Abstract
Low-level viremia (LLV) was defined as persistent or intermittent episodes of detectable hepatitis B virus (HBV) DNA (<2000 IU/mL, detection limit of 10 IU/mL) after 48 weeks of antiviral treatment. Effective antiviral therapies for chronic hepatitis B (CHB) patients, such as entecavir (ETV), tenofovir disoproxil fumarate (TDF), and tenofovir alafenamide (TAF), have been shown to inhibit the replication of HBV DNA and prevent liver-related complications. However, even with long-term antiviral therapy, there are still a number of patients with persistent or intermittent LLV. At present, the research on LLV to address whether adversely affect the clinical outcome is limited, and the follow-up treatment for these patients is open to question. At the same time, the mechanism of LLV is not clear. In this review, we summarize the incidence of LLV, the association between LLV and long-term outcomes, possible mechanisms, and management strategies in these patient populations.
Keywords: Chronic hepatitis B, Nucleoside/nucleotide analog treatment, Low-level viremia, Long-term outcomes
Introduction
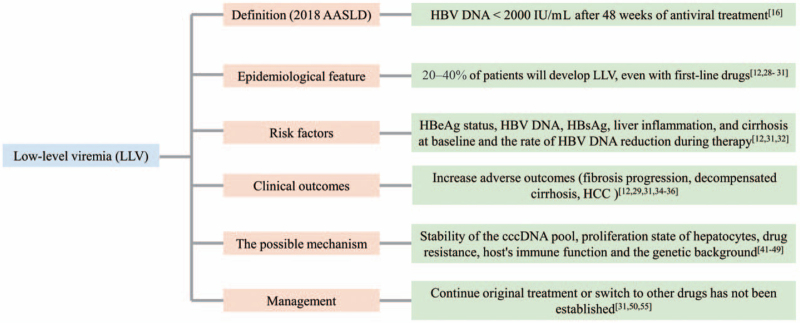
Hepatitis B virus (HBV) is a hepatotropic DNA virus that primarily infects hepatocytes and causes liver disease.[1] Although the widespread implementation of universal neonatal vaccination has dramatically reduced the incidence of HBV infection, 257 million people worldwide live with chronic infection, of whom almost 25% die from liver-related complications of liver cancer or liver failure.[2] In China, which has an HBV epidemic, the hepatitis B surface antigen (HBsAg)-positive rate in the general population has been reported to be approximately 10%.[3] Chronic hepatitis B (CHB) remains the leading cause of liver-related morbidity and mortality worldwide. Understanding the natural history of HBV has resulted in dramatic progress in the development of antiviral therapies and the management of HBV patients. Effective antiviral therapies for CHB patients using potent nucleoside/nucleotide analog (NUC) drugs with a high genetic barrier, such as entecavir (ETV), tenofovir disoproxil fumarate (TDF), and tenofovir alafenamide (TAF), have been shown to regress hepatic fibrosis, prevent liver-related complications, and improve patient survival.[4–9] However, the risk of hepatic complications, particularly the development of hepatocellular carcinoma (HCC), in CHB patients has not been fully eliminated, even with potent agents.[10] Low-level viremia (LLV) has been suggested to be a possible cause of HCC in patients receiving NUC treatment.[11,12] This review reports the current incidence of LLV with oral antiviral therapy, its association with long-term outcomes, and management strategies in this special patient population [Figure 1].
Figure 1.
The main content of LLV. AASLD: American Association for the Study of Liver Diseases; cccDNA: Covalently closed circular DNA; HBeAg: Hepatitis B e antigen; HBsAg: Hepatitis B surface antigen; HBV: Hepatitis B virus; HCC: Hepatocellular carcinoma; LLV: Low-level viremia.
Definition of LLV
At present, partial virological response (PVR) is clearly defined in the guidelines of various countries. The guidelines of the Asian-Pacific Association for the Study of the Liver (APASL) point out that a decrease in HBV DNA of >1 log10 IU/mL can still be detected in CHB patients with good compliance after NUC treatment for at least 6 months or 12 months.[13] The European Association for the Study of the Liver (EASL) defined patients with good compliance as having a “partial virological response” when HBV DNA levels decrease by >1 log10 IU/mL after NUC treatment for at least 12 months with positive HBV DNA.[14] The guidelines for the prevention and treatment of CHB (2019 version) also mention that CHB patients showing HBV DNA levels >2000 IU/mL after treatment with first-line antiviral drugs for 48 weeks are regarded as “patients with bad response” after the exclusion of compliance and detection error.[15] Meanwhile, both the US and European guidelines state that even with first-line antiviral therapies, the complete inhibition rate of HBV DNA in treatment-naive CHB patients who are hepatitis B e antigen (HBeAg)-positive is only approximately 70%.[14,16] However, LLV is not mentioned in most of the guidelines, except the guidelines of the American Association for the Study of Liver Diseases (AASLD) (2018), which define LLV as HBV DNA < 2000 IU/mL that is detectable (detection limit of 10 IU/mL) after 48 weeks of antiviral treatment.[16] Currently, some studies have classified LLV into persistent LLV and intermittent LLV according to the detectable duration of HBV DNA (<2000 IU/mL) after 1 year of NUC treatment.[12] Persistent LLV is defined as LLV without a complete virological response (CVR) (HBV DNA < 12 IU/mL), with HBV DNA levels remaining between 12 IU/mL and 1999 IU/mL throughout the follow-up period. Other patients achieved CVR but had intermittent episodes of detectable HBV DNA levels in the serum (between 12 IU/mL and 1999 IU/mL), which was considered intermittent LLV. With the improvement of detection techniques, LLV may be further stratified into LLV (2000–20 IU/mL) and very low-level viremia (10–19 IU/mL) according to the viral load. The clinical outcomes of the two groups may also be different. At present, there is no unified definition of LLV; more research and guidelines are needed to standardize this definition. Moreover, in the clinical diagnosis of LLV, factors that cause HBV DNA fluctuations (such as missed antiviral drugs, reduced drug dosage, and improper medication methods) should be eliminated. Drug–drug and drug–food interactions may also affect the antiviral effects of NUC.[17] Meanwhile, virus detectability caused by cross-resistance, site mutation, or sample contamination should also be excluded.[18]
Virus replication and disease progression
Oral administration of NUC remains the main form of global antiviral therapy for CHB. At present, ETV, TDF, and TAF are widely used as the first-line recommended drugs in the clinic. In the guidelines of all countries, the replication of HBV DNA should be greatly inhibited for a long time to prevent disease progression and HCC occurrence and to improve the quality of life and prolong the survival time of patients with CHB, which are the main goals of antiviral therapy.[14,16,19] The REVEAL study of Taiwan of China has evaluated more than 3500 CHB patients, of which 2925 are HBeAg-negative patients, with an average follow-up of 11 years.[20,21] In the complete cohort, it was found that the cumulative incidence rate of HCC increased with increasing serum HBV DNA levels. In HBeAg-negative patients, patients with HBV DNA > 2000 IU/mL had a 2.5 times higher risk of liver cirrhosis (95% confidence interval [CI] 1.600–3.800) and a 2.7 times higher risk of HCC (95% CI 1.300–5.600) than those with HBV DNA ≤ 2000 IU/mL. The increase in serum HBV DNA levels was accompanied by an increased risk of liver cirrhosis and HCC. This research lays the foundation for antiviral therapy. In another study from Greece, 399 HBV DNA-positive patients with negative HBeAg and ALT greater than 1-fold ULN were included and based on the liver biopsy results, 62% of the patients with HBV DNA ≤ 2000 IU/mL had significant histological changes (Ishak score system: inflammatory necrosis grade ≥ 7 and/or fibrosis degree ≥ 2).[22]
The above findings suggest that patients, even those with a low viral load, are still at risk of disease progression and should be considered for treatment. If timely and comprehensive biopsies are not performed, some patients who need antiviral treatment may be missed. Moreover, HBeAg-negative patients with normal ALT levels are often considered inactive carriers and are thought to need no treatment. However, previous studies have found that gene mutation in the HBV pre-C region or BCP region can lead to the failure of HBeAg expression, resulting in negative HBeAg detection.[23–25] In this case, HBV DNA is still replicating or is even in a high viral load state. This phenomenon may mask some patients’ need for treatment, resulting in disease progression. Furthermore, a study from China showed that 72.60% of patients with positive HBV DNA and negative HBeAg had pre-C mutations.[26] These patients may not be real inactive carriers, but they need to be considered for treatment. Especially for HBeAg-negative patients with ALT level fluctuations, the indications should be considered to be relaxed for antiviral therapy because clinical long-term ALT monitoring is difficult. The new version of the Chinese guidelines for the prevention and treatment of CHB in 2019 relaxed the antiviral guidelines for CHB patients, which could potentially benefit more patients.[27] The guidelines proposed that antiviral therapy should be recommended for patients with positive serum HBV DNA and continuously abnormal ALT (>ULN) with the exclusion of other causes. Antiviral therapy is recommended for patients with compensated cirrhosis and positive HBV DNA or patients with decompensated cirrhosis and positive HBsAg. Antiviral therapy is also recommended for the following patients: (1) patients with positive serum HBV DNA, normal ALT, and significant inflammation and/or fibrosis [G ≥ 2 and/or S ≥ 2] by liver biopsy; (2) patients (age > 30 years old) with a family history of hepatitis B cirrhosis or HCC; and (3) patients (age > 30 years old) with normal ALT and obvious liver inflammation or fibrosis by noninvasive diagnosis of liver fibrosis or liver histological examination or HBV-related extrahepatic manifestations.
Epidemiological features and risk factors of LLV
Meanwhile, with the progress of nucleic acid detection technology, it helps the detection limit of HBV DNA has gradually expanded from 500 IU/mL to 1000 IU/mL to 10 to 30 IU/mL. The guidelines for the detection limit of serum HBV DNA in CHB patients have made new provisions in each country, and a CVR is defined according to the current detection limit [Table 1]. With the improvement of the sensitivity of HBV DNA detection, continuous or intermittent low levels of HBV DNA (HBV DNA < 2000 IU/mL, LLV) are detected in an increasing number of patients in the process of antiviral therapy, though this phenomenon has not been widely studied; moreover, the difference between LLV and PVR also needs more research and comparison. In a large-scale epidemiological survey of 21,614 CHB patients with antiviral therapy in China, it was found that approximately 20% of the 10,538 treated patients developed LLV.[28] Another Chinese longitudinal cohort study included 163 treatment-naive CHB patients with significant liver fibrosis (Ishak ≥ 3) at baseline.[29] All patients received ETV-based treatment for 78 weeks, and HBV DNA levels were detected at 78 weeks. The results showed that a low viral load (normal HBV DNA was 20–200 IU/mL) could still be detected in 23% of patients. In general, real-world studies have suggested that 20% to 40% of patients will still develop LLV, even with first-line drugs.[12,28,30,31] Therefore, in clinical work, the dynamic monitoring of HBV DNA levels in patients should be considered to identify LLV in a timely manner.
Table 1.
Guidelines for the determination of CVR, defined according to the current detection limits.
| Guidelines | Definition of virological response to NA therapy |
| Guidelines for prevention and treatment of CHB (2019 version)[27] | HBV DNA cannot be detected (no specified detection limit) |
| An expert consensus for the adjustment of treatment strategies in patients with CHB treated with non-first-line nucleos(t)ide analogs (2019)[56] | HBV DNA cannot be detected (<20 IU/mL). The lower the HBV DNA in serum, the better. |
| Guidelines of the AASLD (2018)[16] | HBV DNA cannot be detected (<10 IU/mL) |
| Guidelines of the EASL (2017)[14] | HBV DNA cannot be detected (<10 IU/mL) |
| Guidelines of the APASL (2015)[13] | HBV DNA cannot be detected (<12 IU/mL) |
| Guidelines of the WHO on CHB (2015)[57] | HBV DNA cannot be detected (<15 IU/mL) |
AASLD: American Association for the Study of Liver Diseases; APASL: Asian-Pacific Association for the Study of the Liver; CHB: Chronic hepatitis B; CVR: Complete virological response; EASL: European Association for the Study of the Liver; HBV: Hepatitis B virus; NA: Nucleoside (acid) analog; WHO: World Health Organization.
So what factors are involved in the development of LLV? In one study, 90 CHB patients (55 HBeAg-positive and 35 HBeAg-negative) who received TDF monotherapy >2 years were enrolled. The cumulative CVR rates in the HBeAg-negative group were significantly higher than those in the HBeAg-positive group (P < 0.001). It was also found that baseline HBV DNA level (P = 0.001) and HBsAg quantification (P < 0.001) were significant predictive factors for a CVR. Therefore, we suspected that the above indicators may also have a certain effect on the occurrence of LLV.[32] Another retrospective study enrolled 875 treatment-naive CHB patients with ETV monotherapy. According to this study, it was found that HBeAg status, HBV DNA levels, presence of cirrhosis, and time to first CVR were associated with LLV.[12] Based on our previous research, treatment containing non-first-line drugs, lower ALT and higher HBV DNA levels at baseline, and HBV DNA levels at 6 months were independent risk factors for LLV.[31] Previous studies have reminded us of the importance of assessing the levels of HBV DNA, HBsAg, liver inflammation, and cirrhosis before antiviral therapy; to facilitate the timely identification of LLV patients, attention should also be paid to the decline of HBV DNA in the course of treatment.
Clinical harm of LLV
The question of whether the occurrence of LLV will lead to disease progression and eventually increase adverse outcomes was investigated. From the existing clinical studies, it was found that LLV is tightly related to adverse outcomes, especially in patients with liver cirrhosis. In a cohort of patients with compensated cirrhosis treated with ETV in Hong Kong, the risk of HCC was noticeably reduced when the CVR was ≥24 months, and the adjusted hazard ratio was 0.3. This study found that a CVR was an independent predictor of a reduction in HCC occurrence.[33] Similarly, another study from South Korea included ETV-treated cirrhosis patients, and the effects of LLV and CVR on long-term clinical outcomes were compared during treatment. The cumulative incidence rates of HCC in 5 years were found to be 23.40% in patients with LLV and 10.30% in patients with maintained virological response (MVR).[12] At present, even though the major international guidelines recommend that people with liver cirrhosis should receive antiviral therapy regardless of the HBV DNA level, the HBV DNA level should be dynamically detected even in liver cirrhosis patients administered strong antiviral drugs, because the occurrence of LLV will increase the risk of HCC. In a longitudinal cohort study in China, 163 treatment-naive CHB patients who underwent liver biopsy before and after treatment and who had significant fibrosis (Ishak ≥ 3) at baseline were included and received 78 weeks of ETV treatment. Thirty-seven patients had low HBV DNA levels (20–200 IU/mL) at week 78. Multivariate analysis showed that the risk of liver fibrosis progression in this group of patients was 4.84 times higher than that in the patients with complete viral inhibition (95% CI: 1.300–17.980).[29] In another retrospective study from Turkey, 139 untreated LLV patients (an average age of 23.78 ± 4.2 years) negative for HBeAg were included. Among these young patients, 30.20% developed liver fibrosis, which suggested that even if patients are young and the HBV DNA level is low, their prognosis will be affected if virus replication is not completely inhibited.[34] Moreover, in a previous study, 325 HBeAg-positive CHB patients who received ETV or TDF monotherapy were included, and it was found that failure to achieve a CVR in the first 2 years of antiviral therapy notably increased the risk of HCC (1-year hazard ratio [HR]: 4.54; 2-year HR: 3.38).[35]
In our study, 674 CHB patients receiving oral antiviral drugs were reviewed. The results showed that patients with LLV had a markedly higher risk of end-stage liver disease (decompensated cirrhosis and HCC) at 5 years and 10 years than patients with MVR (P < 0.050). Meanwhile, four risk prediction models of HCC (the Chinese university HCC score [CU-HCC], the guide with age, gender, HBV DNA, core promoter mutations and cirrhosis HCC score [GAG-HCC], risk estimation for hepatocellular carcinoma in chronic hepatitis B [REACH-B], and platelet age gender-B [PAGE-B]) were introduced. In the high-risk population, patients with LLV had a higher risk of developing HCC than patients with MVR (P < 0.050). According to the subgroup analysis of 200 patients with compensated cirrhosis, between the two groups, the incidence rates of cirrhosis reversal in the MVR group were 39.83% and 63.62% at 5 years and 10 years, respectively, which were higher than those in the LLV group (10.63% and 16.21% at 5 years and 10 years, respectively; P < 0.001). Therefore, we believe that LLV not only leads to adverse clinical outcomes but also affects the reversal of liver cirrhosis after antiviral therapy.[31] Furthermore, in a Korean retrospective cohort study, 565 CHB patients with LLV and confirmed HCC were included, with an average follow-up of 4.5 years. Among them, 25.30% of the patients received antiviral therapy at baseline. The study found that a considerable proportion of patients with HCC complicated with LLV may have had HBV relapse. Meanwhile, multivariate analysis showed that the survival rate of LLV patients with HBV relapse was markedly lower than that of patients with sustained virological inhibition (HR = 1.71, 95% CI: 1.150–2.550).[36] From the above, whether in young untreated patients, in patients with liver fibrosis and cirrhosis who are using the currently recommended first-line antiviral drugs, or even in patients with end-stage liver disease who have developed HCC, failure to achieve complete virological suppression may greatly affect the long-term prognosis of patients and endanger their lives.
Possible mechanism of LLV
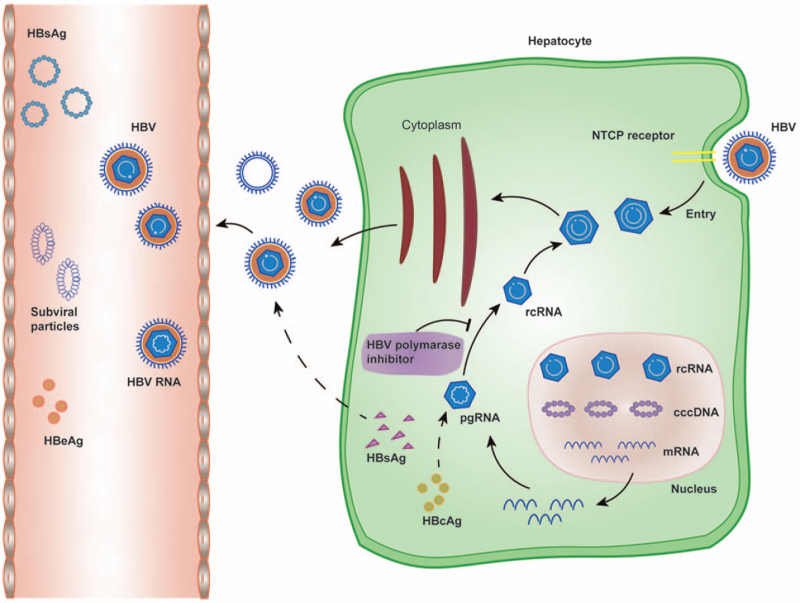
At present, the mechanism of LLV remains unclear. Nevertheless, based on the life cycle of HBV, we found that the key to chronic and refractory HBV infection lies in the stable existence of covalently closed circular DNA (cccDNA) in the infected liver nuclei.[37,38] After HBV enters hepatocytes, the viral nucleocapsid carries HBV relaxed circular DNA (rcDNA) into the hepatocyte nucleus and transforms it into cccDNA under the action of the host DNA repair system. Meanwhile, pregenomic RNA (pgRNA) (core antigen and P protein mRNA) transcribed from cccDNA can be used as reverse transcription templates to form new rcDNA.[39] On the one hand, the newly synthesized rcDNA can be packaged as a complete virus particle to infect new normal hepatocytes. On the other hand, rcDNA can also enter the nucleus and complement cccDNA in the nucleus after repair to maintain the stability of the cccDNA pool in the liver nucleus [Figure 2].[40] The main mechanism of NUC is to competitively bind to the P protein with dNTPs in cells, by which NUC inhibit the synthesis of rcDNA of progeny virus. However, in the presence of a large amount of dNTPs, NUC cannot completely inhibit HBV replication and prevent the formation of cccDNA in newly infected hepatocytes.[41,42] Therefore, NUC cannot completely block the synthesis of the DNA strand. As a result, the HBV DNA level in the serum of some patients with antiviral therapy is continuously or intermittently higher than the detection limit, as manifested as LLV.
Figure 2.
Life cycle of HBV. cccDNA: Covalently closed circular DNA; HBcAg: Hepatitis B c antigen; HBeAg: Hepatitis B e antigen; HBsAg: Hepatitis B surface antigen; HBV: Hepatitis B virus; NTCP: Sodium taurocholate cotransporting polypeptide; pgRNA: Pre-genomic RNA; rcDNA: Relaxed circular DNA.
Moreover, some studies have found that the expression levels of all virological indexes, including cccDNA copy number, in HBV-infected primary human hepatocytes (PHHs) are rapidly decreased during rapid compensatory proliferation. In contrast, as the compensatory proliferation of hepatocytes slows, the above virological markers begin to rebound.[43] When the inflammatory injury occurs in the liver, HBV-infected hepatocytes also participate in compensatory proliferation after injury.[44] However, due to the lack of centromeres that are specific to chromosomes in cccDNA in the form of episomes in infected hepatocytes, it is difficult to enter the newly formed nuclei of progeny cells during mitosis, resulting in the loss of cccDNA and the dilution of the cccDNA pool in progeny cells. We also observed that in clinical work, the serum HBV DNA loads of patients with significantly elevated ALT levels were notably decreased during NUC antiviral therapy, which suggests that patients with liver inflammatory activity are more likely to achieve a CVR during NUC antiviral therapy.[45,46] The study also found that sodium taurocholate cotransporting polypeptide (NTCP) expression and the localization of the cell membrane were significantly downregulated in proliferating hepatocytes, which hindered the de novo infection of HBV.[46] However, hepatocytes in a low proliferation state are easily infected by HBV, which is conducive to HBV replication and cccDNA accumulation.[47] In our study, we also observed that patients with ALT < 100 U/L receiving antiviral therapy were more likely to develop LLV than patients with baseline ALT > 100 U/L, and the ALT level was an independent risk factor for LLV (odds ratio [OR] = 2.885, 95% CI 1.831–4.546, P < 0.001).[31] Therefore, more clinical and basic research is needed to verify whether the low proliferation state is related to the occurrence of LLV in CHB patients.
Drug resistance may also be associated with the development of LLV. Currently, there is no research on host-virus development in LLV patients. Therefore, it is hard to assess whether drug resistance could cause LLV and whether the risk of resistance would increase as LLV develops. According to previous studies on antiviral therapy of HBV, lamivudine (LAM)-resistant patients could still develop resistance even when HBV DNA was <60 IU/mL at 24 weeks and 48 weeks after switching to ADV monotherapy. Moreover, the degree of HBV DNA decline during the treatment course is tightly linked with the development of ADV resistance.[48] In another study, 69 CHB patients receiving ETV treatment were followed, with 13 exhibiting PVR to ETV. No known resistance mutations were detected among the PVR patients. The clinical isolates from PVR patients were susceptible to ETV the same as wild-type HBV. Most PVR patients achieved virological response after long-term ETV monotherapy.[49] In a large retrospective study in Korea, only a minor proportion of LLV patients were documented with drug-resistance mutations. At the same time, drug-resistance testing was not systematically performed in patients showing LLV. Therefore, there remains a possibility that the development of resistance-associated mutations is related to LLV.[12] Moreover, the issue of whether the host's immune function and the genetic background affect LLV occurrence needs further validation.
Management of LLV
When a patient develops LLV, it has not yet been established whether they should continue their original treatment or switch to other drugs. Guidelines recommend that patients with PVR who use non-first-line medication should switch to the most effective antiviral agent that does not share cross-resistance.[13,14,16,27] The AASLD recommendation for patients with LLV suggests that patients treated with ETV or TDF monotherapy should continue monotherapy, although the quality and certainty of evidence are low.[16] EASL does not recommend changing the initial treatment strategy in patients with low HBV DNA levels (HBV DNA < 69 IU/mL) and/or declining HBV DNA concentrations on potent NUC monotherapy; if the HBV DNA has plateaued (69 < DNA < 2000 IU/mL), the possibility of switching to another drug or a combination of ETV+TDF/TAF should be considered.[14] In our study, HBV DNA was intermittently detected in 203 patients in the LLV group, so only 24 patients changed their treatment regimens. We found that patients who changed their treatment were more likely to achieve complete virological suppression than those who continued the original treatment and that their long-term clinical outcomes were better. At the same time, we found no significant difference in the CVR between switching to another drug and adding on a drug[31]; however, due to our limited data, these conclusions may be biased and require further validation in a larger population. In a recent study in which patients with LLV were treated with ETV or NUC combination therapy, almost all patients achieved complete virological suppression when switching to TAF treatment after 48 weeks; at the same time, patients with CKD converted to TAF, and their eGFR also improved (+ 0.40 mL · min−1 · 1.73m−2), suggesting that patients with LLV need more effective treatment and that TAF is a safe and effective choice.[30] Another Chinese study enrolled 211 ETV-treated patients with LLV who were switching to TAF or continuing ETV therapy. After 24 weeks of treatment, the CVR and ALT normalization in the TAF group were 62.70% and 47.60%, which were higher than the 9.30% and 10.50% in the ETV group (OR 16.4, 95% CI 6.600–40.000), respectively. For ETV-treated patients with LLV, switching to TAF is safe enough and superior compared with continuing ETV monotherapy regarding both virological and biochemical benefits.[50] All the above studies have suggested that in the context of the occurrence of LLV, switching to another drug is more conducive to attaining a CVR.
However, the efficacy of NUC in the combination therapy of LLV is still controversial. In treatment-naïve patients, initial combined NUC therapy was found to be associated with a higher complete virological inhibition rate in HBeAg-positive patients with a high viral load. In the subgroup analysis of randomized controlled trials, in HBeAg-positive patients (baseline HBV DNA > 8 log IU/mL) with ETV and TDF combined antiviral therapy, 78.80% had HBV DNA < 50 IU/mL at 96 weeks, while only 62% of patients with ETV monotherapy had HBV DNA < 50 IU/mL at 96 weeks. However, this study lacked patients with TDF monotherapy as controls.[51] In another randomized controlled trial, patients with positive HBeAg, baseline HBV DNA > 7 log IU/mL, and normal ALT were included. In the group with ETV combined with enteltabine antiviral therapy group, 69.40% of the patients achieved HBV DNA < 29 IU/mL, while only 45.30% of the patients in the TDF monotherapy group achieved HBV DNA < 29 IU/mL.[52] For drug-resistant patients, multiple studies have shown that combination therapy (TDF combined with ETV or TDF combined with enteltabine) does not increase the complete inhibition rate of the virus.[53,54]
Moreover, a previous study included 894 CHB patients treated with ETV, and the impact of patients with good compliance and poor compliance on LLV occurrence and long-term clinical prognosis was compared. The study found that the incidence of LLV in patients with good compliance was lower than that in patients with poor compliance. Furthermore, no significant difference was found in the risk of the development of HCC between LLV and MVR in patients with good compliance.[55] Therefore, when LLV occurs, the compliance of patients should be determined before considering the immediate adjustment of antiviral therapy. For patients with good compliance, adjusting the antiviral therapy may be unnecessary.
Conclusion
A certain proportion of CHB patients develop LLV in the treatment of NUC with strong and high resistance barriers. The reason may be that the current antiviral drugs are all reverse transcriptase inhibitors, which competitively inhibit the replication of HBV DNA rather than directly acting on cccDNA. The specific mechanism of LLV is still unclear and needs further research to confirm. With the improvement of nucleic acid detection technology, the monitoring of HBV DNA in CHB patients may help to identify more LLV patients. Since there is insufficient comparative evidence for the benefit of continuing the original treatment or changing the strategy in patients with LLV, further clinical studies are needed to clarify these options. Considering that LLV may be associated with the progression of liver fibrosis cirrhosis, and even the development of HCC, adjusting the therapy plan when necessary will help to reduce the occurrence of adverse prognoses.
Funding
This work was supported by the National Science and Technology Major Project of China (Nos 2017ZX10202203-007, 2017ZX10202203-008, and 2018ZX10302-206-003).
Conflicts of interest
None.
Footnotes
How to cite this article: Zhang Q, Cai DC, Hu P, Ren H. Low-level viremia in nucleoside analog-treated chronic hepatitis B patients. Chin Med J 2021;134:2810–2817. doi: 10.1097/CM9.0000000000001793
References
- 1.Seto WK, Lo YR, Pawlotsky JM, Yuen MF. Chronic hepatitis B virus infection. Lancet 2018; 392:2313–2324. doi: 10.1016/S0140-6736(18)31865-8. [DOI] [PubMed] [Google Scholar]
- 2.Hutin Y, Nasrullah M, Easterbrook P, Nguimfack BD, Burrone E, Averhoff F, et al. Access to treatment for hepatitis B virus infection - Worldwide, 2016. MMWR Morb Mortal Wkly Rep 2018; 67:773–777. doi: 10.15585/mmwr.mm6728a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Polaris Observatory Collaborators. Global prevalence, treatment, and prevention of hepatitis B virus infection in 2016: A modelling study. Lancet Gastroenterol Hepatol 2018; 3:383–403. doi: 10.1016/S2468-1253(18)30056-6. [DOI] [PubMed] [Google Scholar]
- 4.Papatheodoridis GV, Lampertico P, Manolakopoulos S, Lok A. Incidence of hepatocellular carcinoma in chronic hepatitis B patients receiving nucleos(t)ide therapy: A systematic review. J Hepatol 2010; 53:348–356. doi: 10.1016/j.jhep.2010.02.035. [DOI] [PubMed] [Google Scholar]
- 5.Wang X, Liu X, Dang Z, Yu L, Jiang Y, Wang X, et al. Nucleos(t)ide analogues for reducing hepatocellular carcinoma in chronic hepatitis B patients: A systematic review and meta-analysis. Gut Liver 2020; 14:232–247. doi: 10.5009/gnl18546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grossi G, Viganò M, Loglio A, Lampertico P. Hepatitis B virus long-term impact of antiviral therapy nucleot(s)ide analogues (NUCs). Liver Int 2017; 37:45–51. doi: 10.1111/liv.13291. [DOI] [PubMed] [Google Scholar]
- 7.Jang JW, Choi JY, Kim YS, Woo HY, Choi SK, Lee CH, et al. Long-term effect of antiviral therapy on disease course after decompensation in patients with hepatitis B virus-related cirrhosis. Hepatology 2015; 61:1809–1820. doi: 10.1002/hep.27723. [DOI] [PubMed] [Google Scholar]
- 8.Coffin CS, Rezaeeaval M, Pang JX, Alcantara L, Klein P, Burak KW, et al. The incidence of hepatocellular carcinoma is reduced in patients with chronic hepatitis B on long-term nucleos(t)ide analogue therapy. Aliment Pharmacol Ther 2014; 40:1262–1269. doi: 10.1111/apt.12990. [DOI] [PubMed] [Google Scholar]
- 9.Wang JL, Du XF, Chen SL, Yu YQ, Wang J, Hu XQ, et al. Histological outcome for chronic hepatitis B patients treated with entecavir vs lamivudine-based therapy. World J Gastroenterol 2015; 21:9598–9606. doi: 10.3748/wjg.v21.i32.9598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hosaka T, Suzuki F, Kobayashi M, Seko Y, Kawamura Y, Sezaki H, et al. Long-term entecavir treatment reduces hepatocellular carcinoma incidence in patients with hepatitis B virus infection. Hepatology 2013; 58:98–107. doi: 10.1002/hep.26180. [DOI] [PubMed] [Google Scholar]
- 11.Sinn DH, Lee J, Goo J, Kim K, Gwak GY, Paik YH, et al. Hepatocellular carcinoma risk in chronic hepatitis B virus-infected compensated cirrhosis patients with low viral load. Hepatology 2015; 62:694–701. doi: 10.1002/hep.27889. [DOI] [PubMed] [Google Scholar]
- 12.Kim JH, Sinn DH, Kang W, Gwak GY, Paik YH, Choi MS, et al. Low-level viremia and the increased risk of hepatocellular carcinoma in patients receiving entecavir treatment. Hepatology 2017; 66:335–343. doi: 10.1002/hep.28916. [DOI] [PubMed] [Google Scholar]
- 13.Sarin SK, Kumar M, Lau GK, Abbas Z, Chan HLY, Chen CJ, et al. Asian-Pacific clinical practice guidelines on the management of hepatitis B: A 2015 update. Hepatol Int 2016; 10:1–98. doi: 10.1007/s12072-015-9675-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.European Association for the Study of the, Liver., EASL., 2017 Clinical Practice Guidelines on the management of hepatitis, B., virus, infection. J Hepatol 2017; 67:370–398. doi: 10.1016/j.jhep.2017.03.021. [DOI] [PubMed] [Google Scholar]
- 15.Chinese Society of Infectious Diseases, Chinese Medical Association; Chinese Society of Hepatology, Chinese Medical Association. The guidelines of prevention and treatment for chronic hepatitis B (2019 version) (in Chinese). Chin J Hepatol 2019; 27:938–961. doi: 10.3760/cma.j.issn.1007-3418.2019.12.007. [DOI] [PubMed] [Google Scholar]
- 16.Terrault NA, Lok ASF, McMahon BJ, Chang KM, Hwang JP, Jonas MM, et al. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 Hepatitis B Guidance. Clin Liver Dis (Hoboken) 2018; 12:33–34. doi: 10.1002/cld.728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lu C, Jia Y, Chen L, Ding Y, Yang J, Chen M, et al. Pharmacokinetics and food interaction of a novel prodrug of tenofovir, tenofovir dipivoxil fumarate, in healthy volunteers. J Clin Pharm Ther 2013; 38:136–140. doi: 10.1111/jcpt.12023. [DOI] [PubMed] [Google Scholar]
- 18.Liu Y, Miller MD, Kitrinos KM. HBV clinical isolates expressing adefovir resistance mutations show similar tenofovir susceptibilities across genotypes B, C and D. Liver Int 2014; 34:1025–1032. doi: 10.1111/liv.12343. [DOI] [PubMed] [Google Scholar]
- 19.Jia JD, Hou JL, Wei L, Zhuang H. Highlights of the guidelines of prevention and treatment for chronic hepatitis B (2019 version) (in Chinese). Chin J Hepatol 2020; 28:21–23. doi: 10.3760/cma.j.issn.1007-3418.2020.01.006. [DOI] [PubMed] [Google Scholar]
- 20.Chen CJ, Yang HI, Su J, Jen CL, You SL, Lu SN, et al. Risk of hepatocellular carcinoma across a biological gradient of serum hepatitis B virus DNA level. JAMA 2006; 295:65–73. doi: 10.1001/jama.295.1.65. [DOI] [PubMed] [Google Scholar]
- 21.Iloeje UH, Yang HI, Su J, Jen CL, You SL, Chen CJ, et al. Predicting cirrhosis risk based on the level of circulating hepatitis B viral load. Gastroenterology 2006; 130:678–686. doi: 10.1053/j.gastro.2005.11.016. [DOI] [PubMed] [Google Scholar]
- 22.Papatheodoridis GV, Manesis EK, Manolakopoulos S, Elefsiniotis IS, Goulis J, Giannousis J, et al. Is there a meaningful serum hepatitis B virus DNA cutoff level for therapeutic decisions in hepatitis B e antigen-negative chronic hepatitis B virus infection? Hepatology 2008; 48:1451–1459. doi: 10.1002/hep.22518. [DOI] [PubMed] [Google Scholar]
- 23.Malik A, Kumar D, Khan AA, Khan AA, Chaudhary AA, Husain SA, et al. Hepatitis B virus precore G1896A mutation in chronic liver disease patients with HBeAg negative serology from North India. Saudi J Biol Sci 2018; 25:1257–1262. doi: 10.1016/j.sjbs.2016.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Colombatto P, Barbera C, Bortolotti F, Maina AM, Moriconi F, Cavallone D, et al. HBV pre-core mutant in genotype-D infected children is selected during HBeAg/anti-HBe seroconversion and leads to HBeAg negative chronic hepatitis B in adulthood. J Med Virol 2018; 90:1232–1239. doi: 10.1002/jmv.25068. [DOI] [PubMed] [Google Scholar]
- 25.Alexopoulou A, Karayiannis P. HBeAg negative variants and their role in the natural history of chronic hepatitis B virus infection. World J Gastroenterol 2014; 20:7644–7652. doi: 10.3748/wjg.v20.i24.7644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang J, Xu WJ, Wang Q, Zhang Y, Shi M. Prevalence of the precore G1896A mutation in Chinese patients with e antigen negative hepatitis B virus infection and its relationship to pre-S1 antigen. Braz J Microbiol 2009; 40:965–971. doi: 10.1590/S1517-838220090004000031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chinese Society of Hepatology, Chinese Medical Association. Chinese Guidelines on the management of liver cirrhosis (in Chinese). Chin J Hepatol 2019; 27:846–865. doi: 10.3760/cma.j.issn.1007-3418.2019.11.008. [DOI] [PubMed] [Google Scholar]
- 28.Xing M, Sen L, Jane D, Andrew GI. Chronic hepatitis B management in clinical practice in Fuzhou Province, China: Retrospective cross-sectional analysis of electronic medical record data. Hepatol Int 2020; 14:1–470. doi: 10.1007/s12072-020-10030-4.31754958 [Google Scholar]
- 29.Sun Y, Wu X, Zhou J, Meng T, Wang B, Chen S, et al. Persistent low level of hepatitis B virus promotes fibrosis progression during therapy. Clin Gastroenterol Hepatol 2020; 18:2582–2591. doi: 10.1016/j.cgh.2020.03.001. [DOI] [PubMed] [Google Scholar]
- 30.Ogawa E, Nomura H, Nakamuta M, Furusyo N, Koyanagi T, Dohmen K, et al. Tenofovir alafenamide after switching from entecavir or nucleos(t)ide combination therapy for patients with chronic hepatitis B. Liver Int 2020; 40:1578–1589. doi: 10.1111/liv.14482. [DOI] [PubMed] [Google Scholar]
- 31.Zhang Q, Peng H, Liu X, Wang H, Du J, Luo X, et al. Chronic hepatitis B infection with low level viremia correlates with the progression of the liver disease. J Clin Transl Hepatol 2021; doi: 10.14218/JCTH.2021.00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yoo EH, Cho HJ. Clinical response to long-term tenofovir monotherapy in Korean chronic hepatitis B patients. Clin Chim Acta 2017; 471:308–313. doi: 10.1016/j.cca.2017.06.019. [DOI] [PubMed] [Google Scholar]
- 33.Wong GLH, Chan HLY, Chan HY, Tse PCH, Tse YK, Mak CWH, et al. Accuracy of risk scores for patients with chronic hepatitis B receiving entecavir treatment. Gastroenterology 2013; 144:933–944. doi: 10.1053/j.gastro.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 34.Yenilmez E, Çetinkaya RA, Tural E. Diagnostic dilemma for low viremia with significant fibrosis; is hepatitis B virus DNA threshold level a good indicator for predicting liver damage? Balkan Med J 2018; 35:326–332. doi: 10.4274/balkanmedj.2017.0888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nam JY, Chang Y, Cho H, Kang SH, Cho YY, Cho EJ, et al. Delayed viral suppression during antiviral therapy is associated with increased hepatocellular carcinoma rates in HBeAg-positive high viral load chronic hepatitis B. J Viral Hepat 2018; 25:552–560. doi: 10.1111/jvh.12838. [DOI] [PubMed] [Google Scholar]
- 36.Kim TS, Sinn DH, Kang W, Gwak GY, Paik YH, Choi MS, et al. Hepatitis B virus DNA levels and overall survival in hepatitis B-related hepatocellular carcinoma patients with low-level viremia. J Gastroenterol Hepatol 2019; 34:2028–2035. doi: 10.1111/jgh.14750. [DOI] [PubMed] [Google Scholar]
- 37.Lai CL, Wong D, Ip P, Kopaniszen M, Seto WK, Fung J, et al. Reduction of covalently closed circular DNA with long-term nucleos(t)ide analogue treatment in chronic hepatitis B. J Hepatol 2017; 66:275–281. doi: 10.1016/j.jhep.2016.08.022. [DOI] [PubMed] [Google Scholar]
- 38.Allweiss L, Dandri M. The role of cccDNA in HBV maintenance. Viruses 2017; 9:156.doi: 10.3390/v9060156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang J, Huang H, Liu Y, Chen R, Yan Y, Shi S, et al. HBV genome and life cycle. Adv Exp Med Biol 2020; 1179:17–37. doi: 10.1007/978-981-13-9151-4_2. [DOI] [PubMed] [Google Scholar]
- 40.Kapoor R, Kottilil S. Strategies to eliminate HBV infection. Future Virol 2014; 9:565–585. doi: 10.2217/fvl.14.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Boyd A, Lacombe K, Lavocat F, Maylin S, Miailhes P, Lascoux-Combe C, et al. Decay of ccc-DNA marks persistence of intrahepatic viral DNA synthesis under tenofovir in HIV-HBV co-infected patients. J Hepatol 2016; 65:683–691. doi: 10.1016/j.jhep.2016.05.014. [DOI] [PubMed] [Google Scholar]
- 42.Koumbi L. Current and future antiviral drug therapies of hepatitis B chronic infection. World J Hepatol 2015; 7:1030–1040. doi: 10.4254/wjh.v7.i8.1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Allweiss L, Volz T, Giersch K, Kah J, Raffa G, Petersen J, et al. Proliferation of primary human hepatocytes and prevention of hepatitis B virus reinfection efficiently deplete nuclear cccDNA in vivo. Gut 2018; 67:542–552. doi: 10.1136/gutjnl-2016-312162. [DOI] [PubMed] [Google Scholar]
- 44.Goyal A, Ribeiro RM, Perelson AS. The role of infected cell proliferation in the clearance of acute HBV infection in humans. Viruses 2017; 9:350.doi: 10.3390/v9110350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wong D, Littlejohn M, Edwards R, Jackson K, Revill P, Gaggar A, et al. ALT flares during nucleotide analogue therapy are associated with HBsAg loss in genotype A HBeAg-positive chronic hepatitis B. Liver Int 2018; 38:1760–1769. doi: 10.1111/liv.13716. [DOI] [PubMed] [Google Scholar]
- 46.Yan Y, Allweiss L, Yang D, Kang J, Wang J, Qian X, et al. Down-regulation of cell membrane localized NTCP expression in proliferating hepatocytes prevents hepatitis B virus infection. Emerg Microbes Infect 2019; 8:879–894. doi: 10.1080/22221751.2019.1625728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Guan G, Zheng L, Xi J, Yang X, Chen X, Lu F. Cell cycle arrest protein CDKN2C is not an HBV host factor. Virol Sin 2021; doi: 10.1007/s12250-020-00337-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sinn DH, Lee HI, Gwak GY, Choi MS, Koh KC, Paik SW, et al. Virological response to adefovir monotherapy and the risk of adefovir resistance. World J Gastroenterol 2011; 17:3526–3530. doi: 10.3748/wjg.v17.i30.3526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li X, Li F, Zhang Y, Kang Y, Yu J, Yang F, et al. Partial virological response to entecavir treatment in nucleos(t)ide-naïve patients with HBeAg-positive chronic hepatitis B is not caused by reduced sensitivity. Biochem Biophys Res Commun 2015; 464:1185–1191. doi: 10.1016/j.bbrc.2015.07.101. [DOI] [PubMed] [Google Scholar]
- 50.Li ZB, Li L, Niu XX, Chen SH, Fu YM, Wang CY, et al. Switching from entecavir to tenofovir alafenamide for chronic hepatitis B patients with low-level viraemia. Liver Int 2021; 41:1254–1264. doi: 10.1111/liv.14786. [DOI] [PubMed] [Google Scholar]
- 51.Lok AS, Trinh H, Carosi G, Akarca US, Gadano A, Habersetzer F, et al. Efficacy of entecavir with or without tenofovir disoproxil fumarate for nucleos(t)ide-naïve patients with chronic hepatitis B. Gastroenterology 2012; 143:619–628. e1. doi: 10.1053/j.gastro.2012.05.037. [DOI] [PubMed] [Google Scholar]
- 52.Chan HLY, Chan CK, Hui AJ, Chan S, Poordad F, Chang TT, et al. Effects of tenofovir disoproxil fumarate in hepatitis B e antigen-positive patients with normal levels of alanine aminotransferase and high levels of hepatitis B virus DNA. Gastroenterology 2014; 146:1240–1248. doi: 10.1053/j.gastro.2014.01.044. [DOI] [PubMed] [Google Scholar]
- 53.Lim YS, Yoo BC, Byun KS, Kwon SY, Kim YJ, An J, et al. Tenofovir monotherapy versus tenofovir and entecavir combination therapy in adefovir-resistant chronic hepatitis B patients with multiple drug failure: Results of a randomised trial. Gut 2016; 65:1042–1051. doi: 10.1136/gutjnl-2014-308435. [DOI] [PubMed] [Google Scholar]
- 54.Fung S, Kwan P, Fabri M, Horban A, Pelemis M, Hann HW, et al. Tenofovir disoproxil fumarate (TDF) vs. emtricitabine (FTC)/TDF in lamivudine resistant hepatitis B: A 5-year randomised study. J Hepatol 2017; 66:11–18. doi: 10.1016/j.jhep.2016.08.008. [DOI] [PubMed] [Google Scholar]
- 55.Lee SB, Jeong J, Park JH, Jung SW, Jeong ID, Bang SJ, et al. Low-level viremia and cirrhotic complications in patients with chronic hepatitis B according to adherence to entecavir. Clin Mol Hepatol 2020; 26:364–375. doi: 10.3350/cmh.2020.0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hepatitis Group, Chinese Society of Hepatology; Chinese Medical Association; Chinese Journal of Hepatology. An expert consensus for the adjustment of treatment strategies in patients with chronic hepatitis B treated with non-first-line nucleos(t)ide analogues (in Chinese). Chin J Hepatol 2019; 27:343–346. doi: 10.3760/cma.j.issn.1007-3418.2019.05.004. [DOI] [PubMed] [Google Scholar]
- 57.Guidelines for the prevention, care and treatment of persons with chronic hepatitis B infection. WHO Guidelines Approved by the Guidelines Review Committee. Geneva: World Health Organization; 2015. [PubMed] [Google Scholar]