Abstract
The Akt family of serine/threonine-directed kinases promotes cellular survival in part by phosphorylating and inhibiting death-inducing proteins. Here we describe a novel functional interaction between Akt and apoptosis signal-regulating kinase 1 (ASK1), a mitogen-activated protein kinase kinase kinase. Akt decreased ASK1 kinase activity stimulated by both oxidative stress and overexpression in 293 cells by phosphorylating a consensus Akt site at serine 83 of ASK1. Activation of the phosphoinositide 3-kinase (PI3-K)/Akt pathway also inhibited the serum deprivation-induced activity of endogenous ASK1 in L929 cells. An association between Akt and ASK1 was detected in cells by coimmunoprecipitation. Phosphorylation by Akt inhibited ASK1-mediated c-Jun N-terminal kinase and activating transcription factor 2 activities in intact cells. Finally, activation of the PI3-K/Akt pathway reduced apoptosis induced by ASK1 in a manner dependent on phosphorylation of serine 83 of ASK1. These results provide the first direct link between Akt and the family of stress-activated kinases.
The cellular decision to undergo apoptosis is determined by the integration of multiple survival and death signals. The Akt (protein kinase B) serine/threonine kinases are critical mediators of cell survival in response to growth factor stimulation and Ca2+ influx (16, 17, 49). A number of pro-apoptotic proteins have been identified as direct Akt substrates, including glycogen synthase kinase 3 (GSK-3), BAD, caspase-9, and Forkhead transcription factors, which are suppressed upon phosphorylation by Akt (5, 6, 10, 12, 14, 21, 28, 36).
The c-Jun N-terminal kinase (JNK) and p38 kinase pathways are two stress-activated mitogen-activated protein kinase modules stimulated by inflammatory cytokines, oxidative stress, and osmotic shock (13, 41). In several cell types, the stress-activated kinases are directly linked to apoptosis (42, 46, 48). Therefore, one mechanism of cell survival may be to inhibit the activity of the stress-activated kinase cascades. Specifically, increased Akt activity might lead to the suppression of the JNK or p38 pathways. In 293 cells, insulin growth factor-1 (IGF-1) has been shown to inhibit anisomycin and tumor necrosis factor α (TNF-α)-induced JNK activation, the former blocked by introduction of kinase-inactive Akt (34). Expression of a constitutively active Akt inhibited JNK activation upon interleukin-4 (IL-4) deprivation in TS1αβ cells (7). Moreover, in HeLa cells, Akt activity indirectly antagonized p38 activation through caspase inhibition (4). However, a direct connection between the Akt and JNK/p38 pathways has not yet been identified.
Among the stress-activated kinases, apoptosis signal-regulating kinase 1 (ASK1) represents a mitogen-activated protein kinase kinase kinase family member that acts upstream of JNK and p38 kinases (25, 45). ASK1 phosphorylates and activates mitogen-activated protein kinase kinase 4 (MKK4) or MKK7 and MKK3 or MKK6, which in turn induce JNK and p38 kinase activities, respectively (24, 25, 45). A variety of stress-related stimuli activate ASK1, including serum or trophic factor withdrawal, TNF-α, reactive oxygen species (ROS), microtubule-interfering agents, genotoxic stress, and possibly FasL (8, 9, 18, 25, 26, 39, 43). ASK1 plays a causal role in cell death induced by a number of these stimuli (8, 9, 25, 26). Furthermore, overexpression of wild-type or constitutively active ASK1 is sufficient to induce cell death through signals involving the mitochondrial cell death pathway in several cell types (8, 20, 25, 26, 47). How ASK1 levels and activity are regulated at a molecular level is not well understood.
In the present study, we demonstrate that ASK1 is a substrate for phosphorylation by Akt and that this phosphorylation is associated with a decrease in stimulated ASK1 kinase activity. This regulatory event has measurable consequences for ASK1 downstream signaling, including apoptosis induced by ASK1. Taken together, these results suggest that ASK1 may be a physiological target of Akt and raise the intriguing possibility that the ability of Akt to inhibit stress-activated kinases in specific cell contexts is a consequence of this interaction.
MATERIALS AND METHODS
Cell culture and transfections.
Human embryonic kidney 293, L929, MCF-7, and HeLa cells were cultured at 37°C in 5% CO2 in Dulbecco's modified Eagle medium (DMEM) containing 10% fetal bovine serum (FBS) and penicillin-streptomycin (GIBCO BRL). For transient transfection of 293 cells (except in the ATF-2 luciferase assay), cells were cultured in 60-mm-diameter dishes and transfected with the indicated plasmids using Fugene 6 (Roche) according to the manufacturer's instructions. For the luciferase assays, 293 cells plated in six-well plates were transfected with the indicated plasmids using the calcium phosphate method. HeLa cell transfections were carried out using Transfast (Promega). For all experiments, the total plasmid DNA amount was equalized by addition of vector pcDNA3.
Constructs, recombinant proteins, and antibodies.
pcDNA3-hemagglutinin (HA)-tagged wild-type and kinase-dead human ASK1 (ASK1-HA and ASK1KD-HA) have been described previously (39). The serine 83-to-alanine mutant of pcDNA3-ASK1-HA (ASK1S83A-HA), pCMV6-myc-tagged wild-type Akt (myc-Akt) and kinase-dead Akt (K179M) (myc-AktKD), EGFP-IRES-HA-Akt (E40K) (constitutively active Akt), pEBG-glutathione-S-transferase (GST)-tagged JNK3 (GST-JNK3), and pRC-lacZ were generated by standard PCR and cloning methods. The EGFP-IRES and pDsRed plasmids were purchased from CLONTECH. The plasmids encoding ATF-2 (amino acids [aa] 1 to 505) fused to the GAL4 DNA binding domain [GAL4-ATF-2 (WT) and GAL4-ATF-2 (T71A)] and the luciferase reporter plasmid driven by five tandem GAL4 DNA binding motifs have been previously described (19). A cDNA sequence encoding a 99-amino-acid segment of human ASK1 (aa 20 to 118) was subcloned distal to a GST sequence (pGEX-6P-1) by standard PCR and cloning procedures (GST-ASK1). A similar construct with a serine-to-alanine point mutation at the site corresponding to ASK1 serine 83 (GST-ASK1S83A) was also generated. Both pGEX-3X-GST-MKK6 (GST-MKK6) and pGEX-GST-kinase-dead p38 (GST-p38KD) have been described previously (23, 37). Bacterially expressed proteins were produced and isolated using standard GST fusion protein protocols (Pharmacia).
Anti-HA antibodies 3F10 and 12CA5 were purchased from Roche. Anti-myc antibody 9E10, anti-Akt C-20 and the corresponding blocking peptide, anti-ASK1 H-300, and anti-GST B-14 were purchased from Santa Cruz Biotechnology. The anti-phospho-Akt(S473) and anti-phospho-JNK antibodies were obtained from New England BioLabs. The anti-ASK1 DAV antibody has been described previously (39).
32P-orthophosphate labeling in cells.
Transfected 293 cells were washed twice and incubated in phosphate-free DMEM for 1 h and then exposed to 100 μCi of [32P]orthophosphate/ml for 2 h in the presence or absence of dialyzed FBS (GIBCO BRL). After treatments, cells were washed twice with ice-cold Tris-buffered saline (10 mM Tris [pH 8], 150 mM NaCl, 1 mM EDTA) and lysed in 1% NP-40 lysis buffer (with 20 mM Tris [pH 8], 200 mM NaCl, 10% glycerol, 1 mM EDTA, 12 mM β-glycerophosphate, 10 mM NaF, 1 mM sodium orthovanadate, 1 mM phenylmethylsulfonyl fluoride, and 1.5% aprotinin). After lysates were clarified, ASK1-HA was immunoprecipitated with anti-HA 12CA5 followed by protein A-Sepharose (Sigma). 32P incorporation into ASK1 was visualized after sodium dodecyl sulfate–8.5% polyacrylamide gel electrophoresis (SDS–8.5% PAGE) and polyvinylidene difluoride (Millipore) transfer by PhosphorImager analysis, and ASK1 protein was detected with anti-HA 3F10. Quantitative densitometric analysis was performed with ImageQuant (Molecular Dynamics).
In vitro phosphorylation.
GST-ASK1 or GST-ASK1S83A (2 μg) was incubated with [γ-32P]ATP (1 μCi; 3,000 Ci/mmol), ATP (5 μM), and 1 mM dithiothreitol (DTT) in a buffer containing 20 mM HEPES (pH 7.4), 10 mM MnCl2, and 10 mM MgCl2 and used as substrates for preactivated recombinant human Akt1 (1 μg; Upstate Biotechnology). [γ-32P]ATP incorporation was then assessed as for in vivo phosphorylation. Proteins were visualized by Coomassie blue staining.
Immunoprecipitation and immunoblotting.
For endogenous coimmunoprecipitations, treated cells were washed with ice-cold phosphate-buffered saline (PBS) and lysed in 0.2% NP-40 lysis buffer with protease and phosphatase inhibitors. Clarified lysates were precleared with protein G-agarose (Roche), and Akt was immunoprecipitated using anti-Akt C-20 (with or without preincubation with the Akt peptide) followed by protein G-agarose. Pellets were washed four times with 10 ml of the lysis buffer plus phosphatase inhibitors. After SDS–8% PAGE and polyvinylidene difluoride transfer, proteins were visualized by immunoblotting and enhanced chemiluminescence (Amersham). For 293 coimmunoprecipitation experiments, cells transfected for 36 h with constructs were washed with ice-cold PBS and lysed in 1% NP-40 lysis buffer with protease and phosphatase inhibitors. Clarified lysates were subjected to immunoprecipitation using the indicated antibodies followed by protein A-Sepharose. Pellets were washed seven times with 1.5 ml of the lysis buffer plus phosphatase inhibitors. The procedure to visualize proteins by immunoblotting was described above.
For phospho-JNK assays, 293 cells were transfected with the indicated constructs for 24 h, and after treatment, samples were processed largely as described above for 293 cell coimmunoprecipitation. To detect JNK activity, GST-JNK3 was immunoprecipitated with glutathione-Sepharose (Pharmacia) and subjected to immunoblotting with anti-phospho-JNK. Membranes were then stripped (10 min each in 0.1 M glycine [pH 2.5], 3.5 M MgCl2, and 1% SDS) and reprobed with anti-GST B-14.
In vitro kinase assay.
Cells were washed with ice-cold PBS and lysed in a buffer containing 20 mM Tris (pH 7.5), 12 mM β-glycerophosphate, 150 mM NaCl, 5 mM EGTA, 10 mM NaF, 1% Triton X-100, 0.5% deoxycholate, 1 mM DTT, 1 mM sodium orthovanadate, 1 mM phenylmethylsulfonyl difluoride, and 1.5% aprotinin (33). Clarified lysates were immunoprecipitated with anti-HA 12CA5 or anti-ASK1 DAV and then incubated with protein A-Sepharose. The beads were washed once with lysis buffer and twice with washing buffer containing 150 mM NaCl, 20 mM Tris (pH 7.5), 5 mM EGTA, and 1 mM DTT and subjected to kinase assays. GST-MKK6 (0.25 μg) was first incubated with the immune complex for 15 min at 30°C in a final volume of 20 μl (20 mM Tris [pH 7.5], 20 mM MgCl2, and 100 μM ATP). Afterwards, the complex was incubated with 0.6 μCi of [γ-32P]ATP and GST-p38KD (1.5 μg) in the same solution for 10 min at 25°C. Kinase reactions were terminated by adding Laemmli buffer, and proteins were resolved by SDS–8.5% PAGE. Phosphorylation of GST-p38KD was measured by PhosphorImager analysis, and the amount of ASK1 protein in the same sample was visualized by immunoblotting with anti-HA 3F10 or anti-ASK1 H-300. Both the extent of phosphorylation and the amount of protein were quantified by densitometric analysis with ImageQuant.
ATF-2 luciferase assay.
Either GAL4-ATF-2(WT) or a transactivation-incompetent mutant, GAL4-ATF-2(T71A), was cotransfected with a GAL4-driven luciferase plasmid and the indicated constructs in 293 cells. One day after transfection, cells were grown for an additional 24 h in DMEM plus 1% FBS, lysed, and assessed for luciferase activity by a luminometer. All values were first standardized to the total protein amount and then normalized to mutant GAL4-ATF-2(T71A) values to account for ATF-2-independent transactivation. Vector fold activity was calculated by normalizing these subsequent values to the vector alone (=1.0) in each experiment. To verify the validity of protein normalization, a lacZ plasmid was cotransfected with the above-described plasmids to standardize values to lacZ transfections instead of protein in separate experiments. These experiments yielded results similar to those obtained with protein normalization.
Cell death assays.
293 cells plated in six-well plates were cotransfected with the indicated constructs and pDsRed marker plasmid, washed twice with DMEM, and incubated in DMEM containing 10 ng of IGF-1/ml for 36 h. Cells were then suspended in Hank's balanced salt solution containing trypsin-EDTA and transferred to 96-well V-bottom plates. Terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling-fluorescein isothiocyanate (TUNEL-FITC) staining was performed according to the manufacturer's instructions (Roche). To assess the percentage of TUNEL-DsRed-double positive cells among DsRed-positive cells, 1,000 events were counted per condition by flow cytometry.
HeLa cells plated in 35-mm dishes were cotransfected with the indicated ASK1 and EGFP-IRES constructs. Cells were then serum starved and, following Hoechst 33342 staining (Molecular Probes), the percentage of green fluorescent protein (EGFP)-positive cells with fragmented and condensed nuclei was assessed by fluorescence microscopy.
RESULTS
ASK1 serine 83 is phosphorylated by Akt.
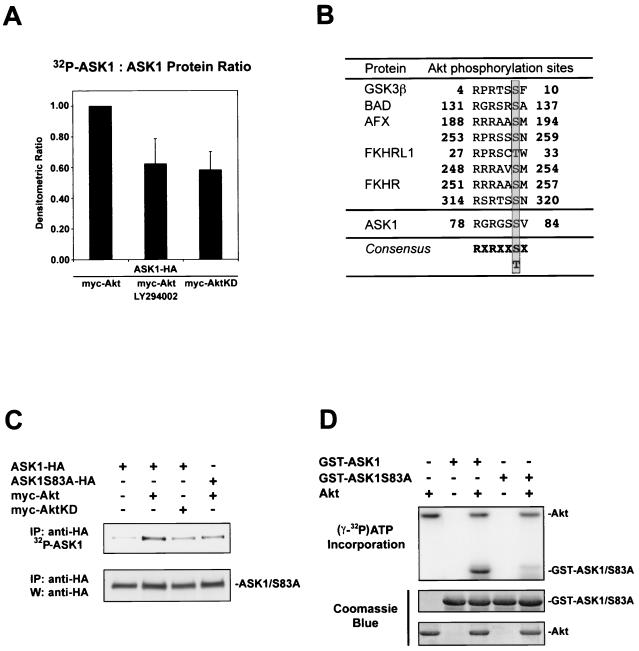
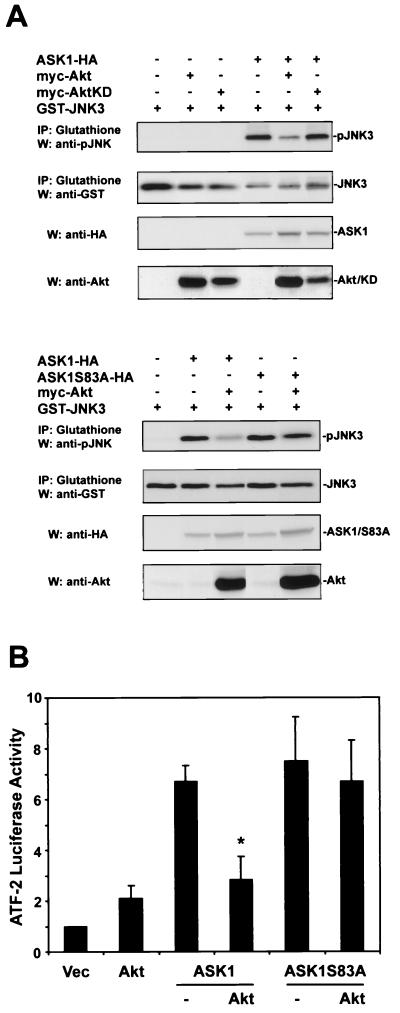
Given that serum, an activator of the P13-K/Akt pathway, inhibits ASK1 activity, we hypothesized that Akt activation might directly affect the phosphorylation status and function of ASK1 (39). To investigate whether ASK1 can act as an Akt substrate, we assessed the extent of [32P]orthophosphate labeling of ASK1 (ASK1-HA) in transfected 293 cells (Fig. 1A). In cells stimulated with serum, cotransfection of Akt (myc-Akt) and ASK1 induced a high level of ASK1 phosphorylation, which was decreased approximately 40% by either the phosphoinositide 3-kinase (PI3-K) inhibitor LY294002 or cotransfection of ASK1 with a kinase-dead Akt construct (myc-AktKD). This suggests that Akt phosphorylates ASK1 in cells.
FIG. 1.
Akt phosphorylates ASK1 in cells and in vitro. (A) ASK1 phosphorylation in cells stimulated by serum. 293 cells transiently transfected for 36 h with 1 μg each of the indicated constructs were deprived of phosphate and serum for 1 h, incubated with or without LY294002 (20 μM) for 1 h, and exposed to [32P]orthophosphate and dialyzed serum for 2 h. Immunoprecipitated ASK1 was assessed for 32P labeling (upper panel) and protein levels with anti-HA 3F10 (lower panel). The histogram shows mean densitometric ratios (32P-labeling/protein) plus standard errors of the mean (SEM) from three independent experiments. (B) A consensus Akt phosphorylation site is found in ASK1. Several known Akt substrates are shown. The phosphorylated serine/threonine site is highlighted. (C) Akt phosphorylates ASK1 on serine 83 in IGF-1-stimulated cells. Samples were processed as for panel A except that cells were stimulated for 10 min with 100 ng of IGF-1/ml after [32P]orthophosphate incubation. Dialyzed serum was not present during labeling. Data are representative of three independent experiments. IP, immunoprecipitation; W, Western blot. (D) In vitro phosphorylation of ASK1 by Akt. GST-ASK1 (aa 20 to 118) and GST-ASK1S83A were used as substrates for preactivated recombinant human Akt1 (Akt). [γ-32P]ATP incorporation (upper panel) and protein levels determined by Coomassie blue staining for substrates (middle panel) and Akt (lower panel) are shown. Data are representative of three independent experiments.
In searching for potential phosphorylation sites in ASK1, we noticed a consensus Akt phosphorylation site at serine 83 (Fig. 1B), which lies within a critical N-terminal regulatory domain (1, 8, 39). Conservation of this consensus motif (RXRXXS/TX) in the murine homolog of ASK1 suggested that regulation of this site may be important for ASK1 function. To test whether this consensus site on ASK1 plays a role in Akt-mediated phosphorylation, we mutated serine 83 to alanine (ASK1S83A-HA) and performed additional 32P labeling experiments on cells stimulated with IGF-1 (Fig. 1C). While a low level of 32P-labeled ASK1 was observed for cells transfected with ASK1 alone, introduction of wild-type Akt enhanced ASK1 32P incorporation, whereas introduction of a kinase-inactive Akt yielded a level of ASK1 phosphorylation comparable to that for ASK1-HA alone. Mutation of ASK1 serine 83 to alanine decreased Akt-dependent phosphorylation, suggesting that ASK1 serine 83 represents a relevant phosphorylation site.
To confirm that this phosphorylation in cells reflects a direct phosphorylation event by Akt, we conducted in vitro phosphorylation experiments. Recombinant Akt phosphorylated a GST fusion protein containing a 99-amino-acid fragment of human ASK1 (aa 20 to 118) (GST-ASK1), which contains the putative Akt phosphorylation site. Mutation of ASK1 serine 83 to alanine in this fusion protein (GST-ASK1S83A) eliminated this phosphorylation event by Akt (Fig. 1D). Taken together, the phosphorylation results suggest that Akt directly phosphorylates ASK1 on serine 83 in cells.
Akt and ASK1 interact in cells.
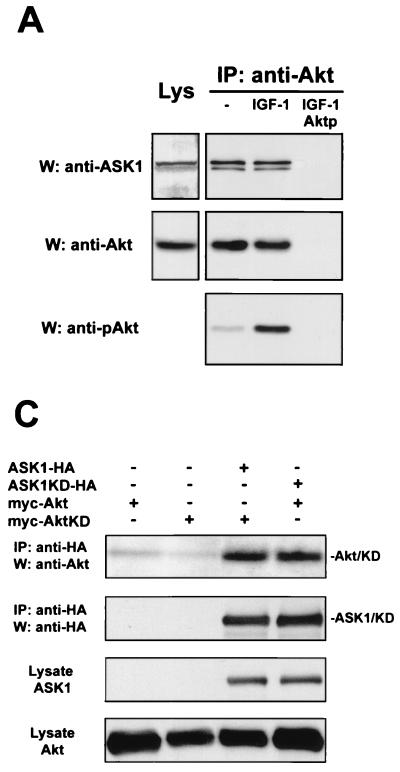
Other substrates of Akt, such as BAD, have been shown to associate with Akt (12). Indeed, an endogenous interaction could also be detected between ASK1 and Akt in L929 cells by coimmunoprecipitation (Fig. 2A). This interaction did not depend on Akt activity, since association occurred in cells with or without IGF-1 treatment. As a test for the specificity of this interaction, a peptide competing for binding to anti-Akt antibody eliminated immunoprecipitation of the complex. A similar interaction was observed in MCF-7 cells (data not shown). The endogenous interaction observed in cultured cells strengthens the possibility that these kinases are functionally associated.
FIG. 2.
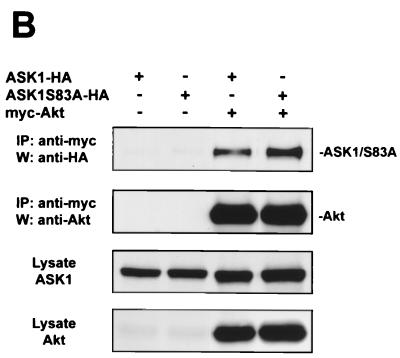
Akt interacts with ASK1 in cells. (A) Endogenous association of Akt and ASK1 in L929 cells. Cells were serum starved for 36 h and then treated with or without 100 ng of IGF-1/ml for 7 min. Endogenous Akt was immunoprecipitated (IP) from lysates with anti-Akt, and proteins were detected with anti-ASK1 H-300 and anti-Akt. To confirm that both active and inactive Akt were immunoprecipitated, membranes were stripped and reprobed with anti-phospho-Akt(S473) (anti-pAkt). As a positive control, crude lysate (left) was probed for ASK1 and Akt by immunoblotting. Aktp represents immunoprecipitation with anti-Akt antibodies preincubated with an Akt peptide. All data are representative of three independent experiments. W, Western blot. (B) Akt and ASK1 associate independently of ASK1 serine 83 phosphorylation in transfected 293 cells. Cells were transfected with 1 μg each of the indicated constructs for 36 h, and lysates were subjected to immunoprecipitation and immunoblotting with the indicated antibodies. (C) Akt and ASK1 binding occurs independently of kinase activity in transfected 293 cells. Samples were processed as for panel B.
We conducted further immunoprecipitation experiments in transfected 293 cells to characterize this interaction in more detail. We verified that transfected Akt and ASK1 interact in 293 cells by coimmunoprecipitation (Fig. 2B). Serine 83 phosphorylation of ASK1 did not appear to regulate this interaction, since ASK1S83A associated with Akt as well. Consistent with the constitutive nature of the endogenous interaction, mutation of the ATP-binding site to eliminate the kinase activity of either Akt or ASK1 did not disrupt binding (Fig. 2C). The physical association between Akt and ASK1 supports the hypothesis that in intact cells, Akt is positioned to utilize ASK1 as a substrate.
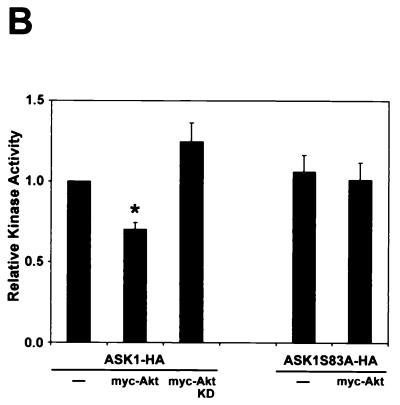
Akt phosphorylation of ASK1 decreases stimulated ASK1 kinase activity.
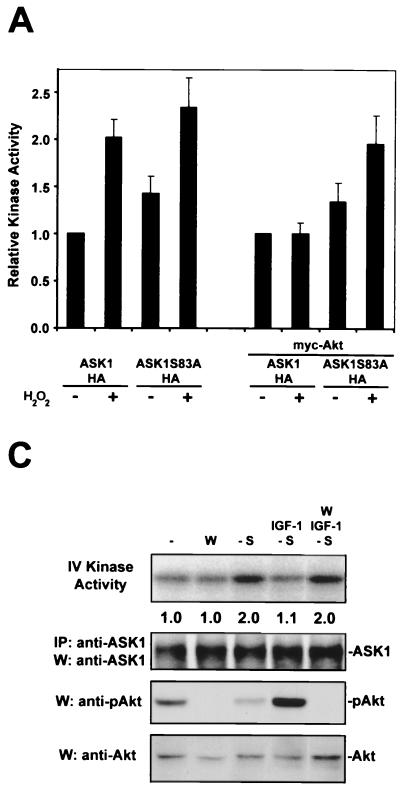
To assess the effects of Akt on ASK1 function, we treated 293 cells expressing a low level of ASK1 with H2O2 and measured ASK1 enzymatic activity by a coupled in vitro kinase assay. ROS are sufficient for ASK1 activation and have been implicated in TNF-α-mediated activation of ASK1 (18, 31, 39). ROS also activate Akt through a PI3-K-dependent mechanism (27, 44). Wild-type ASK1 was stimulated approximately twofold by H2O2 treatment, but introduction of Akt abolished H2O2-mediated activation of ASK1 (Fig. 3A). The activity of the mutant ASK1S83A could be induced to levels comparable to those for the wild type when stimulated with H2O2. However, unlike wild-type ASK1, this point mutant could still be stimulated by H2O2 when cotransfected with Akt. This suggests that ASK1 serine 83 represents a functionally relevant Akt phosphorylation site in cells.
FIG. 3.
Regulation of ASK1 kinase activity by Akt is dependent on ASK1 serine 83. (A) H2O2-stimulated ASK1 activity is inhibited by Akt. 293 cells were transfected with 0.25 μg of ASK1-HA or ASK1S83A-HA with or without 1 μg of myc-Akt for 20 h. Where indicated, cells were then exposed to 300 μM H2O2 for 10 min. ASK1-HA was immunoprecipitated with anti-HA and subjected to a coupled in vitro kinase assay. After samples were resolved by SDS-PAGE, kinase activity was visualized by PhosphorImager analysis and normalized to ASK1 protein levels in the same sample by anti-HA immunoblotting. Values from single transfections were normalized to ASK1-HA without H2O2 (=1.0), and those from double transfections were normalized to ASK1-HA and myc-Akt cotransfection without H2O2 (=1.0). The histogram shows the means plus the SEM from three to five independent experiments. (B) The activity of high ectopic ASK1 is diminished by Akt. 293 cells were transiently transfected with 1 μg each of the indicated constructs for 20 h. Cells were processed for the ASK1 in vitro kinase assay as for panel A. The histogram shows the means plus the SEM from five independent experiments. All values were calculated in reference to ASK1 transfection alone (=1.0). The asterisk indicates a significant difference from ASK1 at P < 0.01 by one-way analysis of variance (ANOVA) followed by the Bonferroni t test. (C) Activation of the P13-K/Akt pathway inhibits serum deprivation-induced ASK1 activity. L929 cells were deprived of serum for 20 min (-S) with or without 100 ng of IGF-1/ml. Where indicated, cells were treated with wortmannin (W)(200 nM) 10 min prior to and during serum deprivation. Wortmannin treatment alone was carried out in DMEM plus 10% FBS. Fold activation was determined in reference to control cells (=1.0). Immunoprecipitated (IP) ASK1 protein in the kinase assay samples was detected with anti-ASK1. To verify the effects of pharmacological treatments, Akt activity was detected with anti-phospho-Akt(S473) (anti-pAkt), and Akt protein was identified with anti-Akt. Data are representative of three independent experiments.
We confirmed these results in cells transfected with high doses of the ASK1 construct (Fig. 3B). As reported previously, high levels of ectopic ASK1 exhibited constitutive activity (31, 39). Introduction of wild-type Akt yielded a moderate 30% reduction in ASK1 kinase activity, whereas kinase-inactive Akt did not have a significant effect on ASK1 activity. On the other hand, ASK1S83A kinase activity was unaffected by wild-type Akt cotransfection. These results indicate that Akt likely decreases ASK1 kinase activity through phosphorylation of ASK1 serine 83.
To determine whether this phosphorylation mechanism occurs in a system endogenously expressing both ASK1 and Akt, we performed in vitro kinase assays for ASK1 using L929 cells. Serum deprivation for 20 min was sufficient to induce the activity of endogenous ASK1 approximately twofold in this cell type (Fig. 3C, lane 3). To test whether activation of the PI3-K/Akt pathway can inhibit ASK1 activity more specifically, we used IGF-1, a well-defined stimulus of the PI3-K/Akt pathway. IGF-1 exposure prevented the activation of ASK1 by serum deprivation, indicating that signaling pathways stimulated by this ligand inhibit ASK1 activity. Consistent with a role for Akt in IGF-1's inhibitory effect, the PI3-K inhibitor wortmannin reversed the ability of IGF-1 to block serum deprivation-induced ASK1 activity. Wortmannin in the presence of a high level of serum did not significantly stimulate ASK1 above control levels (Fig. 3C). These results suggest that ASK1 inhibition by Akt occurs in transfected cells as well as in an endogenous system.
Akt phosphorylation decreases ASK1 signaling to JNK.
ASK1 stimulates JNK by phosphorylating and activating MKK4 and MKK7, two JNK kinases. To test the effect of Akt phosphorylation upon ASK1-induced JNK activation, 293 cells were cotransfected with GST-tagged JNK3 and the indicated constructs, and JNK3 activity was evaluated (Fig. 4A). ASK1 strongly activated JNK3 in this assay. ASK1-mediated JNK activity was markedly suppressed by wild-type Akt, while kinase-inactive Akt had no effect on this JNK activity. ASK1S83A activated JNK3 to an extent comparable to that for wild-type ASK1. However, expression of Akt did not significantly inhibit ASK1S83A activity, indicating that Akt inhibition of ASK1, not signals downstream of ASK1, predominantly accounts for the decrease in JNK activation.
FIG. 4.
Akt diminishes ASK1-mediated JNK and ATF-2 activation. (A) Akt activity decreased ASK1-dependent JNK activity. 293 cells were transiently transfected for 24 h with 1 μg each of the indicated constructs, serum starved for 6 h, and treated with 100 ng of IGF-1/ml for 15 min. GST-JNK3 was immunoprecipitated (IP) from cell lysates and immunoblotted (W) with anti-phospho-JNK (anti-pJNK). After immunoblots were stripped, GST-JNK3 protein was identified with anti-GST. Crude lysates were probed with anti-HA to determine ASK1 protein levels and with anti-Akt to determine Akt protein levels. Data are representative of three independent experiments. (B) Akt decreased ASK1-induced ATF-2 activity. 293 cells were transiently transfected with ATF-2 luciferase plasmids and the indicated constructs for 24 h and then incubated in 1% FBS for 24 h. Cells were lysed and assayed for luciferase activity. The fold activation was determined in reference to the vector (pcDNA3) alone (=1.0). All bars represent means plus SEM from three to five independent experiments. The asterisk indicates a significant difference from ASK1 at P < 0.01 by one-way ANOVA followed by the Bonferroni t test.
Akt phosphorylation inhibits ASK1-induced ATF-2 activity.
To investigate whether Akt regulation of ASK1 influences downstream gene transcriptional events, we examined activating transcription factor 2 (ATF-2), a transcription factor activated by JNK and p38 kinases (Fig. 4B). An ATF-2 reporter assay was used to measure ATF-2 activity in response to transfected ASK1 or ASK1S83A in 293 cells. Although ASK1 and ASK1S83A stimulated ATF-2 activity to a comparable extent, coexpression of Akt inhibited wild-type ASK1's ability to activate ATF-2 by ∼60%, while ATF-2 activated by the ASK1S83A mutant was not affected by Akt cotransfection.
Activation of the PI3-K/Akt pathway suppresses ASK1-induced apoptosis.
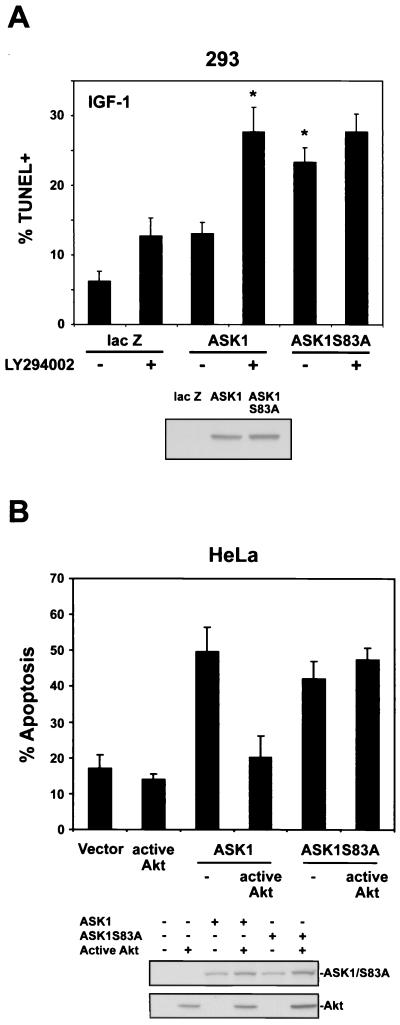
To determine whether cell death induced by ASK1 can be regulated by Akt, we examined the ability of ASK1 to induce apoptosis under conditions of growth factor-stimulated Akt activity (Fig. 5A). After transient transfection of ASK1 constructs with a red fluorescent protein plasmid (pDsRed), 293 cells were incubated with IGF-1 and then assessed for cell death by TUNEL and flow cytometry. In the presence of IGF-1, ASK1 induced a low level of apoptosis (13.1%), which was doubled by pretreatment with LY294002. However, with IGF-1 treatment, ASK1S83A induced a markedly higher degree of cell death (23.3%) than wild-type ASK1. This level of cell death was not significantly altered by LY294002, suggesting that ASK1S83A is not regulated by IGF-1-stimulated Akt activity.
FIG. 5.
Activation of the PI3-K/Akt pathway decreases ASK1-mediated apoptosis. (A) 293 cells were cotransfected with 1.75 μg of lacZ or HA-tagged ASK1 constructs with 0.1 μg of pDsRed as a marker for 24 h. Cells were incubated with or without LY294002 (10 μM) for 1 h, and then all cells were exposed to 10 ng of IGF-1/ml for 36 h. Cell death was assessed by TUNEL-FITC staining and flow cytometry for both TUNEL positivity and DsRed positivity to assay transfected cells only (upper panel). All bars represent means plus SEM from four to seven independent experiments. The asterisk indicates a significant difference from ASK1 at P < 0.05 by one-way ANOVA followed by the Bonferroni t test. In parallel, sister cultures were transfected with solutions identical to those used in cultures later assessed for cell death. Cells were lysed in 1% NP-40 buffer after 24 h, and lysates were probed for expression of ASK1 constructs with anti-HA (bottom panel). (B) HeLa cells were cotransfected with 0.75 μg of the indicated ASK1 constructs plus 0.25 μg of EGFP-IRES-HA-Akt (E40K) (active Akt) or EGFP-IRES (Vector) for 24 h and then serum starved for 24 h. Cells were labeled with Hoechst 33342, and EGFP-positive cells were scored for apoptosis by nuclear morphology. In each experiment, 100 EGFP-positive cells were counted per condition. The histogram represents means plus SEM from four independent experiments. As described for panel A, sister cultures were transfected with solutions identical to those used in cultures assessed for cell death. Cell lysates were probed for expression of ASK1 constructs and active Akt with anti-HA (bottom panel).
To test the ability of Akt to inhibit ASK1-mediated cell death in another cell context, we performed cell death assays with HeLa cells (Fig. 5B). We utilized a bicistronic expression plasmid encoding EGFP (EGFP-IRES) to identify transfected cells. HeLa cells were transfected with ASK1 constructs and either EGFP-IRES (vector) or EGFP-IRES-HA-Akt(E40K) (active Akt), a constitutively active Akt construct. Cells were then deprived of serum for 24 h, and EGFP-positive cells were assessed for apoptosis. ASK1 or ASK1S83A expression alone induced comparable levels of apoptosis. In agreement with the 293 cell results, ASK1-induced death was inhibited ∼60% by Akt cotransfection, whereas death induced by ASK1S83A was unaltered by Akt. Taken together, these results indicate that both IGF-1-induced Akt activity and expression of a constitutively active Akt can suppress ASK1-induced cell death.
DISCUSSION
Our results suggest that ASK1 activity and consequent activation of downstream signaling molecules can be negatively regulated by Akt stimulation. In support of this hypothesis, Akt phosphorylated ASK1 on serine 83, a site similar to the consensus sequence previously identified for other Akt substrates. An association between Akt and ASK1 in cells endogenously expressing these two proteins further suggests a functional link between these kinases. The interaction between these proteins is reminiscent of the Akt-BAD interaction observed in transfected cells (12). ASK1, therefore, can interact constitutively with Akt, but ASK1 phosphorylation is influenced by Akt activity. Collectively, the phosphorylation and coimmunoprecipitation results suggest that ASK1 is an Akt substrate.
Support for a physiological role for Akt-mediated ASK1 phosphorylation comes from several lines of evidence. First, Akt was able to suppress ASK1's response to oxidative stress. Akt, however, had little effect on the H2O2 responsiveness of the ASK1 point mutant, ASK1S83A, suggesting a specific phosphorylation event in Akt-mediated inhibition. Moreover, in cells endogenously expressing ASK1 and Akt, ASK1 activation by serum deprivation was inhibited by IGF-1 in a manner dependent on PI3-K/Akt activity. In confirmation of these findings, the kinase activity of highly expressed ASK1 was decreased by Akt coexpression, and this inhibition was also dependent on ASK1 serine 83. The moderate extent of ASK1 inhibition by Akt (30%) under this stimulus-independent condition perhaps reflects the decreased responsiveness of high ectopic ASK1 to physiological regulation. Indeed, in our experiments, the kinase activity of highly overexpressed ASK1 could not be stimulated further by H2O2 exposure (data not shown).
Modification of ASK1 function would be predicted to alter JNK- and p38-mediated gene transcriptional events as well as cell viability according to the cellular context. The JNK and p38 kinase cascades can initiate or modify transcription by activating several transcription factors, such as members of the AP-1 family and ATF-2 (30, 41). A constitutively active ASK1 has, for instance, been shown to induce differentiation through p38 activation in PC12 cells (40). Given Akt's inhibitory effects on ASK1-mediated JNK and ATF-2 activities, Akt regulation of ASK1 may therefore represent an additional role for Akt in transcriptional control. Akt was initially suggested to influence gene transcription because of its ability to translocate into the nucleus (2, 32). More recently, Akt has been demonstrated to activate transcription factors, such as NF-κB and CREB (15, 35, 38).
An alternative—but not mutually exclusive—role for Akt in regulating ASK1 function may be to suppress ASK1-induced cell killing. In our experiments, we found that ASK1 phosphorylation by the PI3-K/Akt pathway could reduce apoptosis induced by transfected ASK1 in 293 and HeLa cells. The cell death induced by ASK1 in certain contexts may, therefore, be ameliorated by simultaneous Akt activation. For instance, in superior cervical ganglion neurons, which die in an ASK1-dependent manner upon withdrawal of nerve growth factor, nerve growth factor-induced survival may in part reflect activation of Akt and subsequent inhibition of ASK1 (11, 26).
Akt suppression of ASK1 remains to be examined in the context of other ASK1 regulators. Among the negative regulators are thioredoxin and the cell cycle inhibitor, p21Cip1/WAF1 (3, 39). The adapter protein, 14-3-3, interacts with ASK1 and inhibits cell killing without altering ASK1 kinase activity (50). Certain TNF receptor-associated factor (TRAF) proteins, such as TRAFs 2, 5, and 6, and ROS can activate ASK1, at least in part, through ASK1 dimerization (18, 22, 31, 33). Both thioredoxin and TRAF2 have been shown to interact with the ASK1 N terminus, a region that contains the Akt consensus site. Akt phosphorylation of ASK1 may therefore alter the binding affinity of ASK1 for TRAF2 or thioredoxin. It is also conceivable that Akt phosphorylation of ASK1 cannot occur unless TRAF2 or thioredoxin dissociation from ASK1 occurs first. The relative impact of these and other ASK1 regulatory proteins on ASK1 function may well be cell context specific.
Other molecular targets of Akt may also be responsible for antagonizing the activity of the stress-activated kinase cascades in certain cell contexts. Rac1, a small G protein activator of the JNK pathway, has been reported to be phosphorylated by Akt, an event which leads to decreased Rac1 GTP-binding affinity (29). However, this effect was directly tested in vitro only, and the outcome of Rac1 phosphorylation on downstream targets in intact cells has not yet been determined.
Given the growing number of Akt substrates, a central question of Akt signaling is whether multiple substrates can be simultaneously phosphorylated by Akt in a given system. To our knowledge, no integrated investigation of the Akt effectors has been carried out in one system. In this vein, we have conducted preliminary coimmunoprecipitation experiments, which demonstrated that Akt immunoprecipitated from L929 cells associates with both Raf-1 and ASK1, suggesting that these Akt substrates are positioned to undergo phosphorylation at the same time (data not shown). Therefore, Akt may have the potential to deploy several of its reported downstream functions in parallel, perhaps acting as a central regulator in a multipathway signaling complex. Clearly, a more thorough characterization of the kinetics and subcellular localization of Akt and its different substrates, as well as more genetic evidence, will be required to assess the relative contribution of a given substrate to Akt function in vivo.
Our results suggest that one point of convergence between the Akt pathway and the stress-activated kinases is ASK1. The involvement of Akt in the inhibition of apoptosis regulatory protein kinases broadens the scope of Akt as a transcriptional modifier and mediator of cell survival decisions.
ACKNOWLEDGMENTS
We thank Hidenori Ichijo for the ASK1 constructs and anti-ASK1 DAV, Roger Davis for providing the GST-MKK6 construct, Jiahuai Han for the GST-p38KD construct, and Edward Skolnik for advice and reagents.
This work was supported by the National Institutes of Health (NS21072 and CA56490 to M.V.C.) and by DAMD17-99-1-9153 (to T.F.F.).
REFERENCES
- 1.Alessi D R, Caudwell F B, Andjelkovic M, Hemmings B A, Cohen P. Molecular basis for the substrate specificity of protein kinase B; comparison with MAPKAP kinase-1 and p70 S6 kinase. FEBS Lett. 1996;399:333–338. doi: 10.1016/s0014-5793(96)01370-1. [DOI] [PubMed] [Google Scholar]
- 2.Andjelkovic M, Alessi D R, Meier R, Fernandez A, Lamb N J, Frech M, Cron P, Cohen P, Lucocq J M, Hemmings B A. Role of translocation in the activation and function of protein kinase B. J Biol Chem. 1997;272:31515–31524. doi: 10.1074/jbc.272.50.31515. [DOI] [PubMed] [Google Scholar]
- 3.Asada M, Yamada T, Ichijo H, Delia D, Miyazono K, Fukumuro K, Mizutani S. Apoptosis inhibitory activity of cytoplasmic p21 (Cip1/WAF1) in monocytic differentiation. EMBO J. 1999;18:1223–1234. doi: 10.1093/emboj/18.5.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berra E, Diaz-Meco M T, Moscat J. The activation of p38 and apoptosis by the inhibition of Erk is antagonized by the phosphoinositide 3-kinase/Akt pathway. J Biol Chem. 1998;273:10792–10797. doi: 10.1074/jbc.273.17.10792. . (Erratum, 273:16630.) [DOI] [PubMed] [Google Scholar]
- 5.Brunet A, Bonni A, Zigmond M J, Lin M Z, Juo P, Hu L S, Anderson M J, Arden K C, Blenis J, Greenberg M E. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96:857–868. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- 6.Cardone M H, Roy N, Stennicke H R, Salvesen G S, Franke T F, Stanbridge E, Frisch S, Reed J C. Regulation of cell death protease caspase-9 by phosphorylation. Science. 1998;282:1318–1321. doi: 10.1126/science.282.5392.1318. [DOI] [PubMed] [Google Scholar]
- 7.Cerezo A, Martinez A C, Lanzarot D, Fischer S, Franke T F, Rebollo A. Role of Akt and c-Jun N-terminal kinase 2 in apoptosis induced by interleukin-4 deprivation. Mol Biol Cell. 1998;9:3107–3118. doi: 10.1091/mbc.9.11.3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang H Y, Nishitoh H, Yang X, Ichijo H, Baltimore D. Activation of apoptosis signal-regulating kinase 1 (ASK1) by the adapter protein Daxx. Science. 1998;281:1860–1863. doi: 10.1126/science.281.5384.1860. [DOI] [PubMed] [Google Scholar]
- 9.Chen Z, Seimiya H, Naito M, Mashima T, Kizaki A, Dan S, Imaizumi M, Ichijo H, Miyazono K, Tsuruo T. ASK1 mediates apoptotic cell death induced by genotoxic stress. Oncogene. 1999;18:173–180. doi: 10.1038/sj.onc.1202276. [DOI] [PubMed] [Google Scholar]
- 10.Cross D A, Alessi D R, Cohen P, Andjelkovich M, Hemmings B A. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature. 1995;378:785–789. doi: 10.1038/378785a0. [DOI] [PubMed] [Google Scholar]
- 11.Crowder R J, Freeman R S. Phosphatidylinositol 3-kinase and Akt protein kinase are necessary and sufficient for the survival of nerve growth factor-dependent sympathetic neurons. J Neurosci. 1998;18:2933–2943. doi: 10.1523/JNEUROSCI.18-08-02933.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Datta S R, Dudek H, Tao X, Masters S, Fu H, Gotoh Y, Greenberg M E. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell. 1997;91:231–241. doi: 10.1016/s0092-8674(00)80405-5. [DOI] [PubMed] [Google Scholar]
- 13.Davis R J. Signal transduction by the c-Jun N-terminal kinase. Biochem Soc Symp. 1999;64:1–12. doi: 10.1515/9781400865048.1. [DOI] [PubMed] [Google Scholar]
- 14.del Peso L, Gonzalez-Garcia M, Page C, Herrera R, Nunez G. Interleukin-3-induced phosphorylation of BAD through the protein kinase Akt. Science. 1997;278:687–689. doi: 10.1126/science.278.5338.687. [DOI] [PubMed] [Google Scholar]
- 15.Du K, Montminy M. CREB is a regulatory target for the protein kinase Akt/PKB. J Biol Chem. 1998;273:32377–32379. doi: 10.1074/jbc.273.49.32377. [DOI] [PubMed] [Google Scholar]
- 16.Dudek H, Datta S R, Franke T F, Birnbaum M J, Yao R, Cooper G M, Segal R A, Kaplan D R, Greenberg M E. Regulation of neuronal survival by the serine-threonine protein kinase Akt. Science. 1997;275:661–665. doi: 10.1126/science.275.5300.661. [DOI] [PubMed] [Google Scholar]
- 17.Franke T F, Kaplan D R, Cantley L C. PI3K: downstream AKTion blocks apoptosis. Cell. 1997;88:435–437. doi: 10.1016/s0092-8674(00)81883-8. [DOI] [PubMed] [Google Scholar]
- 18.Gotoh Y, Cooper J A. Reactive oxygen species- and dimerization-induced activation of apoptosis signal-regulating kinase 1 in tumor necrosis factor-alpha signal transduction. J Biol Chem. 1998;273:17477–17482. doi: 10.1074/jbc.273.28.17477. [DOI] [PubMed] [Google Scholar]
- 19.Gupta S, Campbell D, Derijard B, Davis R J. Transcription factor ATF2 regulation by the JNK signal transduction pathway. Science. 1995;267:389–393. doi: 10.1126/science.7824938. [DOI] [PubMed] [Google Scholar]
- 20.Hatai T, Matsuzawa A, Inoshita S, Mochida Y, Kuroda T, Sakamaki K, Kuida K, Yonehara S, Ichijo H, Takeda K. Execution of apoptosis signal-regulating kinase 1 (ASK1)-induced apoptosis by the mitochondria-dependent caspase activation. J Biol Chem. 2000;275:26576–26581. doi: 10.1074/jbc.M003412200. [DOI] [PubMed] [Google Scholar]
- 21.Hetman M, Cavanaugh J E, Kimelman D, Xia Z. Role of glycogen synthase kinase-3beta in neuronal apoptosis induced by trophic withdrawal. J Neurosci. 2000;20:2567–2574. doi: 10.1523/JNEUROSCI.20-07-02567.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoeflich K P, Yeh W C, Yao Z, Mak T W, Woodgett J R. Mediation of TNF receptor-associated factor effector functions by apoptosis signal-regulating kinase-1 (ASK1) Oncogene. 1999;18:5814–5820. doi: 10.1038/sj.onc.1202975. [DOI] [PubMed] [Google Scholar]
- 23.Huang S, Jiang Y, Li Z, Nishida E, Mathias P, Lin S, Ulevitch R J, Nemerow G R, Han J. Apoptosis signaling pathway in T cells is composed of ICE/Ced-3 family proteases and MAP kinase kinase 6b. Immunity. 1997;6:739–749. doi: 10.1016/s1074-7613(00)80449-5. [DOI] [PubMed] [Google Scholar]
- 24.Ichijo H. From receptors to stress-activated MAP kinases. Oncogene. 1999;18:6087–6093. doi: 10.1038/sj.onc.1203129. [DOI] [PubMed] [Google Scholar]
- 25.Ichijo H, Nishida E, Irie K, ten Dijke P, Saitoh M, Moriguchi T, Takagi M, Matsumoto K, Miyazono K, Gotoh Y. Induction of apoptosis by ASK1, a mammalian MAPKKK that activates SAPK/JNK and p38 signaling pathways. Science. 1997;275:90–94. doi: 10.1126/science.275.5296.90. [DOI] [PubMed] [Google Scholar]
- 26.Kanamoto T, Mota M, Takeda K, Rubin L L, Miyazono K, Ichijo H, Bazenet C E. Role of apoptosis signal-regulating kinase in regulation of the c-Jun N-terminal kinase pathway and apoptosis in sympathetic neurons. Mol Cell Biol. 2000;20:196–204. doi: 10.1128/mcb.20.1.196-204.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Konishi H, Fujiyoshi T, Fukui Y, Matsuzaki H, Yamamoto T, Ono Y, Andjelkovic M, Hemmings B A, Kikkawa U. Activation of protein kinase B induced by H(2)O(2) and heat shock through distinct mechanisms dependent and independent of phosphatidylinositol 3-kinase. J Biochem (Tokyo) 1999;126:1136–1143. doi: 10.1093/oxfordjournals.jbchem.a022559. [DOI] [PubMed] [Google Scholar]
- 28.Kops G J, de Ruiter N D, De Vries-Smits A M, Powell D R, Bos J L, Burgering B M. Direct control of the Forkhead transcription factor AFX by protein kinase B. Nature. 1999;398:630–634. doi: 10.1038/19328. [DOI] [PubMed] [Google Scholar]
- 29.Kwon T, Kwon D Y, Chun J, Kim J H, Kang S S. Akt protein kinase inhibits Rac1-GTP binding through phosphorylation at serine 71 of Rac1. J Biol Chem. 2000;275:423–428. doi: 10.1074/jbc.275.1.423. [DOI] [PubMed] [Google Scholar]
- 30.Kyriakis J M. Activation of the AP-1 transcription factor by inflammatory cytokines of the TNF family. Gene Expr. 1999;7:217–231. [PMC free article] [PubMed] [Google Scholar]
- 31.Liu H, Nishitoh H, Ichijo H, Kyriakis J M. Activation of apoptosis signal-regulating kinase 1 (ASK1) by tumor necrosis factor receptor-associated factor 2 requires prior dissociation of the ASK1 inhibitor thioredoxin. Mol Cell Biol. 2000;20:2198–2208. doi: 10.1128/mcb.20.6.2198-2208.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meier R, Alessi D R, Cron P, Andjelkovic M, Hemmings B A. Mitogenic activation, phosphorylation, and nuclear translocation of protein kinase Bbeta. J Biol Chem. 1997;272:30491–30497. doi: 10.1074/jbc.272.48.30491. [DOI] [PubMed] [Google Scholar]
- 33.Nishitoh H, Saitoh M, Mochida Y, Takeda K, Nakano H, Rothe M, Miyazono K, Ichijo H. ASK1 is essential for JNK/SAPK activation by TRAF2. Mol Cell. 1998;2:389–395. doi: 10.1016/s1097-2765(00)80283-x. [DOI] [PubMed] [Google Scholar]
- 34.Okubo Y, Blakesley V A, Stannard B, Gutkind S, Le Roith D. Insulin-like growth factor-I inhibits the stress-activated protein kinase/c-Jun N-terminal kinase. J Biol Chem. 1998;273:25961–25966. doi: 10.1074/jbc.273.40.25961. [DOI] [PubMed] [Google Scholar]
- 35.Ozes O N, Mayo L D, Gustin J A, Pfeffer S R, Pfeffer L M, Donner D B. NF-kappaB activation by tumour necrosis factor requires the Akt serine-threonine kinase. Nature. 1999;401:82–85. doi: 10.1038/43466. [DOI] [PubMed] [Google Scholar]
- 36.Pap M, Cooper G M. Role of glycogen synthase kinase-3 in the phosphatidylinositol 3-kinase/Akt cell survival pathway. J Biol Chem. 1998;273:19929–19932. doi: 10.1074/jbc.273.32.19929. [DOI] [PubMed] [Google Scholar]
- 37.Raingeaud J, Whitmarsh A J, Barrett T, Derijard B, Davis R J. MKK3- and MKK6-regulated gene expression is mediated by the p38 mitogen-activated protein kinase signal transduction pathway. Mol Cell Biol. 1996;16:1247–1255. doi: 10.1128/mcb.16.3.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Romashkova J A, Makarov S S. NF-kappaB is a target of AKT in anti-apoptotic PDGF signalling. Nature. 1999;401:86–90. doi: 10.1038/43474. [DOI] [PubMed] [Google Scholar]
- 39.Saitoh M, Nishitoh H, Fujii M, Takeda K, Tobiume K, Sawada Y, Kawabata M, Miyazono K, Ichijo H. Mammalian thioredoxin is a direct inhibitor of apoptosis signal-regulating kinase (ASK) 1. EMBO J. 1998;17:2596–2606. doi: 10.1093/emboj/17.9.2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Takeda K, Hatai T, Hamazaki T S, Nishitoh H, Saitoh M, Ichijo H. Apoptosis signal-regulating kinase 1 (ASK1) induces neuronal differentiation and survival of PC12 cells. J Biol Chem. 2000;275:9805–9813. doi: 10.1074/jbc.275.13.9805. [DOI] [PubMed] [Google Scholar]
- 41.Tibbles L A, Woodgett J R. The stress-activated protein kinase pathways. Cell Mol Life Sci. 1999;55:1230–1254. doi: 10.1007/s000180050369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tournier C, Hess P, Yang D D, Xu J, Turner T K, Nimnual A, Bar-Sagi D, Jones S N, Flavell R A, Davis R J. Requirement of JNK for stress-induced activation of the cytochrome c-mediated death pathway. Science. 2000;288:870–874. doi: 10.1126/science.288.5467.870. [DOI] [PubMed] [Google Scholar]
- 43.Wang T H, Wang H S, Ichijo H, Giannakakou P, Foster J S, Fojo T, Wimalasena J. Microtubule-interfering agents activate c-Jun N-terminal kinase/stress-activated protein kinase through both Ras and apoptosis signal-regulating kinase pathways. J Biol Chem. 1998;273:4928–4936. doi: 10.1074/jbc.273.9.4928. [DOI] [PubMed] [Google Scholar]
- 44.Wang X, McCullough K D, Franke T F, Holbrook N J. Epidermal growth factor receptor-dependent Akt activation by oxidative stress enhances cell survival. J Biol Chem. 2000;275:14624–14631. doi: 10.1074/jbc.275.19.14624. [DOI] [PubMed] [Google Scholar]
- 45.Wang X S, Diener K, Jannuzzi D, Trollinger D, Tan T H, Lichenstein H, Zukowski M, Yao Z. Molecular cloning and characterization of a novel protein kinase with a catalytic domain homologous to mitogen-activated protein kinase kinase kinase. J Biol Chem. 1996;271:31607–31611. doi: 10.1074/jbc.271.49.31607. [DOI] [PubMed] [Google Scholar]
- 46.Xia Z, Dickens M, Raingeaud J, Davis R J, Greenberg M E. Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science. 1995;270:1326–1331. doi: 10.1126/science.270.5240.1326. [DOI] [PubMed] [Google Scholar]
- 47.Yamamoto K, Ichijo H, Korsmeyer S J. BCL-2 is phosphorylated and inactivated by an ASK1/Jun N-terminal protein kinase pathway normally activated at G(2)/M. Mol Cell Biol. 1999;19:8469–8478. doi: 10.1128/mcb.19.12.8469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yang D D, Kuan C Y, Whitmarsh A J, Rincon M, Zheng T S, Davis R J, Rakic P, Flavell R A. Absence of excitotoxicity-induced apoptosis in the hippocampus of mice lacking the Jnk3 gene. Nature. 1997;389:865–870. doi: 10.1038/39899. [DOI] [PubMed] [Google Scholar]
- 49.Yano S, Tokumitsu H, Soderling T R. Calcium promotes cell survival through CaM-K kinase activation of the protein-kinase-B pathway. Nature. 1998;396:584–587. doi: 10.1038/25147. [DOI] [PubMed] [Google Scholar]
- 50.Zhang L, Chen J, Fu H. Suppression of apoptosis signal-regulating kinase 1-induced cell death by 14-3-3 proteins. Proc Natl Acad Sci USA. 1999;96:8511–8515. doi: 10.1073/pnas.96.15.8511. [DOI] [PMC free article] [PubMed] [Google Scholar]