Abstract
The present study aims to analyze the effectiveness of ovitraps in the capture of Hg leucocelaenus eggs and evaluate the influence of the dry and rainy seasons on their abundance and hatching rates. The eggs were collected in the Atlantic Forest of State of Rio de Janeiro, Brazil, an area in which the yellow fever virus is known to circulate. We distributed 15 ovitraps in three sampling points, with five ovitraps per point. We distributed 15 ovitraps in three sampling points on trees within a forested area, which were sequentially numbered, monitored, and replaced every two weeks from October 2016 to April 2018. There was a high dominance of Hg. leucocelaenus eggs (98.4%) and a variation in egg hatching rates between the wet and dry seasons. These rates were 1.5 times higher in the rainy season than in the dry season. The rainy season also showed a greater abundance of eggs and higher values of ovitrap positivity and egg density indexes in the installed ovitraps. The abundances of Hg. leucocelaenus eggs were positively correlated with mean monthly temperature and air humidity but not significantly correlated with accumulated precipitation. These results, as well as their implications for the possible use of ovitraps to monitor vector mosquitoes of yellow fever in the study region, are discussed.
Introduction
Haemagogus leucocelaenus (Dyar and Shannon, 1924) is a mosquito species with a geographic distribution that extends from Argentina to Trinidad [1, 2]. It is commonly found in Brazil, especially in the central-western, southeastern, and southern regions [3]. This species is epidemiologically important since it is considered one of the main vectors of the yellow fever virus (YFV) in forest areas, along with Haemagogus janthinomys Dyar (1921), the primary vector of this virus [4]. Recently, the Zika virus was detected from Hg. leucocelaenus, suggesting that it may also be involved in the transmission cycles of other arboviruses [5].
According to Alencar et al. [6, 7], Hg. leucocelaenus is eclectic in terms of feeding habits and oviposition patterns. These mosquitoes lay their eggs in hollow trees or bamboo internodes in the canopy and near the forest floor. The breeding sites used by Hg. leucocelaenus are affected by water level fluctuations, with potential breeding areas running out of water in drier and hotter periods. Therefore, eggs from this species are laid on moist plant substrates near the water surface where they need to come into contact with water repeatedly in order to hatch [8, 9].
Schaeffer et al. [10] studied two species of Aedes that breed in tree holes and found that precipitation is the primary limiting factor for egg hatching and larval development in such breeding sites. Campos and Sy [11] observed that precipitation and temperature strongly influence the hatching rate of Ochlerotatus albifasciatus (Macquart, 1838) eggs. Climate change can affect biodiversity at different levels by accelerating some individuals’ metabolism and affecting the food chains and ecological interactions of populations and communities [12]. According to Alencar et al. [13], the activity level of different mosquito species is directly influenced by climatic variables, such as temperature and air humidity. Since there are not just some vector species affected by these abiotic factors but entire populations of many Culicidae species and other arthropods as well. However, while climate has been shown to influence the abundance of vector mosquitoes, little is known about the effects of climatic variations on the dynamics of oviposition and the hatching rates of eggs, particularly those of Hg. leucocelaenus.
Population monitoring of Hg. leucocelaenus in the nature, especially in areas with the occurrence of YFV, is performed invariably through the collection of adults using entomological nets, manual vacuums, and CDC traps [14, 15]. However, collecting immature forms of these mosquitoes is challenging since it is not always possible to locate holes in trees in the forest. In addition, when breeding sites are located, the visualization of eggs is very difficult, hampering the adoption of this practice by monitoring programs of YFV vectors in Brazil. Ovitraps are traps that simulate potential breeding sites for mosquitoes, basically consisting of a black plastic pot, water, and a wooden straw. They are widely used in Aedes aegypti monitoring programs of Brazilian urban areas [16]. However, this trap also seems to work well for some groups of mosquitoes that occur in wild areas, especially those that use phytotelmata breeding sites, such as mosquitoes of the genera Aedes, Haemagogus, Limatus, and Toxorhynchites [17, 18].
The present study aimed to analyze the effectiveness of ovitraps for capturing Hg leucocelaenus eggs, as well as evaluate the influence of the dry and rainy seasons on the abundance and hatching rates of this mosquito species.
Materials and methods
Ethics statement
The permanent license for collecting, capturing, and transporting biological material was granted by the Biodiversity Authorization and Information System (SISBIO)/ Chico Mendes Institute for Biodiversity Conservation (ICMBio) under the number 34911–1. All team members were previously vaccinated against YF.
Study area
The Sana Environmental Protection Area (SPA) is a 15.7 ha reserve located in an area of secondary Atlantic Forest in the municipality of Macaé, State of Rio de Janeiro, Brazil. This SPA is situated in a valley surrounded by mountains and consists of a large area of dense ombrophilous vegetation, including several waterfalls and a high diversity of fauna and flora. The Sana River basin, present in most of the SPA territory, is the largest and most important water source of the Macaé River [19].
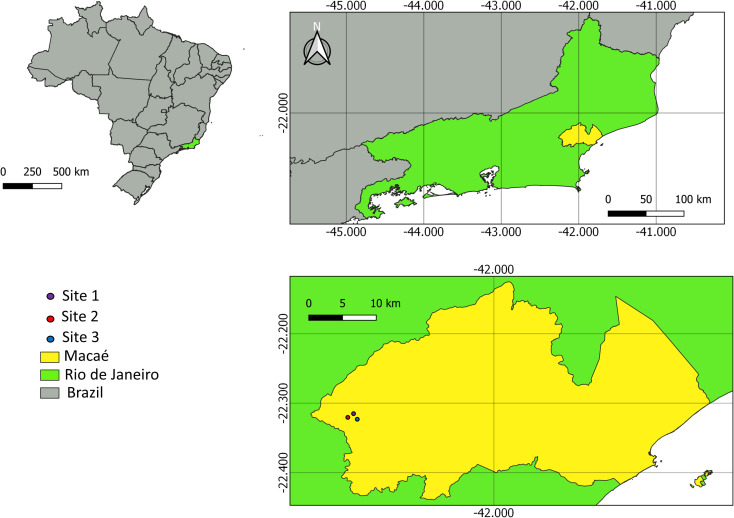
The sampling sites and their characteristics were as follows: site 1, with a dense shrub layer and tall trees very close to each other (22°20’01.3’’S, 42°12’24.0’’W); site 2, located on the banks of the Sana River, with a mosquito fauna influenced by bamboo vegetation (22°20’02.9’’S, 42°12’28.3’’W); and site 3, with vegetation cover similar to site 1 (22°20’02.9’’S, 42°12’31.1’’W). Maps were prepared in ArcGIS 10 software and edited in Adobe Photoshop CS5 and CorelDraw X5. The sampling locations are shown in (Fig 1).
Fig 1. Sampling sites in the Sana Environmental Protection Area, located in the sixth district of Macaé, Rio de Janeiro, Brazil.
Maps were prepared in QGIS 3.14.16software and edited in Adobe Photoshop CS5 and CorelDraw X5. Reprinted from QGIS 3.14.16, a program under a CC BY license, with permission from Jeronimo Alencar—Fiocruz, original copyright 2021.
The state of Rio de Janeiro is characterized by having a hot and rainy climate between November and April (rainy period) and a cold and dry climate between May and October (dry period) [20].
Collections and laboratory procedures
We used ovitraps to collect eggs, which consisted of 500 mL black containers without a lid, resembling a plant vase. Inside, the ovitraps contained four wooden oviposition paddles (2.5 cm × 14 cm), vertically held in place with a clip and textured surfaces to facilitate oviposition. We added 300 ml of natural water from water bodies close to the collection sites and approximately 100g of leaf litter in each ovitrap in an effort to recreate a microecosystem resembling the natural environment (S1 Fig). We distributed 15 ovitraps in three sampling points on trees within a forested area, which were sequentially numbered, monitored, and replaced every two weeks from October 2016 to April 2018 [21]. Ovitraps were installed in trees at a height of 2.50 meters from the ground in the forest, with nylon ropes and wire to hold the ovitraps to the trees [22, 23]. After the ovitraps were collected, they were packed in a polyethylene box and sent to the Diptera Laboratory of the Oswaldo Cruz Institute in the city of Rio de Janeiro. Positive paddles (containing eggs) were separated in the laboratory, where the eggs were counted and immersed in transparent trays containing dechlorinated water. The eggs were then placed in a controlled experimental environment in a laboratory greenhouse with a thermoperiod regulated at a temperature of 28°C ± 1°C, relative air humidity of 75–90%, and a 12 h day/12 h night cycle. After three days, the paddles were removed from the water and left to dry in the air for another three days to quantify the hatched larvae. Immature forms were reared as described by Alencar [6]. Positive paddles were immersed as many times as needed to allow hatching of all eggs present. Thus, the eggs were subjected to repeated cycles of immersions and drying until all of them had hatched. These conditions allowed us to keep the specimens alive until adulthood for specific identification following the methodology described by Arnell [24].
Adult identification was carried out at the species level by direct observation of morphological characters using a stereomicroscope. We consulted the respective specific descriptions/diagnoses of dichotomous keys elaborated by Arnell [24], and Forattini [25]. Following species identification, all specimens were deposited in the Entomological Collection of the Oswaldo Cruz Institute, Fiocruz, Rio de Janeiro, under the “Sana Environmental Protection Area Collection” designation. The abbreviations for mosquito genera adopted are those proposed by Reinert [26].
Data analysis
After quantifying the collected eggs, we calculated the ovitrap positivity index (OPI = No. of positive traps/No. of examined traps x 100%) and egg density index (EDI = No. of eggs/No. of positive traps) [27].
We first obtained descriptive statistics, including absolute and relative frequencies, and then performed the Shapiro–Wilk test to verify the normality of the data. Subsequently, we used Welch’s modified 2-sample t-test, which does not assume equal variances between samples, to test for differences in the abundance of eggs collected between the dry and rainy periods. The number of hatches (fertility) was compared to the total number of eggs collected in the dry and rainy periods using Yates’s chi-squared test, estimates of odds ratios, and 95% confidence intervals. The Spearman’s rank correlation coefficient was used to analyze the correlation between eggs abundance and monthly mean temperature, monthly mean relative air humidity, and monthly accumulated precipitation. We conducted the statistical analysis in GraphPad Prism version 8.01 for Windows (San Diego, California, USA), with the significance level of p < 0.05.
The average values of temperature and relative humidity of the air and accumulated precipitation were obtained from the period of 30 days prior to the day of each collection from daily readings of climatic conditions. These climatic parameters were obtained from Brazil’s Institute of Meteorology (INMET). Furthermore, the beginning, end, and duration of each period were determined using the criterion proposed by Marcuzzo et al. [28], which was based on a series of data spanning 30 years.
Results
We collected 13,419 mosquito eggs, of which 11,129 hatched and were identified as Hg. leucocelaenus (n = 10,946; 98.4%), Ae. terrens (Walker, 1856) (n = 172; 1.5%), and Hg. janthinomys (n = 11; 0.1%). A total of 2,290 eggs did not hatch and consequently could not be identified (S1 Table).
Of all the eggs collected during the rainy season, 83.5% hatched and 16.5% did not hatch. Meanwhile, 77.6% of the eggs collected during the dry period hatched, whereas 22.4% did not (Table 1). The chi-square test showed that the hatching rate was significantly higher during the rainy season (Yates corrected x2 = 30.60, df = 1, p < 0.0001). The odds ratio was 1.5 (CI 95%, 1.281 to 1.676), indicating that eggs are 1.5 times more likely to hatch in the rainy season than in the dry season.
Table 1. Abundance of mosquito eggs collected during dry and rainy periods in the SANA Environmental Protection Area, Macaé, Rio de Janeiro, Brazil, from 2016 to 2018.
| Eggs | Dry period | Rainy period | Total collected |
|---|---|---|---|
| Hatched eggs | 1,074 | 10,055 | 11,129 |
| Unhatched eggs | 310 | 1980 | 2,290 |
| Total collected | 1,384 | 12,035 | 13,419 |
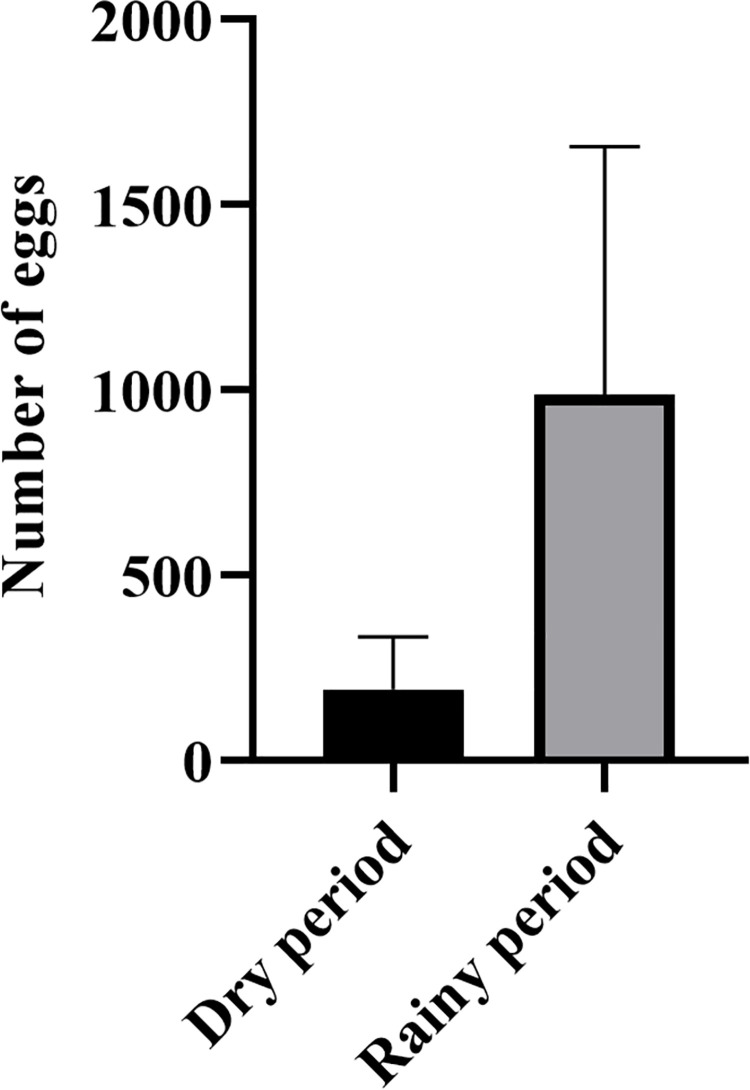
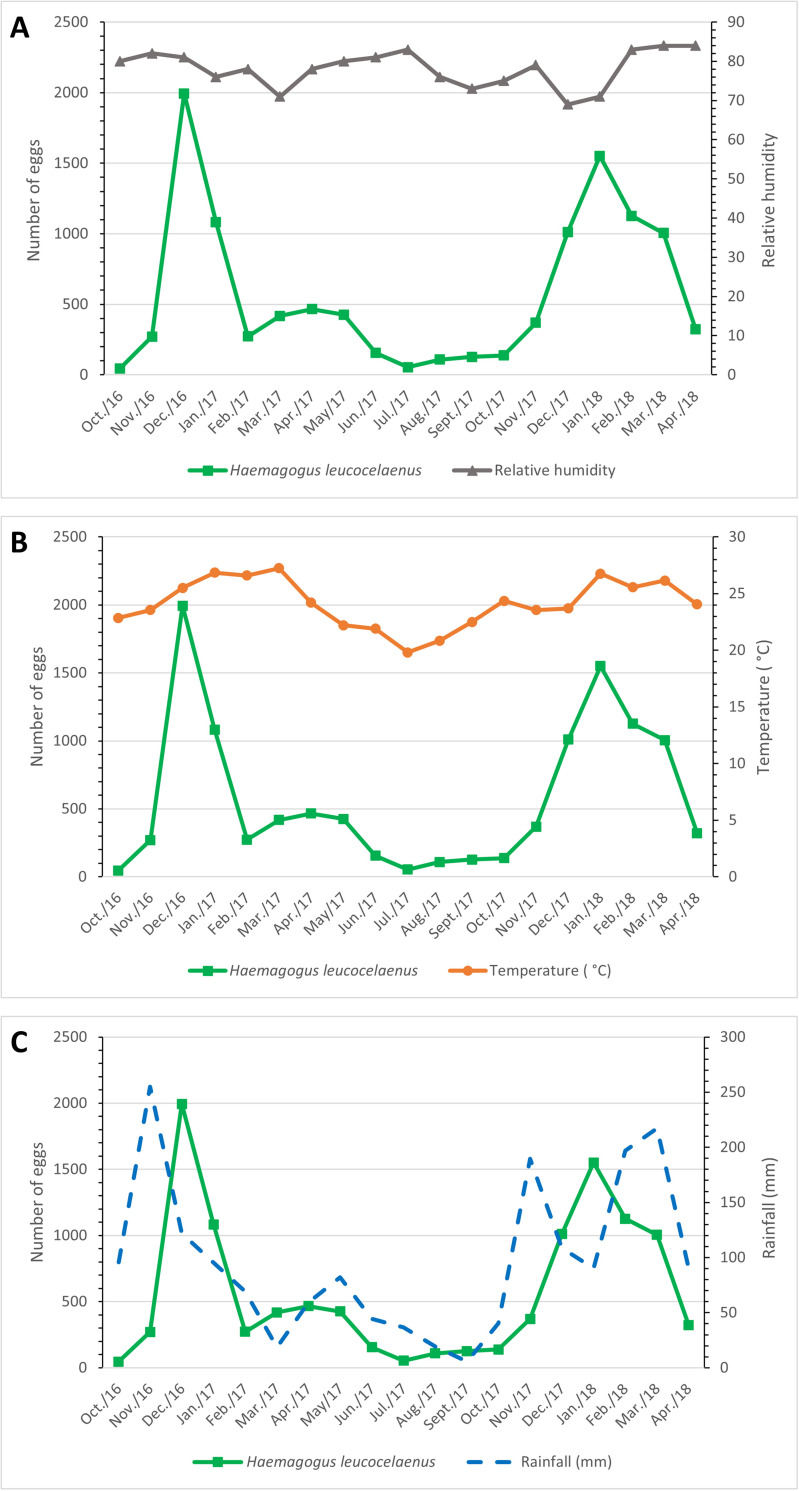
The monthly average (± S.D.) of Hg. leucocelaenus eggs collected in all ovitraps was 193.1 (±141.1) in the dry season and 989.4 (±667.9) in the rainy season. These values are significantly different according to Welch’s t-test (t = 3.980, df = 12.610, p = 0.0017) (Fig 2). The abundance of Hg. leucocelaenus eggs was significantly and positively correlated with relative air humidity (r = 0.507; p< 0.027) (Fig 3A) and temperature (r = 0.670; p = 0.002) (Fig 3B). On the other hand, we found no significant correlation between egg abundance and precipitation (r = -0.068; p> 0.0783) (Fig 3C).
Fig 2. Comparative analysis of the Hg. leucocelaenus mean number of eggs (± S.D.) collected during the dry (Aug/18, Jun/19, Jul/19) and rainy (Dec/18, Jan/19 and Feb/19) periods in the SANA Environmental Protection Area, Macaé, Rio de Janeiro, Brazil, from 2016 to 2018.
Fig 3.
Variation in the number of Hg. leucocelaenus eggs concerning (A) relative humidity, (B) temperature, and (C) precipitation recorded in the SANA Environmental Protection Area, Macaé, Rio de Janeiro, Brazil, from 2016 to 2018.
Each of the 15 installed ovitraps captured, on average (±S.D.), 892 (±1,002) eggs of Hg. leucocelaenus during the study period. The values of OPI and EDI (CI 95%) were 80.5% (74.5–86.5) and 36.6 (22.8–49.9), respectively. In the dry and rainy seasons, the OPI values (CI 95%) were 69.3% (61.5–77.2) and 91.7% (87.0–96.3), respectively. The EDI values (CI 95%) were 14.3 (10.2–18.4) and 58.4 (36.0–80.8), respectively.
Discussion
There has been a growing interest in studying the biology and ecology of Hg. leucocelaenus populations due to their role in the wild cycle of YFV and, potentially, other arboviruses [29]. The species is abundant in the state of Rio de Janeiro, where the circulation of YFV in wild environments has been widely recorded [30]. In the present study, Hg. leucocelaenus was the dominant species in the ovitraps installed. Along with the other two species collected (Ae. terrens and Hg. janthinomys), Hg. leucocelaenus has been found to reproduce naturally in the breeding areas formed by tree holes [3, 31].
The hatching rates of mosquito eggs showed marked differences between the two periods analyzed, with eggs from the rainy period 1.5 times more likely to hatch than those from the dry period. This pattern in hatching rates between dry and rainy periods probably reflects what occurs for Hg. leucocelaenus in the nature since this species represented almost all individuals identified in the present study.
Although the species’ eggs have a certain tolerance to desiccation [32], their viability may be negatively affected in drier periods when the amount of rain is insufficient to keep the breeding site moist or when it may not allow the water level to reach the eggs repeatedly. The resistance level to dissection varies among mosquito species. The period of up to three hours after oviposition, at the beginning of embryogenesis and before the formation of the waxy cuticle, is when the egg is most susceptible to lack of moisture. Furthermore, even after the formation of a waxy cuticle, which prevents water loss from the interior of the egg to the external environment, some species remain highly susceptible to desiccation in the breeding site, as is the case of Culex species [33]. In addition to the moisture of the breeding site, the number of immersions that Hg. leucocelaenus eggs undergo during embryogenesis seems to be an important factor in hatching rates [7]. Thus, rainy and hot periods offer greater possibilities for multiple immersions of eggs. According to Evangelista et al. [34], the combination of high temperatures and precipitation recorded during the rainy summer favors YFV transmission and geographic dissemination of epizootic waves due to the positive influence of the increased hatching rates and the acceleration of larval development.
In many natural breeding sites formed by tree holes, their aquatic content may be less exposed to climatic variations than the content of the ovitraps used in the present study, which have a wide opening in their upper part. Some tree holes may have small openings to the external environment that could limit water loss water through evaporation and reduce temperature variations in their interior [35, 36]. In addition, many breeding sites formed by tree holes have their aquatic content maintained by the plant’s physiological water [37], difficulting desiccation even in drier periods. Therefore, the differences in hatching rates of mosquito eggs observed between the dry and rainy season in the present study could have been overestimated by the use of traps. Instead, such differences could have been associated with variations in the water level in the breeding sites or with variations in humidity at particular stages in the embryogenesis of the eggs.
The present study demonstrated that Hg. leucocelaenus produces more eggs during the rainy season, which is similar to the observations made by Alencar et al. [38], who verified population spikes in Hg. leucocelaenus during the rainy season in a study in the state of Rio de Janeiro carried out in the Ecological Reserve of Guapiaçu. In a study in the Amazon, [39] found higher abundance and richness of mosquitoes in the rainy season compared to the dry season, with Hg. leucocelaenus only present during the rainy season. These findings are also in line with [40, 41], who note that Haemagogus species are generally captured in the wettest and hottest months.
The abundance of eggs identified in the ovitraps was positively correlated with climatic factors such as temperature and relative humidity but not with precipitation. Although it is known that climatic factors influence the abundance of vector mosquitoes and, consequently, affect the dynamics of vector-borne diseases, it is not always easy to establish this relationship [10]. Factors associated with environmental characteristics such as the type and density of vegetation cover, as well as the abundance of hosts and available breeding sites, can overcome the effect of climate on mosquito populations [42]. Other studies on the relationship between the abundance of immature or adult forms of Hg. leucocelaenus and climatic variables are controversial [6, 9, 42], reinforcing that the variations in this species have multifactorial causes. In addition, the fact that we purposefully added 300 ml of water to each ovitrap may have influenced our findings, especially in the absence of correlation between the abundance of Hg. leucocelaenus and the accumulated precipitation of the month. In drier months, such as October 2016 and July 2017, no ovitraps were found to be completely devoid of water.
In the present study, we show that ovitraps are effective for collecting Hg. leucocelaenus eggs in a wild environment. The ovitraps simulate breeding sites formed by tree holes, which retain rainwater, enabling the development of the mosquito fauna [31]. Over the 19 sampling months, each ovitrap captured an average of approximately 900 eggs. This figure was reflected in the high OPI (80.5%) and EDI (36.6) values, especially in the rainy season, where OPI and EDI values were 91.7% and 58.4, respectively. This may indicate that ovitraps can be used to capture and monitor Hg. leucocelaenus populations in wild environments, similarly to Ae. aegypti in urban areas [43]. Because they are easy to install and can be evenly distributed in the environment, ovitraps are a method that offers standardized sampling and is particularly useful in monitoring Hg. leucocelaenus in areas with YFV circulation. This finding was previously supported by Alencar et al. [42], who observed that ovitraps were able to collect Hg. leucocelaenus eggs at different heights in the forest and seasons.
Monitoring YFV vector populations is important since there is a degree of periodicity in disease outbreaks, which occur about 1.4 to 2.7 years apart, depending on the type of environment [24]. We found that ovitraps can be effective for monitoring Hg. leucocelaenus populations in the study region, especially in the rainy season. In addition, our data suggest that this season is more important than the dry season for the production and probably hatching of Hg. leucocelaenus eggs in the study region, which can be considered in the elaboration of surveillance programs of YFV.
Supporting information
(TIF)
(XLS)
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
JA - (Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro - FAPERJ; http://www.faperj.br/; grant number E-26/202.658/2018; E-26/010.101076/2018) The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Chadee DD, Ganesh R, Hingwan JO, Tikasingh ES. Seasonal abundance, biting cycle and parity of the mosquito Haemagogus leucocelaenus in Trinidad, West Indies. Med Vet Entomol. 1995; 9: 372–376. doi: 10.1111/j.1365-2915.1995.tb00006.x [DOI] [PubMed] [Google Scholar]
- 2.Mangudo C, Aparicio JP, Rossi GC, Gleiser RM. Tree hole mosquito species composition and relative abundances differ between urban and adjacent forest habitats in northwestern Argentina. Bull Entomol Res. 2018; 108: 203–212. doi: 10.1017/S0007485317000700 [DOI] [PubMed] [Google Scholar]
- 3.Consoli RAGB, Lourenço-de-Oliveira R. Principais mosquitos de importância sanitária no Brasil. 1st ed. Rio de Janeiro: Fiocruz; 1994. Available: https://www.arca.fiocruz.br/bitstream/icict/2708/1/Rotraut_Consoli_Oliveira.pdf [Google Scholar]
- 4.Abreu FVS, Ribeiro IP, Ferreira-de-Brito A, Santos AAC, Miranda RM, Bonelly MSAS, et al. Haemagogus leucocelaenus and Haemagogus janthinomys are the primary vectors in the major yellow fever outbreak in Brazil, 2016–2018. Emerg Microbes Infect. 2019; 8(1): 218–231. doi: 10.1080/22221751.2019.1568180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alencar J, Ferreira de Mello C, Marcondes CB, Guimarães AÉ, Toma HK, Bastos AQ, et al. Natural Infection and Vertical Transmission of Zika Virus in Sylvatic Mosquitoes Aedes albopictus and Haemagogus leucocelaenus from Rio de Janeiro, Brazil. Trop Med Infect Dis. 2021; 6(99): 1–10. doi: 10.3390/tropicalmed6020099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alencar J, Dégallier N, Hannart A, Silva JS, Pacheco JB, Guimarães AE. Circadian and seasonal preferences for hematophagy among Haemagogus capricornii, Hg. janthinomys, and Hg. leucocelaenus (Diptera: Culicidae) in different regions of Brazil. J Vector Ecol. 2008; 33 (2): 389–392. doi: 10.3376/1081-1710-33.2.389 [DOI] [PubMed] [Google Scholar]
- 7.Tátila-Ferreira A, Maia DA, Abreu FVS, Rodrigues WC, Alencar J. Oviposition behavior of Haemagogus leucocelaenus (Diptera: Culicidae), a vector of the wild yellow fever in Brazil. Rev Inst Med Trop S Paulo. 2017; 59: 1–6. doi: 10.1590/S1678-9946201759060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marcondes CB, Alencar J. Revisão de mosquitos Haemagogus Williston (Diptera: Culicidae) do Brasil. Rev Biomed. 2010; 21: 221–238. [Google Scholar]
- 9.Silva SOF, Mello CF, Figueiró R, Maia DA, Alencar J. Distribution of the mosquito communities (Diptera: Culicidae) in oviposition traps introduced into the Atlantic Forest in the state of Rio de Janeiro, Brazil. Vector Borne and Zoonotic Dis. 2018; 18(4): 214–221. doi: 10.1089/vbz.2017.2222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schaeffer B, Mondet B, Touzeau S. Using a climate-dependent model to predict mosquito abundance: application to Aedes (Stegomyia) africanus and Aedes (Diceromyia) furcifer (Diptera: Culicidae). Infection Genetics and Evolution. 2008; 8(4): 422–432. doi: 10.1016/j.meegid.2007.07.002 [DOI] [PubMed] [Google Scholar]
- 11.Campos RE, Sy VE. Variation in the hatching response of Ochlerotatus albifasciatus egg batches (Diptera: Culicidae) in temperate Argentina. Mem Inst Oswaldo Cruz. 2006; 101(1): 47–53. doi: 10.1590/s0074-02762006000100009 [DOI] [PubMed] [Google Scholar]
- 12.Hughes L. Biological consequences of global warming: is the signal already apparent? Trends Eco Evol. 2000; 15(2): 56–61. doi: 10.1016/s0169-5347(99)01764-4 [DOI] [PubMed] [Google Scholar]
- 13.Alencar J, Fereira ZM, Lopes CM, Serra-Freire NM, et al. Biodiversity and times of activity of mosquitoes (Diptera: Culicidae) in the Biome of the Atlantic Forest in the State of Rio de Janeiro, Brazil. J Med Entomol. 2011; 48(2): 223–231. doi: 10.1603/me09214 [DOI] [PubMed] [Google Scholar]
- 14.Cunha MS, Tubaki RM, de Menezes RMT, Pereira M, Caleiro GS, Coelho E, et al. Possible non-sylvatic transmission of yellow fever between non-human primates in São Paulo city, Brazil, 2017–2018. Sci Rep. 2020; 10: 1–11. doi: 10.1038/s41598-019-56847-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wilk-da-Silva R, Mucci LF, Ceretti-Junior W, Duarte AMRC, Marrelli MT, Medeiros-Sousa AR. Influence of landscape composition and configuration on the richness and abundance of potential sylvatic yellow fever vectors in a remnant of Atlantic Forest in the city of São Paulo, Brazil. Acta Trop. 2020; 204: 1–9. doi: 10.1016/j.actatropica.2020.105385 [DOI] [PubMed] [Google Scholar]
- 16.Nascimento KLC, da Silva JFM, Zequi JAC, Lopes J. Comparison Between Larval Survey Index and Positive Ovitrap Index in the Evaluation of Populations of Aedes (Stegomyia) aegypti (Linnaeus, 1762) North of Paraná, Brazil. Environ Health Insights. 2020; 14: 1–8. doi: 10.1177/1178630219886570 PMCID: PMC6945453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Silva-Inacio CL, Paiva AAP, Araújo JMG, Ximenes MFFM. Ecological relationships of Haemagogus spegazzinii (Diptera: Culicidae) in a semiarid area of Brazil. Rev. Soc. Bras. Med. Trop. 2020; 53: 1–8. doi: 10.1590/0037-8682-0502-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stahlhöfer BD, Borsekowsky AR, Müller GA. Mosquitoes (Diptera: Culicidae) in oviposition traps set in forest fragments of a semideciduous seasonal forest, Atlantic Forest domain, in the state of Rio Grande do Sul, Brazil. Rev. Chil. Entomol. 2021; 47 (2): 375–383. doi: 10.35249/rche.47.2.21.20 [DOI] [Google Scholar]
- 19.Prefeitura Municipal Macaé. Secretaria de Ambiente e Sustentabilidade: Sana 2019. [cited 28 Feb 2019]. Available: http://www.macae.rj.gov.br/sema/conteudo/titulo/sana.
- 20.Couto-Lima D, Andreazzi CS, Leite PJ, Bersot MIL, Alencar J, Lourenço-de-Oliveira R. Seasonal population dynamics of the primary yellow fever vector Haemagogus leucocelaenus (Dyar & Shannon) (Diptera: Culicidae) is mainly influenced by temperature in the Atlantic Forest, southeast Brazil. Mem Inst Oswaldo Cruz. 2020; 115: 1–13. doi: 10.1590/0074-02760200218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kenkel NC, Juhász-Nagy, Podani J. On sampling procedures in population and community ecology. Vegetatio. 1989; 83: 195–207. doi: 10.1007/BF00031692 [DOI] [Google Scholar]
- 22.Silver JB. Mosquito ecology: field sampling methods. Springer, New York; 2008. [Google Scholar]
- 23.Alencar J, de Mello CF, Gil-Santana HR, Guimarães AÉ, de Almeida AS, Gleiser RM. Vertical oviposition activity of mosquitoes in the Atlantic Forest of Brazil with emphasis on the sylvan vector, Haemagogus leucocelaenus (Diptera: Culicidae). J Vector Ecol. 2016; 41(1):18–26. doi: 10.1111/jvec.12189 [DOI] [PubMed] [Google Scholar]
- 24.Arnell JH. Mosquito studies (Diptera, Culicidae) XXXII. A revision of the genus Haemagogus. Contrib Am Entomol Inst. 1973; 10: 1–174. [Google Scholar]
- 25.Forattini OP. Culicidologia Médica: Identificação, biologia, epidemiologia. Usp; 2002. [Google Scholar]
- 26.Reinert JF. List of abbreviations for currently valid generic-level taxa in family Culicidae (Diptera). Eur. Mosq. Bull. 2009; 27: 68–76. [Google Scholar]
- 27.Gomes AC. Medidas dos níveis de infestação urbana para Aedes (Stegomyia) aegypti e Aedes (Stegomyia) albopictus em programa de Vigilância Entomológica. Inf Epidemiol Sus. 1998; 7(3): 49–57. doi: 10.5123/S0104-16731998000300006 [DOI] [Google Scholar]
- 28.Marcuzzo FF, Goularte ER. Caracterização do Ano Hidrológico e Mapeamento Espacial das Chuvas nos Períodos Úmido e Seco do Estado do Tocantins. Rev. Bras. de Geogr. Fís. 2013; 6: 91–99. [Google Scholar]
- 29.Cunha MS, Costa AC, Fernandes NCCA, Guerra JM, Santos FCP, Nogueira JS, et al. Epizootics due to Yellow Fever Virus in São Paulo State, Brazil: viral dissemination to new areas (2016–2017). Sci. Rep. 2019; 9: 1–13. doi: 10.1038/s41598-018-37186-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Giovanetti M, de Mendonça MCL, Fonseca V, Mares-Guia MA, Fabri A, Xavier J, et al. Yellow fever virus re-emergence and spread in southeast Brazil, 2016–2019. J Virol. 2019; 94(11): e01623–19. doi: 10.1128/JVI.01623-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tubaki RM, Menezes RMT, Vesgueiro FT, Cardoso RP Jr. Observations on Haemagogus janthinomys Dyar (Diptera: Culicidae) and other mosquito populations within tree holes in a gallery forest in the northwestern region of São Paulo state, Brazil. Neotrop. Entomol. 2010; 39(4): 664–670. doi: 10.1590/s1519-566x2010000400030 [DOI] [PubMed] [Google Scholar]
- 32.Galindo P, Carpenter SJ, Trapido H. A contribution to the ecology and biology of tree hole breeding mosquitoes of Panama. Ann Entomol Soc Am. 1955; 48: 158–164. doi: 10.1093/aesa/48.3.158 [DOI] [Google Scholar]
- 33.Farnesi LC, Vargas HCM, Valle D, Rezende GL. Darker eggs of mosquitoes resist more to dry conditions: Melanin enhances serosal cuticle contribution in egg resistance to desiccation in Aedes, Anopheles and Culex vectors. PLoS Negl Trop Dis. 2017; 11(10): 1–20. doi: 10.1371/journal.pntd.0006063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Evangelista E, Medeiros-Sousa AR, Ceretti-Junior W, Christe R, Wilk R, Duarte AMRC, et al. Relationship between vertical stratification and feeding habits of mosquito (Diptera: Culicidae) assemblages collected in conservation units in the green belt of the city of São Paulo, Brazil. Acta Tropica. 2021; 221(4): 1–12. doi: 10.1016/j.actatropica.2021.106009 [DOI] [PubMed] [Google Scholar]
- 35.Lin YS and Kam YC. Nest Choice and Breeding Phenology of an Arboreal-Breeding Frog, Kurixalus eiffingeri (Rhacophoridae), in a Bamboo Forest. Zool. Stud. 2008; 47(2): 129–137. http://zoolstud.sinica.edu.tw/Journals/47.2/129.pdf [Google Scholar]
- 36.Kerkow A, Wieland R, Koban MB, Hölker F, Jeschke JM, Werner D, et al. What makes the Asian bush mosquito Aedes japonicus japonicus feel comfortable in Germany? A fuzzy modelling approach. Parasites Vectors. 2019; 12(106): 1–17. doi: 10.1186/s13071-019-3368-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mogi M. Phytotelmata: hidden freshwater habitats supporting unique faunas. Freshwater Invertebrates of the Malaysian Region. 2004; 13–22. Available: https://www.researchgate.net/publication/233727086 [Google Scholar]
- 38.Alencar J, Mello CF, Guimarães AE, Gil-Santana HR, Silva JdS, Santos-Mallet JR, et al. Culicidae Community Composition and Temporal Dynamics in Guapiaçu Ecological Reserve, Cachoeiras de Macacu, Rio de Janeiro, Brazil. PLoS One. 2015; 10: 1–10. doi: 10.1371/journal.pone.0122268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Araújo WS, Vieira TM, Souza GA, Bezerra IC, Corgosinho PHC, Borges MAZ. Nocturnal Mosquitoes of Pará State in the Brazilian Amazon: Species Composition, Habitat Segregation, and Seasonal Variation. J Med Entomol. 2020; 57(6): 1913–1919. doi: 10.1093/jme/tjaa103 [DOI] [PubMed] [Google Scholar]
- 40.Galindo P, Trapido H, Carpenter SJ. Observations on diurnal forest mosquitoes in relation to sylvan yellow fever in Panama. Am J Trop Med Hyg. 1950; 30: 533–574. doi: 10.4269/ajtmh.1950.s1-30.533 [DOI] [PubMed] [Google Scholar]
- 41.Trapido H, Galindo P. Mosquitoes associated with sylvan yellow fever near Almirante Panama. Am J Trop Med Hyg. 1957; 6: 114–144 doi: 10.4269/ajtmh.1957.6.114 [DOI] [PubMed] [Google Scholar]
- 42.Alencar J, Morone F, Mello CF, Dégallier N, Lucio PS, Serra-Freire NM, et al. Flight height preference for oviposition of mosquito (Diptera: Culicidae) vectors of sylvatic yellow fever virus near the hydroelectric reservoir of Simplício, Minas Gerais, Brazil. J Med Entomol. 2013; 50: 791–795. doi: 10.1603/me12120 [DOI] [PubMed] [Google Scholar]
- 43.Tikasingh ES, Laurent E. Use of ovitraps in monitoring Haemagogus equinus populations. Mosquito News. 1981; 41(4): 677–679 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(TIF)
(XLS)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.