Akkermansia muciniphila (A. muciniphila), a type of Gram-negative anaerobe, was first isolated and identified from the feces of a healthy Caucasian woman at Wageningen University in 2004.[1] It can stably colonize the human gut 1 year after birth and accounts for 1%–4% of the total gut microbiota. A. muciniphila specializes in the degradation of mucin (the glycoprotein in mucus) and uses the mucin as the sole source of carbon and nitrogen. A. muciniphila is abundant in the host intestinal mucosal layer, with the greatest abundance in the cecum, where most mucin is produced.[2] Although degradation of mucin is a pathogen-like behavior; A. muciniphila does not show any pathogenicity, it resides only in the outer mucosal layer and does not reach the inner mucosal layer. Recently, several studies have revealed that A. muciniphila has important regulatory effects on gut homeostasis.
Dysbiosis of the gut microbiota has been associated with the development and progression of inflammatory bowel disease (IBD), including ulcerative colitis (UC) and Crohn's disease (CD). Colorectal cancer (CRC), including colitis-associated colorectal cancer (CAC), is inseparably associated with IBD, which is an important influencing factor for the occurrence of CRC. The abundance of A. muciniphila is inversely associated with metabolic disorders, which could increase the risk of CRC. Interestingly, supplementation with A. muciniphila, specific proteins from the outer membrane (Amuc_1100), secreted proteins (glucagon-like peptide-1-inducing protein), or extracellular vesicles could alleviate metabolic disease in humans and mice.[3,4] These results indicate that A. muciniphila is a member of beneficial microbiota in terms of modulating disorders. However, the role of A. muciniphila in IBD and CRC remains inconclusive. Environmental factors, such as diet, probiotics, and medications, play a substantial role in shaping the gut microbiome composition. Therefore, we explored the association between A. muciniphila and IBD and CRC and addressed the important role of A. muciniphila in the anti-inflammatory and anti-cancer effects of dietary agents, probiotics, and medications.
Richness of A. muciniphila in intestinal inflammation and tumor: Lower colonization and abundance of A. muciniphila were also observed in patients with UC and CD.[5] In agreement with data regarding humans, the abundance of A. muciniphila was also decreased in experimental colitis induced by dextran sulfate sodium (DSS) or 2,4,6 trinitrobenzenesulfonic acid (TNBS).[6] Moreover, disruption of the gut microbiota was observed in azoxymethane (AOM)/DSS-induced CAC, reflected by a higher abundance of harmful bacteria (such as Desulfovibrio and Helicobacter) and a lower abundance of beneficial bacteria (such as Lactobacillus, Bifidobacterium, A. muciniphila, and Faecalibaculum). Moreover, the abundance of A. muciniphila in colon biopsies was modestly and inversely correlated with baseline trimethylamine N-oxide content that has been identified as a risk factor for CRC.[7] Our previous study revealed that A. muciniphila could distinguish CRC and AOM/DSS-induced CAC from healthy controls due to a reduced abundance.[8] However, several studies on the effects of A. muciniphila in IBD and CRC have drawn opposite conclusions. Limited human studies have revealed an increased abundance of A. muciniphila in patients with CRC [Supplementary Table 1]. In addition, the abundance of A. muciniphila has been found to be significantly increased in colitis-prone mice with certain gene mutations (muc2) or knockouts, such as interleukin (IL)-10.[6] This discrepancy could be partly explained by health state, disease activity, and gene functions. Consequently, large-scale human studies, animal studies, and clinical trials are needed to verify the causality between A. muciniphila and IBD and CRC.
A. muciniphila, dietary target for the treatment of IBD and CRC: Diet may have a role in the modulation of the microbiota, the metabolome, and gut immunity. Ketogenic diets have been found to alleviate colitis by reducing the number of colonic group 3 innate lymphoid cells through altering the gut microbiome, with a reproducible increase in A. muciniphila abundance. Fermented foods could protect against colitis induced by pathogenic bacteria or DSS by increasing the abundance of A. muciniphila. Extracts from various fruits, vegetables, and meat exhibit inhibitory effects on colitis and augment the abundance of A. muciniphila [Supplementary Table 2]. Moreover, enrichment of A. muciniphila in association with beneficial effects was observed in colitis mice treated with phytochemicals, such as caffeic acid derivatives, myricetin, resveratrol, teasaponin, and polysaccharides. Barley leaf insoluble dietary fiber and egg white peptides ameliorated colitis, while they inhibited the expansion of A. muciniphila [Supplementary Table 2]. Host-derived substances, such as primary bile acids, secondary bile acids, vitamin D, and α-ketoglutarate, could boost the abundance of A. muciniphila. They have been proven to protect against DSS-induced colitis or inhibit the occurrence and development of CRC by regulating A. muciniphila-mediated colon barrier integrity and immunomodulatory effects [Supplementary Table 3].
An increasing number of probiotics are being used to improve IBD and CRC by regulating the immune response and altering the gut microbiota. Single probiotic treatments, such as Lactobacillus pentosus could alleviate the colitis symptoms via modulation of the immune response, accompanied by increased A. muciniphila abundance and short-chain fatty acid (SCFA) [Supplementary Table 2]. Moreover, probiotic mixtures of Bifidobacterium infantis, Lactobacillus acidophilus, Enterococcus faecalis, and Bacillus cereus have been found to improve DSS-induced colitis by rebuilding the structure of the gut microbiota, especially increased abundances of Bifidobacterium, Akkermansia, and Lactobacillus.[9] In addition, several studies have revealed that probiotics alone or in combination with therapeutic proteins or plant extracts reduced tumor volume, inhibited tumor growth, and increased the abundance of A. muciniphila in CAC mice [Supplementary Table 3]. However, several probiotics exert anti-inflammatory activities, while their administration significantly decreases the abundance of A. muciniphila.[10] These results indicate that probiotic effectiveness can be species- and disease-specific. The underlying mechanism involved in direct interaction with intestinal epithelial cells and immune cells has been proposed. In addition, they may also interact with other gut microbiota constituents, such as A. muciniphila, to support gut homeostasis by producing beneficial metabolites.
A. muciniphila is a potential target for pharmaceutical interventions: A major known stressor of the intestinal microbiota is exposure to antibiotics, which significantly modulate the relative abundance of A. muciniphila.[6] The interaction between gut microbes and commonly used non-antibiotic drugs is complex and bidirectional. Enrichment of A. muciniphila following metformin treatment has been found to frequently coincide with alleviated colonic inflammation in colitis mice.[11] TAK-242 (resatorvid), an inhibitor of Toll-like receptor 4 (TLR4), was reported to exhibit therapeutic potential in UC by regulating the gut microbiota and promoting the growth of A. muciniphila. Hyaluronic acid-bilirubin nanomedicine has been found to be associated with enrichment of A. muciniphila, regulate innate immune responses, and exert potent therapeutic efficacy against colitis. HuR inhibition of human antigen R by MS-444 could increase the abundance of A. muciniphila and attenuate tumorigenesis in APCMin/+ mice, a model of familial adenomatosis polyposis and CRC. Expansion of A. muciniphila is associated with the anti-inflammatory and anti-tumor effects of various traditional Chinese medicines [Supplementary Table 2]. Berberine and alisol B 23-acetate prevented the development of CAC, whereas they decreased the relative abundance of A. muciniphila. Although these results support the positive or negative influences of medication on the gut microbiota in hosts with IBD and CRC, A. muciniphila can serve as an intermediary for pharmaceutical interventions of IBD and CRC.
Intervention of A. muciniphila in IBD and CRC: Emerging evidence indicates that A. muciniphila may be a potential probiotic agent for ameliorating colitis and CRC. Gavage of A. muciniphila (strain DSM 22959) accelerated the recovery from colitis in the mice fed the casein diet by decreasing the disease activity index and increasing the mucus thickness and muc2 mRNA level.[12]A. muciniphila type strain ATCC BAA-835 has been shown to alleviate DSS-induced colitis via microbe–host interactions and improving the microbial community.[13] Both the murine A. muciniphila strain (designated 139) and the A. muciniphila type strain ATCC BAA-835 have been reported to exert anti-inflammatory effects on chronic colitis.[14] However, the anti-inflammatory effects of strain ATCC BAA-835 were stronger than those of strain 139, emphasizing the importance of the further study of the function of A. muciniphila at the strain level. Oral administration of A. muciniphila to low-cellulose diet-fed mice elevated crypt length, increased goblet cell numbers, and ameliorated colitis induced by DSS.[15]A. muciniphila-derived extracellular vesicles, Amuc_1100, and pasteurized bacteria (strain ATCC BAA-835) could improve colitis via regulation of intestinal barrier integrity and the immune response.[8,16] Oral administration of A. muciniphila elevated systemic anti-aging and anti-cancer metabolite levels, such as SCFAs, polyamines, and multiple bile acids. These effects were more pronounced after pasteurized A. muciniphila treatment than after live bacterium treatment. Our previous study showed that pasteurized A. muciniphila and Amuc_1100 could blunt AOM/DSS-induced CAC by expanding CD8+ T cells and enhancing their cytotoxic effects.[8] Targeted regulation of the intestinal flora, especially A. muciniphila, might be a novel and promising therapeutic strategy for preventing and curing IBD and CRC. However, A. muciniphila (strain ATCC BAA-835) failed to promote short-term intestinal inflammation in gnotobiotic IL10-deficient mice. Moreover, repeated oral gavage of A. muciniphila could induce spontaneous colitis in germ-free IL10-deficient mice, suggesting that A. muciniphila can act as a pathobiont to promote colitis in a genetically susceptible host.[17] Thus, genotypes and disease states should be considered to evaluate the validity and feasibility of microbiota-based therapies for IBD or CRC.
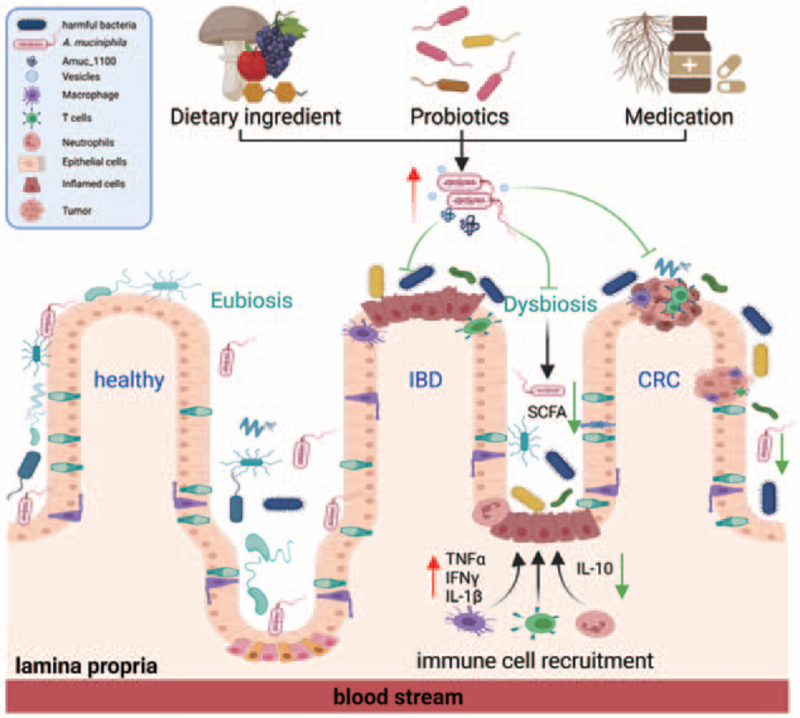
Conclusions and outlook: Dysbiosis of the gut microbiota is a hallmark of intestinal disorders, and the host-A. muciniphila symbiotic equilibrium is disrupted in IBD and CRC [Figure 1]. Increased levels of A. muciniphila are associated with the prevention of and protection against IBD and CRC following dietary ingredients, nutrients, probiotics, and medication intervention. Despite these impressive therapeutic advantages, a negative correlation between their beneficial effects and A. muciniphila has also been observed in some animal studies. Thus, it would be best to propose A. muciniphila as both a “friend and foe” until additional research and clinical data have emerged. However, the application of A. muciniphila, A. muciniphila-derived extracellular vesicles, and the therapeutic protein Amuc_1100 has been revealed to effectively protect against IBD and CRC, and the cellular and molecular mechanism needs extensive further investigation. In addition, the discovery of new strains or derivatives of A. muciniphila will reveal new approaches for biomolecule isolated probiotics to treat intestinal disorders. Overall, A. muciniphila has brought new hope for the use of probiotics for the prevention and treatment of intestinal diseases.
Figure 1.
The role of A. muciniphila in IBD and CRC. Decreased abundance of A. muciniphila was observed in patients and animal models with IBD or CRC. Increased level of A. muciniphila was associated with the prevention and protection in IBD and CRC following dietary ingredients, nutrients, probiotics, and medication intervention. Supplementation with A. muciniphila, A. muciniphila-derived extracellular vesicles, and the therapeutic protein Amuc_1100 could also protect against IBD and CRC by increasing the production of SCFA and modulation of an immune response. A. muciniphila: Akkermansia muciniphila; CRC: Colorectal cancer; IBD: Inflammatory bowel disease; SCFA: Short-chain fatty acid; TNFα: Tumor necrosis factor α; IFNγ: Interferon γ; IL: Interleukin.
Funding
The work was supported by the grants from the National Natural Science Foundation of China (Nos. 81973096 and 81502801) and the Jiangsu Province Natural Science Foundation (No. BK20201354).
Conflicts of interest
None.
Supplementary Material
Footnotes
How to cite this article: Gu ZY, Pei WL, Zhang Y, Zhu J, Li L, Zhang Z. Akkermansia muciniphila in inflammatory bowel disease and colorectal cancer. Chin Med J 2021;134:2841–2843. doi: 10.1097/CM9.0000000000001829
Supplemental digital content is available for this article.
References
- 1.Zhou JC, Zhang XW. Akkermansia muciniphila: a promising target for the therapy of metabolic syndrome and related diseases. Chin J Nat Med 2019; 17:835–841. doi: 10.1016/S1875-5364(19)30101-3. [DOI] [PubMed] [Google Scholar]
- 2.Derrien M, Van Baarlen P, Hooiveld G, Norin E, Muller M, de Vos WM. Modulation of mucosal immune response, tolerance, and proliferation in mice colonized by the Mucin-Degrader Akkermansia muciniphila. Front Microbiol 2011; 2:166.doi: 10.3389/fmicb.2011.00166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Depommier C, Everard A, Druart C, Plovier H, Van Hul M, Vieira-Silva S, et al. Supplementation with Akkermansia muciniphila in overweight and obese human volunteers: a proof-of-concept exploratory study. Nat Med 2019; 25:1096–1103. doi: 10.1038/s41591-019-0495-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yoon HS, Cho CH, Yun MS, Jang SJ, You HJ, Kim JH, et al. Akkermansia muciniphila secretes a glucagon-like peptide-1-inducing protein that improves glucose homeostasis and ameliorates metabolic disease in mice. Nat Microbiol 2021; 6:563–573. doi: 10.1038/s41564-021-00880-5. [DOI] [PubMed] [Google Scholar]
- 5.Zhang T, Li P, Wu X, Lu G, Marcella C, Ji X, et al. Alterations of Akkermansia muciniphila in the inflammatory bowel disease patients with washed microbiota transplantation. Appl Microbiol Biotechnol 2020; 104:10203–10215. doi: 10.1007/s00253-020-10948-7. [DOI] [PubMed] [Google Scholar]
- 6.Zhang T, Ji X, Lu G, Zhang F. The potential of Akkermansia muciniphila in inflammatory bowel disease. Appl Microbiol Biotechnol 2021; 105:5785–5794. doi: 10.1007/s00253-021-11453-1. [DOI] [PubMed] [Google Scholar]
- 7.Griffin LE, Djuric Z, Angiletta CJ, Mitchell CM, Baugh ME, Davy KP, et al. A Mediterranean diet does not alter plasma trimethylamine N-oxide concentrations in healthy adults at risk for colon cancer. Food Funct 2019; 10:2138–2147. doi: 10.1039/c9fo00333a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang L, Tang L, Feng Y, Zhao S, Han M, Zhang C, et al. A purified membrane protein from Akkermansia muciniphila or the pasteurised bacterium blunts colitis associated tumourigenesis by modulation of CD8(+) T cells in mice. Gut 2020; 69:1988–1997. doi: 10.1136/gutjnl-2019-320105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen Y, Zhang L, Hong G, Huang C, Qian W, Bai T, et al. Probiotic mixtures with aerobic constituent promoted the recovery of multi-barriers in DSS-induced chronic colitis. Life Sci 2020; 240:117089.doi: 10.1016/j.lfs.2019.117089. [DOI] [PubMed] [Google Scholar]
- 10.Fan L, Qi Y, Qu S, Chen X, Li A, Hendi M, et al. B. adolescentis ameliorates chronic colitis by regulating Treg/Th2 response and gut microbiota remodeling. Gut Microbes 2021; 13:1–17. doi: 10.1080/19490976.2020.1826746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu Z, Liao W, Zhang Z, Sun R, Luo Y, Chen Q, et al. Metformin affects gut microbiota composition and diversity associated with amelioration of dextran sulfate sodium-induced colitis in mice. Front Pharmacol 2021; 12:640347.doi: 10.3389/fphar.2021.640347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yu L, Zhao D, Nian Y, Li C. Casein-fed mice showed faster recovery from DSS-induced colitis than chicken-protein-fed mice. Food Funct 2021; 12:5806–5820. doi: 10.1039/d1fo00659b. [DOI] [PubMed] [Google Scholar]
- 13.Bian X, Wu W, Yang L, Lv L, Wang Q, Li Y, et al. Administration of Akkermansia muciniphila ameliorates dextran sulfate sodium-induced ulcerative colitis in mice. Front Microbiol 2019; 10:2259.doi: 10.3389/fmicb.2019.02259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhai R, Xue X, Zhang L, Yang X, Zhao L, Zhang C. Strain-specific anti-inflammatory properties of two Akkermansia muciniphila strains on chronic colitis in mice. Front Cell Infect Microbiol 2019; 9:239.doi: 10.3389/fcimb.2019.00239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim Y, Hwang SW, Kim S, Lee YS, Kim TY, Lee SH, et al. Dietary cellulose prevents gut inflammation by modulating lipid metabolism and gut microbiota. Gut Microbes 2020; 11:944–961. doi: 10.1080/19490976.2020.1730149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chelakkot C, Choi Y, Kim DK, Park HT, Ghim J, Kwon Y, et al. Akkermansia muciniphila-derived extracellular vesicles influence gut permeability through the regulation of tight junctions. Exp Mol Med 2018; 50:e450.doi: 10.1038/emm.2017.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seregin SS, Golovchenko N, Schaf B, Chen J, Pudlo NA, Mitchell J, et al. NLRP6 protects Il10(−/−) mice from colitis by limiting colonization of Akkermansia muciniphila. Cell Rep 2017; 19:733–745. doi: 10.1016/j.celrep.2017.03.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.