Abstract
Aims/Introduction
Hyperglycemia, hypoglycemia, and blood glucose fluctuation are associated with the outcome in critically ill patients, but the target of blood glucose control is debatable especially in patients with diabetes regarding the status of blood glucose control before admission to ICU. This study aimed to investigate the association between the glycemic gap which is calculated as the mean blood glucose level during the first 7 days after admission to ICU minus the A1C‐derived average glucose and the outcome of critically ill patients with diabetes.
Method
This study was undertaken in two intensive care units (ICUs) with a total of 30 beds. Patients with diabetes who were expected to stay for more than 24 h were enrolled, the HbA1c was tested within 3 days after admission and converted to the A1C‐derived average glucose (ADAG) by the equation: ADAG = [(HbA1c * 28.7) – 46.7 ] * 18‐1, arterial blood glucose measurements were four per day routinely during the first 7 days after admission, the APACHE II score within the first 24 h, the mean blood glucose level (MGL), standard deviation (SD), and coefficient of variation (CV) during the first 7 days were calculated for each person, the GAPadm and GAPmean were calculated as the admission blood glucose and MGL minus the ADAG, respectively, the incidence of moderate hypoglycemia (MH) and severe hypoglycemia (SH), the total dosage of glucocorticoids and average daily dosage of insulin within 7 days, the duration of renal replacement therapy (RRT), ventilator‐free hours, and non‐ICU stay days within 28 days were also collected. The enrolled patients were divided into a survival group and a nonsurvival group according to survival or not at 28 days and 1 year after admission, and the relationship between parameters derived from blood glucose and mortality in the enrolled critically ill patients was explored.
Results
Five hundred and two patients were enrolled and divided into a survival group (n = 310) and a nonsurvival group (n = 192). It was shown that the two groups had a comparable level of HbA1c, the nonsurvivors had a greater APACHE II, MGL, SD, CV, GAPadm, GAPmean, and a higher incidence of hypoglycemia. A lesser duration of ventilator‐free, non‐ICU stay, and a longer duration of RRT were recorded in the nonsurvival group, who received a lower carbohydrate intake, a higher daily dosage of insulin and glucocorticoid. GAPmean had the greatest predictive power with an AUC of 0.820 (95%CI: 0.781–0.850), the cut‐off value was 3.60 mmol/L (sensitivity 78.2% and specificity 77.3%). Patients with a low GAPmean tended to survive longer than the high GAPmean group 1 year after admission.
Conclusions
Glycemic GAP between the mean level of blood glucose within the first 7 days after admission to ICU and the A1C‐derived average glucose was independently associated with a 28 day mortality of critically ill patients with diabetes, the predictive power extended to 1 year. The incidence of hypoglycemia was associated with mortality either.
Keywords: Diabetes, Glycemic gap, Glycosylated hemoglobin
Acute hyperglycemia in patients with diabetes could result from acute physiological stress, a high baseline blood glucose, or both. The absolute hyperglycemia is not directly associated with the mortality of critically ill patients with diabetes. GAPmean had a greater predictive power than GAPadm with an AUC of 0.820 (95%CI: 0.781–0.850), the cut‐off value was 3.60 mmol/L (sensitivity 78.2% and specificity 77.3%). Patients with a low GAPmean tended to survive longer than the high GAPmean group 1 year after admission.

Background
A disturbance of metabolism is commonplace in critically ill patients, hyperglycemia and hypoglycemia are proved to be risk factors for adverse outcomes in populations of acutely ill patients 1 , 2 , 3 . Nevertheless, Marik 4 , 5 suggested that hyperglycemia and insulin resistance might be preserved adaptive responses and beneficial to patients suffering from acute diseases. The association between the occurrence of hypoglycemia and poor outcome has been reported repeatedly 6 , however, whether hypoglycemia including an iatrogenic episode of hypoglycemia is of itself harmful or not remains unclear, especially corrected for baseline risk factors and for the duration of ICU stay 7 . Besides, glycemic fluctuation might be much more harmful than both hyperglycemia and hypoglycemia 8 , 9 , the variability of blood glucose as the most used index is confirmed to be associated with the mortality of critically ill patients 10 , which is controversial in some studies 11 , because this association is more commonly proven in the non‐DM cohort but not in the DM cohort 12, , 3 .
Acute hyperglycemia in patients with diabetes could result from acute physiological stress, a high baseline blood glucose, or both. Glycosylated hemoglobin (HbA1c) is used to represent the premorbid glycemia in the 3 months prior to intensive care unit (ICU) admission 13 and is used widely to judge the adequacy of diabetes treatment and to adjust therapy 14 . Furthermore, the glycemic gap, the difference between the admission blood glucose and the A1C‐derived average glucose (ADAG) levels, which has been used to evaluate disease severity, predict outcomes and explore the relationship between stress‐induced hyperglycemia (SIH) and mortality in critically ill patients with diabetes. It is confirmed that the glycemic gap can depress the impact of chronic hyperglycemia on the assessment of disease severity in patients with diabetes and optimally improve the value of the assessment consequently 15 .
However, we found the highest level of blood glucose occurred within the first 7 days mostly in preliminary experiment, which means the level of admission blood glucose could not reflect the severity of disease and SIH precisely. The objective of this study was to identify whether GAPmean, defined as the difference between the mean blood glucose level within the first 7 days after admission to ICU and the ADAG is independently associated with mortality in critically ill patients with diabetes or not and to evaluate the predictive power on outcomes compared with GAPadm, the difference between the admission blood glucose and ADAG.
Methods
Study design and setting
We conducted a prospective observational cohort study of consecutive patients with type 2 diabetes admitted to the general ICU between June 1, 2017 and May 31, 2020 in two general ICUs of two tertiary hospitals in Beijing with a total of 30 beds. The institutional review board for human investigation approved this study and waived the need for informed consent. The protocol was formulated by the director, performed by all the staff and closely supervised by a group of intensivists who were charged with this study.
Cohort and data collection
Adult patients admitted to our ICU during the 3 year period of the study, with a diagnosis of type 2 diabetes (in accordance with 1999 WHO diagnostic criteria for type 2 diabetes) estimated to stay over 24 h without oral feeding were enrolled, regardless of whether insulin or oral antidiabetic agents had been prescribed previously. Patients were excluded based on the following criteria: (i) an admission diagnosis of diabetic ketoacidosis or a hyperosmolar hyperglycemic state, (ii) treatment with corticosteroids or had been admitted to ICU within 3 months prior to admission, (iii) the patients or their representatives had signed informed consent of the withdrawal of life‐sustaining treatment within 28 days after admission, (iv) the level of HbA1c was not obtained and the number of blood glucose values obtained was no more than three during the period of study, (v) the ICU stay was no more than 24 h.
The medical records of enrolled patients were reviewed for the following data: age, sex, body mass index (BMI), whether receiving regular insulin therapy before admission, primary disorders, underlying comorbidities, the APACHE II score within the first 24 h after admission, laboratory data including arterial blood glucose level during the first 7 days, and HbA1c levels measured within 24 h after admission. The average daily amount of carbohydrate intake, average daily dosage of insulin (Novolin R) and total dosage of glucocorticoid (converted into the dosage of methylprednisolone) for the first 7 days were obtained.
Outcome indicators including the duration of ventilator‐free hours, renal replacement therapy (RRT), and non‐ICU length of stay during 28 days, survival or not at 28 days and 1 year and the survival time after admission were recorded. Survival or not at 28 days was the primary endpoint, which was the criterion for patients being separated into different groups.
Data of blood glucose level, HbA1c value and glycemic gap
We tested the arterial blood glucose level at least every 6 h during the first 7 days after admission to ICU for each patient using a blood‐gas analyzer (GEM PRIMIER3000) equipped with the current method. HbA1c in venous blood was detected within the first 24 h by high‐performance liquid chromatography.
Parameters including the mean blood glucose level (MGL), standard deviation (SD), and coefficient of variation (CV), which was the MGL divided by SD, during the first 7 days after admission were calculated based on measurements of blood glucose level for each patient. The incidence of moderate hypoglycemia (MH) defined as a blood glucose level in the range 2.2–3.3 mmol/L and severe hypoglycemia (SH) defined as a blood glucose level lower than 2.2 mmol/L were documented.
The HbA1c levels were converted into the A1C‐derived average glucose (ADAG) to represent chronic average blood glucose levels within 3 months before admission to ICU using the following equation: A1C‐derived average glucose (ADAG) = [(HbA1c * 28.7) – 46.7] * 18‐1[ 16 ]. The GAPadm was calculated as the admission blood glucose minus the ADAG as follows: GAPadm = [admission BG – ADAG], GAPmean was calculated as MGL minus ADAG as follows: GAPmean = [MGL – ADAG].
Statistical analysis
Consecutive data normally distributed are expressed as mean ± standard deviation and represented by quartiles in non‐normally distributed data, categorical data are expressed as frequencies (percentage). Analyses were performed by the 2‐tailed Student’s t‐test and the Chi‐square test or Fisher exact test. The factors associated with mortality at 28 days were analyzed using binary logistic regression and receiver operating characteristic (ROC) curves were plotted to analyze the discernibility of the predictive parameters, the area under the ROC curve (AUC), and the 95% confidence interval (CI) was calculated simultaneously to identify the relationship between the glycemic gap and the 28 day mortality. Youden’s index was applied to ascertain the preponderant value of the glycemic gap as an independently predictive factor of 28 day mortality. Survival analysis was shown as a Kaplan‐Meier survival curve. Data were analyzed using SPSS Statistics, Version 24.0. Graphs were built using Medcalc, Version 19.6.1 and GraphPad Prism, Version 8.0. A value of P <0.05 was considered statistically significant.
Results
Study population and baseline characteristics
In total, 1867 patients were admitted to the two general ICUs during the study period, 502 patients were enrolled, of which 192 (38.25%) died at 28 days after inclusion, based on which the patients were separated into two groups, survival and nonsurvival (Figure 1). Blood glucose samples (14552 in total and 28.99 per capita) were collected. The nonsurvivors tended to be older and had a higher APACHE II score compared with the survivors. The proportion of patients undergoing surgery in the nonsurvivors was lower than that of the survivors. There was no statistically significant difference in sex, BMI, insulin therapy before admission between the two groups. Nonsurvivors had higher rates of sepsis and postoperative care as the main reason for admission and were accompanied by cardiac and vascular disease and chronic renal disease (Table 1).
Figure 1.
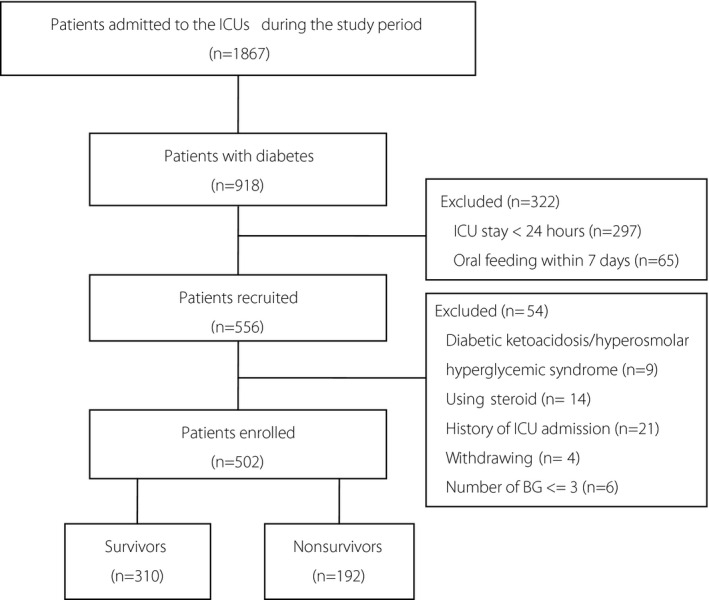
Flow chart of the study. 1867 patients were admitted to the two general ICUs during the study period, 918 patients were diagnosed with diabetes, 276 patients were excluded, 502 patients were enrolled, of which 310 (61.75%) survived and 192 (38.25%) had died at 28 days.
Table 1.
Baseline characteristics of the diabetic ICU survivors and nonsurvivors
|
Survivors (n = 310) |
Nonsurvivors (n = 192) |
All patients (n = 502) | P ‐ value | |
|---|---|---|---|---|
| Sex (male), n (%) | 184 (59.4) | 101 (52.6) | 285 (56.8) | 0.140 |
| Age (years) | 79 (68, 85) | 83 (77, 86.8) | 81 (71.8, 85.3) | <0.001* |
| BMI (kg/m2) | 24.2 (21.7, 26.1) | 23.9 (21.5, 26.1) | 24.2 (21.6, 26.1) | 0.245 |
| APACHE II score | 19 (14, 24) | 25.4 ± 8.7 | 21 (15, 27) | <0.001* |
| Surgical patients, n (%) | 43 (13.9) | 15 (7.8) | 58 (11.6) | 0.044* |
| Insulin therapy before ICU, n (%) | 133 (42.9) | 86 (44.8) | 219 (43.6) | 0.711 |
| Reasons for ICU admission, n (%) | ||||
| Sepsis | 74 (23.9) | 83 (43.2) | 157 (31.3) | <0.001* |
| Thoracic or respiratory disease | 97 (31.3) | 45 (23.4) | 142 (28.3) | 0.066 |
| Cardiac and vascular disease | 59 (19.0) | 42 (21.9) | 101 (20.1) | 0.492 |
| Neurologic disease | 20 (6.5) | 5 (2.6) | 25 (6.5) | 0.059 |
| Renal dysfunction | 19 (6.1) | 8 (4.2) | 27 (5.4) | 0.418 |
| Gastrointestinal disease | 19 (6.1) | 6 (3.1) | 25 (5.0) | 0.146 |
| Postoperative care | 14 (4.5) | 2 (1.0) | 16 (3.2) | 0.036* |
| Other | 8 (2.6) | 1 (0.5) | 9 (1.8) | 0.163 |
| Patient comorbidities | ||||
| Respiratory disease | 63 (20.3) | 47 (24.5) | 110 (21.9) | 0.318 |
| Cardiac and vascular disease | 272 (87.7) | 167 (95.3) | 390 (90.6) | 0.004* |
| Cerebrovascular disease | 205 (66.1) | 113 (58.9) | 318 (63.3) | 0.106 |
| Chronic renal disease | 113 (36.5) | 91 (47.4) | 178 (40.6) | 0.019* |
| Gastrointestinal disease | 20 (6.5) | 16 (8.3) | 36 (7.2) | 0.478 |
| Malignancy | 64 (20.6) | 31 (16.1) | 95 (18.9) | 0.241 |
P < 0.05. APACHE II scores, Acute Physiology and Chronic Health Evaluation II score.
Relevant data of blood glucose level
There were no significant differences in the HbA1c value and ADAG between the two groups, a greater level of BG at admission, MGL, SD, and CV were found in nonsurvivors, the incidence of MH and SH were more common among nonsurvivors who had a higher GAPadm and GAPmean (P < 0.05, Table 2).
Table 2.
Relevant data of plasma glucose levels and GAP
|
Survivors (n = 310) |
Nonsurvivors (n = 192) |
All patients (n = 502) | P ‐ value | |
|---|---|---|---|---|
| BG at admission (mmol/L) | 10.2 (7.7, 13.8) | 11.9 (9.0, 15.0) | 10.8 (8.2,14.3) | 0.002* |
| MGL (mmol/L) | 10.5 ± 3.1 | 12.7 ± 2.4 | 11.6 (9.4,13.3) | <0.001* |
| SD (mmol/L) | 2.6 (1.9, 3.5) | 3.9 ± 1.5 | 2.9 (2.1, 4.1) | <0.001* |
| CV (%) | 25.7 (20.3, 33.3) | 30.7 ± 10.4 | 27.6 (21.2, 34.9) | <0.001* |
| HbA1c (mmol/L) | 6.9 (6.1,7.9) | 7.0 (6.2, 7.8) | 6.9 (6.2,7.9) | 0.763 |
| ADAG (mmol/L) | 8.4 (7.1, 10.0) | 8.6 (7.3, 9.8) | 8.4 (7.3, 10.0) | 0.784 |
| GAPadm (mmol/L) | 1.8 (−0.6,4.4) | 3.3 (0.9, 6.3) | 2.3 (−0.2, 5.3) | <0.001* |
| GAPmean (mmol/L) | 2.4 (−0.1, 3.5) | 4.2 (3.7, 5.0) | 3.3 (1.2, 4.2) | <0.001* |
| MH, n (%) | 14 (4.5) | 45 (23.4) | 59 (11.8) | <0.001* |
| SH, n (%) | 4 (1.3) | 21 (10.9) | 25 (5.0) | <0.001* |
P < 0.05. ADAG, A1C‐derived average glucose; BG, blood glucose; GAPadm, glycemic gap between blood glucose at admission and ADAG; GAPmean, glycemic gap between MGL and ADAG; MGL, mean glucose level; MH, moderate hypoglycemia, blood glucose: 2.2–3.3 mmol/L; SH, severe hypoglycemia, blood glucose:<2.2 mmol/L.
Patients with different levels of HbA1c – whether higher than 6.5 mmol/L or not – were regarded as being under different blood glucose control. Patients were divided into four groups with interquartile ranges of MGL, the distribution of GAPmean moved to a higher level in nonsurvivors, regardless of the level of HbA1c or ADAG (Figure 2).
Figure 2.
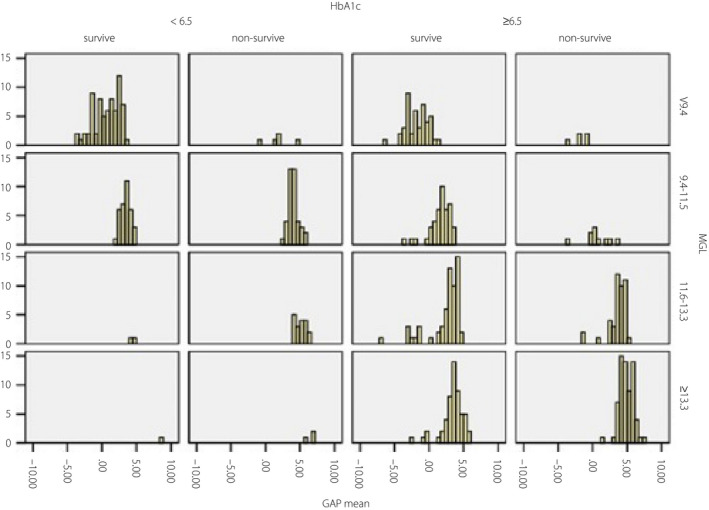
Frequency of GAPmean in survivors and nonsurvivors based on MGL categories. Patients were divided into four groups with interquartile ranges of MGL, the distribution of GAPmean moved to a higher level in nonsurvivors, regardless of the level of HbA1c or ADAG.
Therapy and outcome data
Nonsurvivors received a lower daily intake of carbohydrate and a higher daily dosage of insulin (Novolin R) and an accumulated dosage of glucocorticoid (converted into dosage of methylprednisolone) during the first 7 days of admission.
Outcome indicators including ventilator‐free hours and non‐ICU stay days during 28 days were longer and the duration of renal replacement therapy (RRT) was shorter among survivors (P < 0.05, Table 3).
Table 3.
Therapy and outcome data
|
Survivors (n = 310) |
Nonsurvivors (n = 192) |
All patients (n = 502) |
P ‐ value | |
|---|---|---|---|---|
| Carbohydrate intake (kcal/kg) | 150.2 (132.4, 171.4) | 141.1 (122.5, 162.2) | 145.6 (127.9, 168.1) | 0.013* |
| Insulin daily dosage (U) | 9.3 (0, 34.3) | 18.6 (2.5, 34.3) | 12.9 (0, 34.3) | 0.010* |
| Glucocorticoid dosage (mg) | 0 (0, 66.7) | 40 (0, 198.3) | 26.7 (0, 106.7) | <0.001* |
| Duration of ventilator‐free (h) | 551 (327.5, 652) | 1 (0, 43.5) | 257.5 (6, 590.3) | <0.001* |
| Duration of RRT (h) | 0 (0,0) | 0 (0, 44) | 0 (0, 11.3) | <0.001* |
| ICU‐free days (days) | 14 (1, 21) | 0 (0,0) | 0 (1,17) | <0.001* |
P < 0.05. MV, mechanical ventilation; RRT, renal replacement therapy.
Relative factors and predictors of 28 day mortality
Variables related to the primary outcome were screened out during single factor analysis and binary logistic regression analysis revealed the correlation between the indexes of age, APACHE II score, MH, SH, and GAPmean and mortality at 28 days (Table 4). The data showed a consistent trend of MH and SH, the two indexes were combined to generate the index ‘MH/SH’ and the OR value was higher than other factors, meaning that the incidences of MH or SH doubled the risk of death.
Table 4.
Predictors for mortality at 28 days
| OR (95%CI) | P ‐ value | ||
|---|---|---|---|
| Age |
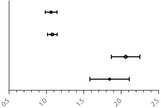
|
1.030 (1.007–1.153) | 0.010* |
| APACHE II score | 1.072 (1.019–1.147) | <0.001* | |
| MH/SH | 2.075 (1.862–2.243) | <0.001* | |
| GAPmean | 1.833 (1.588–2.115) | <0.001* |
P < 0.05. APACHE II scores, Acute Physiology and Chronic Health Evaluation II score; ADAG, A1C‐derived average glucose; GAPmean, glycemic gap between MGL and ADAG; MGL, mean glucose level; MH, moderate hypoglycemia, blood glucose: 2.2–3.3 mmol/L; SH, severe hypoglycemia, blood glucose: <2.2 mmol/L
The AUC of APACHE II, GAPadm, and GAPmean within the first 7 days to predict the mortality at 28 days were plotted, the AUC of GAPmean was the highest, which reflected a greater predictive power. The optimal cut‐off value of GAPmean to predict the 28 day mortality was 3.6 mmol/L (sorted by Youden index), which provided a sensitivity and specificity of 78.2% and 77.3%. APACHE II incorporated with GAPadm were performed as well, of which AUC was not improved markedly (Figure 3).
Figure 3.
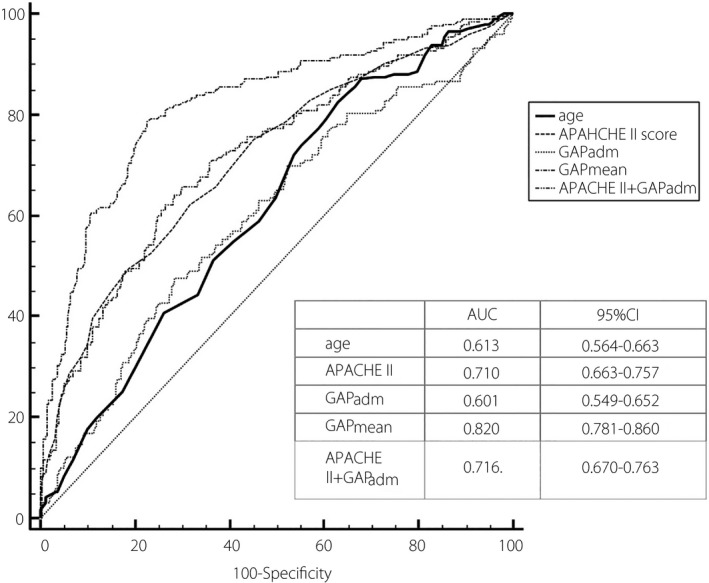
ROC curves for GAP and APACHE II score for predicting 28 day mortality. The AUC of GAPmean was the highest among that of other predictors for 28 day death. APACHE II incorporated with GAPadm were performed as well, of which AUC was not improved markedly.
Survival analysis
The Kaplan‐Meier survival curve shows that patients with a GAPmean higher than 3.6 mmol/L was associated with a significantly shorter survival than patients with a lower GAPmean, and the level of HbA1c did not make any difference to the survival at 1 year after admission (Figure 4).
Figure 4.
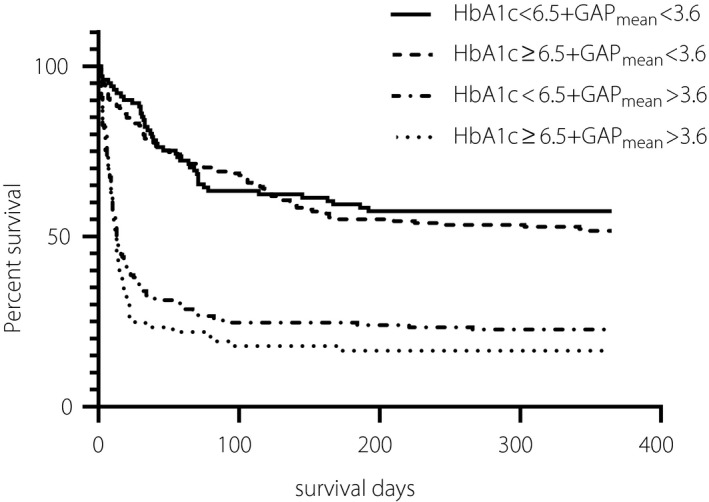
Kaplan‐Meier survival curve of HbA1c and GAPmean at 1 year. The Kaplan‐Meier survival curve shows that patients with a GAPmean higher than 3.6 mmol/L was associated with significantly shorter survival than patients with lower GAPmean at 1 year after admission, which is regardless of the level of HbA1c.
Discussion
Dysglycemia commonly occurs in critically ill patients with and without a history of diabetes, hyperglycemia being one of the common manifestations, with the degree of hyperglycemia being associated with a progressively worse outcome 17 . We actually found that the mean glucose level in nonsurvivors was higher than that in the survivors, which was not an independent predictor for the 28 day mortality. Some researchers dispute whether a raised blood glucose concentration is independently associated with a poor prognosis or that it may indicate a more severe illness with an increased response to stress 18 . These findings indicate that the absolute hyperglycemia level is not directly associated with the mortality of critically ill patients with diabetes. Evidence showed that hyperglycemia in critically ill patients could not be totally attributed to a stress response 19 . Researchers have concluded that admission hyperglycemia results from a combination of acute physiological stress or a higher baseline blood glucose 20 . Stress induced hyperglycemia presents secondarily to an elevated level of counter‐regulatory hormones (cortisol, catecholamines, glucagon, and growth hormone) and an impaired response, which results in increased gluconeogenesis and decreased glycogenolysis 21 , 22 . Moritoki et al. reported that in patients with critical illness‐associated hyperglycemia (CIAH) and ‘adequately controlled’ diabetes, acute hyperglycemia is associated with increased mortality, whereas in patients with ‘insufficiently controlled’ diabetes it is not 23 . Critically ill patients with an elevated level of HbA1c seem to better tolerate hyperglycemia and large glucose fluctuations compared with patients with a normal level of HbA1c 24 . Therefore, quantification of the level of chronic glycemia in critically ill patients is helpful for confirming the severity of critical illness‐associated dysglycemia 24 . The level of HbA1c represents premorbid chronic hyperglycemia before admission and is not affected by stress or fasting status, it is inconsiderable within‐day and in day‐to‐day variations 25 , HbA1c thus can be regarded as a parameter for distinguishing SIH and diabetic hyperglycemia 26 . Further evidence shows the difference between the blood glucose level at admission and ADAG was associated with adverse outcomes 27 , 28 .
Fawzy 29 reported that the elevated glycemic gap between the admission blood glucose and the previous glycemic level was associated with an increased ICU mortality, and the predictive power for the mortality of critically ill patients was improved effectively with the APACHE II score incorporated 30 . Meanwhile, the stress hyperglycemia ratio, that is, the fasting glucose concentration at admission divided by the ADAG, confirmed by Fabbri et al. 31 , 32 , was predictive of mortality following admission for sepsis, and it may be used to refine the prediction of an unfavorable outcome. Nevertheless, this study had witnessed the unparalleled predictive power of GAPmean on 28 day mortality in critically ill patients with diabetes compared with other parameters, GAPadm with smaller AUC than previous studies, despite improvement attributed to the incorporation of APACHE II, the AUC was lower than 0.70. The reasons might refer to diverse reactivities among individuals, severity, and progression of the disease, a single point blood glucose value could not reflect the reality and variation of the disease veritably and timely with numerous impacted factors and unforeseen circumstances, whereas the mean level of blood glucose during several days after admission to ICU might provide more comprehensive clinical information. This difference between the mean level of blood glucose after admission to ICU and the level of chronic glycemia demonstrates the situation of the patients virtually, and sequentially shows the prominent predictive power for mortality of the critically ill patients enrolled, which persists up to a year later.
Hypoglycemia is proved to be an independent predictor for mortality of critically ill patients with diabetes in this study, which is consistent with previous studies, and we did not find a correlation with the amount of carbohydrate intake and the dosage of insulin and glucocorticoid. Patients with diabetes tend to be tolerant of prolonged hyperglycemia and might be adaptive to a wider and individualized range of blood glucose 24 . In critically ill patients, chronic pre‐morbid hyperglycemia increases the risk of hypoglycemia and modifies the association between acute hypoglycemia and mortality 33 , the association between hypoglycemia and outcome is confounded by the severity of illness, with sicker patients being more prone to spontaneous hypoglycemia, patients with poorly controlled diabetes, as expressed by a high HbA1c level, appear to be more vulnerable to hypoglycemia 33 , tight glucose control makes it worse with the use of insulin‐providing medications 34 . Hypoglycemia is confirmed repeatedly to be associated with ICU mortality, regardless of whether the patients are diagnosed with diabetes, which may result in a drastic fluctuation of blood glucose and induce more serious cellular impairment 35 . This might be the reason why studies fail to replicate the benefit of tight glycemic control on ICU mortality 36 . The above conclusion leads us to implement a more rational and effective protocol to monitor and control the level of blood glucose to avoid or balance the two extremes which are ‘uncontrolled hyperglycemia’ and ‘over tightly controlled glucose’ 37 . Therefore, hypoglycemia may indicate the severity of acute illnesses and it seems prudent to prevent long‐lasting hypoglycemia as much as possible by frequent and accurate blood glucose measurements and by the use of a proper insulin protocol with safe and rational blood glucose range.
Limitations
There are limitations to the study. First, this is a study conducted in two general ICUs with a limited number of samples, the patients enrolled were admitted for medical diseases more than for surgical diseases, thus a selection bias may exist. Second, there remains a controversy about the strategy of controlling and the target range of blood glucose. Insulin was administered intravenously continuously or subcutaneously intermittently to achieve the target blood glucose level in the range 8.0–10.0 mmol/L, which might not be appropriate for the cohort. It is approved that long‐lasting hypoglycemia is associated with short term mortality in critically ill patients 38 , but we did not assess the duration of hypoglycemia. Third, we did not exclusively analyze the impact on blood glucose, that the type of nutritional support, or medication such as catecholamine, diuretic or antibiotics may have, the level of lactic acid was not documented either. Fourth, no consensus has been reached to point to the foremost tool for identifying the severity of critically ill patients 39 . More studies are required to evaluate various scoring tools for predicting mortality in ICU, such as sequential organ failure assessment (SOFA). Finally, the detection method of HbA1c is not uniform in our country, which is the reason that HbA1c cannot be used as a diagnostic criterion and be compared between different hospitals at present. It is necessary to standardize the testing method for HbA1c and to carry out multi‐center studies to increase the sample size and to balance the process of monitoring and controlling the level of blood glucose in the future, subgroup analysis of the effects of related medication and classifications of adverse outcome may be needed.
Conclusions
In this study, an elevated glycemic gap between the mean blood glucose level in the first 7 days after admission to ICU and A1C‐derived average glucose( ADAG) was independently associated with 28 day mortality in critically ill patients with diabetes, the predictive power on mortality was superior to GAPadm (the difference between the value of blood glucose at admission and ADAG) even incorporated with APACHE II score, patients with a lower GAPmean survived longer than patients with a higher GAPmean 1 year after admission. Hypoglycemia is also an independent predictor for the mortality of critically ill patients with diabetes.
Disclosure
The authors declare no conflict of interest.
Acknowledgments
This research was approved by Fu Xing Hospital, Capital Medical University IRB with the approval notice number 2015FXHEC‐KY012 on May 10, 2017.
J Diabetes Investig 2021; 12: 2212–2220
References
- 1. Finfer S, Liu B, Chittock DR, et al. Hypoglycemia and risk of death in critically ill patients. N Engl J Med 2012; 367: 1108–1118. [DOI] [PubMed] [Google Scholar]
- 2. Krinsley JS, Schultz MJ, Spronk P, et al. Mild hypoglycemia is independently associated with increased mortality in the critically ill. Crit Care 2011; 15: R173–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mamtani M, Kulkarni H, Bihari S, et al. Degree of hyperglycemia independently associates with hospital mortality and length of stay in critically ill, nondiabetic patients: results from the ANZICS CORE binational registry. J Crit Care 2020; 55: 149–156. [DOI] [PubMed] [Google Scholar]
- 4. Marik PE. Tight glycemic control in acutely ill patients: low evidence of benefit, high evidence of harm! Intensive Care Med 2016; 42: 1475–1477. [DOI] [PubMed] [Google Scholar]
- 5. Marik PE, Bellomo R. Stress hyperglycemia: an essential survival response! Crit Care 2013; 17: 305–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Krinsley JS, Egi M, Kiss A, et al. Diabetic status and the relation of the three domains of glycemic control to mortality in critically ill patients: an international multicenter cohort study. Crit Care 2013; 17: R37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mesotten D, Gielen M, Sterken C, et al. Neurocognitive development of children 4 years after critical illness and treatment with tight glucose control: a randomized controlled trial. JAMA 2012; 308: 1641–1650. [DOI] [PubMed] [Google Scholar]
- 8. Deane AM, Horowitz M. Dysglycaemia in the critically ill – significance and management. Diab Obes Metab 2013; 15: 792–801. [DOI] [PubMed] [Google Scholar]
- 9. Hans DeVries J. Glucose variability: where it is important and how to measure it. Diabetes 2013; 62: 1405–1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hermanides J, Vriesendorp TM, Bosman RJ, et al. Glucose variability is associated with intensive care unit mortality. Crit Care Med 2010; 38: 838–842. [DOI] [PubMed] [Google Scholar]
- 11. Falciglia M, Freyberg RW, Almenoff PL, et al. Hyperglycemia related mortality in critically ill patients varies with admission diagnosis. Crit Care Med 2009; 37: 3001–3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sechterberger MK, Bosman RJ, Oudemans‐van Straaten HM, et al. The effect of diabetes mellitus on the association between measures of glycemic control and ICU mortality: a retrospective cohort study. Crit Care 2013; 17: R52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Oriot P, Hermans MP. "Mind the gap please…": estimated vs. measured A1c from continuous measurement of interstitial glucose over a 3‐month period in patients with type 1 diabetes. Acta Clin Belg 2020; 75 2:109–115. [DOI] [PubMed] [Google Scholar]
- 14. Ahmed S, Fawzia A, Vincenzo DS, et al. Translating the HbA1c assay into estimated average glucose values in children and adolescents with type 1 diabetes mellitus. Acta bio‐medica: Atenei Parmensis 2020 2018; 89: 22–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Liao W‐I, Wang J‐C, Chang W‐C, et al. Usefulness of glycemic gap to predict ICU mortality in critically ill patients with diabetes. Medicine 2015; 94: e1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nathan DM, Kuenen J, Borg R, et al. Translating the A1C assay into estimated average glucose values. Diab Care 2008; 31: 1473–1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gunst J, Doig GS. The optimal blood glucose target in critically ill patients: more questions than answers. Intensive Care Med 2017; 43: 110–112. [DOI] [PubMed] [Google Scholar]
- 18. Riyaz M, Imran I, Jaiswal RK, et al. A study to establish association of hyperglycemia and inpatient mortality in patients with undiagnosed diabetes mellitus. J Evid Based Med Healthc 2015; 2: 3338–3344. [Google Scholar]
- 19. Zhang H‐Y, Cai‐jun W, Li C‐S. Glycated hemoglobin A1C and diabetes mellitus in critically ill patients. World J Emerg Med 2013; 4: 201–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. McAlister FA, Majumdar SR, Blitz S, et al. The relation between hyperglycemia and outcomes in 2,471 patients admitted to the hospital with community‐acquired pneumonia. Diab Care 2005; 28: 810–815. [DOI] [PubMed] [Google Scholar]
- 21. Aleman L, Guerrero J. Sepsis hyperglycemia in the ICU: from the mechanism to the clinic. Rev Med Chil 2018; 146: 502–510. [DOI] [PubMed] [Google Scholar]
- 22. Shebiny AAE, Elewa GM, Gouda EAG, et al. Glucose intolerance in intensive care patients: Incidence and outcome. Egyp J Anaesthesia 2021; 37: 28–34. [Google Scholar]
- 23. Egi M, Bellomo R, Stachowski E, et al. Dysglycaemia in the critically ill and the interaction of chronic and acute glycaemia with mortality. Intensive Care Med 2014; 40: 973–980. [DOI] [PubMed] [Google Scholar]
- 24. Balintescu A, Mårtensson J. Hemoglobin A1c and permissive hyperglycemia in patients in the intensive care unit with diabetes. Crit Care Clin 2019.35 2:289–300. [DOI] [PubMed] [Google Scholar]
- 25. Adamska E, Waszczeniuk M, Goscik J, et al. The usefulness of glycated hemoglobin A1c (HbA1c) for identifying dysglycemic states in individuals without previously diagnosed diabetes. Adv Med Sci 2012; 57: 296–301. [DOI] [PubMed] [Google Scholar]
- 26. Kundu D, Roy A, Mandal T, et al. Relation of microalbuminuria to glycosylated hemoglobin and duration of type 2 diabetes. Niger J Clin Pract 2013; 16: 216–220. [DOI] [PubMed] [Google Scholar]
- 27. Chih‐Jen Yang, Wen‐I Liao, Jen‐Chun Wang, et al. Usefulness of glycated hemoglobin A1c‐based adjusted glycemic variables in diabetic patients presenting with acute ischemic stroke. Am J Emerg Med 2017; 35: 1240–1246. [DOI] [PubMed] [Google Scholar]
- 28. Donagaon S, Dharmalingam M. Association between glycemic gap and adverse outcomes in critically ill patients with diabetes. Indian J Endocrinol Metab 2018; 22: 208–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. El‐Toony, El‐Zohri, Sc MH, et al. Admission hyperglycemia and its implications on outcome in patients attending medical intensive. Care Units at Assiut University Hospital 2018: 869; 2217–2222. [Google Scholar]
- 30. Fawzy F, Saad MSS, ElShabrawy AM, et al. Effect of glycemic gap on short term outcome in critically ill patient: In zagazig university hospitals. Diab Metab Syndrome: Clin Res Rev 2019; 13: 1325–1328. [DOI] [PubMed] [Google Scholar]
- 31. Fabbri A, Marchesini G, Benazzi B, et al. Stress hyperglycemia and mortality in subjects with diabetes and sepsis. Crit Care Explor 2020; 2: e0152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lee TF, Drake SM, Roberts GW, et al. Relative hyperglycemia is an independent determinant of in‐hospital mortality in patients with critical illness. Crit Care Med 2020; 48: e115–e122. [DOI] [PubMed] [Google Scholar]
- 33. Egi M, Krinsley JS, Maurer P, et al. Pre‐morbid glycemic control modifies the interaction between acute hypoglycemia and mortality. Intensive Care Med 2016; 42: 562–571. [DOI] [PubMed] [Google Scholar]
- 34. Arnold SV, Lipska KJ, Wang J, et al. Use of intensive glycemic management in older adults with diabetes mellitus. J Am Geriatr Soc 2018; 66: 1190–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhou J, Jia W, Bao Y, et al. Glycemic variability and its responses to intensive insulin treatment in newly diagnosed type 2 diabetes. Med Sci Monitor Int Med J Exp Clin Res 2008; 14: CR552. [PubMed] [Google Scholar]
- 36. Uyttendaele V, Dickson JL, Shaw GM, et al. Untangling glycaemia and mortality in critical care. Crit Care 2017; 21: 152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lou R, Jiang L. Association between glucose variability and poor prognosis in critically ill patients. Zhonghua Yi Xue Za Zhi 2015; 95: 523–526. [PubMed] [Google Scholar]
- 38. Yamada T, Shojima N, Noma H, et al. Glycemic control, mortality, and hypoglycemia in critically ill patients: a systematic review and network meta‐analysis of randomized controlled trials. Intensive Care Med 2017; 43: 1–15. [DOI] [PubMed] [Google Scholar]
- 39. Kumar S, Gattani SC, Baheti AH, et al. Comparison of the performance of APACHE II, SOFA, and mNUTRIC scoring systems in critically Ill patients: a 2‐year cross‐sectional study. Indian J Crit Care Med 2020; 24: 1057–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]


