Abstract
Aims/Introduction
Diabetes patients develop a variety of metabolic abnormalities in addition to hyperglycemia. However, details regarding change in various metabolites after comprehensive diabetes treatment remain unknown. This study aimed to identify the short‐term change in metabolome in inpatients who were subject to comprehensive diabetes treatment, using gas chromatography/mass spectrometry‐based non‐target metabolomics techniques.
Materials and Methods
Participants of the present study were randomly recruited from the patients with type 2 diabetes hospitalized due to problems with glycemic control (n = 31) and volunteers without diabetes (n = 30), both of whom were aged between 20 and 75 years. A metabolomic analysis of fasting plasma samples on the 2nd (pre‐treatment) and 16th hospital (post‐treatment) day with gas chromatography/mass spectrometry using a multiple reaction monitoring mode was carried out.
Results
A principal component analysis showed that metabolome of fasting plasma was different between individuals with and without diabetes. The metabolome of fasting plasma in diabetes patients after treatment was different from that of pre‐treatment, as well as individuals without diabetes. Many amino acids (proline, glycine, serine, threonine, methionine, pyroglutamic acid, glutamine and lysine) were significantly increased by >10% after administering the inpatient diabetes treatment. A hierarchical clustering analysis showed that in the case of patients with markedly decreased monosaccharide levels and increased 1,5‐anhydroglucitol, the levels of amino acids increased more significantly.
Conclusions
After a 2‐week comprehensive treatment, the plasma levels of various amino acids increased in conjunction with the reduction in monosaccharide levels in poorly controlled type 2 diabetes patients.
Keywords: Amino acids, Diabetes treatment, Metabolomics
This study aimed to identify the short‐term change in metabolome in inpatients who were subject to comprehensive diabetes treatment, using gas chromatography/mass spectrometry‐based non‐target metabolomics techniques. After a 2‐week comprehensive treatment, the plasma levels of various amino acids increased in conjunction with the reduction in monosaccharide levels in poorly controlled type 2 diabetes patients.

INTRODUCTION
Diabetes mellitus, which is caused by relative or absolute deficiency of insulin, is a group of chronic metabolic disorders characterized by hyperglycemia. Diabetes patients also develop a variety of metabolic abnormalities, such as amino acid and lipid metabolic disorders, in addition to hyperglycemia.
Metabolomics attempts to identify and quantify small molecule metabolites 1 . Recently, improvements in the metabolomics analytical platform have enabled the identification of the pathophysiology of diabetes 2 and its complication 3 , and many clinical studies examining patients with diabetes have been carried out. In metabolomics studies, a variety of analytical platforms, such as nuclear magnetic resonance, gas chromatography/mass spectrometry (GC/MS) and liquid chromatography/mass spectrometry, are used. GC/MS is a highly sensitive and specific method, and we identified metabolites related to atherosclerosis in patients with type 2 diabetes, using this platform 4 .
Several metabolomics analyses have evaluated the temporal change in metabolism of diabetes patients 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 . Most of such analyses were related to the long‐term (several months to years) changes brought about by a specific antidiabetic agent. Nevertheless, diabetes patients often receive not only glycemic treatment, but also treatment to control other risk factors, such as lipid level, blood pressure and bodyweight. However, details regarding short‐term change in metabolism caused by real‐world diabetes treatment, which is not limited to glycemic control, remain unknown. In the present study, we aimed to identify the short‐term change in metabolism in inpatients who were subject to comprehensive diabetes treatment, using GC/MS‐based non‐target metabolomics techniques.
MATERIALS AND METHODS
Study population
This is a prospective observational study of diabetes patients hospitalized due to problems with glycemic control. Participants were randomly recruited from the patients with type 2 diabetes who were admitted in Osaka University Hospital, Osaka, Japan, to improve glycemic control and aged between 20 and 75 years. Patients who did not receive intensive glycemic control, such as those with severe retinopathy, patients with serum creatinine >176.80 μmol/L (2.0 mg/dL), severe infection or severe trauma, and patients in the pre‐ and postoperative period were excluded. Volunteers without diabetes aged between 20 and 75 years were also recruited on the website of the Department of Metabolic Medicine, Osaka University Graduate School of Medicine. Overall, 33 diabetes patients and 30 adults without diabetes were enrolled in the study during 2017 to 2019.
This study was approved by the Ethics Committee of the Osaka University Hospital, Japan (approval number: 16374), and was carried out in accordance with the principals of the Declaration of Helsinki and current legal regulations in Japan. Written informed consent was obtained from all participants after a full explanation of the study.
Study protocol
For all participants, patients’ medical history was obtained and height was measured. Diabetes patients received a comprehensive diabetes treatment, including intensive glycemic control, as well as blood pressure, dyslipidemia and bodyweight control, in the hospital. Fasting blood and urine samples were taken, and vital signs and weight were measured on the 2nd and 16th hospital day (hereinafter, referred to pre‐treatment and post‐treatment period, respectively). For participants without diabetes, their fasting blood and urine samples were obtained, and vital signs and weight were measured only once. The estimated glomerular filtration rate (mL/min 1.73 m−2) was calculated according to the Statement of the Japanese Society of Nephrology 13 . C‐peptide immunoreactivity index was calculated as 100 × C‐peptide immunoreactivity (ng/mL) / fasting plasma glucose (mg/dL) 14 .
Some portions of the blood samples, for metabolomics analysis, were cooled in a freezer at 4°C immediately after collection. The samples were then centrifuged (3,000 g, 10 min), and plasma was stored at −80°C within 4 h.
Metabolomics measurements
A metabolomic analysis of plasma samples collected from diabetes patients on the 2nd and 16th hospital day with GC/MS was carried out. Plasma samples of volunteers without diabetes were also analyzed.
All the samples were divided into eight batches after randomization of sample sequences. For each batch, a series of samples was prepared, as described previously 4 , and subsequent metabolite measurements were carried out on a Shimadzu TQ8040 GC system (Shimadzu Corporation, Kyoto, Japan) connected to a mass spectrometer in multiple reaction monitoring mode.
N‐alkane mix C9–C40 (GL Science Inc., Tokyo, Japan) was injected in the beginning part of each batch and was analyzed in scan mode. The result was used to correct the retention time of each batch. A quality control (QC) sample, which was generated by mixing the same volume of plasma randomly selected from the collected plasma samples, was injected after every three study samples. Each batch also contained a blank sample. Multiple reaction monitoring transitions are described in Table S1. The other analytical conditions, such as the column and the instrument, were described previously 4 .
Peak detection was carried out using the LabSolutions Insight (Shimadzu Corporation). Peak height was then used for quantification. Subsequently, each metabolite was calibrated using the LOWESS/Spline normalization method (http://prime.psc.riken.jp/compms/others/main.html) based on the QC value 15 .
Statistical analysis
Descriptive analysis
Clinical data were expressed as the mean and standard deviation if normally distributed, or as the median and interquartile range if log‐normally distributed. Categorical variables were expressed as counts and percentages. P‐values <0.05 were considered significant. Welch’s t‐tests were used to evaluate the differences between the two groups at baseline for continuous variables. Paired t‐tests were used to differentiate the diabetes group between the pre‐treatment period and post‐treatment period. If the variables were log‐normally distributed, the tests were used after log‐transformation. The χ2‐test was used for categorical variables.
Metabolomic analysis
The metabolites that met the following criteria were included in the analysis: (i) detection rate in QC samples was ≥70%; (ii) the residual standard deviation of intensity in QC samples was <30%; and (iii) the maximum value of intensity in samples, except for blank samples, divided by the average value of intensity in blank samples was ≥2, meaning not derived from the blank samples. The detection rate and residual standard deviation of QC samples were obtained to estimate the precision of the quantification of each metabolite. Based on these inclusion criteria, the metabolites with low measurement precision or blank‐related metabolites were removed. In the values of samples without detection, half of the minimum value of the metabolite’s intensity in all the samples was substituted. All metabolite data were then log‐transformed.
A principal component analysis was carried out with a statistical analysis tool (http://prime.psc.riken.jp/compms/others/main.html) using the metabolite data of the diabetes group in the pre‐treatment period and of the non‐diabetes group. The metabolite data were used after autoscaling (standardization with a mean of 0 and a standard deviation of 1). Then, the metabolite data of the diabetes group in the post‐treatment period were transformed according to the loading score and compared with the principal component analysis data of the diabetes group in the pre‐treatment period and the non‐diabetes group.
To evaluate the effect of inpatient treatment, each metabolite datum of the diabetes group was compared between the pre‐treatment period and post‐treatment period using paired t‐tests. To account for multiple testing, we used the Benjamini–Hochberg method; that is, we calculated the adjusted P‐values (q‐values), with the significance level set at 0.05. A volcano plot was constructed using the q‐values and fold‐change values (ratios between the median of post‐treatment values and the median of pre‐treatment values). We then selected the metabolites with a significant difference in the paired t‐tests and whose fold‐change value was not >90% and <110%.
Finally, to evaluate the association of metabolites from the perspective of the change caused by treatment, hierarchical clustering with cosine similarity was carried out using the group average method. Post‐treatment value/pre‐treatment value ratio was used for the analysis after log‐transforming. The analysis was carried out using SPSS Statistics (IBM Corporation, Armonk, NY, USA).
RESULTS
Participants’ characteristics and effect of a comprehensive diabetes treatment
Two diabetes patients withdrew the consent after participation; hence, a total of 31 diabetes patients and 30 volunteers without diabetes, none of whom had missing data, were included in the final analysis. Their clinical data are shown in Table 1. Diabetes patients were older, had a higher body mass index and glycated hemoglobin level, and had a higher prevalence of hypertension and dyslipidemia than the participants without diabetes. A comprehensive diabetes treatment carried out in the hospital resulted in significant changes in bodyweight, liver function, lipid profile, highly sensitive C‐reactive protein level and glycemic control of diabetes patients.
Table 1.
Clinical data of study participants
| Diabetes (n = 31) | Non‐diabetes (n = 30) | ||||
|---|---|---|---|---|---|
| Pre‐treatment | Post‐treatment | P‐value (pre vs post) | P‐value (vs diabetes) | ||
| Sex: male | 13 (41.9) | – | – | 21 (70.0) | 0.027 |
| Age (years) | 63.9 ± 10.5 | – | – | 42.1 ± 11.2 | <0.001 |
| Diabetes duration (year) | 16.8 ± 11.1 | – | – | – | – |
| Ever smoker | 15 (48.4) | – | – | 4 (13.3) | 0.0031 |
| BMI (kg/m2) | 26.7 ± 5.1 | – | – | 22.6 ± 3.8 | <0.001 |
| Bodyweight (kg) | 67.8 ± 13.5 | 66.6 ± 13.1 | <0.001 | – | – |
| Systolic BP (mmHg) | 128.6 ± 17.4 | 125.0 ± 12.6 | 0.249 | – | – |
| Hypertension | 22 (71.0) | – | – | 2 (6.7) | <0.001 |
| Dyslipidemia | 26 (83.9) | – | – | 1 (3.3) | <0.001 |
| AST (U/L) | 27.9 ± 17.1 | 24.5 ± 13.7 | 0.014 | 22.1 ± 5 | 0.082 |
| ALT (U/L) | 30.6 ± 27.4 | 27.0 ± 23.1 | 0.101 | 23.7 ± 11.5 | 0.205 |
| γ‐GTP (U/L) | 39.1 ± 30.4 | 30.9 ± 26.9 | <0.001 | 27.5 ± 18.0 | 0.074 |
| eGFR (mL/min/1.73 m2) | 70.79 ± 21.49 | 66.72 ± 18.96 | 0.0072 | 85.86 ± 9.66 | <0.001 |
| FPG (mmol/L) | 8.1 ± 2.6 | 6.1 ± 1.1 | <0.001 | 5.0 ± 0.5 | <0.001 |
| HbA1c (mmol/mol) | 76.4 ± 22.6 | 68.68 ± 17.53 | <0.001 | 35.6 ± 3.5 | <0.001 |
| HbA1c (%) | 9.1 ± 2.1 | 8.4 ± 1.6 | <0.001 | 5.4 ± 0.3 | <0.001 |
| Glycoalbumin (%) | 23.7 ± 5.7 | 19.4 ± 3.4 | <0.001 | 13.8 ± 1.4 | <0.001 |
| CPR index | 1.07 ± 0.63 | 1.31 ± 0.70 | 0.019 | – | – |
| Total cholesterol (mmol/L) | 5.20 ± 1.58 | 4.10 ± 0.81 | <0.001 | 5.47 ± 1.04 | 0.429 |
| Triglycerides (mmol/L) | 1.41 (0.92–2.59) | 1.06 (0.79–1.45) | <0.001 | 0.79 (0.59–1.15) | <0.001 |
| HDL cholesterol (mmol/L) | 1.37 ± 0.31 | 1.30 ± 0.27 | 0.030 | 1.79 ± 0.53 | <0.001 |
| LDL cholesterol (mmol/L) | 3.11 ± 1.26 | 2.27 ± 0.75 | <0.001 | 3.24 ± 0.93 | 0.650 |
| hs‐CRP (mg/L) | 0.710 (0.465–1.255) | 0.478 (0.200–0.739) | <0.001 | 0.336 (0.132–0.668) | 0.005 |
| Uric acid (mmol/L) | 0.34 ± 0.07 | 0.33 ± 0.06 | 0.248 | 0.33 ± 0.07 | 0.739 |
| u‐Alb/Cr (mg/mmol) | 1.4 (0.4–4.2) | 1.2 (0.6–3.2) | 0.493 | 0.5 (0.4–1.0) | 0.032 |
Data are presented as the mean ± standard deviation if normally distributed or as the median (interquartile range) if log‐normally distributed. Categorical data are presented as counts (percentage). Welch’s t‐tests and χ2‐tests were used to compare the baseline characteristics between the two groups for continuous and categorical variables, respectively. Paired t‐tests were used to compare differences between pre‐treatment and post‐treatment in the diabetes group.
BMI, body mass index; eGFR, estimated glomerular filtration rate; FPG, fasting plasma glucose; CPR index, C‐peptide immunoreactivity index; HbA1c, glycated hemoglobin; HDL, high‐density lipoprotein; hs‐CRP, highly‐sensitive C‐reactive protein; LDL, low‐density lipoprotein; u‐Alb/Cr, urine albumin/creatinine ratio.
Metabolomic analysis
The residual standard deviation of ribitol in all study samples and QC samples was 6.4%, indicating an extremely high stability of GC/MS measurement. Among the 379 target metabolites, the levels of 135 metabolites peaked. Among these 135 metabolites, 78 met the inclusion criteria and were included in the analysis.
The principal component analysis results with the data of the diabetes group in the pre‐treatment period and non‐diabetes group are shown in Figure 1a, which revealed that the metabolomes of the two groups were different.
Figure 1.
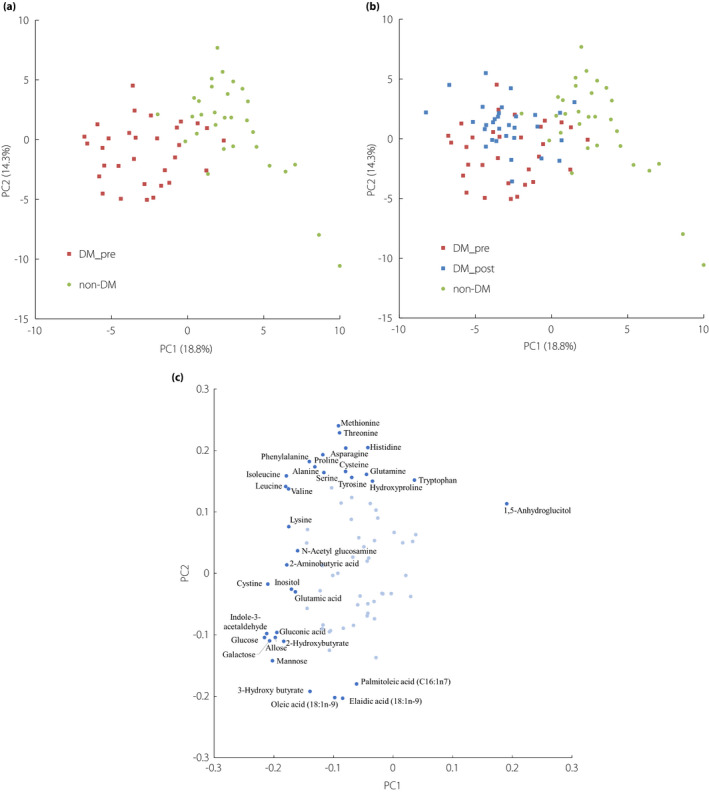
Principal component analysis score scatter plots (a) with the metabolites of the diabetes group in the pre‐treatment period and non‐diabetes group. (b) The data of the diabetes group in the post‐treatment period were transformed according to the loading score and plotted. (c) The loading plots of principal component analysis (first principal component [PC1] and second principal component [PC2]). Red squares, blue squares and green circles in (a) and (b) indicate the diabetes group in the pre‐treatment period, in the post‐treatment period and the non‐diabetes group, respectively. Proportions of variance are presented in parentheses of each principal component. Dark blue points in (c) represent the metabolites with high absolute value (>0.15) of the loading score of PC1 or PC2. Light blue points represent the other metabolites. The metabolite names only of the dark blue points are described. The loading scores of all metabolites are shown in Tables S2 and S3. DM_post, diabetes group in the post‐treatment period; DM_pre, diabetes group in the pre‐treatment period; non‐DM, non‐diabetes group.
The results along with the data of the diabetes group in the post‐treatment period are shown in Figure 1b, which indicated that the metabolome of the post‐treatment period was different from that of the pre‐treatment period, as well as that of the non‐diabetes group. As shown in Figure 1b, the first principal component was likely to be associated with whether the participants had diabetes, whereas the second principal component was likely to be associated with whether the plasma samples were taken before or after inpatient treatment. The metabolites with low loading scores of the first principal component include monosaccharides and branched‐chain amino acids (Figure 1c and Table S2). In contrast, several amino acids had high loading scores of the second principal component, whereas fatty acids had low loading scores (Figure 1c and Table S3), indicating that the changes in these metabolites were probably induced by the inpatient treatment.
The volcano plot (Figure 2) shows the results of the paired t‐tests comparing between metabolite’s intensities in the pre‐treatment and post‐treatment period. This analysis indicates that the metabolites, which decreased over a period of 2 weeks during the inpatient treatment, consisted of several monosaccharides, whereas those that increased consisted of several amino acids.
Figure 2.
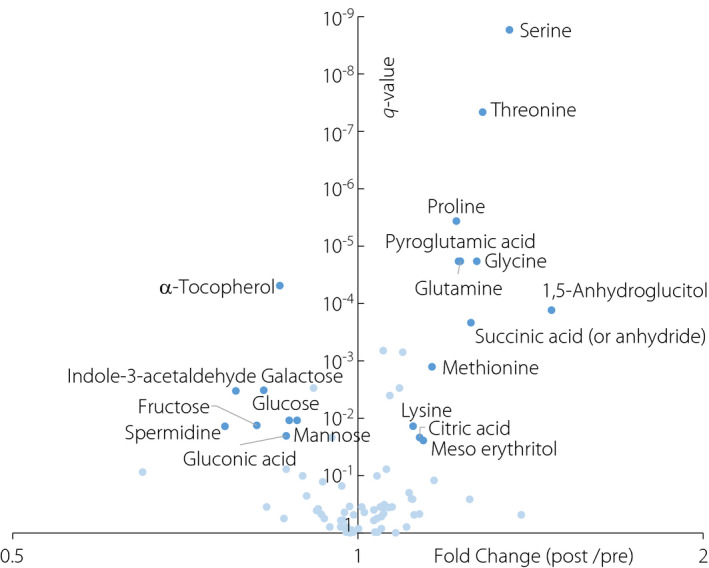
Volcano plot showing the results of the paired t‐tests comparing the metabolite intensities of the pre‐treatment group and post‐treatment group. The x‐axis represents the fold‐change values (ratios between the median of post‐treatment values/the median of pre‐treatment values), whereas the y‐axis represents the q‐values (adjusted P‐values using the Benjamini–Hochberg method). Dark blue points represent the metabolites with a significant difference (q‐value ≤ 0.05) and 10% change (fold change ≥1.1 or ≤0.9). Light blue points represent the other metabolites.
The dendrograms with a heat map (Figure 3) show the results of the hierarchical clustering. The cluster in the lower part consisted mostly of the amino acids, which increased during the inpatient treatment (amino acids of red characters), indicating that they changed in the same way. On the contrary, the cluster in the upper part consisted mostly of the monosaccharides, which decreased (monosaccharides of blue characters). In the case of patients with markedly decreased monosaccharide levels and increased 1,5‐anhydroglucitol (shown in bold letters in Figure 3) levels, the levels of amino acids increased more significantly.
Figure 3.
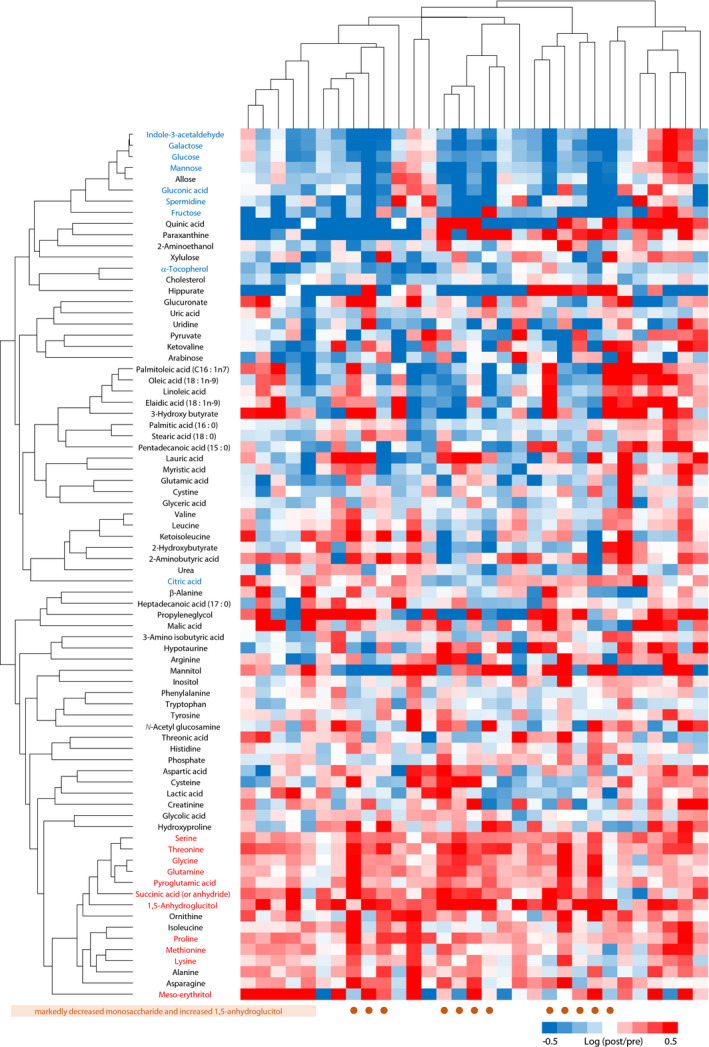
The dendrograms with heat map showing the results of the hierarchical clustering to evaluate the association of metabolites from the perspective of the change caused by the treatment. Heat map represents the log of fold‐change values (post‐treatment intensity/pre‐treatment intensity ratios). Metabolites of red or blue characters indicate the metabolites with a significant increase or decrease (10%), respectively (the metabolites of a dark blue point in Figure 2). The brown circles represent the patients with decreased monosaccharide and increased 1,5‐anhydroglucitol.
Furthermore, the plasma levels of the amino acids with a significant increase after inpatient treatment were compared among the groups. There were significant differences in plasma levels between the non‐diabetes group and the diabetes group in the post‐treatment period, but there was no significant difference between the non‐diabetes group and the diabetes group in the pre‐treatment period (Figure S1).
DISCUSSION
In the present GC/MS‐based non‐target metabolomics study, we found that the plasma metabolome of the diabetes patients with poor glycemic control were different from that of the individuals without diabetes. We also found that the plasma metabolome of the participants with diabetes had significantly changed after the comprehensive treatment against diabetes, although it was still different from that of the participants without diabetes. After the treatment, the plasma levels of many monosaccharides (e.g., glucose, galactose, fructose and mannose) decreased, but those of several amino acids (e.g., proline, glycine, serine, threonine, methionine, pyroglutamic acid, glutamine and lysine) increased.
In the present study, the levels of monosaccharides, such as galactose, fructose and mannose, in addition to glucose, decreased after treatment. This result is partially consistent with that of a previous study, which reported that the serum level of α‐galactose decreased by rosiglitazone or repaglinide treatment, mannose decreased by repaglinide treatment, and fructose increased by rosiglitazone or repaglinide treatment 6 . In the present study, the dendrograms with a heat map (Figure 3) show that the decrease in the level of monosaccharides belong to the same cluster, indicating that they decreased in the same way. Monosaccharides, not restricted to glucose, are catabolized through glycolysis 16 , which is promoted by insulin 17 . Our present result of the hierarchical clustering implies that an improvement in the action of insulin after the treatment promoted glycolysis, resulting in the reduction in the levels of various monosaccharides.
Many previous studies reported the relationships between circulating levels of certain amino acids and diabetes. Several of them showed that elevated blood concentrations of branched‐chain amino acids, such as valine, leucine and isoleucine, and aromatic amino acids, such as phenylalanine and tyrosine, and reduced blood concentration of glycine predict the risk of developing type 2 diabetes 18 , 19 , 20 . A clinical study using the Framingham Heart Study Offspring Cohort identified the valine, leucine, isoleucine, phenylalanine and tyrosine as predictors of type 2 diabetes 18 . These branched‐chain amino acids and aromatic amino acids were also identified as predictors of insulin resistance in healthy individuals 21 . A lower level of glycine was found to be a predictor of impaired glucose tolerance and type 2 diabetes in the Cooperative Health Research in the Region of Augsburg cohort 19 , and of type 2 diabetes in the European Prospective Investigation into Cancer and Nutrition‐Potsdam cohort 20 . As glycine is an acceptor of the acyl groups that buffer the excess acyl groups generating acyl‐glycine derivatives, excessive amounts of fatty acyl group with diabetes leads to glycine consumption followed by a reduction of the free glycine pool 22 . However, it remains unclear whether most of the other amino acids are associated with the pathophysiology of diabetes. The present study showed that comprehensive diabetes treatment increased the plasma levels of many amino acids, and that no amino acids were decreased. This is the first study to report that the plasma levels of proline, threonine, methionine and pyroglutamic acid increased after the diabetes treatment.
Interestingly, the increase in amino acids during diabetes treatment seems to have been extremely large, as the differences between pre‐ and post‐treatment were relatively large compared with those between diabetes patients in the pre‐treatment period and participants without diabetes. The possible underlying mechanisms of the increase in the levels of amino acids in the present study were as follows: (i) an increase in the release of amino acids from the skeletal muscles; and (ii) a reduction in the use of amino acids in the liver as a consequence of a reduction of gluconeogenesis.
Muscles and circulating proteins are the major reservoir of amino acids within the body, and the proteins in the skeletal muscle and the circulating system are in continuous turnover. Protein degradation and synthesis of skeletal muscles are controlled by circulating anabolic and catabolic molecules 23 . Relative or absolute deficiency of insulin, which is one of the major anabolic molecules that induces protein synthesis, is the main pathological condition of diabetes. On the contrary, the expression of catabolic molecules, such as inflammatory cytokines (e.g., tumor necrosis factor‐α and interleukin‐1β) and hormones (catecholamines and cortisol), which induce protein degradation, increases in patients with diabetes 24 . Thus, the protein metabolism of skeletal muscle in diabetes patients, both the reduction in anabolic molecules and increase in catabolic molecules, could lead to protein degradation and release of the amino acids. Amino acids released from muscles are, then, used in the liver to produce glucose by gluconeogenesis 24 . The increased release from muscles and the increased use through enhanced gluconeogenesis might be balanced, and thus, there was no difference in plasma levels of most of the amino acids with an increase after the inpatient treatment between diabetes patients in the pre‐treatment and participants without diabetes. Diabetes treatment is considered to improve the insulin action of skeletal muscle, and reduce the expression of inflammatory cytokines and hormones, attenuating the protein degradation and the release of the amino acids from skeletal muscle. In the liver, it might reduce the usage of the amino acids through the reduction of gluconeogenesis. In the present study, the hierarchical clustering analysis showed that an increase in plasma levels of amino acids was observed in accordance with the improvement in glycemic control (Figure 3), supporting the hypothesis that the reduction of gluconeogenesis caused by diabetes treatment increased the plasma amino acid levels. The changes in amino acid and protein metabolism in the skeletal muscle might have been relatively small. This implies that the short‐term (approximately 2 weeks) comprehensive diabetes treatment was insufficient to improve amino acid and protein metabolism in skeletal muscle, although it might have resulted in the improvement of various metabolic profiles, such as glycemic control, lipid profiles and liver function. In such a scenario, a relatively long‐term glycemic control might be required. Furthermore, the simultaneous performance of resistance exercise seems to be important for the improvement of amino acid and protein metabolism, as it enhances the synthesis of muscle proteins 25 .
A previous randomized controlled study including obese adults with impaired fasting glucose or diabetes reported that a 3‐month treatment of insulin sensitizer with pioglitazone and metformin increased the plasma glycine and serine concentration, and decreased the plasma lysine concentration compared with a placebo 5 . Another study showed that 48 weeks of rosiglitazone treatment increased the serum levels of lysine in diabetes patients 6 . Rosiglitazone was also reported to increase plasma levels of glutamine after 6 weeks of treatment 11 , as well as the plasma levels of serum glutamine after 16 weeks of treatment 12 . Furthermore, rosiglitazone treatment might diminish the demand for amino acids as substrate for hepatic gluconeogenesis 11 . These findings suggest that elevation in the plasma levels of glycine, serine, glutamine and lysine observed in the present study might be induced by improvement in glycemic control or insulin resistance itself, but not by diet or exercise.
The present study also had some potential limitations. First, the present study was a pilot study, and the sample size was insufficient to draw conclusions. In addition, not diabetes, but other factors, might cause the difference in metabolome between the diabetes and the non‐diabetes groups, as we were unable to match clinical characteristics of the two groups.
Second, it remains unclear whether the detected metabolites and glycemic control have a causal relationship; the present findings did not necessarily mean that glycemic control caused the changes in the plasma levels of metabolomes and vice versa. Therefore, experimental studies are necessary to investigate the mechanisms underlying this change.
Third, the possibility that a change in the dietary composition and physical activity during the hospitalization affected the plasma levels of amino acids cannot be denied, as we do not have detailed information about these factors. Blood metabolomes differ between groups who have different dietary composition 26 , and diet influences the metabolism of amino acids 27 . In the present study, physical therapy was not provided based on a standardized protocol; but was prescribed to the patients by their attending physician according to their respective conditions of glycemic control and diabetic complications. Therefore, the study might have included patients whose amount of exercise was reduced. Reduction in the amount of exercise might induce imbalance of protein synthesis and degradation of skeletal muscles, and accelerate the release of amino acids into the blood. However, most of the amino acids released from the skeletal muscles into the blood as a result of the reduction in the amount of exercise is alanine 28 , 29 . Considering the results that the plasma level of alanine did not increase in the present study, the degradation of skeletal muscles might have had little influence.
In conclusion, after a 2‐week comprehensive treatment, the plasma levels of various amino acids increased in conjunction with the reduction in monosaccharide levels in poorly controlled type 2 diabetes patients.
DISCLOSURE
The authors declare no conflict of interest.
Supporting information
Figure S1 | Comparison in the intensities of the amino acids with a significant increase during the inpatient diabetes treatment among the diabetes group in the pre‐ and post‐treatment period and the non‐diabetes group.
Table S1 | Multiple reaction monitoring transitions for analysis.
Table S2 | Loading scores in the principal component analysis of the first principle component.
Table S3 | Loading scores in the principal component analysis of the second principle component.
ACKNOWLEDGMENTS
The authors thank study participants for their participation. This study was supported by AMED‐CREST, Japan Agency for Medical Research and Development (AMED; grant number: JP19gm0710005).
J Diabetes Investig 2021; 12: 2232–2241
REFERENCES
- 1. Pauling L, Robinson AB, Teranishi R, et al. Quantitative analysis of urine vapor and breath by gas‐liquid partition chromatography. Proc Natl Acad Sci USA 1971; 68: 2374–2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sas KM, Karnovsky A, Michailidis G, et al. Metabolomics and diabetes: analytical and computational approaches. Diabetes 2015; 64: 718–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wu T, Qiao S, Shi C, et al. Metabolomics window into diabetic complications. J Diabetes Investig 2018; 9: 244–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Omori K, Katakami N, Arakawa S, et al. Identification of plasma inositol and indoxyl sulfate as novel biomarker candidates for atherosclerosis in patients with type 2 diabetes – findings from metabolome analysis using GC/MS. J Atheroscler Thromb 2020; 27: 1053–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Irving BA, Carter RE, Soop M, et al. Effect of insulin sensitizer therapy on amino acids and their metabolites. Metabolism 2015; 64: 720–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bao Y, Zhao T, Wang X, et al. Metabonomic variations in the drug‐treated type 2 diabetes mellitus patients and healthy volunteers. J Proteome Res 2009; 8: 1623–1630. [DOI] [PubMed] [Google Scholar]
- 7. Safai N, Suvitaival T, Ali A, et al. Effect of metformin on plasma metabolite profile in the Copenhagen Insulin and Metformin Therapy (CIMT) trial. Diabet Med 2018; 35: 944–953. [DOI] [PubMed] [Google Scholar]
- 8. Xu T, Brandmaier S, Messias AC, et al. Effects of metformin on metabolite profiles and LDL cholesterol in patients with type 2 diabetes. Diabetes Care 2015; 38: 1858–1867. [DOI] [PubMed] [Google Scholar]
- 9. Zhang Y, Hu C, Hong J, et al. Lipid profiling reveals different therapeutic effects of metformin and glipizide in patients with type 2 diabetes and coronary artery disease. Diabetes Care 2014; 37: 2804–2812. [DOI] [PubMed] [Google Scholar]
- 10. Adam J, Brandmaier S, Leonhardt J, et al. Metformin effect on nontargeted metabolite profiles in patients with type 2 diabetes and in multiple murine tissues. Diabetes 2016; 65: 3776–3785. [DOI] [PubMed] [Google Scholar]
- 11. van Doorn M, Vogels J, Tas A, et al. Evaluation of metabolite profiles as biomarkers for the pharmacological effects of thiazolidinediones in type 2 diabetes mellitus patients and healthy volunteers. Br J Clin Pharmacol 2007; 63: 562–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Badeau RM, Honka M‐J, Lautamäki R, et al. Systemic metabolic markers and myocardial glucose uptake in type 2 diabetic and coronary artery disease patients treated for 16 weeks with rosiglitazone, a PPARγ agonist. Ann Med 2014; 46: 18–23. [DOI] [PubMed] [Google Scholar]
- 13. Matsuo S, Imai E, Horio M, et al. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis 2009; 53: 982–992. [DOI] [PubMed] [Google Scholar]
- 14. Goto A, Takaichi M, Kishimoto M, et al. Body mass index, fasting plasma glucose levels, and C‐peptide levels as predictors of the future insulin use in Japanese type 2 diabetic patients. Endocr J 2010; 57: 237–244. [DOI] [PubMed] [Google Scholar]
- 15. Tsugawa H, Kanazawa M, Ogiwara A, et al. MRMPROBS suite for metabolomics using large‐scale MRM assays. Bioinformatics 2014; 30: 2379–2380. [DOI] [PubMed] [Google Scholar]
- 16. Dashty M. A quick look at biochemistry: carbohydrate metabolism. Clin Biochem 2013; 46: 1339–1352. [DOI] [PubMed] [Google Scholar]
- 17. Probst I, Unthan‐Fechner K. Activation of glycolysis by insulin with a sequential increase of the 6‐phosphofructo‐2‐kinase activity, fructose‐2,6‐bisphosphate level and pyruvate kinase activity in cultured rat hepatocytes. Eur J Biochem 1985; 153: 347–353. [DOI] [PubMed] [Google Scholar]
- 18. Wang TJ, Larson MG, Vasan RS, et al. Metabolite profiles and the risk of developing diabetes. Nat Med 2011; 17: 448–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wang‐Sattler R, Yu Z, Herder C, et al. Novel biomarkers for pre‐diabetes identified by metabolomics. Mol Syst Biol 2012; 8: 615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Floegel A, Stefan N, Yu Z, et al. Identification of serum metabolites associated with risk of type 2 diabetes using a targeted metabolomic approach. Diabetes 2013; 62: 639–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wurtz P, Soininen P, Kangas AJ, et al. Branched‐chain and aromatic amino acids are predictors of insulin resistance in young adults. Diabetes Care 2013; 36: 648–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Adeva‐Andany M, Souto‐Adeva G, Ameneiros‐Rodríguez E, et al. Insulin resistance and glycine metabolism in humans. Amino Acids 2018; 50: 11–27. [DOI] [PubMed] [Google Scholar]
- 23. Pasini E, Corsetti G, Aquilani R, et al. Protein‐amino acid metabolism disarrangements: the hidden enemy of chronic age‐related conditions. Nutrients 2018; 10: 391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pasini E, Aquilani R, Dioguardi FS. Amino acids: chemistry and metabolism in normal and hypercatabolic states. Am J Cardiol 2004; 93: 3A–5A. [DOI] [PubMed] [Google Scholar]
- 25. Bolster DR, Jefferson LS, Kimball SR. Regulation of protein synthesis associated with skeletal muscle hypertrophy by insulin‐, amino acid‐ and exercise‐induced signaling. Proc Nutr Soc 2004; 63: 351–356. [DOI] [PubMed] [Google Scholar]
- 26. O’Donovan CB, Walsh MC, Woolhead C, et al. Metabotyping for the development of tailored dietary advice solutions in a European population: the Food4Me study. Br J Nutr 2017; 118: 561–569. [DOI] [PubMed] [Google Scholar]
- 27. Vazquez JA, Morse EL, Adibi SA. Effect of dietary fat, carbohydrate, and protein on branched‐chain amino acid catabolism during caloric restriction. J Clin Invest 1985; 76: 737–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Felig P, Wahren J. Amino acid metabolism in exercising man. J Clin Invest 1971; 50: 2703–2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pozefsky T, Felig P, Tobin JD, et al. Amino acid balance across the tissue of the forearm in postabsorptive man: effects of insulin at two dose levels. J Clin Invest 1969; 48: 2273–2282. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 | Comparison in the intensities of the amino acids with a significant increase during the inpatient diabetes treatment among the diabetes group in the pre‐ and post‐treatment period and the non‐diabetes group.
Table S1 | Multiple reaction monitoring transitions for analysis.
Table S2 | Loading scores in the principal component analysis of the first principle component.
Table S3 | Loading scores in the principal component analysis of the second principle component.


