ABSTRACT
Diabetic kidney disease (DKD) is a critical microvascular complication of diabetes. With the continuous increase in the prevalence of diabetes since 2000, the prevalence of DKD has also been increasing in past years. The prevalence of DKD among individuals with type 2 diabetes in Taiwan increased from 13.32% in 2000 to 17.92% in 2014. The cumulative incidence of DKD among individuals with type 1 diabetes in Taiwan was higher than 30% during 1999–2012. DKD is the leading cause of end‐stage renal disease (ESRD), with a prevalence of approximately 45% in a population on chronic dialysis in Taiwan. Among individuals with type 2 diabetes, the prevalence of ESRD in the receipt of dialysis also increased from 1.32% in 2005 to 1.47% in 2014. Risk factors for DKD development are age, race, family history, hyperglycemia, hypertension, dyslipidemia, dietary patterns, and lifestyles. Prognostic factors that aggravate DKD progression include age, family history, sex, glycemic control, blood pressure (BP), microvascular complications, and atherosclerosis. This review summarizes updated information on the onset and progression of DKD, particularly in the Taiwanese population. Translating these epidemiological features is essential to optimizing the kidney care and improving the prognosis of DKD in Asian populations.
Keywords: Diabetic kidney disease, Prognostic factors, Risk factors
This review summarizes updated information on the onset and progression of DKD, particularly in Taiwan. Translating these epidemiological features is essential to optimizing kidney care and improving the prognosis of DKD in Asian populations.
INTRODUCTION
Diabetic kidney disease (DKD) is a devastating diabetic microvascular complication, leading to considerably high burdens on health care costs. With the global increase in the prevalence of diabetes, the number (prevalence) of people with chronic kidney disease due to type 2 diabetes has increased from 81,514,189 (1.39%) in 1999, 101,027,301 (1.52%) in 2009 to 129,560,073 (1.74%) in 2019 1 , DKD has been increasing over past decades. According to a report of the Developing Education on Microalbuminuria for Awareness of Renal and Cardiovascular risk in Diabetes study 2 , approximately 49% and 22% of patients with type 2 diabetes worldwide, respectively, had a urine albumin to creatinine ratio (UACR) of ≥30 mg/g and an estimated glomerular filtration rate (eGFR) of <60 mL/min/1.73 m2. In Taiwan, DKD is currently the leading cause of end‐stage renal disease (ESRD), accounting for approximately 45% of the population on chronic dialysis 3 .
Diabetic kidney disease is associated with high risks of cardiovascular diseases and mortality. In particular, it is a risk multiplier in people with hypertension and diabetes 4 . The United Kingdom Prospective Diabetes Study (UKPDS) disclosed that annually, 2.3% of people with type 2 diabetes and macroalbuminuria progressed to a plasma creatinine level of ≥1.98 mg/dL or received renal replacement therapy (RRT); these patients also had a 4.6% annual rate of all‐cause death and 3.5% annual rate of cardiovascular mortality 5 . A study from Steno Diabetes Center indicated that annually, 2.7% of persons with type 2 diabetes and nephropathy progressed to ESRD, with a 2.6% annual rate of death and 7.5% annual rate of cardiovascular disease 6 . In an Asian study in Hong Kong, So et al. reported that annually, 3.5% of Chinese individuals with type 2 diabetes at stage 3 chronic kidney disease (CKD) progressed to renal end points (reduction in eGFR by >50%, progression to stage 5 CKD, dialysis, or death), with a 1.1% annual rate of all‐cause mortality and 2.1% annual rate of cardiovascular diseases (Figure 1) 7 . Wen et al. investigated Taiwanese people with type 2 diabetes and early CKD (stage 1–3) and found a 2.8% annual rate of all‐cause death and 0.6% annual rate of cardiovascular mortality 8 . According to the aforementioned epidemiological evidence, DKD deserves thorough investigation to enable the development of strategies for preventing serious health consequences.
Figure 1.
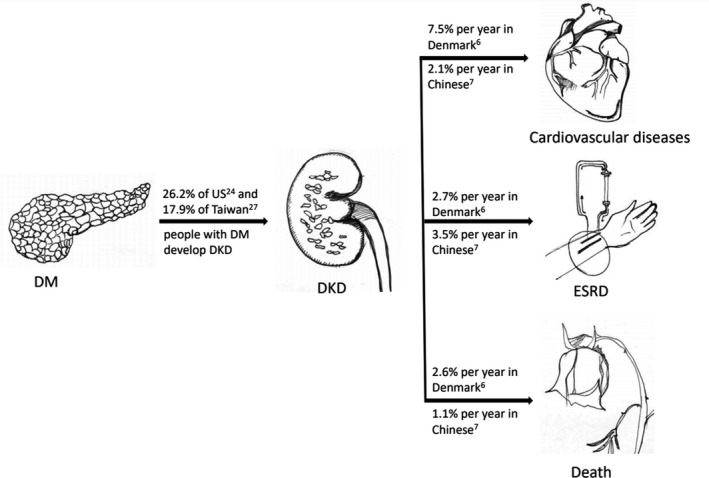
Rates of progression from diabetes mellitus (DM) to diabetic kidney disease (DKD), end stage renal disease (ESRD), cardiovascular disases, and death.
Ethnicity has been shown to play a crucial role in the development and progression of diabetes and DKD. Compared with their Caucasian counterparts, Asians with diabetes usually exhibit a higher DKD prevalence, and those with DKD present a rapid deterioration and a high mortality rate 9 , 10 , 11 , 12 , 13 , 14 . The ethnic disparity may be caused by a distinct genetic predisposition, socioeconomic background, lifestyle patterns, and environmental hazard exposures; however, most published epidemiological studies have focused mainly on epidemiological features in Western countries. Therefore, this study delineated the unique epidemiological pattern of DKD in Asian populations, especially focusing on studies conducted with Taiwanese participants.
Diabetic kidney disease refers to kidney disease that is specific to diabetes mellitus. Although kidney biopsy is required to definitively diagnose diabetic glomerulopathy, it is an interventional procedure and cannot be used routinely to diagnose DKD. Therefore, the presence of UACR ≥ 30 mg/g or eGFR < 60 mL/min/1.73 m2 for more than 3 months in patients with diabetes is used to define DKD 15 . The DKD outcome determinants include risk factors that promote the initiation of DKD development and prognostic factors that may aggravate the progression of DKD to worse renal dysfunction, ESRD, composite cardiovascular diseases risk, or even death. Most risk factors and prognostic factors of DKD are identical (e.g., poor glycemic control); however, some indicators have different mechanisms by which they initiate or worsen DKD status (e.g., obesity is a risk factor for DKD development but it may prevent those with late DKD/ESRD from deterioration). However, most review articles have not elucidated distinctions between risk factors for and prognostic factors of DKD, which make interpretation difficult. In this review, we discuss these two ‘seemingly similar but not exactly the same’ factors separately to aid clinical practice.
EPIDEMIOLOGY OF DIABETES AND DKD
The prevalence of diabetes has been increasing worldwide since 2000, with 463 million people aged 20–79 years having diabetes worldwide in 2019, corresponding to a prevalence of 9.3% 16 . These numbers, respectively, are estimated to reach nearly 700 million and 10.9% by 2045 16 . Among the 10 geographic regions of the world, the Western Pacific region has the highest number of people with diabetes 16 . The prevalence of diabetes among people older than 20 years in Mainland China was 9.7% in 2007–2008 17 , and it increased to 11.6% in 2010 18 . According to a recent analysis based on the National Health Insurance database, the incidence of diabetes in Taiwan among people aged 20–79 years showed a modest increase from 2005 (0.79%) to 2014 (0.88%) 19 . However, the prevalence of diabetes increased from 7.15% to 10.10% during the same period 19 , possibly due to the prolonged life expectancy of the Taiwanese population. The prevalence of diabetes among adults (20–79 years) in Taiwan was estimated to be 10.0% and 10.9%, respectively, in the 2015 and 2017 IDF Diabetes Atlas 20 , 21 ; however, it was reported as 6.6% in the 2019 IDF Diabetes Atlas 16 . From the study 19 that used the reliable national insurance database in Taiwan and the previous 2015 and 2017 IDF reports, the prevalence of diabetes in Taiwan may be underestimated in the 2019 IDF Diabetes Atlas.
Diabetic kidney disease is a chronic complication of diabetes and an important risk factor for CKD 22 . Because of the continual increase in diabetes prevalence in Taiwan, CKD and ESRD have become serious burdens on the national health care system. Taiwan has the highest incidence and prevalence of ESRD requiring chronic dialysis in the world, with a 52.5% increase in incidence for the past 18 years (from 331 in 2000 to 504 per million population in 2017) and a 140% increase in prevalence during the same period (from 1,448 in 2000 to 3,480 per million population in 2017). Of the nearly 90,000 patients under chronic dialysis, more than 46% had received a diagnosis of DKD as the primary cause of dialysis initiation 23 .
According to several cross‐sectional studies in the United States, no obvious change in the prevalence of DKD was observed from 1988–1994 (28.4%) to 2009–2014 (26.2%) 24 . Although a significant decrease in the prevalence of albuminuria was observed (from 20.8% to 15.9%) in the United States during the same period, the rate of renal dysfunction, defined as an eGFR of <60 mL/min/1.73 m2, increased significantly from 9.2% to 14.1% 24 . Compared with Caucasians, the Chinese population had a higher risk (prevalence 27.6% vs 24.8%) of proteinuric DKD but a lower risk (6.3% vs 11.7%) of nonproteinuric DKD 25 . Based on the studies that used the National Health Insurance database, the prevalence of DKD among persons with diabetes in Taiwan increased from 13.32% in 2000 to 15.42% in 2009 26 and further increased to 17.92% in 2014 27 . The prevalence of ESRD requiring dialysis in patients with diabetes also increased from 1.32% in 2005 to 1.47% in 2014 27 . The cumulative incidence of DKD among patients with type 1 diabetes in Taiwan was even higher than 30% during 1999–2012 28 . Although the overall DKD prevalence among patients with diabetes was lower in Taiwan than in the United States (Figure 1), the increasing trend and the persistent burden attributable to ESRD requiring dialysis confirm that DKD is an urgent public health concern.
RISK FACTORS FOR DKD DEVELOPMENT
According to some systemic reviews 29 , 30 , 31 , the risk factors for DKD development may be divided into two categories: unmodifiable risk factors such as age, ethnicity, family history, and genetic susceptibility and potentially modifiable ones such as hyperglycemia, hypertension, dyslipidemia, dietary patterns, and lifestyles (Table 1).
Table 1.
Studies on the risk factors for DKD development
| Authors (year)reference | Study design | Number of patients | Main findings |
|---|---|---|---|
| Hsu et al. (2010) 54 | Cross‐sectional | 509 men with type 2 diabetes | Dose‐response effect of cigarette smoking on the development of proteinuria |
| Hsu et al. (2011) 55 | Prospective | 738 patients with normoalbuminuric type 2 diabetes | Insulin resistance predicted the development of microalbuminuria in patients with type 2 diabetes |
| Hsu et al. (2012) 40 | Prospective | 821 patients with type 2 diabetes and normoalbumiuria | HbA1c variability, even measured as early as 2 years, was independently associated with the development of microalbuminuria |
| Chang et al. (2013) 48 | Prospective | 864 patients with type 2 diabetes | Stable and higher mean HDL‐C levels were associated with lower risks of DKD development |
| Hsu et al. (2013) 56 | Prospective | 851 patients with type 2 diabetes | Hyperferritinemia may be an independent risk factor of nephropathy in patients with type 2 diabetes |
| Liao et al. (2014) 32 | Case–control | 217 diabetic nephropathy cases and 357 controls | SNPs rs11647932, rs11645214, and rs6499323 located at 16q22.1 were associated with 2‐fold increased risk of DKD. 11 Haplotypes (4, 3, and 4 haplotypes in window size of 3‐SNP, 4‐SNP, and 5‐SNP) located in the chromosome 16q22.1 region increased the DKD risk |
| Chung et al. (2014) 34 | Prospective | 566 type 2 diabetes with normoalbuminuria | ADIPOQ genetic polymorphisms were correlated with incident DKD in Taiwanese men with type 2 diabetes |
| Sheen et al. (2014) 44 | Prospective | 215 outpatients with type 2 diabetes | SBP is a powerful modifiable risk factor for incident albuminuria and a rapid renal function decline |
| Tsai et al. (2014) 49 | Case–control | 6,406 patients with type 2 diabetes | A positive temporal relationship was found between nonsteroidal anti‐inflammatory drug use and increased risk of DKD |
| Lin et al. (2014) 51 | Prospective | 559 patients with type 2 diabetes without renal disease | Physical activity is a potential treatment for reducing incident DKD |
| Chang et al. (2016) 33 | Prospective | 568 patients with type 2 diabetes and normoalbumiuria | IL‐6 gene polymorphisms rs1800796 and rs1524107 can be used as predictors of the development of nephropathy in Taiwanese patients with type 2 diabetes |
| Yeh et al. (2017) 45 | Retrospective | 789 patients with newly diagnosed type 2 diabetes | Variability in SBP and DBP was correlated with DKD |
| Chung et al. (2017) 53 | Prospective | 1,187 patients with type 2 diabetes | Obese persons with excessive central fat, large weight gain (>10%), and increases in WC (>15%) were independently associated with incident DKD |
| Lin et al. (2020) 57 | Prospective | 2,797 patients with type 2 diabetes | Higher intake of pickled foods was associated with renal function decline (≧40% drop in the estimated glomerular filtration rate) |
DBP, diastolic blood pressure; DKD, diabetic kidney disease; HDL‐C, high density lipoprotein‐cholesterol; SBP, systolic blood pressure; T2D, type 2 diabetes; WC, waist circumference.
Demographic and hereditary factors
Older age, diabetes onset at young age, prolonged diabetes duration, and male sex are some globally recognized demographic factors related to DKD initiation 30 . Asians as well as black people, American Indians, Hispanics, and Pacific Islanders were reported to be more susceptible to DKD 30 . With regard to family history and genetic predisposibility 29 , some hereditary markers have recently been identified in Chinese diabetic patients. Liao et al. conducted a case–control study to identify diabetic nephropathy‐related susceptible variants in Han Chinese persons with type 2 diabetes. They indicated that some novel single‐nucleotide polymorphisms (SNPs) and haplotypes located at the 16q22.1 region are involved in the biological pathways (these SNP polymorphisms are related to production of glucose, inflammation, cytokines, and transforming growth factor‐beta and may lead to DKD) of DKD (Table 1) 32 . These SNP polymorphisms have not been reported for DKD in Han Chinese. The proportions of minor alleles of SNPs rs11647932 and rs6499323 among Taiwanese were similar to those of Han Chinese in China, but higher than those of Japanese, Europeans, and Africans 32 . Chang et al. examined IL‐6 polymorphisms and the development of DKD in a prospective Taiwanese type 2 diabetes cohort and revealed that Taiwanese populations with type 2 diabetes and IL‐6 gene polymorphisms [rs1800796 GG, adjusted hazard ratio (aHR) 1.98 (1.05–3.73); and rs1524107 CC, aHR 1.95 (1.03–3.68)] may be more likely to experience chronic inflammation and exhibit higher risks of incident DKD 33 . The evidence for the correlation between IL‐6 polymorphisms and the risk of DKD has also been found in Japanese, Korean, and Caucasian patients 33 . Chung et al. also investigated the association of 18 ADIPOQ polymorphisms with DKD development in the same type 2 diabetes cohort. Their study revealed that ADIPOQ genetic polymorphisms rs2241766, rs1063537, rs2241767, and rs2082940 were correlated with the progression of incident DKD in Taiwanese men with type 2 diabetes 34 . The correlation of these SNPs with the progression of DKD in this study has also been reported among Korean, and European patients with some discrepancies 34 .
Hyperglycemia
Persistently poor glycemic control is one of the most important risk factors for DKD. The reduction in the incidence of DKD in the United States since 1997 was attributed to the sustained improvement in diabetes care over the decades 35 . The benefit of better glycemic control in reducing DKD incidence was evident in both type 1 and type 2 diabetes cohorts. In the UKPDS, which recruited persons with new diagnoses of type 2 diabetes, after a 10‐year intensive glycemic intervention targeted to achieve a hemoglobin A1c (HbA1c) of <7%, a 24% reduction was achieved in the development of microvascular complications, including DKD, compared with the conventional therapy 36 . After 12 years, the UKPDS group receiving intensive glycemic intervention exhibited a 33% reduction in the risk of proteinuria and a significant reduction in the proportion of patients with a doubling of the blood creatinine level (0.9% vs 3.5%) relative to the conventional therapy group 37 . Similarly, the Diabetes Control and Complications Trial (DCCT) recruited persons with early stage type 1 diabetes; intensive glucose intervention targeted to achieve a HbA1C level <7% reduced the 9‐year risks of microalbuminuria and overt proteinuria by 34% and 56%, respectively, compared with standard care 38 . After a median follow‐up of 22 years, the intensive intervention group in the DCCT exhibited approximately 50% lower risk of eGFR <60 mL/min per 1.73 m2 39 . In accordance with the results of previous studies, Hsu et al. serially measured HbA1c over 5 years to assess the risk of microalbuminuria in Taiwanese patients with type 2 diabetes and found that in addition to the mean HbA1c values, HbA1c variability was independently and more significantly associated with the development of microalbuminuria 40 .
Hypertension
High blood pressure is a crucial risk factor for DKD in individuals with both type 1 diabetes and type 2 diabetes. Persons with type 1 diabetes usually have a normal blood pressure at diagnosis but exhibit hypertension near the onset of microalbuminuria 41 . The UKPDS trial treated persons with type 2 diabetes to a target blood pressure of 150/85 mmHg over a median of 15 years and revealed a significant 37% reduction in microvascular complications compared with those treated to a target blood pressure of 180/105 mmHg 42 . The Appropriate Blood Pressure Control in Diabetes trial randomly assigned 480 persons with type 2 diabetes to intensive (target BP of approximately 128/75 mmHg) and moderate (target blood pressure of approximately 137/81 mmHg) control. After a 5‐year follow‐up period, intensive control in normotensive type 2 diabetes persons delayed the progression to incipient and overt diabetic nephropathy but did not demonstrate any benefit for creatinine clearance reduction 43 . In line with the results of previous studies, Sheen et al. investigated 215 type 2 diabetes outpatients without symptomatic cardiovascular disease for 12 months in Taiwan and revealed that systolic blood pressure (SBP) is a powerful modifiable independent risk factor [adjusted odds ratio (OR) 1.023 (1.001–1.046)] for incident albuminuria and a rapid renal function decline 44 . Yeh et al. evaluated the relationship between the variability in blood pressure and the magnitude of renal function impairment in Taiwanese type 2 diabetes patients with normal renal function. They found that the visit‐to‐visit variabilities in SBP and diastolic blood pressure (DBP) were correlated with new‐onset DKD in the first decade after type 2 diabetes diagnosis 45 .
Dyslipidemia
Dyslipidemia, including high triglycerides, low‐density lipoprotein (LDL) cholesterol, apolipoprotein‐B‐100, and low high‐density lipoprotein (HDL) cholesterol levels, is independently associated with the development of DKD in both type 1 diabetes and type 2 diabetes cohorts 29 . However, which lipids play the most important role in the pathogenesis of DKD remain unclear. The Fenofibrate Intervention and Event Lowering in Diabetes 46 and Action to Control Cardiovascular Risk in Diabetes studies 47 have disclosed that the use of fenofibrate reduced albuminuria and attenuated eGFR decline. Chang et al. assessed the association of mean values and variability in metabolic parameters (blood pressure, blood glucose, and lipid levels) with the development of DKD in a Taiwanese type 2 diabetes cohort and demonstrated that persons with stable and higher mean HDL‐C levels had lower risk [aHR 0.971 (0.953–0.989)] of incident DKD 48 .
Renal injuries
Acute kidney injury, medications, toxins, smoking, and recurrent infections are potential initiators of DKD 29 , 30 . Tsai et al. investigated the temporal relationship between nonsteroidal anti‐inflammatory drug (NSAID) use and the development of CKD in a type 2 diabetes cohort 49 and found that compared with persons who did not take any NSAIDs, those who took NSAIDs for at least 90 days had a 37% higher risk of DKD development.
Lifestyles, dietary patterns, obesity, and insulin resistance
Sedentary lifestyle, high protein or sodium intake, obesity, and insulin resistance are associated with the development of DKD 29 , 30 . The Look AHEAD study revealed a significant reduction in incident albuminuria after a multifactorial diet and lifestyle intervention 50 . Several empirical studies have conducted in Taiwan illustrated a clear relationship between healthy lifestyle (physical activity, body weight loss, and abstinence of smoking) and DKD development in patients with type 2 diabetes. Lin et al. assessed the effect of physical activity [The questionnaire asked patients whether they had activities (Yes) such as walking, running, cycling, and so on, or not (No)] in persons with type 2 diabetes on the prevention of DKD. They demonstrated that physical activity reduced the probability of new‐onset DKD by approximately 70% 51 . Obesity alone can promote the development of focal and segmental glomerulosclerosis, resulting in high proteinuria 52 . Chung et al. conducted a cohort study and indicated that obese persons with excessive central fat (measured by waist circumference) were more likely to have DKD; a weight gain of >10% and waist circumference increase of >15% independently predicted new‐onset DKD 53 . In a cross‐sectional study, Hsu et al. demonstrated a dose–response effect of cigarette smoking (especially those who smoked 15–30 or more than 30 pack‐years) on the development of DKD and suggested that diabetic patients should quit smoking regardless of age, duration of diabetes mellitus, and status of blood pressure control 54 . Insulin resistance (IR) is also independently associated with DKD irrespective of its association with body weight, glucose, BP, and lipid control. Hsu et al. assessed the relationship between IR [homeostasis model assessment of insulin resistance (HOMA‐IR)] and microalbuminuria in a prospective Taiwanese cohort of type 2 diabetes and confirmed that insulin resistance could significantly predict the development of microalbuminuria in persons with type 2 diabetes 55 . Hsu et al. surveyed 851 patients with type 2 diabetes and found hyperferritinemia, a chronic inflammatory marker with an impact on IR, was a predictor of incident DKD in Taiwan 56 . Lin et al. evaluated the relationship of unhealthy dietary behaviors with renal function decline in 2797 patients with type 2 diabetes and concluded that eating more pickled foods (The frequency of eating pickled foods was quantified as score 1: no eating, score 2: ≦1 time/month, score 3: 2–3 times/month, score 4: 1–2 times/week, score 5: 3–4 times/week, score 6: 5–6 times/week, score 7: 1 time/day, score 8: 2–3 times/day, score 9: 4–6 times/day) was associated with renal function decline defined as a ≥40% drop in the eGFR 57 .
PROGNOSTIC FACTORS INFLUENCING DKD PROGRESSION OR MORTALITY
Identifying the prognostic factors of DKD and delaying DKD progression at early stages are crucial strategies for preventing the development of ESRD, cardiovascular complications, and premature death (Table 2) 58 . The bad outcomes of DKD included mortality, cardiovascular events, renal dysfunction progression, and ESRD. According to a meta‐analysis, to better predict cardiovascular outcomes (such as cardiovascular mortality, heart failure, coronary disease, and stroke) in patients with CKD, researchers should include both eGFR and albuminuria in the study designs in addition to traditional risk factors. The outcome prediction power of eGFR and albuminuria was especially important in individuals with diabetes or hypertension 59 .
Table 2.
Studies on the prognostic factors of DKD
| Authors (year)reference | Study design | Number of cases | Main findings |
|---|---|---|---|
| Hsu et al. (2014) 81 | Cohort | 28,497 patients with type 2 diabetes and advanced CKD | ACEi or ARB were associated with 6% lower risk of long‐term dialysis or death |
| Chung et al. (2014) 85 | Prospective | 376 patients with type 2 diabetes | High PUFA concentrations, especially n‐3 or higher n‐3/n‐6 PUFA ratio, may exert protection against renal function impairment |
| Hsu et al. (2014) 87 | Prospective | 635 patients with type 2 diabetes | Frequent intake of fish and vegetables may be related to better kidney function |
| Hung et al. (2014) 88 | Prospective | 105 patients with type 2 diabetes and CKD stage 3 or 4 | BMI ≧ 25 kg/m2 was a protective factor for renal function deterioration |
| Liao et al. (2015) 68 | Cohort | 51,681 patients aged ≧30 years with type 2 diabetes | HbA1c level ≧ 7.0% and HbA1c < 6.0% were linked with increased ESRD risk |
| Chang et al. (2015) 75 | Prospective | 362 type 2 diabetes and DKD patients | ABI < 0.9 was associated with higher risk of adverse events (mortality, CVD, and diabetic foot) |
| Lee et al (2015) 69 | Retrospective | 101 patients with type 2 diabetes and DKD | Severe hypoglycemia was associated with deterioration of renal function |
| Chen et al. (2015) 70 | Prospective | 2,632 haemodialysis patients with diabetes as comorbidity and 13,160 matched patients with DKD | Patients with diabetes as the primary kidney disease have worse survival than chronic hemodialysis patients with comorbid diabetes |
| Chen et al. (2015) 83 | Cohort | 12,350 diabetic patients with advanced CKD | TZD users were associated with lower risk of ESRD and death than TZD nonusers |
| Kuo et al. (2016) 66 | Prospective | 2,401 patients with type 2 diabetes with stage 3–5 CKD | Higher HbA1c level is associated with higher risks of clinical outcomes in diabetic patients with stage 3–4 CKD but not stage 5 CKD |
| Wen at al. (2017) 8 | Cohort | 9,067 patients with type 2 diabetes with early DKD | Physical inactivity, smoking, alcohol drinking, and obesity could amplify the mortality risk |
| Hung et al. (2017) 71 | Cohort | 1,330 patients with type 2 diabetes and DKD | Diabetic retinopathy was associated with poorer renal outcomes |
| Shen et al. (2017) 91 | Retrospective | 196 patients with newly diagnosed type 2 diabetes | Intensive short‐term multidisciplinary interventions may reduce coronary heart disease and nephropathy |
| Kuo et al. (2018) 67 | Cohort | 1,558 patients with type 2 diabetes with stages 3–4 CKD | Higher HbA1c in patients with Hb ≧ 10 g/dL was associated with worse clinical outcomes (ESRD, all‐cause mortality, and composite endpoint). The relationship did not exist in patients with Hb < 10 g/dL |
| Zhang et al.(2018) 73 | Cross‐sectional | 250 patients with type 2 diabetes and biopsy‐proven DKD | Diabetic retinopathy remained an independent risk factor for progression to ESRD after adjustment for important clinical variables |
| Lin et al. (2018) 79 | Cohort | 1,958 patients with type 2 diabetes and CKD stages 1–5 | Hematuria was associated with an increased risk of ESRD |
| Chou et al. (2019) 61 | Cohort | 55 patients with biopsy‐proven DKD compared with 48 patients with glomerulonephritis and 82 patients with lupus nephritis | Patients older than 65 years and with lower serum albumin levels were independently associated with overall death |
| Lin et al. (2019) 74 | Cohort | 4,050 patients with type 2 diabetes with CKD | Diabetic retinopathy was a risk factor for CKD progression |
| Chen et al. (2019) 81 | Cohort | 125,490 patients with incident DKD | Traditional Chinese medicine users were associated with a 52% reduction of mortality risk and 19% reduction of ESRD risk |
| Chuang et al. (2019) 86 | Retrospective cohort | 935 patients with type 2 diabetes | The presence of metabolic syndrome independently predicted DKD progression |
ABI, ankle brachial index; ACEI, angiotensin‐converting enzyme inhibitor; ARB, angiotensin II receptor blocker; BMI, body mass index; CKD, chronic kidney disease; CVD, cardiovascular disease; DKD, diabetic kidney disease; ESRD, end‐stage renal disease; Hb, hemoglobin; PUFA, polyunsaturated fatty acid; T2D, type 2 diabetes.
Age, sex, family history, and ethnicity
The clinical and biochemical characteristics associated with the progression of DKD include older age, family history, male sex, diabetes onset at young age, glycemic control, BP control, duration of diabetes, baseline albuminuria, CKD stage, retinopathy, neuropathy, and increased pulse wave velocity of brachial‐ankle or carotid‐femoral arteries 60 . Chou et al. compared the mortality risks in a Taiwanese kidney biopsy cohort between patients with pathology‐proven DKD (PP‐DKD) and those with isolated crescentic glomerulonephritis (GN) or lupus nephritis (LN). They found a significantly higher overall death in PP‐DKD than in patients with GN or LN if patients were aged above 65 years or had lower serum albumin levels 61 . In another Chinese cohort with 8301 diabetic participants with no CVDs with a median follow‐up of 8.05 years, Wang et al. reported that diabetic women with proteinuria had a higher risk of all‐cause mortality than diabetic men (HR: 3.96 vs 2.15) 62 .
HBA1c, duration of diabetes, and complications
Glycemic control in patients with DKD was reported to be able to predict the risk of ESRD development 60 . The DCCT study revealed that intensified glycemic intervention did not reduce the rate of progression to macroalbuminuria in persons with type 1 diabetes and microalbuminuria 38 . For type 2 diabetes, positive results were found in the Kumamoto study and the Action in Diabetes and Vascular Disease: Preterax and Diamicron MR Controlled Evaluation (ADVANCE) study; in both, an approximately 21% risk reduction of conversion from micro‐ to macroalbuminuria was found in persons with type 2 diabetes receiving intensive treatments 63 , 64 . However, intensive glucose therapy (targeting low HbA1C 6–6.9%) in the ADVANCE study for persons with longstanding diabetes and DKD for 3.5 years increased the mortality and cardiovascular complications due to the risk of severe hypoglycemia 65 .
Kuo et al. prospectively enrolled 2401 diabetic patients with stage 3–5 CKD and evaluated the changes of risks of adverse clinical outcomes (ESRD, all‐cause mortality and combined CV events) with increasing HbA1c. They reported, in the group with a higher HbA1c (>9.0), the HRs for adverse clinical outcomes were 1.6 (95% CI, 1.07–2.38) for ESRD, 1.52 (95% CI, 0.97–2.38) for all‐cause mortality, and 1.46 (95% CI, 1.02–2.09) for combined CV events with mortality, respectively. The results demonstrated the association between incremental HbA1c and hazard ratios was only in diabetic patients with stage 3–4 CKD but not with stage 5 CKD (Table 2) 66 . In follow‐up research, the authors revealed that anemia modified the prognostic value of glycated hemoglobin in patients with DKD. They also reported that higher HbA1c in patients with hemoglobin (Hb) ≥ 10 g/dL was associated with worse clinical outcomes (ESRD, all‐cause mortality, and composite endpoint). The relationship did not exist in patients with Hb < 10 g/dL 67 . Liao et al. recruited 51618 patients with type 2 diabetes aged ≥30 years without ESRD and followed them up for an average of 8.1 years. In addition, under a competing risk model against death, they evaluated the association between HbA1c levels and ESRD and reported that HbA1c levels ≥7.0 and <6.0% were associated with higher risks of ESRD. They emphasized that not only high HbA1c but also low HbA1c was associated with ESRD. The authors provided a possible explanation for the increased risk of ESRD for HbA1c < 6.0%; that is, diabetic patients with low HbA1c levels are likely to have hypoglycemia and increased risk of following hyperglycemia, which might worsen endothelial function, increase oxidative stress and inflammation. The results suggested that an appropriate glycemic level was essential for diabetes care 68 . Lee et al. conducted a retrospective cohort study to assess the impact of severe hypoglycemia on renal dysfunction in persons with type 2 diabetes and DKD and showed that severe hypoglycemia was associated with the deterioration of renal function and patients with higher baseline creatinine and longer duration of type 2 diabetes might present greater aggravation of renal function decline 69 .
In addition to glycemic control, Chen et al. compared the survival of all patients on maintenance hemodialysis in Taiwan Renal Registry Database with DKD versus diabetes as a comorbid disease. They concluded that patients with DKD were associated with higher first‐year and overall mortality than patients with diabetes as a comorbid disease 70 . Hung et al. evaluated whether clinical parameters favoring diabetic nephropathy could predict clinical outcomes in 1330 persons with type 2 diabetes and DKD and concluded that diabetic retinopathy (DR) was significantly associated with a higher risk of ESRD 71 . Zhang et al. assessed the relationship between DR and the progression of DKD to ESRD in 250 patients with type 2 diabetes with biopsy‐proven DKD. The authors evaluated glomerular lesions according to the glomerular classifications of the Renal Pathology Society in 2010 72 , which included (a) mild changes by light microscopy or glomerular basement membrane thickening by electron microscopy were both Class I; (b) mild mesangial expansion (Class IIa); (c) severe mesangial expansion (Class IIb); (d) Kimmelstiel–Wilson lesion (Class III); (e) global glomerulosclerosis (Class IV). Their results revealed that the severity of glomerular lesions was significantly associated with DR; DR may predict the renal prognosis of patients with type 2 diabetes and DKD 73 . Lin et al. investigated the relationship between DR and the progression of renal dysfunction in 4050 diabetic patients with CKD from 14 hospitals. They reported that the presence and severity of DR were risk factors for CKD progression among Taiwanese CKD patients with diabetes 74 . Chang et al. retrospectively evaluated the influence of peripheral arterial disease (PAD) on 362 patients with type 2 diabetes with DKD. Patients with albuminuria plus ABI<0.9 were associated with a higher risk of composite events than those with albuminuria but normal ABI (P < 0.05). The only trend difference between the two groups was in the all‐cause mortality. Albuminuria plus ABI < 0.9 was associated with a risk of composite events [aHR 4.20 (1.77–9.92)] and an all‐cause mortality (aHR 17.77 (1.93–162.20)]. They concluded that patients with albuminuria and an ankle brachial index (ABI) < 0.9 had a higher risk of composite events (all‐cause mortality, hospitalization for coronary artery disease, stroke, re‐vascularization, amputation, and diabetic foot) and all‐cause mortality than those with albuminuria but normal ankle brachial index 75 .
Wang et al. evaluated the relationship between dipstick proteinuria and the risk of myocardial infarction (MI) and all‐cause mortality in a cohort of 16,573 Chinese patients with diabetes or prediabetes, with a follow‐up of 8.03 years. They concluded that the presence of trace dipstick proteinuria or worse was associated with increased risks of MI and all‐cause mortality 76 . Based on a Chinese cohort of 8,301 diabetic participants with no CVD and a median follow‐up of 8.05 years, Wang et al. reported that both lower eGFR and proteinuria were independent risk factors for all‐cause mortality 77 . Focusing on proteinuria, Sun et al. reported increased mortality risks with elevated proteinuria (HR: 1.54) and decreased risks with reduced proteinuria (HR: 0.70) in 17,878 Chinese participants with diabetes mellitus or prediabetes, with a median follow‐up of 6.69 years 78 . In a prospective Chinese cohort study with 17,380 participants with diabetes mellitus or prediabetes and a median follow‐up of 6.9 years, the authors reported an increased stroke risk in participants with persistent, incident, and remittent proteinuria compared with those without proteinuria. Moreover, proteinuria reduction contributed to a 12% decrease in incident stroke 77 .
Lin et al. investigated the prognostic value of hematuria in a cohort of 1958 patients with type 2 diabetes and CKD stages 1–5. The results revealed that hematuria was associated with an increased risk [aHR 1.39 (1.10–1.76)] of ESRD 79 .
Hypertension and other medications
The 2021 Kidney Disease Improving Global Outcomes guideline recommends a SBP of <120 mmHg in most subgroups of high BP and CKD, including DKD, by using standardized office blood pressure measurement. Although medication with an angiotensin‐converting enzyme inhibitor (ACEi) or an angiotensin II receptor blocker (ARB) was recommended, the combined use of ACEi with ARB or direct renin inhibitors was discouraged 80 . Hsu et al. assessed the effectiveness and safety of ACEi or ARB use for advanced (predialysis) CKD in patients with hypertension and anemia (approximately 50% of these patients have type 2 diabetes). They indicated that patients with advanced CKD using ACEi or ARB were associated with 6% lower risk of long‐term dialysis or all‐cause death 81 .
Chen et al. compared, in a Taiwanese cohort of 125,490 patients with incident DKD, the risks of ESRD and mortality among patients who received traditional Chinese medicine (TCM) prescriptions under Taiwan National Health Insurance versus other patients who did not receive TCM prescription. There was a 52% risk reduction of mortality and a 19% risk reduction of ESRD in TCM users. Once the patients progressed to ESRD, the cumulative incidence of mortality increased rapidly compared with TCM users without ESRD. The TCM users who had used TCM longer or initiated TCM treatments after being diagnosed as having DKD were associated with a lower risk of mortality 82 . Chen et al. selected 12,350 diabetic patients with advanced CKD (serum creatinine levels greater than 6 mg/dL but not yet receiving renal replacement therapy) and compared the risk of ESRD in patients receiving thiazolidinediones (TZDs) with those who did not use TZDs. During a median follow‐up of 6 months, among these diabetic patients with advanced CKD, the TZD users were associated with a lower risk of ESRD and death 83 .
Dyslipidemia and metabolic syndrome
Lipid‐lowering therapy is widely recommended in patients with DKD to reduce the risk of cardiovascular diseases and mortality 84 . However, whether lipid lowering treatment attenuates renal function decline remains controversial. Chung et al. analyzed the concentrations of erythrocyte polyunsaturated fatty acids (PUFA), including total PUFA, n‐3 PUFA, n‐6 PUFA and n‐3/n‐6 PUFA ratio, and inflammatory markers (interleukin‐6) in 2008 and the eGFR changes in 2008 and 2012. The quartile levels of n‐3/n‐6 PUFA ratio were <0.277, 0.277–0.319, 0.320–0.368, and >0.368. The results revealed that high PUFA concentrations, especially n‐3 or a higher n‐3/n‐6 PUFA ratio, may exert protection against renal function decline in patients with type 2 diabetes 85 . Chuang et al. conducted a retrospective cohort study for approximately 5 years including 935 patients with type 2 diabetes and reported that the presence of metabolic syndrome was an independent predictor of DKD progression 86 .
Lifestyles, dietary factors, obesity, and insulin resistance
Intensive diet and lifestyle interventions are frequently recommended to persons with DKD, which include a low‐protein diet, sodium restriction, body weight reduction, increased physical activity, and smoking cessation 30 . Wen et al. studied a cohort of 512,700 adults in Taiwan participating in a health surveillance program and identified that diabetic patients with early DKD were associated with high mortality risks. They found that patients with early DKD presented more lifestyle risks such as inactivity or obesity and were up to five times more vulnerable to mortality risks. Moreover, such patients exhibited a 3‐fold increase in all‐cause mortality (HR: 3.16) and a 16‐year loss in life expectancy, which is much worse than that in patients with early CKD (6‐year loss) or diabetes (10‐year loss) alone. They proposed that identifying early proteinuria among diabetic patients and realizing the importance of reducing lifestyle risks such as inactivity are crucial in increasing life expectancy 8 . Hsu et al. explored the relationships between dietary patterns and renal function in adults with type 2 diabetes. They revealed that healthy diet habits, such as a frequent intake of fish and vegetables, may be associated with better renal function 87 . Hung et al. prospectively observed the relationship between body mass index (BMI) and the progression of renal function decline in 105 patients with type 2 diabetes with CKD stage 3 or 4. They revealed that BMI ≥ 25 kg/m2 was a protective prognostic factor for renal function decline in these patients 88 . In a retrospective study of 188 Chinese patients with type 2 diabetes and biopsy‐proven DKD followed up for at least 1 year, Zhang et al. reported 6.2‐ and 7.37‐fold increased risks of progression to ESRD in patients with moderate and severe hypoalbuminemia, respectively, independent of their histopathological characteristics 89 .
Multifactorial intervention
The Steno‐2 study randomized 160 persons with type 2 diabetes with microalbuminuria to multifactorial intervention (a low‐fat diet, a light‐to‐moderate exercise program three to five times a week, smoking cessation, prescription of ACE inhibitors or ARBs and aspirin, achieved BP < 130/80 mmHg, fasting serum cholesterol < 175 mg/dL, fasting serum triglycerides <150 mg/dL, and HAB1c < 6.5%) and conventional treatment. The multifactorial intervention group exhibited a 61% reduction in the risk of macroalbuminuria and a 55% reduction in the risk of cardiovascular events 90 . Shen et al. retrospectively compared 196 persons with newly diagnosed type 2 diabetes receiving intensive multidisciplinary interventions with 206 persons receiving conventional treatment for 1 year. After 10 years of follow‐up, they demonstrated that the initiation of earlier intensive short‐term multidisciplinary interventions may reduce coronary heart disease and DKD progression 91 .
PERSPECTIVES
Despite the advanced health care in the past decades, the prevalence of DKD in Taiwan is still increasing. Moreover, the incidence and prevalence of ESRD in Taiwan have been among the highest in the world for more than 10 years. Regarding the studies on the risk factors for DKD, Taiwan’s studies (Table 1) echo the results of those from countries around the world; however, studies in Taiwan have also indicated unique genetic factors for the onset of DKD. Many comprehensive studies (Table 2) on the prognostic factors of DKD have been conducted in Taiwan, which provide empirical evidence for caring for Taiwanese patients with DKD. These results must be translated to other populations to optimize renal care and to modify national health policies. In persons who present susceptible factors of incident DKD, clinicians should regularly monitor the occurrence of DKD. Moreover, implementing early and optimal intervention is essential to reverse or attenuate disease progression. For persons who present modifiable risk factors for DKD development, adequate treatment must be given actively to reduce the probability of DKD development. For persons who present worsening factors of DKD, care providers must adopt intensive multifactorial intervention to delay the progression of DKD and even reduce the incidence of cardiovascular events and premature death.
DISCLOSURE
The authors have no financial competing interests.
Approval of the research protocol: N/A.
Informed consent: N/A.
Approval date of registry and the registration no. of the study/trial: N/A.
Animal studies: N/A.
ACKNOWLEDGMENTS
We deeply thank Mr Owen Liu for drawing the pictures in Figure 1. This manuscript was edited by Wallace Academic Editing.
J Diabetes Investig 2021; 12: 2112–2123
Contributor Information
Yueh‐Han Hsu, Email: john.yh.hsu@gmail.com.
Chih‐Cheng Hsu, Email: cch@nhri.edu.tw.
REFERENCES
- 1. Institute for Health Metrics and Evaluation, Global Health Data Exchange, Global Burden of Disease Study 2019 (GBD 2019) Data resources, GBD results tool, terms and conditions. 2019. Available from: http://ghdx.healthdata.org/gbd‐results‐tool Assessed August 13, 2021.
- 2. Parving H‐H, Lewis JB, Ravid M, et al. Prevalence and risk factors for microalbuminuria in a referred cohort of type II diabetic patients: a global perspective. Kidney Int 2006; 69: 2057–2063. [DOI] [PubMed] [Google Scholar]
- 3. Taiwan Society of Nephrology . 2019 Annual Report on Kidney Disease in Taiwan. Available from: https://www.tsn.org.tw/enVersion/TWRDS.aspx Accessed April 6, 2021.
- 4. Couser WG, Remuzzi G, Mendis S, et al. The contribution of chronic kidney disease to the global burden of major noncommunicable diseases. Kidney Int 2011; 80: 1258–1270. [DOI] [PubMed] [Google Scholar]
- 5. Adler AI, Stevens RJ, Manley SE, et al. Development and progression of nephropathy in type 2 diabetes: the United Kingdom Prospective Diabetes Study (UKPDS 64). Kidney Int 2003; 63: 225–232. [DOI] [PubMed] [Google Scholar]
- 6. Andrésdóttir G, Jensen ML, Carstensen B, et al. Improved survival and renal prognosis of patients with type 2 diabetes and nephropathy with improved control of risk factors. Diabetes Care 2014; 37: 1660–1667. [DOI] [PubMed] [Google Scholar]
- 7. So WY, Kong AP, Ma RC, et al. Glomerular filtration rate, cardiorenal end points, and all‐cause mortality in type 2 diabetic patients. Diabetes Care 2006; 29: 2046–2052. [DOI] [PubMed] [Google Scholar]
- 8. Wen CP, Chang CH, Tsai MK, et al. Diabetes with early kidney involvement may shorten life expectancy by 16 years. Kidney Int 2017; 92: 388–396. [DOI] [PubMed] [Google Scholar]
- 9. Earle KA, Porter KK, Ostberg J, et al. Variation in the progression of diabetic nephropathy according to racial origin. Nephrol Dial Transplant 2001; 16: 286–290. [DOI] [PubMed] [Google Scholar]
- 10. de Zeeuw D, Ramjit D, Zhang Z, et al. Renal risk and renoprotection among ethnic groups with type 2 diabetic nephropathy: a post hoc analysis of RENAAL. Kidney Int 2006; 69: 1675–1682. [DOI] [PubMed] [Google Scholar]
- 11. Dreyer G, Hull S, Aitken Z, et al. The effect of ethnicity on the prevalence of diabetes and associated chronic kidney disease. QJM 2009; 102: 261–269. [DOI] [PubMed] [Google Scholar]
- 12. Young BA, Katon WJ, Von Korff M, et al. Racial and ethnic differences in microalbuminuria prevalence in a diabetes population: the pathways study. J Am Soc Nephrol 2005; 16: 219–228. [DOI] [PubMed] [Google Scholar]
- 13. Dreyer G, Hull S, Mathur R, et al. Progression of chronic kidney disease in a multi‐ethnic community cohort of patients with diabetes mellitus. Diabetes Med 2013; 30: 956–963. [DOI] [PubMed] [Google Scholar]
- 14. Ali O, Mohiuddin A, Mathur R, et al. A cohort study on the rate of progression of diabetic chronic kidney disease in different ethnic groups. BMJ Open 2013; 3: e001855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tuttle KR, Bakris GL, Bilous RW, et al. Diabetic kidney disease: a report from an ADA Consensus Conference. Am J Kidney Dis 2014; 64: 510–533. [DOI] [PubMed] [Google Scholar]
- 16. International Diabetes Federation . IDF Diabetes Atlas, 9th edn. 2019. Available from: https://www.diabetesatlas.org/en/ Accessed February 6, 2020.
- 17. Yang W, Lu J, Weng J, et al. Prevalence of diabetes among men and women in China. N Engl J Med 2010; 362: 1090–1101. [DOI] [PubMed] [Google Scholar]
- 18. Xu Y, Wang L, He J, et al. Prevalence and control of diabetes in Chinese adults. JAMA 2013; 310: 948–959. [DOI] [PubMed] [Google Scholar]
- 19. Sheen Y‐J, Hsu C‐C, Jiang Y‐D, et al. Trends in prevalence and incidence of diabetes mellitus from 2005 to 2014 in Taiwan. J Formos Med Assoc 2019; 118: S66–S73. [DOI] [PubMed] [Google Scholar]
- 20. International Diabetes Federation . IDF Diabetes Atlas, 7th edn. 2015. Available from: https://diabetesatlas.org/upload/resources/previous/files/7/IDF%20Diabetes%20Atlas%207th.pdf Accessed April 20, 2021.
- 21. International Diabetes Federation . IDF Diabetes Atlas, 8th edn. 2017. Available from: http://fmdiabetes.org/wp‐content/uploads/2018/03/IDF‐2017.pdf Accessed April 20, 2021.
- 22. Webster AC, Nagler EV, Morton RL, et al. Chronic kidney disease. Lancet 2017; 389: 1238–1252. [DOI] [PubMed] [Google Scholar]
- 23. National Health Research Institutes . 2018 Annual Repoorton Kidney Disease in Taiwan. Available from: http://w3.nhri.org.tw/nhri_org/rl/lib/NewWeb/nhri/ebook/39000000448683.pdf Accessed April 20, 2021.
- 24. Afkarian M, Zelnick LR, Hall YN, et al. Clinical manifestations of kidney disease among US adults with diabetes, 1988–2014. JAMA 2016; 316: 602–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bhalla V, Zhao B, Azar KMJ, et al. Racial/ethnic differences in the prevalence of proteinuric and nonproteinuric diabetic kidney disease. Diabetes Care 2013; 36: 1215–1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Huang Y‐Y, Lin K‐D, Jiang Y‐D, et al. Diabetes‐related kidney, eye, and foot disease in Taiwan: an analysis of the nationwide data for 2000–2009. J Formos Med Assoc 2012; 111: 637–644. [DOI] [PubMed] [Google Scholar]
- 27. Lin K‐D, Hsu C‐C, Ou H‐Y, et al. Diabetes‐related kidney, eye, and foot disease in Taiwan: an analysis of nationwide data from 2005 to 2014. J Formos Med Assoc 2019; 118: S103–S110. [DOI] [PubMed] [Google Scholar]
- 28. Ou H‐T, Lee T‐Y, Li C‐Y, et al. Incidence of diabetes‐related complications in Chinese patients with type 1 diabetes: a population‐based longitudinal cohort study in Taiwan. BMJ Open 2017; 7: e015117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Thomas MC, Brownlee M, Susztak K, et al. Diabetic kidney disease. Nat Rev Dis Primers 2015; 1: 15018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Alicic RZ, Rooney MT, Tuttle KR. Diabetic kidney disease: challenges, progress, and possibilities. Clin J Am Soc Nephrol 2017; 12: 2032–2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gross JL, de Azevedo MJ, Silveiro SP, et al. Diabetic nephropathy: diagnosis, prevention, and treatment. Diabetes Care 2005; 28: 164–176. [DOI] [PubMed] [Google Scholar]
- 32. Liao L‐N, Chen C‐C, Wu F‐Y, et al. Identified single‐nucleotide polymorphisms and haplotypes at 16q22.1 increase diabetic nephropathy risk in Han Chinese population. BMC Genet 2014; 15: 113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chang W‐T, Huang M‐C, Chung H‐F, et al. Interleukin‐6 gene polymorphisms correlate with the progression of nephropathy in Chinese patients with type 2 diabetes: a prospective cohort study. Diabetes Res Clin Pract 2016; 120: 15–23. [DOI] [PubMed] [Google Scholar]
- 34. Chung H‐F, Long KZ, Hsu C‐C, et al. Adiponectin gene (ADIPOQ) polymorphisms correlate with the progression of nephropathy in Taiwanese male patients with type 2 diabetes. Diabetes Res Clin Pract 2014; 105: 261–270. [DOI] [PubMed] [Google Scholar]
- 35. Burrows NR, Li Y, Geiss LS. Incidence of treatment for end‐stage renal disease among individuals with diabetes in the U.S. continues to decline. Diabetes Care 2010; 33: 73–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Holman RR, Paul SK, Bethel MA, et al. 10‐year follow‐up of intensive glucose control in type 2 diabetes. N Engl J Med 2008; 359: 1577–1589. [DOI] [PubMed] [Google Scholar]
- 37. Bilous R. Microvascular disease: what does the UKPDS tell us about diabetic nephropathy? Diabetes Med 2008; 25: 25–29. [DOI] [PubMed] [Google Scholar]
- 38. Nathan DM, DCCT/EDIC Research Group . The diabetes control and complications trial/epidemiology of diabetes interventions and complications study at 30 years: overview. Diabetes Care 2014; 37: 9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. DCCT/EDIC Research Group , de Boer IH, Sun W, et al. Intensive diabetes therapy and glomerular filtration rate in type 1 diabetes. N Engl J Med 2011; 365: 2366–2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hsu CC, Chang HY, Huang MC, et al. HbA1c variability is associated with microalbuminuria development in type 2 diabetes: a 7‐year prospective cohort study. Diabetologia 2012; 55: 3163–3172. [DOI] [PubMed] [Google Scholar]
- 41. Ayodele OE, Alebiosu CO, Salako BL. Diabetic nephropathy — a review of the natural history, burden, risk factors and treatment. J Natl Med Assoc 2004; 96: 1445–1454. [PMC free article] [PubMed] [Google Scholar]
- 42. Retnakaran R, Cull CA, Thorne KI, et al. Risk factors for renal dysfunction in type 2 diabetes: U.K. Prospective Diabetes Study 74. Diabetes 2006; 55: 1832–1839. [DOI] [PubMed] [Google Scholar]
- 43. Schrier RW, Estacio RO, Esler A, et al. Effects of aggressive blood pressure control in normotensive type 2 diabetic patients on albuminuria, retinopathy and strokes. Kidney Int 2002; 61: 1086–1097. [DOI] [PubMed] [Google Scholar]
- 44. Sheen Y‐J, Lin J‐L, Li T‐C, et al. Systolic blood pressure as a predictor of incident albuminuria and rapid renal function decline in type 2 diabetic patients. J Diabetes Complications 2014; 28: 779–784. [DOI] [PubMed] [Google Scholar]
- 45. Yeh C‐H, Yu H‐C, Huang T‐Y, et al. The risk of diabetic renal function impairment in the first decade after diagnosed of diabetes mellitus is correlated with high variability of visit‐to‐visit systolic and diastolic blood pressure: a case control study. BMC Nephrol 2017; 18: 99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Davis TME, Ting R, Best JD, et al. Effects of fenofibrate on renal function in patients with type 2 diabetes mellitus: the Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) Study. Diabetologia 2011; 54: 280–290. [DOI] [PubMed] [Google Scholar]
- 47. Mychaleckyj JC, Craven T, Nayak U, et al. Reversibility of fenofibrate therapy‐induced renal function impairment in ACCORD type 2 diabetic participants. Diabetes Care 2012; 35: 1008–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Chang Y‐H, Chang D‐M, Lin K‐C, et al. High‐density lipoprotein cholesterol and the risk of nephropathy in type 2 diabetic patients. Nutr Metab Cardiovasc Dis 2013; 23: 751–757. [DOI] [PubMed] [Google Scholar]
- 49. Tsai H‐J, Hsu Y‐H, Huang Y‐W, et al. Use of non‐steroidal anti‐inflammatory drugs and risk of chronic kidney disease in people with Type 2 diabetes mellitus, a nationwide longitudinal cohort study. Diabetes Med 2015; 32: 382–390.48. [DOI] [PubMed] [Google Scholar]
- 50. The Look AHEAD Research Group . Effect of a long‐term behavioural weight loss intervention on nephropathy in overweight or obese adults with type 2 diabetes: a secondary analysis of the Look AHEAD randomised clinical trial. Lancet Diabetes Endocrinol 2014; 2: 801–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Lin H‐C, Peng C‐H, Chiou J‐Y, et al. Physical activity is associated with decreased incidence of chronic kidney disease in type 2 diabetes patients: a retrospective cohort study in Taiwan. Prim Care Diabetes 2014; 8: 315–321. [DOI] [PubMed] [Google Scholar]
- 52. Choung H‐Y, Bomback AS, Stokes MB, et al. The spectrum of kidney biopsy findings in patients with morbid obesity. Kidney Int 2019; 95: 647–654. [DOI] [PubMed] [Google Scholar]
- 53. Chung H‐F, Al Mamun A, Huang M‐C, et al. Obesity, weight change, and chronic kidney disease in patients with type 2 diabetes mellitus: a longitudinal study in Taiwan. J Diabetes 2017; 9: 983–993. [DOI] [PubMed] [Google Scholar]
- 54. Hsu CC, Hwang SJ, Tai TY, et al. Cigarette smoking and proteinuria in Taiwanese men with Type 2 diabetes mellitus. Diabet Med 2010; 27: 295–302. [DOI] [PubMed] [Google Scholar]
- 55. Hsu C‐C, Chang H‐Y, Huang M‐C, et al. Association between insulin resistance and development of microalbuminuria in type 2 diabetes: a prospective cohort study. Diabetes Care 2011; 34: 982–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Hsu YH, Huang MC, Chang HY, et al. Association between serum ferritin and microalbuminuria in type 2 diabetes in Taiwan. Diabetes Med 2013; 30: 1367–1373. [DOI] [PubMed] [Google Scholar]
- 57. Lin C‐W, Chen I‐W, Lin Y‐T, et al. Association of unhealthy dietary behaviors with renal function decline in patients with diabetes. BMJ Open Diabetes Res Care 2020; 8: e000743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. GBD Chronic Kidney Disease Collaboration . Global, regional, and national burden of chronic kidney disease, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2020; 395: 709–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Matsushita K, Coresh J, Sang Y, et al. Estimated glomerular filtration rate and albuminuria for prediction of cardiovascular outcomes: a collaborative meta‐analysis of individual participant data. Lancet Diabetes Endocrinol 2015; 3: 514–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Radcliffe NJ, Seah J‐M, Clarke M, et al. Clinical predictive factors in diabetic kidney disease progression. J Diabetes Investig 2017; 8: 6–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Chou YH, Lin WC, Chen YM. Clinical outcomes in patients with biopsy‐proved diabetic nephropathy compared to isolated lupus or crescentic glomerulonephritis. Diabetes Res Clin Pract 2019; 148: 144–151. [DOI] [PubMed] [Google Scholar]
- 62. Wang A, Chen G, Cao Y, et al. Estimated glomerular filtration rate, proteinuria, and risk of cardiovascular diseases and all‐cause mortality in diabetic population: a community‐based Cohort study. Sci Rep 2017; 7: 17948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Shichiri M, Kishikawa H, Ohkubo Y, et al. Long‐term results of the Kumamoto Study on optimal diabetes control in type 2 diabetic patients. Diabetes Care 2000; 23: B21–B29. [PubMed] [Google Scholar]
- 64. ADVANCE Collaborative Group , Patel A, MacMahon S, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 2008; 358: 2560–2572. [DOI] [PubMed] [Google Scholar]
- 65. Action to Control Cardiovascular Risk in Diabetes Study Group , Gerstein HC, Miller ME, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008; 358: 2545–2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Kuo I‐C, Lin H‐H, Niu S‐W, et al. Glycated hemoglobin and outcomes in patients with advanced diabetic chronic kidney disease. Sci Rep 2016; 6: 20028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Kuo I‐C, Lin H‐H, Niu S‐W, et al. Anemia modifies the prognostic value of glycated hemoglobin in patients with diabetic chronic kidney disease. PLoS One 2018; 13: e0199378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Liao L‐N, Li C‐I, Liu C‐S, et al. Extreme levels of HbA1c increase incident ESRD risk in Chinese patients with type 2 diabetes: competing risk analysis in national cohort of Taiwan diabetes study. PLoS One 2015; 10: e0130828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Lee Y‐L, Chen BK, Lin K‐D, et al. The impact of severe hypoglycemia on renal impairment in type 2 diabetes. Diabetes Res Clin Pract 2015; 108: 448–455. [DOI] [PubMed] [Google Scholar]
- 70. Chen H‐C, Chou C‐Y, Hsiao Y‐T, et al. Patients with diabetes as the primary kidney disease have a worse survival than patients with comorbid diabetes in chronic haemodialysis patients. Nephrology 2015; 20: 155–160. [DOI] [PubMed] [Google Scholar]
- 71. Hung C‐C, Lin H‐H, Hwang D‐Y, et al. Diabetic retinopathy and clinical parameters favoring the presence of diabetic nephropathy could predict renal outcome in patients with diabetic kidney disease. Sci Rep 2017; 7: 1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Tervaert TWC, Mooyaart AL, Amann K, et al. Pathologic classification of diabetic nephropathy. J Am Soc Nephrol 2010; 21: 556–563. [DOI] [PubMed] [Google Scholar]
- 73. Zhang J, Wang Y, Li LI, et al. Diabetic retinopathy may predict the renal outcomes of patients with diabetic nephropathy. Ren Fail 2018; 40: 243–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Lin H‐T, Zheng C‐M, Wu Y‐C, et al. Diabetic retinopathy as a risk factor for chronic kidney disease progression: a multicenter case‐control study in Taiwan. Nutrients 2019; 11: 509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Chang L‐H, Chu C‐H, Lin H‐D, et al. The ankle brachial index is associated with prognosis in patients with diabetic kidney disease. Diabetes Res Clin Pract 2015; 108: 316–322. [DOI] [PubMed] [Google Scholar]
- 76. Wang J, Li J, Wang A, et al. Dipstick proteinuria and risk of myocardial infarction and all‐cause mortality in diabetes or pre‐diabetes: a population‐based cohort study. Sci Rep 2017; 7: 11986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Wang A, Chen G, Cao Y, et al. Estimated glomerular filtration rate, proteinuria, and risk of cardiovascular diseases and all‐cause mortality in diabetic population: a community‐based cohort study. Sci Rep 2017; 7: 17948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Sun Y, Wang A, Liu X, et al. Changes in proteinuria on the risk of all‐cause mortality in people with diabetes or prediabetes: a prospective cohort study. J Diabetes Res 2017; 2017: 8368513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Lin H‐H, Niu S‐W, Kuo I‐C, et al. Hematuria and renal outcomes in patients with diabetic chronic kidney disease. Am J Med Sci 2018; 356: 268–276. [DOI] [PubMed] [Google Scholar]
- 80. KDIGO . 2021 Clinical practice guideline for the management of blood pressure in chronic kidney disease. Kidney Int 2021; 99: S1–S87. 10.1016/j.kint.2020.11.003 [DOI] [PubMed] [Google Scholar]
- 81. Hsu TW, Liu JS, Hung SC, et al. Renoprotective effect of renin‐angiotensin‐aldosterone system blockade in patients with predialysis advanced chronic kidney disease, hypertension, and anemia. JAMA Intern Med 2014; 174: 347–354. [DOI] [PubMed] [Google Scholar]
- 82. Chen H‐Y, Pan H‐C, Chen Y‐C, et al. Traditional Chinese medicine use is associated with lower end‐stage renal disease and mortality rates among patients with diabetic nephropathy: a population‐based cohort study. BMC Complement Altern Med 2019; 19: 81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Chen Y‐H, Chiang M‐H, Liu J‐S, et al. Thiazolidinediones and risk of long‐term dialysis in diabetic patients with advanced chronic kidney disease: a nationwide cohort study. PLoS One 2015; 10: e0129922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. American Diabetes Association . Standards of medical care in diabetes — 2019. Diabetes Care 2019; 42: S124–S138. [DOI] [PubMed] [Google Scholar]
- 85. Chung HF, Long KZ, Hsu CC, et al. Association of n‐3 polyunsaturated fatty acids and inflammatory indicators with renal function decline in type 2 diabetes. Clin Nutr 2015; 34: 229–234. [DOI] [PubMed] [Google Scholar]
- 86. Chuang S‐M, Shih H‐M, Chien M‐N, et al. Risk factors in metabolic syndrome predict the progression of diabetic nephropathy in patients with type 2 diabetes. Diabetes Res Clin Pract 2019; 153: 6–13. [DOI] [PubMed] [Google Scholar]
- 87. Hsu C‐C, Jhang H‐R, Chang W‐T, et al. Associations between dietary patterns and kidney function indicators in type 2 diabetes. Clin Nutr 2014; 33: 98–105. [DOI] [PubMed] [Google Scholar]
- 88. Huang W‐H, Chen C‐Y, Lin J‐L, et al. High body mass index reduces glomerular filtration rate decline in type II diabetes mellitus patients with stage 3 or 4 chronic kidney disease. Medicine 2014; 93: e41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Zhang J, Zhang R, Wang Y, et al. The level of serum albumin is associated with renal prognosis in patients with diabetic nephropathy. J Diabetes Res 2019; 2019: 7825804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Gæde P, Vedel P, Larsen N, et al. Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N Engl J Med 2003; 348: 383–393. [DOI] [PubMed] [Google Scholar]
- 91. Shen ZZ, Huang YY, Hsieh CJ. Early short‐term intensive multidisciplinary diabetes care: a ten‐year follow‐up of outcomes. Diabetes Res Clin Pract 2017; 130: 133–141. [DOI] [PubMed] [Google Scholar]


