Abstract
Background:
Obesity and insulin resistance (IR) are common features of polycystic ovary syndrome (PCOS). Metformin (MET) increases insulin sensitivity, but it is associated with unsatisfactory weight loss. The glucagon-like peptide-1 receptor agonist exenatide has been shown to reduce weight and IR in patients with diabetes. This study aimed to explore the therapeutic effects of exenatide once-weekly (QW) combined with MET on body weight, as well as metabolic and endocrinological parameters in overweight/obese women with PCOS.
Methods:
Fifty overweight/obese women with PCOS diagnosed via the Rotterdam criteria were randomized to one of two treatment groups: MET (500 mg three times a day [TID]) or combination treatment (COM) (MET 500 mg TID, exenatide 2 mg QW) for 12 weeks. The primary outcomes were anthropometric changes associated with obesity, and the secondary outcomes included changes in reproductive hormone levels, glucose and lipid metabolism, and C-reactive protein.
Results:
Forty (80%) patients completed the study. COM therapy was superior to MET monotherapy in reducing weight (P = 0.045), body mass index (BMI) (P = 0.041), and waist circumference (P = 0.023). Patients in the COM group on an average lost 3.8 ± 2.4 kg compared with 2.1 ± 3.0 kg in the MET group. In the COM group, BMI and waist circumference decreased by 1.4 ± 0.87 kg/m2 and 4.63 ± 4.42 cm compared with 0.77 ± 1.17 kg/m2 and 1.72 ± 3.07 cm in the MET group, respectively. Moreover, levels of fasting glucose, oral glucose tolerance test (OGTT) 2-h glucose, and OGTT 2-h insulin were significantly lower with COM therapy than with MET (P < 0.050). Mild and moderate gastrointestinal reactions were the most common adverse events in both groups.
Conclusions:
COM therapy was more effective than MET alone in reducing body weight, BMI, and waist circumference, and improving insulin sensitivity in overweight/obese women with PCOS, with acceptable short-term side effects.
Trial registration:
ClinicalTrials.gov, NCT04029272. https://clinicaltrials.gov/ct2/show/NCT04029272
Keywords: Exenatide once-weekly, Insulin resistance, Metformin, Obesity, Polycystic ovary syndrome
Introduction
Polycystic ovary syndrome (PCOS) is a common endocrine disorder characterized by chronic anovulation, hyperandrogenism, polycystic ovary morphology, obesity, and insulin resistance (IR); in addition, obesity contributes substantially to reproductive and metabolic abnormalities in patients with PCOS.[1,2] Multiple studies have shown that weight loss can help women with PCOS resume spontaneous menstruation, reduce circulating androgen levels, and improve glucose and lipid metabolism.[3–5] Moreover, weight loss leads to an increased pregnancy rate in women with PCOS.[6] Obese people with PCOS can obtain these benefits by losing as little as 5% of the initial weight.[7] Increased exercise and dietary habit changes can help patients lose weight and reduce the risk of cardiovascular diseases and diabetes, but it is often challenging for patients to persist with them. Metformin (MET), an insulin sensitizer, is commonly used in combination with lifestyle modification to treat PCOS. The improvement of insulin sensitivity by MET is associated with its ability to decrease androgen levels, increase ovulation rate, and improve glucose tolerance. However, the effects of MET on weight loss are not satisfactory to patients.[8]
Glucagon-like peptide-1 (GLP-1) receptor agonists are a class of novel anti-diabetic agents that share similar effects with incretin mimetics, including glucose-dependent enhancement of insulin secretion and islet B cell proliferation.[9] GLP-1 receptor agonists have shown efficacy in improving IR and impaired glucose tolerance,[10] which is also associated with weight loss due to delayed gastric emptying and increased satiety via a central action.[11] Short-acting exenatide and long-acting liraglutide have been recently used in the treatment of PCOS. Treatment with exenatide or liraglutide led to significant weight loss and improved glucose metabolism in patients with PCOS, and a combined treatment with a GLP-1 receptor agonist and MET was superior to GLP-1 receptor agonist and MET monotherapies in improving menstrual cyclicity, insulin sensitivity, and weight loss[12–14]; however, this combined therapy requires twice-daily (BID) or once-daily subcutaneous administration. Exenatide once-weekly (QW) is the newest member of GLP-1 receptor agonists class. At the initial stage after subcutaneous injection, the surface-bound exenatide was released, followed by the gradual release of exenatide in the microspheres.[15] The plasma concentration of exenatide reached a steady state after multiple QW injections. Moreover, QW dosing regimen is convenient for patients owing to the reduced frequency of injections.
However, there are currently no reported studies on the effect of exenatide QW on patients with PCOS. We hypothesized that the combination of exenatide QW and MET has better efficacy in inducing weight loss and improving IR in PCOS patients. Thus, we conducted a clinical trial to assess the therapeutic efficacy of exenatide QW combined with MET on body weight, as well as metabolic and endocrinological parameters in overweight/obese women with PCOS. We present the following article in accordance with the CONSORT reporting checklist.
Methods
Ethical approval
The study was conducted in accordance with the Declaration of Helsinki and was approved by the Ethics Committee of Peking Union Medical College Hospital, Peking Union Medical College, Chinese Academy of Medical Sciences (No. HS-2032). All participants signed an informed consent form before the start of the study. The study protocol was registered on www.clinicaltrials.gov (NCT04029272).
Study design and patients
The present study was an open-label prospective, randomized, outpatient clinical trial with two treatment groups performed over 12 weeks. All eligible women with PCOS were recruited from the outpatients from Department of Obstetrics and Gynecology, Peking Union Medical College Hospital, and diagnosed according to the Rotterdam criteria. All subjects were 18 to 40 years old and overweight/obese (body mass index [BMI] ≥25 kg/m2). The exclusion criteria included patients with diabetes; history of cancer; personal or family history of multiple endocrine neoplasia type 2; severe cardiovascular, kidney, or liver diseases; and use of statins or other drugs known or suspected to affect reproductive or metabolic functions within 3 months before entering the study.
Eligible patients were assigned to one of the two treatment groups through a simple computer-generated randomization process to allocate an average of 25 patients to each group. The randomized codes in this study were generated electronically using a two-block randomization technique to create a treatment allocation spreadsheet.
Experimental protocol
Fifty women with PCOS were randomly distributed into one of two treatment arms: (1) MET 500 mg three times a day combined with exenatide QW 2 mg combination treatment (COM) or (2) MET alone for 12 weeks. MET was administered at an initial dose of 500 mg/day and gradually increased to a final dose of 1500 mg/day within 2 weeks. Patients in both groups were treated with Diane-35 (ethinylestradiol 0.035 mg and cyproterone acetate 2 mg, Bayer, Leverkusen, Germany) for 21 consecutive days from the first day of menstruation or progesterone withdrawal hemorrhage, and the next cycle began after 7 days of withdrawal. The specific medication and menstrual bleeding were recorded in a diary issued by the patient when the drugs were distributed. Lifestyle intervention was not actively promoted.
Vital signs, anthropometric measurements, and clinical and laboratory evaluations were performed before and after the 12-week treatment. Waist circumference was measured in a standing position midway between the lower costal margin and the iliac crest. BMI was calculated as weight in kilograms divided by square of height in meters. At baseline and after the 12-week treatment, a standard 75 g oral glucose tolerance test (OGTT) was performed in the morning after a 12-h overnight fast. Blood glucose and insulin levels were measured at 0, 30, 60, and 120 min, respectively. Fasting blood samples were also used for measurement of reproductive hormones, blood lipids, complete blood count, C-reactive protein, and markers of hepatic and renal functions.
The primary outcomes were anthropometric changes associated with obesity, and the secondary outcomes included changes in reproductive hormone levels, glucose and lipid metabolism, and C-reactive protein. Throughout the study, adverse events were recorded through direct questioning, patient self-report, physical examination, and clinical laboratory tests.
Assays
The levels of follicle-stimulating hormone, luteinizing hormone, estradiol, total testosterone (TT), and dehydroepiandrosterone sulfate (DHEAS) were measured via chemiluminescence immunoassay (DXI 800; Beckman Coulter, Brea, CA, USA). Total cholesterol (TC), triglycerides (TGs), high-density lipoprotein cholesterol (HDL-c), low-density lipoprotein cholesterol (LDL-c), apolipoprotein A1 (ApoA1), apolipoprotein B (ApoB), free fatty acid (FFA), high-sensitivity C-reactive protein (hsCRP), and fasting plasma glucose (FPG) concentrations were detected using standard automated procedures (AU automated chemistry analyzers; Beckman Coulter). Serum insulin concentrations were measured by using chemiluminescence immunoassay (Siemens Centaur® XP, Tarrytown, NY, USA). Several mathematical models or indices were used to assess insulin sensitivity and pancreatic β-cell function. We used a homeostasis model of insulin resistance (HOMA-IR) = fasting serum insulin (mIU/L) × FPG (mmol/L)/22.5.[16] Insulin sensitivity was assessed using the following formula: Quantitative Insulin Sensitivity Check Index (QUICKI) = 1/(log (fasting insulin [mIU/L]) + log (fasting glucose [mg/dL])).[17] To evaluate β-cell function, we used a homeostasis model of β cell function (HOMA-β) = 20 × fasting insulin (mIU/L)/(FPG [mmol/L] − 3.5) [15]. The Matsuda index was calculated based on the results of 2-h OGTT. Matsuda index = 10,000/square root of ([fasting glucose (mg/dL) × fasting insulin (mIU/L)] × [mean glucose × mean insulin]).[18]
Statistical analysis
Sample size analysis was performed using Power Analysis and Sample Size 11. Mean change in body weight, as the primary end point, was used to determine the required sample size. In accordance with previous studies, we calculated that at least ten patients in each group were required to determine a significant difference in weight loss of approximately 3.4 ± 0.5 kg between groups with statistical power of 90%. Considering the stability, accuracy, and dropout rate of the study, 25 subjects were enrolled in each treatment group. The intention-to-treat (ITT) analysis included all 50 randomized participants, and the per-protocol (PP) analysis included those who completed the allocated treatment.
The Shapiro-Wilk test was used to test the data normality of all variables. The results were expressed as mean ± standard deviation, or median (interquartie range). Then, t-test was used for comparison of data with normal distribution (paired-sample t-test and independent-sample t-test was used for intra- and inter-group comparisons, respectively). For comparison of data with abnormal distribution, Mann-Whitney test and Wilcoxon rank-sum test were used. For analysis of classified data, Pearson χ2 tests or Fisher exact tests were used. All data were analyzed using SPSS 26.0 (IBM, Armonk, NY, USA), and P < 0.050 was considered significant.
Results
Participants and baseline characteristics
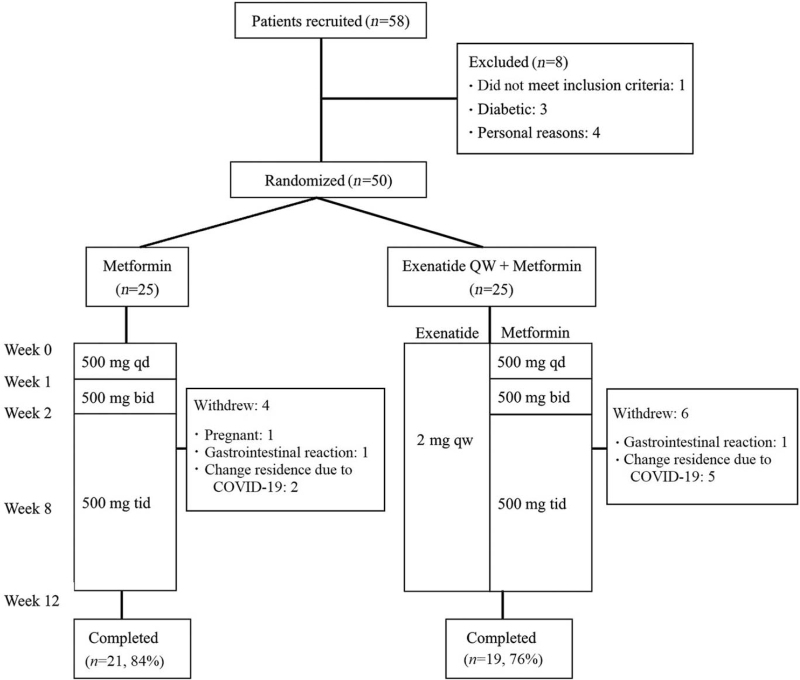
Fifty overweight/obese women with PCOS were randomized and received treatment, and 40 patients (80%) completed the study [Figure 1]. The baseline characteristics of all randomized patients (ITT) are presented in Table 1, showing that the age; baseline anthropometric, hormonal, and metabolic parameters; and baseline hsCRP were similar between the two groups.
Figure 1.
Study flow chart. COVID-19: Coronavirus disease 2019; QW: Once-weekly; bid: Twice a day; tid: Three times a day; qd: One a day.
Table 1.
Baseline characteristics of the study participants with polycystic ovary syndrome.
| Parameters | MET (n = 25) | COM (n = 25) | P values |
| Age (years) | 28.17 ± 4.40 | 30.10 ± 4.52 | 0.097 |
| Weight (kg) | 79.25 ± 10.75 | 84.14 ± 12.82 | 0.109 |
| BMI (kg/m2) | 30.62 ± 3.42 | 31.51 ± 4.20 | 0.404 |
| Waist circumference (cm) | 96.18 ± 8.56 | 98.94 ± 10.77 | 0.194 |
| Hip circumference (cm) | 105.75 ± 5.85 | 108.66 ± 8.12 | 0.150 |
| Total testosterone (ng/mL) | 0.76 ± 0.23 | 0.73 ± 0.28 | 0.705 |
| DHEAS (μg/dL) | 257.91 ± 110.81 | 259.96 ± 111.71 | 0.773 |
| FPG (mmol/L) | 5.19 ± 0.45 | 5.18 ± 0.55 | 0.895 |
| OGTT 2 h PG | 7.11 ± 1.58 | 7.79 ± 1.67 | 0.143 |
| Fasting insulin (μIU/mL) | 20.01 ± 7.35 | 23.47 ± 12.77 | 0.460 |
| OGTT 2 h insulin | 144.08 ± 71.34 | 177.28 ± 81.14 | 0.062 |
| HOMA-IR | 4.41 (3.91–5.28) | 4.91 (3.05–6.79) | 0.478 |
| HOMA-β | 228.19 (169.70–326.51) | 240.65 (194.84–345.03) | 0.437 |
| QUICKI | 0.31 (0.30–0.31) | 0.30 (0.29–0.32) | 0.593 |
| Matsuda index | 2.03 (1.71–2.37) | 1.83 (1.56–2.85) | 0.884 |
| TC (mmol/L) | 5.18 ± 0.84 | 4.77 ± 0.74 | 0.114 |
| TG (mmol/L) | 1.60 (1.02–2.65) | 1.34 (0.97–1.96) | 0.271 |
| HDL-c (mmol/L) | 1.16 ± 0.24 | 1.18 ± 0.24 | 0.699 |
| LDL-c (mmol/L) | 3.40 ± 0.67 | 3.06 ± 0.59 | 0.060 |
| ApoA1 (g/L) | 1.26 ± 0.13 | 1.26 ± 0.15 | 0.852 |
| ApoB (g/L) | 1.04 ± 0.19 | 0.95 ± 0.14 | 0.127 |
| FFA (μmol/L) | 659.33 ± 206.62 | 592.29 ± 223.02 | 0.286 |
| hsCRP (mg/L) | 2.24 (1.45–5.34) | 3.74 (1.86–9.05) | 0.312 |
Data are presented as mean ± standard deviation, or median (interquartile range). ApoA1: Apolipoprotein A1; ApoB: Apolipoprotein B; BMI: Body mass index; COM: Combination treatment; DHEAS: Dehydroepiandrosterone sulphate; FPG: Fasting plasma glucose; FFA: Free fatty acid; HOMA-IR: Homeostasis model of assessment for insulin resistance; HOMA-β: Homeostasis model assessment β cell function; HDL-c: High-density lipoprotein cholesterol; hsCRP: High-sensitivity C-reactive protein; LDL-c: Low-density lipoprotein cholesterol; MET: Metformin; OGTT: Oral glucose tolerance test; QUICKI: Quantitative insulin sensitivity check index; TC: Total cholesterol; TG: Triglyceride.
Changes after drug intervention
The anthropometric and laboratory study results at baseline and after the 12-week treatment of the 40 patients who completed treatment (PP) are summarized in Table 2. Data comparison results of all parameters before and after treatment between the two groups are provided in Table 3.
Table 2.
Baseline and 12-week post-treatment parameters of the participants with polycystic ovary syndrome in every group.
| MET (n = 21) | COM (n = 19) | |||||
| Parameters | Pre-treatment | Post-treatment | P | Pre-treatment | Post-treatment | P |
| Weight (kg) | 79.10 ± 10.80 | 77.05 ± 9.75 | 0.008 | 82.34 ± 11.42 | 78.57 ± 10.94 | <0.001 |
| BMI (kg/m2) | 30.40 ± 3.16 | 29.63 ± 2.80 | 0.010 | 30.80 ± 3.41 | 29.40 ± 3.32 | <0.001 |
| Waist circumference (cm) | 96.63 ± 9.16 | 94.98 ± 8.13 | 0.009 | 97.32 ± 9.60 | 92.70 ± 8.71 | <0.001 |
| Hip circumference (cm) | 105.63 ± 6.02 | 105.05 ± 6.74 | 0.506 | 108.63 ± 6.85 | 106.10 ± 6.46 | 0.002 |
| Total testosterone (ng/mL) | 0.78 ± 0.22 | 0.56 ± 0.20 | <0.001 | 0.74 ± 0.29 | 0.57 ± 0.25 | 0.003 |
| DHEAS (μg/dL) | 265.50 ± 119.60 | 261.55 ± 120.16 | 0.733 | 263.94 ± 119.55 | 261.60 ± 133.86 | 0.818 |
| FPG (mmol/L) | 5.20 ± 0.46 | 5.19 ± 0.51 | 0.889 | 5.21 ± 0.57 | 4.93 ± 0.47 | 0.023 |
| OGTT 2 h PG | 7.13 ± 1.73 | 8.54 ± 1.74 | 0.002 | 8.03 ± 1.62 | 6.66 ± 1.41 | 0.006 |
| Fasting insulin (μIU/mL) | 20.50 ± 7.51 | 21.81 ± 8.26 | 0.394 | 26.55 ± 14.70 | 23.0 ± 10.16 | 0.355 |
| OGTT 2 h insulin | 150.37 ± 73.44 | 131.71 ± 61.96 | 0.232 | 209.33 ± 81.26 | 124.82 ± 82.54 | 0.001 |
| HOMA-IR | 4.49 (3.93–5.43) | 4.80 (3.47–6.39) | 0.414 | 5.29 (3.64–7.77) | 4.70 (4.20–6.21) | 0.212 |
| HOMA-β | 232.63 (177.02–334.68) | 272.0 (179.27–370.19) | 0.181 | 249.47 (196.67–387.50) | 289.33 (202.86–445.45) | 0.601 |
| QUICKI | 0.31 (0.30–0.31) | 0.30 (0.29–0.32) | 0.608 | 0.30 (0.29–0.32) | 0.30 (0.29–0.31) | 0.397 |
| Matsuda index | 1.75 (1.46–2.24) | 1.81 (1.42–2.76) | 0.590 | 1.65 (1.08–1.88) | 1.92 (1.44–2.47) | 0.033 |
| TC (mmol/L) | 5.15 ± 0.87 | 5.64 ± 0.88 | 0.007 | 4.94 ± 0.78 | 5.13 ± 0.99 | 0.356 |
| TG (mmol/L) | 1.70 (1.22–2.97) | 2.46 (1.56–3.61) | 0.027 | 1.30 (0.92–2.20) | 2.0 (1.59–3.20) | 0.005 |
| HDL-c (mmol/L) | 1.14 ± 0.22 | 1.45 ± 0.36 | <0.001 | 1.19 ± 0.24 | 1.44 ± 0.34 | <0.001 |
| LDL-c (mmol/L) | 3.38 ± 0.71 | 3.37 ± 0.78 | 0.970 | 3.22 ± 0.61 | 2.98 ± 0.83 | 0.064 |
| ApoA1 (g/L) | 1.25 ± 0.12 | 1.65 ± 0.33 | <0.001 | 1.27 ± 0.15 | 1.61 ± 0.28 | <0.001 |
| ApoB (g/L) | 1.03 ± 0.20 | 1.11 ± 0.18 | 0.057 | 0.98 ± 0.18 | 0.97 ± 0.18 | 0.663 |
| FFA (μmol/L) | 660.09 ± 192.93 | 711.90 ± 227.86 | 0.455 | 596.68 ± 216.38 | 643.89 ± 226.41 | 0.616 |
| hsCRP (mg/L) | 3.20 (1.72–5.63) | 2.57 (2.18–5.30) | 0.931 | 4.98 (2.29–9.53) | 4.18 (1.74–9.99) | 0.658 |
Data are presented as mean ± standard deviation, or median (interquartile range). ApoA1: Apolipoprotein A1; ApoB: Apolipoprotein B; BMI: Body mass index; COM: Combination treatment; DHEAS: Dehydroepiandrosterone sulphate; FPG: Fasting plasma glucose; FFA: Free fatty acid; HOMA-IR: Homeostasis model of assessment for insulin resistance; HOMA-β: Homeostasis model assessment β cell function; hsCRP: High-sensitivity C-reactive protein; HDL-c: High-density lipoprotein cholesterol; LDL-c: Low-density lipoprotein cholesterol; MET: Metformin; OGTT: Oral glucose tolerance test; PP: Per-protocol; QUICKI: Quantitative insulin sensitivity check index; TC: Total cholesterol; TG: Triglyceride.
Table 3.
Changes of parameters in patients with polycystic ovary syndrome in each group.
| Parameters | MET (n = 21) | COM (n = 19) | P |
| Weight (kg) | 2.05 ± 3.02 | 3.76 ± 2.41 | 0.045 |
| BMI (kg/m2) | 0.77 ± 1.17 | 1.40 ± 0.87 | 0.041 |
| Waist circumference (cm) | 1.72 ± 3.07 | 4.63 ± 4.42 | 0.023 |
| Hip circumference (cm) | 0.58 ± 3.71 | 2.56 ± 3.64 | 0.101 |
| Total testosterone (ng/mL) | 0.22 ± 0.18 | 0.17 ± 0.22 | 0.400 |
| DS (μg/dL) | 3.94 ± 48.25 | 2.34 ± 42.57 | 0.917 |
| FPG (mmol/L) | 0.01 ± 0.31 | 0.26 ± 0.45 | 0.040 |
| OGTT 2 h PG | −1.41 ± 1.64 | 0.85 ± 2.85 | <0.001 |
| Fasting insulin (μIU/mL) | −1.31 ± 7.52 | 2.43 ± 10.23 | 0.118 |
| OGTT 2 h insulin | 18.65 ± 85.03 | 67.96 ± 109.23 | 0.016 |
| HOMA-IR | −0.35 (−2.12–1.05) | 0.55 (−1.06–3.32) | 0.063 |
| HOMA-β | −15.36 (−77.34–29.28) | 2.0 (−150.11–98.17) | 0.851 |
| QUICKI | 0.01 (−0.01–0.01) | −0.01 (−0.02–0.01) | 0.361 |
| Matsuda index | −0.01 (−0.7–0.52) | –0.6 (−0.76–0.26) | 0.282 |
| TC (mmol/L) | −0.49 ± 0.80 | −0.18 ± 0.87 | 0.270 |
| TG (mmol/L) | −0.59 (−1.20–0.11) | −0.54 (−1.06 to −0.05) | 0.893 |
| HDL-c (mmol/L) | −0.31 ± 0.24 | −0.25 ± 0.21 | 0.373 |
| LDL-c (mmol/L) | 0.01 ± 0.68 | 0.23 ± 0.75 | 0.328 |
| ApoA1 (g/L) | −0.40 ± 0.29 | −0.33 ± 0.25 | 0.434 |
| ApoB (g/L) | −0.07 ± 0.16 | 0.02 ± 0.18 | 0.103 |
| FFA (μmol/L) | −51.80 ± 287.57 | −47.21 ± 250.48 | 0.957 |
| hsCRP (mg/L) | −0.26 (−1.22–1.30) | 0.38 (−1.0–1.37) | 0.742 |
Data are presented as mean ± standard deviation, or median (interquartile range). ApoA1: Apolipoprotein A1; ApoB: Apolipoprotein B; BMI: Body mass index; COM: Combination treatment; DHEAS: Dehydroepiandrosterone sulphate; FFA: Free fatty acid; FPG: Fasting plasma glucose; HOMA-IR: Homeostasis model of assessment for insulin resistance; HOMA-β: Homeostasis model assessment β cell function; hsCRP: High-sensitivity C-reactive protein; HDL-c: High-density lipoprotein cholesterol; LDL-c: Low-density lipoprotein cholesterol; MET: Metformin; NS: Not significant; OGTT: Oral glucose tolerance test; QUICKI: Quantitative insulin sensitivity check index; TC: Total cholesterol; TG: Triglyceride.
Weight changes
Body weight significantly decreased in both COM and MET groups after 12 weeks of treatment (P < 0.001 and P = 0.008), accompanied with significantly reduced BMI (P < 0.001 and P = 0.010) and waist circumference (P < 0.001 and P = 0.009) [Table 2]. The mean weight loss was 3.8 ± 2.4 kg in the COM group and 2.1 ± 3.0 kg in the MET group [Table 3]. Seven patients (36.8%) in the COM group and four patients (19.1%) in the MET group lost ≥5% of the initial weight (odds ratio = 2.38, 95% confidence interval = 0.60–9.37). Hip circumference was only decreased in the COM group (P = 0.002) [Table 2]. The COM treatment exhibited higher efficacy than MET in lowering body weight, BMI, waist circumference, and hip circumference (P < 0.050) [Table 3].
Endocrine changes
TT was significantly decreased in both COM and MET groups (P = 0.003 and P < 0.001) [Table 2], but the decrease was similar between the treatments [Table 3]. The DHEAS level was not significantly reduced by the treatments [Table 2].
Metabolic changes
After 12 weeks of treatment, FPG, OGTT 2-h glucose, and OGTT 2-h insulin (P = 0.023, P = 0.006, and P = 0.001, respectively) [Table 2] were significantly decreased by COM therapy but not by MET treatment. Accordingly, the levels of FPG, OGTT 2-h glucose, and OGTT 2-h insulin were reduced to a significantly higher degree with COM therapy than with MET (P < 0.050) [Table 3]. Fasting insulin level was not significantly altered by both treatments [Table 2].
Matsuda index score, which reflects systemic insulin sensitivity, was significantly improved by COM therapy (P = 0.033) [Table 2], but not changed by MET treatment. However, there were no significant differences in the changes within-treatment between the two groups [Table 3]. The HOMA-IR, HOMA-β, and QUICKI values were not significantly changed by both treatments [Table 2].
HDL-c, ApoA1, and TG levels increased significantly with both treatments (P < 0.050) [Table 2]. TC levels increased significantly with only MET monotherapy (P = 0.007) [Table 2]. LDL-c, ApoB, and FFA levels did not significantly change with both treatments [Table 2], and no distinct between-treatment differences were found in all these parameters.
HsCRP level, which is an inflammatory marker, was insignificantly decreased by both treatments. Moreover, the reduction was not significantly different between the therapies [Table 3].
Adverse events
Mild to moderate gastrointestinal (GI) reactions were the most common adverse events. Among these GI side effects, nausea was the most frequent, with an incidence of 40% (10/25) and 44% (11/25) in the MET and COM groups, respectively. The second-most frequent was diarrhea, with an incidence of 44% (11/25) in the MET group, which was higher but not significantly than that in the COM group (36%, 9/25). The incidence of other reported adverse reactions, such as abdominal distension, vomiting, headache, constipation, and fatigue, was similar in the two treatment groups. GI adverse events usually occurred within the first 8 weeks of therapy. Two patients withdrew from therapy because of diarrhea: one in the MET group and one in the COM group. No other patient withdrew from therapy because of GI adverse events [Table 4].
Table 4.
Incidence of adverse events in each group.
| Items | MET | COM | P |
| Nausea | 10/25 (40) | 11/25 (44) | 0.774 |
| Diarrhea | 11/25 (44) | 9/25 (36) | 0.564 |
| Bloating | 2/25 (8) | 625 (24) | 0.247 |
| Vomiting | 3/25 (12) | 2/25 (8) | >0.999 |
| Headache | 1/25 (4) | 2/25 (8) | >0.999 |
| Stomachache | 2/25 (8) | – | – |
| Constipation | 1/25 (4) | 2/25 (8) | >0.999 |
| Fatigue | 2/25 (8) | 3/25 (12) | >0.999 |
| Dizzy | 1/25 (4) | 1/25 (4) | >0.999 |
| Urticaria | – | 1/25 (4) | – |
| Injection site pain | – | 2/25 (8) | – |
| Injection site itchy | – | 12/25 (48) | – |
| Subcutaneous induration | – | 11/25 (44) | – |
Data are presented as n/N (%). COM: Combination treatment; MET: Metformin.
Pruritus and subcutaneous induration with a diameter of <0.5 cm at the injection site were common adverse events after subcutaneous injection of exenatide QW. None of the participants treated with exenatide QW withdrew from treatment owing to these side effects, though itching and induration persisted at the injection site.
Discussion
In this study, we showed that combined therapy with exenatide QW and MET was superior to MET monotherapy in reducing weight and improving blood glucose and insulin response in overweight/obese women with PCOS.
MET and exenatide were associated with weight loss, and the combination therapy showed a synergistic effect. In this study, after the 12-week treatment, patients in the MET group lost an average of 2.1 kg of weight compared with 3.8 kg in the COM group. However, Elkind-Hirsch et al[14] reported that exenatide BID combined with MET led to an average weight loss of 6.0 kg after a 24-week treatment. The difference in weight change may be due to the different treatment durations, as Wysham et al[19] reported that exenatide QW was associated with similar weight loss compared with exenatide BID in patients with type 2 diabetes. Abdominal obesity was associated with greater risks of metabolic abnormalities. In this study, the COM therapy was superior to MET monotherapy in reducing waist circumference; therefore, the COM therapy may provide better control of abdominal obesity.
Total T levels were significantly decreased in both groups, with no significant between-treatment difference. This finding differed from that of Zheng et al[20] and Liu et al,[21] in which overweight/obese PCOS patients were randomized to receive exenatide BID or MET for 12 weeks, and no obvious reductions in the total T levels were observed in both groups. This difference was most likely caused by the administration of Diane-35 to all patients in our present study. Diane-35 can effectively reduce the levels of serum testosterone and is superior to MET in improving the hyperandrogenic state of patients with PCOS.[22] Nonetheless, in another study by Elkind-Hirsch et al,[14] the total T levels significantly decreased after 24 weeks of treatment with either exenatide BID-MET combination or single MET therapy; thus, the efficacy of exenatide QW in reducing androgen needs further investigation.
In this short-term trial, COM significantly reduced FPG as well as OGTT 2-h blood glucose and insulin in patients with PCOS, whereas MET monotherapy did not achieve the same therapeutic effect. Thus, the COM therapy may be beneficial to shorten the course of treatment. MET has been the gold standard in the treatment of type 2 diabetes for many years. It lowers blood glucose level and improves IR by inhibiting gluconeogenesis in the liver and increasing glucose uptake by peripheral tissues (muscle and adipose tissue). It can also reduce the risk of progression from prediabetes to diabetes. Moreover, MET induces the secretion of GLP-1 by intestinal L cells, and increases the sensitivity of pancreatic cells to its effect by regulating the expression of GLP-1 receptor on pancreatic cells.[23] Therefore, there is a synergistic effect between MET and GLP-1 receptor agonists, as Derosa et al[24] reported that exenatide plus MET was effective not only in maintaining glycemic control but also in protecting β-cells.
HOMA-IR and HOMA-β are simple surrogate indexes for insulin sensitivity/resistance and calculated from FPG and fasting insulin.[25] In this study, the HOMA values did not decrease significantly in both groups. This finding was inconsistent with that of Zheng et al[20] who reported that after 12 weeks of exenatide BID in patients with PCOS, HOMA-IR value significantly decreased in both groups, especially in the exenatide BID group. A possible cause of this difference is that all patients in that study were provided diet and exercise instruction. Matsuda index correlates reasonably well with estimates of whole-body insulin sensitivity determined using glucose clamp.[25] In this study, the Matsuda index of patients in the COM group increased significantly after treatment, although there was no significant difference between the two treatment groups. However, this result still suggested that the COM therapy could improve the overall insulin sensitivity more quickly than MET monotherapy.
We found that serum TG, HDL-c, and ApoA1 levels increased significantly in both groups, with no significant difference between the treatments. This finding was inconsistent with previous reports, in which 12 weeks of single or combined treatment with exenatide QW and MET did not result in lipid profile changes.[14,26] This difference was also likely caused by the administration of Diane-35, as Diane-35 has been reported to increase blood TC, TG, LDL-c, and ApoB levels with increasing number of therapy cycles in patients with PCOS.[27,28] The effect of exenatide QW combined with Diane-35 on lipid metabolism needs further evaluation in a long-term trial.
Adverse events were an important part in our research. Mild and moderate nausea and diarrhea were the most common side effects in both groups, with similar incidence. Moreover, itching and induration at the injection site were common side effects after subcutaneous administration of exenatide QW, but none of the patients discontinued the study because of this. Considering that exenatide QW was formulated to increase patient convenience by decreasing the frequency of injections, this lack of severe side effects might also contribute to increasing patient compliance to treatment.
Conclusions
This study provides preliminary evidence that the combination of exenatide QW and MET is superior to MET monotherapy in reducing body weight, BMI, waist circumstance, and improving blood glucose and insulin in overweight/obese Chinese women with PCOS, with acceptable short-term side effects. Nevertheless, the small simple size, open-label design, and short study duration limit the generalizability of the results. More massive-scale, long-term randomized clinical trials focusing on the comparison of exenatide QW with MET, as well as cost-effectiveness analysis are definitively needed to guide the use of exenatide QW as a treatment of PCOS.
Conflicts of interest
None.
Footnotes
How to cite this article: Ma RL, Deng Y, Wang YF, Zhu SY, Ding XS, Sun AJ. Short-term combined treatment with exenatide and metformin for overweight/obese women with polycystic ovary syndrome. Chin Med J 2021;134:2882–2889. doi: 10.1097/CM9.0000000000001712
References
- 1.Escobar-Morreale HF. Polycystic ovary syndrome: definition, aetiology, diagnosis and treatment. Nat Rev Endocrinol 2018; 14:270–284. doi: 10.1038/nrendo.2018.24. [DOI] [PubMed] [Google Scholar]
- 2.Barber TM, Hanson P, Weickert MO, Franks S. Obesity and polycystic ovary syndrome: implications for pathogenesis and novel management strategies. Clin Med Insights Reprod Health 2019; 13:1179558119874042.doi: 10.1177/1179558119874042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lim SS, Hutchison SK, Van Ryswyk E, Norman RJ, Teede HJ, Moran LJ, et al. Lifestyle changes in women with polycystic ovary syndrome. Cochrane Database Syst Rev 2019; 3:CD007506.doi: 10.1002/14651858.CD007506.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marzouk TM, Sayed Ahmed WA. Effect of dietary weight loss on menstrual regularity in obese young adult women with polycystic ovary syndrome. J Pediatr Adolesc Gynecol 2015; 28:457–461. doi: 10.1016/j.jpag.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 5.Wang QY, Song Y, Huang W, Xiao L, Wang QS, Feng GM. Comparison of drospirenone- with cyproterone acetate-containing oral contraceptives, combined with metformin and lifestyle modifications in women with polycystic ovary syndrome and metabolic disorders: a prospective randomized control trial. Chin Med J (Engl) 2016; 129:883–890. doi: 10.4103/0366-6999.179783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Legro RS, Dodson WC, Kris-Etherton PM, Kunselman AR, Stetter CM, Williams NI, et al. Randomized controlled trial of preconception interventions in infertile women with polycystic ovary syndrome. J Clin Endocrinol Metab 2015; 100:4048–4058. doi: 10.1210/jc.2015-2778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Glueck CJ, Goldenberg N. Characteristics of obesity in polycystic ovary syndrome: etiology, treatment, and genetics. Metabolism 2019; 92:108–120. doi: 10.1016/j.metabol.2018.11.002. [DOI] [PubMed] [Google Scholar]
- 8.Dumitrescu R, Mehedintu C, Briceag I, Purcărea VL, Hudita D. Metformin-clinical pharmacology in PCOs. J Med Life 2015; 8:187–192. [PMC free article] [PubMed] [Google Scholar]
- 9.Sfairopoulos D, Liatis S, Tigas S, Liberopoulos E. Clinical pharmacology of glucagon-like peptide-1 receptor agonists. Hormones (Athens) 2018; 17:333–350. doi: 10.1007/s42000-018-0038-0. [DOI] [PubMed] [Google Scholar]
- 10.Htike ZZ, Zaccardi F, Papamargaritis D, Webb DR, Khunti K, Davies MJ. Efficacy and safety of glucagon-like peptide-1 receptor agonists in type 2 diabetes: a systematic review and mixed-treatment comparison analysis. Diabetes Obes Metab 2017; 19:524–536. doi: 10.1111/dom.12849. [DOI] [PubMed] [Google Scholar]
- 11.Vilsbøll T, Christensen M, Junker AE, Knop FK, Gluud LL. Effects of glucagon-like peptide-1 receptor agonists on weight loss: systematic review and meta-analyses of randomised controlled trials. BMJ 2012; 344:d7771.doi: 10.1136/bmj.d7771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sever MJ, Kocjan T, Pfeifer M, Kravos NA, Janez A. Short-term combined treatment with liraglutide and metformin leads to significant weight loss in obese women with polycystic ovary syndrome and previous poor response to metformin. Eur J Endocrinol 2014; 170:451–459. doi: 10.1530/EJE-13-0797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frøssing S, Nylander M, Chabanova E, Frystyk J, Holst JJ, Kistorp C, et al. Effect of liraglutide on ectopic fat in polycystic ovary syndrome: a randomized clinical trial. Diabetes Obes Metab 2018; 20:215–218. doi: 10.1111/dom.13053. [DOI] [PubMed] [Google Scholar]
- 14.Elkind-Hirsch K, Marrioneaux O, Bhushan M, Vernor D, Bhushan R. Comparison of single and combined treatment with exenatide and metformin on menstrual cyclicity in overweight women with polycystic ovary syndrome. J Clin Endocrinol Metab 2008; 93:2670–2678. doi: 10.1210/jc.2008-0115. [DOI] [PubMed] [Google Scholar]
- 15.Grossman SS. Pathophysiological and pharmacological rationale for the use of exenatide once weekly in patients with type 2 diabetes. Adv Ther 2014; 31:247–263. doi: 10.1007/s12325-014-0101-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985; 28:412–419. doi: 10.1007/bf00280883. [DOI] [PubMed] [Google Scholar]
- 17.Katz A, Nambi SS, Mather K, Baron AD, Follmann DA, Sullivan G, et al. Quantitative insulin sensitivity check index: a simple, accurate method for assessing insulin sensitivity in humans. J Clin Endocrinol Metab 2000; 85:2402–2410. doi: 10.1210/jcem.85.7.6661. [DOI] [PubMed] [Google Scholar]
- 18.Seltzer HS, Allen EW, Herron AL, Jr, Brennan MT. Insulin secretion in response to glycemic stimulus: relation of delayed initial release to carbohydrate intolerance in mild diabetes mellitus. J Clin Invest 1967; 46:323–335. doi: 10.1172/jci105534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wysham CH, Rosenstock J, Vetter ML, Dong F, Öhman P, Iqbal N. Efficacy and tolerability of the new autoinjected suspension of exenatide once weekly versus exenatide twice daily in patients with type 2 diabetes. Diabetes Obes Metab 2018; 20:165–172. doi: 10.1111/dom.13056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zheng S, Zhang Y, Long T, Lu J, Liu X, Yan J, et al. Short term monotherapy with exenatide is superior to metformin in weight loss, improving insulin resistance and inflammation in Chinese overweight/obese PCOS women. Obes Med 2017; 7:15–20. doi: 10.1016/j.obmed.2017.06.003. [Google Scholar]
- 21.Liu X, Zhang Y, Zheng SY, Lin R, Xie YJ, Chen H, et al. Efficacy of exenatide on weight loss, metabolic parameters and pregnancy in overweight/obese polycystic ovary syndrome. Clin Endocrinol (Oxf) 2017; 87:767–774. doi: 10.1111/cen.13454. [DOI] [PubMed] [Google Scholar]
- 22.Mhao NS, Al-Hilli AS, Hadi NR, Jamil DA, Al-Aubaidy HA. A comparative study to illustrate the benefits of using ethinyl estradiol-cyproterone acetate over metformin in patients with polycystic ovarian syndrome. Diabetes Metab Syndr 2016; 10:S95–S98. doi: 10.1016/j.dsx.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 23.Glintborg D, Mumm H, Holst JJ, Andersen M. Effect of oral contraceptives and/or metformin on GLP-1 secretion and reactive hypoglycaemia in polycystic ovary syndrome. Endocr Connect 2017; 6:267–277. doi: 10.1530/ec-17-0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Derosa G, Franzetti IG, Querci F, Carbone A, Ciccarelli L, Piccinni MN, et al. Exenatide plus metformin compared with metformin alone on β-cell function in patients with type 2 diabetes. Diabet Med 2012; 29:1515–1523. doi: 10.1111/j.1464-5491.2012.03699.x. [DOI] [PubMed] [Google Scholar]
- 25.Muniyappa R, Lee S, Chen H, Quon MJ. Current approaches for assessing insulin sensitivity and resistance in vivo: advantages, limitations, and appropriate usage. Am J Physiol Endocrinol Metab 2008; 294:E15–E26. doi: 10.1152/ajpendo.00645.2007. [DOI] [PubMed] [Google Scholar]
- 26.Su N, Li Y, Xu T, Li L, Kwong JS, Du H, et al. Exenatide in obese or overweight patients without diabetes: a systematic review and meta-analyses of randomized controlled trials. Int J Cardiol 2016; 219:293–300. doi: 10.1016/j.ijcard.2016.06.028. [DOI] [PubMed] [Google Scholar]
- 27.Prelević GM, Würzburger MI, Trpković D, Balint-Perić L. Effects of a low-dose estrogen-antiandrogen combination (Diane-35) on lipid and carbohydrate metabolism in patients with polycystic ovary syndrome. Gynecol Endocrinol 1990; 4:157–168. doi: 10.3109/09513599009009803. [DOI] [PubMed] [Google Scholar]
- 28.Villaseca P, Hormaza P, Cárdenas I, Oestreicher E, Arteaga E. Ethinylestradiol/cyproterone acetate in polycystic ovary syndrome: lipid and carbohydrate changes. Eur J Contracept Reprod Health Care 2004; 9:155–165. doi: 10.1080/13625180400007751. [DOI] [PubMed] [Google Scholar]