Abstract
Aims/Introduction
Differences in the glucose‐lowering mechanisms of glucagon‐like peptide‐1 receptor agonists (GLP‐1RAs) have been noted. Clarifying these differences could facilitate the choice of optimal drugs for individuals with type 2 diabetes and requires investigation in a clinical setting.
Materials and Methods
A single‐arm, prospective, observational study was conducted to evaluate the effects of various GLP‐1RAs on postprandial glucose excursion, secretions of insulin and glucagon as well as on the gastric emptying rate. Participants were subjected to meal tolerance tests before and 2 weeks and 12 weeks after GLP‐1RA initiation. Effects on postprandial secretions of glucose‐dependent insulinotropic polypeptide (GIP) and apolipoprotein B48 were also investigated.
Results
Eighteen subjects with type 2 diabetes received one of three GLP‐1RAs, i.e., lixisenatide, n = 7; liraglutide, n = 6; or dulaglutide, n = 5. While 12‐week administration of all of the GLP‐1RAs significantly reduced HbA1c, only lixisenatide and liraglutide, but not dulaglutide, significantly reduced body weight. Postprandial glucose elevation was improved by all of the GLP‐1RAs. Postprandial insulin levels were suppressed by lixisenatide, while insulin levels were enhanced by liraglutide. Postprandial glucagon levels were suppressed by lixisenatide. The gastric emptying rate was significantly delayed by lixisenatide, while liraglutide and dulaglutide had limited effects on gastric emptying. GIP secretion was suppressed by lixisenatide and liraglutide. Apolipoprotein B48 secretion was suppressed by all of the GLP‐1RAs.
Conclusions
All of the GLP‐1RAs were found to improve HbA1c in a 12‐week prospective observational study in Japanese individuals with type 2 diabetes. However, differences in the mechanisms of the glucose‐lowering effects and body weight reduction were observed.
Keywords: Gastric emptying, GLP‐1 receptor agonist, islet hormones
GLP‐1 receptor agonists (lixisenatide, liraglutide, and dulaglutide) were found to improve postprandial glucose excursions in Japanese individuals with type 2 diabetes. However, differences in the mechanisms of the glucose‐lowering effects were observed.

INTRODUCTION
The glucagon‐like peptide‐1 (GLP‐1) receptor agonist (GLP‐1RA) has been gaining attention as an anti‐diabetes drug with cardiovascular and renal benefits 1 , 2 , 3 , 4 . Preclinical and clinical studies have demonstrated that GLP‐1 ameliorates secretions of insulin and glucagon and delays gastric emptying, thereby improving postprandial glucose excursion 5 , 6 , 7 . Since GLP‐1 is highly susceptible to degradation by the dipeptidyl‐peptide‐4 (DPP‐4) exenatide, a 39‐amino acid peptide isolated from the venom of Gila monster that potently activates the GLP‐1 receptor and is resistant to DPP‐4 degradation, and its derivative lixisenatide, were developed as GLP‐1RAs having a relatively short half‐life (exenatide, 1.4 h and lixisenatide 2.5 h) 8 , 9 . Liraglutide was also developed as a GLP‐1RA derived from human GLP‐1 and is similarly resistant to DPP‐4 degradation but has a relatively long half‐life in circulation (i.e., 13.5 h) due to its binding to albumin via its fatty acid moiety 10 , 11 . According to their half‐lives in circulation, exenatide and lixisenatide are categorized as short‐acting GLP‐1RAs, while liraglutide is categorized as a long‐acting GLP‐1RA. More recently, exenatide LAR, dulaglutide, and semaglutide have been developed as once‐weekly injectable GLP‐1RAs having much longer half‐lives in circulation (exenatide LAR, >24 h; dulaglutide, approximately 90 h; and semaglutide approximately 1 week) to lessen the burden of injections 12 , 13 , 14 ; exenatide, lixisenatide, and liraglutide require once or twice daily injection. On the other hand, exenatide LAR, dulaglutide, and semaglutide are categorized as ultralong‐acting GLP‐1RAs. The Meier study has demonstrated differing glucose‐lowering mechanisms of the short‐acting GLP‐1RA lixisenatide and the long‐acting GLP‐1RA liraglutide in individuals with type 2 diabetes on optimized insulin glargine with and without metformin 15 . As ≥50% of the patients in that study were Caucasians with type 2 diabetes and obesity, the findings remain to be established in east Asians, who typically have a lean diabetes phenotype 16 , 17 . Several studies have evaluated the effects of GLP‐1RAs on the secretion of insulin and glucagon and on the gastric emptying rate in Japanese individuals with type 2 diabetes 18 , 19 . In addition, similarities and differences between the glucose‐lowering mechanisms of ultralong‐acting GLP‐1RAs and short‐ and long‐acting GLP‐1RAs need to be addressed. As clarification of these differences might facilitate the clinical choice of optimal drugs for diabetes patients, we investigated these differences in a clinical setting.
MATERIALS AND METHODS
Method
The protocol (UMIN registration number: UMIN000042797) was approved by the ethics committee of Kansai Electric Power Hospital (IRB approval no. 25‐34) and written informed consent was obtained from all participants. The study was conducted according to the principles expressed in the Declaration of Helsinki.
Participants
Japanese individuals with type 2 diabetes, untreated or receiving biguanides or sulfonylureas, were recruited at a private hospital in Osaka. Inclusion criteria were as follows: Age 40–75 years, HbA1c 12.0% or less, and BMI 40 kg/m2 or less. Exclusion criteria were as follows: Subjects with type 1 diabetes, severe diabetic autonomic neuropathy, gastrointestinal tract disease including gastroparesis, history of gastrointestinal operation, cardiac disease, pulmonary disease, pancreatic disease, thyroid disease, liver disease, renal disease, alcohol or drug abuse, anti‐diabetes drugs other than biguanides or sulfonylureas, diabetogenic medication, malignancy, or pregnancy. Diagnosis of type 2 diabetes accorded to the criteria of the Japanese Diabetes Society 20 . The patients were subjected to meal tolerance tests before and 2 weeks and 12 weeks after GLP‐1RA initiation. GLP‐1RAs were chosen by investigators based on patient preference and clinical characteristics. Doses of co‐administered drugs (i.e., sulfonylureas or metformin) were not changed during the observation period. Once daily lixisenatide was given at 10 μg during week 1 after the MTT at week 0, 15 μg during week 2 and 20 μg on the day of the MTT at week 2 and thereafter. Similarly, once daily liraglutide was given at 0.3 mg during week 1, 0.6 mg during week 2 and 0.9 mg on the day of the MTT at week 2 and thereafter. Once weekly dulaglutide was given at 0.75 mg throughout the study.
Meal tolerance test (MTT)
Participants were subjected to meal tolerance tests in the morning after an overnight fast. Briefly, a Japanese standard meal (480 kcal, carbohydrate: protein: fat = 2.8:1:1) was ingested within 10 min. To measure the levels of glucose and selected hormones, blood samples were withdrawn at 0, 10, 20, 30, 60, 90, 120, and 240 min and stored as described previously 21 . To analyze the gastric emptying rate, end‐tidal breath samples were collected into small exhalation bags (PYLORI‐BAG20, Otsuka Electronics Co., Ltd, Osaka, Japan) at −15 min, 0 min, and every 5 min until 120 min and every 30 min until 240 min, as recommended by the Japan Society of Smooth Muscle Research 22 . End‐tidal breath samples were also collected into three large exhalation bags (PYLORI‐BAG1.3L, Otsuka Electronics Co., Ltd, Osaka, Japan) at −15 min to use as a reference for exhalation gas analysis. The rice was prepared by steaming with C13‐labeled sodium acetate (Catalog number CLM‐156‐0, Cambridge Isotope Inc., MA, USA) at a ratio of 150 g steamed rice and 200 mg of C13‐labeled sodium acetate and was stored at −20°C until use. The steamed rice and side dishes were microwaved before being served.
Laboratory determinations
Hormones were measured using the following assays as described previously 23 , 24 . Total GIP, Human GIP (total) ELISA (Catalog number EZHGIP‐54K; Merck Millipore, Darmstadt, Germany); Glucagon, Glucagon enzyme‐linked immunosorbent assay (Catalog number 10‐1271‐01; Mercodia, Uppsala, Sweden); and Insulin, lumipulse presto insulin (Fujirebio Inc., Tokyo, Japan). The gastric emptying rate was determined by mathematical modeling based on changes of the 13CO2/12CO2 ratio in breath samples measured by an infrared spectral analyzer (POCone, Otsuka Electronics Co., Ltd, Osaka, Japan) 25 . Stable 13C‐labeled sodium acetate is emptied from the stomach following the trituration and liquefaction of the steamed rice and is transported to the liver via the portal vein, where it is oxidized to 13CO2 and exhaled into breath. The Wagner‐Nelson method was applied to adjust the time for 13CO2 distribution 26 , 27 . Other laboratory measurements including HbA1c, plasma glucose (PG), triglycerides, apolipoprotein (ApoB48), and non‐esterified fatty acids (NEFA) were by standard assays.
Statistical analyses
The results are reported as mean ± standard error. The AUC of each measurement was calculated according to the trapezoidal rule. All statistical calculations were performed using IBM SPSS for Windows ver. 25 (Armonk, NY). Repeated measures were analyzed by repeated measured two‐way ANOVA for all, lixisenatide, liraglutide, and dulaglutide. Other parameters including AUCs and half time for GEs were compared by a paired t‐test. Multiplicity was not accounted for because all analyses were performed in an exploratory manner. No data imputation was performed as there were no missing data. A P value <0.05 indicates a significant difference.
RESULTS
Eighteen subjects with type 2 diabetes received one of three GLP‐1RAs, i.e., lixisenatide n = 7 (monotherapy n = 5, biguanide‐combination n = 1, and sulfonylurea‐combination n = 1), liraglutide n = 6 (monotherapy n = 5 and sulfonylurea‐combination n = 1), and dulaglutide n = 5 (monotherapy n = 4 and biguanide‐combination n = 1) (Table 1). The subjects had a relatively short duration of type 2 diabetes and did not have diabetic neuropathy based on the simplified diagnostic criteria of diabetic polyneuropathy proposed by the Conference on Diabetic Polyneuropathy (revised January 18, 2002) 28 . Subjects receiving dulaglutide tended to be older than those receiving lixisenatide or liraglutide (Table 1). Subjects receiving lixisenatide tended to have a higher body weight and BMI as well as HOMA‐β and HOMA‐IR than those receiving liraglutide or dulaglutide (Table 1). However, age, duration, height, body weight, BMI, HbA1c, FPG, insulin, HOMA‐β, HOMA‐IR, C‐peptide, and CPI at baseline did not show statistically significant differences among subjects receiving lixisenatide, liraglutide, and dulaglutide (Table 1).
Table 1.
Clinical characteristics before and 12 weeks after initiation of glucagon‐like polypeptide‐1 receptor agonists
| All | Lixisenatide | Liraglutide | Dulaglutide | ||
|---|---|---|---|---|---|
| n | 18 | 7 | 6 | 5 | |
| % female | 27.8 | 14.3 | 16.7 | 60.0 | |
| % Biguanides | 11.1 | 14.3 | 0 | 20.0 | |
| % Sulfonylureas | 11.1 | 14.3 | 16.7 | 0 | |
| Age (years) | 48.4 ± 1.8 | 45.6 ± 0.9 | 49.0 ± 4.7 | 51.8 ± 3.4 | |
| Duration (years) | 5.8 ± 1.5 | 4.6 ± 1.9 | 8.2 ± 3.8 | 4.6 ± 2.1 | |
| Height (cm) | 168.6 ± 2.3 | 173.0 ± 3.4 | 168.2 ± 3.2 | 163.0 ± 5.4 | |
| Bodyweight (kg) | Baseline | 86.1 ± 5.1 | 98.9 ± 9.0 | 81.9 ± 5.0 | 73.2 ± 9.1 |
| Week 12 | 81.9 ± 4.7* | 94.4 ± 8.8* | 75.9 ± 3.7 | 71.8 ± 8.4 | |
| BMI (kg/m2) | Baseline | 29.9 ± 1.2 | 32.8 ± 2.3 | 28.9 ± 1.2 | 27.0 ± 1.8 |
| Week 12 | 28.5 ± 1.1* | 31.2 ± 2.2* | 26.8 ± 1* | 26.6 ± 1.7 | |
| HbA1c (%) | Baseline | 8.58 ± 0.28 | 8.21 ± 0.61 | 9.02 ± 0.31 | 8.58 ± 0.39 |
| Week 12 | 6.45 ± 0.16* | 6.27 ± 0.14* | 6.57 ± 0.45* | 6.56 ± 0.15 | |
| FPG (mg/dL) | Baseline | 139.4 ± 5.4 | 122.6 ± 5.7 | 149.3 ± 8.5 | 151.2 ± 10.7 |
| Week 12 | 121.3 ± 6.4* | 117.9 ± 8.8 | 124.3 ± 16.8 | 122.4 ± 5.9 | |
| Insulin (mU/L) | Baseline | 12.0 ± 1.6 | 15.0 ± 3.2 | 10.0 ± 1.8 | 10.1 ± 2.5 |
| Week 12 | 14.1 ± 1.7 | 18.8 ± 3.4* | 12.1 ± 2.0* | 10.0 ± 1.9 | |
| HOMA‐β (%) | Baseline | 61.6 ± 8.9 | 89.7 ± 15.3 | 41.7 ± 5.6 | 46.2 ± 14.8 |
| Week 12 | 102.0 ± 15.7* | 132.4 ± 25.2 | 97.8 ± 32.7 | 64.4 ± 13.2 | |
| HOMA‐IR | Baseline | 4.12 ± 0.55 | 4.72 ± 1.17 | 3.81 ± 0.79 | 3.67 ± 0.80 |
| Week 12 | 4.32 ± 0.63 | 5.67 ± 1.26* | 3.85 ± 0.95 | 2.99 ± 0.55 | |
| C‐peptide (ng/mL) | Baseline | 2.55 ± 0.18 | 2.83 ± 0.29 | 2.57 ± 0.25 | 2.12 ± 0.37 |
| Week 12 | 2.71 ± 0.18 | 3.03 ± 0.26 | 2.41 ± 0.21 | 2.63 ± 0.49 | |
| CPI | Baseline | 1.88 ± 0.15 | 2.32 ± 0.23 | 1.72 ± 0.12 | 1.46 ± 0.30 |
| Week 12 | 2.31 ± 0.18* | 2.67 ± 0.31 | 2.01 ± 0.16 | 2.19 ± 0.42 |
BMI, body mass index; FPG, fasting plasma glucose; HOMA, homeostatic minimal model of assessment; IR, insulin resistance; CPI, C‐peptide index. Note that age, duration, height, body weight, BMI, HbA1c, FPG, insulin, HOMA‐β, HOMA‐IR, C‐peptide, and CPI at baseline did not show a statistically significant difference among subjects receiving lixisenatide, liraglutide, and dulaglutide (Kruskal‐Wallis test). The subjects had a relatively short duration of type 2 diabetes and did not have diabetic neuropathy based on the simplified diagnostic criteria of diabetic polyneuropathy proposed by the Conference on Diabetic Polyneuropathy (revised January 18, 2002) 28 .
Indicates P < 0.05 vs baseline (paired t‐test).
HbA1c, body weight and BMI
The 12‐week GLP‐1RA administration significantly reduced HbA1c, body weight, and BMI (Table 1, All). While all three GLP‐1RAs significantly reduced HbA1c, only lixisenatide and liraglutide significantly reduced body weight and BMI; dulaglutide had limited effects on body weight and BMI (Table 1).
Glucose during MTT
The GLP‐1RA administration significantly reduced glucose levels during MTT (Figure 1a, All). Interestingly, postprandial glucose elevation was barely observed after initiation of lixisenatide (Figure 1a, Lixi), while some peaks were observed after initiation of liraglutide and dulaglutide (Figure 1a, Lira and Dula). The AUC0‐240‐Glucose was significantly reduced by all three GLP‐1RAs (Figure 1a, All, Lixi, Lira, and Dula).
Figure 1.
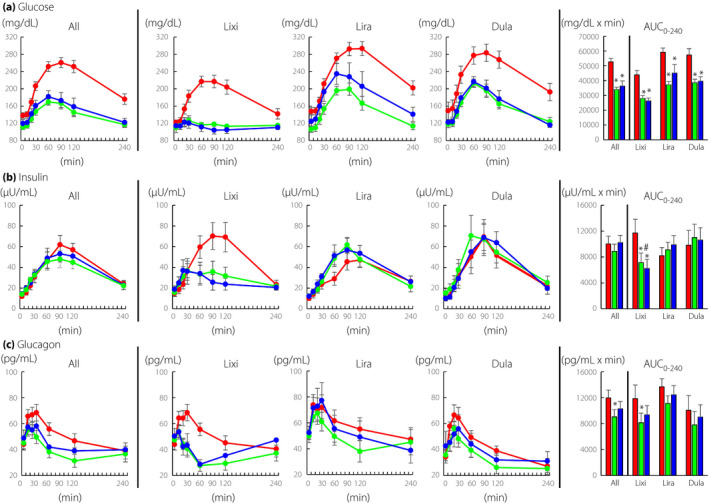
Time course curves are indicated for glucose, insulin, and glucagon during meal tolerance test before and after initiation of glucagon‐like peptide‐1 receptor agonists (0 week, red circles; 2 weeks, green circles; and 12 weeks, blue circles). P values for differences due to week (X), time (Y), and the interaction of week and time (Z) were calculated by mixed effects models. Glucose, All X0.000, Y0.000, and Z0.000; lixisenatide, X0.000, Y0.000, and Z0.000; liraglutide, X0.000, Y0.000, and Z0.000; dulaglutide, X0.001, Y0.000, and Z0.001. Insulin, All X0.718, Y0.000, and Z0.021; lixisenatide, X0.018, Y0.000, and Z0.008; liraglutide, X0.002, Y0.000, and Z0.000; dulaglutide, X0.407, Y0.000, and Z0.565. Glucagon, All X0.032, Y0.000, and Z0.003; lixisenatide, X0.061, Y0.000, and Z0.008; liraglutide, X0.556, Y0.000, and Z0.627; dulaglutide, X0.614, Y0.000, and Z0.107. Area‐under‐the curves (AUC)0min‐240min are indicated (0 week, red bar; 2 weeks green bar; and 12 weeks, blue bar). AUCs were analyzed by paired t‐test, and * indicates P < 0.05 (vs 0 week). Lixi, lixisenatide; Lira, liraglutide; and Dula, dulaglutide
Insulin during MTT
The GLP‐1RA administration significantly reduced insulin levels during MTT (Figure 1b, All). Postprandial insulin levels were significantly suppressed by lixisenatide (Figure 1b, Lixi), while insulin levels were significantly enhanced by liraglutide (Figure 1b, Lira). Insulin levels were slightly increased by dulaglutide but the difference did not reach statistical significance (Figure 1b, Dula). AUC0‐240‐Insulin at 2 weeks and 12 weeks were similar to that at 0 week (Figure 1b, All). AUC0‐240‐Insulin at 2 weeks and 12 weeks were significantly reduced by lixisenatide compared with that at 0 week (Figure 1b, Lixi). AUC0‐240‐Insulin at 2 weeks and 12 weeks after initiation of liraglutide or dulaglutide were increased compared to that at 0 week, but the differences did not reach statistical significance (Figure 1b, Lira and Dula). Since insulin levels are generally affected by glucose, AUC0‐240‐Insulin/AUC0‐240‐Glucose was also evaluated. AUC0‐240‐Insulin/AUC0‐240‐Glucose was statistically increased by liraglutide and dulaglutide at 2 weeks (All, 0 week 0.20 ± 0.03/2 weeks 2 0.27 ± 0.03*/12 weeks 0.25 ± 0.03; Lixisenatide, 0 week 0.27 ± 0.05/2 weeks 0.26 ± 0.05/12 weeks 0.24 ± 0.05; Liraglutide, 0 week 0.14 ± 0.02/2 weeks 0.25 ± 0.04*/12 weeks 0.24 ± 0.05; Dulaglutide, 0 week 0.19 ± 0.06/2 weeks 0.30 ± 0.07*/12 weeks 0.29 ± 0.06; *, P < 0.05 vs. 0 week).
Glucagon during MTT
The GLP‐1RA administration significantly reduced glucagon levels during MTT (Figure 1c, All). Postprandial glucagon levels were significantly suppressed by lixisenatide (Figure 1c, Lixi). Postprandial glucagon levels also tended to be reduced by liraglutide and dulaglutide, but the differences did not reach statistical significance (Figure 1b, Lira and Dula). The AUC0‐240‐Glucagon was reduced significantly by all GLP‐1RAs and lixisenatide at 2 weeks (Figure 1c, All and Lixi). AUC0‐240‐Glucagon tended to be reduced by liraglutide and dulaglutide, but the differences did not reach statistical significance (Figure 1c, Lira and Dula).
Gastric emptying during MTT
The GLP‐1RA administration significantly delayed gastric emptying during MTT (Figure 2, All). The gastric emptying rate was delayed significantly by lixisenatide (Figure 2, Lixi) and by liraglutide at a much lesser degree but with statistical significance (Figure 2, Lira), while dulaglutide administration had little effect on gastric emptying during MTT (Figure 2, Dula). Half times for gastric emptying (Time50%) were extended by GLP‐1RA administration with statistical significance at week 2 and week 12 (Figure 2, All). Times50% were extended by lixisenatide, with statistical significance at week 12. Times50% were largely unaffected by liraglutide and dulaglutide.
Figure 2.
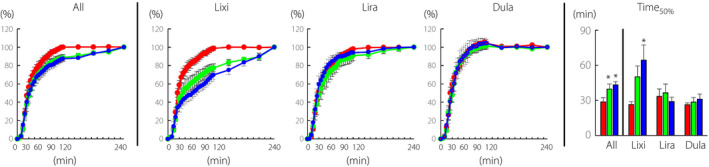
Time course curves are indicated for gastric emptying during meal tolerance test before and after initiation of glucagon‐like peptide‐1 receptor agonists (0 week, red circles; 2 weeks, green circles; and 12 weeks blue circles). P values for differences due to week (X), time (Y), and the interaction of week and time (Z) were calculated by mixed effects models. All X0.022, Y0.000, and Z0.000; lixisenatide, X0.003, Y0.000, and Z0.000; liraglutide, X0.374, Y0.000, and Z0.006; dulaglutide, X0.908, Y0.000, and Z1.000. Half times for emptying the ingested steamed rice (Time50%) are indicated. Time50% was analyzed by paired t‐test, and * indicates P < 0.05 (vs 0 week). Lixi, lixisenatide; Lira, liraglutide; and Dula, dulaglutide
GIP during MTT
The GLP‐1RA administration significantly reduced GIP levels during MTT (Figure 3, All). Postprandial GIP levels were significantly suppressed by lixisenatide and liraglutide (Figure 3, Lixi and Lira). Postprandial GIP levels also tended to be suppressed by dulaglutide without statistical significance (Figure 3, Dula). The AUC0‐240‐GIP was also reduced at 2 weeks and 12 weeks by GLP‐1RA administration compared with that at 0 week (Figure 3, All). AUC0‐240‐GIP was reduced at 2 weeks and 12 weeks by all three GLP‐1RAs (Figure 3, Lixi, Lira and Dura), but with statistical significance only by lixisenatide at 2 and 12 weeks and liraglutide at 12 weeks.
Figure 3.
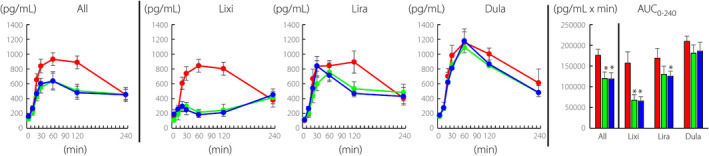
Time course curves are indicated for glucose‐dependent insulinotropic polypeptide (GIP) during meal tolerance test before and after initiation of glucagon‐like peptide‐1 receptor agonists (0 week, red circles; 2 weeks, green circles; and 12 weeks, blue circles). P values for differences due to week (X), time (Y), and the interaction of week and time (Z) were calculated by mixed effects models. All X0.000, Y0.000, and Z0.000; lixisenatide, X0.002, Y0.000, and Z0.000; liraglutide, X0.152, Y0.000, and Z0.000; dulaglutide, X0.449, Y0.000, and Z0.978. Area‐under‐the curves (AUC)0min‐240min are indicated (0 week, red bar; 2 weeks, green bar; and 12 weeks, blue bar). AUCs were analyzed by paired t‐test, and * indicates P < 0.05 (vs 0 week). Lixi, lixisenatide; Lira, liraglutide; and Dula, dulaglutide
Triglycerides, ApoB48, and NEFA during MTT
The GLP‐1RA administration had little effect on the levels of triglycerides during MTT (Figure 4a). The AUC0‐240‐triglycerides was largely unaffected by GLP‐1RA administration (Figure 4a). Postprandial ApoB48 levels were significantly suppressed by all three GLP‐1RAs (Figure 4b). The AUC0‐240‐ApoB48 was significantly reduced by GLP‐1RA administration at 2 weeks and 12 weeks compared with that at 0 week (Figure 3, All). The AUC0‐240‐ApoB48 was reduced by lixisenatide at 2 weeks and 12 weeks and by liraglutide at 2 weeks (Figure 3, Lxi and Lira). The GLP‐1RA administration had little effect on the levels of NEFA during MTT (Figure 4c). The AUC0‐240‐NEFA was largely unaffected by GLP‐1RA administration (Figure 4c).
Figure 4.
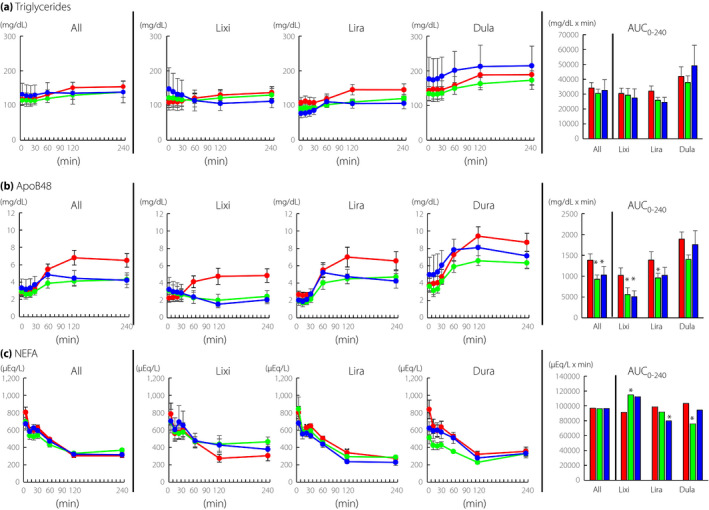
Time course curves are indicated for triglycerides, apolipoprotein B48 (ApoB48), and non‐esterified fatty acids (NEFA) during meal tolerance test before and after initiation of glucagon‐like peptide‐1 receptor agonists (0 week, red circles; 2 weeks, green circles; and 12 weeks, blue circles). P values for differences due to week (X), time (Y), and the interaction of week and time (Z) were calculated by mixed effects models. Triglycerides, All X0.681, Y0.000, and Z0.043; lixisenatide, X0.944, Y0.953, and Z0.162; liraglutide, X0.180, Y0.003, and Z0.01; dulaglutide, X0.540, Y0.000, and Z0.705. ApoB48, All X0.084, Y0.000, and Z0.000; lixisenatide, X0.337, Y0.928, and Z0.000; liraglutide, X0.157, Y0.000, and Z0.004; dulaglutide, X0.466, Y0.000, and Z0.045. NEFA, All X0.425, Y0.000, and Z0.000; lixisenatide, X0.710, Y0.000, and Z0.011; liraglutide, X0.096, Y0.000, and Z0.288; dulaglutide, X0.009, Y0.000, and Z0.000. Area‐under‐the curves (AUC)0min‐240min are indicated (0 week, red bar; 2 weeks, green bar; and 12 weeks, blue bar). AUCs were analyzed by paired t‐test, and * indicates P < 0.05 (vs 0 week). Lixi, lixisenatide; Lira, liraglutide; and Dula, dulaglutide
DISCUSSION
In this prospective observational study, we demonstrate that 12‐week GLP‐1RA administration significantly improves HbA1c and postprandial glucose excursion during MTT in Japanese individuals with type 2 diabetes in a clinical setting. We also find that the effects on insulin and gastric emptying rate differ among the GLP‐1RAs examined, indicating different mechanisms of improvement of glucose excursion by various GLP‐1RAs.
There were significant differences in the effects of the three GLP‐1RAs on insulin secretion and gastric emptying. Postprandial insulin elevation was barely observable after initiation of lixisenatide, while insulin levels were enhanced after liraglutide after meal ingestion. Postprandial glucagon elevation was suppressed by lixisenatide immediately after meal ingestion, while glucagon levels also tended to be suppressed by liraglutide. Gastric emptying was delayed after lixisenatide initiation, while the magnitude of the effects of liraglutide on gastric emptying was subtle. Once daily 0.9 mg was the maximum dose of liraglutide approved in Japan when the current study was performed; it is possible that once daily administration of 1.8 mg liraglutide in Japanese individuals with type 2 diabetes might slightly delay gastric emptying as it does in Caucasians with type 2 diabetes 15 . However, our current results indicate that the short‐acting GLP‐1RA lixisenatide exerts its glucose‐lowering effects mainly by delaying the gastric emptying rate and by suppressing glucagon secretion, while the long‐acting GLP‐1RA liraglutide does so mainly by enhancing insulin secretion and possibly by suppressing glucagon secretion. Postprandial insulinotropic and glucagonostatic effects of dulaglutide have been suggested 29 , but its effects on gastric emptying were found to be minimal in the current study. Thus, the ultralong‐acting GLP‐1RA dulaglutide exerts its glucose‐lowering effects likely by enhancement of insulin secretion and by suppression of glucagon secretion.
It was demonstrated in healthy volunteers that acute GLP‐1 infusion delays gastric emptying robustly 30 . Interestingly, the effect was largely attenuated by prolonged GLP‐1 infusion, while intermittent GLP‐1 infusion maintained the effect 30 . These results suggest that delayed gastric emptying by GLP‐1 is subject to desensitization/tachyphylaxis, which may underlie the differential effects of short‐acting, long‐acting, and ultralong‐acting GLP‐1RAs on gastric emptying. Indeed, it was previously demonstrated in rats that the effect of long‐acting GLP‐1RA liraglutide on gastric emptying was largely attenuated after 2‐week treatment of the drug while those of short‐acting GLP‐1RA exenatide was maintained 31 . Moreover, it was demonstrated that the effects of long‐acting or ultralong‐acting GLP‐1RAs on gastric emptying were much weaker than those of short‐acting GLP‐1RAs in individuals with type 2 diabetes 15 , 32 . Consistently, we found that the effects of liraglutide on gastric emptying were much less than those of lixisenatide, while those of dulaglutide were negligible (Figure 2).
It has been demonstrated that postprandial insulin secretion is enhanced by the long‐acting GLP‐1RA liraglutide, while it is suppressed by the short‐acting GLP‐1RA lixisenatide 15 , 33 . It has been postulated that the delayed gastric emptying by short‐acting GLP‐1RA lixisenatide delays the absorption of nutrients, thereby not only reducing postprandial glucose excursions but also lowering the postprandial secretion of insulin and glucagon. While it needs to be validated, possibly in individuals with gastrectomy and type 2 diabetes, this model could well explain the reduced postprandial secretions of GIP and ApoB48 found in the current study.
In the current study, we found that lixisenatide and liraglutide significantly suppressed meal‐induced GIP; dulaglutide tended to suppress GIP but the differences did not reach statistical significance. Suppression of GIP by lixisenatide began immediately after meal ingestion, suggesting that delay in gastric emptying and subsequent nutrition absorption plays a prominent role. On the other hand, suppression of GIP by liraglutide and dulaglutide began at a relatively later time point after meal ingestion. It was previously reported that fat‐induced GIP secretion is inhibited by insulin 34 . Thus, enhanced insulin secretion by liraglutide and dulaglutide may well contribute to the suppression of meal‐induced GIP.
In the current study, we found that lixisenatide and liraglutide significantly suppressed meal‐induced GIP; dulaglutide tended to suppress GIP but the differences did not reach statistical significance. Suppression of GIP by lixisenatide began immediately after meal ingestion, suggesting that delay in gastric emptying and subsequent nutrition absorption plays a prominent role. On the other hand, suppression of GIP by liraglutide and dulaglutide began at a relatively later time point after meal ingestion. It was previously reported that fat‐induced GIP secretion is inhibited by insulin 34 . Thus, enhanced insulin secretion by liraglutide and dulaglutide may well contribute to the suppression of meal‐induced GIP. Since insulin secretory capacity may differ among the three groups, the mechanisms underlying suppression of GIP secretion clearly requires further investigation in randomized clinical trials.
The levels of preprandial and postprandial serum levels of triglycerides did not differ before and after GLP‐1RA initiation in the present study. It is known that postprandial triglyceride levels are affected by various factors including the amount of fat in meals 35 . The mixed meal used in the current study contains a small amount of fat (i.e., 11.3 g). It was found recently that postprandial triglyceride elevation was not prominent after the ingestion of a similar mixed meal, and that the GLP‐1RA dulaglutide had few effects in Japanese subjects with type 2 diabetes 29 . Thus, postprandial triglyceride levels reflect intestinally derived chylomicrons and hepatically derived very low‐density lipoprotein. To address the effects of GLP‐1RAs specifically on intestinal lipid absorption, we therefore measured apoB48, a unique protein found in chylomicrons, that is suppressed by all three GLP‐1RAs. It has been demonstrated that GLP‐1 suppresses postprandial production and the release of chylomicron from enterocytes 36 , 37 , 38 . These findings lend support to the possibility that GLP‐1RAs suppress fat absorption from the intestine. While the underlying mechanisms of ApoB48 secretion suppression by GLP‐1RAs are not fully understood, it was reported that GLP‐1RA liraglutide treatment reduced the ApoB48 pool in individuals with type 2 diabetes 36 . It was also demonstrated that GLP‐1RA liraglutide treatment reduced the expression of genes involved in chylomicron synthesis (e.g., ApoB48, diglyceride acyltransferase, and microsomal transfer protein) in mouse jejunum slices 36 . Notably, the suppression of ApoB48 by lixisenatide began immediately after meal ingestion, suggesting that delayed gastric emptying and nutrition absorption also plays a prominent role.
We found significant weight reduction in individuals receiving lixisenatide and liraglutide, while a decrease by dulaglutide did not reach statistical significance. Questions remain as to why there are differences in body weight reduction among the different GLP‐RAs. Most relevant to this issue, dulaglutide is a larger molecule, i.e., an approximately 60 kDa fusion protein of human GLP‐1 with some amino‐acid replacements and a human immunoglobulin G Fc portion; the sizes of the other GLP‐1RAs available today are significantly smaller, in the range 4–5 kDa. It has been shown in rodents that some GLP‐1RAs (e.g., liraglutide and semaglutide) bind to GLP‐1 receptors in the hypothalamus and hindbrain, which are involved in appetite control 39 , 40 . It has been speculated that the differences in molecular weight might affect the uptake of GLP‐1RAs into the brain. Further studies are required to understand the differing effects of the various GLP‐1RAs on appetite suppression and body weight reduction.
The present study had several limitations. The current study is not a randomized clinical trial comparing each of the GLP‐1RAs. Differences in various baseline characteristics (e.g., BMI, HOMA‐β, and HOMA‐IR) make it difficult to compare the outcomes of each GLP‐1RAs quantitatively; their effects on the secretions of insulin and glucagon and on gastric emptying are therefore described qualitatively. Differences in the secretions of insulin, glucagon and GIP as well as on the gastric emptying rate and body weight reduction need to be compared in randomized clinical trials with adequate sample size. In addition, the maximum daily dose of liraglutide in Japan was limited to 0.9 mg, half of the maximum daily dose approved globally today, when the current study was conducted. Nevertheless, the current study provides valuable insight on the clinical use of the various GLP‐1RAs in Japanese individuals with type 2 diabetes.
In conclusion, GLP‐1RAs were found to improve HbA1c in a 12‐week prospective observational study in Japanese individuals with type 2 diabetes. Differences in the glucose‐lowering mechanisms and body weight reduction among different GLP‐1RAs are noted.
DISCLOSURE
The current study protocol (UMIN registration number: UMIN000042797) was approved by the ethics committee of Kansai Electric Power Hospital (IRB approval no. 25‐34 and approval date August 28, 2013). DY received consulting or speaker fees from Astellas Pharma Inc., Eli Lilly Japan, MSD, Novo Nordisk Pharma, Nippon Boehringer Ingelheim, Ono Pharmaceutical, Sumitomo Dainippon Pharma, Takeda Pharmaceutical. DY also received clinically commissioned/joint research grants from Ono Pharmaceutical, Novo Nordisk Pharma, Taisho Pharmaceutical, Arklay, and Terumo. YH received consulting or speaker fees from Novo Nordisk Pharma. TK received consulting or speaker fees from Sanofi. YuiY received consulting or speaker fees from MSD, Novo Nordisk Pharma, Ono Pharmaceutical, Sumitomo Dainippon Pharma, Takeda Pharmaceutical, Sanofi, Daiichi Sankyo, and Mitsubishi Tanabe Pharma. YuiY also received clinically commissioned/joint research grants from Novo Nordisk Pharma, Ono Pharmaceutical, Sumitomo Dainippon Pharma, Takeda Pharmaceutical, Daiichi Sankyo, and Mitsubishi Tanabe Pharma. YutS received consulting or speaker fees from Eli Lilly Japan, Sanofi, Novo Nordisk Pharma, Glaxo‐Smith‐Kline, Taisho Pharmaceutical, Taisho Pharmaceutical, Astellas Pharma, BD, Nippon Boehringer Ingelheim, Johnson & Johnson and Takeda Pharmaceutical. YutS also received clinically commissioned/joint research grants from Nippon Boehringer Ingelheim, Eli Lilly, Taisho Pharmaceutical, MSD, Ono Pharmaceutical, Novo Nordisk Pharma, Arkla and Terumo. KM, YF, SK, SKO, MI, RU, and YujY declare no conflict of interest.
ACKNOWLEDGMENTS
HK, DY, and YS contributed to study design and analysis, collection, and interpretation of data and writing of the manuscript. KM, YF, TH, SK, S K‐O, MI, RU, YujY, YH, TK, YusS, and YuiY contributed to analysis, collection, and interpretation of data and critical revisions of the manuscript for important intellectual content. All authors approved the version to be published. HK, DY, and YutS are the guarantors of this work. The authors thank the participants and colleagues who contributed to this study.
J Diabetes Investig 2021; 12: 2162–2171
REFERENCES
- 1. Yabe D, Kuwata H, Seino Y. The journey to understanding incretin systems: theory, practice and more theory. J Diabetes Investig 2019; 10: 1171–1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nauck MA, Quast DR, Wefers J, et al. GLP‐1 receptor agonists in the treatment of type 2 diabetes ‐ state‐of‐the‐art. Mol Metab 2020; 46: 101102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Holst JJ. Incretin therapy for diabetes mellitus type 2. Curr Opin Endocrinol Diabetes Obes 2020; 27: 2–10. [DOI] [PubMed] [Google Scholar]
- 4. Baggio LL, Drucker DJ. Glucagon‐like peptide‐1 receptor co‐agonists for treating metabolic disease. Mol Metab 2020; 46: 101090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Seino Y, Fukushima M, Yabe D. GIP and GLP‐1, the two incretin hormones: similarities and differences. J Diabetes Investig 2010; 1: 8–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Drucker DJ, Habener JF, Holst JJ. Discovery, characterization, and clinical development of the glucagon‐like peptides. J Clin Invest 2017; 127: 4217–4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Müller TD, Finan B, Bloom SR, et al. Glucagon‐like peptide 1 (GLP‐1). Mol Metab 2019; 30: 72–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. McCarty D, Coleman M, Boland CL. Lixisenatide: a new daily GLP‐1 agonist for type 2 diabetes management. Ann Pharmacother 2017; 51: 401–409. [DOI] [PubMed] [Google Scholar]
- 9. McCormack PL. Exenatide twice daily: a review of its use in the management of patients with type 2 diabetes mellitus. Drugs 2014; 74: 325–351. [DOI] [PubMed] [Google Scholar]
- 10. Yabe D, Seino Y. Liraglutide in adults with type 2 diabetes: global perspective on safety, efficacy and patient preference. Clin Med Insights: Endocrinol Diabetes 2011; 4: 47–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Knudsen LB. Liraglutide: the therapeutic promise from animal models. Int J Clin Pract Suppl 2010; 167: 4–11. [DOI] [PubMed] [Google Scholar]
- 12. Cai Y, Wei L, Ma L, et al. Long‐acting preparations of exenatide. Drug Des Devel Ther 2013; 7: 963–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Amblee A. Dulaglutide for the treatment of type 2 diabetes. Drugs Today (Barc) 2014; 50: 277–289. [DOI] [PubMed] [Google Scholar]
- 14. Semaglutide . LiverTox: Clinical and Research Information on Drug‐Induced Liver Injury. Bethesda (MD), 2012.
- 15. Meier JJ, Rosenstock J, Hincelin‐Méry A, et al. Contrasting effects of lixisenatide and liraglutide on postprandial glycemic control, gastric emptying, and safety parameters in patients with type 2 diabetes on optimized insulin glargine with or without metformin: a randomized, open‐label trial. Diabetes Care 2015; 38: 1263–1273. [DOI] [PubMed] [Google Scholar]
- 16. Yabe D, Seino Y. Type 2 diabetes via beta‐cell dysfunction in east Asian people. Lancet Diabetes Endocrinol 2016; 4: 2–3. [DOI] [PubMed] [Google Scholar]
- 17. Yabe D, Seino Y, Fukushima M, et al. beta cell dysfunction versus insulin resistance in the pathogenesis of type 2 diabetes in East Asians. Curr Diab Rep 2015; 15: 602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Suganuma Y, Shimizu T, Sato T, et al. Magnitude of slowing gastric emptying by glucagon‐like peptide‐1 receptor agonists determines the amelioration of postprandial glucose excursion in Japanese patients with type 2 diabetes. J Diabetes Investig 2020; 11: 389–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nagai Y, Hashimoto E, Oikawa R, et al. Differing effects of liraglutide on gastric emptying in Japanese patients with type 2 diabetes. Diabetes Obes Metab 2014; 16: 573–576. [DOI] [PubMed] [Google Scholar]
- 20. Seino Y, Nanjo K, Tajima N, et al. Report of the committee on the classification and diagnostic criteria of diabetes mellitus. J Diabet Investig 2010; 1: 212–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yabe D, Watanabe K, Sugawara K, et al. Comparison of incretin immunoassays with or without plasma extraction: incretin secretion in Japanese patients with type 2 diabetes. J Diabet Investig 2012; 3: 70–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sanaka M, Nakada K. Stable isotope breath tests for assessing gastric emptying: a comprehensive review. J Smooth Muscle Res 2010; 46: 267–280. [DOI] [PubMed] [Google Scholar]
- 23. Yabe D, Eto T, Shiramoto M, et al. Effects of DPP‐4 inhibitor linagliptin and GLP‐1 receptor agonist liraglutide on physiological response to hypoglycaemia in Japanese subjects with type 2 diabetes: a randomized, open‐label, 2‐arm parallel comparative, exploratory trial. Diabetes Obes Metab 2017; 19: 442–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Matsuo T, Miyagawa J, Kusunoki Y, et al. Postabsorptive hyperglucagonemia in patients with type 2 diabetes mellitus analyzed with a novel enzyme‐linked immunosorbent assay. J Diabetes Investig 2016; 7: 324–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kuwata H, Iwasaki M, Shimizu S, et al. Meal sequence and glucose excursion, gastric emptying and incretin secretion in type 2 diabetes: a randomised, controlled crossover, exploratory trial. Diabetologia 2016; 59: 453–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Phillips LK, Deane AM, Jones KL, et al. Gastric emptying and glycaemia in health and diabetes mellitus. Nat Rev Endocrinol 2014; 11: 112–128. [DOI] [PubMed] [Google Scholar]
- 27. Shin AS, Camilleri M. Diagnostic assessment of diabetic gastroparesis. Diabetes 2013; 62: 2667–2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Araki E, Goto A, Kondo T, et al. Japanese clinical practice guideline for diabetes 2019. J Diabetes Investig 2020; 11: 1020–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Inoue M, Shiramoto M, Oura T, et al. Effect of once‐weekly dulaglutide on glucose levels in Japanese patients with type 2 diabetes: findings from a phase 4, randomized controlled trial. Diabetes Ther 2019; 10: 1019–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Umapathysivam MM, Lee MY, Jones KL, et al. Comparative effects of prolonged and intermittent stimulation of the glucagon‐like peptide 1 receptor on gastric emptying and glycemia. Diabetes 2014; 63: 785–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jelsing J, Vrang N, Hansen G, et al. Liraglutide: short‐lived effect on gastric emptying – long lasting effects on body weight. Diabetes Obes Metab 2012; 14: 531–538. [DOI] [PubMed] [Google Scholar]
- 32. Drucker DJ, Buse JB, Taylor K, et al. Exenatide once weekly versus twice daily for the treatment of type 2 diabetes: a randomised, open‐label, non‐inferiority study. Lancet 2008; 372: 1240–1250. [DOI] [PubMed] [Google Scholar]
- 33. Kapitza C, Forst T, Coester HV, et al. Pharmacodynamic characteristics of lixisenatide once daily versus liraglutide once daily in patients with type 2 diabetes insufficiently controlled on metformin. Diabetes Obes Metab 2013; 15: 642–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Creutzfeldt W, Talaulicar M, Ebert R, et al. Inhibition of gastric inhibitory polypeptide (GIP) release by insulin and glucose in juvenile diabetes. Diabetes 1980; 29: 140–145. [DOI] [PubMed] [Google Scholar]
- 35. Lairon D, Lopez‐Miranda J, Williams C. Methodology for studying postprandial lipid metabolism. Eur J Clin Nutr 2007; 61: 1145–1161. [DOI] [PubMed] [Google Scholar]
- 36. Vergès B, Duvillard L, Pais de Barros JP, et al. Liraglutide reduces postprandial hyperlipidemia by increasing ApoB48 (apolipoprotein B48) catabolism and by reducing ApoB48 production in patients with type 2 diabetes mellitus. Arterioscler Thromb Vasc Biol 2018; 38: 2198–2206. [DOI] [PubMed] [Google Scholar]
- 37. Hsieh J, Longuet C, Baker CL, et al. The glucagon‐like peptide 1 receptor is essential for postprandial lipoprotein synthesis and secretion in hamsters and mice. Diabetologia 2010; 53: 552–561. [DOI] [PubMed] [Google Scholar]
- 38. Sakurai K, Lee EY, Morita A, et al. Glucagon‐like peptide‐1 secretion by direct stimulation of L cells with luminal sugar vs non‐nutritive sweetener. J Diabetes Investig 2012; 3: 156–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gabery S, Salinas CG, Paulsen SJ, et al. Semaglutide lowers body weight in rodents via distributed neural pathways. JCI Insight 2020; 5: e133429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Knudsen LB, Secher A, Hecksher‐Sorensen J, et al. Long‐acting glucagon‐like peptide‐1 receptor agonists have direct access to and effects on pro‐opiomelanocortin/cocaine‐ and amphetamine‐stimulated transcript neurons in the mouse hypothalamus. J Diabetes Investig 2016; 7: 56–63. [DOI] [PMC free article] [PubMed] [Google Scholar]


