Abstract
Backgrounds:
Previous surveys have found that children with iron deficiency (ID) were likely to suffer from early childhood caries (ECC). We aimed to assess the scientific evidence about whether ID is intrinsically related to ECC.
Methods:
The medical subject headings (MeSH) terms and free words were searched on PubMed, Web of Science, Cochrane, China National Knowledge Infrastructure, Wanfang, and the Database for Chinese Technical Periodicals from March 2020 to September 2020. Two researchers independently screened the articles. Data extraction and cross-checking were performed for the studies that met the inclusion criteria. Meta-analysis was performed using the Cochrane Collaboration's Review Manager 5.3 software.
Results:
After excluding duplication and irrelevant literature, 12 case-control studies were included in the study. The meta-analysis demonstrated that children with ECC were more likely to have ID (odds ratio [OR] = 2.63, 95% confidence interval [CI]: [1.85, 3.73], P < 0.001). There was no statistically significant association found between the level of serum ferritin and ECC (weighted mean difference (WMD) = −5.80, 95% CI: [−11.97, 0.37], P = 0.07). Children with ECC were more likely to have iron-deficiency anemia (OR = 2.74, 95% CI: [2.41,3.11], P < 0.001). The hemoglobin (HGB) levels in the ECC group were significantly lower compared with that in the ECC-free group (WMD = −9.96, 95% CI: [−15.45, −4.46], P = 0.0004). The mean corpuscular volume (MCV) levels in the ECC group were significantly lower compared with that in the ECC-free group (WMD = −3.72, 95% CI: [−6.65, −0.79], P = 0.01).
Conclusions:
ID was more prevalent in children with ECC, and the markers of iron status in the ECC group, such as serum ferritin, HGB, and MCV, were relatively lower than the ECC-free group.
Keywords: Iron deficiency, Iron deficiency anemia, Early childhood caries
Introduction
Early childhood caries (ECC), affecting the primary tooth of preschool children, is one of the most common chronic diseases in childhood. Recently, clinical and epidemiological surveys have found that children with iron deficiency (ID) or iron deficiency anemia (IDA) were associated with dental caries in childhood.[1–5] Some studies have also shown that children with caries have lower serum ferritin, hemoglobin (HGB), and mean corpuscular volume (MCV) levels than caries-free children.[6–12] However, these studies were performed with limited sample size or only investigated the relationship between one or a few indexes of iron status and ECC. In addition, it was reported that ID was not significantly associated with the number of decayed and filled surfaces or decayed and filled teeth.[13] Therefore, we performed a systematic review and meta-analysis to investigate the association between ID and ECC.
Methods
The systematic review was registered in PROSPERO under protocol number CRD42020215611.
Literature search strategy
The two data researchers carried on article retrieval from March 2020 to September 2020, with the language of Chinese and English. The suitable searching words (medical suject headings [MeSH] terms and free words) were indexed in the electronic databases of PubMed, Web of Science, Cochrane, China National Knowledge Infrastructure (CNKI), Wanfang, and the Database for Chinese Technical Periodicals (VIP).
The MeSH terms used were “dental caries,” “preschool child,” “infant,” and “iron deficiency.” The following strategies were used to search in the PubMed: (“dental caries” [MeSH terms] OR (“dental” [all fields] AND “caries” [all fields]) OR “dental caries” [all fields]) AND ((“infant” [MeSH terms] OR “infant” [all fields]) OR ((“child” [MeSH terms] OR “child” [all field] AND “preschool” [all fields]) OR (“child” [MeSH terms] OR “child” [all fields] OR “children ” [all fields])) AND (“iron deficiency” [MeSH terms] OR (“anemia, iron deficiency” [all fields] AND “iron-deficiency anemia” [all fields]) OR “anemias, iron-deficiency” [all fields] OR “anemias, iron deficiency” [all fields]). The similar search method (MeSH terms and free words) was also applied in the other electronic databases. If additional data and articles were needed, we wrote e-mails to contact the corresponding authors. References of included studies were checked to find suitable articles.
Inclusion and exclusion criteria
Observational studies were included in this study if they met the following criteria: (1) the study investigated the relationship between ECC and ID or IDA; (2) the study population consisted of children < 6 years old or preschool children; (3) ID was defined in the studies or serum ferritin levels were <30 μg/L without inflammation; IDA was defined as two of the three abnormal blood tests determining serum ferritin, HGB, and MCV or defined in related articles;[14] and (4) the primary outcomes investigated between ECC and ECC-free groups were the number of ID or IDA. If the number of ID or IDA did not show in the studies, the corresponding indicators of iron status, such as serum ferritin, HGB, and MCV, were recorded with means with standard deviation (SD).
The exclusion criteria were: (1) no direct comparison between ECC and ECC-free groups or no mention of the proportion of ID or IDA in ECC and ECC-free groups; (2) incomplete data; and (3) reviews, letters, abstracts, and unpublished or inaccessible full-text articles.
Data extraction and quality assessment
The following information from the included literature was collected and summarized by the two independent researchers: first author, published year, country, age, gender, sample size of case and control group, number of ID or IDA kids in two groups, mean value of HGB, serum ferritin, and MCV in two groups. Disagreements were discussed with the senior authors and resolved by them. The Newcastle-Ottawa scale was used for the assessment of including articles’ quality. Studies with seven or more points were considered to have high methodological quality. Studies with four to six points were considered to have medium methodological quality. Studies under three points were considered to have low methodological quality. Two reviewers assessed all these data, and dissents were settled by discussion or consultation with a third author.
Statistical analysis
The meta-analysis was performed using Review Manager 5.3 (Cochrane Collaboration, Oxford, England) to analyze the correlation between ID and ECC. A random-effects model was used for meta-analysis to reduce the heterogeneity when the I2 statistic was >50% or P value <0.10. Sensitivity analyses were also adopted by eliminating articles one by one to determine the heterogeneity. The funnel plot was used to analyze whether there was publication bias.
Results
Study characteristics
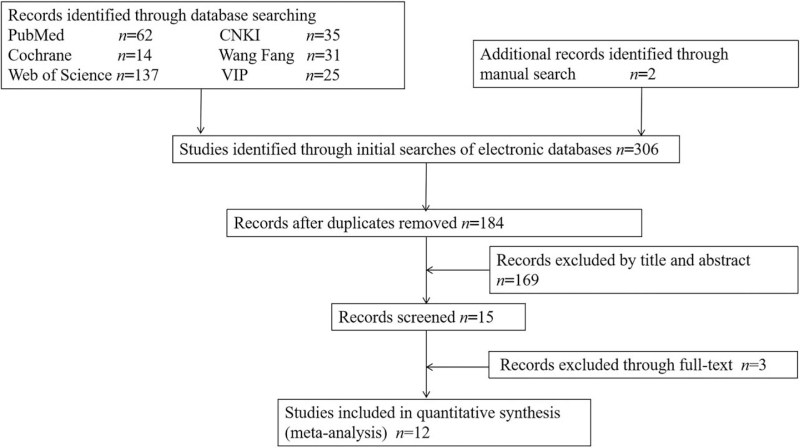
A total of 12 case-control studies published between 2002 and 2019 were included in this meta-analysis [Figure 1, Supplementary Table 1], including four Indian articles, four Chinese articles, one American article, one Canadian article, one Iran article, and one Egyptian article. Ten studies included 9807 children (5351 boys and 4456 girls), with additional 174 children in the two studies of Iranna-Koppal et al[8] and Jayakumar and Gurunathan[10] that did not record gender data. Almost all preschool children were aged <6 years except the study of Lü et al,[11] involving children from 1 to 7 years old. There were no apparent sex and age differences between ECC and ECC-free groups in the following ten articles: Bansal et al,[1] Abed et al,[2] Deane et al,[3] He and Wei,[4] Gao,[5] Sadeghi et al,[7] Guan et al,[9] Lü et al,[11] Shamsaddin et al,[12] and Ramos-Gomez et al.[13] The two studies, Iranna-Koppal et al[8] and Jayakumar and Gurunathan,[10] had not provided single-gender or age information between ECC and ECC-free groups. All included studies had excluded the other diseases that may influence the index of ID or IDA by questionnaire survey or medical records.
Figure 1.
Flowchart of studies identified, included, and excluded.
Risk of bias appraisal
Supplementary Table 2 shows the results of the quality appraisal of the articles. Only three studies, Bansal et al,[1] Abed et al,[2] and Deane et al,[3] received high-quality scores. Nine studies, He and Wei,[4] Gao,[5] Sadeghi et al,[7] Iranna-Koppal et al,[8] Guan et al,[9] Jayakumar and Gurunathan,[10] Lü et al,[11] Shamsaddin et al,[12] and Ramos-Gomez et al,[13] had medium-quality scores.
The proportion of ID between ECC and ECC-free groups
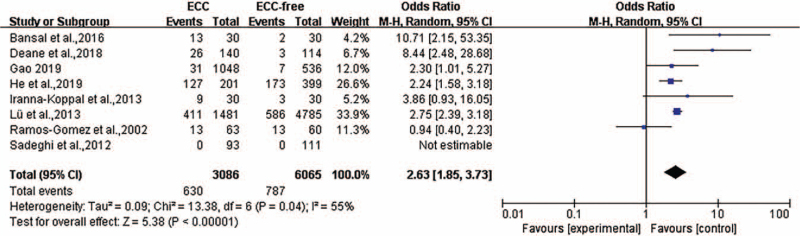
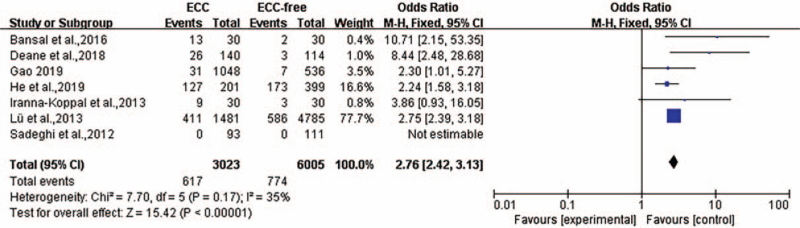
Eight articles described the relationship between ID and ECC, including one article about ID, five articles about IDA, and two articles about serum ferritin with <30 μg/L. The heterogeneity was P = 0.04, I2 = 55%, and a random-effects model was used for meta-analysis. The results of the meta-analysis suggested that children with ECC were more likely to have ID (odds ratio [OR] = 2.63, 95% confidence interval [CI]: [1.85, 3.73]; P < 0.001) [Figure 2]. After eliminating the studies of Ramos-Gomez et al,[13] the heterogeneity was P = 0.170, I2 = 35%, showing statistical significance (P < 0.001) [Figure 3].
Figure 2.
Forest plot shows the meta-analysis outcomes of the correlation between ID and ECC. CI: Confidence interval; ECC: Early childhood caries; ID: Iron deficiency; OR: Odds ratio.
Figure 3.
Forest plot shows the meta-analysis outcomes of the correlation between ID and ECC after sensitive analysis. CI: Confidence interval; ECC: Early childhood caries; ID: Iron deficiency; OR: Odds ratio.
Comparison of serum ferritin between ECC and ECC-free groups
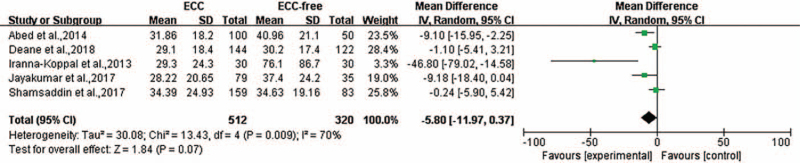
Five of the 12 studies reported the serum ferritin presented as mean with the SD. The heterogeneity was P = 0.009, I2 = 70%, and a random-effects model was used for meta-analysis. The level of heterogeneity could not be minimized <50% through sensitivity analysis. There was no statistically significant association found between the level of serum ferritin and ECC (weighted mean difference [WMD] = −5.80, 95% CI: [−11.97, 0.37], P = 0.07) [Figure 4].
Figure 4.
Forest plot shows the meta-analysis outcomes of the correlation between serum ferritin and ECC. CI: Confidence interval; ECC: Early childhood caries; SD: Standard deviation.
The proportion of IDA in ECC and ECC-free groups
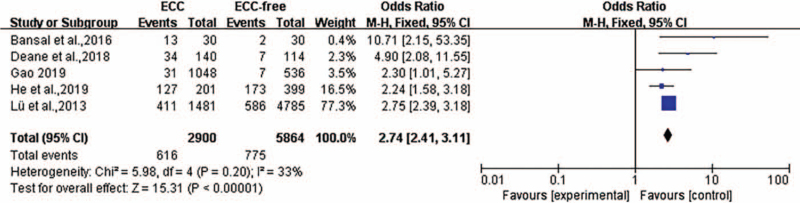
Five articles studied the correlation between IDA and ECC. The heterogeneity was P = 0.20, I2 = 33%, and a fixed-effect model was used for meta-analysis. The results of the meta-analysis suggested that children with ECC were more likely to have IDA (OR = 2.74, 95% CI: [2.41, 3.11], P < 0.001) [Figure 5].
Figure 5.
Forest plot shows the meta-analysis outcomes of the correlation between IDA and ECC. CI: Confidence interval; ECC: Early childhood caries; IDA: Iron deficiency anemia; OR: Odds ratio.
HGB comparison between ECC and ECC-free groups
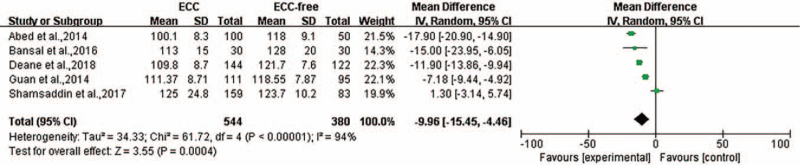
Five of the 12 studies reported the HGB level presented as the mean with the SD. The heterogeneity was P = 0.00001, I2 = 94%, and a random-effect model was used for meta-analysis. The level of heterogeneity could not be minimized <50% through sensitivity analysis. The HGB levels in the ECC group were significantly lower compared with that in the ECC-free group (WMD = −9.96, 95% CI: [−15.45, −4.46], P = 0.0004) [Figure 6].
Figure 6.
Forest plot shows the meta-analysis outcomes of the correlation between HGB and ECC. CI: Confidence interval; ECC: Early childhood caries; HGB: Hemoglobin; SD: Standard deviation.
MCV comparison between ECC and ECC-free groups
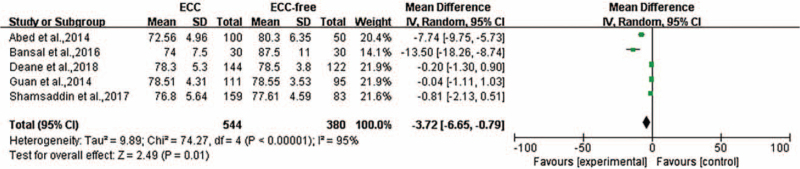
Five of the 12 studies reported the MCV level presented as the mean with the SD. The heterogeneity was P = 0.00001, I2 = 95%, and a random-effect model was used for meta-analysis. The level of heterogeneity could not be minimized <50% through sensitivity analysis. The MCV levels in the ECC group were significantly lower compared with that in the ECC-free group (WMD = −3.72, 95% CI: [−6.65, −0.79], P = 0.01) [Figure 7].
Figure 7.
Forest plot shows the meta-analysis outcomes of the correlation between MCV and ECC. CI: Confidence interval; ECC: Early childhood caries; MCV: Mean corpuscular volume; SD: Standard deviation.
Analysis of publication bias
There were too few included literatures about the relationship between ID and ECC, so funnel plots had no obvious significance.
Discussion
We analyzed 12 studies that used data of 9981 children to investigate the association between ID and ECC. The results of the meta-analysis suggested that children with ECC were more likely to have ID. The level of heterogeneity could be apparently reduced through sensitive analysis of eliminating the study of Ramos-Gomez et al[13]. That is probably because this study divided the children into three groups: ECC, incipient lesions, and ECC-free groups. Only ECC and ECC-free groups were included in our investigation.
Iron storage in vivo in the form of serum ferritin is gradually reduced during the period of ID. That may be the reason why the serum ferritin in the ECC group was also at lower levels compared with the ECC-free group. However, serum ferritin is an acute-phase reactant, with high levels present in an inflammatory state. The level of serum ferritin was not different between the ECC and ECC-free groups in the studies of Deane et al[3] and Shamsaddin et al[12], which may be the source of high heterogeneity in this analysis.
Serum iron level as the important indicator of iron levels in the body is dependent on hepcidin and is not related to iron storage.[15] Hepcidin is easily affected by inflammation, as a result, reducing the level of serum iron. The corresponding studies about the relationship between serum iron and ECC were so few that we do not collect and analyze the data.
We also found that children with ECC were more likely to have IDA. ID goes through three complex phases to become IDA: iron depletion, iron-deficient erythropoiesis, and IDA.[16] In the third phase, red blood cells showed small-cell changes, and serum ferritin, serum iron, HGB, and MCV levels were decreased.[17] Our results suggest that the HGB levels in the ECC group were significantly lower than those in the ECC-free group. The HGB difference between the two groups in the studies of Guan et al[9] and Shamsaddin et al[12] was relatively small compared with the other three studies. That may be the source of high heterogeneity in this analysis. There were two diagnostic criteria for IDA in this study, making the result not entirely convincing. This study showed that the level of MCV in the ECC group was significantly lower than that in the ECC-free group. This is probably due to MCV levels reduce in long-term ID or other kinds of anemia.[18] In another case, IDA would not occur until the iron of red blood cells or iron stores are fully exhausted, and the MCV level may be normal in the earlier stages of IDA.
Two common risk factors between ECC and ID were found in some studies: social-economic status and malnutrition.[19–21] IDA under the age of 5 years accounted for 41.7% worldwide,[22] mainly due to nutritional deficiency.[23] The limitations of economic conditions could bring many children to the edge of malnutrition. Eruption and replacement of primary teeth could be delayed in chronic malnutrition cases, which may increase the prevalence of ECC.[24] However, we cannot verify that assumption due to missing background data in the analyzed articles.
There are two assumptions to interpret the increased risk of ECC when there is ID. The first theory is that salivary gland function is often impaired in ID, influencing salivary secretion and buffering capacity vs. dental caries.[25] Second, ferric ions in blood decrease during ID.[26] Iron has anti-caries characteristics: iron ions can inhibit the activity of the virulence factor of Streptococcus mutans, and iron ions can supplement the minerals dissolved in the acidic environment by combining with calcium and phosphate ions.[27,28]
A mutual relationship between ID and ECC was found, which suggests that many children diagnosed with ECC may have inflammation and necrosis in the pulp of their primary teeth, and the agony and discomfort could change their chewing habits, resulting in decreases in meat intake and fruit frequency, affecting the intake and supplementation of iron.[29] This can lead to nutritional IDA. A study suggested that the treatment of dental caries would remove or relieve the status of IDA with iron remedy.[6] This adds to evidence that an association between ECC and IDA is associated with nutritional status. A closer connection between ID and ECC is found when children suffering from IDA. The inflammatory response accompanied by ECC can produce cell factors that could restrain the production of HGB and further reduce the iron storage levels.[30,31]
The present meta-analysis has the following limitations. First, the criteria of ID diagnosis are inconsistent across studies, and the severe stage of ID–IDA also does not have a consistent norm. Second, the sample sizes varied widely between the different studies (from 60 to 6266). In addition, the population of included article almost were yellow race, and the subgroup analysis would analyze specific indicators meaninglessly. Finally, the main study strategy that used the method from effect to cause testified that the ID was related to ECC, so it would be more credible to carry out a study about the caries experience between ID and ID-free groups.
Conclusions
ID was more prevalent in children with ECC, and the markers of iron status in the ECC group, such as serum ferritin, HGB, and MCV, were relatively lower than in the ECC-free group.
Funding
The work is supported by a grant from Qingdao People's Livelihood Science and Technology Plan Project Description (No.19-6-1-33-nsh).
Conflicts of interest
None.
Supplementary Material
Footnotes
How to cite this article: Ji SQ, Han R, Huang PP, Wang SY, Lin H, Ma L. Iron deficiency and early childhood caries: a systematic review and meta-analysis. Chin Med J 2021;134:2832–2837. doi: 10.1097/CM9.0000000000001729
Supplemental digital content is available for this article.
References
- 1.Bansal K, Goyal M, Dhingra R. Association of severe early childhood caries with iron deficiency anemia. J Indian Soc Pedod Prev Dent 2016; 34:36–42. doi: 10.4103/0970-4388.175508. [DOI] [PubMed] [Google Scholar]
- 2.Abed NT, Aly IA, Deyab SM, Ramoon FM. The relation between early dental caries and iron deficiency anaemia in children. Med Res J 2014; 13:108–114. doi: 10.1097/01.MJX.0000457299.68683.0b. [Google Scholar]
- 3.Deane S, Rodd C, Sharma A, Schroth R. Combined deficiencies of 25-hydroxyvitamin D and anemia in preschool children with severe early childhood caries: a case-control study. Paediatr Child Health 2018; 23:e40–e45. doi: 10.1093 /pch/pxx086.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.He J, Wei L. Investigation on physical development and health level of children with iron deficiency anemia aged 3-6 years in Ji’an. Med Theory Pract Chin 2018; 31:143–144. doi: CNKI:SUN:YXLL.0.2018-08-082. [Google Scholar]
- 5.Gao W. Correlation Between Iron Deficiency and Caries in Young Children. Qingdao, China: Qingdao University of China; 2019. [Google Scholar]
- 6.Shaoul R, Gaitini L, Kharouba J, Darawshi G, Maor I, Somri M. The association of childhood iron deficiency anaemia with severe dental caries. Acta Paediatr 2012; 101:e76–e79. doi: 10.1111/j.1651-2227.2011.02448.x. [DOI] [PubMed] [Google Scholar]
- 7.Sadeghi M, Darakhshan R, Bagherian A. Is there an association between early childhood caries and serum iron and serum ferritin levels? Dent Res J (Isfahan) 2012; 9:294–298. doi: 10.4103/1735-3327.99819. [PMC free article] [PubMed] [Google Scholar]
- 8.Iranna-Koppal P, Sakri MR, Akkareddy B, Hinduja DM, Gangolli RA, Patil BC. Iron deficiency in young children: a risk marker for early childhood caries. Int J Clin Pediatr Dent 2013; 6:1–6. doi: 10.5005/jp-journals-10005-1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guan YR, Wei YR, Liu Y. Correlation between iron deficiency anemia, vitamin D deficiency and caries in children. Maternal Child Health Care China 2015; 30:4986–4988. doi: 10.7620/zgfybj.j.issn.1001-4411.2015.29.24. [Google Scholar]
- 10.Jayakumar A, Gurunathan D. Estimation of ferritin levels in children with and without early childhood caries — a case-control study. J Adv Pharm Educ Res 2017; 7:15–17. doi: 10.5958/0976-5506.2018.01424.9. [Google Scholar]
- 11.Lü GX, Wu SY, Liang M, Zhang XZ, Yan Q. Research on caries and related factors among 1-7 years old children in Weibin District of Xinxiang City. Occup Health 2013; 14:1780–1781. doi:10.13329/j.cnki.zyyjk.2013.14.012. [Google Scholar]
- 12.Shamsaddin H, Jahanimoghadam F, Poureslami H, Haghdoost AA. The association between growth factors and blood factors with early childhood caries. J Oral Health Oral Epidemiol 2017; 6:196–202. [Google Scholar]
- 13.Ramos-Gomez FJ, Weintraub JA, Gansky SA, Hoover CI, Featherstone JD. Bacterial, behavioral and environmental factors associated with early childhood caries. J Clin Pediatr Dent 2002; 26:165–173. doi: 10.17796/jcpd.26.2.t6601j3618675326. [DOI] [PubMed] [Google Scholar]
- 14.Clarke M, Locker D, Berall G, Pencharz P, Kenny DJ, Judd P. Malnutrition in a population of young children with severe early childhood caries. Pediatr Dent 2006; 28:254–259. doi: 10.1063/1.3509332. [PubMed] [Google Scholar]
- 15.Restrepo-Gallego M, Díaz LE, Rondó PHC. Classic and emergent indicators for the assessment of human iron status. Crit Rev Food Sci Nutr 2020; 7:1–14. doi: 10.1080/10408398.2020.1787326. [DOI] [PubMed] [Google Scholar]
- 16.Domellöf M, Braegger C, Campoy C, Colomb V, Decsi T, Fewtrell M, et al. Iron requirements of infants and toddlers. J Pediatr Gastroenterol Nutr 2014; 58:119–129. doi: 10.1097/MPG.0000000000000206. [DOI] [PubMed] [Google Scholar]
- 17.Cappellini WMD, Musallam KM, Taher AT. Iron deficiency anaemia revisited. J Intern Med 2020; 287:153–170. doi: 10.1111/joim.13004. [DOI] [PubMed] [Google Scholar]
- 18.Lopez A, Cacoub P, Macdougall IC, Peyrin-Biroulet L. Iron deficiency anaemia. Lancet 2016; 387:907–916. doi: 10. 1016/S0140-6736 (15)60865-0. [DOI] [PubMed] [Google Scholar]
- 19.Seow WK. Early childhood caries. Pediatr Clin North Am 2018; 65:941–954. doi:10.1016/j.pcl.2018.05.004. [DOI] [PubMed] [Google Scholar]
- 20.Tsang C, Sokal-Gutierrez K, Patel P, Lewis B, Huang D, Ronsin K, et al. Early childhood oral health and nutrition in urban and rural Nepal. Int J Environ Res Public Health 2019; 16:2456.doi: 10.3390/ijerph16142456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tinanoff N, Baez R, Diaz Guillory C, Donly KJ, Feldens CA, McGrath C, et al. Early childhood caries epidemiology, aetiology, risk assessment, societal burden, management, education, and policy: global perspective. Int J Paediatr Dent 2019; 29:238–248. doi: 10.1111/ipd.12484. [DOI] [PubMed] [Google Scholar]
- 22.Pasricha SR, Tye-Din J, Muckenthaler MU, Swinkels DW. Iron deficiency. Lancet 2021; 397:233–248. doi: 10.1016/S0140-6736(20)32594-0. [DOI] [PubMed] [Google Scholar]
- 23.Juffrie M, Helmyati S, Hakimi M. Nutritional anemia in Indonesia children and adolescents: diagnostic reliability for appropriate management. Asia Pac J Clin Nutr 2020; 29: (Suppl 1): S18–S31. doi: 10.6133/apjcn.202012_29(S1).03. [DOI] [PubMed] [Google Scholar]
- 24.Alvarez JO, Lewis CA, Saman C, Caceda J, Montalvo J, Figueroa ML, et al. Chronic malnutrition, dental caries, and tooth exfoliation in Peruvian children aged 3-9 years. Am J Clin Nutr 1988; 48:368–372. doi: 10.1093/ajcn/48.2.368. [DOI] [PubMed] [Google Scholar]
- 25.Mahantesha T, Parveen Reddy KM, Kamavaram Ellore VP, Ramagoni NK, Iitagi V, Anitha KS. Evaluation and association of iron deficiency anemia with salivary pH and buffering capacity in children aged 6-12 years. Natl J Physiol Pharm Pharmacol 2014; 4:229–232. doi: 10.5455/njppp.2014.4.230420142. [Google Scholar]
- 26.Ozdemir N. Iron deficiency anemia from diagnosis to treatment in children. Turk Pediatri Ars 2015; 50:11–19. doi: 10.5152/tpa.2015.2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martinhon C, Italiani Fde M, Padilha Pde M, Bijella MF, Delbem AC, Buzalaf MA. Effect of iron on bovine enamel and on the composition of the dental biofilm formed “in situ”. Arch Oral Biol 2006; 51:471–475. doi: 10.1016/j.archoralbio.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 28.Ribeiro CC, Ccahuana-Vásquez RA, Carmo CD, Alves CM, Leitão TJ, Vidotti LR, et al. The effect of iron on Streptococcus mutans biofilm and on enamel demineralization. Braz Oral Res 2012; 26:300–305. doi: 10.1590 /s1806-83242012000400003. [DOI] [PubMed] [Google Scholar]
- 29.Schroth RJ, Levi J, Kliewer E, Friel J, Moffatt ME. Association between iron status, iron deficiency anaemia, and severe early childhood caries: a case-control study. BMC Pediatr 2013; 13:22.doi: 10.1186/1471-2431-13-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gaur S, Nayak R. Underweight in low socioeconomic status preschool children with severe early childhood caries. J Indian Soc Pedod Prev Dent 2011; 29:305–309. doi: 10.4103/0970-4388.86375. [DOI] [PubMed] [Google Scholar]
- 31.Tang RS, Huang MC, Huang ST. Relationship between dental caries status and anemia in children with severe early childhood caries. Kaohsiung J Med Sci 2013; 29:330–336. doi: 10.1016/j.kjms.2012.10.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.