Abstract
Aims/Introduction
Despite there being several meta‐analyses on the effects of antidiabetic agents in patients with gestational diabetes mellitus, the reliability of their findings is a concern, mainly due to undetermined methodological quality of these studies. This study aimed to assess the methodological quality of available meta‐analyses and provide a summary estimation of the effectiveness of treatments modalities.
Materials and Methods
PubMed, Web of Science and Scopus databases were comprehensively searched for retrieving relevant meta‐analyses published in English up to May 2020. A Measurement Tool to Assess Systematic Reviews (AMSTAR‐2) was applied to evaluate methodological quality of eligible meta‐analyses. A network meta‐analysis was used to calculate the pooled odds ratio of maternal and neonatal outcomes in gestational diabetes mellitus patients treated with metformin or glyburide compared with those treated with insulin. The rank network analysis was carried out for ranking of the treatments and reporting the most efficient treatment.
Results
A total of 27 and 17 studies were included for qualitative and quantitative syntheses, respectively; of these, just four studies were classified as high quality. The results showed that metformin had the highest probability of being the best treatment, compared with insulin and glyburide, for the majority of adverse neonatal outcomes, whereas glyburide was the best treatment in reducing the risk of adverse maternal outcomes. The results were not significantly changed after excluding low‐quality studies.
Conclusions
This review study of available literature shows that metformin can be a superior option in most neonatal and maternal adverse pregnancy outcomes in women with gestational diabetes mellitus; the results need to be further updated by including future more qualified studies.
Keywords: Gestational diabetes, Antidiabetes agents, Network meta‐analysis
Despite there being several meta‐analyses on the effects of the antidiabetic agents in patients with gestational diabetes mellitus, the reliability of their findings is a concern, mainly due to undetermined methodological quality of these studies. This study aimed to assess the methodological quality of available meta‐analyses and provide a summary estimation of the effectiveness of treatments modalities.

INTRODUCTION
Gestational diabetes mellitus (GDM) is a common endocrinopathy during pregnancy affecting 4–11% of all pregnancies, defined as glucose intolerance recognized at any time in pregnancy based on defined thresholds that are less than those considered for overt diabetes 1 , 2 . Earlier studies have shown that GDM is associated with a higher risk of adverse feto‐maternal and neonatal complications 3 , 4 , 5 , 6 . Furthermore, there is a general consensus that treatment should be initiated using individual medical nutrition therapy, exercise and pharmacological therapies if required 7 , 8 , 9 . It is well documented that appropriate management of GDM minimizes the risk of adverse perinatal outcomes through controlling glycemic level 10 . Insulin therapy has been accepted as a gold standard for managing GDM, and can achieve tight maternal glucose control without the risk of transferring across the placenta 7 , 11 , 12 . Oral antidiabetic agents, mainly metformin and glyburide, are attractive alternatives to insulin for various reasons, including their ease of use, low cost and unwanted side‐effects, such as hypoglycemia and weight gain, which increases the compliance to treatment regimens 13 .
However, the results of efficacy and safety of oral antidiabetic agents are controversial; traditionally, these medications have been avoided by women with GDM because of the potential risks of neonatal hypoglycemia and teratogenicity associated with placental transfer to the fetus 14 .
Network meta‐analysis, which is also called multiple‐treatments meta‐analysis, is considered as high‐level evidence for developing guidelines, in particular when there is an inconsistency among studies; these studies can provide the most robust and reliable evidence on a specific topic 15 , 16 . Several meta‐analyses have been published on GDM and its related treatments, including oral antidiabetic agents and insulin, and the reliability of their findings is a concerning matter, mainly due to the lack of methodological quality assessment of these studies. In addition, most of these meta‐analyses’ results are inconclusive, mainly due to the diversity of study populations, diagnostic thresholds for GDM, and the lack of multiple comparisons between oral antidiabetic agents and insulin 6 , 11 , 13 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 .
In the present study, we aimed to assess the methodological quality of the included meta‐analyses using A Measurement Tool to Assess Systematic Reviews (AMSTAR‐2) scale 30 . Then, we identified the pooled odds ratio (OR) of maternal and neonatal outcomes in GDM patients treated with oral antidiabetic agents (metformin or glyburide), compared with those treated with insulin, and also reported the most efficient modality in terms of reducing the risk of maternal and neonatal adverse outcomes in women with GDM. We repeated our analysis by excluding low‐quality studies assessed based on the AMSTAR‐2.
MATERIALS AND METHODS
The present systematic review and network meta‐analysis were designed according to the guidelines for the Preferred Reporting Items for Systematic Reviews and Meta‐analyses (PRISMA) 31 and the Cochrane Handbook for Systematic Reviews of Interventions 32 to identify the pooled OR of maternal and neonatal outcomes in GDM patients treated with oral antidiabetic agents (metformin or glyburide), compared with those treated with insulin, and reported the most efficient modality for managing GDM. Furthermore, we also planned to report the most efficient treatment by including only high‐ and moderate‐quality studies. No ethics approval was required for this systematic review; however, the review was prospectively registered with the National Institute for Health Research Prospero International Prospective Register of Systematic Reviews (CRD42020185909).
Search strategy
PubMed (including Medline), Web of Science and Scopus databases were searched for retrieving relevant meta‐analyses published in the English language from inception to May 2020. Two authors carried out searches separately (SBG and RBY), and any disagreements were resolved by consensus through discussing with senior authors (MA and FRT). The following keywords, alone or in combination, were used for the search process: (“Gestational diabetes” OR “gestational diabetes mellitus” OR “GDM” OR “pregnancy induced diabetes”) AND (“oral antidiabetic agent” OR “hypoglycemic agent” OR “oral anti‐hyperglycemic agent” OR “oral antidiabetic drugs” OR “pharmacological therapy” OR “antidiabetic medication” OR “metformin” OR “glucophage” OR “biguanide” OR “glibenclamide” OR “glyburide”) AND (“review” OR “systemic review” OR “meta‐analysis”). The text words and MeSH terms were entered depending on the databases characteristics. The reference lists from retrieved articles were also screened for additional applicable studies.
Eligibility criteria, study selection and data extraction
Studies were eligible if they fulfilled all the following conditions: (i) they were a meta‐analysis published in the English language; (ii) study populations were women with GDM treated with antidiabetic pharmacological therapy; (iii) studies comparing the effects of glyburide (glibenclamide) with insulin, metformin with insulin, or metformin with glyburide; and (iv) studies assessing at least one of feto‐maternal or neonatal outcomes. We also excluded all studies assessing patients with pre‐existing diabetes, non‐human studies, original studies, reviews, commentaries, editorials, letters, meeting abstracts and case reports. To run network meta‐analysis, we extracted the number of each event from studies, papers that did not report those data were excluded 10 , 14 , 17 , 19 , 23 , 25 , 27 , 33 , 34 , 35 .
The screening of titles and abstracts was carried out independently by all authors to exclude studies that clearly did not fulfill the inclusion criteria. Then, they reviewed the full text of the remaining papers to identify the final eligibility criteria. Disagreements were resolved through discussions.
Data were extracted from full‐text papers by all authors; they checked precisely extracted data to minimize errors. For each study, the following data were extracted: the first author’s name, publication year, outcomes of interest, sample size, type of treatment, risk of bias assessment and summary results. A control check between the final extracted data and the original publications’ data was carried out.
Quality assessment
Quality assessment was carried out independently by two authors (RBY and SBG). Any discrepancies were resolved by discussion, and the third reviewer (MA) was consulted if necessary. We applied the AMSTAR‐2 appraisal tool to evaluate the methodological quality of eligible meta‐analyses. This tool evaluates the quality of systematic reviews with both randomized and non‐randomized studies included 36 . The characteristics of the included studies were reported in Table S1. In addition, Tables 1 and 2 were designed to show results of the AMSTAR‐2 domain (yes, partial yes and no) of each included study. Furthermore, the secular trend of the number and quality of included reviews is shown in Figure 1.
Table 1.
Summary results of methodological quality of meta‐analyses using A Measurement Tool to Assess Systematic Reviews (AMSTAR‐2)
| No. | Reference | Publication year | AMSTAR‐2 quality items | ΨAMSTAR‐2 classification | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | ||||
| 1 | Amin M | 2015 | Y | N | Y | Y | Y | Y | Y | Y | Y | Y | Y | N | Y | Y | N | Y | Low |
| 2 | Balsells M | 2015 | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | High |
| 3 | Brown J | 2017 | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | High |
| 4 | Brown J | 2017 | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | High |
| 5 | Butalia S | 2017 | Y | N | Y | Y | Y | Y | Y | Y | Y | Y | Y | N | N | N | N | Y | Low |
| 6 | Dhulkotia JS | 2010 | Y | N | Y | Y | Y | Y | Y | Y | N | N | Y | N | N | N | N | N | Low |
| 7 | Farrar D | 2017 | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | N | Y | Y | PY | Y | Moderate |
| 8 | Farrar D | 2016 | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | PY | Y | Y | PY | Y | High |
| 9 | Feng Y | 2017 | Y | N | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | PY | Y | Y | Y | Low |
| 10 | Gui J | 2013 | Y | N | Y | Y | Y | Y | Y | Y | N | Y | Y | N | N | Y | PY | Y | Low |
| 11 | Jiang YF | 2015 | Y | N | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | PY | Y | Y | Y | Low |
| 12 | Kitwitee P | 2015 | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | N | Y | PY | Y | Y | Moderate |
| 13 | Li G | 2014 | Y | N | Y | Y | Y | Y | Y | Y | Y | N | Y | PY | Y | Y | Y | Y | Low |
| 14 | Liang HL | 2017 | Y | N | Y | Y | Y | Y | Y | Y | Y | Y | Y | N | Y | Y | Y | Y | Low |
| 15 | Moretti ME | 2008 | Y | N | Y | N | N | N | N | N | N | Y | Y | N | N | Y | N | N | Critically low |
| 16 | Nicholson W | 2009 | Y | N | Y | Y | Y | Y | Y | Y | Y | Y | Y | N | N | N | Y | Y | Low |
| 17 | Poolsup N | 2014 | Y | N | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Low |
| 18 | Song R | 2017 | Y | N | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Low |
| 19 | Su DF | 2017 | Y | N | Y | Y | Y | Y | Y | Y | Y | Y | Y | N | N | N | Y | Y | Low |
| 20 | Zeng YCh | 2014 | Y | N | Y | Y | Y | Y | Y | Y | Y | Y | Y | N | N | N | Y | Y | Low |
| 21 | Zhao LP | 2015 | Y | N | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Low |
| 22 | Zhu B, | 2016 | Y | N | Y | Y | Y | Y | Y | Y | Y | Y | Y | N | N | N | N | Y | Low |
| 23 | L. Tarry‐Adkins J | 2019 | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | High |
| 24 | Bao Le‐xin | 2019 | Y | Y | Y | N | Y | Y | Y | Y | Y | N | Y | N | N | N | Y | Y | Low |
| 25 | Helal, K.F | 2020 | Y | N | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | N | Y | Low |
| 26 | Guo L | 2019 | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | PY | Y | Moderate |
| 27 | Kalafat ER | 2018 | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | High |
Critical domains include items 2, 4, 7, 9, 11, 13 and 15.
ΨAMSTAR‐2 classification: High: no or one non‐critical weakness; the systematic review provides an accurate and comprehensive summary of the results of the available studies that address the question of interest. Moderate: more than one non‐critical weakness; the systematic review has more than one weakness, but no critical flaws. It might provide an accurate summary of the results of the available studies that were included in the review. Low: one critical flaw with or without non‐critical weaknesses; the review has a critical flaw, and might not provide an accurate and comprehensive summary of the available studies that address the question of interest. Critically low: more than one critical flaw with or without non‐critical weaknesses; the review has more than one critical flaw, and should not be relied on to provide an accurate and comprehensive summary of the available studies.
AMSTAR‐2, A Measurement Tool to Assess Systematic Reviews; N, no; PY, partial yes; Y, yes.
Table 2.
Summary results of methodological quality of the included meta‐analyses through A Measurement Tool to Assess Systematic Reviews (AMSTAR‐2) items
| Items | n (%) | |
|---|---|---|
| 1 | Did the research questions and inclusion criteria for the review include the components of PICO (population, intervention, control group and outcome)? | Yes = 27 (100) |
| 2 | Did the report of the review contain an explicit statement that the review methods were established prior to conduct of the review and did the report justify any significant deviations from the protocol? |
Yes = 10 (37) No = 17 (63) |
| 3 | Did the review authors explain their selection of the study designs for inclusion in the review? | Yes = 27 (100) |
| 4 | Did the review authors use a comprehensive literature search strategy? |
Yes = 25 (93) No = 2 (7) |
| 5 | Did the review authors perform study selection in duplicate? |
Yes = 26 (96) No = 1 (4) |
| 6 | Did the review authors perform data extraction in duplicate? |
Yes = 26 (96) No = 1 (4) |
| 7 | Did the review authors provide a list of excluded studies and justify the exclusions? |
Yes = 26 (96) No = 1 (4) |
| 8 | Did the review authors describe the included studies in adequate detail? |
Yes = 26 (96) No = 1 (4) |
| 9 | Did the review authors use a satisfactory technique for assessing the RoB in individual studies that were included in the review? |
Yes = 24 (89) No = 3 (11) |
| 10 | Did the review authors report on the sources of funding for the studies included in the review? |
Yes = 24 (89) No = 3 (11) |
| 11 | If meta‐analysis was justified did the review authors use appropriate methods for statistical combination of results? | Yes = 27 (100) |
| 12 | If meta‐analysis was performed did the review authors assess the potential impact of RoB in individual studies on the results of the meta‐analysis or other evidence synthesis? |
Yes = 12 (44) Partial yes=2 (7) No = 13 (48) |
| 13 | Did the review authors account for RoB in individual studies when interpreting/discussing the results of the review? |
Yes = 16 (59) Partial yes=2 (7) No = 9 (34) |
| 14 | Did the review authors provide a satisfactory explanation for, and discussion of, any heterogeneity observed in the results of the review? |
Yes = 19 (70) Partial yes=1 (4) No = 7 (26) |
| 15 | If they performed quantitative synthesis did the review authors carry out an adequate investigation of publication bias (small study bias) and discuss its likely impact on the results of the review? |
Yes = 17 (63) Partial yes=4 (15) No = 6 (22) |
| 16 | Did the review authors report any potential sources of conflict of interest, including any funding they received for conducting the review? |
Yes = 25 (93) No = 2 (7) |
RoB, risk of bias.
Figure 1.
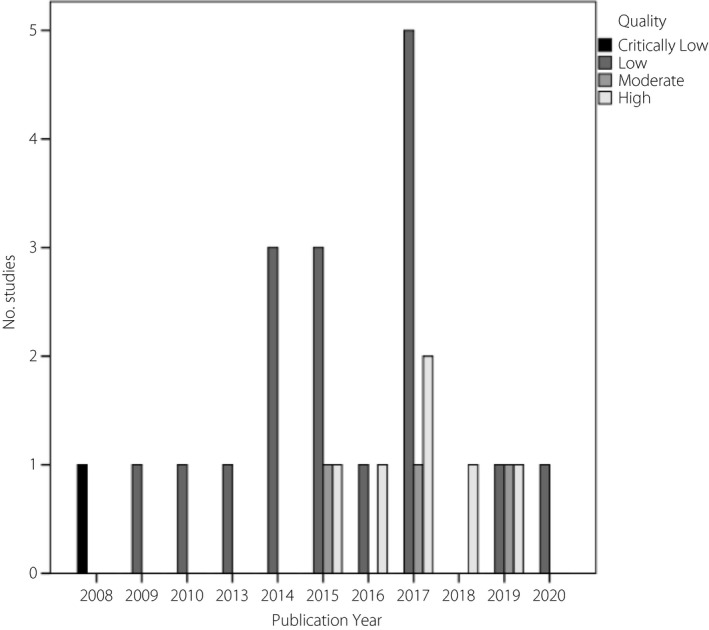
Secular trend of publications by their quality.
Statistical analysis
Statistical analysis was carried out using Stata software (version 13; StataCorp, College Station, TX, USA). For dichotomous outcomes, the pooled OR comparing each group of treatments (metformin, glyburide vs insulin), with its 95% confidence interval (CI), was calculated from the numbers of outcome events in each meta‐analysis study. We carried out a network meta‐analysis to merge information across multiple treatments, simultaneously, through direct and indirect data to calculate the pooled OR of maternal and neonatal outcomes in GDM patients treated with oral antidiabetic agents (metformin or glyburide), compared with those treated with insulin. Both consistency and inconsistency (additional variation in the true treatment effect between designs, where a design is the set of treatments compared in a study) models were run to obtain the results. A random effects model was applied to overcome heterogeneity. Network rank analysis 37 was used for ranking of treatments and reporting the most efficient modality for managing GDM. Applying the cumulative probabilities, the surface under the cumulative ranking curves and the mean rank (the mean of the distribution of the ranking probabilities) were estimated. A network map, network forest and rankogram plots were drawn for a graphical illustration of data 38 . In the network map, the size of the nodes is proportional to the number of studies evaluating each treatment, and the thickness of the edges is proportional to the precision of each direct comparison.
Outcome measures
Fetal, maternal and neonatal outcomes of interest were classified in seven indices composited, including adverse maternal outcomes, adverse neonatal outcomes, excessive fetal growth (large for gestational age [LGA] and macrosomia), hypertensive disorders of pregnancy (pregnancy ‐induced hypertension [PIH] and pre‐eclampsia), neonatal metabolic disturbance (hyperbilirubinemia, and neonatal hypoglycemia), serious neonatal conditions (neonatal intensive care unit [NICU] admission and respiratory distress syndrome) and abnormal delivery (cesarean section, induction of labor). The present study also assessed separate outcomes, including LGA, macrosomia, hyperbilirubinemia, induction of labor, NICU admission, pre‐eclampsia, PIH, preterm birth, neonatal hypoglycemia, small for gestational age (SGA), maternal hypoglycemia, respiratory distress syndrome, shoulder dystocia, congenital abnormality, cesarean section and perinatal mortality.
RESULTS
Search results, study selection, study characteristics and quality assessment
Figure 2 shows the PRISMA flow diagram of the study. Of 234 studies retrieved through searching databases, 27 studies were included for methodology quality assessment, and 17 of them were considered for quantitative synthesis through network meta‐analysis. Of these, eight studies 6 , 11 , 18 , 20 , 21 , 26 , 28 , 29 assessed metformin versus insulin, two studies 22 , 24 assessed glyburide versus insulin, two studies 13 , 39 assessed metformin versus glyburide, three studies 12 , 40 , 41 assessed metformin versus insulin, glyburide versus insulin and metformin versus glyburide, and two studies 42 , 43 assessed metformin versus insulin and glyburide versus insulin. Figure 3 shows map plots of network meta‐analysis of the effects of antidiabetic agents on adverse maternal and neonatal outcomes in patients with GDM. Also, Figures S1 and S2 show these map plots for other composite and separate outcomes. Table S1 presents the summary of included meta‐analyses assessing the effects of antidiabetic agents for treatment of GDM on pregnancy outcomes.
Figure 2.
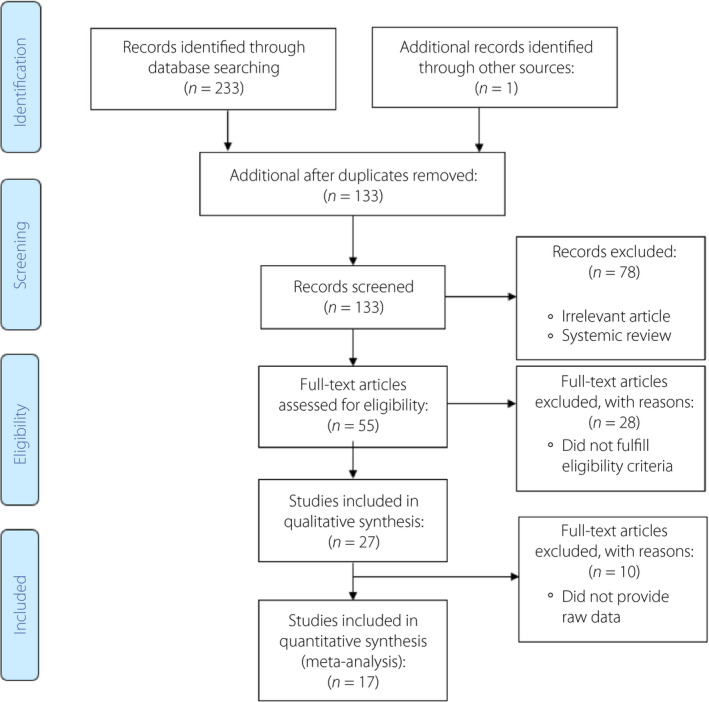
Preferred Reporting Items for Systematic Reviews and Meta‐analyses (PRISMA) flow diagram of the study.
Figure 3.
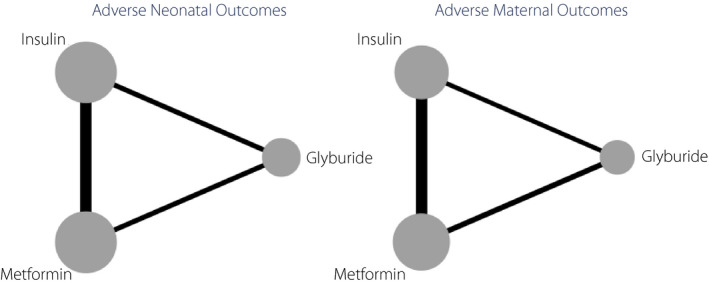
Map plots of network meta‐analysis of the effects of antidiabetic agents on adverse maternal and neonatal outcomes. The size of the nodes is proportional to the number of studies evaluating each treatment, and the thickness of the edges is proportional to the precision (the inverse of the variance) of each direct comparison.
Tables 1 and 2 present the results of the methodological quality assessment of the studies included. Of a total of 27 included studies for quality assessment, 1 (4%), 17 (63%), 3 (11%) and 6 (22%) obtained critically low, low, moderate and high quality, respectively (Table 1). Among them, 17 (63%), 2 (7%), 1 (4%), 3 (11%), 0 (0%), 9 (34%) and 6 (22%) did not fulfill critical domains, including items 2, 4, 7, 9, 11, 13, 15, respectively, leading to low quality of the study (Table 2). Figure 1 shows the secular trend of publications based on their quality; there was no specific trend regarding the studies’ quality over time.
Results of network meta‐analysis
Figure 4 shows the forest plots of the antidiabetic agents’ network for adverse maternal and neonatal outcomes. The results showed that use of metformin and glyburide was associated with a lower pooled OR for adverse maternal outcomes compared with insulin (pooled OR 0.74, 95% CI 0.59–0.92 and pooled OR 0.78, 95% CI 0.66–0.92), respectively. There were no significant differences in the pooled OR of adverse maternal/neonatal outcomes among patients treated with metformin compared with those treated with glyburide. Figures S3‐S10 show the forest plots for other outcomes of interest. The results of the estimated probability (%) for being a treatment that is the most effective remedy in reducing the risk of an adverse outcome for all studies are presented in Table 3. The results showed that metformin had the highest probability of being the best treatment, compared with insulin and glyburide for adverse neonatal outcomes (90.6%), excessive fetal growth (99.9%), hypertensive disorders of pregnancy (99.7%), neonatal metabolic disturbance (99.7%), LGA (99.8%), macrosomia (97.9%), hyperbilirubinemia (75.4%), induction of labor (88.9%), NICU admission (88%), pre‐eclampsia (62.9%), PIH (84.7%), preterm birth (56.8%) and neonatal hypoglycemia (99.9%), whereas glyburide was the best treatment in reducing the risk of adverse maternal outcomes (70.5%), serious neonatal conditions (98.2%), abnormal delivery (100%), SGA (45.5%), maternal hypoglycemia (98.9%), respiratory distress syndrome (88.2%), shoulder dystocia (48%), congenital abnormality (67.2%) and caesarean section (79.7%). The results also showed that insulin had the greatest probability of being the best treatment in reducing the risk of perinatal mortality (48.4%).
Figure 4.
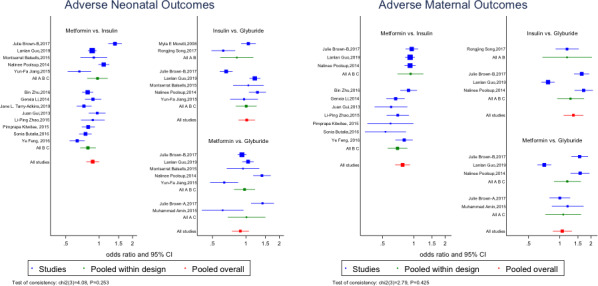
The forest plot of the network meta‐analysis of the effects of antidiabetic agents on adverse maternal and neonatal outcomes. Pooled results within design (from the inconsistency model) are shown as a green square. Overall pooled results are also shown as a red square. The non‐similarity of the “pooled within design” and “pooled overall” results supports the consistency model. A, Glyburide; B, insulin; C, metformin.
Table 3.
Estimated probability (%) of a treatment being the most effective in reducing the risk of a dichotomous outcome for all studies
| Outcomes | Treatments | ||
|---|---|---|---|
| Metformin | Glyburide | Insulin | |
| Adverse maternal outcomes † | 29.5 | 70.5 | 0.0 |
| Adverse neonatal outcomes ‡ | 90.6 | 9.3 | 0.1 |
| Excessive fetal growth | 99.9 | 0.1 | 0.0 |
| Hypertensive disorders of pregnancy | 99.7 | 0.2 | 0.1 |
| Neonatal metabolic disturbance | 99.7 | 0.2 | 0.1 |
| Serious neonatal conditions | 1.8 | 98.2 | 0.0 |
| Abnormal delivery | 0.0 | 100.0 | 0.0 |
| LGA | 99.8 | 0.2 | 0.0 |
| Macrosomia | 97.9 | 1.8 | 0.4 |
| Hyperbilirubinaemia | 75.4 | 22.3 | 2.3 |
| Induction of labor | 88.9 | 8.4 | 2.8 |
| NICU admission | 88.0 | 12.0 | 0.0 |
| Pre‐eclampsia | 62.9 | 32.5 | 4.6 |
| PIH | 84.7 | 15.3 | 0.0 |
| Preterm birth | 56.8 | 33.2 | 10.0 |
| Neonatal hypoglycemia | 99.9 | 0.1 | 0.0 |
| SGA | 43.6 | 45.5 | 10.9 |
| Maternal hypoglycemia | 1.1 | 98.9 | 0.0 |
| Respiratory distress syndrome | 7.1 | 88.2 | 4.7 |
| Shoulder dystocia | 38.9 | 48.0 | 13.1 |
| Congenital abnormality | 28.7 | 67.2 | 4.2 |
| Cesarean section | 18.3 | 79.7 | 2.0 |
| Perinatal mortality | 38.9 | 12.6 | 48.4 |
Adverse maternal outcomes including: induction of labor, pre‐eclampsia, pregnancy‐induced hypertension (PIH), maternal hypoglycemia and cesarean section.
Adverse neonatal outcomes including: large for gestational age (LGA), macrosomia, hyperbilirubinemia, neonatal intensive care unit (NICU) admission, preterm birth, small for gestational age (SGA), neonatal hypoglycemia, respiratory distress syndrome, shoulder dystocia and congenital abnormality.
The results of rankograms for antidiabetic agents on the adverse maternal and neonatal outcomes are also presented in Figure 5; the results show that the probability of metformin being the best treatment is much higher than two other modalities for the risk reduction of adverse neonatal outcomes; however, for adverse maternal outcomes, glyburide was the best. Figures S11‐S13 show the rankograms of other outcomes of interest. Table S2 provides pooled OR (95% CI) for a combination of treatments.
Figure 5.
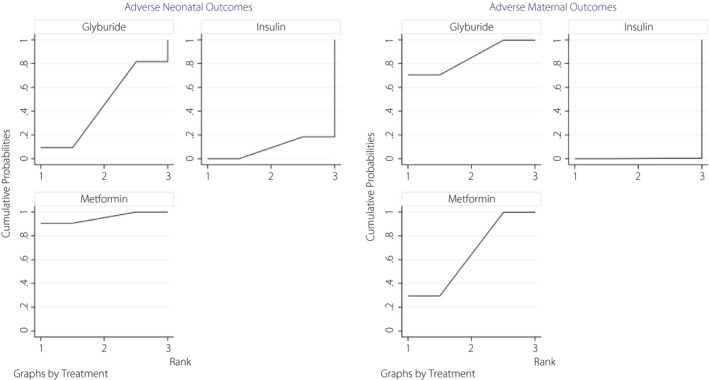
Rankograms of the network meta‐analysis of the effects of antidiabetic agents on adverse maternal and neonatal outcomes, showing the probability for every treatment being in a particular order with predictive probabilities in neonatal and maternal adverse events.
Results of subgroup analysis based on the quality assessment
The results of subgroup analysis by excluding low‐quality studies showed that metformin had the highest probability of being the best treatment in reducing the risk of adverse neonatal outcomes (91.8%), excessive fetal growth (55.7%), hypertensive disorders of pregnancy (81.3%), neonatal metabolic disturbance (93.2%), LGA (85.2%), macrosomia (89%) and neonatal hypoglycemia (91%), whereas glyburide was the best treatment in reducing the risk of adverse maternal outcomes (70.5%), serious neonatal conditions (97.2%), abnormal delivery (97.8%), SGA (82.8%) and cesarean section (94.3%; Table 4).
Table 4.
Estimated probability (%) of a treatment being the most effective in reducing the risk of a dichotomous outcome for high‐ and moderate‐quality studies
| Outcomes | Treatment | ||
|---|---|---|---|
| Metformin | Glyburide | Insulin | |
| LGA | 85.2 | 14.8 | 0.0 |
| Adverse maternal outcomes | 29.5 | 70.5 | 0.0 |
| Adverse neonatal outcomes | 91.8 | 8.1 | 0.0 |
| Excessive fetal growth | 55.7 | 44.3 | 0.0 |
| Hypertensive disorders of pregnancy | 81.3 | 17.4 | 1.3 |
| Neonatal metabolic disturbance | 93.2 | 6.8 | 0.0 |
| Serious neonatal conditions | 2.5 | 97.2 | 0.3 |
| Abnormal delivery | 0.3 | 97.8 | 1.9 |
| Macrosomia | 89.0 | 11.0 | 0.0 |
| Neonatal hypoglycemia | 91.0 | 0.9 | 0.0 |
| SGA | 7.3 | 82.8 | 9.9 |
| Cesarean section | 4.3 | 94.3 | 1.4 |
LGA, large for gestational age; SGA, small for gestational age.
DISCUSSION
This is the first study that provided a methodological quality assessment of published meta‐analysis on the effect of antidiabetic agents for the treatment of GDM. In addition, we provided a summary result of 17 eligible studies through a network meta‐analysis that identified metformin as the best antidiabetic agent for the treatment of GDM by reducing the risk of the most adverse maternal and neonatal outcomes, and findings remained consistent, even after excluding low‐quality studies.
Traditionally, insulin has been considered as a gold standard for managing hyperglycemic in patients with GDM that have failed to achieve normal glycemic levels through lifestyle changes, as it can achieve tight maternal glucose control without the risk of transferring across the placenta 21 , 44 . There is also evidence suggesting that oral antidiabetic agents, mainly metformin and glyburide, might be effective and safe alternatives to insulin for GDM women, especially for those who could not tolerate the injection, which can be better accepted than insulin 4 , although these agents have not been approved by the Food and Drug Administration for this indication yet 23 , and the use of oral antidiabetic agents for the treatment of GDM remains controversial 27 . It has been shown that a multiple‐treatments meta‐analysis (network meta‐analysis) is considered as high‐level evidence for developing guidelines, in particular, when there is an inconsistency among studies. These studies can provide the most robust and reliable evidence on a specific topic 15 , 16 .
In the present network meta‐analysis, we found that metformin had the highest probability of being the best treatment, compared with insulin and glyburide for most outcomes of interest, such as adverse neonatal outcomes, excessive fetal growth, hypertensive disorders of pregnancy, neonatal metabolic disturbance, LGA, macrosomia, hyperbilirubinemia, induction of labor, NICU admission, preeclampsia, PIH, preterm birth and neonatal hypoglycemia. To minimize biases due to a low quality of studies, we excluded these meta‐analyses 6 , 13 , 21 , 22 , 24 , 28 , 29 , 41 , 43 , 45 , 46 , and analyzed only moderate‐ 11 , 40 and high‐quality studies 12 , 26 , 39 , 42 ; however, our preliminary findings remained unchanged after this subgroup analysis. Although metformin can cross the placenta, previous studies showed that it is less likely to cause severe neonatal hypoglycemia compared with insulin, because metformin is not associated with stimulating pancreatic insulin release or increasing serum insulin levels. Therefore, metformin can be an effective and safe alternative to insulin for GDM patients 17 , 46 .
Several meta‐analyses have compared the effects of antidiabetic agents on adverse pregnancy outcomes with inconclusive results 6 , 10 , 11 , 12 , 13 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 33 , 34 , 35 , 39 , 40 , 41 , 43 . For example, similarly to the present results, a recent meta‐analysis of 24 studies suggested that metformin might have potential benefits for pregnant women and newborns with no obvious adverse effects 17 . Likewise, another meta‐analysis of 42 studies showed that metformin had the highest probability of being the most effective treatment in reducing the risk of most adverse pregnancy outcomes, compared with insulin or glyburide 10 . Furthermore, a meta‐analysis of five studies comparing the effects of metformin versus insulin on maternal and neonatal outcomes reported that metformin is an effective and safe alternative to insulin for GDM patients. They reported better glycemic control and lower maternal weight gain with metformin compared with insulin, making metformin worth using, even when metformin is insufficient and supplementary insulin is required. Their data also showed that metformin significantly reduced the gestational hypertension disorders in GDM patients, probably through modifying the endothelial activation and maternal inflammatory response of insulin resistance 20 . Another meta‐analysis of five studies showed that metformin is comparable with insulin in glycemic control and neonatal outcomes, and suggested it as more suitable for women with mild GDM 6 . Furthermore, one meta‐analysis of 41 studies showed that metformin could be a safe and effective treatment for GDM 40 . Another meta‐analysis of five studies showed that metformin is comparable with insulin in glycemic control and neonatal outcomes, and suggested it as more suitable for women with mild GDM 6 . Moreover, one meta‐analysis of 41 studies showed that metformin could be a safe and effective treatment for GDM 40 . Another meta‐analysis of 15 studies showed that metformin use can be associated with a reduced incidence of hypertensive disorders during pregnancy 35 . Meta‐analysis carried out by Kitwitee et al. 11 showed that although GDM patients treated with both metformin and insulin have comparable glycemic control profile, metformin use was associated with a lower risk of neonatal hypoglycemia. Also, Li et al. 21 , during a meta‐analysis of 11 studies, found that metformin can significantly reduce most adverse maternal and neonatal outcomes. Meta‐analysis carried out by Poolsup et al. 43 suggested that because of the favorable effects of metformin in treating GDM, this remedy can be considered as an efficacious alternative to insulin, especially for patients with a mild form of disease. Zhao et al. 28 suggested that although there is no clinically relevant difference in the efficacy or safety between metformin and insulin, metformin might be a good choice for GDM because of the lower risk of PIH. Balsells et al. 12 , during a meta‐analysis of 15 studies on patients with GDM, showed that although metformin (plus insulin when required) seems to be slightly better than insulin, glyburide is clearly inferior to both insulin and metformin. Although most meta‐analyses showed higher efficacy of metformin in comparison with insulin therapy, a limited number of meta‐analyses showed superiority of glyburide 19 , or similar efficacy of insulin and oral antidiabetic agents 14 , 23 , 42 .
The present network meta‐analysis also showed that glyburide was the best treatment in reducing the risk of some adverse maternal outcomes, such as maternal hypoglycemia, abnormal delivery, shoulder dystocia and caesarean section, and neonatal outcomes, such as serious neonatal conditions, SGA, respiratory distress syndrome and congenital abnormality, findings that remained significant even after excluding low‐quality studies. Similar to the present results, a meta‐analysis of 24 studies showed that glyburide is an effective drug compared with insulin in the management of some adverse pregnancy outcomes, such as cesarean section, findings that might suggest the potential clinical benefits of glyburide, compared with metformin or insulin 19 . Also, a network meta‐analysis of 32 randomized controlled trials (RCTs) carried out by Liang et al. 34 showed that the incidence of admission to NICU was higher with insulin therapy compared with glyburide, whereas this drug has the highest incidence of macrosomia, pre‐eclampsia, hyperbilirubinemia, neonatal hypoglycemia, shortest gestational age at delivery and lowest mean birthweight. In contrast, Jiang et al. 41 , during a meta‐analysis of 18 studies, showed that glyburide treatment is associated with an increased risk of neonatal hypoglycemia, high maternal weight gain, high neonatal birthweight and macrosomia. Also, a meta‐analysis carried out by Zeng et al. 27 showed that although glyburide is as effective as insulin, the risk of neonatal hypoglycemia, high fetal birthweight and macrosomia were higher with its use. However, insufficient data were available to compare the efficacy of glyburide with metformin and insulin; it seems that the effectiveness and safety of glyburide require future evaluation by well‐designed RCTs with appropriate sample sizes.
Despite there being several meta‐analyses of trials investigating the effects of oral antidiabetic agents and insulin therapy on adverse maternal and neonatal outcomes in patients with GDM, their results are often conflicting, and there is insufficient high‐quality evidence to be able to draw any meaningful conclusions as to the benefits of oral antidiabetic pharmacological agents over insulin therapy due to including non‐randomized studies, small sample size of trials, limited reporting of data for the adverse outcomes, diversities in thresholds for diagnosis of GDM and or the definition of outcomes, and the lack of sufficient data on the long‐term offspring outcomes in patients with GDM treated with these treatments 6 , 10 , 11 , 12 , 13 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 33 , 34 , 35 , 39 , 40 , 41 , 43 . It is also important to note that in most RCTs, a significant proportion of patients who failed to achieve adequate control with oral antidiabetic agents might then switch to insulin; it is difficult to determine whether enhanced response is solely attributable to oral antidiabetic agents (metformin or glyburide) or if insulin also contributed to the improvement 27 , 43 . Future well‐designed randomized placebo‐controlled double‐blind multicenter trials with an approach of intention‐to‐treat are still required for a more accurate conclusion, and to provide additional evidence for the safety and efficacy of antidiabetic agents in the treatment of GDM.
The greatest strength of the present study was its design, as the first network meta‐analysis consisted of all meta‐analyses comparing the effects of oral antidiabetic agents and insulin therapy, assessing the methodological quality of the studies using a specific tool for assessing multiple systematic reviews, and a then carrying out a subgroup analysis based on the studies’ quality. Although we aimed to minimize all possible biases in this meta‐analysis, it should be noted that there was significant heterogeneity among studies. These heterogeneities were partly predictable and might have resulted from clinical heterogeneity related to variability in diagnostic thresholds for GDM, age, BMI, methods of outcomes assessment, race and ethnicity; however, using a random effects model somehow adjusted these issues by assigning lower weights to studies with higher uncertainty. In addition, sparse data in some outcomes, such as shoulder dystopia, prenatal mortality and SGA, caused higher uncertainty appearing in wide 95% CIs and misleading inferences. It should also be considered that in many RCTs, patients might require insulin therapy after treatment with metformin due to failure to achieve adequate control with oral antidiabetic agents. However, most RCTs did not mention the details of the methods and whether they used an intention‐to‐treat approach for their data analysis. Also, pooling meta‐analyses can result in the duplication of studies, which might affect studies’ assigned weight on the pooling process and leads to misestimating the overall measure of interest. All these limitations should be considered when interpreting the findings. Data and software codes are available on request.
In conclusion, although most of the available meta‐analyses were of low quality, the results of the available literature showed that metformin can be considered as the best alternative to insulin therapy for women with GDM because of maternal and perinatal outcomes comparable with insulin. The results need to be further updated by including future more qualified studies.
DISCLOSURE
The authors declare no conflict of interest.
Supporting information
Figure S1 | Map plots of the anti‐gestational diabetes mellitus network for maternal and neonatal adverse events. The size of the nodes is proportional to the number of studies evaluating each treatment, and the thickness of the edges is proportional to the precision (the inverse of the variance) of each direct comparison.
Figure S2 | Map plots of the anti‐gestational diabetes mellitus network for maternal and neonatal hypoglycemia. The size of the nodes is proportional to the number of studies evaluating each treatment, and the thickness of the edges is proportional to the precision (the inverse of the variance) of each direct comparison.
Figures S3‐S10 | The forest plot for the anti‐gestational diabetes mellitus network for adverse events. The individual study results, grouped by treatment contrast and design. Pooled results within the design (from the inconsistency model) are shown as a green square. Overall pooled results are also shown as a red square. The non‐similarity of the “pooled within design” and “pooled overall” results supports the consistency model.
Figures S11‐S13 | Rankograms for the anti‐gestational diabetes mellitus network showing the probability for every treatment being in a particular order with predictive probabilities in different adverse events.
Table S1 | Characteristics of included meta‐analyses studies in methodological quality assessment
Table S2 | Pooled odds ratios (95% confidence intervals) obtained from meta‐analyses for pregnancy outcomes by different treatment design.
J Diabetes Investig 2021; 12: 2247–2258
REFERENCES
- 1. Behboudi‐Gandevani S, Amiri M, Yarandi RB, et al. The impact of diagnostic criteria for gestational diabetes on its prevalence: a systematic review and meta‐analysis. Diabetol Metab Syndr 2019; 11: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. http://apps.who.int/iris/bitstream/handle/10665/85975/WHO_NMH_MND_13.2_eng.pdf;jsessionid=FD8DC8872A84924274CB92855D70888A?sequence=1 Accessed October 12, 2018.
- 3. Poolsup N, Suksomboon N, Amin M. Effect of treatment of gestational diabetes mellitus: a systematic review and meta‐analysis. PLoS One 2014; 9: e92485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Horvath K, Koch K, Jeitler K, et al. Effects of treatment in women with gestational diabetes mellitus: systematic review and meta‐analysis. BMJ 2010; 340: c1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Feig DS, Zinman B, Wang X, et al. Risk of development of diabetes mellitus after diagnosis of gestational diabetes. Can Med Assoc J 2008; 179: 229–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gui J, Liu Q, Feng L. Metformin vs insulin in the management of gestational diabetes: a meta‐analysis. PLoS One 2013; 8: e64585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Introduction: Standards of Medical Care in Diabetes—2018. Diabetes Care 2018.41: S1–S2. [DOI] [PubMed] [Google Scholar]
- 8. Bulletins–Obstetrics CoP . ACOG practice bulletin no. 190: gestational diabetes mellitus. Obstet Gynecol 2018; 131: e49‐e64. [DOI] [PubMed] [Google Scholar]
- 9. https://www.nice.org.uk/guidance/ng3/resources/diabetes‐in‐pregnancy‐management‐from‐preconception‐to‐the‐postnatal‐period‐pdf‐51038446021 Accessed May 25, 2020.
- 10. Farrar D, Simmonds M, Bryant M, et al. Treatments for gestational diabetes: a systematic review and meta‐analysis. BMJ Open 2017; 7: e015557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kitwitee P, Limwattananon S, Limwattananon C, et al. Metformin for the treatment of gestational diabetes: an updated meta‐analysis. Diabetes Res Clin Pract 2015; 109: 521–532. [DOI] [PubMed] [Google Scholar]
- 12. Balsells M, Garcia‐Patterson A, Sola I, et al. Glibenclamide, metformin, and insulin for the treatment of gestational diabetes: a systematic review and meta‐analysis. Obstet Gynecol Surv 2015; 70: 305–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Amin M, Suksomboon N, Poolsup N, et al. Comparison of glyburide with metformin in treating gestational diabetes mellitus: a systematic review and meta‐analysis. Clin Drug Invest 2015; 35: 343–351. [DOI] [PubMed] [Google Scholar]
- 14. Dhulkotia JS, Ola B, Fraser R, et al. Oral hypoglycemic agents vs insulin in management of gestational diabetes: a systematic review and metaanalysis. Am J Obstet Gynecol 2010; 203: 457.e1–457.e9. [DOI] [PubMed] [Google Scholar]
- 15. Impellizzeri FM, Bizzini M. Systematic review and meta‐analysis: a primer. Int J Sports Phys Therapy 2012; 7: 493. [PMC free article] [PubMed] [Google Scholar]
- 16. Leucht S, Chaimani A, Cipriani AS, et al. Network meta‐analyses should be the highest level of evidence in treatment guidelines. Eur Arch Psychiatry Clin Neurosci. 2016; 266: 477–480. [DOI] [PubMed] [Google Scholar]
- 17. Bao L‐X, Shi W‐T, Han Y‐X. Metformin versus insulin for gestational diabetes: a systematic review and meta‐analysis. J Matern Fetal Neonatal Med 2019; 26: 1–13. [DOI] [PubMed] [Google Scholar]
- 18. Butalia S, Gutierrez L, Lodha A, et al. Short‐and long‐term outcomes of metformin compared with insulin alone in pregnancy: a systematic review and meta‐analysis. Diabet Med 2017; 34: 27–36. [DOI] [PubMed] [Google Scholar]
- 19. Helal KF, Badr MS, Rafeek ME‐S, et al. Can glyburide be advocated over subcutaneous insulin for perinatal outcomes of women with gestational diabetes? A systematic review and meta‐analysis. Arch Gynecol Obstet 2020; 301: 1–14. [DOI] [PubMed] [Google Scholar]
- 20. Feng Y, Yang H. Metformin–a potentially effective drug for gestational diabetes mellitus: a systematic review and meta‐analysis. J Matern Fetal Neonatal Med 2017; 30: 1874–1881. [DOI] [PubMed] [Google Scholar]
- 21. Li G, Zhao S, Cui S, et al. Effect comparison of metformin with insulin treatment for gestational diabetes: a meta‐analysis based on RCTs. Arch Gynecol Obstet 2015; 292: 111–120. [DOI] [PubMed] [Google Scholar]
- 22. Moretti ME, Rezvani M, Koren G. Safety of glyburide for gestational diabetes: a meta‐analysis of pregnancy outcomes. Ann Pharmacother 2008; 42: 483–490. [DOI] [PubMed] [Google Scholar]
- 23. Nicholson W, Bolen S, Witkop CT, et al. Benefits and risks of oral diabetes agents compared with insulin in women with gestational diabetes: a systematic review. Obstet Gynecol 2009; 113: 193–205. [DOI] [PubMed] [Google Scholar]
- 24. Song R, Chen L, Chen Y, et al. Comparison of glyburide and insulin in the management of gestational diabetes: a meta‐analysis. PLoS One 2017; 12: e0182488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Su DF, Wang XY. Metformin vs insulin in the management of gestational diabetes: a systematic review and meta‐analysis. Diabetes Res Clin Pract 2014; 104: 353–357. [DOI] [PubMed] [Google Scholar]
- 26. Tarry‐Adkins JL, Aiken CE, Ozanne SE. Neonatal, infant, and childhood growth following metformin versus insulin treatment for gestational diabetes: a systematic review and meta‐analysis. PLoS Medicine 2019; 16: e1002848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zeng Y‐C, Li M‐J, Chen Y, et al. The use of glyburide in the management of gestational diabetes mellitus: a meta‐analysis. Adv Med Sci 2014; 59: 95–101. [DOI] [PubMed] [Google Scholar]
- 28. Zhao L‐P, Sheng X‐Y, Zhou S, et al. Metformin versus insulin for gestational diabetes mellitus: a meta‐analysis. Br J Clin Pharmacol 2015; 80: 1224–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zhu B, Zhang L, Fan YY, et al. Metformin versus insulin in gestational diabetes mellitus: a meta‐analysis of randomized clinical trials. Ir J Med Sci (1971‐) 2016; 185: 371–381. [DOI] [PubMed] [Google Scholar]
- 30. Shea BJ, Reeves BC, Wells G, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non‐randomised studies of healthcare interventions, or both. BMJ. 2017; 358: j4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Moher D, Shamseer L, Clarke M, et al. Preferred reporting items for systematic review and meta‐analysis protocols (PRISMA‐P) 2015 statement. Syst Rev 2015; 4: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Higgins JP, Green S. Cochrane handbook for systematic reviews of interventions . 2006.
- 33. Farrar D, Simmonds M, Griffin S, et al. The identification and treatment of women with hyperglycaemia in pregnancy: an analysis of individual participant data, systematic reviews, meta‐analyses and an economic evaluation. Health Technol Assess 2016; 20: 1–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Liang H‐L, Ma S‐J, Xiao Y‐N, et al. Comparative efficacy and safety of oral antidiabetic drugs and insulin in treating gestational diabetes mellitus: an updated PRISMA‐compliant network meta‐analysis. Medicine 2017; 96: e7939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kalafat E, Sukur YE, Thilaganathan B, et al. Metformin for the prevention of hypertensive disorders of pregnancy in women with gestational diabetes and obesity: a systematic review and meta‐analysis. Ultrasound Obstet Gynecol 2018; 52: 706–714. [DOI] [PubMed] [Google Scholar]
- 36. Shea BJ, Bouter LM, Peterson J, et al. External validation of a measurement tool to assess systematic reviews (AMSTAR). PLoS One 2007; 2: e1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. White IR. Network meta‐analysis. Stata J 2015; 15: 951–985. [Google Scholar]
- 38. Chaimani A, Higgins JP, Mavridis D, et al. Graphical tools for network meta‐analysis in STATA. PLoS One 2013; 8: e76654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Brown J, Martis R, Hughes B, et al. Oral anti‐diabetic pharmacological therapies for the treatment of women with gestational diabetes. Cochrane Database Syst Rev 2017; 1:1–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Guo L, Ma J, Tang J, et al. Comparative efficacy and safety of metformin, glyburide, and insulin in treating gestational diabetes mellitus: a meta‐analysis. J Diabetes Res. 2019; 2019: 1–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Jiang Y‐F, Chen X‐Y, Ding T, et al. Comparative efficacy and safety of OADs in management of GDM: network meta‐analysis of randomized controlled trials. J Clin Endocrinol Metab 2015; 100: 2071–2080. [DOI] [PubMed] [Google Scholar]
- 42. Brown J, Grzeskowiak L, Williamson K, et al. Insulin for the treatment of women with gestational diabetes. Cochrane Database Syst Rev 2017; 11: 1–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Poolsup N, Suksomboon N, Amin M. Efficacy and safety of oral antidiabetic drugs in comparison to insulin in treating gestational diabetes mellitus: a meta‐analysis. PLoS One 2014; 9: e109985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Association AD. Standards of medical care in diabetes–2012. Diabetes Care 2012; 35: S11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Butalia S, Gutierrez L, Lodha A, et al. Short‐ and long‐term outcomes of metformin compared with insulin alone in pregnancy: a systematic review and meta‐analysis. Diabet Med 2017; 34: 27–36. [DOI] [PubMed] [Google Scholar]
- 46. Feng Y, Yang HX. Metformin ‐ a potentially effective drug for gestational diabetes mellitus: a systematic review and meta‐analysis. J Matern Fetal Neonatal Med 2017; 30: 1874–1881. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 | Map plots of the anti‐gestational diabetes mellitus network for maternal and neonatal adverse events. The size of the nodes is proportional to the number of studies evaluating each treatment, and the thickness of the edges is proportional to the precision (the inverse of the variance) of each direct comparison.
Figure S2 | Map plots of the anti‐gestational diabetes mellitus network for maternal and neonatal hypoglycemia. The size of the nodes is proportional to the number of studies evaluating each treatment, and the thickness of the edges is proportional to the precision (the inverse of the variance) of each direct comparison.
Figures S3‐S10 | The forest plot for the anti‐gestational diabetes mellitus network for adverse events. The individual study results, grouped by treatment contrast and design. Pooled results within the design (from the inconsistency model) are shown as a green square. Overall pooled results are also shown as a red square. The non‐similarity of the “pooled within design” and “pooled overall” results supports the consistency model.
Figures S11‐S13 | Rankograms for the anti‐gestational diabetes mellitus network showing the probability for every treatment being in a particular order with predictive probabilities in different adverse events.
Table S1 | Characteristics of included meta‐analyses studies in methodological quality assessment
Table S2 | Pooled odds ratios (95% confidence intervals) obtained from meta‐analyses for pregnancy outcomes by different treatment design.


