Abstract
Background.
Adverse Childhood Experiences (ACEs) consistently predict poor mental and physical health as well as early all-cause mortality. Much work examines health harming behaviors that may be used to cope with ACEs associated stress responses and dysregulation. Limited research has been conducted assessing plant-based dietary intake on the ACEs and mortality relationship. We investigate moderators of ACEs and mortality association including plant-based dietary intake.
Objective.
Therefore, the purpose of this study is to examine if the association between ACEs and early mortality is potentially moderated by plant-based dietary intake.
Participants.
An observational, prospective cohort study that included 9,301 Seventh-day Adventists were assessed from 2006 to 2017 in the Biopsychosocial Religion and Health Study (BRHS).
Methods.
We examined the potential impact of plant-based intake frequency on the ACEs and all-cause mortality relationship, while adjusting for potential confounders (e.g., demographics, health risks, mental and physical health) in a cox regression survival analysis.
Results.
ACEs were adversely associated with survival time (HR=2.76, 95% CI: 1.15–6.64). Plant-based intake was associated with a reduction in the association of 4+ ACEs with early mortality (HR = .73, 95% CI: .59–.90) above and beyond demographics, animal-based intake, physical health, mental health, BMI, exercise, and worship. We estimate that after 4+ ACEs, those eating a high versus a low plant-based dietary intake may live 5.4 years longer.
Conclusion.
Plant-based dietary intake may potentially moderate the ACEs and early mortality relationship; however, observational studies cannot determine causality.
Evidence links Adverse Childhood Experiences with chronic diseases that ultimately decrease lifespan by 9.5 to 13 years (1–4). Since the landmark Adverse Childhood Experiences (ACEs) study (5), researchers have associated child abuse (physical, emotional, sexual), neglect, and household dysfunction (caregiver mental illness, addiction, incarceration, domestic violence) to chronic illnesses such as diabetes, depression, addiction, cancer, chronic pulmonary disease, cardiovascular disease, obesity, and a shortened lifespan (5–8). Many posit that the mechanism of action may be health harming behaviors such as high calorie diet, sedentary behavior, smoking, and substance use to cope with dysfunctions in the stress response system that eventually lead to even greater increases in systemic inflammation and ultimately adverse health outcomes (1, 4, 9). Specifically, over or under activation of the body’s stress response system related to dysfunctions in the Hypothalamic Pituitary Adrenal (HPA) axis and Sympathetic Nervous System (SNS) can heighten threat responses and negative emotions (2, 10, 11). Maladaptive coping strategies can be used to compensate for these heightened negative responses. Such maladaptive strategies may include a myriad of health risk behaviors (e.g., substance use, overeating foods with saturated fats and sugars, sedentary lifestyle) to compensate for these faulty HPA axis derived stress responses. Health-harming behaviors used in excess along with a faulty stress response system may lead to inflammatory and metabolic comorbidities as well as early mortality (12–14).
ACEs are associated with health risk behaviors, morbidity, and premature mortality (15–18). However, it is not clear whether health promoting behaviors in response to ACEs’ related dysregulations can moderate the association of ACEs on mortality. For example, there is evidence that religious engagement can lead to healthier lifestyle choices and thereby buffer the association of ACEs with poor mental and physical health outcomes (19–23). There is also evidence of bidirectional positive relationships between health behaviors like diet and exercise and mental health (24–27). If risky health behaviors are associated with poor mental health; it follows that better health behaviors could be associated with better mental and physical health leading to greater longevity. Finally, there is evidence that dietary intake patterns with higher levels of polyphenols (found in high concentrations in plant-based foods) may buffer stress reactivity and depression after ACEs (28). Polyphenols are posited to buffer oxidative damage following chronic stress by decreasing free radicals (ROS-reactive oxygen species) that harm cells and ultimately erode health. Polyphenols in plant-based diets likely have anti-inflammatory and antioxidant properties to protect cells from the oxidative load created after ACEs dysregulate the HPA axis. Conversely, animal-based dietary intake is linked to increased systemic inflammation. Meat consumption, especially red meat, is associated with growth of specific gut microbiota that influence Trimethylamine N-oxide (TMAO) production, a metabolite linked to systemic inflammation (29), cardiovascular disease, and early mortality (30–32). Specifically, processed red meats (ham, sausages, bacon, frankfurters, salami, etc.) that undergo treatments to preserve shelf life (curing, smoking, salting, or chemical additives) are associated with a significantly increased risk of chronic illness, cancer, cardiovascular disease, and early mortality in metanalyses and cohort studies (33, 34).
In sum, ACEs are associated with HPA axis deficits and resulting stress reactivity linked with increased risky health behaviors (e.g., inflammatory diet, sedentary lifestyle, obesity) which may be reasonable, short term coping strategies; however, chronic use is associated with poor mental health, morbidity, and early mortality. In a Seventh-day Adventist community, doctrinal belief encourages consumption of more plant-based foods which may be health promoting after ACEs. Turning towards a high polyphenol/antioxidant rich, plant-based diet may potentially moderate the HPA axis deficits to buffer the risk of early mortality after ACEs. Less plant-based dietary intake may be associated with riskier intake of calorie dense foods such as animal-based foods that may increase oxidative stress while providing short-term stress regulation. Overall, ACEs are harmful to health across the lifespan, however, there may be resilience behaviors associated with less harm. As such, the present investigation tests the following hypotheses:
ACEs will be positively associated with early mortality in a sample of older, Seventh-day Adventists.
The association of ACEs on early mortality will be moderated by plant-based dietary intake.
Plant-based dietary intake will remain a significant moderator in the ACEs and early mortality relationship after controlling for potentially confounding variables including demographics, animal-based dietary intake, physical health, mental health, exercise, BMI, and worship attendance.
Methods
Participants and Procedures.
The data were from the Biopsychosocial Religion and Health Study (BRHS), a substudy of the Adventist Health Study-2 (AHS-2) cohort study that investigated lifestyle and health in Black and White Seventh-day Adventists (SDAs; 35). The methods for the BRHS are described in Lee, Morton (36). In brief, after IRB approval, the AHS-2 sampled approximately 96,000 SDAs across Canada and the United States with a 50-page diet and medical history questionnaire between 2001 and 2006. A random sample of approximately 21,000 of these participants were sent a 20-page, wave-one BRHS questionnaire in 2006–7. Of the 10,869 who returned a completed questionnaire, 67.7% were female; 59.7% were White, 34.1% were Black, and 6.2% were of another ethnicity. The proportion of each ethnic group was the result of the baseline AHS-2 oversampling Blacks; we excluded the small number of other ethnicities from further analyses. Including our control variables, we had 17 variables of interest and included only cases missing <10% of primary variables (ACEs, plant-based dietary intake, animal-based dietary intake, kilocalories, age, mortality, age at death, gender, ethnicity, education, difficulty meeting expenses for basic needs in the last year and when under age 18 years, physical health, mental health, body mass index (BMI), exercise, and worship attendance) resulting in 9,301 cases for analysis. We used 42 auxiliary variables with cases that had <20% missing data for multiple imputation (e.g., variables relating to all aspects of mental health, religious engagement, job stress and satisfaction, marital quality, discrimination, alcohol consumption, smoking, physical illness symptoms and comorbid conditions). The 1,568 participants excluded differed from those included as being older, less educated, more likely to be female or Black, and having more difficulty meeting basic expenses in the last year and when under age 18.
Measures
Adverse Childhood Experiences (ACEs).
ACEs research has varied in the numbers of ACEs categories included from seven to ten (5, 7, 37). Most researchers include child abuse (psychological, physical, or sexual), neglect, and household dysfunction (household substance abuse, parental mental illness, mother treated violently, family member imprisoned, and parental divorce). Our ACEs assessment in BRHS wave 1 (2006–7) omits parental mental illness and incarceration. Therefore, seven ACEs were assessed before age 15 years: (a) psychological abuse (2 items; 38), (b) physical abuse (4 items; 38), (c) sexual abuse before age 13 (1 item; 39), (d) living with a substance abuser (1 item; 5), (e) family member treated violently (1 item; 40), (f) neglect (1 item; 38), and (g) parental separation or divorce (1 item; 40). For each, a score of 1 was given if any response indicated an ACE occurred; else 0. Specifically, if the Likert scale response on any item within the ACEs category was rated as anything other than “Never”, the ACE was assumed to have occurred and was counted as 1 in that particular ACEs category. This resulted in a 0–7-point, ACEs score which we further categorized into zero, 1 to 3, or 4 or more. Researchers have concluded that a typical cut point for negative consequences after ACEs is four or more (41) since most report some form of trauma by adolescence (42).
Dietary Intake.
There were 13 questions related to dietary intake in BRHS (e.g., “Fruits of any kind? Include frozen, canned or dried fruits, as well as raw or cooked fruits when they are in season” and “Red meats (steak, hamburgers, sausage, organ meats, etc.)?” Individuals rated how often they consumed each dietary component on an 8-point scale: Never or rarely; 1–3 times per month; 1, 2 to 4, or 5 to 6 times per week; or 1, 2 to 3, or 4+ times per day. These items were factor analyzed using the program Factor—version 10.10.01 (43). Parallel Analysis based on minimum rank factor analysis (44) suggested two factors which were extracted using Robust Unweighted Least Squares, and rotated using Robust Promin (45). Two independent dietary factors (r=−.26) emerged: (a) plant-based dietary intake including fruits, vegetables, nuts, and legumes; and (b) animal-based dietary intake including turkey or chicken, red meats, fish, and dairy (see Table 1 for factor loadings). For data analysis, we computed factor scores using Ferrando and Lorenzo-Seva’s approach to Bayes’ expected a posteriori estimation method (46). This created scale scores with a mean of 50 and a standard deviation of 10. The Adventist Health Study-2 has a more extensive list of foods they sampled in their 50-page nutrition and lifestyle questionnaire that were used to classify individuals into five dietary pattern types--vegan, lacto-ovo vegetarian, pesco-vegetarian, semi-vegetarian, and nonvegetarian (47). Since our participants had completed the AHS-2 questionnaire 0 to 6 years before completing the BRHS questionnaire, we used that earlier classification to validate our factor scores. Table 2 shows the means on the plant-based intake and animal-based intake factor scores for the five dietary patterns. All factor scores are as expected—vegan, lacto-ovo, and pesco-vegetarians have scores on plant-based intake above the mean of 50 and below the mean on animal-based intake, and pesco-vegetarians eat more animal-based foods than the other two vegetarian types. Semi-vegetarians are very close to the mean on both plant and animal-based intake while nonvegetarians are the highest on animal-based dietary intake.
Table 1.
Factor Analysis of Dietary Intake Components
| Variable | Factor Loadings |
|
|---|---|---|
| Plant Intake | Animal Intake | |
| Other leafy green vegetables | 0.77 | 0.09 |
| Broccoli, cabbage, etc | 0.69 | 0.11 |
| Beans | 0.51 | −0.10 |
| Nuts | 0.49 | −0.19 |
| Fruits | 0.48 | −0.15 |
| Turkey or chicken | 0.02 | 0.88 |
| Red meats | −0.06 | 0.69 |
| Fish | 0.13 | 0.61 |
| Dairy-based cheeses | 0.06 | 0.37 |
| Butter on bread | 0.11 | 0.29 |
| Vegetarian protein foods | 0.21 | −0.28 |
| Soy milks | 0.24 | −0.38 |
Note: Extraction using robust unweighted least squares and rotated using robust promin (Lorenzo-Seva & Ferrando, 2019).
Table 2.
Relationship of current dietary intake factor score to primary diet determined from Adventist Health Study-2 measured 0 to 6 years earlier.
| Primary Diet Type | N | Plant Intake Factor Score (T Score) |
Animal Intake Factor Score (T Score) |
||||
|---|---|---|---|---|---|---|---|
| Mean | 95% CI |
95% CI |
|||||
| Lower | Upper | Mean | Lower | Upper | |||
| Vegan | 782 | 53.83 | 53.14 | 54.51 | 41.17 | 40.91 | 41.43 |
| Lacto-ovo | 2928 | 52.24 | 51.88 | 52.60 | 42.75 | 42.60 | 42.91 |
| Pesco | 978 | 53.14 | 52.50 | 53.79 | 45.70 | 45.32 | 46.07 |
| Semi-vegetarian | 475 | 49.03 | 48.11 | 49.96 | 49.41 | 48.74 | 50.07 |
| Nonvegetarian | 4138 | 46.97 | 46.63 | 47.30 | 58.25 | 57.97 | 58.53 |
| Total | 9301 | 49.96 | 49.71 | 50.21 | 50.16 | 49.94 | 50.39 |
Note: Classification into the five diet types from Orlich and Fraser (47).
We also used kilocalorie intake based on the AHS-2 diet reports as a control variable as simply eating more food could artificially increase your scores on the intake variables. For the cox regression analyses we divided this by 1,000 to make it easier to interpret the hazard ratios and their confidence limits for kilocalories.
Mortality.
Participants were followed from the time of survey completion to death. All-cause mortality and date of death were determined by matching personal identifiers with the National Death Index through December 31, 2017. This information was converted into two variables for use in Cox regression—a dichotomous variable indicating whether or not the person had died and a continuous variable indicating age at death for those who had died. There were 1,328 deaths and the mean age at death for those 1,328 was 81.8 years.
Control Variables.
In addition to demographic controls of gender (male, female), ethnicity (Black, White) and difficulty meeting expenses for basic needs at two time points, we also included five health risks: physical health, mental health, exercise, worship attendance, Body Mass Index (BMI; kg/m2) based on self-reported height and weight.
Physical and Mental Health.
These two variables were composite variables calculated from the 12 items of version 2 of the Medical Outcomes Study SF-12v2 quality of life scale (48). This scale is widely used and has been validated across samples of adults (49–51).
Difficulty Meeting Expenses.
Based on Pudrovska, Schieman (52), individuals were asked “On average how difficult was it for your family to meet expenses for basic needs like food, clothing, and housing in each of the following time periods?” “When you were under 18” and “In the last year.” Responses were Not at all, A little, Somewhat, Fairly, and Very.
Exercise Hours per Week.
Exercise was assessed with two items: (a) “How many times per week do you usually engage in regular vigorous activities, such as brisk walking, jogging, bicycling, etc., long enough or with enough intensity to work up a sweat, get your heart thumping, or get out of breath?” (never to 6 or more times/week), (b) “On average, how many minutes do you exercise each session?” (None to >1 hour). The number of sessions was multiplied by the mid-point of each of the 8 time periods as in Singh, Tonstad (53) and the resulting minutes divided by 60.
Religious engagement.
Worship attendance was based on a single item from the Duke University Religion Index (54) rated on a 6-point scale (Never to More than once/week).
Data Analysis.
Analyses were conducted with SPSS v27. Items likely to be correlated with key variables were retained as “auxiliary” variables (55) and then multiple imputation was applied to produce 5 imputed datasets.
The hazard of mortality at each age was regressed on the variables of interest using the Cox proportional hazards model (SPSS COX). All variables in the final model were assessed with respect to meeting the requirements of proportional hazard assumptions by checking the log [-log(survival)] curves against the log time variable, and four variables violated this assumption: ethnicity, difficulty meeting expenses when the respondent was under age 18, ACEs, and worship attendance. The interactions of each of these variables with the time-dependent covariate (e.g., proportional hazard of dying at each age) were included in the Cox regression analyses to control for this violation. Additionally, all variables were assessed for multicollinearity, prior to inclusion in the final models. Lastly, all confounders and our exposure of interest variables are fixed across time because they are measured at a single point in time in our study.
Results
Description of Participants
The BRHS study cohort consisted of 9,301 individuals. Table 3 shows that in the 2006–2007 collection period the majority of our sample were female and White, about 60 years old, and had no trouble meeting expenses in the last year. More reported having difficulty meeting expenses in their family when they were under 18. They tended to report eating plant-based more often than animal-based foods and more were overweight and obese than were normal or underweight. About 90% attended worship services once a week or more.
Table 3.
Differences on study variables by three levels of Adverse Childhood Experiences (N = 9301)
| Zero ACEs (n = 3620, 38.9%) |
1 to 3 ACEs (n = 4616, 49.6%) |
4 or more (n = 1065, 11.5%) |
All (n = 9301) |
p | p linear | p quadratic | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 95% CI |
95% CI |
95% CI |
95% CI |
||||||||||||
| Mean | Lower | Upper | Mean | Lower | Upper | Mean | Lower | Upper | Mean | Lower | Upper | ||||
| Demographics | |||||||||||||||
| Age | 62.99 | 62.54 | 63.44 | 59.82 | 59.44 | 60.20 | 57.09 | 56.40 | 57.78 | 60.74 | 60.47 | 61.01 | .001 | .001 | .438 |
| Educationa | 6.06 | 6.00 | 6.12 | 5.75 | 5.69 | 5.81 | 5.43 | 5.32 | 5.54 | 5.83 | 5.79 | 5.87 | .001 | .001 | .841 |
| Difficulty meeting expensesb | |||||||||||||||
| in last year | 1.42 | 1.39 | 1.45 | 1.58 | 1.55 | 1.61 | 1.78 | 1.71 | 1.85 | 1.54 | 1.52 | 1.56 | .001 | .001 | .592 |
| when under 18 | 2.44 | 2.40 | 2.48 | 2.79 | 2.75 | 2.83 | 3.34 | 3.25 | 3.43 | 2.72 | 2.69 | 2.75 | .001 | .001 | .004 |
| Diet | |||||||||||||||
| Plant Intakec | 50.57 | 50.21 | 50.92 | 49.67 | 49.33 | 50.01 | 49.16 | 48.49 | 49.83 | 49.96 | 49.71 | 50.21 | .001 | .001 | .428 |
| Animal Intakec | 48.83 | 48.52 | 49.15 | 50.67 | 50.36 | 50.97 | 52.51 | 51.83 | 53.19 | 50.16 | 49.94 | 50.39 | .001 | .001 | .853 |
| Kilocalories | 1883 | 1853 | 1912 | 1942 | 1916 | 1968 | 2000 | 1934 | 2067 | 1926 | 1904 | 1947 | .001 | .001 | .930 |
| Health Risk Controls | |||||||||||||||
| SF-12 Physicalc | 48.63 | 48.26 | 48.99 | 48.25 | 47.93 | 48.57 | 47.44 | 46.75 | 48.13 | 48.30 | 48.07 | 48.53 | .008 | .003 | .404 |
| SF-12 Mentalc | 53.92 | 53.65 | 54.19 | 52.10 | 51.84 | 52.36 | 49.58 | 48.95 | 50.21 | 52.52 | 52.34 | 52.71 | .001 | .001 | .104 |
| BMI | 26.45 | 26.28 | 26.63 | 27.51 | 27.34 | 27.68 | 28.60 | 28.20 | 29.00 | 27.22 | 27.10 | 27.35 | .001 | .001 | .894 |
| Exercise Hrs/Wk | 1.33 | 1.28 | 1.38 | 1.26 | 1.22 | 1.31 | 1.43 | 1.33 | 1.52 | 1.31 | 1.28 | 1.34 | .003 | .075 | .001 |
| Worship Attendanced | 5.27 | 5.25 | 5.30 | 5.28 | 5.26 | 5.30 | 5.19 | 5.13 | 5.25 | 5.27 | 5.25 | 5.28 | .004 | .010 | .015 |
| Dichotomous Demographics | % | n | % | n | % | n | n | ||||||||
|
| |||||||||||||||
| Female | 63.8% | 2310 | 66.9% | 3086 | 74.2% | 790 | 66.5% | 6186 | .001 | ||||||
| White | 73.8% | 2671 | 58.4% | 2698 | 60.2% | 641 | 64.6% | 6010 | .001 | ||||||
1=Grade School to 9 = Doctoral Degree
1=Not at all to 5=Very
T score (Mean 50, SD 10)
1=Never, 6=More than once a week
Linear and nonlinear associations of ACEs with study variables.
In our sample, 49.5% reported at least one ACE, and 11.5% reported 4 or more ACEs. There were significant differences in the study variables across the three levels of ACEs (see Table 3). Almost all the relationships were linear. In 2006–2007 individuals with more ACEs were younger, less educated, had more difficulty meeting expenses in the last year and when under 18, lower plant-based intake but more animal-based intake and consumed more kilocalories. As ACEs increased physical and mental health scores were lower and BMI higher. However, two relationships were not linear and can be explicated by examining the confidence intervals for each group and by examining the p value for quadratic effects. Hours of exercise dropped from the zero to the 1 to 3 ACEs groups but then went up again in the 4 or more ACEs group. There was also an interesting nonlinear pattern for worship attendance. Attendance was basically the same for the zero and 1 to 3 ACEs groups but then dropped for the 4 or more ACEs group.
Since there was a linear association of age with number of ACEs one possibility is that many of the linear associations of ACEs with the other variables in table 3 were due to confounding with age. Therefore, we tested all associations with ACEs after controlling for age (not shown). Controlling for age slightly reduced the p value for the linear association of plant-based intake with ACEs (from p = .001 to .029) however, with two exceptions, controlling for age had no effect on the relationship of other variables with age. The two exceptions were exercise and worship attendance; controlling for age eliminated any linear association but strengthened the quadratic associations for these two variables. It should be noted that controlling for age in the Cox regression we report in table 4 in the next section would be inappropriate since in Cox regression the outcome variables are mortality and age at death.
Table 4.
Relationship of Diet and ACEs to Age at Death and Survival (Cox Regression)
| Model 1 | Model 2 | Model 3 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Demographics, ACEs & Diet |
+ ACEs × Diet Interactions |
+ Lifestyle Controls |
||||||||||
| Hazard Ratio | 95% CI |
p | Hazard Ratio | 95% CI |
p | Hazard Ratio | 95% CI |
p | ||||
| Lower | Upper | Lower | Upper | Lower | Upper | |||||||
| Demographics | ||||||||||||
| Male | 1.31 | 1.16 | 1.49 | .001 | 1.31 | 1.16 | 1.48 | .001 | 1.44 | 1.28 | 1.63 | .001 |
| Black | 2.36 | 0.94 | 5.98 | .069 | 2.32 | 0.92 | 5.87 | .075 | 3.11 | 1.21 | 8.01 | .019 |
| Educationa | 0.95 | 0.93 | 0.98 | .001 | 0.95 | 0.93 | 0.98 | .001 | 0.96 | 0.93 | 0.98 | .002 |
| Difficulty Meeting Basic Needsb | ||||||||||||
| Last year | 1.14 | 1.08 | 1.22 | .001 | 1.14 | 1.08 | 1.22 | .001 | 1.10 | 1.03 | 1.17 | .004 |
| When under 18 | 0.97 | 0.92 | 1.02 | .184 | 0.97 | 0.92 | 1.02 | .192 | 0.96 | 0.92 | 1.01 | .081 |
| ACEs Contrasted with Zero ACEs | ||||||||||||
| 1 to 3 ACEs | 2.76 | 1.15 | 6.64 | .023 | 2.93 | 1.21 | 7.08 | .017 | 2.51 | 1.03 | 6.09 | .042 |
| 4 or more ACEs | 3.97 | 0.94 | 16.84 | .061 | 2.95 | 0.66 | 13.21 | .157 | 2.22 | 0.49 | 10.03 | .300 |
| Diet | ||||||||||||
| Plant Intakec | 0.92 | 0.86 | 0.98 | .017 | 0.86 | 0.79 | 0.94 | .001 | 0.89 | 0.82 | 0.97 | .006 |
| Animal Intakec | 1.11 | 1.04 | 1.18 | .002 | 1.10 | 1.01 | 1.19 | .025 | 1.02 | 0.94 | 1.11 | .603 |
| Thousand Kilocalories | 1.05 | 0.98 | 1.14 | .174 | 1.06 | 0.98 | 1.14 | .166 | 1.04 | 0.97 | 1.13 | .281 |
| Diet × ACEs Interactions | ||||||||||||
| Plant Intake × | ||||||||||||
| 1 to 3 ACEs | 0.94 | 0.81 | 1.10 | .439 | 0.95 | 0.82 | 1.11 | .540 | ||||
| ≥4 ACEs | 0.73 | 0.59 | 0.90 | .003 | 0.74 | 0.60 | 0.91 | .004 | ||||
| Animal Intake | ||||||||||||
| × 1 to 3 ACEs | 0.90 | 0.79 | 1.03 | .131 | 0.90 | 0.79 | 1.03 | .127 | ||||
| ≥4 ACEs | 0.89 | 0.72 | 1.11 | .299 | 0.87 | 0.70 | 1.08 | .219 | ||||
| Health risk controls | ||||||||||||
| SF-12 Physical Healthc | 0.81 | 0.74 | 0.87 | .001 | ||||||||
| SF-12 Mental Healthc | 0.92 | 0.85 | 1.00 | .043 | ||||||||
| BMI | 1.02 | 1.01 | 1.04 | .004 | ||||||||
| Exercise (Hours per week) | 0.96 | 0.91 | 1.02 | .146 | ||||||||
| Worship Attendanced | 0.45 | 0.30 | 0.68 | .001 | ||||||||
Note: In models 2 and 3 the ethnicity and ACEs variables violated the proportional hazards assumption. In model 3 worship attendance also violated this assumption. Thus, in these analyses, we controlled how these variables modified the proportional hazard of dying across age.
1=Grade School to 9 = Doctoral Degree
1=Not at all to 5=Very
Standard Deviation Units (Mean=0, SD=1)
1=Never, 6=More than once a week
ACEs, Mortality, and Healthy Diet
Table 4 reports three Cox regression models predicting mortality. For these analyses we converted the T scores on plant-based intake, animal-based intake, physical health, and mental health to standard deviation units with a mean of zero and standard deviation of 1. This was done to make it easier to explicate the HR and HR confidence intervals across the different models. While these transformations did not change the significance values, they made it easier to see how the HRs for these variables differed from 1 where there was a difference. Model 1 examines the demographic controls, ACEs, and main effects of the two dietary intake factors. Higher education was protective; being male or having difficulty meeting expenses in the last year were risks. Having four or more ACEs was associated with a higher hazard ratio. Plant-based dietary intake was protective and animal-based dietary intake was a risk.
Model 2 adds the interaction of ACEs and dietary intake. The relationships found in Model 1 were stronger. What is notable, however, is that there was an interaction of plant-based dietary intake and having 4 or more ACEs potentially indicating the plant-based dietary intake was protective (see Figure 1).
Figure 1. Plant-Based Intake Moderates the Association of Adverse Childhood Experiences with Mortality.
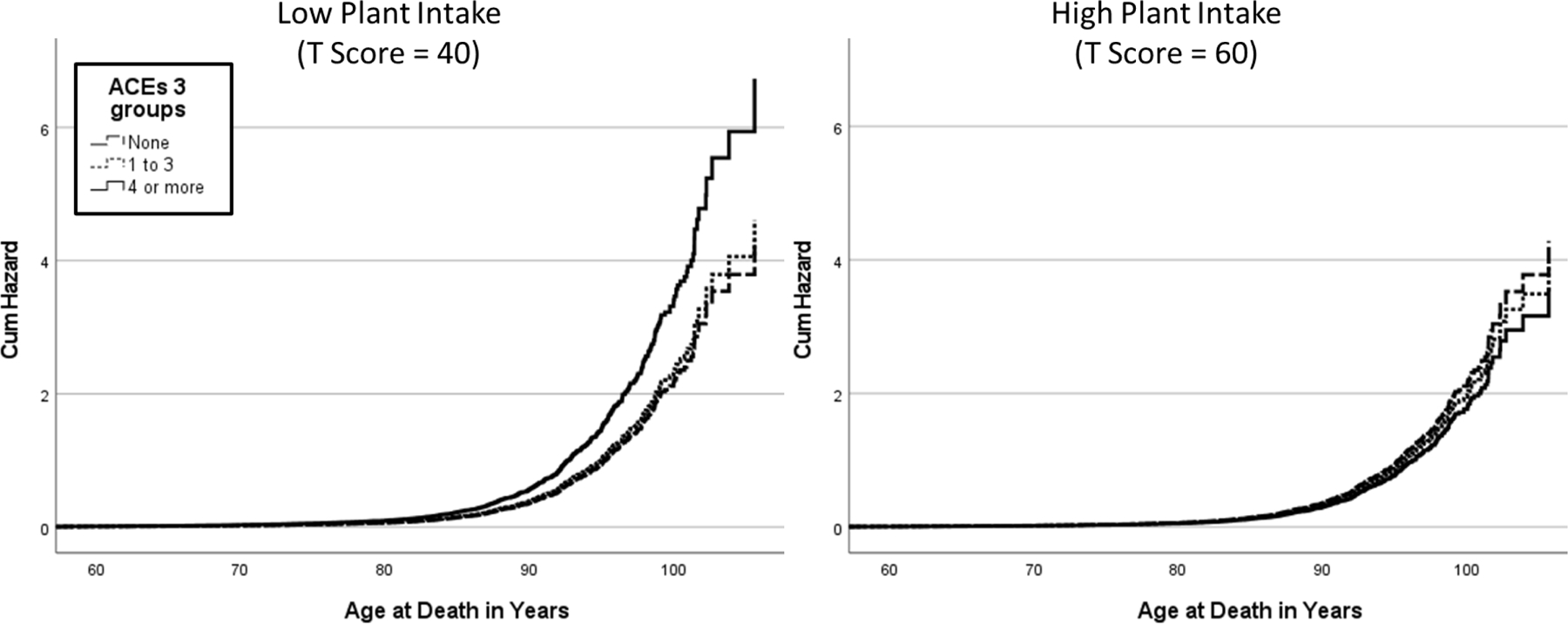
Note. High and low plant intake factor score as one standard deviation above or below the mean.
Model 3 adds four health risk controls that could be confounding variables in the association we are testing. Physical and mental health and worship attendance were all associated with a lower hazard ratio; however, worship attendance was associated with the smallest hazard ratio. BMI was associated with a greater hazard ratio while exercise showed no association with the hazard ratio. While adding these controls eliminated the association of animal-based dietary intake with mortality risk, it is notable that these controls had no effect on the association of plant-based dietary intake with ACEs or on the interaction of plant-based dietary intake with ACEs in the reduction of the hazard ratio.
To better understand how the health risk controls were involved we did an additional analysis adding the interactions of each health risk variable with ACEs (not shown). The addition of these interactions did not appreciably change any of the relationships seen in Model 3. Although the interaction of plant-based dietary intake with ≥4 ACEs hazard ratio was reduced slightly it remained significant in the model after these five additional interaction terms were entered—HR= .88, (95% CI: .81, .96), p = .005). The only significant interaction of the four was with physical health and ≥4 ACEs (p = .033); better physical health slightly reduced the association of ≥4 ACEs with mortality.
A Plant-Based Diet and Age of Death
To better understand how plant-based dietary intake related to actual age of death we split the sample at below and above the mean (mean=50) on plant intake and then, using the generalized linear model, predicted mean age at death for high and low plant intake versus three levels of ACEs. The results are in table 5. This analysis paralleled the analysis for model two in table 4 but with the dichotomous categorical variable substituting for plant intake. Since this analysis only included those who had died, the sample size and power were considerably reduced. As ACEs increased the drop in mean age at death was considerably more for those with low plant intake than for those with high plant intake. We carried out pairwise comparisons for each of the pair of means in Table 5 using the sequential Sidak method to control for multiple comparisons. Examining just the means in the low plant intake column, individuals with four or more ACEs died younger (73.2 years) than those with 1 to 3 ACEs (78.5 years; p = .003) or those with no ACEs (82.4 years; p < .001). None of the means in the high plant intake column differed significantly from each other for age at death.
Table 5.
Mean Age at death by Plant Intakea and Adverse Childhood Experiences (n = 1328)
| Low Plant Intake (< 50) |
High Plant Intake (<=50) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Mean | 95% CI |
n | Mean | 95% CI |
n | |||
| Lower | Upper | Lower | Upper | |||||
| ACEs | ||||||||
| None | 82.4 | 81.1 | 83.8 | 268 | 82.6 | 81.3 | 83.9 | 365 |
| 1 to 3 | 78.5 | 77.1 | 79.9 | 290 | 79.3 | 77.9 | 80.7 | 292 |
| 4 or more | 73.2 | 70.4 | 75.9 | 69 | 78.6 | 75.0 | 82.2 | 44 |
Plant intake was a factor score set as a T score with mean = 50 and SD = 10.
Note: Mean values are controlling for gender, ethnicity, difficulty meeting expenses in the past year and when under age 18, meat intake and kilocalorie consumption.
Discussion
Our models examine plant-based and animal-based dietary intake interactions with three levels of ACEs exposure (none, 1–3 and 4+ ACEs). We concur with other researchers that ACEs are associated with earlier mortality above and beyond demographic controls (15). In a novel examination of dietary intake, our findings suggest that plant-based intake may potentially moderate the ACEs and early mortality risk relationship. The interaction of plant-based dietary intake with ACEs; even after adjusting for other known health risk factors, potentially moderated the association of ACEs with early mortality in the most vulnerable, ≥4 ACEs group. This finding suggests a possible resiliency pathway after early trauma exposure though causality cannot be determined in an observational cohort study. Much further research is needed to understand the mechanism that underlies this association. Other variables also associated with plant-based dietary intake may also associated with the study findings that were not controlled in the model tested.
These study findings contribute to our understanding of plant-based dietary intake as a health behavior that may potentially moderate the association between early mortality and four or more ACEs. Though other studies have found that ACEs do predict the risk of early mortality (2, 4, 5, 16, 19) and obesity (10, 14); only one other study examined ACEs and any fruit and vegetable intake (12). The current findings add to this literature by focusing on diet as a health behavior after ACEs in association with early mortality risk in a large observational cohort study. This study has several strengths including a large cohort of Adventists from North America that allows findings to be generalized to other groups of religious adults. In addition, we employed validated measures of each construct of interest. Finally, we have standardized National Death Index data to track our outcome of mortality.
This study is driven by Felitti’s Adverse Childhood Experiences theory (5) that has revolutionized research on childhood adversity exposures. Felitti posits that after ACEs a survivor’s brain is structurally changed to disrupt neurodevelopment in the HPA axis causing stress reactivity dysregulation that impairs psychosocial functioning (see ACEs Pyramid of Lifespan Impact). Psychosocial impairments may then increase health risk behaviors (high calorie diets, sedentary behavior, obesity, substance use) to cope in the short term with mental health symptoms (6, 56). These health risk behaviors may be initially adaptive, but overreliance can result in early morbidity and mortality (14). Two recent ACEs research reviews posit that ACEs research has changed our conceptualization of trauma. We recognize now that trauma is pervasive, has a dose response relationship with lifelong negative outcomes, especially after four or more ACEs (41, 42). What is needed is further work to explain the mechanisms that may underlie these relationships.
We propose a possible inflammatory mechanism that derives from disruptions in the HPA axis that could possibly be moderated by plant-based dietary intake. Though ACEs may act synergistically (e.g., sexual abuse coupled with any other abuse has exponentially greater harm than any ACE alone); protective factors are of interest to help us understand such long term and distal outcomes as early mortality. We studied one potential protective factor, plant-based dietary intake though there are clearly other potential protective factors that we have not examined here.
Few researchers examine health behaviors after ACEs in relation to health outcomes. The earliest paper on ACEs includes Felitti’s observations that Kaiser patients in a weight loss program typically regained weight because they perceived their diet choices and resulting morbid obesity as aiding in the management of emotional distress and social concerns after child abuse during critical periods of development (57). Others have examined obesity, comorbidities, smoking and substance use disorders after ACEs and conclude that health risk behaviors follow ACEs in a dose response relationship particularly after four or more ACEs (1, 2, 4, 9, 12, 58–61). However, few examine specific diet intake behaviors in relation to risks for early mortality after ACEs. Determining whether a health behavior is associated with resilience after the ravages of ACEs related deficits could lead to cost effective interventions. Since 11.5% of this sample reported four or more ACEs, such an intervention could potentially be related to less health-related risks after ACEs for many individuals. This investigation of this cohort of older Adventists who have low rates of smoking (.6%) and alcohol use (3.8%) allows us to focus on dietary intake as a potential resilience behavior associated with ACEs and mortality six to seven years later (excluding participants who smoked or drank did not change the outcomes and are not shown). We go beyond the typical examination of more than one fruit or vegetable a day and include overall plant-based food intake. Plant-based food intake that is low in fat and sugar and high in fiber could theoretically improve multiple health related factors including the gut microbiome, oxidative stress, and inflammation and these factors should be examined further in future research (62).
SDAs demonstrate longevity and our academic center is located in a “blue zone” of longevity (63). SDAs like other denominations who attend worship services frequently live longer than the general U.S. population (64). In addition, SDA doctrinal beliefs like some other fundamentalist denominations promote healthy lifestyle choices including exercise, worship attendance, and avoiding of substance use (65). SDAs also have a doctrinal belief to avoid animal-based dietary intake and this makes them a unique study sample. The findings here may most readily generalize to other religious groups; however, the efficacy of plant-based dietary intake on moderating the association between four or more ACEs on longevity should be examined in other non-religious samples that have a wider range of dietary intake patterns (66).
These findings demonstrate that plant-based dietary intake be associated with the relationship between ACEs and early mortality in the ≥4 ACEs group. Theoretically we posit that plant-based intake may operate via polyphenols that may have antioxidant properties as in our previous work (28). In the Tan et al. (1) study, we found that flavonoid consumption mediated the relationship of ACEs and depression when stress reactivity was also high. The present study extends this work to examine ACEs, plant-based intake and early mortality and suggests plant-based intake may be associated with stress reactivity, depression and a change in the association between ACEs and early mortality risk. Support for this interpretation includes the finding that plant-based intake remained significant after controlling for other potentially correlated risks of early mortality including mental and physical health as well as other lifestyle behaviors of exercise, BMI, and worship.
BRHS studies indicate mental health is associated with health behaviors like exercise, diet, and religious engagement (25, 27, 67). It is established that ACEs likely disrupts both mental health and health behaviors, however, in this model plant-based intake remained significant in the ACEs and mortality model beyond controls for these accepted risk factors. Morton, Lee (21) found that religious engagement in Adventists led to both mental health and indirectly to plant-based intake to predict longevity. As such, how mental health, physical health, and health behaviors (that may include religious engagement in some groups) is associated with the ACEs and mortality relationship should be examined further.
This study is limited by the religious SDA and higher SES sample. Though this observational cohort study allows us to focus our investigation on diet with the natural controls of participants avoiding substance use, it may be less generalizable to nonreligious and lower income populations. In addition, though this sample includes a wide range of SES levels during childhood, the current higher SES levels of study participants may indicate potentially easier access to plant-based foods than lower SES samples. Since, except for mortality assessment, all variables are self-reported, our ability to make causal inferences about these associations is limited. Also, such self-reports may include a social desirability bias as well as a retrospective bias for ACEs reports that may be under or overreported which may have weakened our associations (68). Other important variables may be missing from the analysis that limit our understanding of the associations including racism and community violence exposures. Further, we do not examine personality or other aspects of the environment that may be important in determining who pursues a plant-based diet after ACEs exposure and why. Though we do examine animal-based dietary intake, this sample has a much lower animal-based intake than the U.S. population. In addition, SDAs may have higher levels of plant-based intake which can restrict the range of intake. Therefore, dietary intake relationships may be restricted, and the relationships may be stronger in other samples.
Conclusions
Overall, these findings are promising and suggest plant intake may be associated with the ACEs and early mortality risk relationship. We posit that plant-based dietary intake may work by decreasing inflammation associated with ACEs exposure in the most vulnerable group reporting four or more ACEs. Our findings, together with those of Tan, Morton (28) suggest plant-based intake could have antioxidant properties associated with wear and tear on the body. Future studies, especially dietary clinical trials, are needed to confirm our findings and expand to include assessing for potential associations in other groups to understand the link between ACEs, diet, mental health, and longevity.
Highlights.
Adverse Childhood Experiences (ACEs) predict early mortality
Plant-based dietary intake moderates the ACEs and early mortality relationship
After 4+ ACEs, those with high vs. low plant-based intake lived 4.6–6.3 years longer
ACEs and diet predict mortality after controlling for mental and physical health
Acknowledgements:
This research was supported by grants from the National Institute on Aging (Biopsychosocial Religion and Health Study, Grant 1R01AG026348), National Cancer Institute for the parent study (Adventist Health Study-2, Grant 5R01CA094594), and Ardmore Health Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bellis MA, Hughes K, Leckenby N, Hardcastle KA, Perkins C, Lowey H. Measuring mortality and the burden of adult disease associated with adverse childhood experiences in England: a national survey. J Public Health (Oxf). 2015;37(3):445–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Castagne R, Gares V, Karimi M, Chadeau-Hyam M, Vineis P, Delpierre C, et al. Allostatic load and subsequent all-cause mortality: which biological markers drive the relationship? Findings from a UK birth cohort. Eur J Epidemiol. 2018;33(5):441–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jia H, Lubetkin EI. Impact of adverse childhood experiences on quality-adjusted life expectancy in the U.S. population. Child Abuse Negl. 2020;102:104418. [DOI] [PubMed] [Google Scholar]
- 4.Lee LO, Aldwin CM, Kubzansky LD, Mroczek DK, Spiro A. The long arm of childhood experiences on longevity: Testing midlife vulnerability and resilience pathways. Psychol Aging. 2019;34(7):884–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Felitti VJ, Anda RF, Nordenberg D, Williamson DF, Spitz AM, Edwards V, et al. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults: The Adverse Childhood Experiences (ACE) Study. Am J Prev Med. 1998;14(4):245–58. [DOI] [PubMed] [Google Scholar]
- 6.Anda RF, Felitti VJ, Bremner JD, Walker JD, Whitfield C, Perry BD, et al. The enduring effects of abuse and related adverse experiences in childhood - A convergence of evidence from neurobiology and epidemiology. Eur Arch Psychiatry Clin Neurosci. 2006;256(3):174–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dong M, Anda RF, Felitti VJ, Dube SR, Williamson DF, Thompson TJ, et al. The interrelatedness of multiple forms of childhood abuse, neglect, and household dysfunction. Child Abuse Negl. 2004;28(7):771–84. [DOI] [PubMed] [Google Scholar]
- 8.Edwards VJ, Holden GW, Felitti VJ, Anda RF. Relationship Between Multiple Forms of Childhood Maltreatment and Adult Mental Health in Community Respondents: Results From the Adverse Childhood Experiences Study. Am J Psychiatry. 2003;160(8):1453–60. [DOI] [PubMed] [Google Scholar]
- 9.Campbell JA, Walker RJ, Egede LE. Associations Between Adverse Childhood Experiences, High-Risk Behaviors, and Morbidity in Adulthood. Am J Prev Med. 2016;50(3):344–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lu S, Gao W, Huang M, Li L, Xu Y. In search of the HPA axis activity in unipolar depression patients with childhood trauma: Combined cortisol awakening response and dexamethasone suppression test. J Psychiatr Res. 2016;78:24–30. [DOI] [PubMed] [Google Scholar]
- 11.Wingenfeld K, Kuehl LK, Boeker A, Schultebraucks K, Ritter K, Hellmann-Regen J, et al. Stress reactivity and its effects on subsequent food intake in depressed and healthy women with and without adverse childhood experiences. Psychoneuroendocrinology. 2017;80:122–30. [DOI] [PubMed] [Google Scholar]
- 12.Bellis MA, Hardcastle K, Ford K, Hughes K, Ashton K, Quigg Z, et al. Does continuous trusted adult support in childhood impart life-course resilience against adverse childhood experiences - a retrospective study on adult health-harming behaviours and mental well-being. BMC Psychiatry. 2017;17(1):110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Farr OM, Ko BJ, Joung KE, Zaichenko L, Usher N, Tsoukas M, et al. Posttraumatic stress disorder, alone or additively with early life adversity, is associated with obesity and cardiometabolic risk. Nutr Metab Cardiovasc Dis. 2015;25(5):479–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sinnott C, Mc Hugh S, Fitzgerald AP, Bradley CP, Kearney PM. Psychosocial complexity in multimorbidity: the legacy of adverse childhood experiences. Fam Pract. 2015;32(3):269–75. [DOI] [PubMed] [Google Scholar]
- 15.Brown DW, Anda RF, Tiemeier H, Felitti VJ, Edwards VJ, Croft JB, et al. Adverse Childhood Experiences and the Risk of Premature Mortality. Am J Prev Med. 2009;37(5):389–96. [DOI] [PubMed] [Google Scholar]
- 16.Hughes K, Bellis MA, Hardcastle KA, Sethi D, Butchart A, Mikton C, et al. The effect of multiple adverse childhood experiences on health: a systematic review and meta-analysis. The Lancet Public Health. 2017;2(8):e356–e66. [DOI] [PubMed] [Google Scholar]
- 17.Kalmakis KA, Chandler GE. Health consequences of adverse childhood experiences: a systematic review. J Am Assoc Nurse Pract. 2015;27(8):457–65. [DOI] [PubMed] [Google Scholar]
- 18.Kelly-Irving M, Lepage B, Dedieu D, Bartley M, Blane D, Grosclaude P, et al. Adverse childhood experiences and premature all-cause mortality. Eur J Epidemiol. 2013;28(9):721–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brewer-Smyth K, Koenig HG. Could spirituality and religion promote stress resilience in survivors of childhood trauma? Issues Ment Health Nurs. 2014;35(4):251–6. [DOI] [PubMed] [Google Scholar]
- 20.Morton KR, Lee JW, Haviland MG, Fraser GE. Religious Engagement in a Risky Family Model Predicting Health in Older Black and White Seventh-Day Adventists. Psycholog Relig Spiritual. 2012;4(4):298–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morton KR, Lee JW, Martin LR. Pathways From Religion to Health: Mediation by Psychosocial and Lifestyle Mechanisms. Psycholog Relig Spiritual. 2017;9(1):106–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morton KR, Tanzini L, Lee JW. Adult Life Satisfaction and the Role of Forgiveness After Childhood Sexual Abuse: Evidence from a Seventh-day Adventist Cohort. Journal for the Scientific Study of Religion. 2019;58(1):138–52. [Google Scholar]
- 23.Superville DJ, Pargament KI, Lee JW. Sabbath Keeping and Its Relationships to Health and Well-Being: A Mediational Analysis. The International Journal for the Psychology of Religion. 2014;24(3):241–56. [Google Scholar]
- 24.Ford PA, Jaceldo-Siegl K, Lee JW, Youngberg W, Tonstad S. Intake of Mediterranean foods associated with positive affect and low negative affect. J Psychosom Res. 2013;74(2):142–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Holt ME, Lee JW, Morton KR, Tonstad S. Mediterranean diet and emotion regulation. Med J Nutrition Metab. 2014;7(3):163–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Holt ME, Lee JW, Morton KR, Tonstad S. Trans fatty acid intake and emotion regulation. J Health Psychol. 2015;20(6):785–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leibow MS, Lee JW, Morton KR. Exercise, Flourishing, and the Positivity Ratio in Seventh-Day Adventists: A Prospective Study. Am J Health Promot. 2020. [DOI] [PubMed]
- 28.Tan A, Morton KR, Lee JW, Hartman R, Lee G. Adverse childhood experiences and depressive symptoms: Protective effects of dietary flavonoids. J Psychosom Res. 2020;131. [DOI] [PubMed] [Google Scholar]
- 29.Yang S, Li X, Yang F, Zhao R, Pan X, Liang J, et al. Gut Microbiota-Dependent Marker TMAO in Promoting Cardiovascular Disease: Inflammation Mechanism, Clinical Prognostic, and Potential as a Therapeutic Target. Front Pharmacol. 2019;10:1360–. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Qi J, You T, Li J, Pan T, Xiang L, Han Y, et al. Circulating trimethylamine N-oxide and the risk of cardiovascular diseases: a systematic review and meta-analysis of 11 prospective cohort studies. J Cell Mol Med. 2018;22(1):185–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang Y, Beydoun MA. Meat consumption is associated with obesity and central obesity among US adults. Int J Obes (Lond). 2009;33(6):621–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.You W, Henneberg M. Meat consumption providing a surplus energy in modern diet contributes to obesity prevalence: an ecological analysis. BMC Nutrition. 2016;2(1):22. [Google Scholar]
- 33.Rohrmann S, Linseisen J. Processed meat: the real villain? Proc Nutr Soc. 2016;75(3):233–41. [DOI] [PubMed] [Google Scholar]
- 34.Wolk A Potential health hazards of eating red meat. J Intern Med. 2017;281(2):106–22. [DOI] [PubMed] [Google Scholar]
- 35.Butler TL, Fraser GE, Beeson WL, Knutsen SF, Herring RP, Chan J, et al. Cohort Profile: the Adventist Health Study-2 (AHS-2). Int J Epidemiol. 2008;37(2):260–5. [DOI] [PubMed] [Google Scholar]
- 36.Lee JW, Morton KR, Walters J, Bellinger DL, Butler TL, Wilson C, et al. Cohort profile: The biopsychosocial religion and health study (BRHS). Int J Epidemiol. 2009;38(6):1470–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Anda RF, Brown DW, Dube SR, Bremner JD, Felitti VJ, Giles WH. Adverse childhood experiences and chronic obstructive pulmonary disease in adults. Am J Prev Med. 2008;34(5):396–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ryff CD, Singer BH, Palmersheim KA. Social inequalities in health and well-being: The role of relational and religious protective factors. In: Brim OG, Ryff CD, Kessler RC, editors. How healthy are we? : a national study of well-being at midlife. Chicago: University of Chicago Press; 2004. p. 90–123. [Google Scholar]
- 39.Cusack KJ, Frueh BC, Brady KT. Trauma history screening in a community mental health center. Psychiatr Serv. 2004;55(2):157–62. [DOI] [PubMed] [Google Scholar]
- 40.Dong M, Anda RF, Felitti VJ, Williamson DF, Dube SR, Brown DW, et al. Childhood Residential Mobility and Multiple Health Risks During Adolescence and Adulthood: The Hidden Role of Adverse Childhood Experiences. Arch Pediatr Adolesc Med. 2005;159(12):1104–10. [DOI] [PubMed] [Google Scholar]
- 41.Briggs EC, Amaya-Jackson L, Putnam KT, Putnam FW. All adverse childhood experiences are not equal: The contribution of synergy to adverse childhood experience scores. Am Psychol. 2021;76(2):243–52. [DOI] [PubMed] [Google Scholar]
- 42.Hamby S, Elm JHL, Howell KH, Merrick MT. Recognizing the cumulative burden of childhood adversities transforms science and practice for trauma and resilience. Am Psychol. 2021;76(2):230–42. [DOI] [PubMed] [Google Scholar]
- 43.Ferrando PJ, Lorenzo-Seva U. Program FACTOR at 10: Origins, development and future directions. Psicothema. 2017;29(2):236–40. [DOI] [PubMed] [Google Scholar]
- 44.Timmerman ME, Lorenzo-Seva U. Dimensionality assessment of ordered polytomous items with parallel analysis. Psychol Methods. 2011;16(2):209–20. [DOI] [PubMed] [Google Scholar]
- 45.Lorenzo-Seva U, Ferrando PJ. Robust Promin: A method for diagonally weighted factor rotation. Liberabit: Revista Peruana de Psicología. 2019;25(1):99–106. [Google Scholar]
- 46.Ferrando PJ, Lorenzo-Seva U. A note on improving EAP trait estimation in oblique factor-analytic and item response theory models. Psicológica. 2016;37(2):235–47. [Google Scholar]
- 47.Orlich MJ, Fraser GE. Vegetarian diets in the Adventist Health Study 2: a review of initial published findings. Am J Clin Nutr. 2014;100 Suppl 1:353S–8S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ware JE, Kosinski M, Turner-Bowker DM, Gandek B. How to Score Version 2 of the SF-12® Health Survey (With a Supplement Documenting Version 1). Lincoln, RI: QualityMetric Incorporated; 2002. [Google Scholar]
- 49.Hayes CJ, Bhandari NR, Kathe N, Payakachat N. Reliability and Validity of the Medical Outcomes Study Short Form-12 Version 2 (SF-12v2) in Adults with Non-Cancer Pain. Healthcare (Basel). 2017;5(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shah CH, Brown JD. Reliability and Validity of the Short-Form 12 Item Version 2 (SF-12v2) Health-Related Quality of Life Survey and Disutilities Associated with Relevant Conditions in the U.S. Older Adult Population. J Clin Med. 2020;9(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kathe N, Hayes CJ, Bhandari NR, Payakachat N. Assessment of Reliability and Validity of SF-12v2 among a Diabetic Population. Value Health. 2018;21(4):432–40. [DOI] [PubMed] [Google Scholar]
- 52.Pudrovska T, Schieman S, Pearlin LI, Nguyen K. The Sense of Mastery as a Mediator and Moderator in the Association Between Economic Hardship and Health in Late Life. J Aging Health. 2005;17(5):634–60. [DOI] [PubMed] [Google Scholar]
- 53.Singh PN, Tonstad S, Abbey DE, Fraser GE. Validity of selected physical activity questions in white Seventh-day Adventists and non-Adventists. Med Sci Sports Exerc. 1996;28(8):1026–37. [DOI] [PubMed] [Google Scholar]
- 54.Koenig HG, Parkerson GR, Meador KG. Religion index for psychiatric research. Am J Psychiatry. 1997;154(6):885–6. [DOI] [PubMed] [Google Scholar]
- 55.Collins LM, Schafer JL, Kam C-M. A comparison of inclusive and restrictive strategies in modern missing data procedures. Psychol Methods. 2001;6(4):330–51. [PubMed] [Google Scholar]
- 56.Herzog JI, Schmahl C. Adverse Childhood Experiences and the Consequences on Neurobiological, Psychosocial, and Somatic Conditions Across the Lifespan. Front Psychiatry. 2018;9:420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Felitti VJ. Childhood sexual abuse, depression, and family dysfunction in adult obese patients: a case control study. South Med J. 1993;86(7):732–6. [DOI] [PubMed] [Google Scholar]
- 58.Isohookana R, Marttunen M, Hakko H, Riipinen P, Riala K. The impact of adverse childhood experiences on obesity and unhealthy weight control behaviors among adolescents. Compr Psychiatry. 2016;71:17–24. [DOI] [PubMed] [Google Scholar]
- 59.Remigio-Baker RA, Hayes DK, Reyes-Salvail F. The Relationship of Adverse Childhood Events to Smoking, Overweight, Obesity and Binge Drinking Among Women in Hawaii. Matern Child Health J. 2017;21(2):315–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schneiderman JU, Mennen FE, Negriff S, Trickett PK. Overweight and obesity among maltreated young adolescents. Child Abuse Negl. 2012;36(4):370–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Windle M, Haardorfer R, Getachew B, Shah J, Payne J, Pillai D, et al. A multivariate analysis of adverse childhood experiences and health behaviors and outcomes among college students. J Am Coll Health. 2018;66(4):246–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Martinez I, Lattimer JM, Hubach KL, Case JA, Yang J, Weber CG, et al. Gut microbiome composition is linked to whole grain-induced immunological improvements. ISME J. 2013;7(2):269–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Spencer-Hwang R, Torres X, Valladares J, Pasco-Rubio M, Dougherty M, Kim W. Adverse Childhood Experiences among a Community of Resilient Centenarians and Seniors: Implications for a Chronic Disease Prevention Framework. Perm J. 2018;22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hummer RA, Ellison CG, Rogers RG, Moulton BE, Romero RR. Religious Involvement and Adult Mortality in the United States: Review and Perspective. South Med J. 2004;97(12):1223–30. [DOI] [PubMed] [Google Scholar]
- 65.Hill TD, Ellison CG, Burdette AM, Musick MA. Religious involvement and healthy lifestyles: Evidence from the survey of Texas adults. Ann Behav Med. 2007;34(2):217–22. [DOI] [PubMed] [Google Scholar]
- 66.Le LT, Sabate J. Beyond meatless, the health effects of vegan diets: findings from the Adventist cohorts. Nutrients. 2014;6(6):2131–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Asamsama OH, Lee JW, Morton KR, Tonstad S. Bidirectional longitudinal study of type 2 diabetes and depression symptoms in black and white church going adults. Journal of Diabetes & Metabolic Disorders. 2015;14(1):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Colman I, Kingsbury M, Garad Y, Zeng Y, Naicker K, Patten S, et al. Consistency in adult reporting of adverse childhood experiences. Psychol Med. 2016;46(3):543. [DOI] [PubMed] [Google Scholar]


