Abstract
Diabetes is a rare, but potentially life‐threatening, adverse event of immune checkpoint inhibitors that requires prompt recognition and treatment. It usually occurs in the first 3 months of treatment and is typically related to programmed cell death‐1 antibodies, alone or in combined therapy. It has rarely been described developing after immunotherapy cessation. We present a 51‐year‐old man with metastatic melanoma, who developed acute‐onset diabetes 52 days after combined immunotherapy cessation with nivolumab and ipilimumab, and 25.6 months after receiving the first dose. He presented with acute hyperglycemic symptoms, ketosis, complete insulin depletion and negative autoimmunity, fulfilling the criteria of fulminant type 1 diabetes. The patient had previously developed hypophysitis with isolated adrenocorticotropic hormone deficiency during immunotherapy. We describe a case of late‐onset fulminant type 1 diabetes developing after immunotherapy cessation. Patient education and active follow up after immunotherapy discontinuation are crucial to warrant a timely intervention.
Keywords: Fulminant type 1 diabetes, Hypophysitis, Immunotherapy
We describe a case of immunotherapy‐related type 1 diabetes developing after combined immunotherapy cessation. The patient developed fulminant type 1 diabetes >2 years after the first immunotherapy dose, and had previously developed immune‐related hypophysitis.

Introduction
Immune checkpoint inhibitors (ICI) have emerged as a new therapeutic strategy for a broad spectrum of malignancies. As a counterpart, ICI impair self‐tolerance and can trigger immune‐related adverse events, of which endocrinopathies are common 1 . Pancreatic insulitis is a rare (1%), but potential life‐threatening, adverse effect. It is related to programmed cell death‐1 (PD‐1) antibodies alone or combined with cytotoxic T‐lymphocyte antigen‐4 (CTLA‐4) antibodies and is extremely rare with CTLA‐4 antibody monotherapy, highlighting the importance of the PD‐1/PD‐ligand pathway in maintaining self‐tolerance against pancreatic islets 2 , 3 .
Time to onset is generally <3 months, ranging up to 16 months after ICI initiation, and usually occurs earlier for combined (CTLA‐4 and PD‐1 antibody) therapy 2 , 4 , 5 . However, new‐onset ICI‐related diabetes diagnosed later and/or after discontinuing immunotherapy has been scarcely documented 6 . We describe a case of late‐onset fulminant type 1 diabetes developing after long‐term combined ICI immunotherapy cessation.
Case report
A 51‐year‐old white man with stage IV–M1c(0) BRAF wild‐type melanoma, with soft tissues and pleural involvement, had been previously referred to the Endocrinology Department in Hospital Clínic of Barcelona (Barcelona, Spain) due to orthostatic hypotension and weakness 37 weeks after starting immunotherapy. The patient had started first‐line treatment with nivolumab plus ipilimumab every 3 weeks for four doses followed by nivolumab flat dose every 4 weeks. The patient was overweight (body mass index 28 kg/m2) and had no other relevant medical issues. He had never received corticoids or chemotherapy. The hormonal tests confirmed the clinical suspicion of adrenocorticotropic hormone (ACTH)‐deficient adrenal insufficiency. Thyroid and gonadal functions, prolactin, electrolytes and blood glucose were all in the normal range. No compressive symptoms were present, and the nuclear magnetic resonance carried out 2 months later did not show significant alterations in the pituitary gland. The patient was diagnosed as grade 2 immune‐related hypophysitis with isolated ACTH deficiency; after an initial intravenous stress dose of hydrocortisone, oral hydrocortisone 20 mg/day was started and maintained through follow up. Symptoms and overall condition improved, and the patient resumed his active lifestyle.
After 2 years (13 cycles) of treatment, the patient maintained a partial response according to Response Evaluation Criteria in Solid Tumors (RECIST) v1.1 criteria and treatment was stopped. Eight weeks later, he abruptly presented with polyuria, polydipsia and weight loss of 6 kg (5% of initial bodyweight), and was referred to the Emergency Department. Blood glucose had previously been normal, including the last blood test carried out 4 weeks before. He was dehydrated, glycemia was 46.4 mmol/L, ketonemia 1.4 mmol/L and osmolality 327 mmol/kg. Acid–base equilibrium and kidney function were normal. The glycated hemoglobin (HbA1c) level was 7.9%, islet autoantibodies were negative and a glucagon tolerance test at 4 weeks showed complete insulin depletion. High‐resolution human leukocyte antigen (HLA) typing showed DRB1*01:01, DRB1*12:01. Detailed evolution of glycemia, including glycemia during admission, is represented in Figure 1. Laboratory findings are summarized in Table 1.
Figure 1.
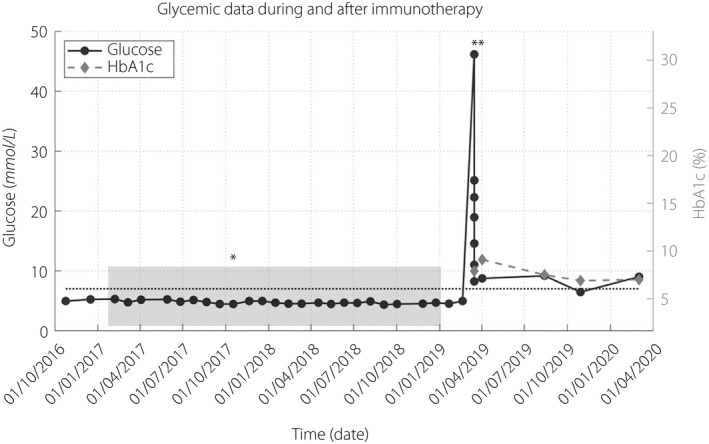
Glycemic data during and after immunotherapy. Shaded area: duration of immunotherapy treatment; dotted line: reference range threshold for diabetes (glycemia 7 mmol/L); *date of hypophysitis diagnosis; **date of diabetes diagnosis. HbA1c, glycated hemoglobin.
Table 1.
Laboratory data on admission at diabetes diagnosis
| Value | Reference range | |
|---|---|---|
| Glucose (mmol/L) | 46.2 | 3.9–5.6 |
| Urea (mmol/L) | 15 | 5.9–17.3 |
| Creatinine (µmol/L) | 99.91 | 61.89–106.1 |
| eGFR, CKD‐EPI (mL/min/1.73 m2) | >60 | >60 |
| Na (mmol/L) | 131.6 | 135–150 |
| K (mmol/L) | 4.9 | 3.5–5 |
| Cl (mmol/L) | 94 | 98–107 |
| pH | 7.37 | 7.33–7.42 |
| HCO3 (mmol/L) | 21.8 | 24–28 |
| Base excess (mmol/L) | −3.9 | −2 to 3 |
| Ketonemia (mmol/L) | 1.4 | <0.1 |
| Osmolality (mmol/kg) | 327 | 280–295 |
| Ca (mmol/L) | 2.32 | 2.12–2.62 |
| P (mmol/L) | 1.07 | 0.74–1.39 |
| CRP (mg/L) | 80 | 0–50 |
| Albumin (g/L) | 44 | 34–48 |
| AST (IU/L) | 18 | <41 |
| ALT (IU/L) | 20 | <40 |
| Total bilirubin (µmol/L) | 18.81 | <20.52 |
| LDH (IU/L) | 177 | <234 |
| Alkaline phosphatase (IU/L) | 112 | 46–116 |
| Lipase (IU/L) | 143 | <393 |
| White blood cell count (×109/L) | 9.2 | 3.6–12.0 |
| Neutrophils (%) | 67.4 | 39.6–67 |
| Hemoglobin (g/L) | 156 | 135–180 |
| Platelet (×109/L) | 192 | 150–350 |
| Thyroid | ||
| TSH (mIU/L) | 3.181 | 0.4–4 |
| FT4 (pmol/L) | 20.98 | 10.3–25.74 |
| TPO antibodies (UI/mL) | <28 | <35 |
| Gonadal axis | ||
| LH (IU/L) | 6.01 | 1.5–7.5 |
| FSH (IU/L) | 6.58 | 1.7–8 |
| Testosterone (nmol/L) | 12.85 | 9.54–29.5 |
| Adrenal axis † | ||
| ACTH (pmol/L) | <1 | 2.2–13.2 |
| Cortisol (nmol/L) | 74.52 | 276–690 |
| 21‐hydroxylase antibodies (IU/mL) | <0.3 | <0.4 |
| Diabetes | ||
| HbA1c (NGSP/DCCT, %) | 7.9 | 4–6 |
| HbA1c (IFCC, mmol/mol) | 63 | <42 |
| GADA (IU/mL) | 0.1 | <1 |
| IA‐2A (IU/mL) | 0.05 | <1 |
| Insulin antibodies (IU/mL) | 0.2 | <0.4 |
| C‐peptide (nmol/L) ‡ | 0.32 | 0.13–0.87 |
| HLA typing (high resolution) | DRB1*01:01, DRB1*12:01 | |
| Glucagon tolerance test (performed 27 days after admission) | ||
| Glucose (mmol/L) | 7.7 | 3.9–5.6 |
| Basal C‐peptide (nmol/L) | <0.07 | 0.13–0.87 |
| C‐peptide at 6 min (nmol/L) | 0.07 | 0.13–0.87 |
ACTH, corticotropin; ALT, alanine aminotransferase; AST, aspartate aminotransferase; Ca, calcium; Cl, chlorine; CRP, C‐reactive protein; eGFR, estimated glomerular filtration rate; FSH, follicle‐stimulating hormone; FT4, free‐thyroxine; GADA, glutamic acid decarboxylase antibodies; HbA1c, glycated hemoglobin; HCO3, bicarbonate; IA‐2A, insulinoma‐associated protein 2 autoantibodies; K, potassium; LDH, lactic acid dehydrogenase; LH, luteinizing hormone; Na, sodium; NGSP, National Glycohemoglobin Standardization Program; P, phosphate; TPO, peroxidase; TSH, thyroid‐stimulating hormone.
6–8 h after the last hydrocortisone administration.
Performed 72 h after admission once acute ketosis was corrected, glucose was 8.9 mmol/L.
The patient did not refer to symptoms of pancreatic exocrine deficiency, pancreatic enzymes were normal, and the computed tomography evaluation of the pancreatic region showed normal pancreatic volume and morphology, and the absence of pancreatic metastases. After correction of the acute ketosis with intravenous insulin and fluids, treatment with multiple insulin injections was started. Twelve months from diabetes onset, the patient was on multiple insulin injections (0.6 units/kg/day), had an optimal metabolic control (HbA1c 7%) and maintained a sustained partial response of his neoplasia.
Informed consent was obtained from the patient. The Research Ethical Committee of Hospital Clínic de Barcelona does not require specific approval for the publication of case reports beyond the patient’s informed consent.
Discussion
We present a case of late‐onset ICI‐related diabetes presenting after immunotherapy discontinuation, 103 weeks after the first dose and 8 weeks after discontinuation. In previously reported cases, the typical clinical presentation (up to 76%) occurs in the first 3 months, especially when receiving combined therapy. Stamatouli et al. 7 described one case developing 228 weeks (54 months) after initial treatment. The patient had a treatment holiday between two rounds of therapy and it is not clarified if the patient was on immunotherapy at the time of diabetes onset. In addition, the patient received treatment with interferon‐α and interleukin‐2. Interferon‐α can cause type 1 diabetes through mediation of β‐cell overexpression of HLA class I, endoplasmic reticulum stress and β‐cell apoptosis, and high‐dose interleukin‐2 has been associated with deterioration in C‐peptide levels in new‐onset type 1 diabetes. Thus, the role of interleukin‐2 and interferon‐α in the development of diabetes onset in this case cannot be ruled out 7 .
Fulminant type 1 diabetes is a subtype of type 1 diabetes first described in Japan (it accounts for up to 20% of type 1 diabetes cases in Japanese patients), and is frequently seen in Asian populations 8 . To date, it is extremely rare in white people 9 . Other features distinctive from ‘classical’ type 1 diabetes are: onset in adulthood, undetectable islet autoantibodies, elevation of pancreatic enzymes in 98% and frequent flu‐like symptoms before diabetes onset 1 . Fulminant type 1 diabetes is defined as severe hyperglycemia with ketosis with concomitant near normal HbA1c and the absence of insulin secretion at disease onset, and is the predominant clinical presentation of ICI‐related diabetes 8 , 10 .
ICI therapy, especially anti‐PD‐1 therapy, causes rapidly progressive diabetes, possibly through an inappropriate activation of T cells that leads to massive β‐cell destruction, although underlying mechanisms remain unclear 11 , 12 . Fulminant type 1 diabetes induced by ICI differs from ‘spontaneous’ fulminant type 1 diabetes. First, a non‐Asian ethnic predominance has been described in ICI‐related diabetes patients. Second, pancreatic islet autoantibodies are detectable in 47% of ICI‐related diabetes patients, which is higher than expected in the general population (12.7%), but less frequent than in ‘classical’ type 1 diabetes (>80% positivity) 7 . Furthermore, elevation of pancreatic enzymes is only observed in 50% of cases of ICI‐related diabetes, and is usually asymptomatic and flu‐like symptoms are uncommon 1 .
Biomarkers that predict ICI‐related diabetes have not yet been identified, but HLA haplotypes could be one. HLA genotypes with increased susceptibility for type 1 diabetes or fulminant type 1 diabetes are found in the majority of patients (61%) with ICI‐related diabetes, with a striking predominance for HLA‐DR4 (up to 76% in some cohorts), higher than in ‘classical’ type 1 diabetes and the white reference population 5 , 7 .
Some clinical and immunological traits have been related to an earlier onset of ICI‐induced diabetes, namely: diabetic ketoacidosis at onset, combined treatment with anti‐CTLA‐4 and anti‐PD‐1 therapies, and positive islet autoantibodies 5 . The median interval from immunotherapy initiation to diagnosis of diabetes was shorter (3–7 weeks) in patients with positive glutamic acid decarboxylase antibodies versus 9–16 weeks in glutamic acid decarboxylase antibodies‐negative patients 2 , 12 . Yet, no differences in HLA expression have been described in early‐ and delayed‐onset ICI‐related diabetes, and no clinical or immunological distinctive phenotype has been identified.
Another possibility is that fulminant type 1 diabetes had developed by chance. Nevertheless, the non‐Asian origin, the non‐predisposing HLA haplotype, the non‐elevated pancreatic enzymes, the absence of flu‐like symptoms, together with the development of clinical features typical of ICI‐related diabetes and the previous development of another ICI‐related endocrinopathy, make this possibility unlikely. The patient had typical clinical features of ICI‐related diabetes – which occurs mainly in men (55–60%), aged in their sixties, treated with an anti‐PD‐1 (96%) alone or in combination for melanoma – and had no personal or family history of prediabetes or diabetes 1 . He did not have a high‐risk HLA haplotype for ‘spontaneous’ classical or fulminant type 1 diabetes, yet HLA DRB1*01 alleles have been described in white patients with ICI‐related diabetes 13 . Despite not presenting with diabetic ketoacidosis, the patient’s glycemia on admission was higher (median 31.4–36.3 mmol/L), HbA1c was similar and C‐peptide levels were as low as most cases of ICI‐related diabetes. He had negative pancreatic antibodies and normal lipase levels, both are found in 50% of cases 2 , 3 , 4 , 5 .
Furthermore, nivolumab persists in circulation and binds to T cells in patients 20 weeks after the last infusion 14 . This adds biological plausibility to the hypothesis that fulminant type 1 diabetes was driven by immunotherapy.
The present patient had previously developed an ICI‐related low‐grade hypophysitis/isolated ACTH deficiency. Hypophysitis incidence ranges from 8.8 to 10.5% in patients treated with combination therapy, occurs mostly in middle‐aged men, and adrenal insufficiency is frequent and persistent. In one series, 44% of patients who developed diabetes had an ICI‐related endocrinopathy previous or concurrent to the development of diabetes, mainly primary thyroid dysfunction 3 . To date, just two previous cases of ICI‐related diabetes and hypophysitis have been published: one case of concomitant nivolumab‐related diabetes and hypophysitis, and another with ipilimumab‐induced hypophysitis followed by pembrolizumab‐induced diabetes 15 , 16 .
The present case shows the importance of follow up after immunotherapy cessation, and patient education to recognize and treat this life‐threatening complication of immunotherapy. The increasing survival rates of these patients will probably reveal further late‐onset immune‐related adverse events in the following years.
Disclosure
The authors declare no conflict of interest.
Acknowledgments
This work has been partially funded by the Resident Award ‘Premi Fi de Residènica Emili Letang’ 2019–2020, granted by Hospital Clínic de Barcelona, Research, Innovation and Education Department to LB
J Diabetes Investig 2021; 12: 2263–2266
References
- 1. Chang LS, Barroso‐Sousa R, Tolaney SM, et al. Endocrine toxicity of cancer immunotherapy targeting immune checkpoints. Endocr Rev 2018; 40: 17–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Akturk HK, Kahramangil D, Sarwal A, et al. Immune checkpoint inhibitor‐induced type 1 diabetes: a systematic review and meta‐analysis. Diabet Med 2019; 36: 1075–1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bluestone JA, Anderson M, Herold KC, et al. Endocrine toxicity of cancer immunotherapy targeting immune checkpoints. Diabetes 2018; 26: 17–65. [Google Scholar]
- 4. Farina KA, Kane MP. Programmed cell death‐1 monoclonal antibody therapy and type 1 diabetes mellitus: a review of the literature. J Pharm Pract 2021; 34: 133–140 [DOI] [PubMed] [Google Scholar]
- 5. de Filette JMK , Pen JJ, Decoster L, et al. Immune checkpoint inhibitors and type 1 diabetes mellitus: a case report and systematic review. Eur J Endocrinol 2019; 181: 363–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mae S, Kuriyama A, Tachibana H. Diabetic ketoacidosis as a delayed immune‐related event after discontinuation of nivolumab. J Emerg Med 2020; 60: 342–344. [DOI] [PubMed] [Google Scholar]
- 7. Stamatouli AM, Quandt Z, Perdigoto AL, et al. Collateral damage: insulin‐dependent diabetes induced with checkpoint inhibitors. Diabetes 2018; 67: 1471–1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Imagawa A, Hanafusa T, Awata T, et al. Report of the Committee of the Japan Diabetes Society on the research of fulminant and acute‐onset type 1 diabetes mellitus: new diagnostic criteria of fulminant type 1 diabetes mellitus (2012). J Diabetes Investig 2012; 3: 536–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Moreau C, Drui D, Arnault‐Ouary G, et al. Fulminant type 1 diabetes in Caucasians: a report of three cases. Diabetes Metab 2008; 34: 529–532. [DOI] [PubMed] [Google Scholar]
- 10. Marchand L, Disse E, Dalle S, et al. The multifaceted nature of diabetes mellitus induced by checkpoint inhibitors. Acta Diabetol 2019; 56: 1239–1245. [DOI] [PubMed] [Google Scholar]
- 11. Okamoto M, Okamoto M, Gotoh K, et al. Fulminant type 1 diabetes mellitus with anti‐programmed cell death‐1 therapy. J Diabetes Investig 2016; 7: 915–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Clotman K, Janssens K, Specenier P, et al. Programmed cell death‐1 inhibitor‐induced type 1 diabetes mellitus. J Clin Endocrinol Metab 2018; 103: 3144–3154. [DOI] [PubMed] [Google Scholar]
- 13. Marchand L, Thivolet A, Dalle S, et al. Diabetes mellitus induced by PD‐1 and PD‐L1 inhibitors: description of pancreatic endocrine and exocrine phenotype. Acta Diabetol 2019; 56: 441–448. [DOI] [PubMed] [Google Scholar]
- 14. Osa A, Uenami T, Koyama S, et al. Clinical implications of monitoring nivolumab immunokinetics in non‐small cell lung cancer patients Find the latest version : clinical implications of monitoring nivolumab immunokinetics in non‐small cell lung cancer patients. JCI Insight 2018; 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Marchand L, Paulus V, Fabien N, et al. Nivolumab‐induced acute diabetes mellitus and hypophysitis in a patient with advanced pulmonary pleomorphic carcinoma with a prolonged tumor response. J Thorac Oncol 2017; 12: e182–e184. [DOI] [PubMed] [Google Scholar]
- 16. Humayun MA, Poole R. A case of multiple immune toxicities from Ipilimumab and pembrolizumab treatment. Hormones 2016; 15: 303–306. [DOI] [PubMed] [Google Scholar]


