Abstract
Reported post-surgery 1-year survival rate for oral canine malignant melanoma (cMM) is around 30%; novel treatments are needed as the role of adjuvant chemotherapy is unclear. This prospective study regards adjuvant electrovaccination with human chondroitin sulfate proteoglycan-4 (hCSPG4)-encoded plasmid in 23 dogs with resected II/III-staged CSPG4-positive oral cMM compared with 19 dogs with resected only II/III-staged CSPG4-positive oral cMM. Vaccination resulted in 6-, 12-, 18- and 24-month survival rate of 95.6, 73.9, 47.8 and 30.4%, respectively [median survival time (MST) 684 days, range 78–1694, 8 of 23 dogs alive] and 6-, 12-, 18- and 24-month disease-free interval (DFI) rate of 82.6, 47.8, 26.1 and 17.4%, respectively (DFI 477 days, range 50–1694). Non-vaccinated dogs showed 6-, 12-, 18- and 24-month survival rate of 63.2, 26.3, 15.8 and 5.3%, respectively (MST 200 days, range 75–1507, 1 of 19 dogs alive) and 6-, 12-, 18- and 24-month DFI rate of 52.6, 26.3, 10.5 and 5.3%, respectively (DFI 180 days, range 38–1250). Overall survival and DFI of vaccinated dogs was longer in those <20 kg. In vaccinated and non-vaccinated dogs local recurrence rate was 34.8 and 42%, respectively while lung metastatic rate was 39 and 79%, respectively.
Keywords: adjuvant immunotherapy, canine oral malignant melanoma, comparative oncology, CSPG4, DNA electroporation
Introduction
Oral cancers account for 6–7% of all canine neoplasms while canine malignant melanoma (cMM) for 30–40% of all oral malignancies.1–3 Over 10-year-old male dogs appear to be predisposed and small dogs seem to be more affected than large dogs.1 CMM may be melanotic, partially melanotic and amelanotic; the latter may be misdiagnosed as undifferentiated sarcoma or as an epithelial cancer but it may be then recognized by immunohistochemistry (Melan A, PNL2).4–7 Oral cMM may develop everywhere in the mouth but mainly at the level of the gingiva, lips and cheek,3 tongue and tonsils may also be affected.5 At presentation oral cMM is often ulcerated, necrotic, odorous and easily bleeding. Oral cMM are almost all malignant, with early local invasion (loss of teeth) and metastasis in up to 80% of cases.1–3 Metastasis to ipsilateral and contralateral regional lymph nodes (LNs) is frequent at presentation (up to 74% of cases); diagnosis should not rely on palpation only, as size may be not predictive, and cytology and histology are necessary.8 Metastasis at distant sites (mainly lungs) is also frequent. Reported clinical prognosticators are age, tumour size and clinical stage, bone lysis and localization (that may also influence surgical resection).9–12 Other prognosticators include mitotic index, percentage of atypical nuclei, Ki-67 value and plateled derived growth factor receptor (PDGFR)-α/-β co-expression.11,13,14 It has been reported that also the degree of pigmentation may have some prognostic value.15 Treatment of oral cMM is delivered either with a curative or palliative intent. Local control of cMM relies mainly on surgery and/or radiotherapy; adjuvant chemotherapy has been used in an attempt to control the systemic spread.12,16–29 Due to the often disappointing results obtained with standard chemotherapy and also to the high immunogenicity of MM, immunotherapy is progressively becoming one of the most attractive adjunctive therapeutic tools,30–43 also in the form of combined protocols.44 Other treatments reported in the literature include the use of lupeol.45–47
The goal of this article is to report both disease-free and overall survival times following adjuvant intramuscular electrovaccination with a plasmid encoding for human (h) chondroitin sulfate proteoglycan-4 (CSPG4) in prospectively enrolled client-owned dogs with en bloc surgically resected stage II and III CSPG4-positive oral cMM. Results of vaccination were compared with those obtained in a second group of dogs with stage II and III CSPG4-positive oral cMM treated with surgery alone. In previous articles, the authors have shown the expression of CSPG4 in about 57% of oral cMM48 and both safety and efficacy of the adjuvant anti-CSPG4 DNA vaccination in prolonging survival of treated dogs.40 CSPG4 is an early cell surface progression marker involved in tumour cell proliferation, migration and invasion49 and has been inserted by the National Cancer Institute among the prioritized cancer antigens being worthy to be investigated in clinical trials.50 As CSPG4 is a self-antigen with poor or no immunogenicity in autologous hosts, in this study we used a plasmid coding for the hCSPG4, that is characterized by 82% homology and 88% similarity in its amino-acid sequence when compared with its canine counterpart (cCSPG4), in order to break immune tolerance. The addition of electroporation to DNA vaccine delivery (electrovaccination) further increases the vaccine immunogenicity and its therapeutic efficacy, and prolongs the duration of the immune response.51–55
Material and methods
Patient enrollment
The study was conducted as a prospective bi-centric trial involving the Veterinary Teaching Hospital of the University of Torino and the Clinica Veterinaria Roma Sud of Rome. Dogs were treated according to both the Good Clinical Practice guidelines for animal clinical studies and the approval of the Ethical Committee of the University of Torino (Italy).
Pretreatment work-up included physical examination, blood count, serum biochemistry and urinalysis. Fine needle aspiration and/or biopsy were used for the preoperative tumour diagnosis. Cytology was the initial preoperative procedure adopted to clinically stage the palpable regional lymph nodes (LN), even in case of not apparent pathologic enlargement. A more complete staging was achieved via the surgical removal of the regional LN at the time of the primary tumour resection and their histologic evaluation. Completion of tumour staging also included a pre-operative total body CT (computerized tomography)-scan evaluation (including skull and neck); alternatively (owners’ decision), skull and three-view chest radiographs and abdominal ultrasound examination were obtained.
Dogs without concurrent life-threatening diseases and with histologically confirmed oral stage II (2–4-cm diameter, negative LN) and III (>4-cm diameter and negative LN or any tumour size with positive LN) surgically resected cMM, and with a minimum of 6 months follow-up on 31 December 2015 were included. Primary tumour en bloc resection (maxillectomy, mandibulectomy, lip/cheek excision followed by reconstruction, etc.) with the inclusion, when feasible, of at least 2 cm of macroscopically normal tissues around the tumour, and regional lymphadenectomy, were performed. Regional lymphadenectomy involved ipsilateral or bilateral mandibular LN (single or multiple LN) resection. For the excision margin evaluation, the cut surface was stained with a specific dye [Tissue Marking Dye (TMD), Triangle Biomedical Sciences, Durham, NC, USA] just after surgery; the sample was then fixed in 10% formalin. The same pathologists (S. I. and L. M.) evaluated histologically all the samples and also checked for tumour or lack of tumour infiltration at the level of excision margins. Samples were also immunohistochemically tested for PNL2 (Santa Cruz Biotech, to confirm cMM diagnosis) and Ki67 expression (polyclonal Ki67 antibody A-047; DAKO; cut-off of 19.5%), mitotic index (<4/10 high-power fields [hpf] or ≥4/10 hpf), and nuclear atypia (% atypical nuclei in 200 cells counted, < or ≥30%).11,13 Immunohistochemical analysis of CSPG4 expression on cMM samples was performed as previously described.48 A total score ranging from 0 to 8 was assigned to each MM sample by adding the value that represented the proportion of CSPG4 positively stained tumour cells (score from 0 to 5) and the average staining intensity of CSPG4-positive tumour cells (score from 0 to 3). Only dogs bearing an oral cMM with a CSPG4 score ≥3/8 were considered as suitable for vaccination and included in the study. Dogs were entered in the vaccination arm based on the owners’ decision; a written consent was obtained from owners before starting the vaccination. Two groups of dogs were formed: group A involving dogs with CSPG4-positive oral cMM treated with surgery plus adjuvant anti-CSPG4 DNA vaccination, and group B involving dogs with CSPG4-positive oral cMM treated with surgery alone.
Electrovaccination
Vaccination started at the 3rd–4th post-operative week and was repeated after 2 weeks and then monthly; dogs surviving over 2 years were then vaccinated every 6 months. Dogs were electrovaccinated with a pcDNA3.1 plasmid coding for hCSPG4 generated as previously described.40,56 Briefly, under a short inhalation anaesthesia, the hCSPG4 plasmid (500 μg in 200 μL of 0.03% NaCl solution) was injected in dogs into the muscles of the caudal thigh. Two minutes after plasmid injection, nine electric pulses (1 high voltage, amplitude 450 V, length 50 ms, frequency 3 HZ; 1 s pause; 8 low-voltage amplitude 110 V, length 20 ms, pause 300 ms) were applied to the injection site using the CLINIPORATOR (Igea, Carpi, Italy). Dogs recovered quickly from anaesthesia and were standing up within 10–15 min from electrovaccination. They were monitored for acute, late local or systemic side effects. At each vaccination, clinical examinations, blood-work and three-view chest radiographs were performed. Sera were also collected, aliquoted and cryopreserved at -80 °C until used.
anti-CSPG4 antibody detection in vaccinated dog sera
Sera collected before the first and after the fourth electrovaccination were analysed for the presence of antibodies against hCSPG4. Sera were analyzed at this time of treatment since our previous results showed a detectable antibody response in all patients after the fourth electrovaccination.40 To specifically quantify in the serum of vaccinated dogs the antibody titer against the hCSPG4 protein an enzyme-linked immunosorbent (ELISA) assay was performed; 96-well plates (Costar®, Sigma-Aldrich, Milano, Italy) were coated in triplicate with 50 ng per well of hCSPG4 recombinant protein (R&D Systems, Minneapolis, MN, USA) overnight (ON) at 4°C. Coated plates were then blocked with 10% fetal bovine serum (FBS; Sigma-Aldrich) in phosphate-buffered saline (PBS; Invitrogen, Carlsbad, CA, USA)-Tween (Sigma-Aldrich) 0.05% buffer for 2 h at 37 °C. Plates were incubated with samples diluted 1:100 in 1% blocking buffer for 1 h at 37 °C. Plates were washed three times with a PBS-Tween buffer. The horseradish peroxidase (HRP)-conjugated anti-dog IgG antibody (AbD Serotec, Raleigh, NC, USA; 1:10.000 dilution in blocking buffer) was incubated for 1 h at 37 °C. Plates were washed six times and chromogenic 3,30,5,50-tetramethylbenzidine substrate was added (TMB; Sigma-Aldrich). The reaction was stopped by the addition of 2 N hydrochloric acid and optical density was measured at 450 nm using a microplate reader (680XR, BioRad, Milano, Italy).
Immunoblot
hCSPG4-positive SK-MEL-28 melanoma cells were purchased from the American Type Culture Collection and cultured in Dulbecco’s modified Eagle’s medium (DMEM, Life Technologies) supplemented with 10% FBS. Cells were seeded at the concentration of 1.0 × 104 per well in a 96-well plate in DMEM medium without serum and incubated at 37 °C for 24h. Cells were then incubated with control CSPG4-specific mAb (0.1 mg mL−1, of 149.53, 225.28, TP61.5, VF1-TP34, VF4-TP108, VF20-T87.41, VF20-VT20 mAb), with canine sera (dilution 1:20) before and after the fourth DNA vaccination or medium alone at 37 °C for an additional 48 h. Cells were lysed in lysis buffer (50 mmol L−1 Tris–HCl, 150 mmol L−1 NaCl, pH 7.4) containing 1% Triton X-100, and an ethylenediaminetetraacetic acid (EDTA)-free protease inhibitor mix (Roche, Monza, Italy). Suspensions were extensively vortexed and incubated on ice for 15 min and centrifuged for 5 min at 15,000 × g at 4 °C. Supernatants were collected and stored at -80 °C until used. Protein concentration was determined using an acid protein assay (Pierce Biotechnology, Rockford, IL, USA). Total cell lysates were separated by sodium dodecyl sulfate–polyacrylamide (SDS–PAGE) gel electrophoresis and transferred to polyvinylidene fluoride membrane of 0.45-μm pore size (Millipore, Bedford, MA, USA). After blocking the membranes with 5% non-fat dry milk with 5% bovine serum albumin (BSA) at 4 °C ON, membranes were incubated ON at 4 °C with CSPG4-specific mAb diluted 1:250 in Tris-buffered saline and Tween 20 (TTBS) 3% non-fat dry milk. After washing with TTBS, HRP goat anti-mouse (1:8000 dilution) was used as secondary antibody and bound antibodies were detected using ECL Plus Western Blotting Detection System (GE Healthcare, Buckinghamshire, UK).
Statistical analysis
Non-normally distributed data are reported as median and range. Other variables are expressed as percentages. All quantitative evaluations were carried out using the Student’s t-test. The Kaplan–Meier method was used to estimate disease-free and survival times. A Wilcoxon rank-sum test was used to compare values of the cCSPG4 expression between groups in disease outcome. Differences in survival distribution were analysed using the log-rank test. Statistical significance was set at P < 0.05. All analyses were conducted in R.57
Results
Patient characteristics
Forty-two dogs bearing an oral cMM with a CSPG4 score >3/8 and without any evidence of metastasis beyond the first LN (stage II and III), were prospectively included in the study. The decision to proceed with the adjuvant vaccination was based on the owner’s consent. Among the included dogs, 29 were males (69%, 18 intact and 11 castrated) and 13 females (31%, 12 spayed and 1 intact). There were 18 mixed breed dogs: 3 Golden Retriever, 3 Doberman Pinscher, 3 Dachshund, 3 Cocker Spaniel, 2 Beagle, 2 German Shepherd, and 1 of each German Bloodhound, dog de Bordeaux, West Highland White Terrier, Pinscher, Dwarf Schnauzer, Yorkshire, Schi-Tzu and Pekingese. Mean and median age of the entire population was 11.1 and 12 years, respectively (range 4–16). Weight ranged between 2.3 and 43 kg; there were 2 dogs <5 kg (4.8%), 9 dogs between 5 and 10 kg (21.4%), 12 dogs between 10 and 15 kg (28.6%), 8 dogs between 20 and 30 kg (19%), and 11 dogs >30 kg (26.2%).
Twenty-three dogs were included in the surgery plus vaccination group (group A), while 19 dogs received surgery alone (group B). In group A there were 16 males (9 castrated and 7 intact) and 7 females (5 spayed and 2 intact), while in group B there were 13 males (11 intact and 2 castrated) and 6 spayed females. Mean and median age of the 23 vaccinated dogs was 11.3 and 12 years, respectively (range 6–14); mean and median age of the 19 non-vaccinated dogs was 11 and 12 years, respectively (range 4–16). Mean and median weight of the 23 vaccinated dogs was 18 and 13 kg, respectively (range 2.3–35); mean and median age of the 19 non-vaccinated dogs was 23.1 and 29 kg, respectively (range 7–43). No statistical differences regarding age or weight distribution in the two groups were observed.
Diagnostic imaging for clinical staging was provided by radiographic evaluation in 4 dogs of group A and 7 of group B, and total body CT in 19 of group A and 12 of group B.
In both groups A and B either an ipsilateral or bilateral regional (mandibular) lymphadenectomy was performed. In group A surgery consisted of: 10 segmental/horizontal and 1 bilateral rostral mandibulectomies, 3 maxillectomies, 7 en bloc excisions of lip and/or cheek followed by plastic reconstruction, 1 bilateral tonsillectomy and 1 en bloc cMM tongue excision. In group B surgery consisted of: 7 segmental/horizontal and 5 (4 bilateral, 1 unilateral) rostral mandibulectomies, 2 maxillectomies and 5 en bloc excisions of lip and/or cheek followed by plastic reconstruction. A summary of the surgical resections performed is listed in Table 1. Histological evaluation of removed regional LN allowed defining the final clinical staging of each cMM included in the study, reported in Table 2. Local bone invasion (determined by radiographs and/or CT) was evident pre-operatively in 9 cases of group A and 7 of group B (Table 2). Histology of the excision margin identified 4 incomplete removals in group A and 4 in group B (Table 2). All of the incomplete resections were at the level of the soft tissue and not bone. Clean margins exceeded 2 mm in all the samples.
Table 1.
Surgical MM resection in canine patients enrolled in the trial
| Type of surgery | Overall population (n = 42) | Group A (n = 23) | Group B (n = 19) |
|---|---|---|---|
|
| |||
| Mandibulectomy | 23/42 (54.77)a | 11/23 (47.83)a | 12/19 (63.16)a |
| Segmental/horizontal | 17 | 10 | 7 |
| Rostral | 6 | 1 | 5 |
| Maxillectomy | 5/42 (11.90) | 3/23 (13.04) | 2/19 (10.53) |
| Tonsillectomy | 1/42 (2.38) | 1/23 (4.35) | 0/19 (0.00) |
| En bloc excision | 13/42 (30.95) | 8/23 (34.78) | 5/19 (26.31) |
| Lip/cheek | 12 | 7 | 5 |
| Tongue | 1 | 1 | 0 |
% in brackets.
Table 2.
Clinical stage and excision margin status of oral cMM enrolled in the trial
| Stage II | Stage III | Metastatic LN | Local bone invasion | Excision margins |
||
|---|---|---|---|---|---|---|
| Complete | Incomplete | |||||
|
| ||||||
| Overall population (n = 42) | 15 (35.71)a | 27 (64.29)a | 18 (42.86)a | 16 (38.09)a | 34 (80.95)a | 8 (19.05)a |
| Group A (n = 23) | 9 (39.13) | 14 (60.87) | 8 (34.78) | 9 (39.13) | 19 (82.61) | 4 (17.39) |
| Group B (n = 19) | 6 (31.58) | 13 (68.42) | 10 (52.63) | 7 (36.84) | 15 (78.95) | 4 (21.05) |
% in brackets.
The CSPG4 immunohistochemistry score for cMM of group A or group B is summarized in Table 3. No significant difference in distribution was found between the two groups.
Table 3.
Immunohistochemical CSPG4 score of MM from dogs included in the study
| CSPG4 score | Overall population (n = 42) | Group A (n = 23) | Group B (n = 19) |
|---|---|---|---|
|
| |||
| 3/8 | 6 (14.29)a | 3 (13.04)a | 3 (15.79)a |
| 4/8 | 8 (19.05) | 5 (21.74) | 3 (15.79) |
| 5/8 | 8 (19.05) | 5 (21.74) | 3 (15.79) |
| 6/8 | 3 (7.14) | 2 (8.70) | 1 (5.26) |
| 7/8 | 14 (33.33) | 6 (26.09) | 8 (42.11) |
| 8/8 | 3 (7.14) | 2 (8.69) | 1 (5.26) |
% in brackets.
Ki67 immunohistochemistry results are summarized in Table 4. It was not available in 3 cases of group A and in 2 of group B. In group A it was <19.5% in 1 case and >19.5% in 19 cases (mean 34%, median 30%, range 21–74%). In group B it was <19.5% in 3 cases and >19.5% in 14 cases (mean 25.4%, median 24.6%, range 20–50%).
Table 4.
Histological and immunohistochemical characterization of the cMM included in the study
| Threshold | Overall population | Group A | Group B | |
|---|---|---|---|---|
|
| ||||
| Ki67 | <19.5 | 4/37a (10.81)b | 1/20a (5.00)b | 3/17a (17.65)b |
| ≥19.5 | 33/37 (89.19) | 19/20 (95.00) | 14/17 (82.35) | |
| Mitotic index (MI) | <4/10 hpf | 1/38a (2.63)b | 0/20a (0.00)b | 1/18a (5.56)b |
| ≥4/10 hpf | 37/38 (97.37) | 20/20 (100.00) | 17/18 (94.44) | |
| Nuclear atypia | <30% | 12/38 (31.58) | 5/20 (25.00) | 7/18 (38.89) |
| ≥30% | 26/38 (68.42) | 15/20 (75.00) | 11/18 (61.11) | |
Number of patients for which the data were available.
% in brackets.
Results regarding mitotic index are summarized in Table 4. It was not available in 3 cases of group A and in 1 case of group B. In 20 cMM of group A it was >4/10 hpf (range 9–40, mean 25.7, median 24). In cMM of group B, it was <4/10 hpf (=2) in 1 case while in the remaining 17 cases it was >4/10 hpf (range 7–92, mean 29.8, median 25).
Results regarding nuclear atypia are summarized in Table 4. It was not available in 3 cases of group A and in 1 of group B. In group A it was <30% in 5 cases and >30 in 15 cases; in group B, it was <30% in 7 cMM and >30% in 11 cases.
Specific humoral response induced by hCSPG4 electrovaccination in cMM vaccinated dogs
The immune response induced by the xenogeneic hCSPG4 DNA vaccine was measured in sera by using a recombinant hCSPG4 ELISA assay. As in the previous study we have shown the presence of anti-hCSPG4 antibodies in all cMM dogs after the fourth hCSPG4-DNA vaccination.40 The humoral response was measured in sera before and after four immunizations. The level of antibody response, assessed spectrophotometrically to reflect the specific antibody binding to the hCSPG4 recombinant protein, was significantly higher in the post-vaccination sera than the pre-immune sera (Fig. 1A), confirming the ability of xenogeneic hCSPG4 vaccination to induce specific antibodies in CSPG4-positive cMM dogs. The vaccine-induced antibody titer was not correlated to the cMM CSPG4 positivity in vaccinated dogs (not shown); however, even if not statistically significant, a trend was evident in relation to body weight (BW) of dogs, being higher in dogs with BW <20 kg when compared with those with BW >20 kg (Fig. 1B).
Figure 1.
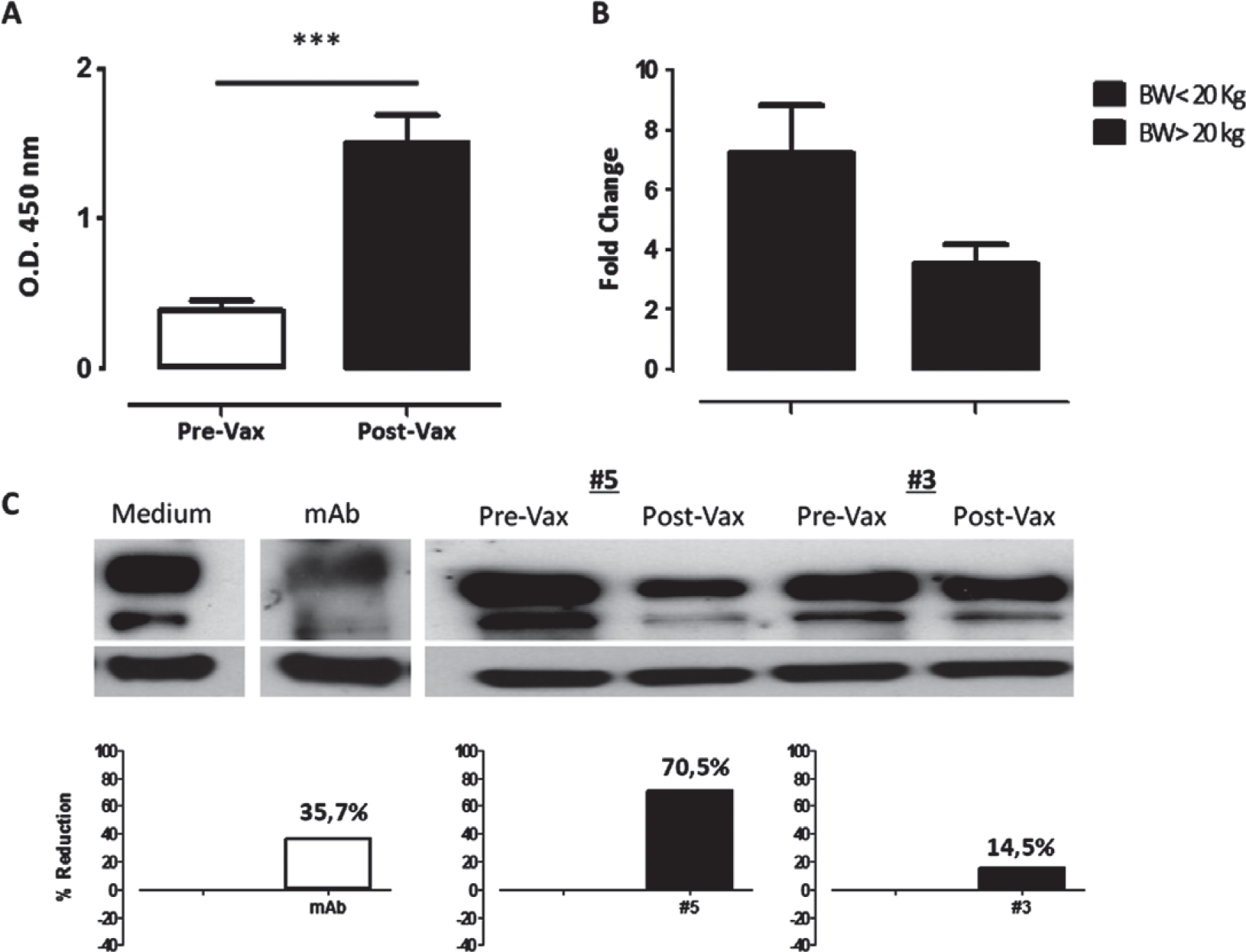
Vaccination-induced anti-hCSPG4 humoral response in sera of cMM dogs. (A) Detection of hCSPG4 antibodies in sera collected before (white bar) and after the fourth DNA vaccination (black bar) by ELISA. Results are expressed as the mean OD at 450 nm ± SD values of all vaccinated dogs. ***P < 0.0001, Student’s t-test. (B) Evaluation of hCSPG4 antibody response in sera collected from vaccinated dogs in relation to BW. Results are shown as fold change values expressing the ratio between post-vaccination OD/pre-vaccination OD values measured by ELISA. (C) Assessment of the effect of vaccine-induced anti-hCSPG4 antibodies on hCSPG4 expression. hCSPG4-positive SK-MEL28 cells were incubated with medium alone, CSPG4-specific mAb or canine sera before and after the fourth immunization at 37°C for 48h. Representative immunoblot analysis of CSPG4 modulation induced by the sera of 2 vaccinated dogs (#5 and #3) and the corresponding percentage of CSPG4 reduction compared with the medium is shown. Actin was used as loading control.
Authors then investigated the effect of vaccination-induced antibodies on antigen expression in a hCSPG4-positive melanoma cell line (SK-MEL28). In vitro incubation of SK-MEL28 cells with canine sera after the fourth vaccination showed a differential decrease in the level of CSPG4 expression as compared with sera before immunization (Fig. 1C). Incubation of SK-MEL28 cells with the medium alone or with CSPG4-specific mAb were used as controls for CSPG4 expression and modulation (Fig. 1C). Interpreting these results as a whole, they indicate the direct effect in vitro of specific antibodies detected in sera of vaccinated dogs in the modulation of CSPG4 expression in a CSPG4-positive human melanoma cell line. As CSPG4 is involved in the regulation of several pathways concerning growth, adhesion and migration of tumour cells,49 these results suggest one of the non-immunological mechanisms by which specific antibodies can affect CSPG4 function in the biology of melanoma cells, hampering tumour progression. As we previously showed the ability of vaccine-induced antibodies to bind the syngenic CSPG4 canine protein expressed on OLGA cells, a canine CSPG4-positive melanoma cell line,40 it can be speculated that the anti-cCSPG4 antibodies may carry out a similar action also in vivo.
Clinical response to hCSPG4 electrovaccination
The median survival time (MST) and disease-free interval (DFI) results of group A and group B dogs are summarized in Table 5.
Table 5.
MST and DFI calculated at 31 December 2015
| Groups | MST (days) | DFI (days) | MST (months) |
DFI (months) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 6 | 12 | 18 | 24 | 6 | 12 | 18 | 24 | |||
|
| ||||||||||
| Group A | 684 (458–∞)a | 477 (207–∞) | 95.6% | 73.9% | 47.8% | 30.4% | 82.6% | 47.8% | 26.1% | 17.4% |
| Group B | 220 (174–∞) | 180 (131–∞) | 63.2% | 26.3% | 15.8% | 5.3% | 52.6% | 26.3% | 10.5% | 5.3% |
(LCL 95%–UCL 95%) lower–upper control limits.
In the CSPG4-positive vaccinated dogs (group A) the 6-, 12-, 18- and 24-month survival rates are 95.6, 73.9, 47.8 and 30.4%, respectively. The 6-, 12-, 18- and 24-month DFI rates are 82.6, 47.8, 26.1 and 17.4%, respectively. At the end of the observation period (31 December 2015), 8 dogs of the group A (35%) were still alive (mean 1064 days, median 1028 days, range 493–1694 days) and 15 (65%) were dead, 11 because of the cMM (mean 416 days, median 385 days, range 78–684 days) and 4 for unrelated causes (1 dog was submitted to euthanasia for very serious orthopaedic/neurological problems at day 278 even though disease-free; 2 castrated male dogs developed a prostatic carcinoma and were submitted to euthanasia at day 447 and 1299, respectively; a fourth dog was euthanized at day 277 for a perianal adenocarcinoma with metastatic sublumbar lymphadenopathy).
In the CSPG4-positive non-vaccinated dogs (group B), the 6-, 12-, 18- and 24-month survival rates were 63.2, 26.3, 15.8 and 5.3%, respectively. The 6-, 12-, 18- and 24-month DFI rates were 52.6, 26.3, 10.5 and 5.3% (1 dog, still disease-free, was lost to follow-up after 694 days), respectively. At the end of the observation period, 18 dogs of the group B (94.7%) were dead. Sixteen died because of cMM (mean 295.1 days, median 184 days, range 75–1507 days), 2 for tumour-unrelated causes (1 for an idiopathic larynx paralysis at day 370, surgery refused by the owner, the second one for an idiopathic megaesophagus and ab ingestis pneumonia at day 367; both dogs were disease-free); the remaining dog of group B was lost to follow-up after 694 days.
A summary of local recurrence (LR) and lung metastasis (LM) in the two groups is presented in Table 6. LR developed in 8 dogs of group A (34.8%; mean 299.6 days, median 203.5 days, range 128–639 days). A second marginal or en bloc surgery was performed in 6 dogs; 5 of these ultimately died of their cMM at day 333, 374, 458, 574 and 684, respectively (four of these with systemic metastasis); the sixth dog was operated at day 179 and it was alive at day 1400. A further dog had two excisions (one en bloc and one marginal) and it was alive at day 875; finally, the last dog had five marginal excisions, two cycles of palliative radiotherapy (October 2014: 2 fractions per week × 5 fractions, 6Gy each fraction; May 2015: 2 fractions per week × 5 fractions, 4Gy each fraction). In the latter dog metronomic chemotherapy was also started since June 2015 (cyclophosphamide, 15 mg m−2 sid and thalidomide, 6 mg kg−1 sid) and it was still alive at day 1040, without any evidence of systemic metastasis. In group B, 8 dogs developed a LR (42%; mean 318.3 days, median 180 days, range 38–1250 days), for 7 of which it was associated with distant metastatic disease (euthanasia or death at day 75, 174, 183, 220, 224, 232 and 621 days) while a further dog had a recurrence at day 1250, it was operated and then irradiated but experienced a second LR after about 200 days and was euthanized at day 1507. After the initial pre-operative staging (8 metastatic LNs in group A and 10 in group B), distant metastasis, which was the cause of the death, developed in 9 dogs of group A (39%; mean 267.2 days, median 196 days, range 50–639 days) and 15 of group B (79%; mean 164 days, median 137 days, range 38–445 days).
Table 6.
Percentage of LR and LM for each group, calculated up to 31 December 2015
| Groups | LR | LM |
|---|---|---|
|
| ||
| Group A (n = 23) | 34.80% | 39.00% |
| Group B (n = 19) | 42.00% | 79.00% |
Kaplan–Meier curves for survival times and DFI were analysed (Fig. 2A,B). The MST in group A is 684 days (range, 78–1694), in group B 220 days (range, 75–1507) (ratio A/B = 3.109). Group A exhibited a significantly longer MST than group B (P = 0.0005; Fig. 2A). The median DFI in group A is 477 days (range, 50–1694), in group B 180 days (range, 38–1250) (ratio A/B = 2.650). The group A DFI was significantly longer than group B (P = 0.0174; Fig. 2B).
Figure 2.
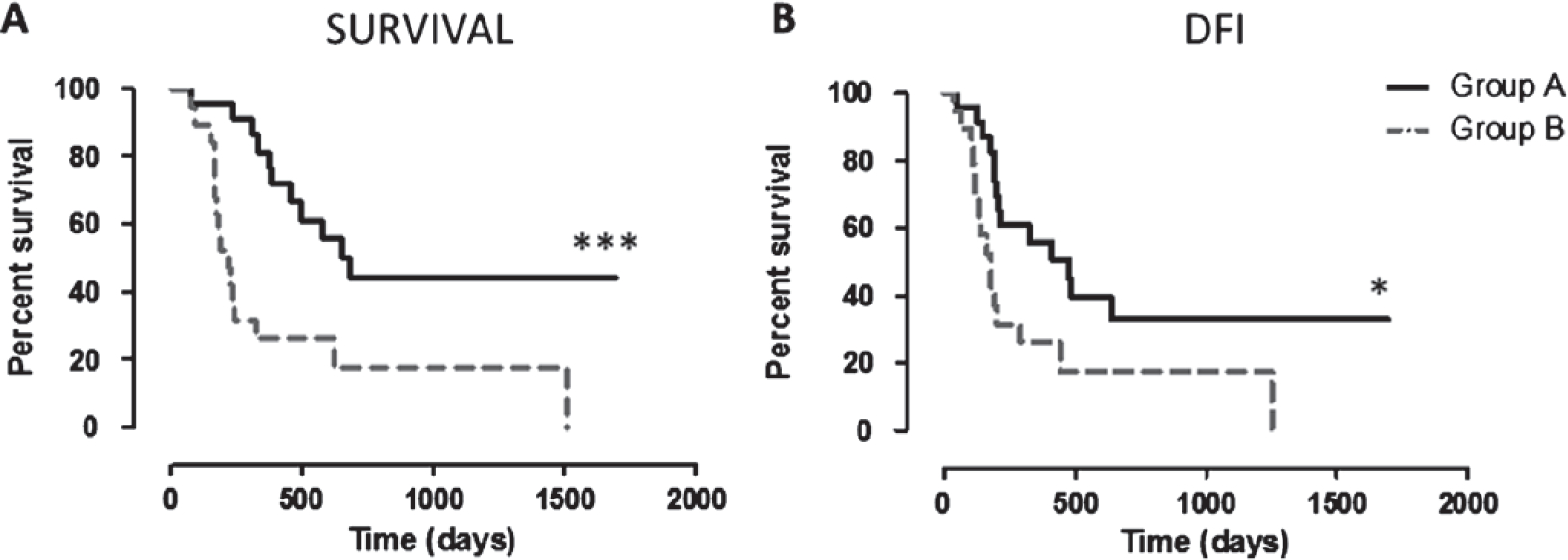
Kaplan–Meier curves comparing survival and DFI in the two groups. (A) Survival (in days) of CSPG4-positive MM, vaccinated dogs (group A, black line) and of CSPG4-positive MM, non-vaccinated dogs (group B, grey dotted line; *** log-rank test P = 0.0005). (B) DFI (in days) of CSPG4-positive MM, vaccinated dogs (group A, black line) and of CSPG4-positive MM, non-vaccinated dogs (group B, grey dotted line; * log-rank test P = 0.0174).
No statistically significant correlation was found between the clinical outcome of vaccinated patients and the excision margin status, percentages of Ki67 positivity, mitotic index and nuclear atypia scores. Nevertheless, taking into account the BW of dogs and selecting 20 kg as a threshold, a differential trend in group A was clearly evident, being the survival of dogs with BW <20 kg longer than that of those with BW >20 kg (Fig. 3A). Although, this is not completely surprising as smaller dogs tend to live longer than larger ones, however, survival of group A dogs with BW <20 kg was significantly longer than both the entire population of group B dogs (Fig. 3B) and dogs of group B with BW <20 kg (Fig. 3C). On the contrary, the survival of group A dogs with BW >20 kg was not significantly different from group B population, neither considered as a whole (Fig. 3D) nor considering only dogs >20 kg (Fig. 3E). The same scenario is evident considering the DFI (Fig. 4A–E), being the DFI of group A dogs with BW <20 kg significantly longer than both the entire population of group B dogs (Fig. 4B) and dogs of group B with BW <20 kg (Fig. 4C).
Figure 3.
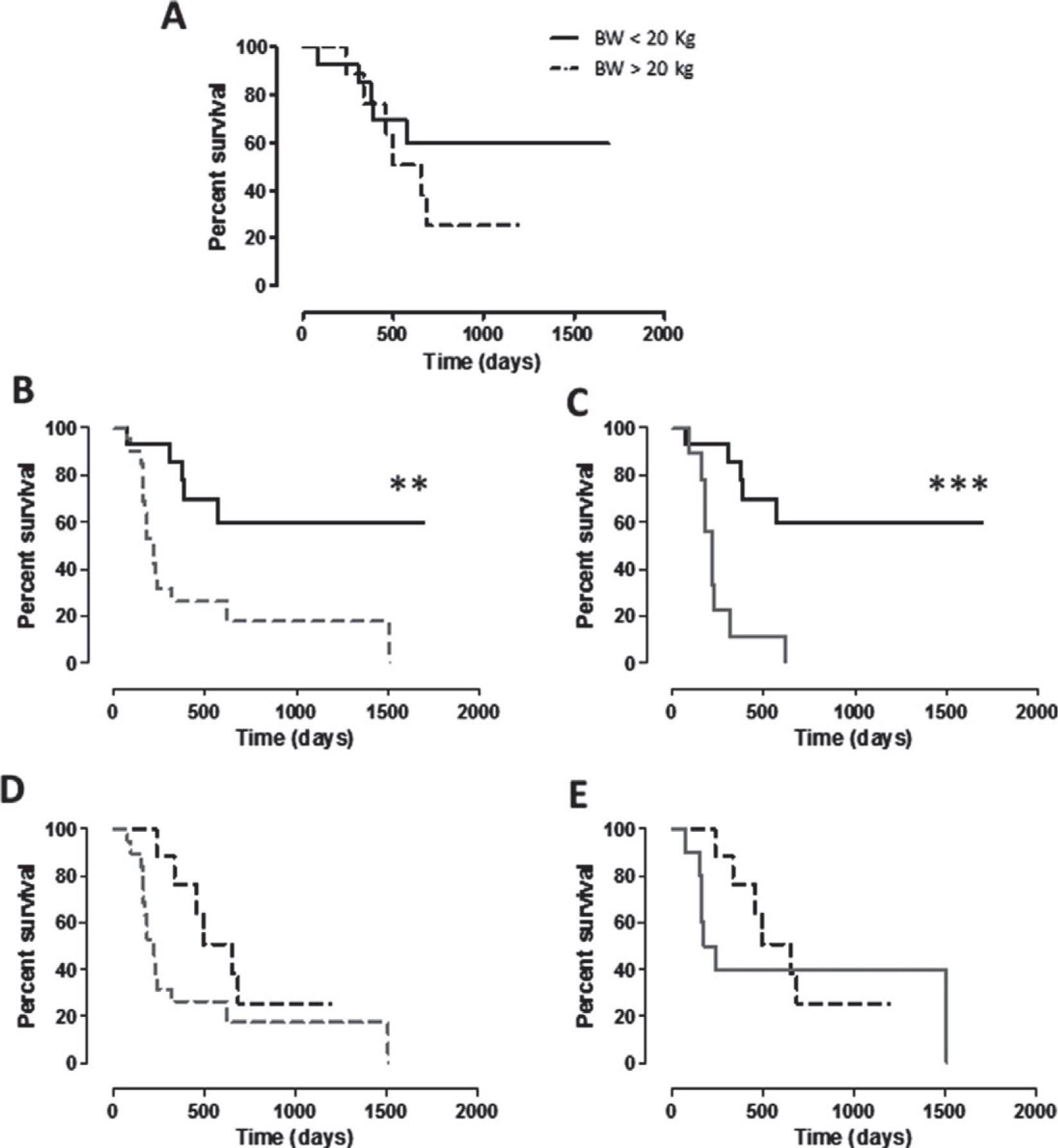
Kaplan–Meier curves comparing survival (in days) in relation to BW of dogs. (A) Survival of CSPG4-positive cMM, vaccinated dogs (group A) with BW <20 kg (black line) and with BW >20 kg (black dotted line). (B, C) Survival of CSPG4-positive cMM, vaccinated dogs (group A) with BW <20 kg (black line) in comparison with survival of (B) the entire population of non-vaccinated dogs (group B, grey dotted line; ** log-rank test P = 0.0015) or of (C) non-vaccinated dogs with BW <20 kg (group B, grey line; *** log-rank test P = 0.0002). (D, E) Survival of CSPG4-positive cMM vaccinated dogs (group A) with BW > 20 kg (black dotted line) in comparison with survival of (D) the entire population of non-vaccinated dogs (group B, grey dotted line) or of (E) non-vaccinated dogs with BW >20 kg (group B, grey line).
Figure 4.
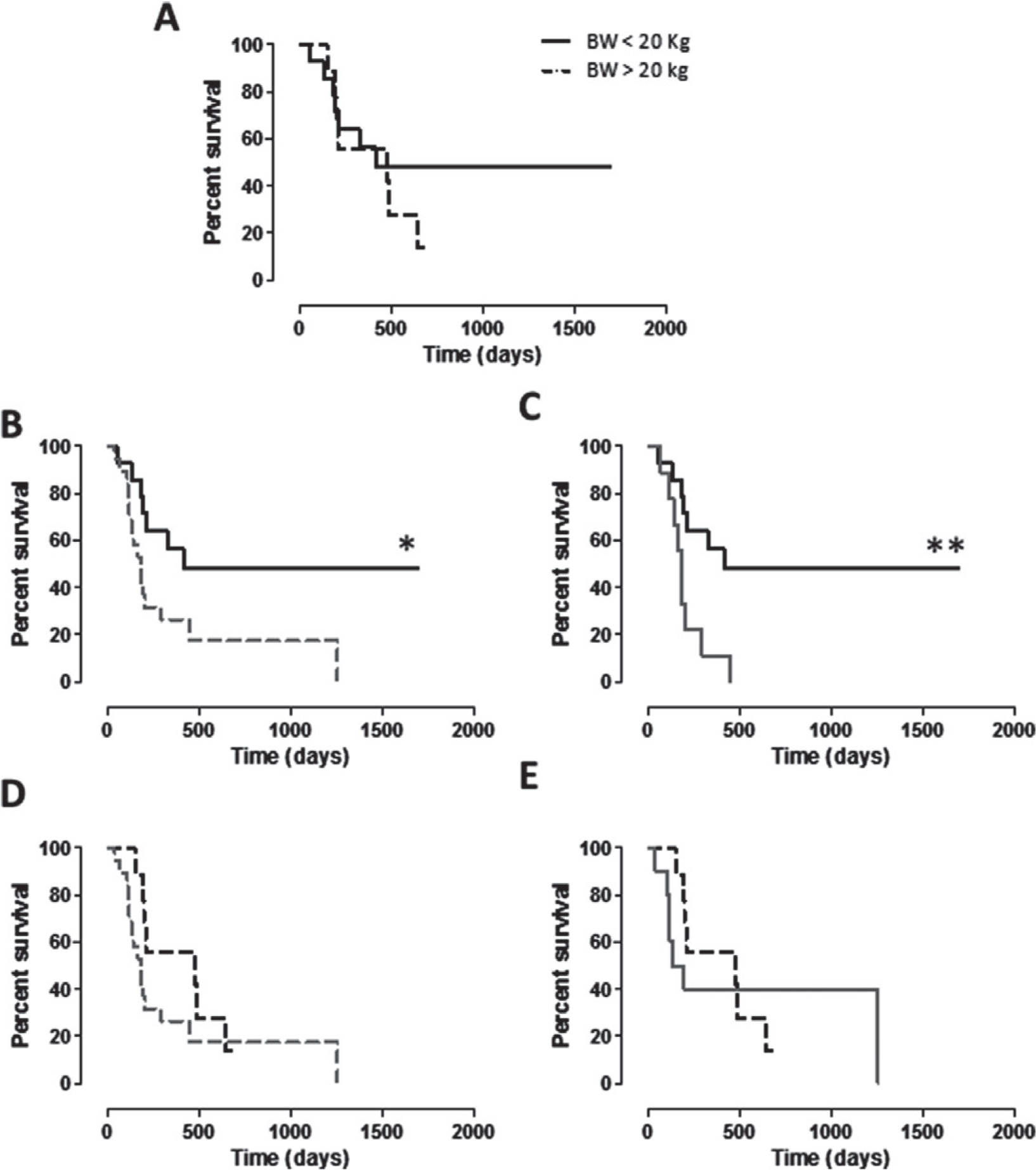
Kaplan–Meier curves comparing DFI (in days) in relation to BW of dogs. (A) DFI of CSPG4-positive cMM vaccinated dogs (group A) with BW <20 kg (black line) and with BW >20 kg (black dotted line). (B, C) DFI of CSPG4-positive cMM vaccinated dogs (group A) with BW <20 kg (black line) in comparison with DFI of (B) the entire population of non-vaccinated dogs (group B, grey dotted line; * log-rank test P = 0.0252) or of (C) non-vaccinated dogs with BW <20 kg (group B, grey line; ** log-rank test P = 0.0070). (D, E) DFI of CSPG4-positive cMM vaccinated dogs (group A) with BW >20 kg (black dotted line) in comparison with DFI of (D) the entire population of non-vaccinated dogs (group B, grey dotted line) or of (E) non-vaccinated dogs with BW >20 kg (group B, grey line).
While the CSPG4 positivity of cMM in dogs of group B is not correlated with the overall survival (not shown), it has a significant impact on survival of dogs of group A. Indeed, even if not statistically significant, vaccinated dogs affected by a cMM with CSPG4 score ≥5 displayed a longer survival as compared with vaccinated dogs with CSPG4-positive cMM with score <5 (Fig. 5A). Moreover, group A dogs with a cMM CSPG4 score ≥5 exhibited a significantly longer MST when compared with both the entire group B population (Fig. 5B) or only with the non-vaccinated dogs affected by a cMM with CSPG4 score ≥5(Fig. 5C). This is not the case of group A vaccinated dogs with a cMM with CSPG4 score <5, as survival is not significantly longer when compared with the entire population of non-vaccinated (group B) dogs (Fig. 5D) or when considering only group B dogs with a cMM with CSPG4 score <5 (Fig. 5E). The same situation is evident considering the DFI and CSPG4 expression (Fig. 6A–E), being the DFI of group A dogs with CSPG4 score ≥5 significantly longer when compared with both the entire group B population (Fig. 6B) or only with non-vaccinated dogs affected with a cMM of CSPG4 score ≥5 (Fig. 6C).
Figure 5.
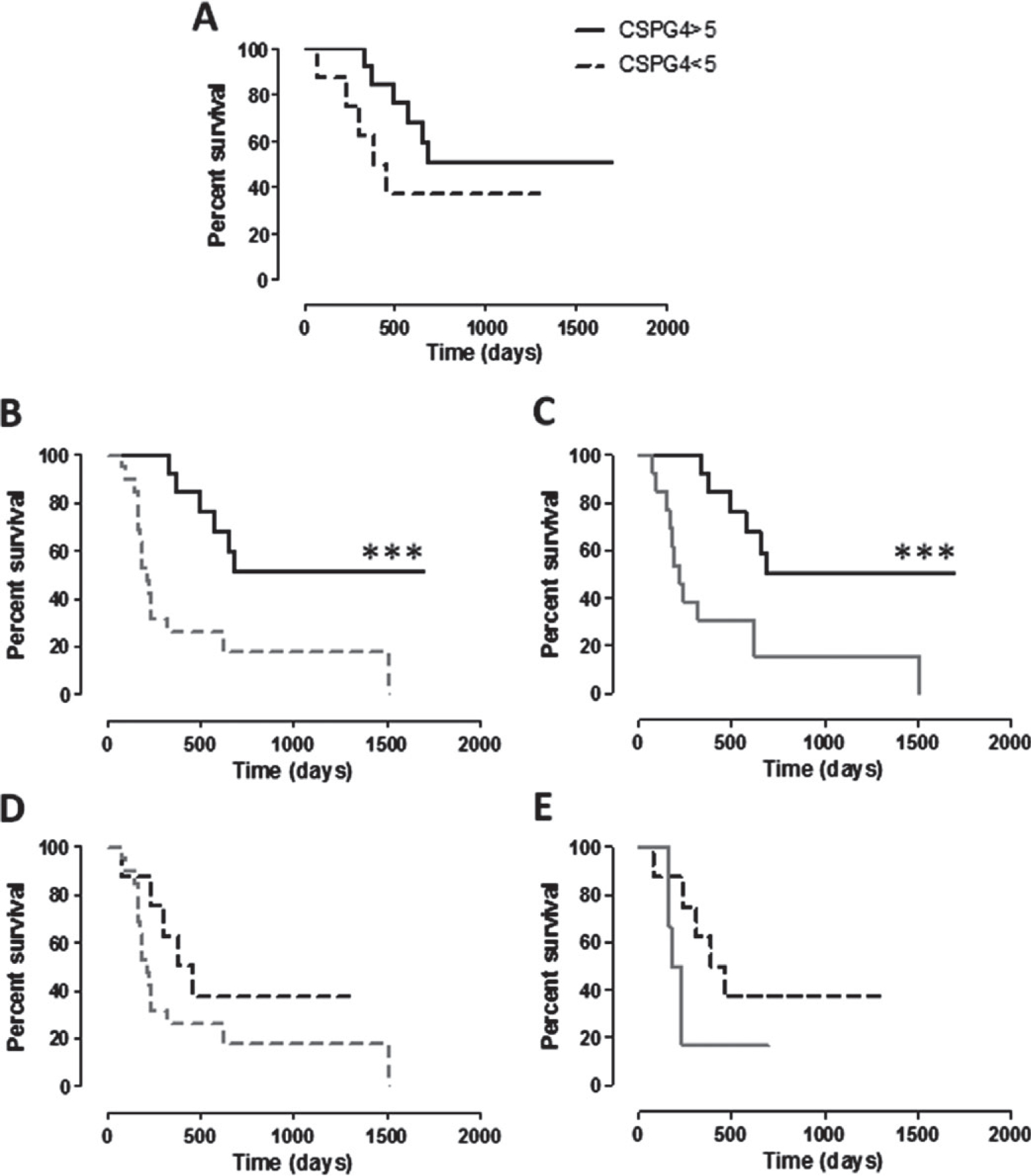
Kaplan–Meier curves comparing survival (in days) of dogs in relation to CSPG4-positivity score of cMM. (A) Survival of vaccinated dogs (group A) bearing a cMM with CSPG4-positivity score ≥5 (black line) or <5 (black dotted line). (B, C) Survival of vaccinated dogs (group A) bearing a cMM with CSPG4-positivity score ≥5 (black line) in comparison with the survival of (B) the entire population of non-vaccinated dogs (group B, grey dotted line; *** log-rank test P = 0.0004) or of (C) non-vaccinated dogs bearing a cMM with CSPG4-positivity score ≥5 (group B, grey line; *** log-rank test P = 0.0006). (D, E) Survival of vaccinated dogs (group A) bearing a cMM with CSPG4-positivity score <5 (black dotted line) in comparison with the survival of (D) the entire population of non-vaccinated dogs (group B, grey dotted line) or of (E) non-vaccinated dogs bearing a cMM with CSPG4-positivity score <5 (group B, grey line).
Figure 6.
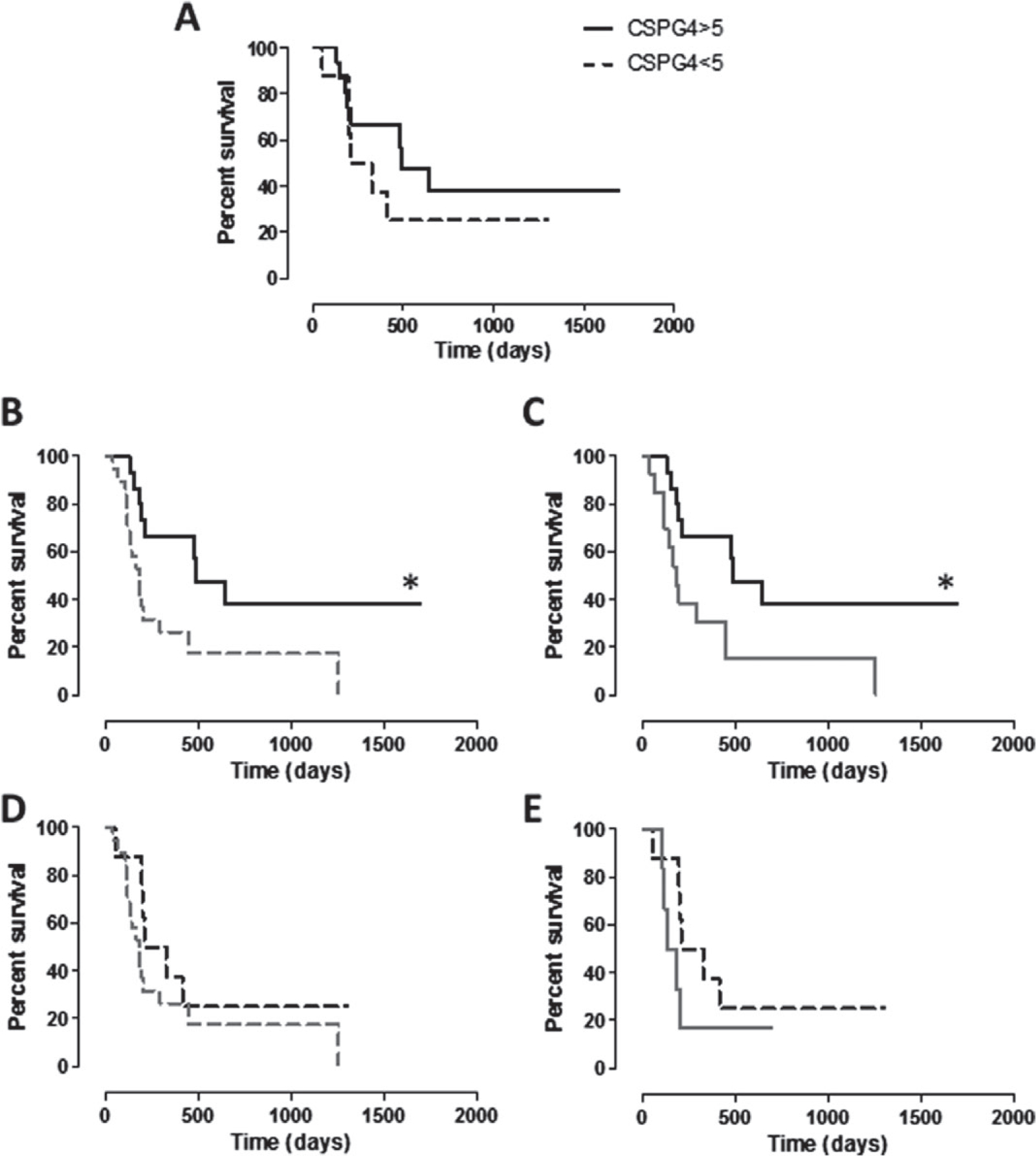
Kaplan–Meier curves comparing DFI (in days) of dogs in relation to cMM CSPG4-positivity score. (A) DFI of vaccinated dogs (group A) bearing a cMM with CSPG4-positivity score ≥5 (black line) or <5 (black dotted line). (B, C) DFI of vaccinated dogs (group A) bearing a cMM with CSPG4-positivity score ≥5 (black line) in comparison with DFI of (B) the entire population of non-vaccinated dogs (group B, grey dotted line; * log-rank test P = 0.0167) or of (C) non-vaccinated dogs bearing a cMM with CSPG4-positivity score ≥5 (group B, grey line; * log-rank test P = 0.0249). (D, E) DFI of vaccinated dogs (group A) bearing a cMM with CSPG4-positivity score <5 (black dotted line) in comparison with DFI of (D) the entire population of non-vaccinated dogs (group B, grey dotted line) or of (E) non-vaccinated dogs bearing a cMM with CSPG4-positivity score <5 (group B, grey line).
Discussion
Dogs with untreated oral cMM usually survive a few months.9 Surgery, when feasible, is important for the local control of oral cMM, especially for stage II and III tumours.1,12,27,28 After surgery alone, a MST up to 352 days and a 1-year survival rate around 30% have been reported.5,12,35 An alternative to surgery is radiotherapy, alone (also for palliation) or adjuvantly, especially in case of incomplete margins; hypofractionated radiation protocols are also used.3,17,18,20,21,23,29,32 Local control after radiation may be as high as 83–100% in up to 70% of oral cMM within some weeks and it seems better when cMM are rostral, small sized and without bone invasion, and when radiation is used in an adjuvant setting.17,18,20,21,32 However, LR may occur, even after a complete response has been reached;17,18,20,32 in the not responding cMM, progression of the disease usually occurs.17 The reported 1- and 2-year survival rate after radiotherapy is 36–48% and 21%, respectively, with a MST ranging from 211 to 363 days.17,18,20,21,32
Despite the local control of oral cMM provided by surgery and/or radiotherapy, distant metastasis represents the cause of death in up to 65–80% of dogs.1,2,20 No statistical increase of survival times was shown when dogs received also an adjuvant treatment.12,27 Chemotherapy for oral cMM is often used as an adjunctive treatment to control distant metastasis. Drugs more frequently utilized are carboplatin, cisplatin (also in conjunction with piroxicam) and melphalan.19,22–24,26,27 Platinum salts have also been used as radiosensitizers20,21; besides, cisplatin has been used intralesionally.58 In a more recent article, metronomic chemotherapy has also been utilized.27
As the role of adjuvant chemotherapy in prolonging survival of dogs bearing an oral cMM and controlling/delaying of the distant metastatic spread is uncertain, many studies have been addressed to alternative adjuvant treatments, such as new drugs59–61 and immunotherapy,3,30,31,33–43 also in form of combined protocols.44
Several immunotherapeutic approaches have been attempted, finally leading, in USA, to the approval of a xenogeneic DNA vaccine against tyrosinase (Oncept, Merial), whose efficacy has been shown in cMM patients when compared with historical controls.38,42 As Oncept efficacy has not been confirmed in two retrospective studies,37,62 further studies should be warranted. In our previous article, we showed both the safety and anti-tumour efficacy of CSPG4-immunotargeting in a group of dogs with surgically resected stage II–III CSPG4-positive oral MM.40 As the authors of this study never used ONCEPT as it has not been approved by the European Medicines Agency, no prospective comparison between the two vaccines was attempted.
In this study the authors report the data of a larger cohort of oral CSPG4-positive cMM patients treated by surgery plus adjuvant electrovaccination with hCSPG4-encoded plasmid (group A) or treated by surgery alone (group B), including both new enrolled dogs (coming also from a second center of vaccination) and those already considered in the previous study but updated in their follow-up. The distribution of age, sex, score of CSPG4 expression, percentage of Ki67 positivity, mitotic index, nuclear atypia and clinical stage within the two groups was uniform. A third group of dogs previously considered in Riccardo et al.,40 i.e. those bearing a CSPG4-negative oral cMM, was not considered here due to the fact that it displayed an intermediate behaviour and could not benefit anyway from the anti CSPG4-immunotargeting. In Riccardo et al.40 we showed that MST of vaccinated dogs was significantly longer when compared with the overall non-vaccinated canine population (both CSPG4-positive and negative); DFI of vaccinated dogs was significantly longer than CSPG4-positive non-vaccinated dogs but not CSPG4-negative non-vaccinated dogs or the entire non-vaccinated canine population; finally, both MST and DFI of CSPG4-positive non-vaccinated dogs showed no significant statistical difference in comparison with CSPG4-negative non-vaccinated dogs.
The results obtained here confirm both safety and immunogenicity of the electrovaccination with the hCSPG4 plasmid in dogs with CSPG4-positive oral cMM. The significant increase of the anti-hCSPG4 antibody titer in the post-vaccination sera as compared with pre-vaccination sera of group A dogs relates favorably with the significant prolongation of both survival times and DFI as compared with dogs of group B, receiving surgery alone, but no direct correlation between the antibody titer and the survival was found. Regarding this, it should be emphasized that endpoints for a clinical trial involving an immunotherapeutic approach (targeted treatment) are more challenging and different from those involving the use of a specific cytotoxic drug; in the latter case, in fact, procedures to evaluate its efficacy are usually more direct and easier to be applied.63 Nevertheless, it is authors’ hypothesis that the humoral anti-CSPG4 immunity has a direct beneficial effect on the clinical course of canine oral cMM. The vaccine-induced antibody titer in dogs with BW <20 kg is higher than that observed in dogs with BW >20 kg and, interestingly, vaccinated dogs with BW <20 kg are those with survival and DFI significantly longer than the population of non-vaccinated dogs (group B). This is not the case when we compare both the survival and DFI of vaccinated dogs with BW >20 kg and the population of non-vaccinated dogs (group B). These data suggest the importance of the level of the antibody titer induced by the vaccine and the potential fundamental role of the humoral response in prolonging both the survival time and DFI of vaccinated dogs; on the other hand, these results raise the question of scaling up doses in dogs with a high BW. Besides, also the CSPG4-positivity score of the cMM may have had an impact on the outcome. We have shown that anti-CSPG4 antibodies induced by the vaccine may act directly on the CSPG4 expression on MM cells, down regulating the protein and, consequently, likely affecting the several cancer-related pathways regulated by CSPG4. Despite the fact that the degree of CSPG4 expression did not correlate with the survival of non-vaccinated dogs,40 vaccinated dogs with a cMM with a CSPG4-positivity score higher than 5 survived longer than vaccinated dogs affected by an oral cMM with a CSPG4-positivity score lower than 5. It is likely that in the latter dogs a greater prevalence of CSPG4-negative tumour clones are present, being able to escape the anti-CSPG4 immunity induced by the vaccine and, ultimately allowing the progression of the disease. Collectively, these results provide not only a mechanistic explanation for the therapeutic effect of anti-CSPG4 antibodies in the treatment of cMM, but also corroborate the role of CSPG4 in the biology of cMM cells.
Clinical stage is a reported prognosticator and, apart from systemic metastasis which makes prognosis worse, the impact of regional lymphatic metastasis at presentation on survival is uncertain. One limitation here may have been that only the mandibular nodes were removed at surgery and histologically examined, with no attempt to identify other potential and/or alternative regional lymphatic stations (e.g. the retropharyngeal LNs)12,64,65; for this important issue, further studies are warranted. Also the decision that the surgeons involved in this study adopted, i.e. not to remove systematically the mandibular nodes bilaterally, may have influenced the final clinical stage (N parameter of the TNM system). In all the cases in which a regional lymphatic spread is demonstrated, the addition of an adjuvant treatment should be advantageous.27
Another important issue here is that in many published series of cases dealing with oral cMM, and also in this series, there are dogs that experience a long survival but it is not clear whether this reflects the efficacy of the treatment (despite the fact that a similar treatment was usually utilized also in dogs surviving for a shorter period of time) or a less aggressive tumoural behaviour.11 It is the authors’ opinion that these less malignant oral cMM may not be recognized clinically, being possible to identify them only by the evaluation of some already known prognostic factors such as Ki67, mitotic index and nuclear atypia.3,11 Therefore, the evaluation of these prognosticators should always be included in all the studies dealing with the results of the different therapeutic approaches applied for oral cMM, in order to better interpret the final outcome. Therefore, according to a recent article published by this group, also the PDGFR-α/-β co-expression in oral cMM should be evaluated and correlated with survival;14 so far, this parameter has not been evaluated yet in the cMM of the vaccinated dogs included in this article and further study are warranted.
Finally, it has been reported that the risk of LR after surgery seems correlated to the size of the primary tumour.27 LR seems more likely after en bloc excision performed at the level of the upper jaw in comparison with the lower jaw (22 versus 48%).66,67 Also the development of a LR should represent a negative prognostic factor for survival but its real impact is not clear. In this series of cases a LR developed mainly in the vaccinated dogs but it did not influence the continuation of vaccination when the recurrent tumours were properly treated (surgery±radiotherapy) provided that systemic metastasis were still under control, likely thanks to the vaccine; this was not the case of the non-vaccinated dogs in which the cause of the death was mainly due to systemic metastasis regardless the development of a LR. Moreover, in this series of cases, in the group of vaccinated dogs (group A), three patients died because of a second tumour (two prostatic adenocarcinomas and, one perianal adenocarcinoma with submandibular metastases), while in group B, all dead dogs succumbed because of the cMM. It can be speculated that the prolongation of the survival in group A dogs induced by the vaccine may have resulted in an increased risk of developing a second malignant tumour of a different histotype.
In conclusion, the results presented here are encouraging and confirm the usefulness of the anti-CSPG4 adjuvant vaccination in dogs with oral cMM. However, they should be still considered cautiously as the number of dogs included is still low.
Acknowledgements
This work was supported by grants from Fondazione Ricerca Molinette Onlus and from Fondazione CRT, Torino, Italy, within the ‘Richieste Ordinarie 2015’ call. F.R. has been supported with a fellowship from Fondazione Italiana per la Ricerca sul Cancro (FIRC).
References
- 1.Liptak JM and Lascelles BDX. Oral tumors. In: Veterinary Surgical Oncology. Kidnig ST and Séguin B Eds., Ames, Wiley-Blackwell, 2012: 119–177. [Google Scholar]
- 2.Liptak JM and Withrow SJ. Oral tumors. In: Small Animal Clinical Oncology. Withrow SJ, Vail DM and Page RL Eds., St. Louis, Elsevier, 2013: 381–398. [Google Scholar]
- 3.Bergman PJ, Kent MS and Farese J. Melanoma. In: Withrow & MacEwen’s Small Animal Clinical Oncology. 5th edn., Withrow SJ, Vail DM and Page RL Eds., St. Louis, Sounders, 2013. [Google Scholar]
- 4.Smedley RC, Lamoureux J, Sledge DG and Kiupel M. Immunohistochemical diagnosis of canine oral amelanotic melanocytic neoplasms. Veterinary Pathology 2011; 48: 32–40. [DOI] [PubMed] [Google Scholar]
- 5.Ramos-Vara JA, Beissenherz ME, Miller MA, Johnson GC, Pace LW, Fard A, et al. Retrospective study of 338 canine oral melanomas with clinical, histologic, and immunohistochemical review of 129 cases. Veterinary Pathology 2000; 37: 597–608. [DOI] [PubMed] [Google Scholar]
- 6.Giudice C, Ceciliani F, Rondena M, Stefanello D and Grieco V. Immunohistochemical investigation of PNL2 reactivity of canine melanocytic neoplasms and comparison with Melan A. Journal of Veterinary Diagnostic Investigation 2010; 22: 389–394. [DOI] [PubMed] [Google Scholar]
- 7.Ramos-Vara JA and Miller MA. Immunohistochemical identification of canine melanocytic neoplasms with antibodies to melanocytic antigen PNL2 and tyrosinase: comparison with Melan A. Veterinary Pathology 2011; 48: 443–450. [DOI] [PubMed] [Google Scholar]
- 8.Williams LE and Packer RA. Association between lymph node size and metastasis in dogs with oral malignant melanoma: 100 cases (1987–2001). Journal of the American Veterinary Medical Association 2003; 222: 1234–1236. [DOI] [PubMed] [Google Scholar]
- 9.Harvey HJ, MacEwen EG, Braun D, Patnaik AK, Withrow SJ and Jongeward S. Prognostic criteria for dogs with oral melanoma. Journal of the American Veterinary Medical Association 1981; 178: 580–582. [PubMed] [Google Scholar]
- 10.MacEwen EG, Patnaik AK, Harvey HJ, Hayes AA and Matus R. Canine oral melanoma: comparison of surgery versus surgery plus Corynebacterium parvum. Cancer Investigation 1986; 4: 397–402. [DOI] [PubMed] [Google Scholar]
- 11.Smedley RC, Spangler WL, Esplin DG, Kitchell BE, Bergman PJ, Ho HY, et al. Prognostic markers for canine melanocytic neoplasms: a comparative review of the literature and goals for future investigation. Veterinary Pathology 2011; 48: 54–72. [DOI] [PubMed] [Google Scholar]
- 12.Boston SE, Lu X, Culp WT, Montinaro V, Romanelli G, Dudley RM, et al. Efficacy of systemic adjuvant therapies administered to dogs after excision of oral malignant melanomas: 151 cases (2001–2012). Journal of the American Veterinary Medical Association 2014; 245: 401–407. [DOI] [PubMed] [Google Scholar]
- 13.Bergin IL, Smedley RC, Esplin DG, Spangler WL and Kiupel M. Prognostic evaluation of Ki67 threshold value in canine oral melanoma. Veterinary Pathology 2011; 48: 41–53. [DOI] [PubMed] [Google Scholar]
- 14.Iussich S, Maniscalco L, Di Sciuva A, Iotti B, Morello E, Martano M, et al. PDGFRs expression in dogs affected by malignant oral melanomas: correlation with prognosis. Veterinary and Comparative Oncology 2016. doi: 10.1111/vco.12190. [DOI] [PubMed] [Google Scholar]
- 15.Esplin DG. Survival of dogs following surgical excision of histologically well-differentiated melanocytic neoplasms of the mucous membranes of the lips and oral cavity. Veterinary Pathology 2008; 45: 889–896. [DOI] [PubMed] [Google Scholar]
- 16.Dewhirst MW, Sim DA, Forsyth K, Grochowski KJ, Wilson S and Bicknell E. Local control and distant metastases in primary canine malignant melanomas treated with hyperthermia and/or radiotherapy. International Journal of Hyperthermia 1985; 1: 219–234. [DOI] [PubMed] [Google Scholar]
- 17.Bateman KE, Catton PA, Pennock PW and Kruth SA. 0–7-21 radiation therapy for the treatment of canine oral melanoma. Journal of Veterinary Internal Medicine 1994; 8: 267–272. [DOI] [PubMed] [Google Scholar]
- 18.Blackwood L and Dobson JM. Radiotherapy of oral malignant melanomas in dogs. Journal of the American Veterinary Medical Association 1996; 209: 98–102. [PubMed] [Google Scholar]
- 19.Rassnick KM, Ruslander DM, Cotter SM, Al-Sarraf R, Bruyette DS, Gamblin RM, et al. Use of carboplatin for treatment of dogs with malignant melanoma: 27 cases (1989–2000). Journal of the American Veterinary Medical Association 2001; 218: 1444–1448. [DOI] [PubMed] [Google Scholar]
- 20.Freeman KP, Hahn KA, Harris FD and King GK. Treatment of dogs with oral melanoma by hypofractionated radiation therapy and platinum-based chemotherapy (1987–1997). Journal of Veterinary Internal Medicine 2003; 17: 96–101. [DOI] [PubMed] [Google Scholar]
- 21.Proulx DR, Ruslander DM, Dodge RK, Hauck ML, Williams LE, Horn B, et al. A retrospective analysis of 140 dogs with oral melanoma treated with external beam radiation. Veterinary Radiology & Ultrasound 2003; 44: 352–359. [DOI] [PubMed] [Google Scholar]
- 22.Boria PA, Murry DJ, Bennett PF, Glickman NW, Snyder PW, Merkel BL, et al. Evaluation of cisplatin combined with piroxicam for the treatment of oral malignant melanoma and oral squamous cell carcinoma in dogs. Journal of the American Veterinary Medical Association 2004; 224: 388–394. [DOI] [PubMed] [Google Scholar]
- 23.Murphy S, Hayes AM, Blackwood L, Maglennon G, Pattinson H and Sparkes AH. Oral malignant melanoma – the effect of coarse fractionation radiotherapy alone or with adjuvant carboplatin therapy. Veterinary and Comparative Oncology 2005; 3: 222–229. [DOI] [PubMed] [Google Scholar]
- 24.Brockley LK, Cooper MA and Bennett PF. Malignant melanoma in 63 dogs (2001–2011): the effect of carboplatin chemotherapy on survival. New Zealand Veterinary Journal 2013; 61: 25–31. [DOI] [PubMed] [Google Scholar]
- 25.Cancedda S, Rohrer Bley C, Aresu L, Dacasto M, Leone VF, Pizzoni S, et al. Efficacy and side effects of radiation therapy in comparison with radiation therapy and temozolomide in the treatment of measurable canine malignant melanoma. Veterinary and Comparative Oncology 2014. doi: 10.0000/vco.12122. [DOI] [PubMed] [Google Scholar]
- 26.Dank G, Rassnick KM, Sokolovsky Y, Garrett LD, Post GS, Kitchell BE, et al. Use of adjuvant carboplatin for treatment of dogs with oral malignant melanoma following surgical excision. Veterinary and Comparative Oncology 2014; 12: 78–84. [DOI] [PubMed] [Google Scholar]
- 27.Tuohy JL, Selmic LE, Worley DR, Ehrhart NP and Withrow SJ. Outcome following curative-intent surgery for oral melanoma in dogs: 70 cases (1998–2011). Journal of the American Veterinary Medical Association 2014; 245: 1266–1273. [DOI] [PubMed] [Google Scholar]
- 28.Culp WT, Ehrhart N, Withrow SJ, Rebhun RB, Boston S, Buracco P, et al. Results of surgical excision and evaluation of factors associated with survival time in dogs with lingual neoplasia: 97 cases (1995–2008). Journal of the American Veterinary Medical Association 2013; 242: 1392–1397. [DOI] [PubMed] [Google Scholar]
- 29.Kawabe M, Mori T, Ito Y, Murakami M, Sakai H, Yanai T, et al. Outcomes of dogs undergoing radiotherapy for treatment of oral malignant melanoma: 111 cases (2006–2012). Journal of the American Veterinary Medical Association 2015; 247: 1146–1153. [DOI] [PubMed] [Google Scholar]
- 30.Moore AS, Theilen GH, Newell AD, Madewell BR and Rudolf AR. Preclinical study of sequential tumor necrosis factor and interleukin 2 in the treatment of spontaneous canine neoplasms. Cancer Research 1991; 51: 233–238. [PubMed] [Google Scholar]
- 31.Quintin-Colonna F, Devauchelle P, Fradelizi D, Mourot B, Faure T, Kourilsky P, et al. Gene therapy of spontaneous canine melanoma and feline fibrosarcoma by intratumoral administration of histoincompatible cells expressing human interleukin-2. Gene Therapy 1996; 3: 1104–1112. [PubMed] [Google Scholar]
- 32.Theon AP, Rodriguez C and Madewell BR. Analysis of prognostic factors and patterns of failure in dogs with malignant oral tumors treated with megavoltage irradiation. Journal of the American Veterinary Medical Association 1997; 210: 778–784. [PubMed] [Google Scholar]
- 33.Dow SW, Elmslie RE, Willson AP, Roche L, Gorman C and Potter TA. In vivo tumor transfection with superantigen plus cytokine genes induces tumor regression and prolongs survival in dogs with malignant melanoma. The Journal of Clinical Investigation 1998; 101: 2406–2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hogge GS, Burkholder JK, Culp J, Albertini MR, Dubielzig RR, Keller ET, et al. Development of human granulocyte-macrophage colony-stimulating factor-transfected tumor cell vaccines for the treatment of spontaneous canine cancer. Human Gene Therapy 1998; 9: 1851–1861. [DOI] [PubMed] [Google Scholar]
- 35.MacEwen EG, Kurzman ID, Vail DM, Dubielzig RR, Everlith K, Madewell BR, et al. Adjuvant therapy for melanoma in dogs: results of randomized clinical trials using surgery, liposome-encapsulated muramyl tripeptide, and granulocyte macrophage colony-stimulating factor. Clinical Cancer Research 1999; 5: 4249–4258. [PubMed] [Google Scholar]
- 36.Bergman PJ, McKnight J, Novosad A, Charney S, Farrelly J, Craft D, et al. Long-term survival of dogs with advanced malignant melanoma after DNA vaccination with xenogeneic human tyrosinase: a phase I trial. Clinical Cancer Research 2003; 9: 1284–1290. [PubMed] [Google Scholar]
- 37.Ottnod JM, Smedley RC, Walshaw R, Hauptman JG, Kiupel M and Obradovich JE. A retrospective analysis of the efficacy of Oncept vaccine for the adjunct treatment of canine oral malignant melanoma. Veterinary and Comparative Oncology 2013; 11: 219–229. [DOI] [PubMed] [Google Scholar]
- 38.Grosenbaugh DA, Leard AT, Bergman PJ, Klein MK, Meleo K, Susaneck S, et al. Safety and efficacy of a xenogeneic DNA vaccine encoding for human tyrosinase as adjunctive treatment for oral malignant melanoma in dogs following surgical excision of the primary tumor. American Journal of Veterinary Research 2011; 72: 1631–1638. [DOI] [PubMed] [Google Scholar]
- 39.Glikin GC and Finocchiaro LM. Clinical trials of immunogene therapy for spontaneous tumors in companion animals. Scientific World Journal 2014; 2014: 718520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Riccardo F, Iussich S, Maniscalco L, Lorda-Mayayo S, La Rosa G, Arigoni M, et al. CSPG4-specific immunity and survival prolongation in dogs with oral malignant melanoma immunized with human CSPG4 DNA. Clinical Cancer Research 2014; 20: 3753–3762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Finocchiaro LM, Fondello C, Gil-Cardeza ML, Rossi UA, Villaverde MS, Riveros MD, et al. Cytokine-enhanced vaccine and interferon-beta plus suicide gene therapy as surgery adjuvant treatments for spontaneous canine melanoma. Human Gene Therapy 2015; 26: 367–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McLean JL and Lobetti RG. Use of the melanoma vaccine in 38 dogs: The South African experience. Journal of the South African Veterinary Association 2015; 86: 1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Paoloni M, Mazcko C, Selting K, Lana S, Barber L, Phillips J, et al. Defining the pharmacodynamic profile and therapeutic index of NHS-IL12 immunocytokine in dogs with malignant melanoma. PloS One 2015; 10: e0129954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Herzog A, Buchholz J, Ruess-Melzer K, Lang J and Kaser-Hotz B. Combined use of irradiation and DNA tumor vaccine to treat canine oral malignant melanoma: a pilot study. Schweizer Archiv für Tierheilkunde 2013; 155: 135–142. [DOI] [PubMed] [Google Scholar]
- 45.Itoh H, Mukaiyama T, Goto T, Hata K, Azuma K, Tsuka T, et al. Non-surgical treatment of canine oral malignant melanoma: a case study of the application of complementary alternative medicine. Oncology Letters 2014; 7: 1829–1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ogihara K, Naya Y, Okamoto Y and Hata K. Differentiation-inducing and anti-proliferative activities of lupeol on canine melanoma cells. Springerplus 2014; 3: 632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yokoe I, Azuma K, Hata K, Mukaiyama T, Goto T, Tsuka T, et al. Clinical systemic lupeol administration for canine oral malignant melanoma. Molecular and Clinical Oncology 2015; 3: 89–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mayayo SL, Prestigio S, Maniscalco L, La Rosa G, Arico A, De Maria R, et al. Chondroitin sulfate proteoglycan-4: a biomarker and a potential immunotherapeutic target for canine malignant melanoma. Veterinary Journal 2011; 190: e26–e30. [DOI] [PubMed] [Google Scholar]
- 49.Price MA, Colvin Wanshura LE, Yang J, Carlson J, Xiang B, Li G, et al. CSPG4, a potential therapeutic target, facilitates malignant progression of melanoma. Pigment Cell & Melanoma Research 2011; 24: 1148–1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cheever MA, Allison JP, Ferris AS, Finn OJ, Hastings BM, Hecht TT, et al. The prioritization of cancer antigens: a national cancer institute pilot project for the acceleration of translational research. Clinical Cancer Research 2009; 15: 5323–5337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bodles-Brakhop AM, Heller R and Draghia-Akli R. Electroporation for the delivery of DNA-based vaccines and immunotherapeutics: current clinical developments. Molecular Therapy 2009; 17: 585–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Heller LC and Heller R. Electroporation gene therapy preclinical and clinical trials for melanoma. Current Gene Therapy 2010; 10: 312–317. [DOI] [PubMed] [Google Scholar]
- 53.Sardesai NY and Weiner DB. Electroporation delivery of DNA vaccines: prospects for success. Current Opinion in Immunology 2011; 23: 421–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cavallo F, Aurisicchio L, Mancini R and Ciliberto G. Xenogene vaccination in the therapy of cancer. Expert Opinion on Biological Therapy 2014; 14: 1427–1442. [DOI] [PubMed] [Google Scholar]
- 55.Impellizeri JA, Ciliberto G and Aurisicchio L. Electro-gene-transfer as a new tool for cancer immunotherapy in animals. Veterinary and Comparative Oncology 2014; 12: 310–318. [DOI] [PubMed] [Google Scholar]
- 56.Yang J, Price MA, Neudauer CL, Wilson C, Ferrone S, Xia H, et al. Melanoma chondroitin sulfate proteoglycan enhances FAK and ERK activation by distinct mechanisms. The Journal of Cell Biology 2004; 165: 881–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.R Development Core Team. R: A Language and Environment for Statistical Computing. Vienna, R Foundation for Statistical Computing, 2010. [Google Scholar]
- 58.Kitchell BE, Brown DM, Luck EE, Woods LL, Orenberg EK and Bloch DA. Intralesional implant for treatment of primary oral malignant melanoma in dogs. Journal of the American Veterinary Medical Association 1994; 204: 229–236. [PubMed] [Google Scholar]
- 59.Breit MN, Kisseberth WC, Bear MD, Landesman Y, Kashyap T, McCauley D, et al. Biologic activity of the novel orally bioavailable selective inhibitor of nuclear export (SINE) KPT-335 against canine melanoma cell lines. BMC Veterinary Research 2014; 10: 160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.London CA, Bernabe LF, Barnard S, Kisseberth WC, Borgatti A, Henson M, et al. Preclinical evaluation of the novel, orally bioavailable Selective Inhibitor of Nuclear Export (SINE) KPT-335 in spontaneous canine cancer: results of a phase I study. PloS One 2014; 9: e87585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Seo KW, Coh YR, Rebhun RB, Ahn JO, Han SM, Lee HW, et al. Antitumor effects of celecoxib in COX-2 expressing and non-expressing canine melanoma cell lines. Research in Veterinary Science 2014; 96: 482–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Treggiari E, Grant JP and North SM. A retrospective review of outcome and survival following surgery and adjuvant xenogeneic DNA vaccination in 32 dogs with oral malignant melanoma. The Journal of Veterinary Medical Science 2016. [Epub ahead of print]. [DOI] [PMC free article] [PubMed]
- 63.Marconato L, Buracco P and Aresu L. Perspectives on the design of clinical trials for targeted therapies and immunotherapy in veterinary oncology. Veterinary Journal 2015; 205: 238–243. [DOI] [PubMed] [Google Scholar]
- 64.Herring ES, Smith MM and Robertson JL. Lymph node staging of oral and maxillofacial neoplasms in 31 dogs and cats. Journal of Veterinary Dentistry 2002; 19: 122–126. [DOI] [PubMed] [Google Scholar]
- 65.Tuohy JL, Milgram J, Worley DR and Dernell WS. A review of sentinel lymph node evaluation and the need for its incorporation into veterinary oncology. Veterinary and Comparative Oncology 2009; 7: 81–91. [DOI] [PubMed] [Google Scholar]
- 66.Kosovsky JK, Matthiesen DT, Marretta SM and Patnaik AK. Results of partial mandibulectomy for the treatment of oral tumors in 142 dogs. Veterinary Surgery 1991; 20: 397–401. [DOI] [PubMed] [Google Scholar]
- 67.Wallace J, Matthiesen DT and Patnaik AK. Hemimaxillectomy for the treatment of oral tumors in 69 dogs. Veterinary Surgery 1992; 21: 337–341. [DOI] [PubMed] [Google Scholar]


