Abstract
Females are more affected by psychiatric illnesses including eating disorders, depression, and post-traumatic stress disorder than males. However, the neural mechanisms mediating these sex differences are poorly understood. Animal models can be useful in exploring such neural mechanisms. Conditioned taste aversion (CTA) is a behavioral task that assesses how animals process the competition between associated reinforcing and aversive stimuli in subsequent task performance, a process critical to healthy behavior in many domains. The purpose of the present study was to identify sex differences in this behavior and associated neural responses. We hypothesized that females would value the rewarding stimulus (Boost®) relative to the aversive stimulus (LiCl) more than males in performing CTA. We evaluated behavior (Boost® intake, LiCl-induced behaviors, ultrasonic vocalizations (USVs), CTA performance) and Fos activation in relevant brain regions after the acute stimuli [acute Boost® (AB), acute LiCl (AL)] and the context-only task control (COT), Boost® only task (BOT) and Boost®-LiCl task (BLT). Acutely, females drank more Boost® than males but showed similar aversive behaviors after LiCl. Females and males performed CTA similarly. Both sexes produced 55 kHz USVs anticipating BOT and inhibited these calls in the BLT. However, more females emitted both 22 kHz and 55 kHz USVs in the BLT than males: the latter correlated with less CTA. Estrous cycle stage also influenced 55 kHz USVs. Fos responses were similar in males and females after AB or AL. Females engaged the gustatory cortex and ventral tegmental area (VTA) more than males during the BOT and males engaged the amygdala more than females in both the BOT and BLT. Network analysis of correlated Fos responses across brain regions identified two unique networks characterizing the BOT and BLT, in both of which the VTA played a central role. In situ hybridization with RNAscope identified a population of D1-receptor expressing cells in the CeA that responded to Boost® and D2 receptor-expressing cells that responded to LiCl. The present study suggests that males and females differentially process the affective valence of a stimulus to produce the same goal-directed behavior.
Introduction
Females are more affected by psychiatric illnesses including eating disorders, depression, post-traumatic stress disorder and other anxiety disorders than males [1–5]. Animal models have provided insight into neural mechanisms mediating sex differences in the behavioral and neural processes that may be involved, like stress and fear-learning [6–12]. However, there has been little exploration of models in which animals must balance the benefits of a reward against the risk of an aversive experience, a process that more closely recapitulates real world decision making.
Conditioned taste aversion is a task which interrogates this situation. This model has been proposed to have special relevance for eating disorders and conditioned/anticipatory nausea, conditions experienced disproportionately by women [13]. CTA is a classical conditioning paradigm which pairs a palatable substance with an aversive visceral experience, such as the emetic agent lithium chloride (LiCl), producing an aversion to the substance at subsequent exposure. Unlike many other classical conditioning paradigms, in which an aversive stimulus is paired with a neutral cue, the use of a palatable substance in CTA requires the animal to process the affective valence of the reward versus the aversion [14–16]. This provides an opportunity to understand neural processes by which this decision-making occurs. Animal studies of sex differences in CTA are mixed but tend to report that females develop less CTA than males [17–23]. While this seems to be discordant with the human situation, careful reviews of sex differences in varying models of classical conditioning suggest that in situations involving stress, fear, or aversive stimulation, including conditioned taste aversion, males and females do not show quantitative differences in responding but utilize different strategies [12, 18, 19].
The purpose of the present study was to test the hypothesis that males and females use different strategies to attain the same degree of CTA, with females exhibiting greater influence of the reinforcing stimulus that is paired with the aversive stimulus than males. We used several modifications of most published approaches to better capture the ability of this task to assess affective valence. We used a nutritive substance (chocolate-flavored Boost®) in non-food deprived animals, which more closely represents CTA outside the confines of a laboratory [24]. While current studies of CTA focus primarily on performance of the task, this study aimed to capture both the emotional valence and the neural mechanisms that respond to each step of the CTA process. We accomplished this goal by evaluating both behavior and Fos responses to the appetitive stimulus (Boost®), the aversive stimulus (LiCl), and the performance of the CTA task. We evaluated a total of 6 conditions: (1) acute Boost® (AB), (2) acute LiCl (AL), (3) NaCl injection only (control for acute conditions) (AN), (4) Boost® previously paired with NaCl (“BOT), (5) Boost® previously paired with LiCl (BLT), and (6) context only task (COT). By including the acute conditions, as well as the control and appetitive task, we were better able to capture previously underappreciated neural activation in brain regions relevant to the CTA paradigm.
In this study, we characterized behavioral and neural responses to CTA in males and females. Behavioral observations of the aversive stimulus included pica, ptosis, and lying on belly (LOB) as indications of nausea in LiCl-treated rats. We also quantitated ultrasonic vocalizations (USVs) as a measure of affective valence on task day. Rats engage in two distinct call patterns: short 55-kHz (which range from 30–70 kHz and are associated with positive emotional valence), and longer 22-kHz calls (associated with negative affective valence). They emit 55 kHz calls during behaviors such as play, mating, social approach, and in anticipation of a reward [25–28]. We therefore hypothesized that animals would engage in these calls on task day when anticipating Boost® that was not devalued with LiCl injection. Conversely, 22-kHz calls are exhibited in contexts of social withdrawal (e.g. after mating [29]) and to warn the colony of potential predators [30–32]. Female rats are more likely than males to exhibit 22-kHz calls in the presence of cat urine [33]. We hypothesized that rats would engage in these calls on task day when anticipating Boost® that was previously paired with LiCl.
We used the immediate early gene Fos as a marker of neuronal activity at each stage of CTA: in response to the acute rewarding, aversive, and neutral stimuli (NaCl injection), and during control task, Boost® task and during CTA expression. We analyzed 11 brain regions known to be associated with the response to reward and/or LiCl. We selected more rostral regions that are associated with the decision-making process in CTA, rather than the more caudal regions which serve to transmit sensory signals that are interpreted by higher-order areas of the brain. Specifically, we included the following regions of interest that have been implicated in CTA. We evaluated the supraoptic and paraventricular nuclei of the hypothalamus (SON, PVN), which respond to LiCl and have been implicated in inhibition of food intake [34–41].We also evaluated the Central nucleus of the Amygdala (CeA) [42], which responds both to LiCl and Boost®, and has been shown to also contribute to the establishment of either positive or negative affective valence of food stimuli, but is not required for acquisition of expression of CTA [37, 38, 42, 43]. We also selected key regions of interests that are critical for establishment of taste memory and CTA including the basolateral amygdala (BLA) and the agranular (aIC) and granular (gIC4 and gIC5/6) insula, the interaction of which are thought to be critical for taste memory and the acquisition and expression of CTA [44–51] as well as the ventromedial prefrontal cortex (vmPFC), a highly-interconnected brain region known promote decision-making and valuation processing [52–55]. We sampled the ventral tegmental area (VTA), implicated in responses to both reinforcing and aversive stimuli [56, 57], as well as targets of dopaminergic projections from the VTA, the nucleus accumbens core and shell, which have been implicated in CTA acquisition and expression [58–64]. We also conducted network analysis to interrogate correlations among the Fos responses to the areas sampled. Finally, our Fos data prompted us to examine more closely specific cell types in the amygdala using mRNA in situ hybridization.
The following studies showed that males and females differentially assign valence to the expectation of a rewarding and aversive stimulus, identified cell types that contribute to the neural processing of these stimuli, and characterized novel behaviors that correlate with predicted CTA expression and experimental condition.
Results
Behavioral responses during conditioned taste aversion
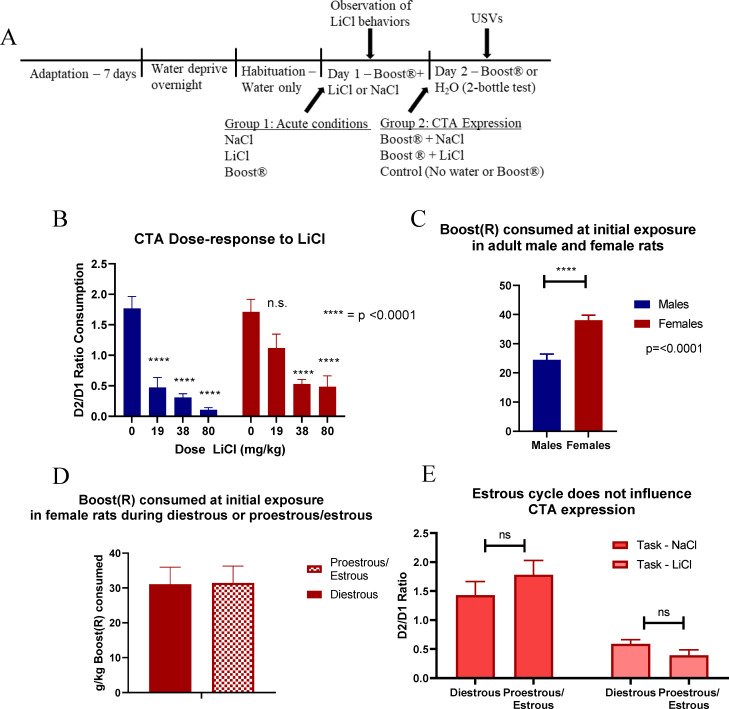
The CTA experimental design is described in Fig 1A. To first characterize CTA expression, a dose-response curve was established in both sexes. Animals received either NaCl (0.15M) or LiCl (19 mg/kg, 38 mg/kg, or 80 mg/kg) (Fig 1B). LiCl-induced behavior was measured as described in Table 4 in Materials and Methods. Statistics show a main effect of sex [F (1,111) = 6.05, p = 0.015] and a main effect of treatment [F (3,111) = 29.31, p< 0.001] but no interaction of treatment x sex. Fisher’s post hoc analysis showed that males inhibited Boost® intake more than females after pairing with the low dose of 19 mg/kg LiCl.
Fig 1.
(A) Protocol for CTA paradigm. (B) Dose response curve to LiCl (n = 7–23). (C) Boost® consumption on first exposure by sex (n = 48–100). (D) Estrous cycle and day 1 Boost® consumption (n = 8–9). (E) Estrous cycle and CTA expression on task day (n = 10–14). All data expressed as mean±SEM. These findings show that females consume more of the reinforcer (Boost®) and show less CTA after a low dose of LiCl, but CTA is not influenced by estrous state.
Table 4. Scoring of LiCl behaviors.
| Behavior | Score = 0 | Score = 1 | Score = 2 | Score = 3 |
|---|---|---|---|---|
| Pica | No pica | Some pica while still moving around | Pica in a crouched position, but still moved or looked around | Remained crouched, engaged in pica for most of 15-second interval |
| LOB | No LOB | Some LOB, still in crouched position | Some LOB, more flattened posture | Entirely flattened posture |
| Ptosis | No ptosis | Ptosis for <5 seconds | Ptosis for most interval (>5 seconds, <10 seconds) | Ptosis for entire interval |
Female rats drank more Boost® than males at initial exposure when adjusting for body weight, with a main effect of sex [F (1,146) = 21.91, p< 0.001, (Fig 1C)] but no effect of estrous cycle (Fig 1D). Both male and female rats generally increased the volume of Boost® they consumed at subsequent exposure. This ratio of consumption was similar in males and females despite the propensity for females to drink more on first exposure.
To determine whether the estrous cycle contributed to CTA behavior, estrous cycle was determined by vaginal lavage on task day. Cycle stage did not have a significant effect on CTA expression (Fig 1E).
LiCl-induced behaviors
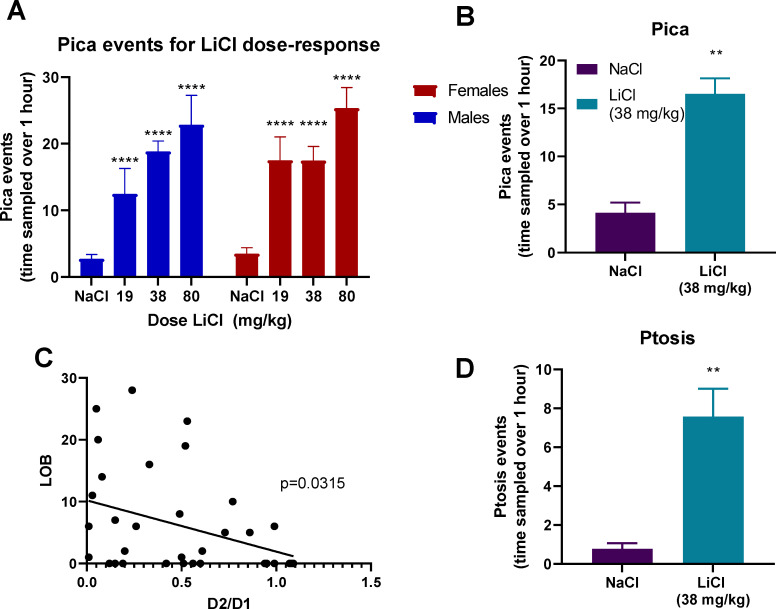
Pica was measured after the same range of doses of LiCl: 0 mg/kg (NaCl), 19, 38, and 80 mg/kg. Both male and female rats engaged in pica equally across doses of LiCl. 2-way ANOVA showed an effect of treatment [F (3,158) = 20.79, p< 0.001] and no effect of sex or interaction of treatment x sex (Fig 2A). Pica events at 80 mg/kg were higher than 38 and 19 mg/kg. Lying on belly (LOB) was the only nausea behavior measured in this study that correlated with CTA expression, Pearson correlation = -0.3701 and p < 0.0434 (Fig 2B and 2C). Rats also exhibited ptosis after LiCl injection (Fig 2D). Neither of these behaviors correlated with CTA behavior as measured by the ratio of Boost® consumption on day 2 as compared to day 1, but both LOB and pica correlated with ptosis ([F(1,43] = 8.00, p < 0.007 and [F(1,43] = 21.5, p < 0.0001) respectively.
Fig 2.
(A) Pica events after increasing doses of LiCl (0, 19, 38, and 80 mg/kg) in male and female rats (n = 6–45) (B) LOB was measured for 1 hour after 38 mg/kg LiCl or NaCl (n = 18–33). Data shown as mean ± SEM. (C) LOB correlation with D2/D1 ratio (D) Ptosis events measured over 1 hour after 38 mg/kg LiCl or NaCl (n = 18–33). All data shown as mean±SEM. * = different from NaCl (0 mg/kg). # = different from 19 mg/kg and 38 mg/kg. *** = p<0.0005. These findings show that among the behaviors induced by LiCl administration, only LOB correlated with CTA, although the other behaviors correlated with each other.
Ultrasonic vocalizations during conditioned taste aversion task
Rats were taken through the CTA protocol as described. We recorded USVs during the first 10 minutes of the habituation process on Day 2, before offering the 2-bottle test, as vocalizations typically occur in anticipation of a reward.
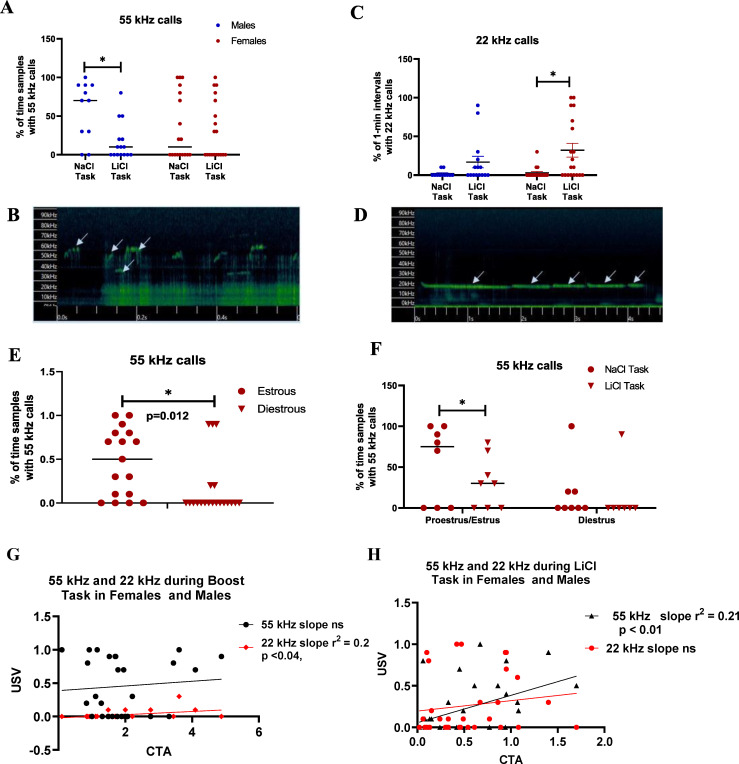
We found an effect of treatment [F (1,59) = 4.52, p< 0.038, Fig 3A and 3B], with BOT showing more 55 kHz calls than the BLT. Dividing by sex due to pre-planned contrast showed that males expressed more 55 kHz calls in the BOT[F (1,24) = 8.53, p = 0.007], while females were not significant.
Fig 3. USVs were recorded for first 10 minutes during CTA task.
(A) 55 kHz calls in male and female rats during CTA expression (n = 11–19). (B) Representative 55 kHz call. (C) 22 kHz warning calls during CTA expression in male and female rats (n = 12–19). (D) Representative 22 kHz call (E) 55 kHz vocalizations in females by cycle only (n = 150–16) (E) 55 kHz calls in females by task and cycle (n = 6–8). Data expressed as mean±SEM. *indicates different by Fisher’s post hoc except where otherwise. (G) Correlation of 55 kHz and 22 kHz USV’s during the BOT with CTA in males and females (combined). (H) Correlation of 55 kHz and 22 kHz USV’s during BLT with CTA in males and females (combined).
There was a significant correlation of 22 kHz calls in the LiCl-paired environment by 2-way ANOVA, with a main effect treatment [F (1,59) = 9.48, p< 0.0032, Fig 3C and 3D]. Dividing by sex due to pre-planned contrast showed that females significantly increased 22 kHz calls in the LiCl-paired environment [F (1,34) = 9.17, p< 0.005], while males were not significant. This effect was not influenced by estrous cycle state.
Female rat 55 kHz vocalizations were substantially influenced by estrous cycle. By task, females showed an effect of estrous cycle with no effect of treatment [F (1,24) = 7.38, p< 0.012, Fig 3D]. One-way ANOVA for each low- and high-estradiol states individually showed females in high-estradiol states increased 55 kHz vocalizations in the COT group compared to active task [F (1,12) = 5.20, p< 0.042, Fig 3E]. There was no effect of treatment in the low-estradiol group. High-estradiol states were associated with an increased likelihood of engaging in 55 kHz calls, and low-estradiol states with decreased the frequency of 55 kHz calls, regardless of experimental condition [F (1,27) = 6.35, p < 0.018, Fig 3F].
We conducted analysis of potential correlations between USVs expressed by all animals (both sexes, irrespective of estrous cycle) and performance in the CTA task (D2/D1 ratio). These results are shown in Fig 3G and 3H. For the BOT group, 22 kHz but not 55 kHz USVs correlated significantly with CTA. Conversely for the BLT group, 55 kHz but not 22 kHz USVs correlated with CTA. We also conducted 2-way ANOVA of CTA for the interaction of the presence and absence of USVs by an individual rat x sex to determine if sex was contributing to the linear regression observed above. For the BOT, there was a nonsignificant association between 22 kHz with CTA ([F(1,27] = 3.53, p < 0.07), and the relationship of CTA to 55 kHz was nonsignificant. These data are consistent with the significant, but minor association depicted in Fig 3G and 3H. For the BLT, sex had a significant impact on the relationship between 55 kHz USVs and CTA ([F(1,34] = 6.43, p < 0.02). CTA in females that expressed 55 kHz USVs was higher (less suppressed) than males.
To further understand the contributions of individual animals, we counted the number of individual animals of each sex that emitted 22 kHz, 55 kHz calls or both under each of the task conditions. Females were chosen irrespective of estrous cycle stage, as animals of each stage were distributed equally among groups. These data are shown in Table 1.
Table 1. Number of individual males and females emitting 22 kHz and/or 55 kHz USVs in different task conditions.
| 22 kHz | 22 + 55 kHz | 55 kHz only | Total | ||||
|---|---|---|---|---|---|---|---|
| F | M | F | M | F | M | ||
| COT | 2 | 0 | 3 | 0 | 1 | 2 | 8 |
| BOT | 3 | 1 | 1 | 1 | 6 | 7 | 19 |
| BLT | 7 | 2 | 6 | 3 | 2 | 4 | 24 |
| Total | 12 | 3 | 11 | 4 | 9 | 13 | 51 |
In summary, overall animals responded as predicted with an increase in 55 kHz USVs when anticipating Boost® and decrease in 55 kHz USV’s after pairing with LiCl (BLT). However, marked sex differences were observed including a significant effect of estrous cycle, an increased expression of dual 22 kHz and 55 kHz among individual females compared to males during BLT and a correlation between 55 kHz USVs during the BLT which correlated with less CTA in females only.
The Fos response to acute and conditioned stimuli
We evaluated Fos expression to better characterize differences in the neural circuits involved in the development and expression of the COT, BOT and BLT in males and females. Fos analysis was performed across 11 brain regions associated with the response to rewarding and aversive stimuli (Table 2).
Table 2. Fos positive neurons±SEM.
| Acute NaCl | Acute Boost® | Acute LiCl | Control Task | Boost® Task | LiCl Task | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F | M | F | M | F | M | F | M | F | M | F | M | |
| vmPFC | 244±37 | 229±72 | 244±20 | 239±33 | 266±41 | 272±51 | 246±37 | 241±32 | 268±52 | 212±31 | 249±44 | 221±43 |
| aIC | 183±32 | 187±40 | 198±20 | 215±22 | 212±50 | 206±27 | 232±32 | 193±14 | 223±31 | 176±26 | 227±21 | 174±24 |
| gIC5/6 | 100±38 | 87±44 | 197±29 | 178±77 | 206±44 | 206±70 | 81±45 | 86±26 | 162±53 | 145±23 | 144±55 | 99±72 |
| gICL4 | 134±66 | 85±28 | 262±23 | 253±82 | 255±23 | 267±89 | 148±82 | 98±64 | 225±69 | 184±27 | 121±45 | 79±43 |
| NAcC | 91±30 | 98±35 | 133±7 | 98±20 | 100±62 | 109±29 | 110±1 | 76±18 | 123±26 | 99±34 | 101±21 | 64±29 |
| NAcS | 117±39 | 123±36 | 125±19 | 107±33 | 171±78 | 141±68 | 120±26 | 90±17 | 153±38 | 113±41 | 124±52 | 118±52 |
| SON | 7±5 | 14±8 | 11±9 | 10±12 | 112±34 | 112±3 | 10±10 | 2±1 | 21±6 | 27±27 | 5±2 | 45±70 |
| BLA | 34±8 | 32±8 | 56±16 | 53±8 | 67±25 | 58±15 | 52±19 | 24±5 | 61±10 | 42±3 | 52±6 | 41±15 |
| CeA | 44±22 | 42±26 | 133±40 | 108±33 | 191±73 | 199±58 | 30±7 | 42±60 | 142±19 | 115±60 | 62±48 | 48±28 |
| PVN | 113±51 | 103±26 | 122±51 | 143±29 | 230±102 | 226±96 | 95±36 | 107±28 | 91±20 | 74±45 | 141±39 | 134±70 |
| VTA | 63±17 | 50±13 | 84±30 | 92±16 | 93±29 | 52±15 | 63±22 | 69±15 | 118±22 | 66±30 | 83±13 | 51±9 |
We performed 3-way repeated measures ANOVA (sex x treatment, area as a repeated measure) on all experimental conditions, which revealed a main effect of sex [F (1,62) = 5.37, p = <0.001, females greater than males], a main effect of treatment [F (5,62) = 9.57, p = <0.01], a main effect of area [F (10,462) = 229.69, p <0.001], and an interaction of treatment x area [F (50,462) = 8.46, p< 0.001]. These results show that Fos expression varies significantly between conditions, and females generally express more Fos across conditions than males.
We then performed 3-way repeated measures ANOVA for the acute and conditioned stimuli separately. For the acute condition, 3-way repeated measures ANOVA (treatment x sex, area as a repeated measure) revealed a main effect of treatment [F (2,31) = 17.36, p < 0.001] and area [F (10,231) = 101.64, p < 0.001], and an interaction of treatment x area [F (20,231) = 9.73, p < 0.001], with no effect of sex for the acute stimuli. AB and AL groups both differ from NaCl control, and different from each other (LiCl greater than Boost®). We conducted lower level 2-way ANOVA (sex x Rx) for areas that are implicated functionally in CTA and/or showed statistically relevant findings. These data show that Fos expression is different between experimental conditions, and Fos expression in individual brain regions shows an effect of treatment. Fos expression between males and females was not different in these conditions.
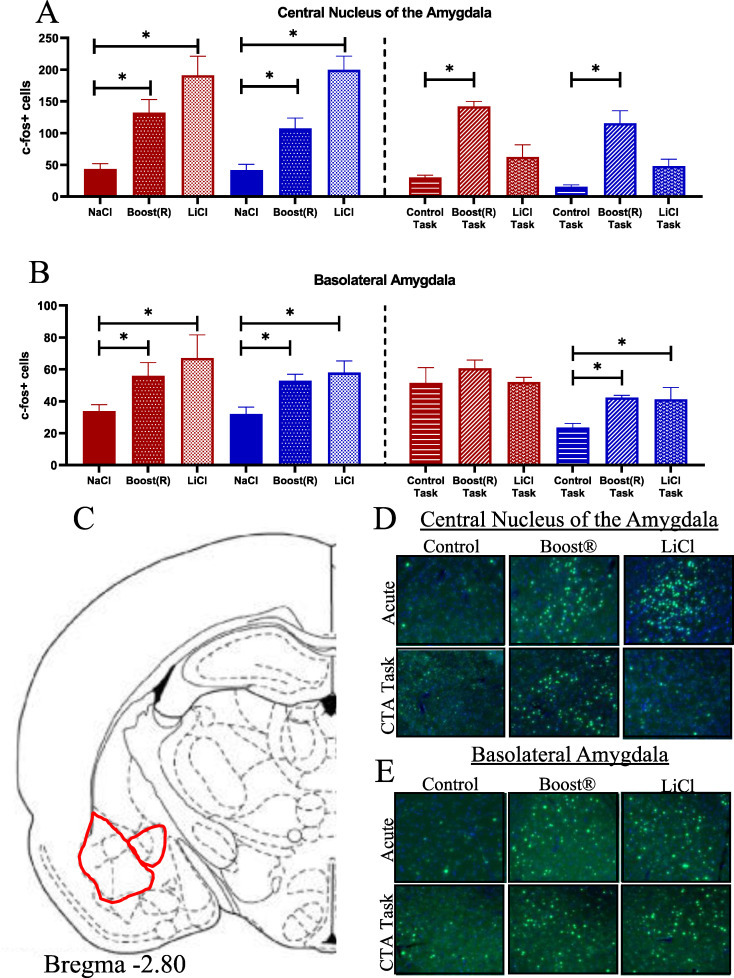
The central nucleus of the amygdala (CeA) responds to aversive visceral stimuli such as LiCl as well as rewarding stimuli [43]. We found Fos in the CeA increased both AB and AL groups [F (2,30) = 31.39, p< 0.001 effect of treatment] similarly in male and female rats (Fig 4A, atlas location shown in Fig 4C, representative images shown in Fig 4D). The basolateral amygdala (BLA), known for its role in assessing the valence of a stimulus [65–68], showed an effect of treatment [F (2,17) = 9.06, p< 0.0021], with no effect of sex (Fig 4B, atlas location shown in Fig 4C, representative images shown in Fig 4E). Post-hoc analysis showed both AB and AL groups were increased compared to NaCl control.
Fig 4.
(A) Fos response in the CeA across conditions. N = 4–7. (B) Fos response in the BLA across conditions. N = 3–4 (C) Atlas image of CeA and BLA. (D-E) Representative images.
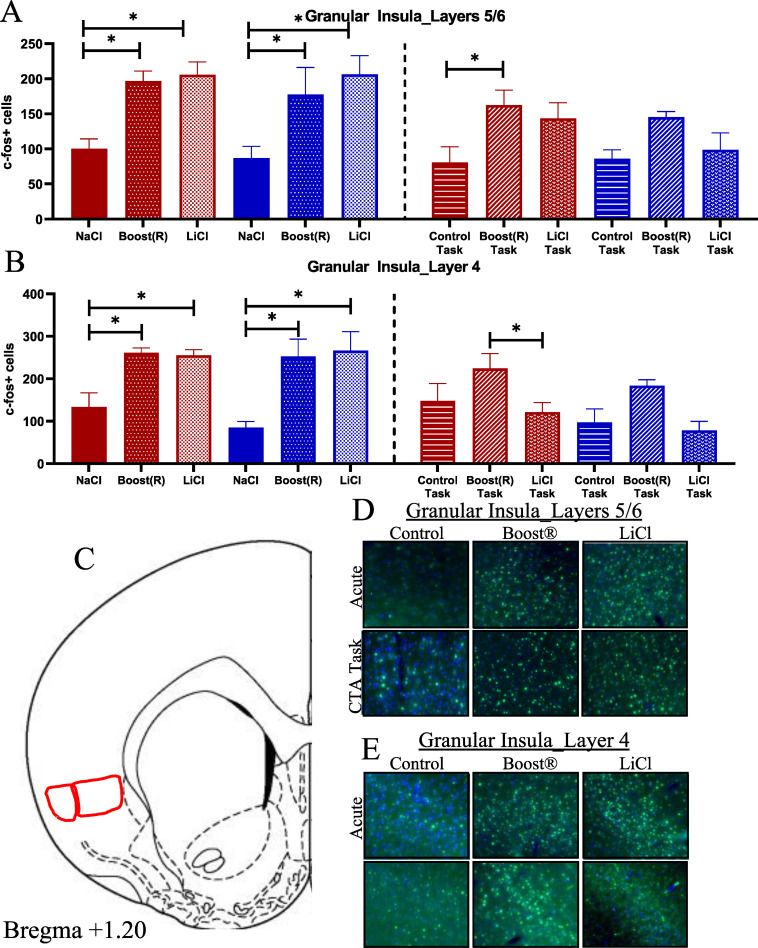
The granular insula (gIC) receives both taste and visceral inputs [69–76], and is divided into several layers. We considered layer IV (gIC4) and layers V/VI (gIC5/6). Fos in gIC5/6 increased in both AB and AL (Fig 5A, atlas location shown in Fig 5C representative images shown in Fig 5D), showing a significant effect of treatment [gIC5/6; F (2,29) = 14.52, p< 0.001]. Fos in gIC4 likewise increased in both AB and AL compared to AN (Fig 5B, atlas location shown in Fig 5C representative images shown in Fig 5E). The gIC4 showed an effect of treatment [F (2,17) = 15.62, p> 0.001]. These results support the hypothesis that the gIC is responsive to both rewarding and aversive visceral stimuli.
Fig 5.
(A) Fos response in the gIC5/6 (N = 4–9) across conditions. (B) Fos response in the gIC4 (N = 3–4) across conditions. (C) Atlas image of gIC5/6 and gIC4. (D,E) Representative images.
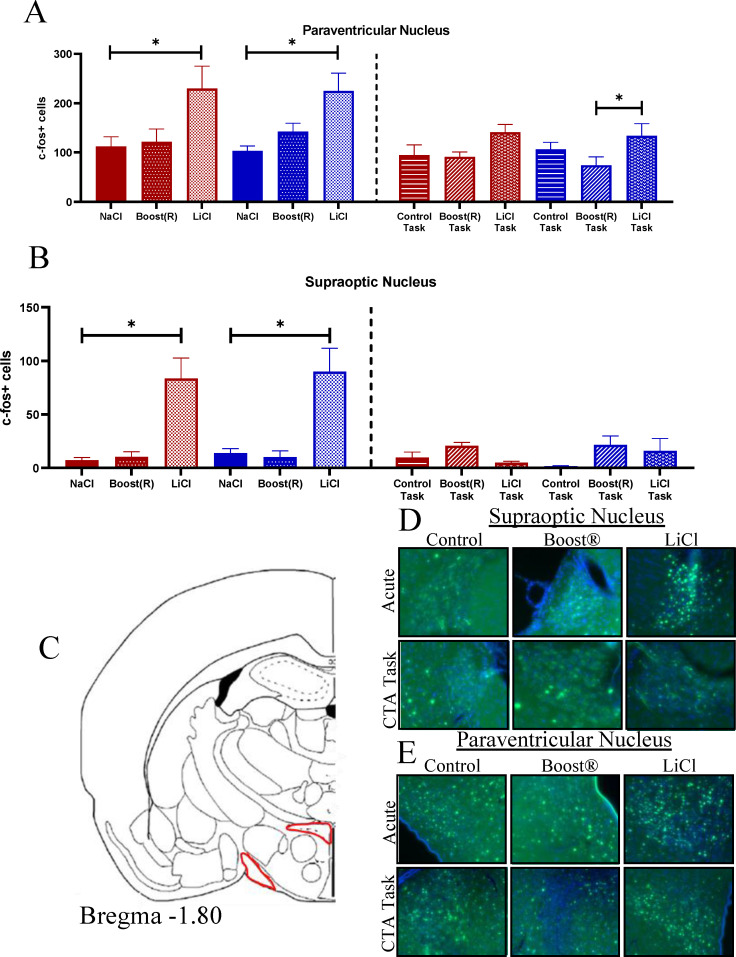
The supraoptic nucleus (SON) responds to acute LiCl [37, 77]. It is the primary site oxytocin production, which is known to have emetic and anorectic properties [40, 78–80]. The Fos response in the SON increased markedly in response to AL [F (2,16) = 54.36, p< 0.001 effect of treatment], with no response to AB (Fig 6A, atlas location shown in Fig 6C, representative images shown in Fig 6D). Fos in the paraventricular nucleus (PVN), which contains receptors for oxytocin and is the primary location for release of stress-related peptides such as corticotropin-releasing hormone and vasopressin [34, 35, 41, 81], likewise increased specifically in response to the aversive stimulus [F(2,27) = 8.44, p< 0.001, Fig 6B, atlas image shown in Fig 6C, representative images shown in Fig 6E]. These data show that the PVN and SON are uniquely activated in the AL group, but not the AB group, and are therefore specific to the nausea response.
Fig 6.
(A) Fos expression in the PVN across conditions. N = 3–8. (B) Fos expression in the SON across conditions. N = 3–4 (C) Atlas image of SON and PVN. (D,E) Representative images.
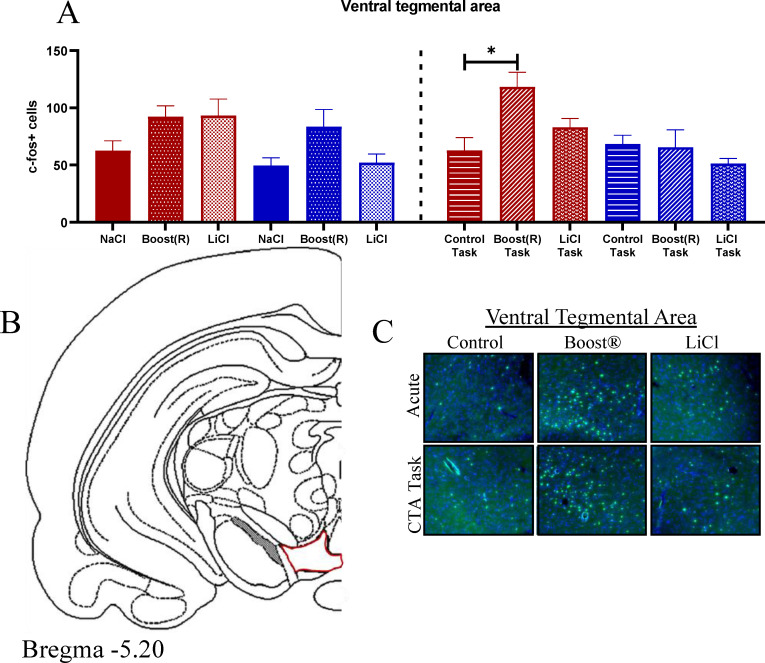
The ventral tegmental area (VTA) is responsive to rewarding stimuli and implicated in addiction [82–84]. Fos in the VTA showed an effect of treatment [F (2,22) = 4.19, p = 0.03], with AB increased compared to AL and AN (Fig 7A, atlas image shown in Fig 7B, representative images shown in Fig 7C). These data show that Boost®, but not LiCl, activates the VTA.
Fig 7.
(A) Fos response across conditions in the VTA. N = 3–4 (B) Atlas image of the VTA. (C) Representative images.
Interoceptive signals and contextual stimuli converge in the ventromedial frontal cortex (vmPFC) and agranular insula (aIC) [44, 55, 73, 85–89]. However, no significant effects were found in the vmPFC or aIC in any condition, and so these will not be discussed further.
The nucleus accumbens responds to rewarding stimuli, typically in behavioral paradigms that utilize deprivation. The accumbens shell is more responsive to rewarding stimuli, while the accumbens core responds to conditioning of rewarding stimuli [63, 90–95]. No significant effects were found in either the accumbens core (NAcC) or shell (NAcS) during the acute stimuli.
3-way repeated measures ANOVA (sex x treatment x area) of the CTA task conditions revealed a main effect of sex [F (1,31) = 7.33, p< 0.011] and area [F (10,231) = 145.72, p< 0.001] and an interaction of treatment x area [F (20,231) = 6.87, p = <0.001]. Females were more responsive than males overall. We then ran second order ANOVAs of treatment x sex in individual areas, and treatment x area in each sex.
Fos in the CeA increased in response to the BOT. It showed an effect of treatment [F (2,29) = 24.95, p< 0.001] and just missed an effect of sex [F (1,29) = 3.96, p< 0.056]. The BOT differed from the COT, both globally and in each sex individually (Fig 4A, atlas location shown in Fig 4C, representative images shown in Fig 4D). Fos in the BLA showed an effect of sex [F (1,18) = 17.34, p < 0.001, Fig 4B, atlas location shown in Fig 4C, representative images shown in Fig 4E]. This was driven largely by high baseline Fos immunoreactivity in the COT for females. Lower-level ANOVA by sex showed a significant effect of condition in males only, with both BOT and BLT differing from control. These results show that the CeA is responsive to the rewarding stimulus (BOT group), but not after association with the visceral stimulus (BLT group). The BLA, meanwhile, was unusually activated in COT for females, but showed increased Fos response in both the BOT and BLT for males.
The gIC5/6 showed an effect of treatment [F (2,31) = 5.29, p< 0.011], with the BOT differing from the COT. This difference is driven by the female rats, which showed increases in Fos response in both the BOT and BLT compared to COT (Fig 5A, atlas location shown in Fig 5C, representative images shown in Fig 5D). Fos in the gIC4 showed an effect of treatment [F (2,17) = 4.39, p< 0.014], and no effect of sex (Fig 5B, atlas location shown in Fig 5C, representative images shown in Fig 5E). The BOT showed increased Fos expression compared to the BLT globally. The females showed a statistically significant increase in Fos expression in the BOT compared to BLT conditions. These results suggest that the gIC is responsive to the reinforcing stimulus, an effect that is driven by high expression in females.
Females had higher Fos expression in the NAcC than males. It showed an effect of sex [F (1,31) = 12.31, p< 0.001] but no effect of treatment (see Table 2). Females had higher Fos expression than males, both globally and in the BOT specifically. Fos in the NAcS showed an effect of sex [n = 4, F (1,18) = 14.52, p< 0.001], with no effect of treatment (see Table 2). These results suggest that, in the non-deprived CTA conditions, the NAc is non-contributory.
Neither the PVN nor the SON responded during the task conditions (Fig 6A and 6B, atlas location in 6C, representative images 6D, E). In the VTA in the task conditions, there was an effect of treatment [F (2,16) = 3.86, p = 0.043], an effect of sex [F (1,16) = 9.18, p = 0.01], and an interaction of sex x treatment [F (2,16) = 3.90, p = 0.04]. By Fisher’s post hoc, females were increased compared to males overall, and BOT in females was increased compared to their control, as well as compared to males in the BOT (Fig 7A, atlas location 7B, representative images 7C). These data show that females engage the VTA more than males in response to the reinforcing stimulus.
Network analysis of Fos responses to control, Boost®, and LiCl tasks
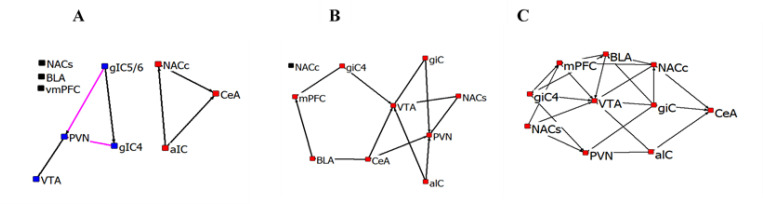
A major goal of the present study was to compare activated neural networks involved in the typical LiCl conditioned taste aversion task (BLT) with those activated when animals were anticipating Boost® presentation without a previous aversive stimulus (BOT) and those activated when animals experienced only the test cage (COT). We first conducted network analysis of these three conditions. We then subtracted correlations contained in the COT from the BOT and BLT networks (Fig 8A and 8B respectively). The two networks are quite distinct. However, in both, the VTA became central to each network, with all brain areas sample involved in the BLT—COT network.
Fig 8.
Network of significant Fos correlations for control task (A), BOT—COT(B) and BLT—COT(C). Each brain region represented as a node in the network. Colors indicate correlations with no other brain areas (black), members of the main network (red) and members of a second network (blue, Control task). Line color indicates positive (black) or negative (magenta) correlations.
Responses of dopamine D1- and D2-receptor expressing cells in the amygdala to rewarding and aversive stimuli
The role of the amygdala in the response to acute aversive stimuli such as LiCl is well-established [37, 63, 77, 96–99]. A study by Kim, et. al., examined the expression patterns and responses to both fear and reward in multiple neuronal cell types in the CeA and BLA [43], laying an important groundwork for further interrogation into the function of specific neuronal cell types. The BLA is responsible for assessing the salience of a signal, whether it be rewarding or aversive [43, 65–67, 100–102]. BLA outputs to the CeA promote defensive and appetitive behaviors [43].
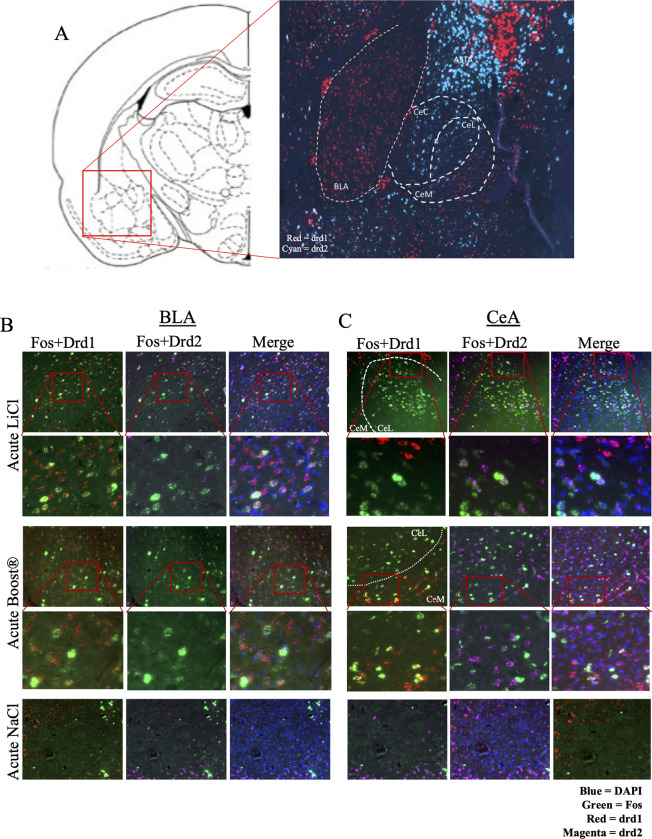
Findings above indicating the centrality of the VTA in the task networks stimulated interest in potential amygdala dopamine involvements in this task. Dopamine receptors are present throughout the amygdala, but little is known about their reactivity to reinforcing or aversive stimuli. We used RNAscope to visualize D1 and D2 dopamine receptors (Drd1 and Drd2) throughout the amygdala. We found that Drd1+ and Drd2+ cells occupy largely distinct anatomical locations within the amygdala. Consistent with previous studies, we found that the CeC and CeL were composed primarily of Drd2+ cells, while the CeM contained primarily Drd1+ cells. A smaller population of Drd1+ cells are present in the CeL (Fig 9A). The BLA, in contrast to the CeA, represents a nearly homogenous population of D1-receptor expressing cells, with few D2-receptor expressing cells (Fig 9A). Using the mRNA in situ hybridization method RNAScope, the co-expression of Fos and D1 or D2 receptors was characterized in each of the acute conditions, as well as during CTA expression, in the BLA and CeA (Fig 9B and 9C)
Fig 9.
(A) Distribution of Drd1+ and Drd2+ cells across the amygdala. BLA = basolateral amygdala. CeA = central nucleus of the amygdala. ASTA = amygdalostriatal transition area. (B) Representative images of RNAScope in acute LiCl and acute Boost® ® conditions in the CeA. (C) Representative images of RNAScope in acute LiCl and Boost® conditions in the BLA.
Responses of D1-receptor expressing cells in the BLA to acute and conditioned rewarding and aversive stimuli
The BLA expressed Fos in response to both rewarding and aversive stimuli, and during BLT expression in male rats only. The Drd2 cells, which represented a small fraction of total cells, did not respond in any stimulus-specific pattern (Fig 10A).
Fig 10.
(A) RNAScope analysis of co-expression of Fos and Drd1+/Drd2+ cells in the basolateral amygdala across conditions. N = 3-4/condition (B) RNAScope analysis of co-expression of Fos and Drd1+/Drd2+ cells in the central nucleus of the amygdala across conditions. N = 3-4/condition. * = p<0.05; ** = p<0.005; *** = p<0.0005; **** = p<0.0001 by Tukey’s post hoc.
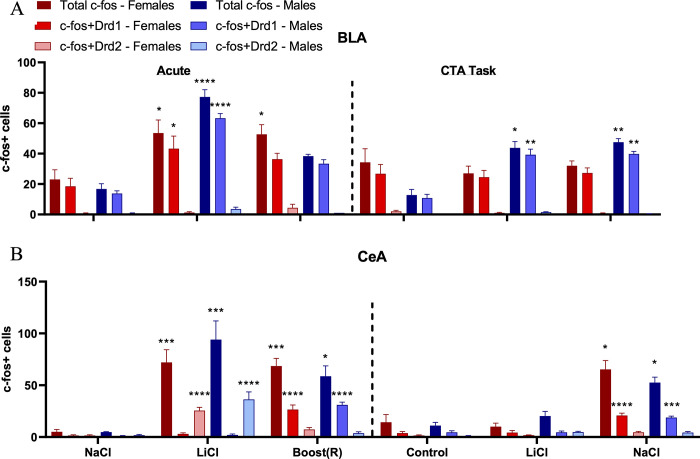
We performed 2-way ANOVA (sex x treatment) for total Fos mRNA. In the acute conditions, total Fos mRNA expression in the BLA showed a main effect of treatment [F (2,17) = 32.00, p< 0.001] and an effect of treatment by sex [F (2,17) = 5.73, p< 0.013] for the acute stimuli. Post hoc analysis showed AB and AL conditions differed from, globally and for both sexes individually. We next considered the co-expression of Fos+/Drd1+ cells. 2-way ANOVA (sex x treatment) revealed an effect of treatment [F (2,22) = 4.03, p< 0.005] and an interaction of treatment x sex [F (2,22) = 4.03, p< 0.037]. Post hoc analyses revealed a difference between the AB and AL conditions compared to AN, both globally and by each sex individually.
For the task condition, 2-way ANOVA revealed a main effect of treatment [F (2,23) = 5.66, p< 0.001] and an interaction of sex x treatment [F (2,23) = 9.46, p< 0.0016]. There was no main effect of sex. Post hoc analysis revealed both the BOT and BLT differed from the COT. By sex, males in the BOT and BLT differed from their respective controls. Females did not differ from controls, which had high levels of baseline Fos.
The same pattern emerged when considering Fos+/Drd1+ co-expressing cells (Fig 10B). There was an effect of treatment [F (2,23) = 8.59, p< 0.0024] and an interaction of sex x treatment [F (2,23) = 9.64, p 0.0014]. There was no main effect of sex. As with total Fos, the BOT and BLT conditions showed greater Fos mRNA expression than the control. As with total Fos only males differed in the BOT and BLT conditions from their respective controls.
The CeA is responsive to rewarding and aversive stimuli in a cell-specific manner
Representative images for the CeA are shown in Fig 9C and results in Fig 10B. Unlike the BLA, the CeA responded to rewarding and aversive stimuli in a cell-specific manner. In the acute condition, ANOVA revealed an effect of treatment [F (2,17) = 30.32 p < 0.001] for total Fos+ cells. There was no main effect of sex or treatment x sex interaction. Post hoc analysis revealed the AN differed from both the AB and AL conditions.
When considering Fos+/Drd1+ co-expressing cells, there was an effect of treatment [F (2,17) = 100.22, p = < 0.001]. There was no main effect of sex or interaction of sex x treatment. The AB condition differed from the AN condition, with robust expression of double positive cells (Fos and Drd1). There was no difference between the AL and AN condition.
The opposite association of Fos and condition was found for Drd2 expressing cells. By ANOVA there was an effect of treatment [F (2,17) = 38.63, p < 0.001]. There was no effect of sex or interaction of treatment x sex. The AL condition showed significant expression of Fos in Drd2+ cells, but not the AB condition.
In the task condition, there was an effect of treatment [F (2,18) = 40.24, p = <0.001], with no effect of sex or treatment x sex interaction. There was significant mRNA expression of Fos in the BOT, but not the BLT condition. When considering Fos+/Drd1+ cells, there was an effect of treatment [F (2,18) = 53.15, p < 0.001]. As with the acute condition, there was significant overlap between Fos+ and Drd1+ cells.
Altogether, these data showed that Drd1+ cells are differentially responsive to the rewarding stimulus in the CeA, while Drd2+ cells are differentially responsive to the aversive stimulus in the CeA. Following treatment with acute LiCl, Fos response showed both anatomical and neuronal cell type specificity, with activation occurring primarily in the CeL and CeC, and co-expressing with Drd2-expressing cells. Conversely, in Fos and Fos/drd1 co-expression were observed in both AB and BOT.
Boost® produced a Fos response in the CeM and CeL and co-expressed with Drd1-expressing cells. Increases in Fos and Fos/Drd1 co-expression were observed in both AB and BOT.
Discussion
Conditioned taste aversion is most often characterized as a task which reflects avoidance/aversion. By assessing behavioral and neural responses to both the reinforcing and aversive stimulus as well as the reinforcing and aversive task (BOT, BLT), the present findings suggest that male and female rats respond behaviorally in a different way and activate different brain areas in anticipation of reinforcing or aversive stimuli. Females demonstrated greater responsiveness to the rewarding stimulus. This responsiveness was reflected in their behavioral tendency to drink more Boost® than males in both AB and BOT conditions, and in Fos activation in the VTA and other areas during the BOT. Furthermore, the correlation between less CTA and 55 kHz USVs in females suggests that their behavior in the BLT was more influenced by memory of the reinforcing aspects of the task than males, as we predicted. Interrogation of the neural network revealed that the reinforcing and aversive stimuli activate circuits in a stimulus- and cell type-specific manner, suggesting that opposing stimuli activate parallel, but distinct, neural pathways.
Behavioral responses to rewarding and aversive stimuli
CTA is an essential behavior to prevent further consumption of foods that may be toxic and should be avoided. The devaluation of the hedonic stimulus when it is paired with an aversive visceral stimulus is critical to the CTA paradigm. For example, delivering only an aversive stimulus like footshock can reduce CS consumption without changing palatability, while aversive visceral experience reduces both consumption and palatability, as measured by CS consumption in the home cage [103–105]. Here, we use USVs to show that the behavior is more complex than simply showing aversion. The literature demonstrates that USVs show high levels of individual variation, including a substantial proportion who do not vocalize at all [27, 64, 106]. Despite this variability, we found that animals were more likely to engage in USVs that reflected the relative reinforcing or aversive qualities of their anticipated stimulus. Indeed, animals whose reinforcing stimulus was previously paired with LiCl were more likely to emit both 55 kHz and 22 kHz calls, suggesting that they are responding in anticipation of both the hedonic and aversive stimulus.
USVs were remarkably sex specific. They proved to be a useful tool to assess sex differences in both positive (55 kHz) and negative (22 kHz) response to stimuli during CTA expression. Males expressed 55 kHz calls in anticipation of Boost® and inhibited 55 kHz calls when the Boost® was devalued with LiCl. The expression of USVs is consistent with the literature on USVs in CTA [107] and our prediction. Increases in 55 kHz in the context previously paired with Boost® suggests animals are assigning a positive valuation to the hedonic stimulus. The converse inhibition of 55 kHz USVs reinforces the notion that USVs can be correlated with an expected outcome. Vocalization patterns in females reflected high-estradiol (more vocal) and low-estradiol (less vocal) states but females were more likely to engage in 22 kHz calls than males. This result could suggest that females are more likely to attach a negative valuation to BLT or that they are more likely, in general, to elicit warning calls to alert the colony of nearby dangers. The latter hypothesis is supported by studies showing females are more likely than males to increase 22 kHz calls in the presence of a predator [108], especially considering their comparable sensitivity to CTA. In addition, during the BLT, more females emitted both 22 kHz and 55 kHz USVs, suggesting as predicted that they would exhibit behaviors indicating greater anticipation of Boost® even after pairing with LiCl. USVs provide additional insight not only into the animal’s valuation of the stimulus, but to how they communicate this valuation.
This is the first study to comprehensively evaluate nausea behaviors in both male and female rats in CTA. We found that male and female rats showed similar nausea behaviors after LiCl administration. Of the three acute nausea behaviors we observed, only lying on belly correlated with CTA expression, suggesting this behavior may be an expression of more severe nausea than pica. Further studies in CTA and other nausea-relevant paradigms may prove useful in characterizing how this behavior correlates to nausea.
The Fos response to rewarding and aversive stimuli
CTA is a unique form of learning in that the stimuli do not need the same temporal proximity as other operant learning and behavioral conditioning paradigms [109, 110]. The nature of digestion is such that visceral malaise may occur minutes to hours after the ingestion of a toxic substance. Hedonic and aversive inputs follow parallel pathways: caudal regions, such as the nucleus of the solitary tract and parabrachial nucleus, increase Fos in response to both LiCl and sucrose [111, 112]. These circuits then converge in brain regions like the amygdala to assess valence. The responses to acute stimuli observed in the present study replicated these basic findings [37, 63, 77, 99, 113–119].
We hypothesized intersection of the neural circuits conveying information about reinforcing and aversive stimuli may be found within the circuit itself, reducing the necessity for events to overlap in time for learning to occur. This schema was borne out in our data. The neural circuit underlying the response to the acute stimuli overlapped in key brain regions, including the BLA, the CeA, the gIC5/6, and the gIC4. In contrast to brain areas that respond to both acute stimuli, the PVN and SON uniquely responded to the aversive stimuli, and the VTA responded uniquely to the reinforcing stimuli. Sampling of more caudal areas may prove useful in delineating the cell type-specific circuit in future studies. Regions such as the NTS [120–127] and PBN [97, 98, 112, 121, 127, 128] which were omitted in the present study to focus on rostral brain areas that are involved in higher-order processing of both aversive (emetic) and rewarding stimuli, and future use of cell-type specific probes will allow more complete dissection of this circuit.
The gustatory cortex (GC, which processes taste [70, 71, 75, 129]) and visceral insular cortex (VIC, which processes visceral stimuli [75, 76, 130, 131]) are topographically distributed throughout the insular cortex, including layers IV, V, and VI [75, 76, 130, 132, 133]. Our data demonstrate that the GC and VIC show significant overlap in Fos expression [75, 134]. The GC reacts to novel taste and lesions are known to cause a decrease in neophobia [58, 71, 129, 135]. Additionally, lesions to the GC can attenuate CTA on first trial, a deficit that disappears with repeated CS-US pairings [72]. These studies raise the question whether the loss of neophobia acts as a latent inhibition, reducing CTA by making the CS appear familiar [42]. An alternative explanation from our studies is the possibility that lesions of the GC likewise destroy cells that are part of the VIC, and thereby reduce the nausea effects of LiCl. Lesion studies that also assess nausea behavior would help to clarify this question. Additionally, our findings of cell-specific Fos responses in the CeA suggested that future studies which interrogate cell-specific responses in areas like the highly heterogenous insular cortex to rewarding and aversive stimuli in a cell-specific manner will provide new insight into integration of affective valence to produce motivated behavior.
In contrast to fear learning, CTA involves the convergence of two stimuli of opposing valence, resulting in complex decision-making rather than a fear-induced defensive response. The RNAScope data showing that the response to rewarding and aversive stimuli show cell-type and anatomical specificity in the CeA suggests that other regions may show similar stimulus-specificity when specific cell populations are evaluated. How these two neural networks converge to produce the memory of the experience, and the subsequent decision-making process that guides the animal’s behavior, is an important area of future study.
We were surprised to see there was no activation of the nucleus accumbens shell after AB, or the core during BOT. One explanation is that most studies showing such activation increased the reward of the hedonic substance by water and/or food deprivation during CTA acquisition, while we did our studies in animals that were not deprived. Studies show deprived animals express higher levels of Fos in these key brain areas compared to satiated animals [92, 94].
We found no sex differences in the neural activation in response to the acute stimuli in any of 11 brain regions we assessed. This was a surprising result, as females drink more Boost® at first exposure and are relatively less sensitive to developing CTA. It is possible that our limited analysis did not sample a critical area that accounts for these differences. A more comprehensive study of the neural circuit that includes other brain regions associated with CTA, such as the bed nucleus of the stria terminalis or parabrachial nucleus may be of value.
The Fos response during CTA expression
Our novel experimental approach included evaluation of the neural circuits engaged during both the acute rewarding and aversive stimuli, as well as during the reinforcing task (expectation of Boost®) and CTA task (expectation of Boost® after pairing with LiCl). Neural activation during BOT was similar to that of AB, while activation during the BLT differed considerably from AL. The rewarding effects of Boost® that were not devalued with LiCl were reinforced with repeated offerings of the hedonic stimulus. We therefore saw similar neural activation during expression of the BOT. There was no increase in Fos with the second pairing, likely because the taste is no longer novel [136, 137]. When this same stimulus was devalued with LiCl, drinking behavior was reduced, and the circuit was inhibited.
During CTA expression, females broadly expressed higher levels of Fos than males. By area, they were significantly more responsive in the gIC5/6 and gIC4 during the BOT. We also found that the VTA increased with the reinforcer (BOT) in only female rats. The VTA is known for its role in reinforcement and addiction, and sends outputs to the nucleus accumbens, amygdala, and frontal cortex [83, 138–140]. Our data support the hypothesis that female rats find the sweet taste more reinforcing and are therefore less sensitive to CTA, especially at lower doses of LiCl. Cell-type analysis would benefit our understanding of how females differentially process sweet taste, a critical area of study in the field of eating disorders [141–149]. This result is particularly interesting considering their relative lack of USV expression in response to the rewarding stimulus. While males are more unequivocal about the “goodness” of Boost®, as evidenced by their consistent expression of 55 kHz USVs, females in low-estradiol states do not express 55 kHz USVs, despite drinking comparably.
Network analysis of Fos correlations
Network analysis of Fos regional correlations during COT, BOT and BLT provided insights into the Fos responses that were not revealed by simple analysis of Fos responses by condition. Each of the three task conditions elicited unique networks of brain area interactions. The control task was the simplest, with two independent small networks and 3 areas with no interactions (vmPFC, NACs and BLA). In the BOT and the BLT, the aIC was a central node in the more complex interactions, a finding consistent with its known role in ingestive tasks and suppression of ingestion associated with sickness [150]. The BLA exhibited correlated responses with aIC and vmPFC in both tasks, also a relationship predicted by BLA’s known role in learned responses (including to taste and ingested stimuli), both appetitive and aversive[48, 101, 151].
The most impressive demonstration from this analysis is the centrality of the VTA in both BOT–COT and BLT–COT networks, and its correlations with PFC, NACs, BLA and CeA with the Boost® task and both NACs and NACc in the LiCl-Control task. A significant body of work has identified specific subpopulations of dopamine neurons in the VTA that respond to positive and negative stimuli through interaction with nucleus accumbens and prefrontal cortex, as well as its innervation of the CeA and BLA [150, 152–156]. All these associations are present in this single model.
Network analysis also revealed previously unexpected relationships. The presence of the PVN and vmPFC in all three task networks in both Boost® and LiCl was not predicted from their Fos levels in individual tasks, given their lack of significant Fos response to task. For PVN, its conflicting negative relationship at least with gIC5/6 and gIC4 suggests that heterogenous populations in the brain area were activated by varying aspects of these conditions. This speculation is strengthened by the more central involvement of the PVN in the BOT–COT and BLT–COT task networks once these Control influences were subtracted. vmPFC Fos was the highest in almost all conditions, but the specificity of its role in each condition might not have been revealed without network analysis.
The multiple interactions revealed from the modest number of brain areas sampled (11) demonstrates the potential value of network analysis, especially the subtraction approach of removing correlations associated with control conditions. For example, it suggests that mechanistic interrogation of dopaminergic influences in CeA and BLA during CTA with approaches like optogenetics and/or DREADDS could be informative. More complex networks can be identified by sampling many brain areas, as has been shown for fear memory [157] and in response to both approach and avoidance associated with social interactions [158].
Dopamine receptors in the amygdala respond to rewarding and aversive stimuli
Our studies showed that Drd1+ neurons were responsive to the rewarding stimulus, while Drd2+ cells were responsive to the aversive stimulus. This is the first time these cell types have been studied in the context of CTA. Kim, et. al. [43] showed that silencing Drd1+ neurons in the CeA inhibited feeding behavior in mice. However, other studies demonstrate that direct blockade of Drd1 in the CeA did not affect feeding behavior, suggesting these neurons are being activated by something other than dopamine. Drd1+ neurons highly co-express with 3 other neuropeptides: tachykinin 2, neurotensin, and somatostatin. Although these neuronal cell types do not affect behavior when manipulated on their own, Kim shows that these cell types may work collectively to coordinate feeding behavior.
Only about 30% of Fos+ neurons in the CeA co-expressed with Drd1+ cells in response to the appetitive stimuli, suggesting additional cell types may also be important for the processing of the reward. A similar percentage of Drd2+ receptors were responsive to LiCl. A more thorough examination of the CeA in response to a food reward and aversive stimulus would be useful in delineating the cell types that process this stimulus.
Contrary to our findings in the CeA, Drd1+ neurons in the BLA responded to both rewarding and aversive stimuli, without input from Drd2+ neurons. These findings suggest that Drd1+ neurons may receive innervation from more caudal areas that respond specifically to these stimuli [159]. Additionally, the BLA is known to respond to rewarding or aversive stimuli in a location-specific manner, with the rostral BLA responding to aversive stimuli and the caudal BLA responding to rewarding stimuli. The dual responses observed in the present study may reflect the location at which we sampled the BLA, which was in the central zone where these two stimuli converge [160].
In summary, these findings support our hypothesis that CTA is best characterized as a task in which animals must balance rewarding and aversive stimuli. As such, it has significant relevance as a task which reveals how affective valence is integrated into decisions about behavior. The present study provides the first comprehensive comparison of how male and female rats respond to the reinforcing stimulus, aversive stimulus and integrate this information to express CTA. These findings support our hypothesis that females may attach more significance to the reinforcing stimulus in assessing the balance of positive and negative affective valence in performing this task.
The USV analysis showing inhibition of 55 kHz calls and increased 22 kHz calls in the LiCl-paired context suggests that additional neural centers may be involved in assigning valence during the CTA task that are not captured by LiCl behaviors or our Fos analysis. Our Fos analysis of rostral brain regions revealed a significant role of the CeA and granular insula, which represents both the gustatory and viscerotopic cortices, in processing of the reinforcing and aversive stimuli and showed increased global Fos expression in females overall during the task. Further characterization of both the acute and conditioned circuits in males and females will help clarify how these stimuli are differentially processed between the sexes. Finally, our data found a novel role for specific cell types in the CeA and BLA that have not previously been studied. These results suggest that similar cell-specific analysis in cortical areas will continue to reveal details of the neural circuit(s) engaged while animals learn this type of task.
Materials and methods
Animals
Male and female Sprague Dawley rats [post-natal day (PN) 60, Charles River Laboratories, Raleigh, NC] were received 1 week before behavior tests. Rats were housed by sex in ventilated plastic cages with ad libitum PM5001 Rodent Chow and water on a 7 am-7pm light/dark cycle. Females were not selected based on estrous cycle stage, but cycle was determined by lavage on the final day of behavioral testing. A single lavage was performed as repeated lavages can establish place preference for the location of lavage and suppress behavioral responses to other reinforcers [161].
Conditioned taste aversion protocol
Animals were conditioned to ip injections with NaCl for 3–4 consecutive days prior to experimental procedures. The conditioned taste aversion protocol (Fig 1A) was conducted over 3 days using a slight modification of the Boost® method described by Anderson and colleagues. [106] On day 0, animals were water deprived overnight and then conditioned to test cage. Water bottles were weighed and placed on cages with spouts pointing way. Rats were allowed to habituate to their new environment for 30 minutes, after which they were given access to water for 60 minutes in the test cage, to condition them to drinking from new bottles. Water bottles were weighed again, and consumption was measured.
On day 1, animals were returned to the test cage and allowed to habituate for 30 minutes. They were then offered Boost® and allowed to drink for 60 minutes. Boost® consumption was measured. If animals drank a minimum of 1 mL of Boost®, they were then injected with LiCl (38 mg/kg, 0.15M) or the equivalent volume of isotonic NaCl. This dose was selected to achieve a robust Fos response for interrogation of the neural network. They were observed for 60 minutes for LiCl-related behaviors. They were then returned to their home cages and allowed to drink and eat ad libitum.
On day 2, non-water deprived animals were returned to the same test cage and allowed to habituate for 30 minutes. They were then given a 2-bottle test, offering either Boost® or water for 60 minutes. Boost® and water consumption were measured, and the ratio of day 2/day 1 (D2/D1) consumption was calculated. After 60 minutes of re-exposure to the Boost®, animals were perfused with formalin and brains collected for Fos analysis. Brains were post-fixed in formalin overnight at 4°C, then cryoprotected in 30% sucrose solution (30% sucrose, 70% PBS) for 3–4 days. Brains were flash-frozen in brain molds in ethanol/dry ice bath, in 2:1 30% sucrose/tissue freezing media solution. They were stored at -80°C until ready to cut for immunohistochemistry or RNAScope.
Animals whose brains were analyzed for the acute stimulus were taken through day 0 as described. On day 1, they were allowed to habituate for 30 minutes in the test cage before receiving LiCl (38 mg/kg) or the equivalent of isotonic NaCl or were allowed to drink Boost® for one hour, after which they were perfused, and brains collected for Fos analysis (see Table 3). Experimental conditions are described in Table 3.
Table 3. Experimental conditions.
| Acute Stimulus | Conditioned Stimulus |
|---|---|
| Boost® | Boost® paired with NaCl |
| LiCl (38 mg/kg) | Boost® paired with LiCl |
| NaCl (control) | Context only control |
Behavior scoring
Three behaviors were time-sampled as a behavioral estimation of nausea [pica, ptosis, lying on belly (LOB)]. As rats do not vomit, they will often attempt to dilute an ingested toxin through pica, the consumption of non-food substances. LiCl behaviors were assessed using a modified version of published measures: Pica and LOB are well known LiCl behaviors [162–164], and ptosis was included following the observation that it occurred frequently in LiCl-treated animals compared to NaCl-treated animals. Animals are observed for two consecutive 15-second blocks (30 seconds total) every 5 minutes for 1 hour. Animals were given a score of 0–3 as described in Table 4.
Scoring of ultrasonic vocalizations
In a within-groups design, rats were split by sex, treatment [NaCl + H2O (Control), NaCl + Boost®, and LiCl + Boost®] on Day 2 of CTA experiment (task day). USVs were recorded with an Echo Meter Touch 2, from Wildlife Acoustics, an accessory that attaches to a lightning port on the Apple iPhone. The Echo Meter Touch Bat Detector iOS app (v 2.7.20) was used to record the vocalizations. Recordings were uploaded from the smartphone to a secure online server. Manual scoring of the USVs during the 10-minute recording period was done using the visualization software from Wildlife Acoustics, Kaleidoscope Pro Analysis. Scoring was performed by a blinded observer in batches of 1-minute intervals. The scorer determined how often the rat engaged in >10 55 kHz vocalizations, or >1 22 kHz vocalizations in each interval. Scores were calculated as a percentage of the 10 1-minute blocks in which the vocalizations occurred.
Collection of brain area slices and immunohistochemistry
Brains were cut on a Leica CM3050S cryostat to produce 30 uM slices at approximately 180 μm intervals (every 6th slice) through the rostral to caudal extent of the region. The coordinates that were sampled are provided in Table 5. Coordinates were based on the Paxinos and Watson Rat Brain Atlas. Three to four slices were stained for each rat, and 2 images (both hemispheres) were taken per slice and counted at 20X. Manual counts of fos+ cells using FIJI were performed under blinded conditions for each animal and averaged. Six slices per region were counted per region for each rat and averaged to provide a single value for each area/rat except for the PVN which yielded only 2 slices using the cutting parameters described above. Slices were stored in 1:1 TBS/glycerol solution at -20°C until ready to stain. To stain, slices were first washed in 0.2% Triton-X in TBS solution 3 times. Slices were then blocked for 1 hour in solution of 0.3% Triton-X and 5% normal goat serum (NGS) in PBS. Slices were stained overnight in 0.3% Triton-X, 5% NGS, and 1:20,000 anti-Fos antibody (Abcam—ab190289) with gentle shaking at 4°C. Slices were then washed 3 times in solution of 0.3% Triton-X and 5% NGS in PBS (10 minutes first wash, 30 minutes second wash, 40 minutes third wash). Slices were then stained with secondary antibody (AlexaFluor 488, Invitrogen–A-11034) in 0.3% Triton-X, 5% NGS, and 1:200 secondary antibody for 2 hours. Slices were then washed in 1X PBS (10 minutes first wash, 30 minutes second wash, and 40 minutes third wash), with DAPI (R&D Systems 5748) added at a dilution of 1:10,000 for last 10 minutes of second wash. Slices were mounted on VWR Superfrost® Plus microscope slides with a drop of Vecta-Shield anti-fade medium (Vector Laboratories 101098–042).
Table 5.
| Brain area | Coordinates |
|---|---|
| vmPFC | Bregma +3.72 to +3.00 |
| aIC | Bregma +1.92 to +1.20 |
| gIC4 | |
| gIC5/6 | |
| NAcC | |
| NAcS | |
| SON | Bregma -1.20 to -1.55 |
| BLA | Bregma -2.16 to -2.80 |
| CeA | Bregma -2.16 to -2.80 |
| PVN | Bregma -1.80 to -2.04 |
| VTA/PBP | Bregma -4.80 to -5.04 |
Vaginal lavage and estrus cycle analysis
A vaginal lavage smear was performed on all female test subjects prior to transcardial perfusion. Vaginal cytology was analyzed to determine the phase of estrous cycle. The method of estrous cycle analysis as previously described [161]. Animals were grouped into high estradiol states (proestrus/estrus) and low estradiol states (metestrus/diestrus).
mRNA In Situ hybridization
Brains were cut at 14 uM on a Leica CM3050S cryostat and placed on a Superfrost® Plus Micro Slide. 3 consecutive slices were placed on each slide. Slices were collected ~90 uM apart. Slides were desiccated at -20°C for 20 minutes, then placed into -80°C with desiccants for long term storage. Slides were stained within 1 month of collection for optimal signal.
When ready to stain, slides were placed in 1X PBS for 5 minutes to remove OTC then placed in the HybEZ™ oven for 30 minutes at 60°C. They were then transferred to cold PFA for 15 minutes at 4°C, after which they were taken through the mRNA in situ hybridization procedure as outlined for RNAscope by ACDBio, using Protease IV. Probes were for Fos (Rn-Fos—403591), Drd1 (Rn-Drd1a-C2, 317031-C2), and Drd2 (Rn-Drd2-C3–315641-C3). The Fos probe contained 20 oligo pairs and targeted region 84–1218 (Acc. No. NM_001256509.1) of the Fos transcript. The Drd1 probe contained 20 oligo pairs and targeted region 104–1053 (Acc. No. NM_012546.2) of the DRD1 transcript. The Drd2 probe contained 20 oligo pairs and targeted region 445–1531 (Acc. No. NM_012547.1) of the DRD2 transcript. Secondary antibodies were obtained from Akoya (OPAL 690, OPAL 520, and OPAL 570) and diluted to a concentration of 1:750. All experiments were run with positive and negative controls. The positive control targeted Polr2a (C1), PPIB (C2), and UBC (C3). The negative control targeted DapB (of Bacillus subtilis strain).
Imaging and analysis
Slides were imaged with an Axio Imager upright microscope at 20x (16 z-stacks 2 uM apart) for IHC or 20x (8 z-stacks 1 uM apart) for mRNA in situ hybridization. Images were z-projected for max intensity using FIJI (ImageJ) software and Fos+ neurons were manually counted. 2–6 slices per region were imaged and counted per animal. Counts were averaged per animal.
Drugs
LiCl was purchased from Sigma Aldrich (203637). LiCl was dissolved in distilled sterile water to a concentration of 0.15M. Sterile isotonic saline (NaCl, 0.9%; 0.15M) was used as a control injection.
Network analysis
Network analysis of the COT, BOT and BLT were conducted using slight modifications from a similar approach used to analyze networks activated by D1 and D2 agonists in developing rat brain [165]. Correlations among Fos levels in brain areas in each task condition were established by developing a matrix of correlations for each pair of brain areas for every subject. Males and females were combined to generate adequate statistical power to conduct the analysis. A symmetrical matrix of statistically significant (p < 0.05 or better) Pearson correlation coefficients (r) was created in NCSS. All other correlations were set at 0. This matrix was loaded into UCINET 6.370 (Analytic Technologies, Lexington KY) [166]. Networks for COT, BOT and BLT were visualized with Netdraw. Each brain area appears as a node, and statistically significant correlations between specific pairs of brain areas are indicated as a line between brain areas. Figures are derived from UCINET graph theoretic layout with distance and node repulsion which provided the clearest illustration of all the correlations detected. Members of independent substructures identified by UCINET software (in COT only) are indicated by the color of the symbols. Brain areas with no Fos correlations with other brain areas are shown in the upper left. A secondary analysis of BLT and BOT networks after subtraction of correlations associated with the COT was conducted. All Pearson correlation coefficients were converted to Z scores using the Fischer transformation (⅟2[ln (1 + r)–ln (1-r]) then Z scores for control conditions were subtracted from BOT and from BLT, Z-scores were back-translated to Pearson correlation coefficients, t values calculated using the formula r*√(n-2)/√(1-r2). Significant correlations in the subtraction networks were visualized as described above. This approach was selected in lieu of more typical heatmaps of Pearson correlation coefficients as it provides more information about relationships among multiple areas and is more rigorous because it includes only statistically significant correlations.
Statistics
All results were analyzed by ANOVA with post hoc Fisher’s exact test corrected for multiple comparisons using the statistical package NCSS. Behavioral results were analyzed by 2-way ANOVA (sex x treatment). Fos responses were analyzed by 3-way repeated measures ANOVA (sex and condition as between measures and brain area as repeated measure). All conditions yielded area as a main effect, and second level ANOVAs were run for each brain area independently. Fisher’s LSD post hoc was used to determine groups that differed from control. Tukey’s post hoc was used in RNAScope experiment. N for all behavior and Fos experiments was estimated by power analysis and variance observed in previous experiments with CTA behavior and Fos analysis of multiple brain regions [120–122, 124, 167–170]. Individual differences shown in estrous cycle and USVs to illustrate individual variability in behavior.
Data Availability
Data have been uploaded to the Duke Data Repository. DOI assigned is: https://doi.org/10.7924/r44q80q5d.
Funding Statement
AA017621 from the National Institute on Alcohol Abuse and Alcoholism (https://www.niaaa.nih.gov) and funds from the Department of Pharmacology and Cancer Biology (https://pharmacology.duke.edu) for CK Grant No. GM007171 from the Duke Medical Scientist Training Program (https://medschool.duke.edu/education/degree-programs-and-admissions/medical-scientist-training-program/about-mstp-program) AB.
References
- 1.Bangasser DA, Valentino RJ. Sex differences in stress-related psychiatric disorders: neurobiological perspectives. Front Neuroendocrinol. 2014;35(3):303–19. doi: 10.1016/j.yfrne.2014.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferrari AJ, Somerville AJ, Baxter AJ, Norman R, Patten SB, Vos T, et al. Global variation in the prevalence and incidence of major depressive disorder: a systematic review of the epidemiological literature. Psychol Med. 2013;43(3):471–81. doi: 10.1017/S0033291712001511 [DOI] [PubMed] [Google Scholar]
- 3.Franceschini A, Fattore L. Gender-specific approach in psychiatric diseases: Because sex matters. Eur J Pharmacol. 2021;896:173895. doi: 10.1016/j.ejphar.2021.173895 [DOI] [PubMed] [Google Scholar]
- 4.Seedat S, Scott KM, Angermeyer MC, Berglund P, Bromet EJ, Brugha TS, et al. Cross-national associations between gender and mental disorders in the World Health Organization World Mental Health Surveys. Arch Gen Psychiatry. 2009;66(7):785–95. doi: 10.1001/archgenpsychiatry.2009.36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Asarian L, Geary N. Sex differences in the physiology of eating. Am J Physiol Regul Integr Comp Physiol. 2013;305(11):R1215–67. doi: 10.1152/ajpregu.00446.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kokras N, Dalla C. Sex differences in animal models of psychiatric disorders. Br J Pharmacol. 2014;171(20):4595–619. doi: 10.1111/bph.12710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hodes GE, Pfau ML, Purushothaman I, Ahn HF, Golden SA, Christoffel DJ, et al. Sex Differences in Nucleus Accumbens Transcriptome Profiles Associated with Susceptibility versus Resilience to Subchronic Variable Stress. J Neurosci. 2015;35(50):16362–76. doi: 10.1523/JNEUROSCI.1392-15.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dalla C, Pitychoutis PM, Kokras N, Papadopoulou-Daifoti Z. Sex differences in animal models of depression and antidepressant response. Basic Clin Pharmacol Toxicol. 2010;106(3):226–33. doi: 10.1111/j.1742-7843.2009.00516.x [DOI] [PubMed] [Google Scholar]
- 9.Dalla C, Antoniou K, Kokras N, Drossopoulou G, Papathanasiou G, Bekris S, et al. Sex differences in the effects of two stress paradigms on dopaminergic neurotransmission. Physiol Behav. 2008;93(3):595–605. doi: 10.1016/j.physbeh.2007.10.020 [DOI] [PubMed] [Google Scholar]
- 10.Delprato DJ, Thompson RW. Effect of electroconvulsive shock on passive avoidance learning with high and low intensity footshock. Psychol Rep. 1965;17(1):209–10. doi: 10.2466/pr0.1965.17.1.209 [DOI] [PubMed] [Google Scholar]
- 11.Dennis M. Sex-dependent and sex-independent neural control of reactivity to electric footshock in the rat. Exp Neurol. 1972;37(2):256–68. doi: 10.1016/0014-4886(72)90072-6 [DOI] [PubMed] [Google Scholar]
- 12.Dalla C, Shors TJ. Sex differences in learning processes of classical and operant conditioning. Physiol Behav. 2009;97(2):229–38. doi: 10.1016/j.physbeh.2009.02.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parker LA. Conditioned flavor avoidance and conditioned gaping: rat models of conditioned nausea. Eur J Pharmacol. 2014;722:122–33. doi: 10.1016/j.ejphar.2013.09.070 [DOI] [PubMed] [Google Scholar]
- 14.Chambers KC. Conditioned taste aversions. World J Otorhinolaryngol Head Neck Surg. 2018;4(1):92–100. doi: 10.1016/j.wjorl.2018.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mediavilla C, Molina F, Puerto A. Concurrent conditioned taste aversion: a learning mechanism based on rapid neural versus flexible humoral processing of visceral noxious substances. Neurosci Biobehav Rev. 2005;29(7):1107–18. doi: 10.1016/j.neubiorev.2005.06.002 [DOI] [PubMed] [Google Scholar]
- 16.Welzl H, D’Adamo P , Lipp HP. Conditioned taste aversion as a learning and memory paradigm. Behav Brain Res. 2001;125(1–2):205–13. doi: 10.1016/s0166-4328(01)00302-3 [DOI] [PubMed] [Google Scholar]
- 17.Schramm-Sapyta NL, Francis R, MacDonald A, Keistler C, O’Neill L, Kuhn CM. Effect of sex on ethanol consumption and conditioned taste aversion in adolescent and adult rats. Psychopharmacology (Berl). 2014;231(8):1831–9. doi: 10.1007/s00213-013-3319-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Angulo R, Arevalo-Romero CA. Sexual dimorphism in classical conditioning? Sex differences in neophobia, latent inhibition, generalization, and extinction for rats (Rattus norvegicus) in a conditioned taste aversion preparation irrespective of housing conditions. J Comp Psychol. 2021;135(3):315–26. doi: 10.1037/com0000275 [DOI] [PubMed] [Google Scholar]
- 19.Angulo R, Bustamante J, Arevalo-Romero CA. Age, sex and pre-exposure effects on acquisition and generalization of conditioned taste aversion in rats. Behav Brain Res. 2020;394:112813. doi: 10.1016/j.bbr.2020.112813 [DOI] [PubMed] [Google Scholar]
- 20.Jones JD, Busse GD, Riley AL. Strain-dependent sex differences in the effects of alcohol on cocaine-induced taste aversions. Pharmacol Biochem Behav. 2006;83(4):554–60. doi: 10.1016/j.pbb.2006.03.017 [DOI] [PubMed] [Google Scholar]
- 21.Rinker JA, Busse GD, Riley AL. An assessment of sex differences in nicotine-induced conditioned taste aversions. Pharmacol Biochem Behav. 2008;88(4):427–31. doi: 10.1016/j.pbb.2007.09.016 [DOI] [PubMed] [Google Scholar]
- 22.Randall-Thompson JF, Riley AL. Morphine-induced conditioned taste aversions: assessment of sexual dimorphism. Pharmacol Biochem Behav. 2003;76(2):373–81. doi: 10.1016/j.pbb.2003.08.010 [DOI] [PubMed] [Google Scholar]
- 23.Kim EU, Spear LP. Sex-dependent consequences of pre-pubertal gonadectomy: Social behavior, stress and ethanol responsivity. Behav Brain Res. 2016;296:260–9. doi: 10.1016/j.bbr.2015.09.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Anderson RI, Agoglia AE, Morales M, Varlinskaya EI, Spear LP. Stress, kappa manipulations, and aversive effects of ethanol in adolescent and adult male rats. Neuroscience. 2013;249:214–22. doi: 10.1016/j.neuroscience.2012.12.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Costa G, Serra M, Marongiu J, Morelli M, Simola N. Influence of dopamine transmission in the medial prefrontal cortex and dorsal striatum on the emission of 50-kHz ultrasonic vocalizations in rats treated with amphetamine: Effects on drug-stimulated and conditioned calls. Prog Neuropsychopharmacol Biol Psychiatry. 2020;97:109797. doi: 10.1016/j.pnpbp.2019.109797 [DOI] [PubMed] [Google Scholar]
- 26.Demaestri C, Brenhouse HC, Honeycutt JA. 22 kHz and 55 kHz ultrasonic vocalizations differentially influence neural and behavioral outcomes: Implications for modeling anxiety via auditory stimuli in the rat. Behav Brain Res. 2019;360:134–45. doi: 10.1016/j.bbr.2018.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mulvihill KG, Brudzynski SM. Effect of microinjections of dopamine into the nucleus accumbens shell on emission of 50kHz USV: Comparison with effects of d-amphetamine. Pharmacol Biochem Behav. 2019;176:23–32. doi: 10.1016/j.pbb.2018.11.006 [DOI] [PubMed] [Google Scholar]
- 28.Willey AR, Spear LP. Development of anticipatory 50 kHz USV production to a social stimuli in adolescent and adult male Sprague-Dawley rats. Behav Brain Res. 2012;226(2):613–8. doi: 10.1016/j.bbr.2011.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cagiano R, Barfield RJ, White NR, Pleim ET, Cuomo V. Mediation of rat postejaculatory 22 kHz ultrasonic vocalization by dopamine D2 receptors. Pharmacol Biochem Behav. 1989;34(1):53–8. doi: 10.1016/0091-3057(89)90352-3 [DOI] [PubMed] [Google Scholar]
- 30.Reno JM, Thakore N, Cormack LK, Schallert T, Bell RL, Maddox WT, et al. Negative Affect-Associated USV Acoustic Characteristics Predict Future Excessive Alcohol Drinking and Alcohol Avoidance in Male P and NP Rats. Alcohol Clin Exp Res. 2017;41(4):786–97. doi: 10.1111/acer.13344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Taylor JO, Urbano CM, Cooper BG. Differential patterns of constant frequency 50 and 22 khz usv production are related to intensity of negative affective state. Behav Neurosci. 2017;131(1):115–26. doi: 10.1037/bne0000184 [DOI] [PubMed] [Google Scholar]
- 32.Thakore N, Reno JM, Gonzales RA, Schallert T, Bell RL, Maddox WT, et al. Alcohol enhances unprovoked 22–28 kHz USVs and suppresses USV mean frequency in High Alcohol Drinking (HAD-1) male rats. Behav Brain Res. 2016;302:228–36. doi: 10.1016/j.bbr.2016.01.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Litvin Y, Blanchard DC, Blanchard RJ. Rat 22kHz ultrasonic vocalizations as alarm cries. Behav Brain Res. 2007;182(2):166–72. doi: 10.1016/j.bbr.2006.11.038 [DOI] [PubMed] [Google Scholar]
- 34.Pirnik Z, Kiss A. Detection of oxytocin mRNA in hypertonic saline Fos-activated PVN neurons: comparison of chromogens in dual immunocytochemical and in situ hybridization procedure. Endocr Regul. 2002;36(1):23–30. [PubMed] [Google Scholar]
- 35.Pirnik Z, Mravec B, Kiss A. Fos protein expression in mouse hypothalamic paraventricular (PVN) and supraoptic (SON) nuclei upon osmotic stimulus: colocalization with vasopressin, oxytocin, and tyrosine hydroxylase. Neurochem Int. 2004;45(5):597–607. doi: 10.1016/j.neuint.2004.04.003 [DOI] [PubMed] [Google Scholar]
- 36.Olson BR, Drutarosky MD, Stricker EM, Verbalis JG. Brain oxytocin receptor antagonism blunts the effects of anorexigenic treatments in rats: evidence for central oxytocin inhibition of food intake. Endocrinology. 1991;129(2):785–91. doi: 10.1210/endo-129-2-785 [DOI] [PubMed] [Google Scholar]
- 37.Spencer CM, Houpt TA. Dynamics of c-fos and ICER mRNA expression in rat forebrain following lithium chloride injection. Brain Res Mol Brain Res. 2001;93(2):113–26. doi: 10.1016/s0169-328x(01)00173-5 [DOI] [PubMed] [Google Scholar]
- 38.St Andre J, Albanos K, Reilly S. C-fos expression in the rat brain following lithium chloride-induced illness. Brain Res. 2007;1135(1):122–8. doi: 10.1016/j.brainres.2006.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jahng JW, Lee JH, Lee S, Lee JY, Kim GT, Houpt TA, et al. N(omega)-nitro-L-arginine methyl ester attenuates lithium-induced c-Fos, but not conditioned taste aversion, in rats. Neurosci Res. 2004;50(4):485–92. doi: 10.1016/j.neures.2004.08.016 [DOI] [PubMed] [Google Scholar]
- 40.Herisson FM, Waas JR, Fredriksson R, Schioth HB, Levine AS, Olszewski PK. Oxytocin Acting in the Nucleus Accumbens Core Decreases Food Intake. J Neuroendocrinol. 2016;28(4). doi: 10.1111/jne.12381 [DOI] [PubMed] [Google Scholar]
- 41.Olszewski PK, Shi Q, Billington CJ, Levine AS. Opioids affect acquisition of LiCl-induced conditioned taste aversion: involvement of OT and VP systems. Am J Physiol Regul Integr Comp Physiol. 2000;279(4):R1504–11. doi: 10.1152/ajpregu.2000.279.4.R1504 [DOI] [PubMed] [Google Scholar]
- 42.Reilly S, Bornovalova MA. Conditioned taste aversion and amygdala lesions in the rat: a critical review. Neurosci Biobehav Rev. 2005;29(7):1067–88. doi: 10.1016/j.neubiorev.2005.03.025 [DOI] [PubMed] [Google Scholar]
- 43.Kim J, Zhang X, Muralidhar S, LeBlanc SA, Tonegawa S. Basolateral to Central Amygdala Neural Circuits for Appetitive Behaviors. Neuron. 2017;93(6):1464–79 e5. doi: 10.1016/j.neuron.2017.02.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Uematsu A, Kitamura A, Iwatsuki K, Uneyama H, Tsurugizawa T. Correlation Between Activation of the Prelimbic Cortex, Basolateral Amygdala, and Agranular Insular Cortex During Taste Memory Formation. Cereb Cortex. 2015;25(9):2719–28. doi: 10.1093/cercor/bhu069 [DOI] [PubMed] [Google Scholar]
- 45.Arieli E, Gerbi R, Shein-Idelson M, Moran A. Temporally-precise basolateral amygdala activation is required for the formation of taste memories in gustatory cortex. J Physiol. 2020;598(23):5505–22. doi: 10.1113/JP280213 [DOI] [PubMed] [Google Scholar]
- 46.Ferreira G, Miranda MI, De la Cruz V, Rodriguez-Ortiz CJ, Bermudez-Rattoni F. Basolateral amygdala glutamatergic activation enhances taste aversion through NMDA receptor activation in the insular cortex. Eur J Neurosci. 2005;22(10):2596–604. doi: 10.1111/j.1460-9568.2005.04440.x [DOI] [PubMed] [Google Scholar]
- 47.Torrealba F, Madrid C, Contreras M, Gomez K. Plasticity in the Interoceptive System. Adv Exp Med Biol. 2017;1015:59–74. doi: 10.1007/978-3-319-62817-2_4 [DOI] [PubMed] [Google Scholar]
- 48.Abe K, Kuroda M, Narumi Y, Kobayashi Y, Itohara S, Furuichi T, et al. Cortico-amygdala interaction determines the insular cortical neurons involved in taste memory retrieval. Mol Brain. 2020;13(1):107. doi: 10.1186/s13041-020-00646-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lavi K, Jacobson GA, Rosenblum K, Luthi A. Encoding of Conditioned Taste Aversion in Cortico-Amygdala Circuits. Cell Rep. 2018;24(2):278–83. doi: 10.1016/j.celrep.2018.06.053 [DOI] [PubMed] [Google Scholar]
- 50.Yamamoto T, Ueji K. Brain mechanisms of flavor learning. Front Syst Neurosci. 2011;5:76. doi: 10.3389/fnsys.2011.00076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nerad L, Ramirez-Amaya V, Ormsby CE, Bermudez-Rattoni F. Differential effects of anterior and posterior insular cortex lesions on the acquisition of conditioned taste aversion and spatial learning. Neurobiol Learn Mem. 1996;66(1):44–50. doi: 10.1006/nlme.1996.0042 [DOI] [PubMed] [Google Scholar]
- 52.Huang ACW, Yu YH, He ABH, Ou CY. Interactions between prelimbic cortex and basolateral amygdala contribute to morphine-induced conditioned taste aversion in conditioning and extinction. Neurobiol Learn Mem. 2020;172:107248. doi: 10.1016/j.nlm.2020.107248 [DOI] [PubMed] [Google Scholar]
- 53.Mickley GA, Hoxha Z, Bacik S, Kenmuir CL, Wellman JA, Biada JM, et al. Spontaneous recovery of a conditioned taste aversion differentially alters extinction-induced changes in c-Fos protein expression in rat amygdala and neocortex. Brain Res. 2007;1152:139–57. doi: 10.1016/j.brainres.2007.03.050 [DOI] [PubMed] [Google Scholar]
- 54.Mickley GA, Kenmuir CL, Yocom AM, Wellman JA, Biada JM. A role for prefrontal cortex in the extinction of a conditioned taste aversion. Brain Res. 2005;1051(1–2):176–82. doi: 10.1016/j.brainres.2005.05.033 [DOI] [PubMed] [Google Scholar]
- 55.Gonzalez MC, Kramar CP, Tomaiuolo M, Katche C, Weisstaub N, Cammarota M, et al. Medial prefrontal cortex dopamine controls the persistent storage of aversive memories. Frontiers in behavioral neuroscience. 2014;8:408. doi: 10.3389/fnbeh.2014.00408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Barragan R, Coltell O, Portoles O, Asensio EM, Sorli JV, Ortega-Azorin C, et al. Bitter, Sweet, Salty, Sour and Umami Taste Perception Decreases with Age: Sex-Specific Analysis, Modulation by Genetic Variants and Taste-Preference Associations in 18 to 80 Year-Old Subjects. Nutrients. 2018;10(10). doi: 10.3390/nu10101539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sanchez-Catalan MJ, Faivre F, Yalcin I, Muller MA, Massotte D, Majchrzak M, et al. Response of the Tail of the Ventral Tegmental Area to Aversive Stimuli. Neuropsychopharmacology. 2017;42(3):638–48. doi: 10.1038/npp.2016.139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Soto A, Gasalla P, Begega A, Lopez M. c-Fos activity in the insular cortex, nucleus accumbens and basolateral amygdala following the intraperitoneal injection of saccharin and lithium chloride. Neurosci Lett. 2017;647:32–7. doi: 10.1016/j.neulet.2017.03.025 [DOI] [PubMed] [Google Scholar]
- 59.Ramirez-Lugo L, Nunez-Jaramillo L, Bermudez-Rattoni F. Taste memory formation: role of nucleus accumbens. Chem Senses. 2007;32(1):93–7. doi: 10.1093/chemse/bjl023 [DOI] [PubMed] [Google Scholar]
- 60.Roitman MF, Wheeler RA, Tiesinga PH, Roitman JD, Carelli RM. Hedonic and nucleus accumbens neural responses to a natural reward are regulated by aversive conditioning. Learn Mem. 2010;17(11):539–46. doi: 10.1101/lm.1869710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fenu S, Bassareo V, Di Chiara G. A role for dopamine D1 receptors of the nucleus accumbens shell in conditioned taste aversion learning. J Neurosci. 2001;21(17):6897–904. doi: 10.1523/JNEUROSCI.21-17-06897.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Marotta R, Fenu S, Scheggi S, Vinci S, Rosas M, Falqui A, et al. Acquisition and expression of conditioned taste aversion differentially affects extracellular signal regulated kinase and glutamate receptor phosphorylation in rat prefrontal cortex and nucleus accumbens. Frontiers in behavioral neuroscience. 2014;8:153. doi: 10.3389/fnbeh.2014.00153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yasoshima Y, Scott TR, Yamamoto T. Memory-dependent c-Fos expression in the nucleus accumbens and extended amygdala following the expression of a conditioned taste aversive in the rat. Neuroscience. 2006;141(1):35–45. doi: 10.1016/j.neuroscience.2006.03.019 [DOI] [PubMed] [Google Scholar]
- 64.Singh T, McDannald MA, Haney RZ, Cerri DH, Schoenbaum G. Nucleus Accumbens Core and Shell are Necessary for Reinforcer Devaluation Effects on Pavlovian Conditioned Responding. Front Integr Neurosci. 2010;4:126. doi: 10.3389/fnint.2010.00126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang X, Kim J, Tonegawa S. Amygdala Reward Neurons Form and Store Fear Extinction Memory. Neuron. 2020;105(6):1077–93 e7. doi: 10.1016/j.neuron.2019.12.025 [DOI] [PubMed] [Google Scholar]
- 66.Kim J, Pignatelli M, Xu S, Itohara S, Tonegawa S. Antagonistic negative and positive neurons of the basolateral amygdala. Nat Neurosci. 2016;19(12):1636–46. doi: 10.1038/nn.4414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.O’Neill PK, Gore F, Salzman CD. Basolateral amygdala circuitry in positive and negative valence. Curr Opin Neurobiol. 2018;49:175–83. doi: 10.1016/j.conb.2018.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Beyeler A, Chang CJ, Silvestre M, Leveque C, Namburi P, Wildes CP, et al. Organization of Valence-Encoding and Projection-Defined Neurons in the Basolateral Amygdala. Cell Rep. 2018;22(4):905–18. doi: 10.1016/j.celrep.2017.12.097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kosar E, Grill HJ, Norgren R. Gustatory cortex in the rat. II. Thalamocortical projections. Brain Res. 1986;379(2):342–52. doi: 10.1016/0006-8993(86)90788-2 [DOI] [PubMed] [Google Scholar]
- 70.Kosar E, Grill HJ, Norgren R. Gustatory cortex in the rat. I. Physiological properties and cytoarchitecture. Brain Res. 1986;379(2):329–41. doi: 10.1016/0006-8993(86)90787-0 [DOI] [PubMed] [Google Scholar]
- 71.Gallo M, Roldan G, Bures J. Differential involvement of gustatory insular cortex and amygdala in the acquisition and retrieval of conditioned taste aversion in rats. Behav Brain Res. 1992;52(1):91–7. doi: 10.1016/s0166-4328(05)80328-6 [DOI] [PubMed] [Google Scholar]
- 72.Lin JY, Arthurs J, Reilly S. Gustatory insular cortex, aversive taste memory and taste neophobia. Neurobiol Learn Mem. 2015;119:77–84. doi: 10.1016/j.nlm.2015.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.King MS. Distribution of Fos-immunoreactive neurons in the gustatory cortex elicited by intra-oral infusion of taste solutions in conscious rats. Brain Res. 2018;1683:67–77. doi: 10.1016/j.brainres.2018.01.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sticht MA, Limebeer CL, Rafla BR, Abdullah RA, Poklis JL, Ho W, et al. Endocannabinoid regulation of nausea is mediated by 2-arachidonoylglycerol (2-AG) in the rat visceral insular cortex. Neuropharmacology. 2016;102:92–102. doi: 10.1016/j.neuropharm.2015.10.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cechetto DF, Saper CB. Evidence for a viscerotopic sensory representation in the cortex and thalamus in the rat. J Comp Neurol. 1987;262(1):27–45. doi: 10.1002/cne.902620104 [DOI] [PubMed] [Google Scholar]
- 76.Allen GV, Saper CB, Hurley KM, Cechetto DF. Organization of visceral and limbic connections in the insular cortex of the rat. J Comp Neurol. 1991;311(1):1–16. doi: 10.1002/cne.903110102 [DOI] [PubMed] [Google Scholar]
- 77.Spencer CM, Eckel LA, Nardos R, Houpt TA. Area postrema lesions attenuate LiCl-induced c-Fos expression correlated with conditioned taste aversion learning. Physiol Behav. 2012;105(2):151–60. doi: 10.1016/j.physbeh.2011.08.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Klockars OA, Klockars A, Levine AS, Olszewski PK. Oxytocin administration in the basolateral and central nuclei of amygdala moderately suppresses food intake. Neuroreport. 2018;29(6):504–10. doi: 10.1097/WNR.0000000000001005 [DOI] [PubMed] [Google Scholar]
- 79.Head MA, Jewett DC, Gartner SN, Klockars A, Levine AS, Olszewski PK. Effect of Oxytocin on Hunger Discrimination. Front Endocrinol (Lausanne). 2019;10:297. doi: 10.3389/fendo.2019.00297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wald HS, Chandra A, Kalluri A, Ong ZY, Hayes MR, Grill HJ. NTS and VTA oxytocin reduces food motivation and food seeking. Am J Physiol Regul Integr Comp Physiol. 2020;319(6):R673–R83. doi: 10.1152/ajpregu.00201.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Piekut DT, Pretel S, Applegate CD. Activation of oxytocin-containing neurons of the paraventricular nucleus (PVN) following generalized seizures. Synapse. 1996;23(4):312–20. doi: [DOI] [PubMed] [Google Scholar]
- 82.Beier KT, Steinberg EE, DeLoach KE, Xie S, Miyamichi K, Schwarz L, et al. Circuit Architecture of VTA Dopamine Neurons Revealed by Systematic Input-Output Mapping. Cell. 2015;162(3):622–34. doi: 10.1016/j.cell.2015.07.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Li Y, Ge S, Li N, Chen L, Zhang S, Wang J, et al. NMDA and dopamine D1 receptors within NAc-shell regulate IEG proteins expression in reward circuit during cocaine memory reconsolidation. Neuroscience. 2016;315:45–69. doi: 10.1016/j.neuroscience.2015.11.063 [DOI] [PubMed] [Google Scholar]
- 84.Tang W, Kochubey O, Kintscher M, Schneggenburger R. A VTA to Basal Amygdala Dopamine Projection Contributes to Signal Salient Somatosensory Events during Fear Learning. J Neurosci. 2020;40(20):3969–80. doi: 10.1523/JNEUROSCI.1796-19.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nasser HM, McNally GP. Neural correlates of appetitive-aversive interactions in Pavlovian fear conditioning. Learn Mem. 2013;20(4):220–8. doi: 10.1101/lm.029744.112 [DOI] [PubMed] [Google Scholar]
- 86.Hernadi I, Karadi Z, Vigh J, Petyko Z, Egyed R, Berta B, et al. Alterations of conditioned taste aversion after microiontophoretically applied neurotoxins in the medial prefrontal cortex of the rat. Brain Res Bull. 2000;53(6):751–8. doi: 10.1016/s0361-9230(00)00361-0 [DOI] [PubMed] [Google Scholar]
- 87.Hefner K, Whittle N, Juhasz J, Norcross M, Karlsson RM, Saksida LM, et al. Impaired fear extinction learning and cortico-amygdala circuit abnormalities in a common genetic mouse strain. J Neurosci. 2008;28(32):8074–85. doi: 10.1523/JNEUROSCI.4904-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ramirez-Lugo L, Penas-Rincon A, Angeles-Duran S, Sotres-Bayon F. Choice Behavior Guided by Learned, But Not Innate, Taste Aversion Recruits the Orbitofrontal Cortex. J Neurosci. 2016;36(41):10574–83. doi: 10.1523/JNEUROSCI.0796-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hurley SW, Carelli RM. Activation of Infralimbic to Nucleus Accumbens Shell Pathway Suppresses Conditioned Aversion in Male But Not Female Rats. J Neurosci. 2020;40(36):6888–95. doi: 10.1523/JNEUROSCI.0137-20.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yim CY, Mogenson GJ. Response of nucleus accumbens neurons to amygdala stimulation and its modification by dopamine. Brain Res. 1982;239(2):401–15. doi: 10.1016/0006-8993(82)90518-2 [DOI] [PubMed] [Google Scholar]
- 91.Prado-Alcala R, Wise RA. Brain stimulation reward and dopamine terminal fields. I. Caudate-putamen, nucleus accumbens and amygdala. Brain Res. 1984;297(2):265–73. doi: 10.1016/0006-8993(84)90567-5 [DOI] [PubMed] [Google Scholar]
- 92.Moscarello JM, Ben-Shahar O, Ettenberg A. Dynamic interaction between medial prefrontal cortex and nucleus accumbens as a function of both motivational state and reinforcer magnitude: a c-Fos immunocytochemistry study. Brain Res. 2007;1169:69–76. doi: 10.1016/j.brainres.2007.06.064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yamamoto T. Central mechanisms of roles of taste in reward and eating. Acta Physiol Hung. 2008;95(2):165–86. doi: 10.1556/APhysiol.95.2008.2.2 [DOI] [PubMed] [Google Scholar]
- 94.Moscarello JM, Ben-Shahar O, Ettenberg A. Effects of food deprivation on goal-directed behavior, spontaneous locomotion, and c-Fos immunoreactivity in the amygdala. Behav Brain Res. 2009;197(1):9–15. doi: 10.1016/j.bbr.2008.07.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Alejandro Borja GP, Alejandro Navarro E, Beatriz GC, Ignacio M, Milagros G. Accumbens and amygdala in taste recognition memory: The role of d1 dopamine receptors. Neurobiol Learn Mem. 2020;174:107277. doi: 10.1016/j.nlm.2020.107277 [DOI] [PubMed] [Google Scholar]
- 96.Lamprecht R, Dudai Y. Differential modulation of brain immediate early genes by intraperitoneal LiCl. Neuroreport. 1995;7(1):289–93. [PubMed] [Google Scholar]
- 97.Yamamoto T, Shimura T, Sako N, Yasoshima Y, Sakai N. Neural substrates for conditioned taste aversion in the rat. Behav Brain Res. 1994;65(2):123–37. doi: 10.1016/0166-4328(94)90097-3 [DOI] [PubMed] [Google Scholar]
- 98.Yamamoto T. Neural mechanisms of taste aversion learning. Neurosci Res. 1993;16(3):181–5. doi: 10.1016/0168-0102(93)90122-7 [DOI] [PubMed] [Google Scholar]
- 99.Olson BR, Freilino M, Hoffman GE, Stricker EM, Sved AF, Verbalis JG. c-Fos Expression in Rat Brain and Brainstem Nuclei in Response to Treatments That Alter Food Intake and Gastric Motility. Mol Cell Neurosci. 1993;4(1):93–106. doi: 10.1006/mcne.1993.1011 [DOI] [PubMed] [Google Scholar]
- 100.Namburi P, Beyeler A, Yorozu S, Calhoon GG, Halbert SA, Wichmann R, et al. A circuit mechanism for differentiating positive and negative associations. Nature. 2015;520(7549):675–8. doi: 10.1038/nature14366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gore F, Schwartz EC, Brangers BC, Aladi S, Stujenske JM, Likhtik E, et al. Neural Representations of Unconditioned Stimuli in Basolateral Amygdala Mediate Innate and Learned Responses. Cell. 2015;162(1):134–45. doi: 10.1016/j.cell.2015.06.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zhang X, Li B. Population coding of valence in the basolateral amygdala. Nat Commun. 2018;9(1):5195. doi: 10.1038/s41467-018-07679-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Inui T, Sugishita T, Inui-Yamamoto C, Yasoshima Y, Shimura T. The Basolateral Nucleus of the Amygdala Executes the Parallel Processes of Avoidance and Palatability in the Retrieval of Conditioned Taste Aversion in Male Rats. eNeuro. 2019;6(4). doi: 10.1523/ENEURO.0004-19.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Pelchat ML, Grill HJ, Rozin P, Jacobs J. Quality of acquired responses to tastes by Rattus norvegicus depends on type of associated discomfort. J Comp Psychol. 1983;97(2):140–53. [PubMed] [Google Scholar]
- 105.Garcia J, Kovner R, Green KF. Cue properties vs palatability of flavors in avoidance learning. Psychonomic Science. 1970;20(5):313–4. [Google Scholar]
- 106.Sangarapillai N, Ellenberger M, Wohr M, Schwarting RKW. Ultrasonic vocalizations and individual differences in rats performing a Pavlovian conditioned approach task. Behav Brain Res. 2021;398:112926. doi: 10.1016/j.bbr.2020.112926 [DOI] [PubMed] [Google Scholar]
- 107.Hamdani S, White NM. Ultrasonic vocalization ratios reflect the influence of motivational state and amygdala lesions on different types of taste avoidance learning. Behav Brain Res. 2011;217(1):88–98. doi: 10.1016/j.bbr.2010.09.026 [DOI] [PubMed] [Google Scholar]
- 108.Blanchard RJ, Blanchard DC, Agullana R, Weiss SM. Twenty-two kHz alarm cries to presentation of a predator, by laboratory rats living in visible burrow systems. Physiol Behav. 1991;50(5):967–72. doi: 10.1016/0031-9384(91)90423-l [DOI] [PubMed] [Google Scholar]
- 109.Hinderliter CF, Misanin JR. Context familiarity and delayed conditioned taste aversion in young-adult and old-age rats. Percept Mot Skills. 1993;77(3 Pt 2):1403–6. doi: 10.2466/pms.1993.77.3f.1403 [DOI] [PubMed] [Google Scholar]
- 110.Foy MR, Foy JG. Reversal of long-delay conditioned taste aversion learning in rats by sex hormone manipulation. Integr Physiol Behav Sci. 2003;38(3):203–13. doi: 10.1007/BF02688854 [DOI] [PubMed] [Google Scholar]
- 111.Chen K, Yan J, Li J, Lv B, Zhao X. c-Fos expression in rat brainstem following intake of sucrose or saccharin. Front Med. 2011;5(3):294–301. doi: 10.1007/s11684-011-0144-8 [DOI] [PubMed] [Google Scholar]
- 112.Yamamoto T, Shimura T, Sako N, Azuma S, Bai WZ, Wakisaka S. C-fos expression in the rat brain after intraperitoneal injection of lithium chloride. Neuroreport. 1992;3(12):1049–52. doi: 10.1097/00001756-199212000-00004 [DOI] [PubMed] [Google Scholar]
- 113.Kwon B, Houpt TA. Phospho-acetylation of histone H3 in the amygdala after acute lithium chloride. Brain Res. 2010;1333:36–47. doi: 10.1016/j.brainres.2010.03.068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Jahng JW, Choi SH, Kim DG, Houpt TA. Central Nomega-nitro-L-arginine methyl ester does not influence lithium-induced c-Fos and conditioned taste aversion. Yonsei Med J. 2003;44(5):869–74. doi: 10.3349/ymj.2003.44.5.869 [DOI] [PubMed] [Google Scholar]
- 115.Koehnle TJ, Rinaman L. Progressive postnatal increases in Fos immunoreactivity in the forebrain and brain stem of rats after viscerosensory stimulation with lithium chloride. Am J Physiol Regul Integr Comp Physiol. 2007;292(3):R1212–23. doi: 10.1152/ajpregu.00666.2006 [DOI] [PubMed] [Google Scholar]
- 116.Rinaman L, Dzmura V. Experimental dissociation of neural circuits underlying conditioned avoidance and hypophagic responses to lithium chloride. Am J Physiol Regul Integr Comp Physiol. 2007;293(4):R1495–503. doi: 10.1152/ajpregu.00393.2007 [DOI] [PubMed] [Google Scholar]
- 117.Bodnar RJ. Conditioned flavor preferences in animals: Merging pharmacology, brain sites and genetic variance. Appetite. 2018;122:17–25. doi: 10.1016/j.appet.2016.12.015 [DOI] [PubMed] [Google Scholar]
- 118.Park TH, Carr KD. Neuroanatomical patterns of fos-like immunoreactivity induced by a palatable meal and meal-paired environment in saline- and naltrexone-treated rats. Brain Res. 1998;805(1–2):169–80. doi: 10.1016/s0006-8993(98)00719-7 [DOI] [PubMed] [Google Scholar]
- 119.Sakai N, Yamamoto T. Conditioned taste aversion and c-fos expression in the rat brainstem after administration of various USs. Neuroreport. 1997;8(9–10):2215–20. doi: 10.1097/00001756-199707070-00025 [DOI] [PubMed] [Google Scholar]
- 120.Swank MW, Schafe GE, Bernstein IL. c-Fos induction in response to taste stimuli previously paired with amphetamine or LiCl during taste aversion learning. Brain Res. 1995;673(2):251–61. doi: 10.1016/0006-8993(94)01421-d [DOI] [PubMed] [Google Scholar]
- 121.Thiele TE, Roitman MF, Bernstein IL. c-Fos induction in rat brainstem in response to ethanol- and lithium chloride-induced conditioned taste aversions. Alcohol Clin Exp Res. 1996;20(6):1023–8. doi: 10.1111/j.1530-0277.1996.tb01941.x [DOI] [PubMed] [Google Scholar]
- 122.Houpt TA, Philopena JM, Joh TH, Smith GP. c-Fos induction in the rat nucleus of the solitary tract by intraoral quinine infusion depends on prior contingent pairing of quinine and lithium chloride. Physiol Behav. 1996;60(6):1535–41. doi: 10.1016/s0031-9384(96)00326-5 [DOI] [PubMed] [Google Scholar]
- 123.Travers SP, Norgren R. Coding the sweet taste in the nucleus of the solitary tract: differential roles for anterior tongue and nasoincisor duct gustatory receptors in the rat. J Neurophysiol. 1991;65(6):1372–80. doi: 10.1152/jn.1991.65.6.1372 [DOI] [PubMed] [Google Scholar]
- 124.Spray KJ, Halsell CB, Bernstein IL. c-Fos induction in response to saccharin after taste aversion learning depends on conditioning method. Brain Res. 2000;852(1):225–7. doi: 10.1016/s0006-8993(99)02203-9 [DOI] [PubMed] [Google Scholar]
- 125.Kinzeler NR, Travers SP. Licking and gaping elicited by microstimulation of the nucleus of the solitary tract. Am J Physiol Regul Integr Comp Physiol. 2008;295(2):R436–48. doi: 10.1152/ajpregu.00189.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Roussin AT, D’Agostino AE, Fooden AM, Victor JD, Di Lorenzo PM. Taste coding in the nucleus of the solitary tract of the awake, freely licking rat. J Neurosci. 2012;32(31):10494–506. doi: 10.1523/JNEUROSCI.1856-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Weiss MS, Victor JD, Di Lorenzo PM. Taste coding in the parabrachial nucleus of the pons in awake, freely licking rats and comparison with the nucleus of the solitary tract. J Neurophysiol. 2014;111(8):1655–70. doi: 10.1152/jn.00643.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Fu O, Iwai Y, Kondoh K, Misaka T, Minokoshi Y, Nakajima KI. SatB2-Expressing Neurons in the Parabrachial Nucleus Encode Sweet Taste. Cell Rep. 2019;27(6):1650–6 e4. doi: 10.1016/j.celrep.2019.04.040 [DOI] [PubMed] [Google Scholar]
- 129.Rosenblum K, Meiri N, Dudai Y. Taste memory: the role of protein synthesis in gustatory cortex. Behav Neural Biol. 1993;59(1):49–56. doi: 10.1016/0163-1047(93)91145-d [DOI] [PubMed] [Google Scholar]
- 130.Sakai N, Yamamoto T. Possible routes of visceral information in the rat brain in formation of conditioned taste aversion. Neurosci Res. 1999;35(1):53–61. doi: 10.1016/s0168-0102(99)00067-x [DOI] [PubMed] [Google Scholar]
- 131.Limebeer CL, Rock EM, Mechoulam R, Parker LA. The anti-nausea effects of CB1 agonists are mediated by an action at the visceral insular cortex. Br J Pharmacol. 2012;167(5):1126–36. doi: 10.1111/j.1476-5381.2012.02066.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Cubero I, Thiele TE, Bernstein IL. Insular cortex lesions and taste aversion learning: effects of conditioning method and timing of lesion. Brain Res. 1999;839(2):323–30. doi: 10.1016/s0006-8993(99)01745-x [DOI] [PubMed] [Google Scholar]
- 133.Livneh Y, Ramesh RN, Burgess CR, Levandowski KM, Madara JC, Fenselau H, et al. Homeostatic circuits selectively gate food cue responses in insular cortex. Nature. 2017;546(7660):611–6. doi: 10.1038/nature22375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Gal-Ben-Ari S, Rosenblum K. Molecular Mechanisms Underlying Memory Consolidation of Taste Information in the Cortex. Frontiers in behavioral neuroscience. 2012;5(87). doi: 10.3389/fnbeh.2011.00087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Geddes RI, Han L, Baldwin AE, Norgren R, Grigson PS. Gustatory insular cortex lesions disrupt drug-induced, but not lithium chloride-induced, suppression of conditioned stimulus intake. Behav Neurosci. 2008;122(5):1038–50. doi: 10.1037/a0012748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Montag-Sallaz M, Welzl H, Kuhl D, Montag D, Schachner M. Novelty-induced increased expression of immediate-early genes c-fos and arg 3.1 in the mouse brain. J Neurobiol. 1999;38(2):234–46. [PubMed] [Google Scholar]
- 137.Lin JY, Roman C, Arthurs J, Reilly S. Taste neophobia and c-Fos expression in the rat brain. Brain Res. 2012;1448:82–8. doi: 10.1016/j.brainres.2012.02.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Lowen SB, Rohan ML, Gillis TE, Thompson BS, Wellons CB, Andersen SL. Cocaine-conditioned odor cues without chronic exposure: Implications for the development of addiction vulnerability. Neuroimage Clin. 2015;8:652–9. doi: 10.1016/j.nicl.2015.06.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Lammel S, Lim BK, Malenka RC. Reward and aversion in a heterogeneous midbrain dopamine system. Neuropharmacology. 2014;76 Pt B:351–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kim Y, Simon NW, Wood J, Moghaddam B. Reward Anticipation Is Encoded Differently by Adolescent Ventral Tegmental Area Neurons. Biol Psychiatry. 2016;79(11):878–86. doi: 10.1016/j.biopsych.2015.04.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Frank GK, Shott ME, Riederer J, Pryor TL. Altered structural and effective connectivity in anorexia and bulimia nervosa in circuits that regulate energy and reward homeostasis. Transl Psychiatry. 2016;6(11):e932. doi: 10.1038/tp.2016.199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Frank GKW, DeGuzman MC, Shott ME, Laudenslager ML, Rossi B, Pryor T. Association of Brain Reward Learning Response With Harm Avoidance, Weight Gain, and Hypothalamic Effective Connectivity in Adolescent Anorexia Nervosa. JAMA Psychiatry. 2018;75(10):1071–80. doi: 10.1001/jamapsychiatry.2018.2151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Drewnowski A, Halmi KA, Pierce B, Gibbs J, Smith GP. Taste and eating disorders. Am J Clin Nutr. 1987;46(3):442–50. doi: 10.1093/ajcn/46.3.442 [DOI] [PubMed] [Google Scholar]
- 144.Sunday SR, Halmi KA. Taste perceptions and hedonics in eating disorders. Physiol Behav. 1990;48(5):587–94. doi: 10.1016/0031-9384(90)90196-b [DOI] [PubMed] [Google Scholar]
- 145.Chao AM, Roy A, Franks AT, Joseph PV. A Systematic Review of Taste Differences Among People With Eating Disorders. Biol Res Nurs. 2020;22(1):82–91. doi: 10.1177/1099800419872824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Drewnowski A. Taste responsiveness in eating disorders. Ann N Y Acad Sci. 1989;575:399–408; discussion -9. doi: 10.1111/j.1749-6632.1989.tb53260.x [DOI] [PubMed] [Google Scholar]
- 147.Frank S, Kullmann S, Veit R. Food related processes in the insular cortex. Frontiers in human neuroscience. 2013;7:499. doi: 10.3389/fnhum.2013.00499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Friederich HC, Wu M, Simon JJ, Herzog W. Neurocircuit function in eating disorders. Int J Eat Disord. 2013;46(5):425–32. doi: 10.1002/eat.22099 [DOI] [PubMed] [Google Scholar]
- 149.Alonso-Alonso M. Brain, reward, and eating disorders: a matter of taste? Am J Psychiatry. 2013;170(10):1082–5. doi: 10.1176/appi.ajp.2013.13070932 [DOI] [PubMed] [Google Scholar]
- 150.Yiannakas A, Rosenblum K. The Insula and Taste Learning. Front Mol Neurosci. 2017;10:335. doi: 10.3389/fnmol.2017.00335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Petrovich GD. Learning and the motivation to eat: forebrain circuitry. Physiol Behav. 2011;104(4):582–9. doi: 10.1016/j.physbeh.2011.04.059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Berman DE, Hazvi S, Neduva V, Dudai Y. The role of identified neurotransmitter systems in the response of insular cortex to unfamiliar taste: activation of ERK1-2 and formation of a memory trace. J Neurosci. 2000;20(18):7017–23. doi: 10.1523/JNEUROSCI.20-18-07017.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.McCutcheon JE, Ebner SR, Loriaux AL, Roitman MF. Encoding of aversion by dopamine and the nucleus accumbens. Frontiers in neuroscience. 2012;6:137. doi: 10.3389/fnins.2012.00137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Dela Cruz JA, Coke T, Bodnar RJ. Simultaneous Detection of c-Fos Activation from Mesolimbic and Mesocortical Dopamine Reward Sites Following Naive Sugar and Fat Ingestion in Rats. J Vis Exp. 2016(114). doi: 10.3791/53897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Dela Cruz JA, Coke T, Karagiorgis T, Sampson C, Icaza-Cukali D, Kest K, et al. c-Fos induction in mesotelencephalic dopamine pathway projection targets and dorsal striatum following oral intake of sugars and fats in rats. Brain Res Bull. 2015;111:9–19. doi: 10.1016/j.brainresbull.2014.11.002 [DOI] [PubMed] [Google Scholar]
- 156.Yuan L, Dou YN, Sun YG. Topography of Reward and Aversion Encoding in the Mesolimbic Dopaminergic System. J Neurosci. 2019;39(33):6472–81. doi: 10.1523/JNEUROSCI.0271-19.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Wheeler AL, Teixeira CM, Wang AH, Xiong X, Kovacevic N, Lerch JP, et al. Identification of a Functional Connectome for Long-Term Fear Memory in Mice. PLOS Computational Biology. 2013;9(1):e1002853. doi: 10.1371/journal.pcbi.1002853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Rogers-Carter MM, Varela JA, Gribbons KB, Pierce AF, McGoey MT, Ritchey M, et al. Insular cortex mediates approach and avoidance responses to social affective stimuli. Nat Neurosci. 2018;21(3):404–14. doi: 10.1038/s41593-018-0071-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Hernandez VS, Hernandez OR, Perez de la Mora M, Gomora MJ, Fuxe K, Eiden LE, et al. Hypothalamic Vasopressinergic Projections Innervate Central Amygdala GABAergic Neurons: Implications for Anxiety and Stress Coping. Front Neural Circuits. 2016;10:92. doi: 10.3389/fncir.2016.00092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.McLaughlin RJ, Floresco SB. The role of different subregions of the basolateral amygdala in cue-induced reinstatement and extinction of food-seeking behavior. Neuroscience. 2007;146(4):1484–94. doi: 10.1016/j.neuroscience.2007.03.025 [DOI] [PubMed] [Google Scholar]
- 161.Walker QD, Nelson CJ, Smith D, Kuhn CM. Vaginal lavage attenuates cocaine-stimulated activity and establishes place preference in rats. Pharmacol Biochem Behav. 2002;73(4):743–52. doi: 10.1016/s0091-3057(02)00883-3 [DOI] [PubMed] [Google Scholar]
- 162.Mitchell D, Wells C, Hoch N, Lind K, Woods SC, Mitchell LK. Poison induced pica in rats. Physiol Behav. 1976;17(4):691–7. doi: 10.1016/0031-9384(76)90171-2 [DOI] [PubMed] [Google Scholar]
- 163.Watson PJ, Leitner C. Patterns of increased and decreased ingestive behavior after injections of lithium chloride and 2-deoxy-D-glucose. Physiol Behav. 1988;43(6):697–704. doi: 10.1016/0031-9384(88)90366-6 [DOI] [PubMed] [Google Scholar]
- 164.Meachum CL, Bernstein IL. Behavioral conditioned responses to contextual and odor stimuli paired with LiCl administration. Physiol Behav. 1992;52(5):895–9. doi: 10.1016/0031-9384(92)90368-c [DOI] [PubMed] [Google Scholar]
- 165.Dwyer JB, Leslie FM. Adolescent Maturation of Dopamine D1 and D2 Receptor Function and Interactions in Rodents. PLoS One. 2016;11(1):e0146966. doi: 10.1371/journal.pone.0146966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Borgatti SP EMaRM. Ucinet 6 for Windows: Software for Social Network Analysis. 6.730 ed. MA: Harvard; 2002. [Google Scholar]
- 167.Houpt TA, Pittman DW, Barranco JM, Brooks EH, Smith JC. Behavioral effects of high-strength static magnetic fields on rats. J Neurosci. 2003;23(4):1498–505. doi: 10.1523/JNEUROSCI.23-04-01498.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Lockwood DR, Kwon B, Smith JC, Houpt TA. Behavioral effects of static high magnetic fields on unrestrained and restrained mice. Physiol Behav. 2003;78(4–5):635–40. doi: 10.1016/s0031-9384(03)00040-4 [DOI] [PubMed] [Google Scholar]
- 169.Swank MW, Bernstein IL. c-Fos induction in response to a conditioned stimulus after single trial taste aversion learning. Brain Res. 1994;636(2):202–8. doi: 10.1016/0006-8993(94)91018-9 [DOI] [PubMed] [Google Scholar]
- 170.Snyder DJ, Jahng JW, Smith JC, Houpt TA. c-Fos induction in visceral and vestibular nuclei of the rat brain stem by a 9.4 T magnetic field. Neuroreport. 2000;11(12):2681–5. doi: 10.1097/00001756-200008210-00015 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data have been uploaded to the Duke Data Repository. DOI assigned is: https://doi.org/10.7924/r44q80q5d.