Abstract
Background:
Central nervous system (CNS) symptoms after efavirenz (EFV) treatment in people living with human immunodeficiency virus (HIV) could persist and impact their quality of life. We assessed the impact of EFV-based regimen replacement with elvitegravir/cobicistat/emtricitabine/tenofovir alafenamide (E/C/F/TAF), which is considered an alternative option for subjects who do not tolerate EFV. Most specifically, we assessed the safety and the efficacy of E/C/F/TAF and its effects on the participants’ neuropsychiatric toxicity symptoms in a real-life setting.
Methods:
A prospective cohort study was conducted among virologic suppressed HIV-positive participants receiving EFV-based regimens with ongoing CNS toxicity ≥ grade 2. The participants were switched to single-pill combination regimens E/C/F/TAF and followed up for 48 weeks. The neuropsychiatric toxicity symptoms were measured using a CNS side effects questionnaire, as well as the Hospital Anxiety and Depression Scale and the Pittsburgh Sleep Quality Index. The primary outcome measure was the proportion of participants experiencing grade 2 or higher CNS toxicity after EFV switch off at weeks 12, 24, and 48. Secondary endpoints included virologic and immunological responses and the effect on fasting lipids at week 48 after switch.
Results:
One hundred ninety-six participants (96.9% men, median age: 37.5 years, median: 3.7 years on prior EFV-containing regimens) were included in the study. Significant improvements in anxiety and sleep disturbance symptoms were observed at 12, 24, and 48 weeks after switching to E/C/F/TAF (P < 0.05). No significant change in depression symptom scores was observed. At 48 weeks after switch, HIV viral load <50 copies/mL was maintained in all of the participants, median fasting lipid levels were moderately increased (total cholesterol [TC]: 8.2 mg/dL, low-density lipoprotein cholesterol [LDL-C]: 8.5 mg/dL, high-density lipoprotein cholesterol [HDL-C]: 2.9 mg/dL, and triglyceride (TG): 1.6 mg/dL, and the TC:HDL-C ratio remained stable.
Conclusions:
The single-pill combination regimens E/C/F/TAF is safe and well tolerated. This study reveals that switching from EFV to E/C/F/TAF significantly reduces neuropsychiatric toxicity symptoms in people living with HIV with grade 2 or higher CNS complaints.
Keywords: HIV, Efavirenz, Elvitegravir, Tenofovir alafenamide, Central nervous system
Introduction
Scaling up of antiretroviral therapy (ART) has dramatically reduced human immunodeficiency virus (HIV)-related mortality and morbidity worldwide, including those in resource-limited countries.[1] Since 2003, the National Free Antiretroviral Treatment Program (NFATP) was launched in China.[2] It has greatly increased the access to ART and significantly led to a substantial decline in HIV incidence and mortality.[3] Consistent with the recommendation of the World Health Organization and the national acquired immune deficiency syndrome (AIDS) control programs in several resource-rich countries,[4,5] the Chinese NFATP currently recommends tenofovir (TDF) + lamivudine (3TC) + efavirenz (EFV) as the preferred first-line antiretroviral regimen due to its efficacy and relative affordability.[3]
Several evidence reports indicate that 40% to 60%[5] of individuals treated with EFV, which is a non-nucleoside reverse transcriptase inhibitor, suffer from its related central nervous system (CNS) adverse events. Indeed, EFV administration has been associated with psychiatric symptoms (e.g., severe depression, anxiety, suicidal ideation) and CNS symptoms (e.g., dizziness, insomnia, trouble concentrating, unusual dreams, and feeling drowsy during the daytime) early after initiation prompting regimen switches.[4,5] In addition, there is a growing awareness that many individuals receiving long-term EFV treatment may have subtler symptoms that substantially affect their quality of life albeit they are not strictly related to EFV. These include subclinical symptoms in mood disorders and ambiguous performances such as irritability and hallucinations.[5,6] Before the availability of potent integrase inhibitors, HIV-positive individuals who do not tolerate EFV were treated with lopinavir/ritonavir (LPV/r), which was the only NFATP-sponsored protease inhibitors (PIs) freely provided by the Chinese government.[3] A significant number of PI-related dyslipidemias have also been reported.[7–10] As such, we can note the elevation of lipid parameters like total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), and triglycerides (TG), which are recognized risk factors for cardiovascular disease.[10]
The fixed-dose combination, oral tablet, of elvitegravir/cobicistat/emtricitabine/tenofovir alafenamide (E/C/F/TAF) for HIV treatment was first approved by the US Food and Drug Administration in 2015[11] and then officially introduced in China for the first time in 2018.[3] E/C/F/TAF is a single-tablet regimen that contains elvitegravir (150 mg)/cobicistat (150 mg)/emtricitabine (200 mg)/tenofovir alafenamide (10 mg). The coformulated E/C/F/TAF is effective in treatment-naive and virologic suppressed individuals,[12–14] along with a favorable safety profile. Furthermore, the Chinese Guidelines for HIV/AIDS Diagnosis and Treatment (2018) recommends E/C/F/TAF as the first-line initial ART regimen for people living with HIV (PLWH).[3] In addition, E/C/F/TAF (once daily dosing) is more convenient for PLWH when compared with LPV/r (twice daily) and currently more affordable as it was the only reimbursement single-tablet HIV drug covered by the Chinese government since January 2020. However, the reimbursement policy varies widely between different regions. The low-cost single-tablet regimen E/C/F/TAF is reasonably selected as an alternative option for PLWH who do not tolerate EFV. Therefore, this study aimed to assess CNS effects and efficacy in Chinese HIV adults experiencing any grade two or higher neuropsychiatric and CNS toxicity who switched from an EFV-based regimen to E/C/F/TAF.
Methods
Ethical approval
The study was conducted in accordance with the Declaration of Helsinki. This study was reviewed and approved by the Tianjin Second People's Hospital Institutional Review Board (No. 2020-03). Written informed consent was provided by each of the recruited participants.
Participants and study design
This observational study was conducted prospectively at Tianjin Second People's Hospital, a tertiary-care teaching hospital in Tianjin (China) from January 2020 to June 2021. The inclusion criteria were as listed: (1) age range of 18 to 65 years; (2) HIV infection confirmed by western blotting; (3) on TDF/3TC/EFV regimen for at least 12 months before enrollment; (4) undetectable HIV viral load for at least 6 months; (5) symptomatic CNS-related toxicity associated with EFV at least grade two using the Division of AIDS (DAIDS) criteria[15]; and (6) consent switching to E/C/F/TAF. On the contrary, the exclusion criteria were as follows: (1) history of CNS opportunistic infections; (2) active CNS infections; (3) history of chronic neurological disorders, such as multiple sclerosis, epilepsy, and structural brain lesions; (4) severe psychiatric disorders (excluding anxiety and depression); (5) substance abuse and/or heavy alcohol use (>12 oz/week); (6) co-infections with hepatitis B and/or hepatitis C; and (7) pregnant and lactating women.
On day one, eligible individuals were switched from TDF/3TC/EFV to E/C/F/TAF (Genvoya®) for the duration of the study period (48 weeks). Participants were followed up at weeks 12, 24, and 48 after switch.
Study assessments
The laboratory assessments were performed at each follow-up visit. Laboratory tests included CD4 count, plasma HIV RNA, biochemical blood index (hepatic and renal profiles, e.g., alanine aminotransferase, creatinine), fasting lipid parameters (TC, LDL-C, high-density lipoprotein cholesterol [HDL-C], and TG), and complete blood count. Adverse events were monitored at each visit.
Neuropsychiatric symptoms and outcome measures
The neuropsychiatric toxicity symptoms were measured using a CNS side effects questionnaire on the EFV label and graded according to the DAIDS grading scale[15] (version 2.1). Study subjects were questioned by a doctor at each visit about any objective neuropsychiatric adverse events. The survey questionnaires were administered and scored following a standardized test procedure. The questionnaire included ten sections ascertaining symptoms as follows: abnormal dreams, aggression, anxiety, confusion, depression, dizziness, headache, impaired concentration, insomnia, and somnolence.[16–18] Each symptom score was calculated as follows: none (0), mild (1), moderate (2), or severe (3).[17] The total CNS score was also summed for calculating all grades of the neuropsychiatric adverse events. A minimum possible score is 0 (none), and a maximum possible score is 30 (severe).[17]
Anxiety and depression symptoms were assessed using the Hospital Anxiety and Depression Scale (HADS), which includes the HADS Anxiety (HADS-A) subscale and HADS Depression (HADS-D) subscale.[19] Sleep quality and disturbances were measured using the Pittsburgh Sleep Quality Index (PSQI).[20]
The primary outcome measure was the proportion of participants experiencing grade two or higher CNS toxicity after EFV switch off at weeks 12, 24, and 48. The change in neuropsychiatric and CNS toxicity parameters within the follow-up time point was also assessed after switching from EFV to E/C/F/TAF.
Secondary endpoints are the proportion of participants with HIV RNA <50 copies/mL, the change from baseline in CD4 cell count, and the effects on fasting lipids (TC, LDL-C, HDL-C, and TG) which were assessed at week 48 after switch.
Statistical analysis
Categorical variables were reported as frequencies (%); continuous variables were presented as median (interquartile range [IQR]) or mean (±standard deviation) as appropriate. At the follow-up time points, changes from the baseline for the total CNS toxicity score were tested using the Wilcoxon signed-rank test. Besides, changes from the baseline to weeks 12, 24, and 48 for the proportion of participants with central CNS toxicities grade two or higher were tested using the McNemar test. Median changes from the baseline in fasting lipid profiles (TC, LDL-C, HDL-C, TG, and TC:HDL-C) and patient self-reported outcome scores (PSQI, HADS-A, HADS-D) were analyzed using the Wilcoxon signed-rank test. A two-tailed P value <0.05 was considered statistically significant. All statistical analyses were performed using SAS, version 9.4, software (SAS Institute, Cary, NC, USA).
Results
Participants
A total of 196 participants were enrolled and completed the study. The median age of the study population was 37.5 (IQR: 30.3–50.8) years, and 96.9% (190/196) were males. At baseline, the median CD4 count was 629 cells/μL (IQR: 450–859 cells/μL), and all subjects had an estimated HIV RNA <50 copies/mL. At the study entry, the median duration on prior EFV-containing regimens was 3.7 (IQR: 1.7–5.6) years [Table 1].
Table 1.
Demographic and clinical characteristics at baseline.
| Parameters | Value |
| Number of participants | 196 |
| Age (years) | 37.5 (30.3–50.8) |
| Male gender | 190 (96.9) |
| Years on EFV | 3.7 (1.7–5.6) |
| CD4 cell count (cells/μL) | 629 (450–859) |
| Plasma HIV RNA < 50 copies/mL | 196 (100) |
Data expressed as median (interquartile range) or number (percentage). EFV: Efavirenz; HIV: Human immunodeficiency virus.
Change in central nervous symptoms and patient self-reported outcomes for anxiety, depression, and sleep disturbance
Rates of CNS symptoms at baseline and weeks 12, 24, and 48 after switch are shown in Table 2. At baseline, all subjects had at least one grade two or higher CNS symptom, including abnormal dreams (65.8%), insomnia (55.1%), and anxiety (47.5%). The median CNS toxicity score at baseline was estimated at 13 (IQR: 11–15). Compared to the baseline CNS toxicity score, the median CNS toxicity score at week 12 (which became 10 [IQR: 8–12]) significantly decreased (compared with baseline, P < 0.001) and remained stable at weeks 24 (9 [IQR: 7–12], P < 0.001) and 48 (9 [IQR: 7–11], P < 0.001).
Table 2.
Proportion of participants with CNS toxicities grade 2 or higher at baseline and weeks 12, 24 and 48.
| Baseline to week 12 | Baseline to week 24 | Baseline to week 48 | |||||
| Variable | Baseline | Week 12 | P value | Week 24 | P value | Week 48 | P value |
| Total CNS toxicity score | 13 (11–15) | 10 (8–12) | <0.001 | 9 (7–12) | <0.001 | 9 (7–11) | <0.001 |
| Abnormal dreams | 129 (65.8) | 61 (31.1) | <0.001 | 57 (29.1) | <0.001 | 53 (27.0) | <0.001 |
| Aggression | 37 (18.9) | 42 (21.3) | 0.492 | 38 (19.4) | 0.869 | 29 (14.8) | 0.276 |
| Anxiety | 93 (47.5) | 78 (39.8) | <0.001 | 67 (34.4) | 0.006 | 81 (41.3) | 0.040 |
| Confusion | 30 (15.3) | 23 (11.7) | 0.127 | 26 (13.3) | 0.433 | 19 (9.7) | 0.012 |
| Depression | 87 (44.4) | 82 (41.8) | 0.398 | 76 (38.8) | 0.101 | 85 (43.4) | 0.763 |
| Dizziness | 79 (40.3) | 35 (17.9) | <0.001 | 28 (14.3) | <0.001 | 33 (16.8) | <0.001 |
| Headache | 65 (33.2) | 41 (20.9) | 0.001 | 35 (17.9) | <0.001 | 36 (18.4) | 0.001 |
| Impaired concentration | 71 (36.2) | 58 (29.6) | 0.085 | 56 (28.6) | 0.051 | 61 (31.1) | 0.114 |
| Insomnia | 108 (55.1) | 65 (33.2) | <0.001 | 56 (28.6) | <0.001 | 63 (32.1) | <0.001 |
| Somnolence | 77 (39.3) | 62 (31.6) | 0.096 | 79 (40.3) | 0.825 | 74 (37.8) | 0.742 |
Data are expressed as median (interquartile range) or number (percentage). CNS: Central nervous system.
Concerning each of the specific grade two or higher CNS symptoms, at week 12, the proportion of patients experiencing abnormal dreams, headache, dizziness, insomnia, and anxiety has significantly decreased (compared to baseline, P < 0.001) by 34.7%, 12.3%, 22.4%, 21.9%, and 7.7%, respectively [Table 2]. The trend remained similar at weeks 24 and 48. On the contrary, at weeks 12, 24, and 48, there were no significant changes in the proportion of individuals experiencing CNS symptoms such as aggression, depression, impaired concentration, and somnolence (compared to baseline, P > 0.050) [Table 2].
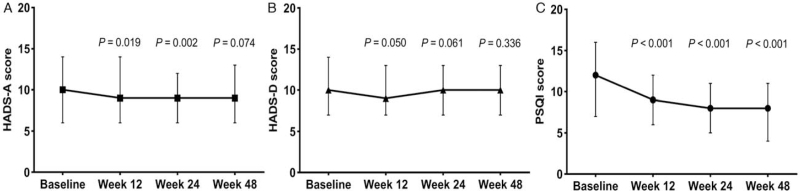
Furthermore, PSQI and HADS-A scores steadily and significantly decreased at weeks 12 and 24 compared with baseline levels (all P ≤ 0.050). At week 48, we noted that the PSQI score remained significantly different compared with the baseline level, while the HADS-A score was not [Figure 1A and 1C]. We did not observe significant changes with depression scores from baseline to weeks 12, 24, or 48 [Figure 1B].
Figure 1.
The trend of changes in patient self-reported outcomes for anxiety, depression, and sleep quality at different time points. The participants’ level of anxiety (A) and quality of sleep (C) have been improving over the time as the reported index scores were decreasing. On the contrary, the level of depression (B) remained stable. Point estimates show the median; error bars are IQR changes using the Wilcoxon signed-rank test. All P-values were obtained when comparing each median obtained at 12, 24, and 48 weeks to the one from the baseline. HADS: Hospital Anxiety and Depression Scale; HADS-A: Hospital Anxiety and Depression Scale-Anxiety subscale; HADS-D: Hospital Anxiety and Depression Scale-Depression subscale; IQR: Interquartile range; PSQI: Pittsburgh Sleep Quality Index.
Efficacy
At week 48 after switch, 100% of participants in the study maintained virologic suppression (HIV RNA <50 copies/mL). Not a single virologic failure was observed during the follow-up period. Median CD4 counts did not show any statistical change at week 48 after switch (P > 0.1).
Safety
During this study, no grade 2 to 4 adverse reactions were reported. Median values for hematologic and biochemical parameters were generally within normal reference ranges (data not shown).
Lipid profiles
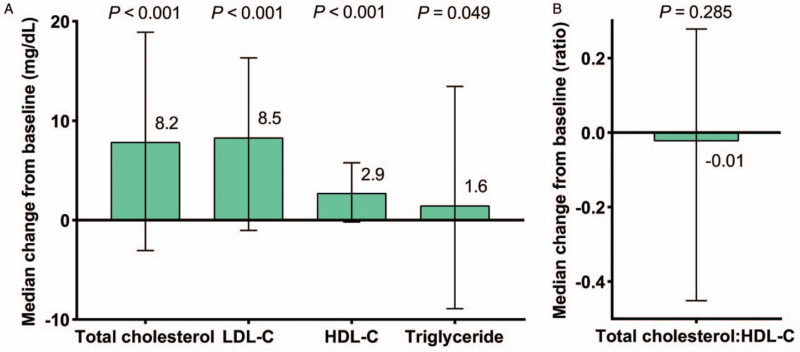
Increases from before switch to 48 weeks after switch were observed in median TC (difference, 8.2 mg/dL; 95% confidence interval [CI], 5.0–9.9; P < 0.001), LDL-C (difference, 8.5 mg/dL; 95% CI, 7.0–10.1; P < 0.001), HDL-C (difference, 2.9 mg/dL; 95% CI, 2.3–3.4; P < 0.001), and TG (difference, 1.6 mg/dL; 95% CI, −0.5 to 3.8; P = 0.049), and the changes were all statistically significant [Figure 2A]. At week 48, no significant difference was found from the baseline in median TC:HDL-C ratio (difference, −0.01; 95% CI, −0.12 to 0.08; P = 0.285) [Figure 2B].
Figure 2.
Fasting lipids changes from baseline to week 48. Error bars represent IQR. HDL-C: High-density lipoprotein cholesterol; IQR: Interquartile range; LDL-C: Low-density lipoprotein cholesterol.
Discussion
This study suggests that switching from EFV-based regimens to E/C/F/TAF, in virologically suppressed PLWH, significantly reduces the CNS symptoms. The most remarkable improvement was observed in anxiety, dizziness, and sleep-associated symptoms when looking at individual CNS symptoms. EFV is still widely used in many resource-limited settings as a first-line ART regimen.[21] Currently, in China, the EFV-based regimen is freely provided by the government to most PLWH through the NFATP. Only a fraction of individuals can afford newer generation ART drugs. Neuropsychiatric side effects in PLWH taking EFV are clinically meaningful, can potentially impact adherence to ART medications, the quality of life, and may weaken their immune system.[21] These results may provide valuable suggestions for recommendations regarding appropriate regimens for PLWH who had suffered chronic CNS side effects from EFV in China.
Several studies have reported significant improvement in PLWH experiencing ongoing CNS symptoms when switching from EFV-containing regimens to alternative ART regimens; the mean duration of previous EFV-based treatment was up to 2 years.[5,18,22] Most EFV-induced CNS adverse events appear early and usually disappear or diminish within the first month of treatment.[4,5] However, in a significant portion of cases (up to 40% in some studies), effects such as anxiety, depression, abnormal dreams, and sleep difficulties persist for much more extended periods, even months or years after exposure to EFV.[4,23] The underlying mechanisms responsible for long-term EFV-associated symptoms remain unclear. Several in vitro studies reported that short-term therapy with clinically relevant concentrations of EFV impair cellular proliferation and/or cell viability;[4] however, short-term treatment and observation cannot reflect the actual situation over a long time in PLWH under chronic treatment. Other studies showed that EFV-induced neuronal mitochondrial inhibition and autophagy may lead to CNS toxicity-related neuropsychiatric adverse events.[24,25]
Our study found that severity of depression did not change significantly after EFV switch. This observation is supported by a recent observational cohort study (n = 270) involving participants on EFV-based regimen for >2 years, in which depression scores were not affected by EFV discontinuation.[26] In another study, 41 subjects who received an EFV-based regimen for 5 years (median) were switched to rilpivirine.[18] We did not report significant improvements in depression score after 24 weeks. In an early randomized study by Tiraboschi et al[27] switching from EFV-containing regimens to darunavir/ritonavir improved anxiety symptoms and sleep quality, but it does not show significant changes in depression after 48 weeks of follow-up. These findings are consistent with our study, indicating that treatment switch may not be effective in resolving all those neuropsychiatric disturbances. Therefore, we believe that further studies on the prevalence of depressive symptoms in PLWH treated with EFV-based regimens are needed.
The CNS toxicity scoring questionnaire has been similarly used in several recent studies.[16–18]Although there is clinical heterogeneity across the studies, the change of CNS scores showed significant improvement for the EFV switch-off participants. This scoring tool is self-reported, self-graded, and shows somewhat subjective symptoms such as dizziness, headache, and abnormal dreams. Researchers generally characterized the psychiatric events by asking participants to self-report mood swings instead of using scales, which are beneficial to identify anxiety, depression, and sleep quality and disturbances more exactly than self-reporting. Consequently, we also assessed the symptoms of anxiety/depression and sleep quality using the HADS and PSQI, respectively. The PSQI includes a self-rated 19-item questionnaire that evaluates sleep quality and disturbances and has already been used to evaluate neuropsychiatric adverse events in Chinese PLWH.[28] The HADS is the most commonly used clinical measure of anxiety and depression in psychiatric studies and has solid psychometric validity and reliability. Thus, in this study, we assessed anxiety, depression, and sleep quality using HADS and PSQI. We found that HADS-A and PSQI scores significantly decreased over time after switching to E/C/F/TAF. This finding was consistent with a previous EFV switching to rilpivirine study,[18] indicating that EFV switch off could improve participants’ long-term tolerance and CNS effect.
In our study, the elevation of lipid profiles such as LDL-C was observed to have significance. Previous studies have also shown significantly increased LDL-C and TG levels after switch to TAF, but the degree of elevation depended on the characteristics of the participants and the follow-up time.[29–31] One significant risk for dyslipidemia after switching to TAF was the elevation in baseline TG or LDL-C levels.[32] The risk population which needs lipid-lowering drugs after switching to TAF should be extensively studied in the future. The adaptation of long-term ART and longer life expectancy have raised concerns about metabolic complications such as lipid abnormality, which is related to cardiovascular risk. Routine surveillance and evaluation of metabolic parameters, monitoring of ART drug side effects, and behavioral interventions are required to optimize the prevention and management of metabolic disorders in PLWH.
This study has several limitations. First, cognitive function was not assessed in our study. The impact of EFV on cognitive function remains controversial, as several studies show a deterioration of cognitive profile related to EFV use,[5,33] while others do not present any improvement in cognitive performances after EFV withdrawal.[22,34] The evaluation of the effect of EFV switch off on neurocognitive disorder with a larger sample size and longer follow-up time should be performed in the future. Second, our open-label study design and the subjective measurement of the CNS toxicity grading/scoring questionnaire may introduce bias. In addition, participants’ baseline mental states were not evaluated in our study. A high rate of depression, anxiety, and sleep disturbance among treatment-naïve PLWH was previously reported in China[28]; chronic HIV infection can directly impair CNS functions.[5,21] However, beneficial effects regarding CNS toxicity were observed in our switching therapy, which indicates that the impaired CNS function in our study may probably be EFV related. Finally, the relatively small sample size, the absence of a control arm, and the fact that most subjects were male limit the generalizability of our results.
To conclude, we found that switching from EFV-based regimens to E/C/F/TAF was associated with improvements in CNS symptoms, maintenance of viral suppression, and a moderate worsening lipid profile. Although these findings support the use of E/C/F/TAF as an alternative ART medication for virologic suppressed PLWH with EFV-induced complaints, long-term follow-up is expected to further evaluate the benefit of the switch.
Funding
This study was funded by the 13th Five-year National Major Project for HIV/AIDS and Hepatitis B Control and Prevention and the Chinese Ministry of Science and Technology (No. 2017ZX10202102005004).
Conflicts of interest
None.
Footnotes
How to cite this article: Xia H, Huang XJ, Hu Y, Gao LY, Wu Y, Wu H, Yan ZF, Ma P. Switching from efavirenz to elvitegravir/cobicistat/emtricitabine/tenofovir alafenamide reduces central nervous system symptoms in people living with HIV. Chin Med J 2021;134:2850–2856. doi: 10.1097/CM9.0000000000001824
Huan Xia and Xiao-Jie Huang contributed equally to this work.
References
- 1.De Cock KM, Jaffe HW, Curran JW. Reflections on 40 years of AIDS. Emerg Infect Dis 2021; 27:1553–1560. doi: 10.3201/eid2706.210284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang FJ, Pan J, Yu L, Wen Y, Zhao Y. Current progress of China's free ART program. Cell Res 2005; 15:877–882. doi: 10.1038/sj.cr.7290362. [DOI] [PubMed] [Google Scholar]
- 3.Cao W, Hsieh E, Li T. Optimizing treatment for adults with HIV/AIDS in China: successes over two decades and remaining challenges. Curr HIV/AIDS Rep 2020; 17:26–34. doi: 10.1007/s11904-019-00478-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Apostolova N, Blas-Garcia A, Galindo MJ, Esplugues JV. Efavirenz: what is known about the cellular mechanisms responsible for its adverse effects. Eur J Pharmacol 2017; 812:163–173. doi: 10.1016/j.ejphar.2017.07.016. [DOI] [PubMed] [Google Scholar]
- 5.Apostolova N, Funes HA, Blas-Garcia A, Galindo MJ, Alvarez A, Esplugues JV. Efavirenz and the CNS: what we already know and questions that need to be answered. J Antimicrob Chemother 2015; 70:2693–2708. doi: 10.1093/jac/dkv183. [DOI] [PubMed] [Google Scholar]
- 6.Ma Q, Vaida F, Wong J, Sanders CA, Kao YT, Croteau D, et al. Long-term efavirenz use is associated with worse neurocognitive functioning in HIV-infected patients. J Neurovirol 2016; 22:170–178. doi: 10.1007/s13365-015-0382-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dai L, Liu A, Zhang H, Wu H, Zhang T, Su B, et al. Impact of lopinavir/ritonavir and efavirenz-based antiretroviral therapy on the lipid profile of Chinese HIV/AIDS treatment-naive patients in Beijing: a Retrospective Study. Curr HIV Res 2019; 17:324–334. doi: 10.2174/1570162X17666191025115508. [DOI] [PubMed] [Google Scholar]
- 8.Sun LQ, Liu JY, He Y, Zhou Y, Xu LM, Zhang LK, et al. Evolution of blood lipids and risk factors of dyslipidemia among people living with human immunodeficiency virus who had received first-line antiretroviral regimens for 3 years in Shenzhen. Chin Med J (Engl) 2020; 133:2808–2815. doi: 10.1097/CM9.0000000000001245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Muya E, Kamuhabwa A. Comparative assessment of the magnitude of hyperlipidemia in HIV-infected patients receiving lopinavir/r- and atazanavir/r-based antiretroviral drugs. J Int Assoc Provid AIDS Care 2019; 18:2325958219841908.doi: 10.1177/2325958219841908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feinstein MJ, Hsue PY, Benjamin LA, Bloomfield GS, Currier JS, Freiberg MS, et al. Characteristics, prevention, and management of cardiovascular disease in people living with HIV: a Scientific Statement from the American Heart Association. Circulation 2019; 140:e98–e124. doi: 10.1161/CIR.0000000000000695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Angione SA, Cherian SM, Ozdener AE. A review of the efficacy and safety of genvoya(R) (elvitegravir, cobicistat, emtricitabine, and tenofovir alafenamide) in the management of HIV-1 infection. J Pharm Pract 2018; 31:216–221. doi: 10.1177/0897190017710519. [DOI] [PubMed] [Google Scholar]
- 12.Sax PE, Wohl D, Yin MT, Post F, DeJesus E, Saag M, et al. Tenofovir alafenamide versus tenofovir disoproxil fumarate, coformulated with elvitegravir, cobicistat, and emtricitabine, for initial treatment of HIV-1 infection: two randomised, double-blind, phase 3, non-inferiority trials. Lancet 2015; 385:2606–2615. doi: 10.1016/S0140-6736(15)60616-X. [DOI] [PubMed] [Google Scholar]
- 13.Mills A, Arribas JR, Andrade-Villanueva J, DiPerri G, Van Lunzen J, Koenig E, et al. Switching from tenofovir disoproxil fumarate to tenofovir alafenamide in antiretroviral regimens for virologically suppressed adults with HIV-1 infection: a randomised, active-controlled, multicentre, open-label, phase 3, non-inferiority study. Lancet Infect Dis 2016; 16:43–52. doi: 10.1016/S1473-3099(15)00348-5. [DOI] [PubMed] [Google Scholar]
- 14.Ma R, Zhang Q, Zhang YS, Xu B, Tong ZW, Zhao CS, et al. Preoperative rapid suppression of viral load by elvitegravir/cobicistat/emtricitabine/tenofovir alafenamide regimen in human immunodeficiency virus-positive fracture patients significantly reduces postoperative complications. Chin Med J 2020; 133:2892–2893. doi: 10.1097/CM9.0000000000001185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.National Institutes of Health. National Institutes of Health Division of AIDS (DAIDS) Table for Grading the Severity of Adult and Pediatric Adverse Events. National Institutes of Health; 2017. Available at https://rsc.niaid.nih.gov/sites/default/files/daidsgradingcorrectedv21.pdf. [Last accessed on 2020 January 1]. [Google Scholar]
- 16.Nelson M, Winston A, Hill A, Mngqibisa R, Bassa A, Orkin C, et al. Efficacy, safety and central nervous system effects after switch from efavirenz/tenofovir/emtricitabine to doravirine/tenofovir/lamivudine. AIDS 2021; 35:759–767. doi: 10.1097/QAD.0000000000002804. [DOI] [PubMed] [Google Scholar]
- 17.Keegan MR, Winston A, Higgs C, Fuchs D, Boasso A, Nelson M. Tryptophan metabolism and its relationship with central nervous system toxicity in people living with HIV switching from efavirenz to dolutegravir. J Neurovirol 2019; 25:85–90. doi: 10.1007/s13365-018-0688-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vera JH, Bracchi M, Alagaratnam J, Lwanga J, Fox J, Winston A, et al. Improved central nervous system symptoms in people with HIV without objective neuropsychiatric complaints switching from efavirenz to rilpivirine containing cART. Brain Sci 2019; 9:195.doi: 10.3390/brainsci9080195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand 1983; 67:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 20.Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res 1989; 28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 21.Nosik M, Lavrov V, Svitich O. HIV infection and related mental disorders. Brain Sci 2021; 11:248.doi: 10.3390/brainsci11020248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Payne B, Chadwick TJ, Blamire A, Anderson KN, Parikh J, Qian J, et al. Does efavirenz replacement improve neurological function in treated HIV infection? HIV Med 2017; 18:690–695. doi: 10.1111/hiv.12503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dalwadi DA, Ozuna L, Harvey BH, Viljoen M, Schetz JA. Adverse neuropsychiatric events and recreational use of efavirenz and other HIV-1 antiretroviral drugs. Pharmacol Rev 2018; 70:684–711. doi: 10.1124/pr.117.013706. [DOI] [PubMed] [Google Scholar]
- 24.Funes HA, Apostolova N, Alegre F, Blas-Garcia A, Alvarez A, Marti-Cabrera M, et al. Neuronal bioenergetics and acute mitochondrial dysfunction: a clue to understanding the central nervous system side effects of efavirenz. J Infect Dis 2014; 210:1385–1395. doi: 10.1093/infdis/jiu273. [DOI] [PubMed] [Google Scholar]
- 25.Purnell PR, Fox HS. Efavirenz induces neuronal autophagy and mitochondrial alterations. J Pharmacol Exp Ther 2014; 351:250–258. doi: 10.1124/jpet.114.217869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li Y, Wang Z, Cheng Y, Becker JT, Martin E, Levine A, et al. Neuropsychological changes in efavirenz switch regimens. AIDS 2019; 33:1307–1314. doi: 10.1097/QAD.0000000000002206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tiraboschi J, Hamzah L, Teague A, Kulasegaram R, Post F, Jendruleck I, et al. Short communication: The impact of switching from atripla to darunavir/ritonavir monotherapy on neurocognition, quality of life, and sleep: results from a Randomized Controlled Study. AIDS Res Hum Retroviruses 2016; 32:1198–1201. doi: 10.1089/AID.2015.0263. [DOI] [PubMed] [Google Scholar]
- 28.Hua W, Wang S, Wang X, Shao Y, Wang Y, Ye J, et al. Neuropsychiatric adverse events during 12 months of treatment with efavirenz in treatment-naive HIV-infected patients in China: a Prospective Cohort Study. Front Psychiatry 2021; 12:579448.doi: 10.3389/fpsyt.2021.579448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schwarze-Zander C, Piduhn H, Boesecke C, Schlabe S, Stoffel-Wagner B, Wasmuth JC, et al. Switching tenofovir disoproxil fumarate to tenofovir alafenamide in a real life setting: what are the implications? HIV Med 2020; 21:378–385. doi: 10.1111/hiv.12840. [DOI] [PubMed] [Google Scholar]
- 30.Huhn GD, Shamblaw DJ, Baril JG, Hsue PY, Mills BL, Nguyen-Cleary T, et al. Atherosclerotic cardiovascular disease risk profile of tenofovir alafenamide versus tenofovir disoproxil fumarate. Open Forum Infect Dis 2020; 7:ofz472.doi: 10.1093/ofid/ofz472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Surial B, Mugglin C, Calmy A, Cavassini M, Gunthard HF, Stockle M, et al. Weight and metabolic changes after switching from tenofovir disoproxil fumarate to tenofovir alafenamide in people living with HIV: a Cohort Study. Ann Intern Med 2021; 174:758–767. doi: 10.7326/M20-4853. [DOI] [PubMed] [Google Scholar]
- 32.Lacey A, Savinelli S, Barco EA, Macken A, Cotter AG, Sheehan G, et al. Investigating the effect of antiretroviral switch to tenofovir alafenamide on lipid profiles in people living with HIV. AIDS 2020; 34:1161–1170. doi: 10.1097/QAD.0000000000002541. [DOI] [PubMed] [Google Scholar]
- 33.Hakkers CS, Hermans AM, van Maarseveen EM, Teunissen CE, Verberk IMW, Arends JE, et al. High efavirenz levels but not neurofilament light plasma levels are associated with poor neurocognitive functioning in asymptomatic HIV patients. J Neurovirol 2020; 26:572–580. doi: 10.1007/s13365-020-00860-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lapadula G, Bernasconi DP, Bai F, Foca E, Di Biagio A, Bonora S, et al. Switching from efavirenz to rilpivirine improves sleep quality and self-perceived cognition but has no impact on neurocognitive performances. AIDS 2020; 34:53–61. doi: 10.1097/QAD.0000000000002377. [DOI] [PubMed] [Google Scholar]