Abstract
Chronic hepatitis B virus (HBV) infection due to vertical transmission remains a critical concern with regards to eliminating HBV infection. Implementation of hepatitis B vaccine, the foundation to prevent perinatal and horizontal transmission, has reduced the prevalence of HBV by >80%. In countries where the hepatitis B immune globulin (HBIG) is available, such as China and the United States, the administration of HBIG and hepatitis B vaccine to the infants of mothers who are positive for hepatitis B surface antigen has become a standard practice and is effective in preventing vertical transmission. Accumulating evidence on the efficacy and safety of antiviral prophylaxis during pregnancy indicates the probability of attaining the goal of the World Health Organization to eliminate hepatitis by 2030. In this review, we discuss the transmission routes, diagnostic criteria, and preventive strategies for vertical transmission. A preventive program that includes screening before pregnancy, antiviral prophylaxis during pregnancy, and postpartum immunoprophylaxis provides “perfect strategies” to eliminate vertical transmission. However, there is still a notable gap between “perfect strategies” and real-world application, including insufficient coverage of timely birth dose vaccine and the efficacy and necessity of HBIG, especially in mothers who are negative for hepatitis B envelope antigen. In particular, there is a clear need for a comprehensive long-term safety profile of antiviral prophylaxis. Therefore, feasible and cost-effective preventive strategies need to be determined across regions. Access also needs to be scaled up to meet the demands for prophylaxis and prevalence targets.
Keywords: Hepatitis B virus, Vertical transmission, Immunoprophylaxis, Antiviral prophylaxis
Introduction
Although the universal hepatitis B virus (HBV) vaccination program has been implemented for almost 20 years, HBV infection is still a public health priority, with approximately 257 million chronic infections worldwide.[1] The annual number of deaths related to HBV-associated cirrhosis or hepatocellular carcinoma surpasses those caused by human immunodeficiency virus (HIV), tuberculosis, and malaria.[2] Vertical transmission, occurring during pregnancy or perinatal periods, remains a major route for the persistence of HBV infection in endemic areas, such as China, Southeast Asia, and sub-Saharan Africa. Moreover, HBV infection at birth is associated with a risk of up to 90% in developing a chronic infection, which is much higher than the infections in later life via horizontal transmission, approximately 20% to 60% in children aged 1 to 5 years and 5% to 10% in older children and adults.[3] In endemic areas, vertical transmission has been estimated to account for 40% to 50% of chronic infections[4,5]; furthermore, the infected offspring become reservoirs for subsequent horizontal infection. More significantly, the population acquiring chronic HBV infection through vertical transmission is more prone to severe liver diseases and poor response to antiviral therapy.[6] In 2016, the World Health Organization (WHO) endorsed a global health strategy to eliminate hepatitis B as a public health threat by 2030, which aims to reduce the incidence of hepatitis and hepatitis-related mortality by 90% and 65%, respectively, by 2030. Consequently, the prevention of vertical transmission has become the most efficient and critical method to meet these objectives.
Based on the mechanisms of vertical transmission, three major strategies have been adopted to prevent transmission during pregnancy and perinatal periods. The primary strategy for preventing vertical transmission is three or more doses of hepatitis B vaccine, including a timely dose at birth. The application of hepatitis B immunoglobulin (HBIG) immediately after birth is recommended for infants born to mothers with chronic HBV infection. The use of antiviral agents to suppress HBV viral activity during pregnancy can theoretically reduce the risk of transmission during pregnancy and delivery. Antiviral agents that exhibit strong effects and high viral mutation barriers are preferred for pregnant women with high HBV viremia. In this concise review, we discuss the significant efforts to disrupt the vertical transmission and highlight these issues that need further attention. By applying such efforts, there is a clear expectation of meeting the goal set by the WHO and eliminating HBV infection.
Routes of vertical transmission
It is proposed that vertical transmission can occur at any stage of pregnancy (intrauterine, peripartum, or postpartum), including each stage from in utero to breastfeeding. The significant success of postpartum immunoprophylaxis addresses that vertical transmission predominantly occurs during the peripartum period. Whereas postpartum combined prophylaxis cannot completely block the viral transmission, this supports the fact that transplacental leakage or HBV-infected villous endothelial cells and infected maternal peripheral blood mononuclear cells represent a separate mechanism for intrauterine transmission. The cellular transfer of HBV via the placenta was demonstrated by a gradual reduction in the proportion of HBV markers and HBV-infected cells in different placental compartments from the maternal side to the fetal side.[7,8] The likelihood of intrauterine infection was increased with a greater proportion of infected HBV cells in the placenta, especially when villous capillary endothelial cells were infected.[8] Furthermore, the positive association between HBV DNA level and intrauterine transmission provided further confirmation for transmission through the placenta and HBV-infected cells.
Previously, researchers have also proposed that HBV can be transmitted by infected germ cells. Hepatitis B surface antigen (HBsAg), hepatitis B core antigen, and HBV DNA have all been identified in oocytes at different developmental stages from female HBV carriers.[9,10] The close relationship between HBV covalently closed circular DNA expression in the human ovary, and the high infection rate in infants provides strong evidence for the role of oocytes in intrauterine transmission.[11] Kong et al[12] demonstrated that HBV could replicate in oocytes and that transmission to the fetus was associated with serum levels of HBV DNA and the status of hepatitis B envelop antigen (HBeAg). In a previous study, integration of HBV genes was detected in the early stages of hamster embryos; the researchers considered that the gene had been transmitted by sperm from male HBV carriers. By using reverse transcription-polymerase chain reaction analysis and fluorescence in situ hybridization analysis, the researchers showed that sperm-mediated HBV genes were capable of replicating and expressing in early embryonic cells.[13] Nevertheless, the integration of viral DNA occurred at multiple sites and was non-specific[14]; furthermore, the transmission was not further confirmed in the offspring. In the postpartum immunoprophylaxis era, with a pooled transmission rate of almost zero following antiviral prophylaxis during pregnancy, the proposed role of germ cells in the transmission of HBV remains doubtful.
Another concern for vertical transmission is breastfeeding. Although HBV DNA and HBsAg can be detected in breast milk, researchers have failed to identify an increased risk of transmission in breastfed infants.[15] Hepatitis experts have also considered the risk of exposure to large daily amounts of breast milk with regards to the fragile nature of the gastrointestinal mucosa and the immature status of digestive function. With appropriate immunoprophylaxis at birth, the increased rate of transmission by contact with bleeding nipples or broken skin on breast sores has not been demonstrated thus far.[16] Considering the gastrointestinal tract not the primary route of HBV transmission and the benefits associated with breastfeeding, guidelines promote, and encourage breastfeeding following the administration of a whole course of passive-active immunoprophylaxis to the newborns of HBsAg-positive mothers.[17–20]
Diagnostic criteria for vertical transmission
A wide range has been reported for the rates of HBV vertical transmission; this variability is due to the previous lack of a universal diagnostic standard for HBV vertical transmission. The detection of HBsAg and/or HBV DNA is regarded as a positive sign of infection. However, the transplacental transfer of maternal antibodies can affect the diagnosis of vertical transmission. With dynamic investigation, the positive rate of serological markers for HBV in infants has reduced over time. It is also possible that positivity in the umbilical cord blood or femoral venous blood at birth could be transient or due to contamination.[21] With systematic review and network meta-analyses, the positive rates of HBsAg and/or HBV DNA were shown to be comparable among infants at the ages of 6, 7, and 12 months; furthermore, these rates were significantly lower than the positive rates at birth.[22] Based upon previous systematic reviews and high-quality clinical trials,[23,24] the positivity of HBsAg and/or HBV DNA at 6 to 12 months of age is considered to demonstrate vertical transmission. Although viral detection in liver tissue is considered as the gold standard of diagnosis, this is not a practical procedure and can be difficult to justify from an ethical point of view. In addition, when tests for HBV serological markers are unavailable at 6 to 12 months of age, an assessment at 12 to 24 months of age is accepted as long as routine vaccination is provided to exclude horizontal transmission.
Interventional strategies for vertical transmission
Screening strategy for HBV
Currently, the pivotal strategies for eliminating vertical transmission include adequate screening and preventive measures during pregnancy and the perinatal periods. Universal screening for HBsAg before or during pregnancy has displayed a significant effect on reducing neonatal infection by identifying HBV-infected mothers in endemic regions, such as China. In such cases, the combined immunoprophylaxis of hepatitis B vaccine and HBIG administered soon after birth can shield infants from peripartum infection. The clinical assessment of liver function and laboratory tests for serological HBV markers and HBV DNA could help to evaluate the status of HBV infection. Joint management should be counseled by obstetricians for the well-being of the fetus and mother, and by hepatologists with regards to their hepatitis disease status during pregnancy, delivery, and the postpartum period. However, there is controversy relating to the routine screening for HBV, because HBIG is unavailable in some countries. In these regions, HBV vaccination is the only postpartum preventive measure against vertical transmission; routine screening is not cost-effective because it does not provide additional management for the infants of HBsAg-positive mothers.
Following a comprehensive systematic review and meta-analysis, the WHO has identified that HBeAg has a high sensitivity to predict cases in which immunoprophylaxis might fail and therefore provides an alternative risk factor for vertical transmission.[25] Under these circumstances, the universal screening of HBsAg and HBeAg is suggested; the newborns of women who are positive for HBsAg and HBeAg could benefit from antiviral intervention. In addition, a test for hepatitis B surface antibody (HBsAb) is also preferred in HBV endemic regions; vaccination could be administered for childbearing women who are HBsAb-negative, as acute infection could occur during pregnancy. The changes in physiological and immune function during this period make pregnant women more susceptible to HBV infection.
Postpartum immunoprophylaxis
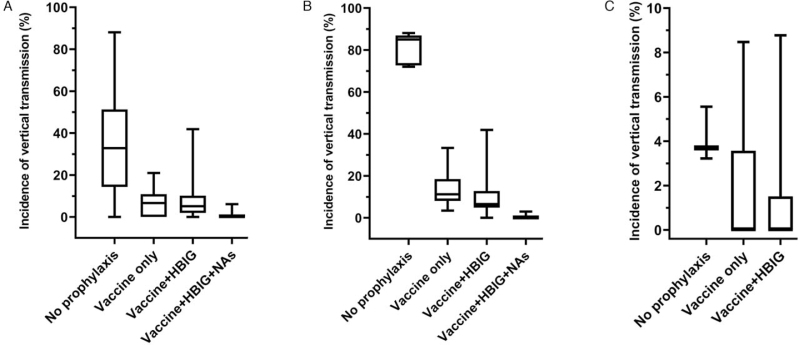
As the most cost-effective measure for eliminating HBV infection, the hepatitis B vaccine was first introduced in the early 1980s and has been recommended as a national immunization program in all countries by the WHO. A dose of hepatitis B vaccine at birth has demonstrated a significant effect in reducing mother-to-newborn transmission [Figure 1A]. A timely dose at birth followed by two or more doses of vaccine has reduced the prevalence of chronic HBV by almost 90% in infants of HBeAg-positive mothers and almost all in HBeAg-negative mothers [Figure 1B and 1C].[1] Timely administration of the vaccine is crucial for interrupting vertical transmission and compliance in completing the vaccination series.[26] In endemic regions with a significant proportion of HBeAg-positive population, such as the Western Pacific Region, the efficacy of hepatitis B vaccine could be reduced to as low as only 50% to 75% without timely administration of vaccines at birth.[27] Unfortunately, only 46% of infants receive a timely dose of vaccine at birth and only 87% of these completed the whole course of vaccination on schedule globally; this deviates significantly from the 90% coverage target set by the WHO.[1] The lowest coverage of strategies involving three doses and a birth dose of hepatitis B vaccine (76% and 4%, respectively) was reported in the African Region, a highly endemic region with a high risk of vertical transmission.[28] Thus, scaling up the coverage of vaccination, particularly, the timely birth dose is the primary requirement towards the global elimination of HBV.
HBIG is the purified product of human immunoglobulin from human plasma that contains a high titer of HBsAb and could reduce or even prevent HBV infection.[29] The efficacy of the combined hepatitis B vaccine and HGIB has been fully confirmed in infants from HBeAg-positive mothers.[1,30] Where resources are available, the combination of HBIG with hepatitis B vaccine may be of additional benefit for newborns whose mothers are HBeAg-positive [Figure 1B]. However, the HBIG option is conditional on the existence of a comprehensive antenatal screening program for HBV infection and the limitations that may be imposed by local resources. As a blood product, the costs of screening for infectious diseases and cold chain storage cause significant limitations with regard to the application of HBIG. In addition, controversy exists as to whether HBIG should be given to the newborns of HBeAg-negative mothers. Some studies have shown that hepatitis B vaccine alone may be sufficient for preventing the transmission of HBV in the neonates of HBeAg-negative mothers.[31,32] Moreover, there is a lack of evidence comparing the risk of immunoprophylaxis failure in neonates of HBeAg-negative mothers receiving vaccine alone or the vaccine combined with HBIG. In addition, the recommended dose for HBIG differs across countries. In the United States, the standard dose of HBIG in infants ranges from 220 U/mL to >312 U/mL (Hyper Hepatitis B vaccine S/D: 220 U/mL, HepaGam: >312 U/mL, Nabi-HB: >312 U/mL)[33]; in contrast, current guidelines recommend a dose of 100 IU in China. A recent prospective study demonstrated that different doses of HBIG exhibited similar efficacies.[34] Considering cost-effectiveness, the combination of 100 IU of HBIG and hepatitis B vaccine is reasonable and can be recommended for the prevention of perinatal transmission. There is only scant information in the existing literature related to the protective effect of HBIG in terms of preventing infantile fulminant hepatitis; such effects are yet to be confirmed in clinical practice.[35]
Antiviral prophylaxis during pregnancy
The combination of passive and active immunization has significantly reduced the rate of vertical transmission; however, there is still approximately 10% immunoprophylaxis failure.[36] Persistent HBV infection can still be detected despite the administration of standard combined immunoprophylaxis; the rate can be as high as 9% to 22% in neonates of mothers with high viremia.[37–40] Accumulating evidence shows that high viremia before delivery acts as a robust predictive factor for vertical transmission.[25] Adopting antiviral therapy to suppress maternal HBV DNA before delivery has become the standard recommendation for HBsAg-positive mothers with a high viral load, as reported in the most recent guidelines from associations for the study of liver disease.[17,18,41,42] However, there is still debate related to the optimal viral load cut-off to initiate HBV antiviral prophylaxis. The previous cut-off was derived from retrospective observational studies,[37,38] or a randomized controlled trial.[43] Recent systematic and meta-analysis commissioned by the WHO showed that the risk of vertical transmission is increasing continuously in mothers with different ranges of viral load. This study has reported the risk of vertical transmission stratified by narrow ranges of viral loads, 2 × 105 U/mL appears to be the optimal threshold for antiviral prophylaxis during pregnancy.[25] Another systematic review and meta-analysis revealed a transmission rate of about 1% in pregnant women with HBV DNA levels of 104 to 106 U/mL.[36] These differences can be explained by the exclusion of peripartum antiviral prophylaxis in the systematic review carried out by the WHO. Given the goal to eliminate hepatitis, antiviral prophylaxis could be suggested to pregnant women with a high risk of vertical transmission, such as a history of vertical transmission, indications of invasive procedures, and a family history of HBV infection; however, patient education and informed consent would also be required. In clinical work, the physician should be cautious when converting from copies/mL to IU/mL and comparing data from different methodologies and platforms.[44] The time points used for HBV DNA quantification may also influence the assessment, as these may be affected by the immune status of pregnancy. In addition, most previous studies did not describe the timing used for the assessment of viral replication.
HBeAg status, which indicates active viral replication and acts as a tolerogen and immunogen, has also been demonstrated to be associated with immunoprophylaxis failure. Previous investigations further confirmed that the HBeAg test is a reliable alternative to HBV DNA quantification and can identify high-risk populations where the use of an HBV DNA assay is unavailable.[25]
Very few studies have compared the risk of transmission between the second and the third trimester. Although infrequent, the cases of HBV vertical transmission are still being reported even if their mothers commenced antiviral prophylaxis in late pregnancy.[45,46] A network meta-analysis revealed that the administration of antiviral agents in early-middle pregnancy appeared superior to that in late pregnancy in terms of preventing vertical transmission.[47] High-quality clinical trials have demonstrated the efficacy and safety profiles of antiviral prophylaxis initiated from 24 weeks of gestation to prevent vertical transmission.[43,48] Based on the available evidence, antiviral prophylaxis initiated before 28 weeks of gestation is preferred to achieve viral suppression below the recommended threshold before delivery.
Randomized controlled studies and data reported from the Antiretroviral Pregnancy Registry have demonstrated the efficacy and fine tolerance of tenofovir disoproxil fumarate (TDF), (GlaxoSmithKline PLC, Middlesex, United Kingdom) for use in pregnant women and fetuses. The frequency of adverse events in pregnant women receiving TDF, such as postpartum hemorrhage, the rate of cesarean section, birth defects, and preterm birth, is similar with the general population.[49] An up to 6 to 7 year follow-up study after delivery showed comparable long-term growth, renal function, and bone development in infants exposed to TDF during pregnancy, thus demonstrating that the administration of TDF during pregnancy is safe, although the number of infants enrolled in this particular study was small.[50] Another first-line oral antiviral agent that is described in the current HBV guidelines is tenofovir alafenamide (TAF) (Gilead Sciences, Inc., CA, the United States). This agent exhibits high levels of antiviral activity and a high barrier to resistance; it is also safe and effective in terms of preventing vertical transmission.[51,52] On-going randomized controlled trails will provide valuable evidence for the application of TAF in pregnant women, particularly in women suffering from kidney and bone diseases.
Achievements in China
As a country with the greatest population of HBsAg-positive individuals, the rate of HBV infection among women of childbearing age currently remains at a medium-high level (6.61%); each year, almost one million neonates are born to HBsAg-positive mothers in China. To reduce the disease burden associated with chronic hepatitis, the Chinese government has made strenuous effort to disrupt the transmission of HBV and has achieved notable success. Since 1992, hepatitis B vaccines have been integrated into the national immunization program, and have been provided free of charge to all neonates since 2005.[53] Among the 16 countries with the greatest number of HBV-infected children, China is the only one with timely birth dose coverage of >90%.[1] The additional administration of HBIG combined with the vaccine within 12 h of birth has been applied as a standard procedure for neonates of HBsAg-positive mothers; this represents a strategy that is available free of charge for this particular population. As the primary strategy of disrupting disease transmission, screening for HIV, syphilis, and HBV during pregnancy has been integrated into routine maternal and child health services. Pregnant women are offered all three tests simultaneously and free of charge.[54] Collectively, these efforts have reduced the prevalence of HBsAg to 0.94% in children of 5 to 15 years of age and 0.32% in children < 5 years of age.[55] In addition, TDF is also a safe and efficient means of interrupting vertical transmission and has led to the probability of reaching zero vertical transmission with <50 US dollars per month; this represents an affordable solution for most families against the burden of chronic hepatitis.
Based on the accumulating evidence, an effective and affordable standardized intervention procedure for HBV vertical transmission has been identified in China and provides a model that shows how countries with a high HBV prevalence can reduce the number of new infections by applying proactive national strategies, and serves as an example for other regions.
Research gap
As a pivotal step towards eliminating HBV infection, we must consider the remaining gaps in our knowledge so that we can completely eradicate vertical transmission. There has been much debate about universal screening for HBV in pregnant women and administering HBIG to newborns, especially those born to HBeAg-negative mothers. Considering the limited access to HBIG and the need to screen for infectious diseases, the use of HBIG-free strategies, such as vaccination in conjunction with antivirals administered peripartum, could be a cheaper and more accessible option compared with HBIG. A range of clinical trials is now underway to provide evidence for this consideration. Furthermore, the transmission of HBV, hepatitis C virus, and HIV can all occur during intrauterine and intrapartum periods. The influence of co-infection by hepatitis C virus and HIV on the vertical transmission of HBV has yet to be fully elucidated. In addition, HBV genotypes have a distinct geographical distribution across continents and are associated with the prevalence of HBeAg, a determinant for the mode of HBV transmission.[56,57] Whether HBV genotypes influence the strategies required for preventing vertical transmission is another significant concern. The Global Alliance for Vaccine and Immunization has been launched since 2000; however, hepatitis B vaccines have still not been widely adopted in low-income countries. Oral antiviral agents administered during pregnancy may provide a less costly option for women whose infants would not receive a timely birth dose vaccine. Finally, the long-term outcomes in offspring experiencing intrauterine TDF exposure, as well as the safety of TAF, require intensive research to address the current gaps in knowledge.
Conclusion
Preventing new infection among infants is pivotal to ensure elimination of HBV infection, which requires reaching a 0.10% prevalence of HBsAg in children under 5 years of age by 2030. As shown in [Figure 1], the administration of vaccines is the key strategy if we are to eliminate vertical transmission; vaccination has led to >80% reduction in prevalence thus far. HBIG provides additional benefit when available and has led to an obvious reduction in the transmission in infants born to HBeAg-positive mothers [Figure 1B]. Screening for HBsAg and HBeAg is strongly recommended as this would guide the use of antiviral prophylaxis. Considering feasibility and cost-effectiveness, vaccines and antiviral agents may be sufficient to prevent HBV transmission, although high-quality evidence is still required. There are substantial gaps in our knowledge with regards to the prevention of vertical transmission between recommendations and routine practices in low-income countries, thus highlighting the importance of the feasibility of present recommendations to eliminate HBV vertical transmission in the endemic regions. In addition, future research is required to explore feasible measures that could be adhered to in low-income regions. In countries with available measures including hepatitis B vaccine and HBIG, such as China, Japan, and the United States, screening pre-pregnancy and standard preventive strategies are pivotal for preventing vertical transmission. Promoting and implementing these strategies is of the greatest importance to eliminate vertical transmission.
Figure 1.
Relationship between the prevalence of vertical transmission and different preventive measures, including hepatitis B vaccine, HBIG, and NAs prophylaxis. (A) The prevalence of vertical transmission with different prophylaxis measures in pregnant women with chronic HBV infection; (B) The prevalence of vertical transmission with different prophylaxis measures in HBeAg-positive pregnant women; (C) The prevalence of vertical transmission with different prophylaxis measures in HBeAg-negative pregnant women. HBeAg: Hepatitis B envelop antigen; HBIG: Hepatitis B immune globulin; HBV: Hepatitis B virus; NAs: Nucleotide analogues.
Funding
This research was funded by the National Natural Science Foundation of China (No. 81670537), the Key R&D Program of Shaanxi (No. 2018ZDXM-SF-037), and the National Science and Technology Projects on Major Infectious Diseases (No. 2017ZX10202202-002-006).
Conflicts of interest
None.
Footnotes
How to cite this article: Liu JF, Chen TY, Zhao YR. Vertical transmission of hepatitis B virus: propositions and future directions. Chin Med J 2021;134:2825–2831. doi: 10.1097/CM9.0000000000001800
References
- 1.Polaris Observatory Collaborators. Global prevalence, treatment, and prevention of hepatitis B virus infection in 2016: a Modelling Study. Lancet Gastroenterol Hepatol 2018; 3:383–403. doi: 10.1016/S2468-1253(18)30056-6. [DOI] [PubMed] [Google Scholar]
- 2.Schweitzer A, Horn J, Mikolajczyk RT, Krause G, Ott JJ. Estimations of worldwide prevalence of chronic hepatitis B virus infection: a systematic review of data published between 1965 and 2013. Lancet 2015; 386:1546–1555. doi: 10.1016/S0140-6736(15)61412-X. [DOI] [PubMed] [Google Scholar]
- 3.Shimakawa Y, Lemoine M, Njai HF, Bottomley C, Ndow G, Goldin RD, et al. Natural history of chronic HBV infection in West Africa: a longitudinal population-based study from The Gambia. Gut 2016; 65:2007–2016. doi: 10.1136/gutjnl-2015-309892. [DOI] [PubMed] [Google Scholar]
- 4.Lamberth JR, Reddy SC, Pan JJ, Dasher KJ. Chronic hepatitis B infection in pregnancy. World J Hepatol 2015; 7:1233–1237. doi: 10.4254/wjh.v7.i9.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nayagam S, Thursz M, Sicuri E, Conteh L, Wiktor S, Low-Beer D, et al. Requirements for global elimination of hepatitis B: a modelling study. Lancet Infect Dis 2016; 16:1399–1408. doi: 10.1016/S1473-3099(16)30204-3. [DOI] [PubMed] [Google Scholar]
- 6.Li J, Dong XQ, Wu Z, Ma AL, Xie SB, Zhang XQ, et al. Unsatisfying antiviral therapeutic effect in patients with mother-to-child transmissed chronic hepatitis B virus infection: a prospective multi-center clinical study. Chin Med J (Engl) 2019; 132:2647–2656. doi: 10.1097/CM9.0000000000000522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu DZ, Yan YP, Choi BC, Xu JQ, Men K, Zhang JX, et al. Risk factors and mechanism of transplacental transmission of hepatitis B virus: a case-control study. J Med Virol 2002; 67:20–26. doi: 10.1002/jmv.2187. [DOI] [PubMed] [Google Scholar]
- 8.Zhang SL, Yue YF, Bai GQ, Shi L, Jiang H. Mechanism of intrauterine infection of hepatitis B virus. World J Gastroenterol 2004; 10:437–438. doi: 10.3748/wjg.v10.i3.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ye F, Yue Y, Li S, Chen T, Bai G, Liu M, et al. Presence of HBsAg, HBcAg, and HBVDNA in ovary and ovum of the patients with chronic hepatitis B virus infection. Am J Obstet Gynecol 2006; 194:387–392. doi: 10.1016/j.ajog.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 10.Chen LZ, Fan XG, Gao JM. Detection of HBsAg, HBcAg, and HBV DNA in ovarian tissues from patients with HBV infection. World J Gastroenterol 2005; 11:5565–5567. doi: 10.3748/wjg.v11.i35.5565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yu MM, Gu XJ, Xia Y, Wang GJ, Kan NY, Jiang HX, et al. Relationship between HBV cccDNA expression in the human ovary and vertical transmission of HBV. Epidemiol Infect 2012; 140:1454–1460. doi: 10.1017/S0950268811002068. [DOI] [PubMed] [Google Scholar]
- 12.Kong Y, Ye F, Jin Y, Shi J, Qiu H, Lin S. Hepatitis b virus expression and replication in ovum and the influencing factors. Saudi J Gastroenterol 2016; 22:215–219. doi: 10.4103/1319-3767.182456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ali BA, Huang TH, Salem HH, Xie QD. Expression of hepatitis B virus genes in early embryonic cells originated from hamster ova and human spermatozoa transfected with the complete viral genome. Asian J Androl 2006; 8:273–279. doi: 10.1111/j.1745-7262.2006.00141.x. [DOI] [PubMed] [Google Scholar]
- 14.Huang JM, Huang TH, Qiu HY, Fang XW, Zhuang TG, Liu HX, et al. Effects of hepatitis B virus infection on human sperm chromosomes. World J Gastroenterol 2003; 9:736–740. doi: 10.3748/wjg.v9.i4.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shi Z, Yang Y, Wang H, Ma L, Schreiber A, Li X, et al. Breastfeeding of newborns by mothers carrying hepatitis B virus: a meta-analysis and systematic review. Arch Pediatr Adolesc Med 2011; 165:837–846. doi: 10.1001/archpediatrics.2011.72. [DOI] [PubMed] [Google Scholar]
- 16.Xiao F, Lan A, Mo W. Breastfeeding from mothers carrying HBV would not increase the risk of HBV infection in infants after proper immunoprophylaxis. Minerva Pediatr 2020; 72:109–115. doi: 10.23736/S0026-4946.17.04798-3. [DOI] [PubMed] [Google Scholar]
- 17.Terrault NA, Lok ASF, McMahon BJ, Chang KM, Hwang JP, Jonas MM, et al. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology 2018; 67:1560–1599. doi: 10.1002/hep.29800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.European Association for the Study of the Liver. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol 2017; 67:370–398. doi: 10.1016/j.jhep.2017.03.021. [DOI] [PubMed] [Google Scholar]
- 19.WHO Guidelines Approved by the Guidelines Review Committee. Prevention of Mother-to-Child Transmission of Hepatitis B Virus: Guidelines on Antiviral Prophylaxis in Pregnancy. Geneva: WHO Guidelines Approved by the Guidelines Review Committee; 2020. [Google Scholar]
- 20.Chinese Society of Infectious Diseases, Chinese Medical Association; Chinese Society of Hepatology, Chinese Medical Associaton. [The guidelines of prevention and treatment for chronic hepatitis B (2019 version)](in Chinese). Chin J Hepatol 2019; 27:938–961. doi: 10.3760/cma.j.issn.1007-3418.2019.12.007. [DOI] [PubMed] [Google Scholar]
- 21.Liu J, Xu B, Chen T, Chen J, Feng J, Xu C, et al. Presence of hepatitis B virus markers in umbilical cord blood: exposure to or infection with the virus? Dig Liver Dis 2019; 51:864–869. doi: 10.1016/j.dld.2018.11.003. [DOI] [PubMed] [Google Scholar]
- 22.Fu S, Yao N, Feng Y, Li J, Zhao Y. Dynamic changes of HBsAg and/or HBV DNA in infants born to HBsAg(+) mothers: a systematic review and network meta-analysis. Hepatology 2019; 70:581A–1581A. [Google Scholar]
- 23.Pan CQ, Duan Z, Dai E, Zhang S, Han G, Wang Y, et al. Tenofovir to prevent hepatitis B transmission in mothers with high viral load. N Engl J Med 2016; 374:2324–2334. doi: 10.1056/NEJMoa1508660. [DOI] [PubMed] [Google Scholar]
- 24.Jourdain G, Ngo-Giang-Huong N, Harrison L, Decker L, Khamduang W, Tierney C, et al. Tenofovir versus placebo to prevent perinatal transmission of hepatitis B. N Engl J Med 2018; 378:911–923. doi: 10.1056/NEJMoa1708131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boucheron P, Lu Y, Yoshida K, Zhao T, Funk AL, Lunel-Fabiani F, et al. Accuracy of HBeAg to identify pregnant women at risk of transmitting hepatitis B virus to their neonates: a systematic review and meta-analysis. Lancet Infect Dis 2021; 21:85–96. doi: 10.1016/S1473-3099(20)30593-4. [DOI] [PubMed] [Google Scholar]
- 26.Centers for Disease Control and Prevention (CDC). Assessing completeness of perinatal hepatitis B virus infection reporting through comparison of immunization program and surveillance data – United States. MMWR Morb Mortal Wkly Rep 2011; 60:410–413. [PubMed] [Google Scholar]
- 27.McMahon BJ. Two key components to address chronic hepatitis B in children: detection and prevention. J Pediatr 2015; 167:1186–1187. doi: 10.1016/j.jpeds.2015.09.015. [DOI] [PubMed] [Google Scholar]
- 28.WHO. WHO-UNICEF estimates of HepB_BD coverage. 15-July-2020. http://apps.who.int/immunization_monitoring/globalsummary/timeseries/tswucoveragehepb3.html. [Accessed July 15, 2020]. [Google Scholar]
- 29.Li XM, Yang YB, Hou HY, Shi ZJ, Shen HM, Teng BQ, et al. Interruption of HBV intrauterine transmission: a clinical study. World J Gastroenterol 2003; 9:1501–1503. doi: 10.3748/wjg.v9.i7.1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang L, Gui XE, Teter C, Zhong H, Pang Z, Ding L, et al. Effects of hepatitis B immunization on prevention of mother-to-infant transmission of hepatitis B virus and on the immune response of infants towards hepatitis B vaccine. Vaccine 2014; 32:6091–6097. doi: 10.1016/j.vaccine.2014.08.078. [DOI] [PubMed] [Google Scholar]
- 31.Lu Y, Liang XF, Wang FZ, Yan L, Li RC, Li YP, et al. Hepatitis B vaccine alone may be enough for preventing hepatitis B virus transmission in neonates of HBsAg (+)/HBeAg (-) mothers. Vaccine 2017; 35:40–45. doi: 10.1016/j.vaccine.2016.11.061. [DOI] [PubMed] [Google Scholar]
- 32.Machaira M, Papaevangelou V, Vouloumanou EK, Tansarli GS, Falagas ME. Hepatitis B vaccine alone or with hepatitis B immunoglobulin in neonates of HBsAg+/HBeAg- mothers: a systematic review and meta-analysis. J Antimicrob Chemother 2015; 70:396–404. doi: 10.1093/jac/dku404. [DOI] [PubMed] [Google Scholar]
- 33.Mast EE, Margolis HS, Fiore AE, Brink EW, Goldstein ST, Wang SA, et al. A comprehensive immunization strategy to eliminate transmission of hepatitis B virus infection in the United States: recommendations of the Advisory Committee on Immunization Practices (ACIP) part 1: immunization of infants, children, and adolescents. MMWR Recomm Rep 2005; 54:1–31. [PubMed] [Google Scholar]
- 34.Wei KP, Zhu FC, Liu JX, Yan L, Lu Y, Zhai XJ, et al. The efficacy of two different dosages of hepatitis B immunoglobulin combined with hepatitis B vaccine in preventing mother-to-child transmission of hepatitis B virus: a prospective cohort study. Vaccine 2018; 36:256–263. doi: 10.1016/j.vaccine.2017.11.037. [DOI] [PubMed] [Google Scholar]
- 35.Chen SCC, Toy M, Yeh JM, Wang JD, Resch S. Cost-effectiveness of augmenting universal hepatitis B vaccination with immunoglobin treatment. Pediatrics 2013; 131:e1135–e1143. doi: 10.1542/peds.2012-1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu JF, Yao NJ, Chen TY, Fu S, Wu YC, Feng YL, et al. Prevalence of mother-to-child transmission of hepatitis B virus: a systematic review and meta-analysis. J Hepatol 2019; 70:E123–E124. doi: 10.1016/S0618-8278(19)30217-8. [Google Scholar]
- 37.Wen WH, Chang MH, Zhao LL, Ni YH, Hsu HY, Wu JF, et al. Mother-to-infant transmission of hepatitis B virus infection: significance of maternal viral load and strategies for intervention. J Hepatol 2013; 59:24–30. doi: 10.1016/j.jhep.2013.02.015. [DOI] [PubMed] [Google Scholar]
- 38.Zou H, Chen Y, Duan Z, Zhang H, Pan C. Virologic factors associated with failure to passive-active immunoprophylaxis in infants born to HBsAg-positive mothers. J Viral Hepat 2012; 19:e18–e25. doi: 10.1111/j.1365-2893.2011.01492.x. [DOI] [PubMed] [Google Scholar]
- 39.Wiseman E, Fraser MA, Holden S, Glass A, Kidson BL, Heron LG, et al. Perinatal transmission of hepatitis B virus: an Australian experience. Med J Aust 2009; 190:489–492. doi: 10.5694/j.1326-5377.2009.tb02524.x. [DOI] [PubMed] [Google Scholar]
- 40.Liu J, Wang J, Yan T, Du D, Qi C, Cao F, et al. Efficacy and safety of telbivudine and tenofovir disoproxil fumarate in preventing hepatitis B vertical transmission: a real-life practice. J Viral Hepat 2019; 26:1170–1177. doi: 10.1111/jvh.13156. [DOI] [PubMed] [Google Scholar]
- 41.Sarri G, Westby M, Bermingham S, Hill-Cawthorne G, Thomas H. Guideline Development Group. Diagnosis and management of chronic hepatitis B in children, young people, and adults: summary of NICE guidance. BMJ 2013; 346:3893–3897. doi: 10.1136/bmj.f3893. [DOI] [PubMed] [Google Scholar]
- 42.Sarin SK, Kumar M, Lau GK, Abbas Z, Chan HLY, Chen CJ, et al. Asian-Pacific clinical practice guidelines on the management of hepatitis B: a 2015 update. Hepatol Int 2016; 10:1–98. doi: 10.1007/s12072-015-9675-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pan CQ, Han G, Wang Y. Prevention of peripartum hepatitis B transmission. N Engl J Med 2016; 375:1497–1498. doi: 10.1056/NEJMc1609991. [DOI] [PubMed] [Google Scholar]
- 44.Shyamala V, Arcangel P, Cottrell J, Coit D, Medina-Selby A, McCoin C, et al. Assessment of the target-capture PCR hepatitis B virus (HBV) DNA quantitative assay and comparison with commercial HBV DNA quantitative assays. J Clin Microbiol 2004; 42:5199–5204. doi: 10.1128/JCM.42.11.5199-5204.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu Y, Wang M, Yao S, Yuan J, Lu J, Li H, et al. Efficacy and safety of telbivudine in different trimesters of pregnancy with high viremia for interrupting perinatal transmission of hepatitis B virus. Hepatol Res 2016; 46:E181–E188. doi: 10.1111/hepr.12525. [DOI] [PubMed] [Google Scholar]
- 46.Yi W, Li MH, Xie Y, Wu J, Hu YH, Zhang D, et al. Prospective cohort study on the efficacy and safety of telbivudine used throughout pregnancy in blocking mother-to-child transmission of hepatitis B virus. J Viral Hepat 2017; 24:49–56. doi: 10.1111/jvh.12788. [DOI] [PubMed] [Google Scholar]
- 47.Wu Y, Liu J, Feng Y, Fu S, Ji F, Ge L, et al. Efficacy and safety of antiviral therapy for HBV in different trimesters of pregnancy: systematic review and network meta-analysis. Hepatol Int 2020; 14:180–189. doi: 10.1007/s12072-020-10026-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Han GR, Cao MK, Zhao W, Jiang HX, Wang CM, Bai SF, et al. A prospective and open-label study for the efficacy and safety of telbivudine in pregnancy for the prevention of perinatal transmission of hepatitis B virus infection. J Hepatol 2011; 55:1215–1221. doi: 10.1016/j.jhep.2011.02.032. [DOI] [PubMed] [Google Scholar]
- 49.Brown RS, Jr, McMahon BJ, Lok ASF, Wong JB, Ahmed AT, Mouchli MA, et al. Antiviral therapy in chronic hepatitis B viral infection during pregnancy: a systematic review and meta-analysis. Hepatology 2016; 63:319–333. doi: 10.1002/hep.28302. [DOI] [PubMed] [Google Scholar]
- 50.Wen WH, Chen HL, Shih TT, Wu JF, Ni YH, Lee CN, et al. Long-term growth and bone development in children of HBV-infected mothers with and without fetal exposure to tenofovir disoproxil fumarate. J Hepatol 2020; 72:1082–1087. doi: 10.1016/j.jhep.2020.01.021. [DOI] [PubMed] [Google Scholar]
- 51.Ding Y, Cao L, Zhu L, Huang Y, Lin C, Wang Y, et al. Efficacy and safety of tenofovir alafenamide fumarate for preventing mother-to-child transmission of hepatitis B virus: a national cohort study. Aliment Pharmacol Ther 2020; 52:1377–1386. doi: 10.1111/apt.16043. [DOI] [PubMed] [Google Scholar]
- 52.Zeng QL, Yu ZJ, Ji F, Li GM, Zhang GF, Xu JH, et al. Tenofovir alafenamide to prevent perinatal hepatitis B transmission: a Multicenter, Prospective, Observational Study. Clin Infect Dis 2021. ciaa1939.doi: 10.1093/cid/ciaa1939. [DOI] [PubMed] [Google Scholar]
- 53.Yang SG, Wang B, Chen P, Yu CB, Deng M, Yao J, et al. Effectiveness of HBV vaccination in infants and prediction of HBV prevalence trend under new vaccination plan: findings of a large-scale investigation. PLoS One 2012; 7:e47808.doi: 10.1371/journal.pone.0047808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang AL, Qiao YP, Wang LH, Fang LW, Wang F, Jin X, et al. Integrated prevention of mother-to-child transmission for human immunodeficiency virus, syphilis and hepatitis B virus in China. Bull World Health Organ 2015; 93:52–56. doi: 10.2471/BLT.14.139626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cui F, Shen L, Li L, Wang H, Wang F, Bi S, et al. Prevention of chronic hepatitis B after 3 decades of escalating vaccination policy, China. Emerg Infect Dis 2017; 23:765–772. doi: 10.3201/eid2305.161477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kramvis A. The clinical implications of hepatitis B virus genotypes and HBeAg in pediatrics. Rev Med Virol 2016; 26:285–303. doi: 10.1002/rmv.1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Singh AE, Plitt SS, Osiowy C, Surynicz K, Kouadjo E, Preiksaitis J, et al. Factors associated with vaccine failure and vertical transmission of hepatitis B among a cohort of Canadian mothers and infants. J Viral Hepat 2011; 18:468–473. doi: 10.1111/j.1365-2893.2010.01333.x. [DOI] [PubMed] [Google Scholar]