. Immunol, 2015. 6: p. 457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Murphy G. and Isenberg D, Effect of gender on clinical presentation in systemic lupus erythematosus. Rheumatology (Oxford), 2013. 52(12): p. 2108–15. [DOI] [PubMed] [Google Scholar]
- 4.Rider V, et al. , Gender Bias in Human Systemic Lupus Erythematosus: A Problem of Steroid Receptor Action? Front. Immunol, 2018. 9: p. 611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fava A. and Petri M, Systemic lupus erythematosus: Diagnosis and clinical management. J Autoimmun, 2019. 96: p. 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Velo-García A, Castro SG, and Isenberg DA, The diagnosis and management of the haematologic manifestations of lupus. J Autoimmun, 2016. 74: p. 139–160. [DOI] [PubMed] [Google Scholar]
- 7.Maria NI and Davidson A, Protecting the kidney in systemic lupus erythematosus: from diagnosis to therapy. Nat Rev Rheumatol, 2020. 16(5): p. 255–267. [DOI] [PubMed] [Google Scholar]
- 8.Kivity S, et al. , Neuropsychiatric lupus: a mosaic of clinical presentations. BMC Med., 2015. 13: p. 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Medlin JL, et al. , Pulmonary manifestations in late versus early systemic lupus erythematosus: A systematic review and meta-analysis. Semin. Arthritis Rheum, 2018. 48(2): p. 198–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dein E, et al. , Pericarditis in Lupus. Cureus, 2019. 11(3): p. e4166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mak A, et al. , Global trend of survival and damage of systemic lupus erythematosus: meta-analysis and meta-regression of observational studies from the 1950s to 2000s. Semin. Arthritis Rheum, 2012. 41(6): p. 830–839. [DOI] [PubMed] [Google Scholar]
- 12.Singh RR and Yen EY, SLE mortality remains disproportionately high, despite improvements over the last decade. Lupus, 2018. 27(10): p. 1577–1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yen EY and Singh RR, Brief Report: Lupus-An Unrecognized Leading Cause of Death in Young Females: A Population-Based Study Using Nationwide Death Certificates, 2000–2015. Arthritis Rheumatol, 2018. 70(8): p. 1251–1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang N. and Perl A, Metabolism as a Target for Modulation in Autoimmune Diseases. Trends Immunol, 2018. 39(7): p. 562–576. [DOI] [PubMed] [Google Scholar]
- 15.Teng X, et al. , Metabolic determinants of lupus pathogenesis. Immunol Rev, 2020. 295(1): p. 167–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li Y. and Wu T, Proteomic approaches for novel systemic lupus erythematosus (SLE) drug discovery. Expert Opin Drug Discov, 2018. 13(8): p. 765–777. [DOI] [PubMed] [Google Scholar]
- 17.Ballestar E, Sawalha AH, and Lu Q, Clinical value of DNA methylation markers in autoimmune rheumatic diseases. Nat Rev Rheumatol, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mohan C. and Putterman C, Genetics and pathogenesis of systemic lupus erythematosus and lupus nephritis. Nat Rev Nephrol, 2015. 11(6): p. 329–41. [DOI] [PubMed] [Google Scholar]
- 19.Almlöf JC, et al. , Novel risk genes for systemic lupus erythematosus predicted by random forest classification. Sci Rep, 2017. 7(1): p. 6236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oparina N, Martínez-Bueno M, and Alarcón-Riquelme ME, An update on the genetics of systemic lupus erythematosus. Curr Opin Rheumatol, 2019. 31(6): p. 659–668. [DOI] [PubMed] [Google Scholar]
- 21.Baechler EC, et al. , Interferon-inducible gene expression signature in peripheral blood cells of patients with severe lupus. Proc. Natl. Acad. Sci. U. S. A, 2003. 100(5): p. 2610–2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bennett L, et al. , Interferon and granulopoiesis signatures in systemic lupus erythematosus blood. J. Exp. Med, 2003. 197(6): p. 711–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O’Gorman WE, et al. , Single-cell systems-level analysis of human Toll-like receptor activation defines a chemokine signature in patients with systemic lupus erythematosus. J. Allergy Clin. Immunol, 2015. 136(5): p. 1326–1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McKinney EF, et al. , A CD8+ T cell transcription signature predicts prognosis in autoimmune disease. Nat. Med, 2010. 16(5): p. 586–91, 1p following 591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McKinney EF, et al. , T-cell exhaustion, co-stimulation and clinical outcome in autoimmunity and infection. Nature, 2015. 523(7562): p. 612–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gupta R, Yadav A, and Aggarwal A, Longitudinal assessment of monocyte chemoattractant protein-1 in lupus nephritis as a biomarker of disease activity. Clin Rheumatol, 2016. 35(11): p. 2707–2714. [DOI] [PubMed] [Google Scholar]
- 27.Arazi A, et al. , The immune cell landscape in kidneys of patients with lupus nephritis. Nat. Immunol, 2019. 20(7): p. 902–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Der E, et al. , Tubular cell and keratinocyte single-cell transcriptomics applied to lupus nephritis reveal type I IFN and fibrosis relevant pathways. Nat. Immunol, 2019. 20(7): p. 915–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Der E, et al. , Single cell RNA sequencing to dissect the molecular heterogeneity in lupus nephritis. JCI Insight, 2017. 2(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Banchereau R, et al. , Personalized Immunomonitoring Uncovers Molecular Networks that Stratify Lupus Patients. Cell, 2016. 165(3): p. 551–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Korbet SM, et al. , Severe lupus nephritis: racial differences in presentation and outcome. J. Am. Soc. Nephrol, 2007. 18(1): p. 244–254. [DOI] [PubMed] [Google Scholar]
- 32.Barnado A, et al. , Phenome-wide association study identifies marked increased in burden of comorbidities in African Americans with systemic lupus erythematosus. Arthritis Res. Ther, 2018. 20(1): p. 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mikdashi J. and Nived O, Measuring disease activity in adults with systemic lupus erythematosus: the challenges of administrative burden and responsiveness to patient concerns in clinical research. Arthritis Res. Ther, 2015. 17: p. 183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Catalina MD, et al. , The pathogenesis of systemic lupus erythematosus: Harnessing big data to understand the molecular basis of lupus. J. Autoimmun, 2019: p. 102359. [DOI] [PubMed] [Google Scholar]
- 35.Kegerreis B, et al. , Machine learning approaches to predict lupus disease activity from gene expression data. Sci. Rep, 2019. 9(1): p. 9617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Haynes WA, et al. , Integrated, multicohort analysis reveals unified signature of systemic lupus erythematosus. JCI Insight, 2020. 5(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Faurschou M, et al. , The renal metallothionein expression profile is altered in human lupus nephritis. Arthritis Res. Ther, 2008. 10(4): p. R76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Papayannopoulos V, et al. , Neutrophil elastase and myeloperoxidase regulate the formation of neutrophil extracellular traps. J. Cell Biol, 2010. 191(3): p. 677–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moulton VR, et al. , Pathogenesis of Human Systemic Lupus Erythematosus: A Cellular Perspective. Trends Mol Med, 2017. 23(7): p. 615–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Muñoz LE, et al. , The role of defective clearance of apoptotic cells in systemic autoimmunity. Nat. Rev. Rheumatol, 2010. 6(5): p. 280–289. [DOI] [PubMed] [Google Scholar]
- 41.Lande R, et al. , Neutrophils activate plasmacytoid dendritic cells by releasing self-DNA-peptide complexes in systemic lupus erythematosus. Sci. Transl. Med, 2011. 3(73): p. 73ra19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Biazar C, et al. , Cutaneous lupus erythematosus: first multicenter database analysis of 1002 patients from the European Society of Cutaneous Lupus Erythematosus (EUSCLE). Autoimmun Rev, 2013. 12(3): p. 444–54. [DOI] [PubMed] [Google Scholar]
- 43.Tan EM, et al. , Deoxybonucleic acid (DNA) and antibodies to DNA in the serum of patients with systemic lupus erythematosus. J Clin Invest, 1966. 45(11): p. 1732–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang X. and Xia Y, Anti-double Stranded DNA Antibodies: Origin, Pathogenicity, and Targeted Therapies. Front. Immunol, 2019. 10: p. 1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stegert M, Bock M, and Trendelenburg M, Clinical presentation of human C1q deficiency: How much of a lupus? Mol. Immunol, 2015. 67(1): p. 3–11. [DOI] [PubMed] [Google Scholar]
- 46.Macedo ACL and Isaac L, Systemic Lupus Erythematosus and Deficiencies of Early Components of the Complement Classical Pathway. Front. Immunol, 2016. 7: p. 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Davis JC Jr., et al. , Recombinant human Dnase I (rhDNase) in patients with lupus nephritis. Lupus, 1999. 8(1): p. 68–76. [DOI] [PubMed] [Google Scholar]
- 48.Burge DJ, et al. , Safety, pharmacokinetics, and pharmacodynamics of RSLV-132, an RNase-Fc fusion protein in systemic lupus erythematosus: a randomized, double-blind, placebo-controlled study. Lupus, 2017. 26(8): p. 825–834. [DOI] [PubMed] [Google Scholar]
- 49.Ugarte-Berzal E, et al. , MMP-9/Gelatinase B Degrades Immune Complexes in Systemic Lupus Erythematosus. Front. Immunol, 2019. 10: p. 538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Frost PG and Lachmann PJ, The relationship of desoxyribonuclease inhibitor levels in human sera to the occurrence of antinuclear antibodies. Clin. Exp. Immunol, 1968. 3(5): p. 447–455. [PMC free article] [PubMed] [Google Scholar]
- 51.Chitrabamrung S, Rubin RL, and Tan EM, Serum deoxyribonuclease I and clinical activity in systemic lupus erythematosus. Rheumatol. Int, 1981. 1(2): p. 55–60. [DOI] [PubMed] [Google Scholar]
- 52.Cauwe B, et al. , Multidimensional degradomics identifies systemic autoantigens and intracellular matrix proteins as novel gelatinase B/MMP-9 substrates. Integr. Biol, 2009. 1(5–6): p. 404–426. [DOI] [PubMed] [Google Scholar]
- 53.Makowski GS and Ramsby ML, Concentrations of circulating matrix metalloproteinase 9 inversely correlate with autoimmune antibodies to double stranded DNA: implications for monitoring disease activity in systemic lupus erythematosus. Mol. Pathol, 2003. 56(4): p. 244–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Enzenauer RJ, West SG, and Rubin RL, D-penicillamine-induced lupus erythematosus. Arthritis Rheum., 1990. 33(10): p. 1582–1585. [DOI] [PubMed] [Google Scholar]
- 55.Miller KK, et al. , Drug-induced subacute cutaneous lupus erythematosus related to doxycycline. Dermatol. Online J, 2011. 17(10): p. 3. [PubMed] [Google Scholar]
- 56.Pedersen HL, et al. , Lupus nephritis: low urinary DNase I levels reflect loss of renal DNase I and may be utilized as a biomarker of disease progression. J Pathol Clin Res, 2018. 4(3): p. 193–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Holl EK, et al. , Scavenging nucleic acid debris to combat autoimmunity and infectious disease. Proc. Natl. Acad. Sci. U. S. A, 2016. 113(35): p. 9728–9733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Merrill JT, et al. , Efficacy and safety of rituximab in moderately-to-severely active systemic lupus erythematosus: the randomized, double-blind, phase II/III systemic lupus erythematosus evaluation of rituximab trial. Arthritis Rheum., 2010. 62(1): p. 222–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rovin BH, et al. , Efficacy and safety of rituximab in patients with active proliferative lupus nephritis: the Lupus Nephritis Assessment with Rituximab study. Arthritis Rheum., 2012. 64(4): p. 1215–1226. [DOI] [PubMed] [Google Scholar]
- 60.Chen B, et al. , Humanised effector-null FcγRIIA antibody inhibits immune complex-mediated proinflammatory responses. Ann. Rheum. Dis, 2019. 78(2): p. 228–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jain K, et al. , Dendrimer toxicity: Let’s meet the challenge. Int J Pharm, 2010. 394(1–2): p. 122–42. [DOI] [PubMed] [Google Scholar]
- 62.Yamaji K, et al. , Long-term clinical outcomes of synchronized therapy with plasmapheresis and intravenous cyclophosphamide pulse therapy in the treatment of steroid-resistant lupus nephritis. Ther. Apher. Dial, 2008. 12(4): p. 298–305. [DOI] [PubMed] [Google Scholar]
- 63.Mistry-Burchardi N, Schönermarck U, and Samtleben W, Apheresis in lupus nephritis. Ther. Apher, 2001. 5(3): p. 161–170. [PubMed] [Google Scholar]
- 64.Danieli MG, et al. , Synchronised therapy and high-dose cyclophosphamide in proliferative lupus nephritis. J. Clin. Apher, 2002. 17(2): p. 72–77. [DOI] [PubMed] [Google Scholar]
- 65.Kuznik A, et al. , Mechanism of endosomal TLR inhibition by antimalarial drugs and imidazoquinolines. J Immunol, 2011. 186(8): p. 4794–804. [DOI] [PubMed] [Google Scholar]
- 66.Liu Y-J, IPC: professional type 1 interferon-producing cells and plasmacytoid dendritic cell precursors. Annu. Rev. Immunol, 2005. 23: p. 275–306. [DOI] [PubMed] [Google Scholar]
- 67.Crow MK, Advances in understanding the role of type I interferons in systemic lupus erythematosus. Curr. Opin. Rheumatol, 2014. 26(5): p. 467–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chasset F. and Arnaud L, Targeting interferons and their pathways in systemic lupus erythematosus. Autoimmun. Rev, 2018. 17(1): p. 44–52. [DOI] [PubMed] [Google Scholar]
- 69.Rönnblom L. and Leonard D, Interferon pathway in SLE: one key to unlocking the mystery of the disease. Lupus Sci Med, 2019. 6(1): p. e000270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yan B, et al. , Dysfunctional CD4+,CD25+ regulatory T cells in untreated active systemic lupus erythematosus secondary to interferon-alpha-producing antigen-presenting cells. Arthritis Rheum., 2008. 58(3): p. 801–812. [DOI] [PubMed] [Google Scholar]
- 71.Blanco P, et al. , Induction of dendritic cell differentiation by IFN-alpha in systemic lupus erythematosus. Science, 2001. 294(5546): p. 1540–1543. [DOI] [PubMed] [Google Scholar]
- 72.Kiefer K, et al. , Role of type I interferons in the activation of autoreactive B cells. Immunol. Cell Biol, 2012. 90(5): p. 498–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Greth W, et al. , Targeting the interferon pathway with sifalimumab for the treatment of systemic lupus erythematosus. Immunotherapy, 2017. 9(1): p. 57–70. [DOI] [PubMed] [Google Scholar]
- 74.Goldberg A, et al. , Dose-escalation of human anti-interferon-α receptor monoclonal antibody MEDI-546 in subjects with systemic sclerosis: a phase 1, multicenter, open label study. Arthritis Res. Ther, 2014. 16(1): p. R57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Houssiau FA, et al. , IFN-α kinoid in systemic lupus erythematosus: results from a phase IIb, randomised, placebo-controlled study. Ann. Rheum. Dis, 2020. 79(3): p. 347–355. [DOI] [PubMed] [Google Scholar]
- 76.Furie R, et al. , Anifrolumab, an Anti-Interferon-α Receptor Monoclonal Antibody, in Moderate-to-Severe Systemic Lupus Erythematosus. Arthritis Rheumatol, 2017. 69(2): p. 376–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Morand EF, et al. , Trial of Anifrolumab in Active Systemic Lupus Erythematosus. N. Engl. J. Med, 2020. 382(3): p. 211–221. [DOI] [PubMed] [Google Scholar]
- 78.Felten R, et al. , Spotlight on anifrolumab and its potential for the treatment of moderate-to-severe systemic lupus erythematosus: evidence to date. Drug Des. Devel. Ther, 2019. 13: p. 1535–1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pellerin A, et al. , Anti-BDCA2 monoclonal antibody inhibits plasmacytoid dendritic cell activation through Fc-dependent and Fc-independent mechanisms. EMBO Mol. Med, 2015. 7(4): p. 464–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Röck J, et al. , CD303 (BDCA-2) signals in plasmacytoid dendritic cells via a BCR-like signalosome involving Syk, Slp65 and PLCgamma2. Eur. J. Immunol, 2007. 37(12): p. 3564–3575. [DOI] [PubMed] [Google Scholar]
- 81.Cao W, et al. , BDCA2/Fc epsilon RI gamma complex signals through a novel BCR-like pathway in human plasmacytoid dendritic cells. PLoS Biol, 2007. 5(10): p. e248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Furie R, et al. , Monoclonal antibody targeting BDCA2 ameliorates skin lesions in systemic lupus erythematosus. J. Clin. Invest, 2019. 129(3): p. 1359–1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tucci M, et al. , Glomerular accumulation of plasmacytoid dendritic cells in active lupus nephritis: role of interleukin-18. Arthritis Rheum, 2008. 58(1): p. 251–62. [DOI] [PubMed] [Google Scholar]
- 84.Vermi W, et al. , Cutaneous distribution of plasmacytoid dendritic cells in lupus erythematosus. Selective tropism at the site of epithelial apoptotic damage. Immunobiology, 2009. 214(9–10): p. 877–86. [DOI] [PubMed] [Google Scholar]
- 85.Dotan E, Aggarwal C, and Smith MR, Impact of Rituximab (Rituxan) on the Treatment of B-Cell Non-Hodgkin’s Lymphoma. P T, 2010. 35(3): p. 148–157. [PMC free article] [PubMed] [Google Scholar]
- 86.Mok CC, Rituximab for the treatment of rheumatoid arthritis: an update. Drug Des. Devel. Ther, 2013. 8: p. 87–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ahuja A, et al. , Depletion of B cells in murine lupus: efficacy and resistance. J. Immunol, 2007. 179(5): p. 3351–3361. [DOI] [PubMed] [Google Scholar]
- 88.Bekar KW, et al. , Prolonged effects of short-term anti-CD20 B cell depletion therapy in murine systemic lupus erythematosus. Arthritis Rheum., 2010. 62(8): p. 2443–2457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gomez Mendez LM, et al. , Peripheral Blood B Cell Depletion after Rituximab and Complete Response in Lupus Nephritis. Clin. J. Am. Soc. Nephrol, 2018. 13(10): p. 1502–1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chavarot N, et al. , Rituximab alone as induction therapy for membranous lupus nephritis: A multicenter retrospective study. Medicine (Baltimore), 2017. 96(27): p. e7429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Falchi L, et al. , An Evidence-based Review of Anti-CD20 Antibody-containing Regimens for the Treatment of Patients With Relapsed or Refractory Chronic Lymphocytic Leukemia, Diffuse Large B-cell Lymphoma, or Follicular Lymphoma. Clin Lymphoma Myeloma Leuk, 2018. 18(8): p. 508–518.e14. [DOI] [PubMed] [Google Scholar]
- 92.Reddy V, et al. , Obinutuzumab induces superior B-cell cytotoxicity to rituximab in rheumatoid arthritis and systemic lupus erythematosus patient samples. Rheumatology, 2017. 56(7): p. 1227–1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Carter LM, Isenberg DA, and Ehrenstein MR, Elevated serum BAFF levels are associated with rising anti-double-stranded DNA antibody levels and disease flare following B cell depletion therapy in systemic lupus erythematosus. Arthritis Rheum, 2013. 65(10): p. 2672–9. [DOI] [PubMed] [Google Scholar]
- 94.Kansal R, et al. , Sustained B cell depletion by CD19-targeted CAR T cells is a highly effective treatment for murine lupus. Sci. Transl. Med, 2019. 11(482). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Daikh DI and Wofsy D, Cutting edge: reversal of murine lupus nephritis with CTLA4Ig and cyclophosphamide. J. Immunol, 2001. 166(5): p. 2913–2916. [DOI] [PubMed] [Google Scholar]
- 96.Finck BK, Linsley PS, and Wofsy D, Treatment of murine lupus with CTLA4Ig. Science, 1994. 265(5176): p. 1225–1227. [DOI] [PubMed] [Google Scholar]
- 97.Wofsy D, Hillson JL, and Diamond B, Abatacept for lupus nephritis: alternative definitions of complete response support conflicting conclusions. Arthritis Rheum., 2012. 64(11): p. 3660–3665. [DOI] [PubMed] [Google Scholar]
- 98.Merrill JT, et al. , The efficacy and safety of abatacept in patients with non-life-threatening manifestations of systemic lupus erythematosus: results of a twelve-month, multicenter, exploratory, phase IIb, randomized, double-blind, placebo-controlled trial. Arthritis Rheum., 2010. 62(10): p. 3077–3087. [DOI] [PubMed] [Google Scholar]
- 99.Furie R, et al. , Efficacy and safety of abatacept in lupus nephritis: a twelve-month, randomized, double-blind study. Arthritis Rheumatol, 2014. 66(2): p. 379–389. [DOI] [PubMed] [Google Scholar]
- 100.Furie R, et al. , Efficacy and safety of abatacept in lupus nephritis: a twelve-month, randomized, double-blind study. Arthritis Rheumatol, 2014. 66(2): p. 379–89. [DOI] [PubMed] [Google Scholar]
- 101.Wofsy D, Hillson JL, and Diamond B, Comparison of alternative primary outcome measures for use in lupus nephritis clinical trials. Arthritis Rheum, 2013. 65(6): p. 1586–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Crowley JE, et al. , Homeostatic niche specification among naïve and activated B cells: a growing role for the BLyS family of receptors and ligands. Semin. Immunol, 2005. 17(3): p. 193–199. [DOI] [PubMed] [Google Scholar]
- 103.Duan JH, et al. , Expression of BAFF and BR3 in patients with systemic lupus erythematosus. Braz. J. Med. Biol. Res, 2016. 49(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Eilertsen GØ, et al. , Increased levels of BAFF in patients with systemic lupus erythematosus are associated with acute-phase reactants, independent of BAFF genetics: a case-control study. Rheumatology, 2011. 50(12): p. 2197–2205. [DOI] [PubMed] [Google Scholar]
- 105.Scapini P, et al. , Myeloid cells, BAFF, and IFN-gamma establish an inflammatory loop that exacerbates autoimmunity in Lyn-deficient mice. J. Exp. Med, 2010. 207(8): p. 1757–1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Huang W, et al. , BAFF/APRIL inhibition decreases selection of naive but not antigen-induced autoreactive B cells in murine systemic lupus erythematosus. J. Immunol, 2011. 187(12): p. 6571–6580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Groom JR, et al. , BAFF and MyD88 signals promote a lupuslike disease independent of T cells. J. Exp. Med, 2007. 204(8): p. 1959–1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Stohl W, et al. , Belimumab reduces autoantibodies, normalizes low complement levels, and reduces select B cell populations in patients with systemic lupus erythematosus. Arthritis Rheum., 2012.64(7): p. 2328–2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Navarra SV, et al. , Efficacy and safety of belimumab in patients with active systemic lupus erythematosus: a randomised, placebo-controlled, phase 3 trial. Lancet, 2011. 377(9767): p. 721–731. [DOI] [PubMed] [Google Scholar]
- 110.Dubey AK, et al. , Belimumab: First targeted biological treatment for systemic lupus erythematosus. J. Pharmacol. Pharmacother, 2011. 2(4): p. 317–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Teng YKO, et al. , Phase III, multicentre, randomised, double-blind, placebo-controlled, 104-week study of subcutaneous belimumab administered in combination with rituximab in adults with systemic lupus erythematosus (SLE): BLISS-BELIEVE study protocol. BMJ Open, 2019. 9(3): p. e025687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Neusser MA, et al. , Intrarenal production of B-cell survival factors in human lupus nephritis. Mod. Pathol, 2011. 24(1): p. 98–107. [DOI] [PubMed] [Google Scholar]
- 113.Gross JA, et al. , TACI and BCMA are receptors for a TNF homologue implicated in B-cell autoimmune disease. Nature, 2000. 404(6781): p. 995–9. [DOI] [PubMed] [Google Scholar]
- 114.Dolff S, et al. , Increased expression of costimulatory markers CD134 and CD80 on interleukin-17 producing T cells in patients with systemic lupus erythematosus. Arthritis Res. Ther, 2010. 12(4): p. R150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Crispín JC, et al. , Expanded double negative T cells in patients with systemic lupus erythematosus produce IL-17 and infiltrate the kidneys. J. Immunol, 2008. 181(12): p. 8761–8766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wong CK, et al. , Elevation of proinflammatory cytokine (IL-18, IL-17, IL-12) and Th2 cytokine (IL-4) concentrations in patients with systemic lupus erythematosus. Lupus, 2000. 9(8): p. 589–593. [DOI] [PubMed] [Google Scholar]
- 117.Wong CK, et al. , Hyperproduction of IL-23 and IL-17 in patients with systemic lupus erythematosus: implications for Th17-mediated inflammation in auto-immunity. Clin. Immunol, 2008. 127(3): p. 385–393. [DOI] [PubMed] [Google Scholar]
- 118.Edgerton C, et al. , IL-17 producing CD4+ T cells mediate accelerated ischemia/reperfusion-induced injury in autoimmunity-prone mice. Clin. Immunol, 2009. 130(3): p. 313–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hsu H-C, et al. , Interleukin 17-producing T helper cells and interleukin 17 orchestrate autoreactive germinal center development in autoimmune BXD2 mice. Nat. Immunol, 2008. 9(2): p. 166–175. [DOI] [PubMed] [Google Scholar]
- 120.Zhang Z, Kyttaris VC, and Tsokos GC, The role of IL-23/IL-17 axis in lupus nephritis. J. Immunol, 2009. 183(5): p. 3160–3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kyttaris VC, et al. , Cutting edge: IL-23 receptor deficiency prevents the development of lupus nephritis in C57BL/6-lpr/lpr mice. J. Immunol, 2010. 184(9): p. 4605–4609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Tokano Y, et al. , Levels of IL-12 in the sera of patients with systemic lupus erythematosus (SLE)--relation to Th1- and Th2-derived cytokines. Clin. Exp. Immunol, 1999. 116(1): p. 169–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lauwerys BR, Van Snick J, and Houssiau FA, Serum IL-12 in systemic lupus erythematosus: absence of p70 heterodimers but presence of p40 monomers correlating with disease activity. Lupus, 2002. 11(6): p. 384–387. [DOI] [PubMed] [Google Scholar]
- 124.van Vollenhoven RF, et al. , Efficacy and safety of ustekinumab, an IL-12 and IL-23 inhibitor, in patients with active systemic lupus erythematosus: results of a multicentre, double-blind, phase 2, randomised, controlled study. Lancet, 2018. 392(10155): p. 1330–1339. [DOI] [PubMed] [Google Scholar]
- 125.van Vollenhoven RF, et al. , Maintenance of Efficacy and Safety of Ustekinumab Through One Year in a Phase II Multicenter, Prospective, Randomized, Double-Blind, Placebo-Controlled Crossover Trial of Patients With Active Systemic Lupus Erythematosus. Arthritis Rheumatol, 2020. 72(5): p. 761–768. [DOI] [PubMed] [Google Scholar]
- 126.Solomou EE, et al. , Molecular basis of deficient IL-2 production in T cells from patients with systemic lupus erythematosus. J. Immunol, 2001. 166(6): p. 4216–4222. [DOI] [PubMed] [Google Scholar]
- 127.Humrich JY, et al. , Homeostatic imbalance of regulatory and effector T cells due to IL-2 deprivation amplifies murine lupus. Proc. Natl. Acad. Sci. U. S. A, 2010. 107(1): p. 204–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lieberman LA and Tsokos GC, The IL-2 defect in systemic lupus erythematosus disease has an expansive effect on host immunity. J. Biomed. Biotechnol, 2010. 2010: p. 740619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.He J, et al. , Efficacy and safety of low-dose IL-2 in the treatment of systemic lupus erythematosus: a randomised, double-blind, placebo-controlled trial. Ann. Rheum. Dis, 2020. 79(1): p. 141–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Lai ZW, et al. , Sirolimus in patients with clinically active systemic lupus erythematosus resistant to, or intolerant of, conventional medications: a single-arm, open-label, phase 1/2 trial. Lancet, 2018. 391(10126): p. 1186–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Schlender L, et al. , Efficacy and safety of metformin in the management of type 2 diabetes mellitus in older adults: a systematic review for the development of recommendations to reduce potentially inappropriate prescribing. BMC Geriatr, 2017.17(Suppl 1): p. 227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lee SY, et al. , Metformin Suppresses Systemic Autoimmunity in Roquin san/san Mice through Inhibiting B Cell Differentiation into Plasma Cells via Regulation of AMPK/mTOR/STAT3. J Immunol, 2017. 198(7): p. 2661–2670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Jensen MA and Niewold TB, Interferon regulatory factors: critical mediators of human lupus. Transl. Res, 2015. 165(2): p. 283–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Cohen SB, et al. , Long-term safety of tofacitinib for the treatment of rheumatoid arthritis up to 8.5 years: integrated analysis of data from the global clinical trials. Ann. Rheum. Dis, 2017. 76(7): p. 1253–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Furumoto Y, et al. , Tofacitinib Ameliorates Murine Lupus and Its Associated Vascular Dysfunction . Arthritis Rheumatol, 2017. 69(1): p. 148–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Wallace DJ, et al. , Baricitinib for systemic lupus erythematosus: a double-blind, randomised, placebo-controlled, phase 2 trial. Lancet, 2018. 392(10143): p. 222–231. [DOI] [PubMed] [Google Scholar]
- 137.Cole S, et al. , Integrative analysis reveals CD38 as a therapeutic target for plasma cell-rich pre-disease and established rheumatoid arthritis and systemic lupus erythematosus. Arthritis Res. Ther, 2018. 20(1): p. 85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Usmani SZ, et al. , Clinical efficacy of daratumumab monotherapy in patients with heavily pretreated relapsed or refractory multiple myeloma. Blood, 2016. 128(1): p. 37–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Toro-Domínguez D, et al. , Stratification of Systemic Lupus Erythematosus Patients Into Three Groups of Disease Activity Progression According to Longitudinal Gene Expression. Arthritis Rheumatol, 2018. 70(12): p. 2025–2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Carlucci PM, et al. , Neutrophil subsets and their gene signature associate with vascular inflammation and coronary atherosclerosis in lupus. JCI Insight, 2018. 3(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Lood C, et al. , Neutrophil extracellular traps enriched in oxidized mitochondrial DNA are interferogenic and contribute to lupus-like disease. Nat Med, 2016. 22(2): p. 146–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Villanueva E, et al. , Netting neutrophils induce endothelial damage, infiltrate tissues, and expose immunostimulatory molecules in systemic lupus erythematosus. J. Immunol, 2011. 187(1): p. 538–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Hakkim A, et al. , Impairment of neutrophil extracellular trap degradation is associated with lupus nephritis. Proc. Natl. Acad. Sci. U. S. A, 2010. 107(21): p. 9813–9818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Patel S, et al. , Nitric oxide donors release extracellular traps from human neutrophils by augmenting free radical generation. Nitric Oxide, 2010. 22(3): p. 226–34. [DOI] [PubMed] [Google Scholar]
- 145.Lai ZW, et al. , N-acetylcysteine reduces disease activity by blocking mammalian target of rapamycin in T cells from systemic lupus erythematosus patients: a randomized, double-blind, placebo-controlled trial. Arthritis Rheum, 2012. 64(9): p. 2937–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Garcia RJ, et al. , Attention deficit and hyperactivity disorder scores are elevated and respond to N-acetylcysteine treatment in patients with systemic lupus erythematosus. Arthritis Rheum, 2013. 65(5): p. 1313–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Wang H, et al. , Neutrophil Extracellular Trap Mitochondrial DNA and Its Autoantibody in Systemic Lupus Erythematosus and a Proof-of-Concept Trial of Metformin . Arthritis Rheumatol, 2015. 67(12): p. 3190–200. [DOI] [PubMed] [Google Scholar]
- 148.Van Roy M, et al. , The preclinical pharmacology of the high affinity anti-IL-6R Nanobody® ALX-0061 supports its clinical development in rheumatoid arthritis. Arthritis Res. Ther, 2015. 17: p. 135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Zhang M, et al. , Development of an ICOSL and BAFF bispecific inhibitor AMG 570 for systemic lupus erythematosus treatment. Clin. Exp. Rheumatol, 2019. 37(6): p. 906–914. [PubMed] [Google Scholar]
- 150.Look M, et al. , The nanomaterial-dependent modulation of dendritic cells and its potential influence on therapeutic immunosuppression in lupus. Biomaterials, 2014. 35(3): p. 1089–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Look M, et al. , Nanogel-based delivery of mycophenolic acid ameliorates systemic lupus erythematosus in mice. J. Clin. Invest, 2013. 123(4): p. 1741–1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Shimizu H, et al. , siRNA-based therapy ameliorates glomerulonephritis. J. Am. Soc. Nephrol, 2010. 21(4): p. 622–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Aberle T, et al. , Use of SLICC criteria in a large, diverse lupus registry enables SLE classification of a subset of ACR-designated subjects with incomplete lupus. Lupus Sci Med, 2017. 4(1): p. e000176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Sheikh SZ, et al. , The State of Lupus Clinical Trials: Minority Participation Needed. J. Clin. Med. Res, 2019. 8(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Somers EC, et al. , Population-based incidence and prevalence of systemic lupus erythematosus: the Michigan Lupus Epidemiology and Surveillance program. Arthritis Rheumatol, 2014. 66(2): p. 369–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Merrill JT, et al. , Lupus community panel proposals for optimising clinical trials: 2018. Lupus Sci Med, 2018. 5(1): p. e000258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Carter P, et al. , Humanization of an anti-p185HER2 antibody for human cancer therapy. Proc Natl Acad Sci U S A, 1992. 89(10): p. 4285–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Grupp SA, et al. , Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N Engl J Med, 2013. 368(16): p. 1509–1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Maude SL, et al. , Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med, 2014. 371(16): p. 1507–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.McDowell S. Cancer Patients May Not Be Told About Costs of Genomic Testing. 2019. [cited 2020 August 25]; Available from: https://www.cancer.org/latest-news/cancer-patients-may-not-be-told-about-costs-of-genomic-testing.html. [Google Scholar]
- 161.Clark CA, Spitzer KA, and Laskin CA, Decrease in pregnancy loss rates in patients with systemic lupus erythematosus over a 40-year period. J Rheumatol, 2005. 32(9): p. 1709–12. [PubMed] [Google Scholar]
- 162.Lateef A. and Petri M, Managing lupus patients during pregnancy. Best Pract Res Clin Rheumatol, 2013. 27(3): p. 435–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Moroni G, et al. , Fetal outcome and recommendations of pregnancies in lupus nephritis in the 21st century. A prospective multicenter study. J Autoimmun, 2016. 74: p. 6–12. [DOI] [PubMed] [Google Scholar]
- 164.Fischer-Betz R. and Specker C, Pregnancy in systemic lupus erythematosus and antiphospholipid syndrome. Best Pract Res Clin Rheumatol, 2017. 31(3): p. 397–414. [DOI] [PubMed] [Google Scholar]
- 165.McDonald EG, et al. , Monitoring of Systemic Lupus Erythematosus Pregnancies: A Systematic Literature Review. J Rheumatol, 2018. 45(10): p. 1477–1490. [DOI] [PubMed] [Google Scholar]
- 166.Kim MY, et al. , Complement activation predicts adverse pregnancy outcome in patients with systemic lupus erythematosus and/or antiphospholipid antibodies. Ann Rheum Dis, 2018. 77(4): p. 549–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Flint J, et al. , BSR and BHPR guideline on prescribing drugs in pregnancy and breastfeeding-Part II: analgesics and other drugs used in rheumatology practice. Rheumatology (Oxford), 2016. 55(9): p. 1698–702. [DOI] [PubMed] [Google Scholar]
- 168.Costedoat-Chalumeau N, et al. , Safety of hydroxychloroquine in pregnant patients with connective tissue diseases: a study of one hundred thirty-three cases compared with a control group. Arthritis Rheum, 2003. 48(11): p. 3207–11. [DOI] [PubMed] [Google Scholar]
- 169.Klinger G, et al. , Ocular toxicity and antenatal exposure to chloroquine or hydroxychloroquine for rheumatic diseases. Lancet, 2001. 358(9284): p. 813–4. [DOI] [PubMed] [Google Scholar]
- 170.Seo MR, et al. , Hydroxychloroquine treatment during pregnancy in lupus patients is associated with lower risk of preeclampsia. Lupus, 2019. 28(6): p. 722–730. [DOI] [PubMed] [Google Scholar]
- 171.Clowse ME, et al. , Hydroxychloroquine in lupus pregnancy. Arthritis Rheum, 2006. 54(11): p. 3640–7. [DOI] [PubMed] [Google Scholar]
- 172.Ruperto N, et al. , International consensus for a definition of disease flare in lupus. Lupus, 2011. 20(5): p. 453–62. [DOI] [PubMed] [Google Scholar]
- 173.Fernandez D. and Kirou KA, What Causes Lupus Flares? Curr Rheumatol Rep, 2016. 18(3): p. 14. [DOI] [PubMed] [Google Scholar]
- 174.Fanouriakis A, et al. , 2019 update of the EULAR recommendations for the management of systemic lupus erythematosus. Ann Rheum Dis, 2019. 78(6): p. 736–745. [DOI] [PubMed] [Google Scholar]
- 175.Alarcón GS, et al. , Effect of hydroxychloroquine on the survival of patients with systemic lupus erythematosus: data from LUMINA, a multiethnic US cohort (LUMINA L). Ann Rheum Dis, 2007. 66(9): p. 1168–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Pons-Estel GJ, et al. , Possible protective effect of hydroxychloroquine on delaying the occurrence of integument damage in lupus: LXXI, data from a multiethnic cohort. Arthritis Care Res (Hoboken), 2010. 62(3): p. 393–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Kaiser R, Cleveland CM, and Criswell LA, Risk and protective factors for thrombosis in systemic lupus erythematosus: results from a large, multi-ethnic cohort. Ann Rheum Dis, 2009. 68(2): p. 238–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Melles RB and Marmor MF, The risk of toxic retinopathy in patients on long-term hydroxychloroquine therapy. JAMA Ophthalmol, 2014. 132(12): p. 1453–60. [DOI] [PubMed] [Google Scholar]
- 179.Kim JW, et al. , Risk of Retinal Toxicity in Longterm Users of Hydroxychloroquine. J Rheumatol, 2017. 44(11): p. 1674–1679. [DOI] [PubMed] [Google Scholar]
- 180.Almaani S, Meara A, and Rovin BH, Update on Lupus Nephritis. Clin J Am Soc Nephrol, 2017. 12(5): p. 825–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Weening JJ, et al. , The classification of glomerulonephritis in systemic lupus erythematosus revisited. Kidney Int, 2004. 65(2): p. 521–30. [DOI] [PubMed] [Google Scholar]
- 182.Created with BioRender.com.