Abstract
IMPORTANCE
Immunotherapy has emerged as a new pillar of cancer therapy over the past decade. Adoptive immunotherapy in particular has become a major area of research interest, with advances seen in the development of T-cell engineering. As a result, chimeric antigen receptor (CAR) T-cell therapy has become a new and highly effective treatment option, especially for patients with refractory or resistant blood cell cancers. However, CAR T-cell therapy has shown limited efficacy for the treatment of solid tumors thus far.
OBSERVATIONS
Combinatorial treatment approaches, such as addition of radiotherapy to CAR T cells, may provide a strategy to prevent resistance to CAR T-cell therapy of solid tumors. These approaches need to overcome obstacles that include abnormal vessels and adhesion molecule expression on tumor vasculature, leading to reduced transmigration of effector immune cells, including CAR T cells, and immunosuppressive cues in the tumor microenvironment, including regional hypoxia.
CONCLUSIONS AND RELEVANCE
This review provides an overview of the current developments in CAR T-cell therapy and highlights the unique opportunities and challenges in combining CAR T-cell therapy with radiotherapy.
Chimeric antigen receptor (CAR) T-cell therapy, which is a result of the evolution of T-cell engineering, represents a significant breakthrough in adoptive T-cell therapies for cancer therapy. The basic principle of CAR T-cell therapy is that T cells are transduced with a synthetic tumor antigen–targeted receptor. This receptor, chimeric antigen receptor, is constructed of a tumor antigen–recognizing antibody; a single-chain variable fragment comprising a heavy and light chain, as well as a flexible peptide linker, called ectodomain; a transmembrane domain; and the signaling domain of a T-cell receptor (TCR) (endodomain) (Figure 1).1
Figure 1. Chimeric Antigen Receptor (CAR) T-Cell Evolution.
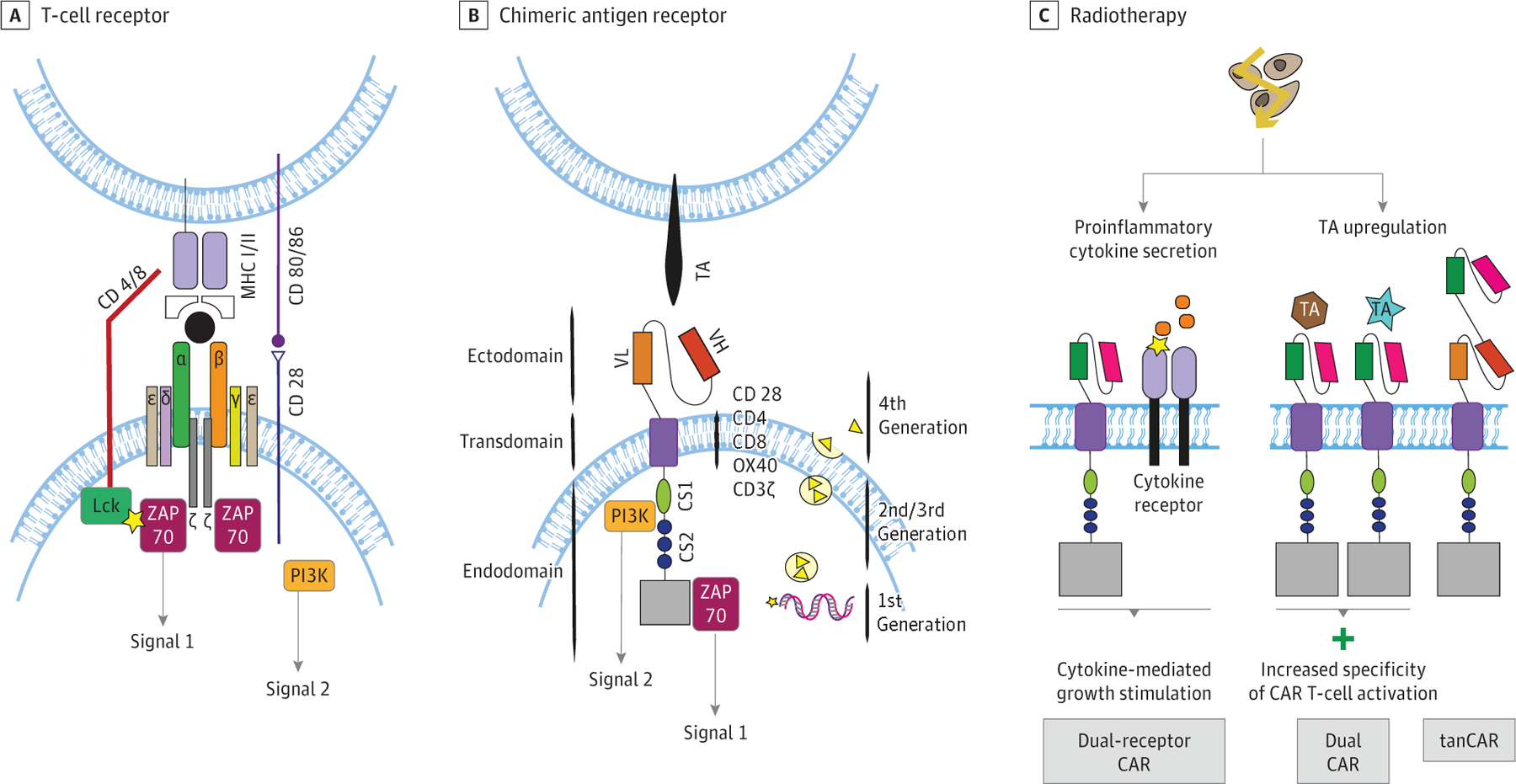
A and B, Illustration of a physiological T-cell receptor and a CAR T-cell receptor. A, Dual-receptor CAR T cells: simultaneous expression of a CAR with a cytokine receptor providing cytokine-mediated growth stimulation. B, T cells redirected for universal cytokine-mediated killing, known as TRUCK (fourth-generation CAR T cells): after activation of CAR T-cell receptor, a promotor is induced, resulting in the production of cytokines, such as IL-12. These cytokines may, in turn, help to overcome the immunosuppressive microenvironment after irradiation. C, Modification of CAR T cells potentially aiding synergy with radiation. Dual CAR T cells: increasing specificity of CAR T cells by expression of 2 different CARs, which can only be fully activated by binding both antigens. Tandem CAR T cells (tanCAR): CARs engineered to express 2 antigen-binding domains resulting in higher probability of activation. CD indicates cluster of differentiation; Lck, lymphocyte-specific protein tyrosine kinase; MHC, major histocompatibility complex; OX40, also known as tumor necrosis factor receptor superfamily member 4 (TNFRSF4) and CD134; TA, tumor antigen; VH, V domain of the heavy chain; VL, V domain of the light chain; ZAP, zeta chain–associated protein kinase.
Whereas TCRs are only able to recognize antigen-derived peptides presented by major histocompatibility complex (MHC) class I molecules, CAR T cells bind to the corresponding antigen without the need of its processing by MHC class I antigen-processing machinery. In addition, while TCRs recognize only proteins and mostly intracellular antigens, CARs bind cell surface antigens and can recognize both proteins and gangliosides.2 The CAR transmembrane domains usually consist of CD28, as this structure increases stability. However, CD4, CD8, OX40, or CD3ζ may be used as well.
First-generation CARs consisted of a single intracellular signaling module including CD3ζ to induce T-cell activation.3–5 Physiologically, to activate and promote expansion of T cells following repeated antigen exposure, a second signal is provided by antigen-presenting cells (APCs) through interactions between CD28 and the CD80/CD86 complex on APCs, resulting in PI3K activation and costimulation of T cells.4 To leverage this mechanism, second-generation and third-generation CARs combine the CD3ζ receptor with either 2 (second generation)6,7 or 3 (third generation)8,9 signaling endodomains, such as CD28, OX40, or 4–1BB (Figure 1). To improve antitumor efficacy, fourth-generation CAR T cells have evolved from second-generation CARs by adding a cytokine expression cassette; they are called T cells redirected for universal cytokine-mediated killing, or TRUCK.10,11 The incorporated cytokines include interleukin (IL)-2, IL-10, and IL-17, among others, and promote enhanced T-cell activation and recruitment of a patient’s own immune cells.12,13
Challenges in the Implementation of CAR T-Cell Therapies in Solid Tumors
Although CAR T-cell treatment has had an important influence on treatment of patients with hematologic cancers, only limited success has been achieved in solid tumors.14 Here, we will discuss several hallmarks of solid tumors that contribute to the limited therapeutic efficacy of CAR T cells,1,2 as well as a potential solution to address treatment resistance by using radiotherapy.
Barriers to Immune Cell Infiltration
Chimeric antigen receptor T-cell trafficking and ability to home and infiltrate into tumor tissue is the first requirement for successful therapy. Chimeric antigen receptor T-cell therapy can be limited in TA upregulation solid tumors by reduced homing to abnormal tumor vessels and tissue infiltration via endothelial transmigration. Tumor infiltration by CAR T cells depends on chemokine receptor expression, which has to match chemokines secreted by targeted tumors. However, there is often a mismatch between these, reducing treatment efficacy.15 Several approaches have been proposed to overcome these limitations. A preclinical study showed that antiangiogenic therapy can enhance T-cell homing and extravasation by upregulation of adhesion molecules on endothelial cells.16 Increased homing of adoptively transferred T cells has also been demonstrated when combined with anti–vascular endothelial growth factor (VEGF) therapy to normalize tumor vasculature or when using VEGF receptor 2–targeted CAR T cells plus exogenous IL-2 in mouse models.17,18 In addition, designing CAR T cells to express a small peptide, called the regulatory subunit I anchoring disruptor, that inhibits the association of protein kinase A with ezrin, a protein serving as a linker between plasma membrane and actin cytoskeleton, led to activation of CAR T cells, enhanced their migration in response to the chemokine CXCL10, and increased their trafficking into tumors.19 The efficacy of these combinatorial strategies remains to be demonstrated in patients.20
Role of Tumor Microenvironment in CAR T-Cell Therapy Resistance
One of the many ways tumor cells shape their microenvironment is through the secretion of cytokines, leading to the recruitment of immunosuppressive cells, such as regulatory T cells (Tregs) (Figure 2). Regulatory T cells play a major role in healthy immune system self-tolerance. In the tumor microenvironment, Tregs suppress effector T-cell activity, leading to exhaustion not only of patient’s own immune defense mechanisms but also of infused CAR T cells. The chemokine CXCL12 or SDF1α is one of the main chemokines recruiting Tregs to tumor sites. Infiltration of Tregs is also induced by the secretion of reactive oxygen species as well as of anti-inflammatory cytokines (IL-10, TGF-β) by myeloid-derived suppressor cells.21 Myeloid-derived suppressor cells are a heterogenous population of cells with myeloid origin, such as immature macrophages, myeloid progenitor cells, and immature dendritic cells. Myeloid-derived suppressor cells can express immune checkpoint molecules, such as programmed cell death ligand 1 and indoleamine-2,3 dioxygenase on their surface, thus promoting T-cell and CAR T-cell exhaustion (a state of functional hyporesponsiveness) and anergy (a state of T-cell dysfunction induced by suboptimal stimulation). Activation of CAR T cells may be further compromised by tumor-associated macrophages (TAMs), which contribute to Treg activity through secretion of prostaglandin E2 and IL-10 as well as of chemokines such as CCL17, CCL18, and CCL22 and potential programmed cell death ligand 1 expression.22 Cytokine release syndrome, a severe adverse effect of CAR T-cell therapy, has been shown to be associated with IL-6, IL-1, and nitric oxide release by TAMs in the tumor microenvironment.23
Figure 2. Process of Chimeric Antigen Receptor (CAR) T-Cell Therapy in Patients and Barriers for Delivery in the Tumor Microenvironment.
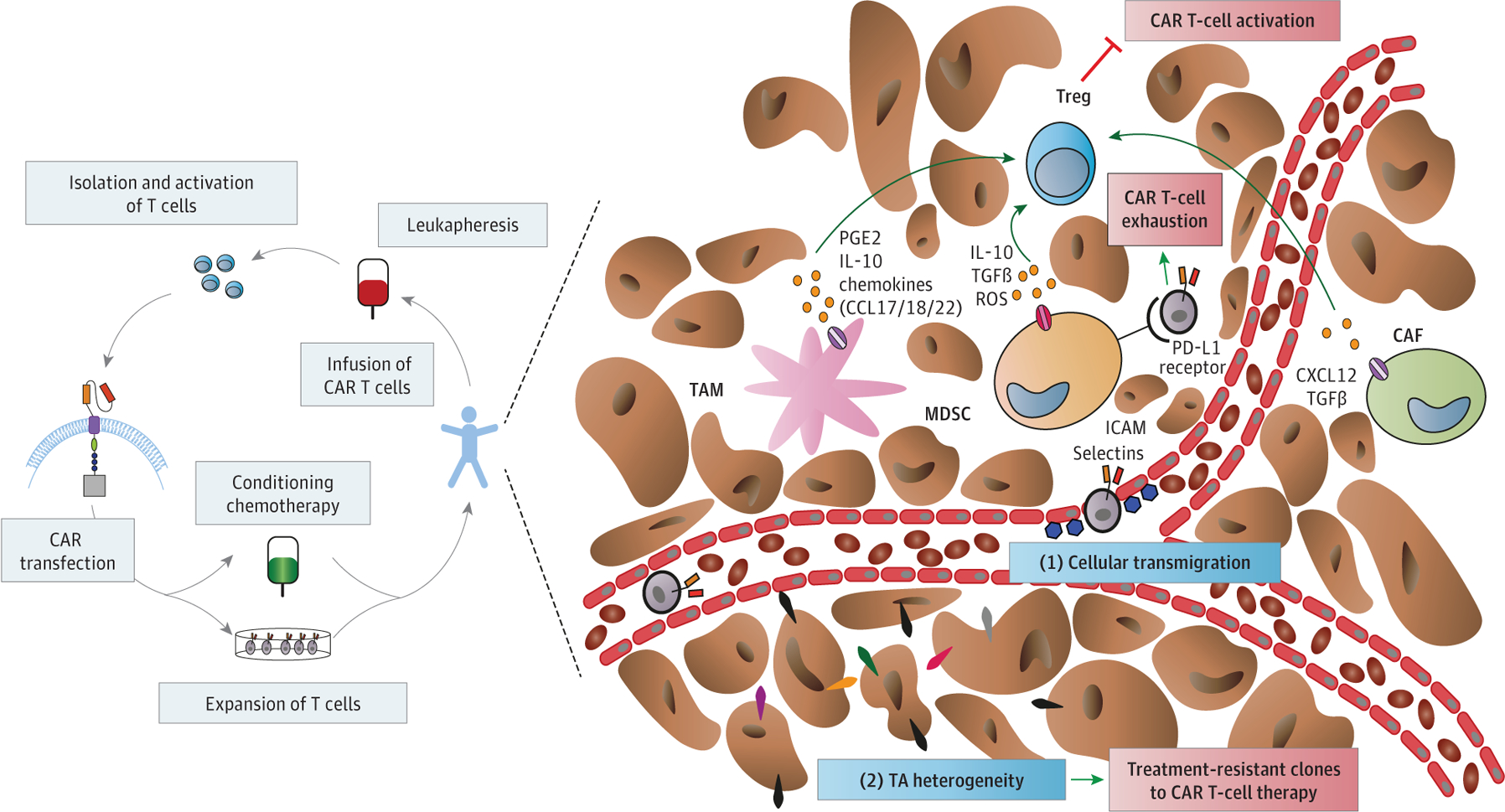
CAF indicates cancer-associated fibroblast; CXCL, chemokine ligand; ICAM, intercellular adhesion molecule; IL, interleukin; LH, L domain of the light chain; MDSC, myeloid-derived suppressor cell; NO, nitric oxide; PD-L1, programmed cell death ligand 1; PGE2, prostaglandin E2; ROS, reactive oxygen species; TAM, tumor-associated macrophage; TGFβ, transforming growth factor beta; Treg, regulatory T cell.
Tumor Antigen Expression Heterogeneity
The selection of targets for CAR T cells in solid tumors is particularly challenging because most of the identified target antigens are also expressed on normal cells, albeit at a lower level. This has caused adverse effects in early clinical trials, so called “on-target off-tumor” toxic effects.24 Furthermore, CAR T-cell treatment efficacy may be limited by heterogeneous targeted tumor antigen expression within tumors, resulting in the generation of treatment-resistant clones.25 Heterogeneity may be intrinsic to the tumor or may arise from treatment owing to immunoediting.26 Unfortunately, the critical questions of the percentage of tumor cells needed to express the targeted tumor antigen and its expression level to allow for a successful CAR T-cell therapy remain unanswered.
However, clinical evidence suggests that recognition of expressed tumor antigen on normal vs malignant cells might depend on the makeup and affinity of distinct Fab fragments used for engineering CARs as well as on targeted epitope.24,27 The influence on the potential of CAR association constant for the targeted structure, the location and density of the epitope on cell surface, and the binding properties of CARs on their antitumoral effects need to be carefully considered when selecting the optimal target. Another important variable is the optimization of costimulation for CAR T cells to achieve the highest activation and expansion rates following infusion, especially in solid tumors, which most likely must be adapted for each individual CAR.
Vascular Abnormalities and Hypoxia in Tumors
In solid tumors, the newly formed vessels are abnormal in their structure and function, leading to regional tissue hypoxia. Hypoxia (defined as low oxygen tension in tissue) is a common mechanism of treatment resistance—including immunotherapy, radiotherapy, and some chemotherapies—in most solid tumors.28 Of note, Berahovich et al29 reported reduced expansion of CAR T cells and cytokine secretion and a less differentiated phenotype when cells were cultured under hypoxic conditions. The tumor vessels are also critical for T-cell trafficking and activation. Thus, we anticipate that the efficacy of CAR T-cell therapy, alone or in combination, will be limited by the abnormal function of the vasculature and the hypoxic conditions. Future studies will need to characterize how the abnormal tumor vessels and hypoxia mediate CAR T-cell function, as well as their role in combination treatment, and to develop effective approaches to prevent hypoxia-mediated treatment resistance in solid tumors—for example, by vascular-normalizing strategies.30
Radioimmunotherapy Involving CAR T Cells
Rationale for Combining Radiotherapy With Immunotherapy
Radiotherapy can increase the local expression of multiple cytokines, including IFN-γ and its inducible chemokines, such as CXCL9, CXCL10, CXCL11, or CXCL16,31 which could chemoattract lymphocytes, including CAR T cells. In addition, radiotherapy may also affect tumor vessels, promoting a more adhesive phenotype, characterized by expression of adhesion molecules on the endothelium. This change facilitates homing and transmigration of immune effector cells and functional normalization of the vasculature. Vascular normalization is a critical mediator of target antigen-specific immunity and might enhance the efficacy of CAR T-cell therapies.30 Adhesion molecule expression on the surface of endothelial cells was significantly increased after conditioning radiotherapy ina mouse model of pancreatic insulinoma (RIP1-Tag5-knockout mice). Furthermore, combination of conditioning radiotherapy with transfer of activated T lymphocytes was associated with normalization of tumor vasculature in this model.32 Lastly, low-dose irradiation with 2 Gywas shown to result in normalization of tumor vasculature and increased T-cell recruitment.33 Because there is a strong association between target antigen density on targeted cells and CAR T-cell recognition and elimination of targeted cells, increased target antigen expression after radiotherapy could be a key effect in the context of CAR T-cell therapy.34
Radiotherapy may also have complementary effects by inducing endogenous target antigen-specific immunity. For example, radiotherapy can promote a so-called inflamed microenvironment, characterized by immunogenic cell death, resulting in the release of danger-associated molecular patterns, including adenosine triphosphate, calreticulin, and HMGB1.35,36 This may also facilitate the recruitment of APCs, such as dendritic cells and TAMs into the tumor.37,38 Radiotherapy has also been shown to induce and/or increase target antigen cross-presentation by MHC class II antigens expressed on APCs.39 Taken together, these results indicate that radiotherapy may be useful to enhance CAR T-cell efficacy directly, by increasing the target antigen expression, CAR T-cell infiltration, and cytokine/chemokine expression, and also indirectly, by enhancing endogenous target antigen–specific immunity to promote more durable responses.
Effects of Radiotherapy Outside the Irradiated Field
The so-called abscopal effects, which describe tumor responses outside the irradiation field after radiotherapy—for example, in patients with metastatic disease—is another important effect of radiotherapy. So far, more than 30 clinical cases have been published describing abscopal effects in patients.40,41 Theinterest in abscopal effects has been renewed by the advent of immunotherapy in the past decade. As early as 1916, tumor control dose was shown to be double for tumors lacking T cells, indicating the importance of T-cell activity for the cytotoxic effects of radiotherapy.42 This conclusion is supported by the growth factor Flt3 ligand–induced increased survival of mice expressing a highly metastatic variant of lung carcinoma in a T-cell–dependent manner after local radiotherapy with 60 Gy.43 The concept of an immune-cell dependence of the abscopal effect has since been confirmed in several other studies evaluating the effect of radiotherapy in combination with different immune-modulating approaches both preclinically and clinically.44 Of note, one case report described aresponse outside the irradiation field in a patient with multiple myeloma following localized radiotherapy of the spinal cord shortly after infusion of B-cell maturation antigen–targeted CAR T cells.45 However, the mechanisms mediating effects outside the radiation field remain poorly understood, and the occurrence of abscopal effects is unpredictable in patients. A 2021 phase 2 randomized clinical trial46 investigated the effect of nivolumab alone or in combination with stereotactic body radiotherapy on untreated (secondary) lesions in patients with metastatic head and neck cancer and reported no evidence of abscopal effects.
Challenges for the Use of Radiotherapy With CAR T Cells
Despite these exciting mechanisms of potential synergy with immunotherapy, it is noteworthy that radiotherapy has the potential to cause immunosuppression—for example, by increasing Treg infiltration in tumors.47 The association of radiotherapy with the immunogenicity of solid tumors has gained tremendous interest with the recent breakthroughs in immunotherapy. Several recent clinical studies have suggested a correlation between the number of circulating Tregs and a more immunosuppressive tumor microenvironment after radiochemotherapy.48 Furthermore, several reports converged to the conclusion that radiotherapy can lead to an increased secretion of immunosuppressive cytokines, such as IL-10 and TGF-β.49 The role of TAMs in the context of radiotherapy remains insufficiently characterized. However, several studies support the concept that M2-like TAMs act predominantly as mediators of immunosuppression after radiotherapy.50 In line with this hypothesis, TAM depletion prior to radiotherapy led to improved tumor control in a melanoma model.51 Similarly, TAM depletion post radiotherapy significantly delayed tumor growth in murine cancer models.52–54 Another immunosuppressive mechanism may be mediated by the inhibition of natural killer cells.55 This effect might be associated with impairment of the immune-mediated effects of radiotherapy aswell as the efficacy of CAR T-cell treatment (Figure 3).
Figure 3. Potential Influence of Radiotherapy on Chimeric Antigen Receptor (CAR) T-Cell Therapy.
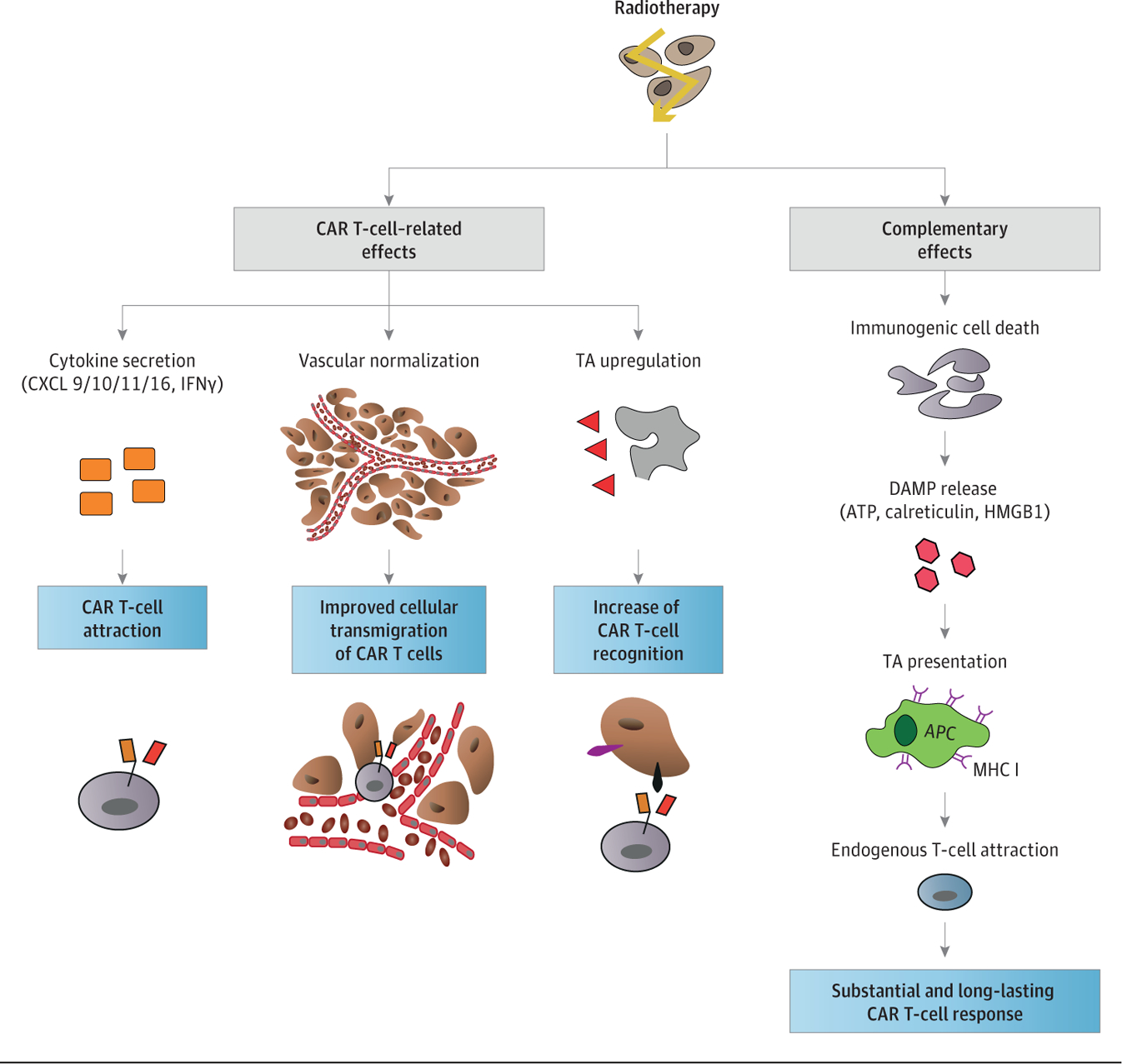
APC indicates antigen-presenting cell; ATP, adenosine triphosphate; CXCL, chemokine ligand; DAMP, damage-associated molecular pattern; HMGB1, high-mobility group box 1; IFN, interferon; MHC, major histocompatibility complex.
The optimal dose and fractionation of radiotherapy for enhancing immunotherapy remain unclear. Preclinical data have suggested that target antigen release after radiotherapy may depend on both fractionation and total dose—with a threshold of 7.5 Gy or higher in a single dose to promote a sufficient immune reaction.56,57 In this context, Lee et al58 compared 20 Gy as a single dose with fractionated radiotherapy using 4 fractions of 5 Gy and showed that the former led to increased T-cell proliferation and tumor regression. Interestingly, Dewan et al59 reported that a hypofractionated irradiation regimen of 3 fractions of 8 Gy was even more effective than a single-dose irradiation with 20 Gy or 5 fractions of 6 Gy in combination with anti–CTLA-4 antibody in a breast cancer model in mice, whereas no difference in tumor control was detected when radiotherapy was used alone. In addition, the regimen of 3 fractions of 8 Gy induced higher numbers of target antigen–specific T cells as well as abscopal effects. In a recent clinical study, fractionated radiotherapy (3 fractions of 9 Gy) improved progression-free survival compared with single-dose stereotactic radiotherapy.60 This effect was most notable in patients simultaneously treated with the anti–programmed cell death 1 monoclonal antibody nivolumab, suggesting an additive effect of combined treatment.
Hypofractionation of radiotherapy may also be associated with increased intratumoral infiltration by immune cells, such as CD8+ T cells; thus, it might potentially increase tumor infiltration by CAR T cells. For example, a 30-Gy single-dose radiotherapy regimen increased the number of tumor-infiltrating CD8+ T cells when compared with a fractionated treatment regimen (10 fractions of 3 Gy) in a preclinical colon cancer model.61
Moreover, Treg infiltration may be increased in a radiation dose–dependent manner.47,56These observations imply that Tregs are more radioresistant than other T-cell subpopulations. In line with this possibility, increased circulating Treg counts have been found following radiochemotherapy in patients with colorectal cancer.62 As Tregs are key mediators of immunosuppression in the tumor microenvironment, combining radiotherapy with CAR T-cell therapies will require strategies to overcome this potential obstacle.
Furthermore, radiation has been shown to upregulate the expression of Fas, a molecule involved in cell death, by sensitizing cancer cells to killing by cytotoxic T cells in several human cancer cell lines.63 However, future studies need to define the optimal dose and fractionation of radiotherapy that most efficiently induce Fas expression in cancer cells.
Taken together, current data point to a therapeutic window for optimal synergistic effects of radiotherapy and immunotherapy that includes CAR T-cell therapy. Identifying the boundaries of the therapeutic window and leveraging it will be crucial to achieve optimal therapeutic outcomes with the lowest risk of adverse effects with these combinatorial strategies in patients.
Optimal Dose/Fractionation of Radiotherapy for CAR T-Cell Therapy
The role of fractionation and radiotherapy dose in CAR T-cell function and receptor–antigen interaction is poorly characterized. In particular, the timing of radiotherapy with respect to CAR T-cell infusion will likely be crucial for the successful treatment of patients with cancer. One of the critical questions in this context is whether ablative radiotherapy doses are required or if a lower dose of radiotherapy could lead to superior efficacy. Indeed, sialyl Lewis-A–targeted CAR T cells were effective in a pancreatic adenocarcinoma mouse model with heterogeneous expression of the targeted antigen when combined with low-dose radiotherapy.64 Finally, another preclinical study showed dose-dependent target antigen release as well as T-lymphocyte priming and killing after radiotherapy using doses between 2 and 8 Gy.65 As the target antigen density on targeted cells is closely associated with CAR T-cell recognition and elimination, target antigen upregulation is likely to promote a stronger treatment response.
Another unanswered question in this context is which type of radiotherapy (ie, brachytherapy, proton therapy, or photon irradiation) results in the best outcome with the least risks of adverse effects when combined with CAR T-cell therapy. To date, most studies have focused on combining CAR T-cell therapy with photon irradiation; however, little is known about other radiotherapy types. Of note, combination of a Robo1 bichimeric antigen receptor–natural killer cell with iodine 125 seed brachytherapy significantly delayed tumor growth compared with brachytherapy alone in an orthotopic model of human pancreatic cancer in mice.66 Robo1 is an axon guidance receptor protein that is highly expressed on vessels during angiogenesis. However, there was no significant effect on overall survival of the tumor-bearing mice after this treatment.
Implementation and Feasibility of Radioimmunotherapies Involving CAR T Cells
The available data on this approach are currently limited (summarized in the Table).45,64,66–70 However, some important insights into the potential benefits and risks of using radiotherapy with CAR T cells have come from pilot studies as well as from studies investigating combinations of radiotherapy with other types of immunotherapy.
Table.
Overview of Current Clinical and Preclinical Studies Investigating Chimeric Antigen Receptor (CAR) T-Cell Therapy in Combination With Radiotherapy (RT)
| Reference | Title | Type |
Target | RT dose/fraction, Gy | Overall dose, Gy | |
|---|---|---|---|---|---|---|
| Cell | Tumor | |||||
| Preclinical studies | ||||||
| Weiss et al,67 2018 | NKG2D-Based CAR T Cells and Radiotherapy Exert Synergistic Efficacy in Glioblastoma | CAR T cells | Glioblastoma | NKG2D | 4 | 4 |
| DeSelm et al,64 2018 | Low-Dose Radiation Conditioning Enables CAR T Cells to Mitigate Antigen Escape | CAR T cells | Pancreatic cancer | sLeA | 2 | 2 |
| Xia et al,66 2019 | Robo1-Specific CAR-NK Immunotherapy Enhances Efficacy of 125I Seed Brachytherapy in an Orthotopic Mouse Model of Human Pancreatic Carcinoma | CAR natural killer cells | Pancreatic carcinoma | Robo1 | NA | Half-life, 59.6 d |
|
| ||||||
| Clinical studies | ||||||
| Sim et al,68 2019 | Radiation Therapy as a Bridging Strategy for CAR T Cell Therapy with Axicabtagene Ciloleucel in Diffuse Large B-Cell Lymphoma | CAR T cells | Diffuse large B-cell lymphoma | CD19 | 2–4 | Median (range) dose, 20 (6–36.5) Gy |
| Imber et al,69 2020 | Early Experience Using Salvage Radiotherapy for Relapsed/Refractory Non-Hodgkin Lymphomas After CD19 Chimeric Antigen Receptor (CAR) T Cell Therapy | CAR T cells | Non-Hodgkin lymphoma | CD19, EGFR | NA | NA |
| Smith et al,45 2019 | BCMA-Targeted CAR T-Cell Therapy Plus Radiotherapy for the Treatment of Refractory Myeloma Reveals Potential Synergy | CAR T cells | Multiple myeloma | BCMA | 4 | 20 |
| Qu et al,70 2020 | Radiation Priming Chimeric Antigen Receptor T-Cell Therapy in Relapsed/Refractory Diffuse Large B-Cell Lymphoma With High Tumor Burden | CAR T cells | Diffuse large B-cell lymphoma | CD19/CD20/CD22 | 2 | 40 |
Abbreviations: BCMA, B-cell maturation antigen; EGFR, epidermal growth factor receptor; NA, not applicable.
A case report showed no notable local adverse effects after palliative radiotherapy (5 fractions of 4 Gy) of a painful lesion with subsequent CD19-specific CAR T-cell therapy in a patient with refractory diffuse large B-cell lymphoma.64 Interestingly, although the patient’s tumor relapsed within 2 months after CAR T-cell therapy, the irradiated area remained disease free forat least 1 year after treatment. Consistent with this observation, palliative radiotherapy to the thoracic spine or whole-brain irradiation after B-cell maturation antigen–targeted CAR T-cell therapy has been reported to be well tolerated.45 Moreover, a study reported that radiotherapy can safely be used as a salvage therapy after CAR T-cell treatment in patients.69 In addition, a direct comparison between tumor priming with chemotherapy vs radiotherapy prior to CAR T-cell therapy in patients with relapsed or refractory diffuse large B-cell lymphoma showed, in addition to favorable response rates, fewer adverse effects after CAR T-cell therapy in the patients treated with radiotherapy.70 Despite these promising safety signals in patients, concerns remain regarding additional challenges when combining CAR T-cell therapy with standard radiochemotherapies for solid tumors. For example, chemotherapies such as paclitaxel and dacarbazine can suppress T-cell responses. Unfortunately, data on such combinations are currently limited. In a retrospective analysis, several cases of cytopenia were reported when bridging radiotherapy before CD19-targeting CAR T-cell therapy was combined with chemotherapy (fludarabine plus cyclophosphamide).68
Some insights have been provided from studies of combination of radiotherapy with other cell-based immunotherapies. A phase 3 clinical trial71 tested the combination of radiochemotherapy with an autologous cytokine-induced killer cell immunotherapy vs radiochemotherapy in patients with newly diagnosed glioblastoma. Study data showed a nonsignificant trend for higher incidence of total and grade 3 and higher adverse events in the immunotherapy group, although there was no difference detected in the quality of life between the 2 groups. In addition, feasibility and synergistic effects of radiochemotherapy (using cisplatin) and intratumoral dendritic cell vaccination have been reported in a murine preclinical model of squamous cell carcinoma.72 Another report showed that addition of autologous T-cell infusion to radiotherapy (3 fractions of 20 Gy) with chemotherapy (cyclophosphamide) was feasible and significantly prolonged survival in a metastatic breast cancer mouse model.73 These data highlight the importance of defining the immunostimulatory vs immunosuppressive effects of different chemotherapy regimens. Finally, radiotherapy has been previously tested in combination with intratumoral or systemic therapies to promote T-cell survival and priming. These studies showed synergy and favorable safety for the combinational treatment.74,75
Taken together, these results support the potential feasibility and efficacy of approaches combining CAR T-cell therapy with radiotherapy. Radiotherapy may induce functional normalization of tumor vessels and increased chemokine expression in tumors, which can result in increased CAR T-cell infiltration. Radiotherapy can also increase target antigen expression and level of cytokines associated with T-cell activation. In turn, effective CAR T-cell therapy could enhance tumor cell killing by radiotherapy. Through these mechanisms, radiotherapy has the potential to improve the limited efficacy of CAR T-cell therapy in solid tumors. In addition, radiotherapy may stimulate endogenous immune responses, potentially promoting more durable responses to CAR T-cell therapy. However, further randomized clinical trials are needed to evaluate these treatment interactions and demonstrate the feasibility and efficacy of this combinatorial approach.
Conclusions
Chimeric antigen receptor T cells are unique tools for immunotherapy. A key advantage over other immunotherapy approaches targeting endogenous effector T cells is the lack of requirement of antigen processing by antigen-processing machinery. In addition, CAR T cells have specificity to the target antigen and can be genetically manipulated. The high efficacy of CAR T cells in treating patients with hematologic cancers may hold great promise for the treatment of solid tumors. But the influence of CAR T-cell therapy on the treatment of solid tumors has been limited thus far. One approach to overcome the treatment resistance observed in solid tumors is to combine CAR T-cell therapy with other treatment modalities, such as radiotherapy. Radiotherapy is widely used and may have unique immunomodulatory effects locally and systemically, which could potentially be leveraged to enhance the efficacy of CAR T-cell therapy. This enhancement by radiotherapy may be through mechanisms involving direct effects on CAR T-cell infiltration and activation in tumors, as well as indirect effects on endogenous immune responses. Achieving this goal will require optimization of fractionation, dosing, and timing with respect to the optimal modality of radiotherapy to be combined with CAR T-cell treatment. It will also require a detailed characterization of nonoverlapping toxic effects. Finally, this approach would greatly benefit from biomarkers to safely identify patients most likely to benefit from these combinatorial strategies and to minimize adverse effects and the risk of overtreatment in patients.
Acknowledgments
Dr Hauth reported receiving nonfinancial research support (provision of activity trackers to institution for studies) from Beurer GmbH outside the submitted work and being supported by a Mildred-Scheel Postdoctoral Fellowship from Deutsche Krebshilfe, Germany; Dr Hauth’s Department of Radiation Oncology, University Hospital Tuebingen, has research agreements with Elekta, Philips, Siemens, Dr Sennewald Medizintechnik, Kaiku Health, TheraPanacea, PTW, ITV. Dr Ho reported receiving personal fees from Seattle Genetics and La Roche-Posay and research grants from Breast Cancer Research Foundation, the Translational Breast Cancer Research Consortium, Merck, and GlaxoSmithKline. Dr Ferrone reported receiving grants from the Department of Defense (W81XWH-16–1-0500 and W81XWH-20–1-0315) and the National Institutes of Health (R01DE028172) during the conduct of the study and serving as a member of the scientific advisory board of Kiromic Biopharma. Dr Duda reported receiving consultant fees from Bayer, Simcere, Surface Oncology, and Bristol Myers Squibb and research grants from Department of Defense (W81XWH-19–1-0284 and W81XWH-19–1-0482), Bayer, Exelixis, and Bristol Myers Squibb during the conduct of the study.
Contributor Information
Franziska Hauth, Department of Radiation Oncology, Massachusetts General Hospital, Harvard Medical School, Boston, Massachusetts, Department of Radiation Oncology, University Clinic Tuebingen, Tuebingen, Germany.
Alice Y. Ho, Department of Radiation Oncology, Massachusetts General Hospital, Harvard Medical School, Boston, Massachusetts.
Soldano Ferrone, Department of Surgery, Massachusetts General Hospital, Harvard Medical School, Boston, Massachusetts.
Dan G. Duda, Department of Radiation Oncology, Massachusetts General Hospital, Harvard Medical School, Boston, Massachusetts.
REFERENCES
- 1.Morgan MA, Schambach A. Engineering CAR-T cells for improved function against solid tumors. Front Immunol. 2018;9:2493. doi: 10.3389/fimmu.2018.02493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chruściel E, Urban-Wójciuk Z, Arcimowicz Ł, et al. Adoptive cell therapy—harnessing antigen-specific T cells to target solid tumours. Cancers (Basel). 2020;12(3):E683. doi: 10.3390/cancers12030683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gong MC, Latouche JB, Krause A, Heston WD, Bander NH, Sadelain M. Cancer patient T cells genetically targeted to prostate-specific membrane antigen specifically lyse prostate cancer cells and release cytokines in response to prostate-specific membrane antigen. Neoplasia. 1999;1(2):123–127. doi: 10.1038/sj.neo.7900018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maher J, Brentjens RJ, Gunset G, Rivière I, Sadelain M. Human T-lymphocyte cytotoxicity and proliferation directed by a single chimeric TCRzeta/CD28 receptor. Nat Biotechnol. 2002;20(1):70–75. doi: 10.1038/nbt0102-70 [DOI] [PubMed] [Google Scholar]
- 5.Eshhar Z, Waks T, Gross G, Schindler DG. Specific activation and targeting of cytotoxic lymphocytes through chimeric single chains consisting of antibody-binding domains and the gamma or zeta subunits of the immunoglobulin and T-cell receptors. Proc Natl Acad SciUS A. 1993;90(2): 720–724. doi: 10.1073/pnas.90.2.720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kowolik CM, Topp MS, Gonzalez S, et al. CD28 costimulation provided through a CD19-specific chimeric antigen receptor enhances in vivo persistence and antitumor efficacy of adoptively transferred T cells. Cancer Res. 2006;66(22): 10995–11004. doi: 10.1158/0008-5472.CAN-06-0160 [DOI] [PubMed] [Google Scholar]
- 7.Milone MC, Fish JD, Carpenito C, et al. Chimeric receptors containing CD137 signal transduction domains mediate enhanced survival of T cells and increased antileukemic efficacy in vivo. Mol Ther. 2009;17(8):1453–1464. doi: 10.1038/mt.2009.83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhong XS, Matsushita M, Plotkin J, Riviere I, Sadelain M. Chimeric antigen receptors combining 4–1BB and CD28 signaling domains augment PI3kinase/AKT/Bcl-XL activation and CD8+ T cell-mediated tumor eradication. Mol Ther. 2010;18 (2):413–420. doi: 10.1038/mt.2009.210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carpenito C, Milone MC, Hassan R, et al. Control of large, established tumor xenografts with genetically retargeted human T cells containing CD28 and CD137 domains. Proc Natl Acad SciUS A. 2009;106(9):3360–3365. doi: 10.1073/pnas.0813101106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chmielewski M, Abken H. TRUCKs: the fourth generation of CARs. Expert Opin Biol Ther. 2015;15 (8):1145–1154. doi: 10.1517/14712598.2015.1046430 [DOI] [PubMed] [Google Scholar]
- 11.Zabel M, Tauber PA, Pickl WF. The making and function of CAR cells. Immunol Lett. 2019;212:53–69. doi: 10.1016/j.imlet.2019.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shahrara S, Pickens SR, Dorfleutner A, Pope RM. IL-17 induces monocyte migration in rheumatoid arthritis. J Immunol. 2009;182(6): 3884–3891. doi: 10.4049/jimmunol.0802246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Raphael I, Nalawade S, Eagar TN, Forsthuber TG. T cell subsets and their signature cytokines in autoimmune and inflammatory diseases. Cytokine. 2015;74(1):5–17. doi: 10.1016/j.cyto.2014.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ahmed N, Brawley VS, Hegde M, et al. Human epidermal growth factor receptor 2 (HER2)-specific chimeric antigen receptor-modified T cells for the immunotherapy of HER2-positive sarcoma. J Clin Oncol. 2015;33(15):1688–1696. doi: 10.1200/JCO.2014.58.0225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harlin H, Meng Y, Peterson AC, et al. Chemokine expression in melanoma metastases associated with CD8+ T-cell recruitment. Cancer Res. 2009;69(7): 3077–3085. doi: 10.1158/0008-5472.CAN-08-2281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dirkx AE, oude Egbrink MG, Castermans K, et al. Anti-angiogenesis therapy can overcome endothelial cell anergy and promote leukocyte-endothelium interactions and infiltration in tumors. FASEB J. 2006; 20(6):621–630. doi: 10.1096/fj.05-4493com [DOI] [PubMed] [Google Scholar]
- 17.Chinnasamy D, Yu Z, Theoret MR, et al. Gene therapy using genetically modified lymphocytes targeting VEGFR-2 inhibits the growth of vascularized syngenic tumors in mice. J Clin Invest. 2010;120(11):3953–3968. doi: 10.1172/JCI43490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shrimali RK, Yu Z, Theoret MR, Chinnasamy D, Restifo NP, Rosenberg SA. Antiangiogenic agents can increase lymphocyte infiltration into tumor and enhance the effectiveness of adoptive immunotherapy of cancer. Cancer Res. 2010;70(15): 6171–6180. doi: 10.1158/0008-5472.CAN-10-0153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Newick K, O’Brien S, Sun J, et al. Augmentation of CAR T-cell trafficking and antitumor efficacy by blocking protein kinase A localization. Cancer Immunol Res. 2016;4(6):541–551. doi: 10.1158/2326-6066.CIR-15-0263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bocca P, Di Carlo E, Caruana I, et al. Bevacizumab-mediated tumor vasculature remodelling improves tumor infiltration and antitumor efficacy of GD2-CAR T cells in a human neuroblastoma preclinical model. Oncoimmunology. 2017;7(1):e1378843. doi: 10.1080/2162402X.2017.1378843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fleming V, Hu X, Weber R, et al. Targeting myeloid-derived suppressor cells to bypass tumor-induced immunosuppression. Front Immunol. 2018;9:398. doi: 10.3389/fimmu.2018.00398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Noguchi T, Ward JP, Gubin MM, et al. Temporally distinct PD-L1 expression by tumor and host cells contributes to immune escape. Cancer Immunol Res. 2017;5(2):106–117. doi: 10.1158/2326-6066.CIR-16-0391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Giavridis T, van der Stegen SJC, Eyquem J, Hamieh M, Piersigilli A, Sadelain M. CAR T cell-induced cytokine release syndrome is mediated by macrophages and abated by IL-1 blockade. Nat Med. 2018;24(6):731–738. doi: 10.1038/s41591-018-0041-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morgan RA, Yang JC, Kitano M, Dudley ME, Laurencot CM, Rosenberg SA. Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol Ther. 2010;18(4): 843–851. doi: 10.1038/mt.2010.24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Newick K, O’Brien S, Moon E, Albelda SM. CAR T cell therapy for solid tumors. Annu Rev Med. 2017;68: 139–152. doi: 10.1146/annurev-med-062315-120245 [DOI] [PubMed] [Google Scholar]
- 26.O’Rourke DM, Nasrallah MP, Desai A, et al. A single dose of peripherally infused EGFRvIII-directed CAR T cells mediates antigen loss and induces adaptive resistance in patients with recurrent glioblastoma. Sci Transl Med. 2017;9 (399):eaaa0984. doi: 10.1126/scitranslmed.aaa0984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ahmed N, Brawley V, Hegde M, et al. HER2-specific chimeric antigen receptor-modified virus-specific T cells for progressive glioblastoma: a phase 1 dose-escalation trial. JAMA Oncol. 2017;3 (8):1094–1101. doi: 10.1001/jamaoncol.2017.0184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jain RK. Antiangiogenesis strategies revisited: from starving tumors to alleviating hypoxia. Cancer Cell. 2014;26(5):605–622. doi: 10.1016/j.ccell.2014.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Berahovich R, Liu X, Zhou H, et al. Hypoxia selectively impairs CAR-T cells in vitro. Cancers (Basel). 2019;11(5):E602. doi: 10.3390/cancers11050602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fukumura D, Kloepper J, Amoozgar Z, Duda DG, Jain RK. Enhancing cancer immunotherapy using antiangiogenics: opportunities and challenges. Nat Rev Clin Oncol. 2018;15(5):325–340. doi: 10.1038/nrclinonc.2018.29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schaue D, Kachikwu EL, McBride WH. Cytokines in radiobiological responses: a review. Radiat Res. 2012;178(6):505–523. doi: 10.1667/RR3031.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ganss R, Ryschich E, Klar E, Arnold B, Hämmerling GJ. Combination of T-cell therapy and trigger of inflammation induces remodeling of the vasculature and tumor eradication. Cancer Res. 2002;62(5):1462–1470. [PubMed] [Google Scholar]
- 33.Klug F, Prakash H, Huber PE, et al. Low-dose irradiation programs macrophage differentiation to an iNOS+/M1 phenotype that orchestrates effective T cell immunotherapy. Cancer Cell. 2013;24(5):589–602. doi: 10.1016/j.ccr.2013.09.014 [DOI] [PubMed] [Google Scholar]
- 34.Watanabe K, Kuramitsu S, Posey AD Jr, June CH. Expanding the therapeutic window for CAR T cell therapy in solid tumors: the knowns and unknowns of CAR T cell biology. Front Immunol. 2018;9:2486. doi: 10.3389/fimmu.2018.02486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Apetoh L, Ghiringhelli F, Tesniere A, et al. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med. 2007;13(9):1050–1059. doi: 10.1038/nm1622 [DOI] [PubMed] [Google Scholar]
- 36.Rapoport BL, Anderson R. Realizing the clinical potential of immunogenic cell death in cancer chemotherapy and radiotherapy. Int J Mol Sci. 2019; 20(4):E959. doi: 10.3390/ijms20040959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Demaria S, Formenti SC. Role of T lymphocytes in tumor response to radiotherapy. Front Oncol. 2012;2:95. doi: 10.3389/fonc.2012.00095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Teitz-Tennenbaum S, Li Q, Rynkiewicz S, et al. Radiotherapy potentiates the therapeutic efficacy of intratumoral dendritic cell administration. Cancer Res. 2003;63(23):8466–8475. [PubMed] [Google Scholar]
- 39.Sharabi AB, Nirschl CJ, Kochel CM, et al. Stereotactic radiation therapy augments antigen-specific PD-1-mediated antitumor immune responses via cross-presentation of tumor antigen. Cancer Immunol Res. 2015;3(4):345–355. doi: 10.1158/2326-6066.CIR-14-0196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Okuma K, Yamashita H, Niibe Y, Hayakawa K, Nakagawa K. Abscopal effect of radiation on lung metastases of hepatocellular carcinoma: a case report. J Med Case Rep. 2011;5:111. doi: 10.1186/1752-1947-5-111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Grimaldi AM, Simeone E, Giannarelli D, et al. Abscopal effects of radiotherapy on advanced melanoma patients who progressed after ipilimumab immunotherapy. Oncoimmunology. 2014;3:e28780. doi: 10.4161/onci.28780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shohan J. Some theoretical considerations on the present status of roentgen therapy. Boston Medical and Surgical Journal. 1916;175:321–327. doi: 10.1056/NEJM191609071751001 [DOI] [Google Scholar]
- 43.Chakravarty PK, Alfieri A, Thomas EK, et al. Flt3-ligand administration after radiation therapy prolongs survival in a murine model of metastatic lung cancer. Cancer Res. 1999;59(24):6028–6032. [PubMed] [Google Scholar]
- 44.Sato H, Suzuki Y, Yoshimoto Y, et al. An abscopal effect in a case of concomitant treatment of locally and peritoneally recurrent gastric cancer using adoptive T-cell immunotherapy and radiotherapy. Clin Case Rep. 2017;5(4):380–384. doi: 10.1002/ccr3.758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Smith EL, Mailankody S, Staehr M, et al. BCMA-targeted CAR T-cell therapy plus radiotherapy for the treatment of refractory myeloma reveals potential synergy. Cancer Immunol Res. 2019;7(7):1047–1053. doi: 10.1158/2326-6066.CIR-18-0551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McBride S, Sherman E, Tsai CJ, et al. Randomized phase II trial of nivolumab with stereotactic body radiotherapy versus nivolumab alone in metastatic head and neck squamous cell carcinoma. J Clin Oncol. 2021;39(1):30–37. doi: 10.1200/JCO.20.00290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kachikwu EL, Iwamoto KS, Liao YP, et al. Radiation enhances regulatory T cell representation. Int J Radiat Oncol Biol Phys. 2011;81 (4):1128–1135. doi: 10.1016/j.ijrobp.2010.09.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Qinfeng S, Depu W, Xiaofeng Y, Shah W, Hongwei C, Yili W. In situ observation of the effects of local irradiation on cytotoxic and regulatory T lymphocytes in cervical cancer tissue. Radiat Res. 2013;179(5):584–589. doi: 10.1667/RR3155.1 [DOI] [PubMed] [Google Scholar]
- 49.Ruffell B, Chang-Strachan D, Chan V, et al. Macrophage IL-10 blocks CD8+ T cell-dependent responses to chemotherapy by suppressing IL-12 expression in intratumoral dendritic cells. Cancer Cell. 2014;26(5):623–637. doi: 10.1016/j.ccell.2014.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Medrek C, Pontén F, Jirström K, Leandersson K. The presence of tumor associated macrophages in tumor stroma as a prognostic marker for breast cancer patients. BMC Cancer. 2012;12:306. doi: 10.1186/1471-2407-12-306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Meng Y, Beckett MA, Liang H, et al. Blockade of tumor necrosis factor alpha signaling in tumor-associated macrophages as a radiosensitizing strategy. Cancer Res. 2010;70(4): 1534–1543. doi: 10.1158/0008-5472.CAN-09-2995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ahn GO, Tseng D, Liao CH, Dorie MJ, Czechowicz A, Brown JM. Inhibition of Mac-1 (CD11b/CD18) enhances tumor response to radiation by reducing myeloid cell recruitment. Proc Natl Acad SciUS A. 2010;107(18):8363–8368. doi: 10.1073/pnas.0911378107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shiao SL, Ruffell B, DeNardo DG, Faddegon BA, Park CC, Coussens LM. TH2-polarized CD4(+) T cells and macrophages limit efficacy of radiotherapy. Cancer Immunol Res. 2015;3(5):518–525. doi: 10.1158/2326-6066.CIR-14-0232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kozin SV, Kamoun WS, Huang Y, Dawson MR, Jain RK, Duda DG. Recruitment of myeloid but not endothelial precursor cells facilitates tumor regrowth after local irradiation. Cancer Res. 2010; 70(14):5679–5685. doi: 10.1158/0008-5472.CAN-09-4446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Melo AM, Maher SG, O’Leary SM, Doherty DG, Lysaght J. Selective effects of radiotherapy on viability and function of invariant natural killer T cells in vitro. Radiother Oncol. 2020;145:128–136. doi: 10.1016/j.radonc.2019.12.016 [DOI] [PubMed] [Google Scholar]
- 56.Schaue D, Ratikan JA, Iwamoto KS, McBride WH. Maximizing tumor immunity with fractionated radiation. Int J Radiat Oncol Biol Phys. 2012;83(4): 1306–1310. doi: 10.1016/j.ijrobp.2011.09.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Verbrugge I, Hagekyriakou J, Sharp LL, et al. Radiotherapy increases the permissiveness of established mammary tumors to rejection by immunomodulatory antibodies. Cancer Res. 2012; 72(13):3163–3174. doi: 10.1158/0008-5472.CAN-12-0210 [DOI] [PubMed] [Google Scholar]
- 58.Lee Y, Auh SL, Wang Y, et al. Therapeutic effects of ablative radiation on local tumor require CD8+ T cells: changing strategies for cancer treatment. Blood. 2009;114(3):589–595. doi: 10.1182/blood-2009-02-206870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dewan MZ, Galloway AE, Kawashima N, et al. Fractionated but not single-dose radiotherapy induces an immune-mediated abscopal effect when combined with anti-CTLA-4 antibody. Clin Cancer Res. 2009;15(17):5379–5388. doi: 10.1158/1078-0432.CCR-09-0265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Minniti G, Anzellini D, Reverberi C, et al. Stereotactic radiosurgery combined with nivolumab or ipilimumab for patients with melanoma brain metastases: evaluation of brain control and toxicity. J Immunother Cancer. 2019;7 (1):102. doi: 10.1186/s40425-019-0588-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Filatenkov A, Baker J, Mueller AM, et al. Ablative tumor radiation can change the tumor immune cell microenvironment to induce durable complete remissions. Clin Cancer Res. 2015;21(16): 3727–3739. doi: 10.1158/1078-0432.CCR-14-2824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schaue D, Comin-Anduix B, Ribas A, et al. T-cell responses to survivin in cancer patients undergoing radiation therapy. Clin Cancer Res. 2008;14(15): 4883–4890. doi: 10.1158/1078-0432.CCR-07-4462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chakraborty M, Abrams SI, Camphausen K, et al. Irradiation of tumor cells up-regulates Fas and enhances CTL lytic activity and CTL adoptive immunotherapy. J Immunol. 2003;170(12):6338–6347. doi: 10.4049/jimmunol.170.12.6338 [DOI] [PubMed] [Google Scholar]
- 64.DeSelm C, Palomba ML, Yahalom J, et al. Low-dose radiation conditioning enables CAR T cells to mitigate antigen escape. Mol Ther. 2018;26 (11):2542–2552. doi: 10.1016/j.ymthe.2018.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Morisada M, Moore EC, Hodge R, et al. Dose-dependent enhancement of T-lymphocyte priming and CTL lysis following ionizing radiation in an engineered model of oral cancer. Oral Oncol. 2017; 71:87–94. doi: 10.1016/j.oraloncology.2017.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Xia N, Haopeng P, Gong JU, et al. Robo1-specific CAR-NK immunotherapy enhances efficacy of 125I seed brachytherapy in an orthotopic mouse model of human pancreatic carcinoma. Anticancer Res. 2019;39(11):5919–5925. doi: 10.21873/anticanres.13796 [DOI] [PubMed] [Google Scholar]
- 67.Weiss T, Weller M, Guckenberger M, Sentman CL, Roth P. NKG2D-based CAR T cells and radiotherapy exert synergistic efficacy in glioblastoma. Cancer Res. 2018;78(4):1031–1043. doi: 10.1158/0008-5472.CAN-17-1788 [DOI] [PubMed] [Google Scholar]
- 68.Sim AJ, Jain MD, Figura NB, et al. Radiation therapy as a bridging strategy for CAR T cell therapy with axicabtagene ciloleucel in diffuse large B-cell lymphoma. Int J Radiat Oncol Biol Phys. 2019;105 (5):1012–1021. doi: 10.1016/j.ijrobp.2019.05.065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Imber BS, Sadelain M, DeSelm C, et al. Early experience using salvage radiotherapy for relapsed/refractory non-Hodgkin lymphomas after CD19 chimeric antigen receptor (CAR) T cell therapy. Br J Haematol. 2020;190(1):45–51. doi: 10.1111/bjh.16541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Qu C, Ping N, Kang L, et al. Radiation priming chimeric antigen receptor T-cell therapy in relapsed/refractory diffuse large B-cell lymphoma with high tumor burden. J Immunother. 2020;43(1): 32–37. doi: 10.1097/CJI.0000000000000284 [DOI] [PubMed] [Google Scholar]
- 71.Kong DS, Nam DH, Kang SH, et al. Phase III randomized trial of autologous cytokine-induced killer cell immunotherapy for newly diagnosed glioblastoma in Korea. Oncotarget. 2017;8(4):7003–7013. doi: 10.18632/oncotarget.12273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Moyer JS, Li J, Wei S, Teitz-Tennenbaum S, Chang AE. Intratumoral dendritic cells and chemoradiation for the treatment of murine squamous cell carcinoma. J Immunother. 2008;31 (9):885–895. doi: 10.1097/CJI.0b013e3181880f1e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Filatenkov A, Baker J, Müller AM, et al. Treatment of 4T1 metastatic breast cancer with combined hypofractionated irradiation and autologous T-cell infusion. Radiat Res. 2014;182(2): 163–169. doi: 10.1667/RR13471.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Multhoff G, Seier S, Stangl S, et al. Targeted natural killer cell-based adoptive immunotherapy for the treatment of patients with NSCLC after radiochemotherapy: a randomized phase II clinical trial. Clin Cancer Res. 2020;26(20):5368–5379. doi: 10.1158/1078-0432.CCR-20-1141 [DOI] [PubMed] [Google Scholar]
- 75.Ho AY, Barker CA, Arnold BB, et al. A phase 2 clinical trial assessing the efficacy and safety of pembrolizumab and radiotherapy in patients with metastatic triple-negative breast cancer. Cancer. 2020;126(4):850–860. doi: 10.1002/cncr.32599 [DOI] [PubMed] [Google Scholar]


