Abstract
Context
The effect of the anti-inflammatory and immunomodulatory actions of vitamin D on the duration of partial clinical remission (PR) in youth with type 1 diabetes (T1D) is unclear.
Objective
This work aimed to determine the effect of adjunctive ergocalciferol on residual β-cell function (RBCF) and PR in youth with newly diagnosed T1D who were maintained on a standardized insulin treatment protocol. The hypothesis was that ergocalciferol supplementation increases RBCF and prolongs PR.
Methods
A 12-month, randomized, double-blind, placebo-controlled trial was conducted of 50 000 IU of ergocalciferol per week for 2 months, and then once every 2 weeks for 10 months, vs placebo in 36 individuals aged 10 to 21 years, with T1D of less than 3 months and a stimulated C-peptide (SCP) level greater than or equal to 0.2 nmol/L (≥ 0.6 ng/mL). The ergocalciferol group had 18 randomly assigned participants (10 male/8 female), mean age 13.3 ± 2.8 years, while the control group had 18 participants (14 male/4 female), aged 14.3 ± 2.9 years.
Results
The ergocalciferol treatment group had statistically significantly higher serum 25-hydroxyvitamin D at 6 months (P = .01) and 9 months (P = .02) than the placebo group. At 12 months, the ergocalciferol group had a statistically significantly lower serum tumor necrosis factor α (TNF-α) concentration (P = .03). There were no statistically significant differences between the groups at each time point from baseline to 12 months for SCP concentration (P = .08), glycated hemoglobin A1c (HbA1c) (P = .09), insulin dose–adjusted A1c (IDAA1c), or total daily dose of insulin. Temporal trends for rising HbA1c (P = .04) and IDAA1c (P = .02) were statistically significantly blunted in the ergocalciferol group.
Conclusion
Ergocalciferol statistically significantly reduced serum TNF-α concentration and the rates of increase both in A1c and IDAA1c, suggesting a protection of RBCF and PR in youth with newly diagnosed T1D.
Keywords: type 1 diabetes, ergocalciferol, partial clinical remission, pediatrics, C-peptide
Type 1 diabetes (T1D) is a syndrome of persistent hyperglycemia resulting from autoimmune destruction of pancreatic β cells causing insulinopenia [1]. Fifty-percent of β-cell function may remain at T1D diagnosis, and this RBCF may persist for months or years [2-4]. Longer duration of the partial clinical remission (PR), or “honeymoon” phase, of T1D improves glycemic control and reduces long-term complications [5, 6]. Efforts to block immune-mediated destruction of β cells with immunomodulatory and immunosuppressive agents have yielded promising trends but insufficient protection [7-10]. Vitamin D is safe and has immunomodulatory functions that could protect RBCF [11]. Studies suggest the possibility that vitamin D supplementation may lengthen PR and increase RBCF [6, 11]. The rationale for this randomized controlled trial (RCT) was to establish the effect of an adequate dose of ergocalciferol on PR and RBCF.
We enrolled 48 individuals aged 10 to 21 years with newly diagnosed T1D in a 12-month RCT of ergocalciferol vs placebo to determine the effect of vitamin D on RBCF and PR in youth with newly diagnosed T1D. The hypothesis was that ergocalciferol would increase RBCF and prolong PR. The primary aim was to determine the effect of adjunctive ergocalciferol on RBCF and PR in youth with T1D. The primary outcome was the longitudinal change in peak stimulated C-peptide concentrations (SCP; a measure of RBCF).
Materials and Methods
The study protocol [12] was approved by the University of Massachusetts Medical School (UMMS) Institutional Review Board on May 27, 2016. The Federal Award Date was July 21, 2017. Study registration at Clinical Trials.gov was completed February 8, 2017, with a clinical trial identification number of NCT03046927. The Food and Drug Administration Regulatory Document Registration and Investigational New Drug approval were finalized June 20, 2017. The first study participant was enrolled October 19, 2017. The last participant completed the study April 12, 2021, and the study was closed April 20, 2021. Food and Drug Administration review from January 3 to 8, 2020, found the RCT to be in full compliance with federal regulations.
Study Design and Setting
This was an investigator-initiated, single-center, randomized, double-blind, parallel trial of ergocalciferol vs placebo treatments in youth with newly diagnosed T1D at a university teaching hospital.
Participants
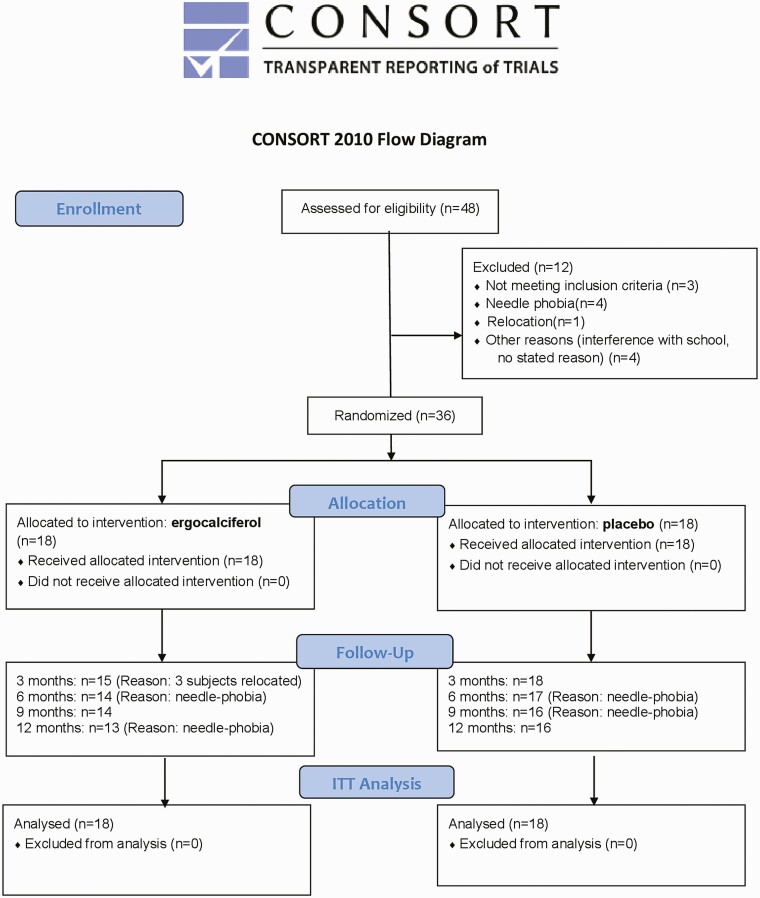
Written informed consent was obtained from each participant’s parent(s) and assent was obtained from minors. Individuals aged 18 years and older signed the consent form. Inclusion criteria were male and female individuals aged 10 to 21 years with new-onset T1D of less than 3 months’ duration. All participants had a fasting C-peptide level of greater than 0.1 nmol/L (0.3 ng/mL) or SCP level greater than or equal to 0.2 nmol/L (≥ 0.6 ng/mL). The diagnosis of T1D was established by the presence of autoantibodies against islet antigens [13]. Individuals were excluded if they had eating disorders; active neoplasms; 25-hydroxyvitamin D [25(OH)D] level greater than 70 ng/mL; had received any investigational drug in the prior 6 months; were on medications other than insulin that could affect glycemia; pregnancy, breastfeeding, treatment with weight-altering therapies, systemic illnesses, recurrent hypoglycemia, or a history of 2 or more episodes of diabetic ketoacidosis in the preceding 3 months (Fig. 1). At enrollment, all participants were receiving once-daily subcutaneous basal insulin injection, and premeal bolus insulin injections using insulin analogues. All participants employed a self-directed treat-to-target insulin regimen (TTIR) (Table 1). Fig. 1 summarizes the study flow from screening to study conclusion.
Figure 1.
CONSORT (Consolidated Standards of Reporting Trials) flow diagram.
Table 1.
Titration algorithm for long-acting insulin
| Titration algorithm for long-acting insulin | |
|---|---|
| Average value of fasting plasma glucose for 3 consecutive d | Recommended long-acting insulin dose adjustments |
| < 5.0 mmol/L (90 mg/dL) | Subtract 1 unit from total dose of detemir |
| 5.0-6.7 mmol/L (90-120 mg/dL) | No adjustments |
| > 6.7 mmol/L (120 mg/dL) | Add 1 unit to total dose of detemir |
Methods
Participants were evaluated between 8 am to 10:30 am following an overnight fast.
Anthropometry
Anthropometric data were collected at each study visit. Height was measured to the nearest 0.1 cm using a wall-mounted stadiometer (Holtain Ltd). Weight was measured to the nearest 0.1 kg using an upright scale. Body mass index (BMI) was calculated using the formula weight/height2 (kg/m2) and expressed as z scores. Waist circumference was measured to the nearest 0.1 cm at the superior border of the iliac crests.
Biochemical studies
Mixed-meal tolerance test for stimulated C-peptide.
A mixed-meal tolerance test was performed between 8:30 am and 10:30 am following an overnight fast, with no injection of bolus insulin in the preceding 6 hours. Boost (formerly Sustacal, Mead Johnson), at a dose of 6 mL/kg (maximum 360 mL), was ingested in less than 10 minutes. Blood draws were obtained for baseline glucose and C-peptide, and at 30 minutes and 90 minutes for post–mixed-meal C-peptide and glucose estimations [14]. Glucose and C-peptide were analyzed by the UMass Memorial Medical Center Biochemistry laboratory. SCP concentrations were analyzed by enzyme-linked immunosorbent assay using Quest Immunoassays on an Atellica IM Analyzer (Siemens) with less than 12% intra-assay and interassay coefficients of variability.
Glycated hemoglobin A 1c .
Blood samples for glycated hemoglobin A1c (HbA1c) were obtained at each visit and were analyzed by the UMass Memorial Medical Center Biochemistry laboratory. HbA1c was measured by high-pressure liquid chromatography, which has an interassay variability of less than 1.5%, an intra-assay variability of less than 2.5%, and a normal range of 4.4% to 6.0% [15, 16].
Cytokine assay methodology.
Plasma proinflammatory and anti-inflammatory cytokine levels (interferon-γ, interleukin [IL]-1β, IL-2, IL-4, IL-6, IL-8, IL-10, IL-12p70, IL-13, and tumor necrosis factor α [TNF-α]) were quantified using the Meso Scale Discovery V-PLEX plus kit (Meso Scale Diagnostics, catalog No. K15049G). The assay was performed according to the manufacturer’s protocol. The intra-assay and interassay coefficients of variability were less than 10%.
Study supplies
Ergocalciferol and placebo were prepared as identical capsules by Boulevard Pharmaceutical Compounding Center [17] and shipped directly to the Investigational Drug Services (IDS) of the UMMS, which maintained accountability logs for receipt and dispensing of study drugs.
Procedure
Following enrollment, all participants entered a run-in phase of 1 to 2 months’ duration and started on TTIR.
Insulin adjustment.
At enrollment, all participants were on multiple daily injections. Long-acting insulin was self-adjusted by participants using TTIR based on a titration algorithm of (–1) to 0 to (+1) units to adjust basal insulin dose every third day to maintain fasting plasma glucose between 90 and 120 mg/dL (range, 5.0-6.7 mmol/L) (see Table 1). Insulin-to-carbohydrate ratio, correction factor, ideal blood glucose, and insulin sensitivity (IS) factor were adjusted as needed for normoglycemia (Table 2).
Table 2.
Summary of daily capillary glucose goals
| Time | Before breakfast | Before lunch or dinner | Before bedtime | 2 h after a meal | At 3 am |
|---|---|---|---|---|---|
| Glucose level, mmol/L | 5.0-6.7 | 4.44-7.22 | > 5.56 | < 12.22 | > 5.56 |
| Glucose level, mg/dL | 90-120 | 80-130 | > 100 | < 220 | > 100 |
Glucose data collection
Fingerstick glucose monitoring: Fasting and nonfasting capillary glucose data were uploaded to the UMass MyCareTeam [18] website every 4 weeks.
Continuous glucose monitoring (CGM): Participants shared their glucose data with the study team via cloud-based data repositories.
Hypoglycemia was classified as nocturnal (plasma glucose ≤ 60 mg/dL [3.3 mmol/L] 11 pm-6 am); symptoms only (plasma glucose > 60 mg/dL [3.3 mmol/L] or no measurement); minor (plasma glucose < 60 mg/dL [3.3 mmol/L]; and major (hypoglycemia requiring third-party assistance) [19]. To prevent nocturnal hypoglycemia, participants targeted a prebedtime and nocturnal glucose level greater than 100 mg/dL (5.6 mmol/L) (see Table 2).
Nutrition and exercise.
A registered dietician instructed all participants on medical nutrition therapy at study entry and at 6 months. No specific exercise regimen was prescribed.
Randomization protocol.
Participants were randomly assigned to either ergocalciferol or placebo at the conclusion of the run-in phase. The random treatment assignments were generated using a permuted block design with random block sizes of 2, 4, or 6 by the Quantitative Methods Core and administered by the IDS, both at UMMS.
Ergocalciferol Treatment
The study group received ergocalciferol 50 000 international units (IU) orally once weekly for 2 months, and then once every other week for 10 months to maintain serum 25(OH)D concentration between 20 and 100 ng/dL. This dose was determined to be below the standard tolerable upper intake level for ergocalciferol for people older than 9 years [20] and thus unlikely to lead to ergocalciferol toxicity. This dosing regimen was chosen to ensure an early increase in the serum 25(OH)D concentration.
Forty-eight participants were enrolled, and 12 individuals were excluded before randomization (see Fig. 1). Thirty-six participants were randomly assigned to either ergocalciferol (n = 18) or placebo (n = 18) after stratification by BMI into normal-weight (BMI < 85th percentile) and overweight/obese individuals (BMI ≥ 85th percentile). Nine individuals from the normal-weight group received ergocalciferol, and 9 individuals from the overweight/obese group received ergocalciferol. Participants received similar-appearing pills and pill-counting dosettes to monitor compliance.
Randomization-Allocation Concealment
Double-blinded treatments were allocated using sequentially numbered drug containers. Concealed treatment allocation was made by the IDS, which also secured blinding codes during the trial. IDS maintained a sealed copy of the randomization sequence at the investigation site in case of the need for emergency unblinding.
Randomization-Implementation
The IDS established the randomization sequence. Trial endocrinologists enrolled patients to the study. A pharmacist, unconnected with the study, assigned participants to the groups.
Blinding
Participants and investigators were blinded to the identity of the study products. IDS maintained blinding information throughout the study duration. At study conclusion, the randomization code was decrypted in a 2-step procedure: first step, treatment A or B; second step, A equal to ergocalciferol and B equal to placebo. All statistical analyses were performed after the second step of unblinding.
Objectives
The primary objective was to determine the effect of adjunctive ergocalciferol on RBCF and PR in youth with newly diagnosed T1D.
The secondary objectives were to determine the longitudinal changes in insulin dose–adjusted A1c (IDAA1c), HbA1c, fasting blood glucose (FBS), total daily dose of insulin (TDDI), and inflammatory cytokines between the groups.
Outcomes
Primary outcome
The primary outcome was the comparison of changes in baseline and SCP levels between the ergocalciferol and placebo groups over 12 months.
Secondary outcomes
The second outcome was the comparison of the changes in glycemia, IDAA1c, and inflammatory markers.
Safety parameters.
Safety parameters included monitoring of 25(OH)D, calcium, phosphorus, urinary calcium-creatinine ratio in all participants, and pregnancy tests in female participants.
Protocol Deviations
COVID-19 pandemic lockdown
Participant study visits were suspended from March 16 through June 19, 2020, because of a temporary closure of the Clinical Research Center for precautionary measures. Following the lifting of the lockdown, 2 individuals left the study because of fear of contracting the virus at the Clinical Research Center. One of these 2 participants was later determined to be in the placebo group, and the other was in the ergocalciferol group.
Compliance Monitoring Measures
Compliance was monitored by frequent review of MyCareTeam software downloads, along with CGM downloads, counting of pills in the dosettes during clinic visits, review of study drug administration diaries, and the review of participants’ home documentation of glucose data. Analysis of compliance parameters and pharmacy records showed no difference in compliance between the 2 groups. Data on CGM metrics are not shown because fewer than 8 participants used the CGM in each group.
Statistical Analyses
Sample size and power calculation
The planned sample size was based on establishing a stable estimate (with a 2-sided 95% CI) for the difference in C-peptide between the 2 treatment groups. Based on the assumption of a meaningful difference of 20% [21] from a baseline C-peptide level of 0.80 ng/mL [6] with an SD at 12 months of follow-up of 0.38 ng/mL [6], a sample size of 24 per group would produce a distance from the mean difference to the limit of the 2-sided 95% CI of ± 0.22 ng/mL. This sample size was also sufficient to produce a Cohen effect size (f2)of almost 0.30, a moderate to large effect size, for the least-squares adjusted treatment differences, which is expected for this size study. In contrast, we were able to randomly assigned 36 participants, rather than the planned 48. This affected the size of the 95% CI, increasing it from ± 0.22 ng/mL for the planned sample size to ± 0.26 ng/mL, but had little effect on the effect size.
Statistical analysis was based on the intent-to-treat principle. Participant characteristics were summarized using means and SDs. Group-specific comparisons of anthropometric and biochemical parameters were performed at baseline using 2-sample t test (or Satterthwaite test for unequal variances) for continuous variables and the chi-square test for dichotomized variables. Differences in treatment effects between the 2 groups were evaluated by comparison of the 5 study time points for the outcomes of interest, using generalized linear regression model on outcome variables, with regressors being treatment group, time, and their interaction term. A statistically significant interaction term indicates different patterns of trend over time between the 2 groups. The generalized estimating equations method was used to account for correlations between repeated measures. All analyses were performed using SAS 9.4.
Results
Baseline Characteristics
Table 3 shows a comparison of the baseline characteristics of the placebo and the ergocalciferol groups. There were no statistically significant differences between the 2 groups at study entry.
Table 3.
Baseline anthropometric and biochemical characteristics of participants
| Parameters | Placebo (n = 18) | Ergocalciferol (n = 18) | P | ||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| Age, y | 14.28 | 2.86 | 13.25 | 2.76 | .28 |
| Height, cm | 158.65 | 11.30 | 156.12 | 12.77 | .53 |
| Height z score | 0.48 | 1.18 | 0.50 | 0.73 | .93 |
| Weight, kg | 56.16 | 14.66 | 53.33 | 15.19 | .58 |
| Weight z score | 0.67 | 0.68 | 0.86 | 0.81 | .45 |
| Body mass index | 22.01 | 4.15 | 22.03 | 5.41 | .99 |
| Body mass index z score | 0.74 | 0.68 | 0.89 | 0.94 | .59 |
| Waist circumference, cm | 76.22 | 11.56 | 76.16 | 14.83 | .99 |
| Systolic blood pressure, mm Hg | 104.94 | 9.06 | 106.44 | 10.60 | .65 |
| Diastolic blood pressure, mm Hg | 64.67 | 6.80 | 64.72 | 9.14 | .98 |
| Fasting plasma glucose, mg/dL | 111.13 | 35.78 | 125.83 | 25.00 | .18 |
| HbA1c, % | 7.47 | 1.69 | 7.62 | 1.35 | .77 |
| TDD insulin, units/d | 27.17 | 14.41 | 37.00 | 29.61 | .23 |
| TDD insulin, units/kg/d | 0.48 | 0.23 | 0.51 | 0.23 | .72 |
| TDD long-acting insulin only, units | 14.14 | 7.30 | 18.50 | 14.81 | .28 |
| n | % | n | % | ||
| Male sex | 14 | 77.9 | 10 | 55.6 | .16 |
| White ethnicity | 15 | 88.2 | 12 | 85.7 | ≥ .999 |
| Pubertal stage, Tanner II-V | 10 | 71.4 | 12 | 85.7 | .65 |
P values for continuous variables were obtained by 2 sample t test, or Satterthwaite test in case variances were not equal, and for dichotomized variables, either chi-square or Fisher exact test, whichever was appropriate.
Abbreviations: HbA1c, glycated hemoglobin A1c; SDS, SD score; TDDI, total daily dose of insulin.
Achieved Serum 25-Hydroxyvitamin D Concentration
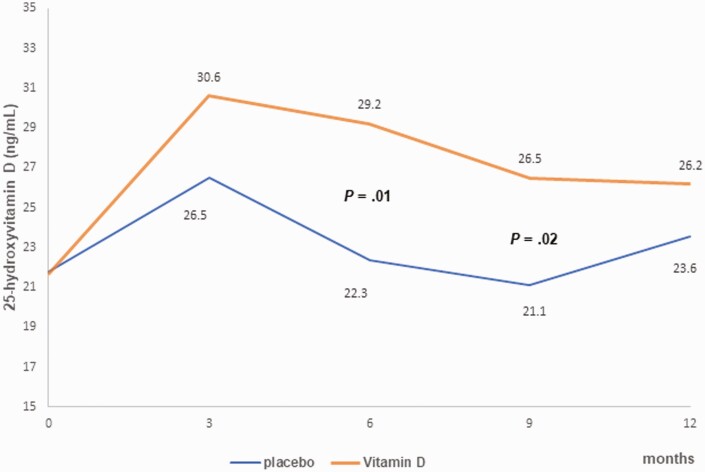
Serum 25(OH)D concentration (Fig. 2) was statistically significantly higher in the ergocalciferol group compared to the placebo group at 6 months (P = .01) and at 9 months (P = .02).
Figure 2.
A graph of the changes in 25-hyroxyvitamin D concentration in a 12-month randomized controlled trial (RCT) of ergocalciferol in youth with new-onset type 1 diabetes. The graph shows a statistically significantly higher 25-hydroxyvitamin D concentration in the ergocalciferol group compared to the placebo at 6 months (P = .01) and at 9 months (P = .02). All P values were adjusted for multiple comparisons.
Anthropometric Parameters and C-Peptide Levels
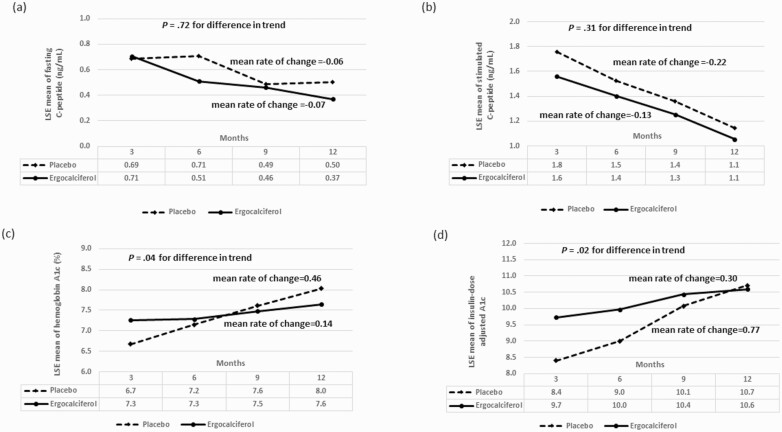
There were no statistically significant differences in change from baseline to 12 months in systolic and diastolic blood pressure, BMI z score, and waist circumference between the 2 groups (Tables 3 and 4). There was no statistically significant difference in the overall mean of fasting C-peptide concentration between the 2 groups during the trial (P = .54) (Table 5, Fig. 3A), nor in fasting C-peptide trend over time (P = .72). Similarly, for SCP neither the overall mean (P = .08) (see Table 5, Fig. 3B), nor temporal trend (P = .31) was different between the groups.
Table 4.
Longitudinal changes in clinical parameters during the trial
| Parameter | Time | Placebo (n = 18) | Ergocalciferol (n = 18) | P, from LSE estimates | ||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | |||
| Systolic blood pressure, mm Hg | Baseline | 104.9 | 9.1 | 106.4 | 10.6 | .64 |
| Mo 3 | 105.0 | 10.5 | 105.0 | 9.1 | .61 | |
| Mo 6 | 108.8 | 9.9 | 107.4 | 13.8 | .91 | |
| Mo 9 | 110.1 | 11.5 | 109.3 | 13.9 | .88 | |
| Mo 12 | 109.1 | 10.4 | 107.3 | 12.6 | .84 | |
| Diastolic blood pressure, mm Hg | Baseline | 64.7 | 6.8 | 64.7 | 9.1 | .98 |
| Mo 3 | 67.2 | 7.4 | 65.6 | 8.5 | .84 | |
| Mo 6 | 66.5 | 7.0 | 67.2 | 9.2 | .62 | |
| Mo 9 | 69.8 | 8.1 | 67.8 | 9.0 | .71 | |
| Mo 12 | 69.6 | 10.3 | 66.3 | 11.3 | .49 | |
| Body mass index | Baseline | 22.0 | 4.2 | 22.0 | 5.4 | .99 |
| Month 3 | 22.2 | 4.7 | 22.1 | 6.1 | .84 | |
| Mo 6 | 22.3 | 4.4 | 22.1 | 6.1 | .59 | |
| Mo 9 | 21.9 | 4.5 | 22.1 | 6.2 | .63 | |
| Mo 12 | 22.0 | 4.8 | 22.6 | 6.0 | .48 | |
| Body mass index, z score | Baseline | 0.74 | 0.68 | 0.89 | 0.94 | .58 |
| Mo 3 | 0.74 | 0.82 | 0.74 | 0.99 | .68 | |
| Mo 6 | 0.66 | 0.80 | 0.65 | 1.11 | .71 | |
| Mo 9 | 0.51 | 0.80 | 0.60 | 1.13 | .69 | |
| Mo 12 | 0.44 | 0.89 | 0.65 | 1.08 | .32 | |
| Waist circumference, cm | Baseline | 76.2 | 11.6 | 76.2 | 14.8 | .96 |
| Mo 3 | 73.2 | 14.4 | 74.1 | 13.2 | .54 | |
| Mo 6 | 76.2 | 13.6 | 73.9 | 12.0 | .88 | |
| Mo 9 | 72.9 | 14.4 | 70.7 | 15.0 | .85 | |
| Mo 12 | 75.2 | 11.6 | 75.3 | 14.3 | .80 |
Abbreviation: LSE, least square estimates, based on generalized linear model with repeated measures.
Table 5.
Longitudinal changes in therapeutic and biochemical parameters during the trial
| Parameter | Placebo (n = 18) | Ergocalciferol (n = 18) | Placebo (n = 18) | Ergocalciferol (n = 18) | P | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | LSE Mean | SE | LSE mean | SE | Difference in overall mean | Overall trend | Difference in trend | Difference at specific time (adjusted for multiple comparisons) | |
| TDDI, units/kg/d | .046 | .001 | .097 | |||||||||
| Baseline | 0.48 | 0.23 | 0.51 | 0.23 | 0.48 | 0.05 | 0.55 | 0.06 | .41 | |||
| Mo 3 | 0.46 | 0.18 | 0.62 | 0.36 | 0.43 | 0.05 | 0.62 | 0.08 | .049 | |||
| Mo 6 | 0.49 | 0.27 | 0.62 | 0.30 | 0.48 | 0.06 | 0.67 | 0.08 | .06 | |||
| Mo 9 | 0.62 | 0.28 | 0.69 | 0.26 | 0.63 | 0.07 | 0.73 | 0.07 | .31 | |||
| Mo 12 | 0.67 | 0.30 | 0.65 | 0.24 | 0.67 | 0.07 | 0.72 | 0.07 | .65 | |||
| FBG, mg/dL | .13 | .03 | .10 | |||||||||
| Baseline | 111 | 36 | 126 | 25 | 115 | 9 | 126 | 6 | .31 | |||
| Mo 3 | 131 | 53 | 157 | 61 | 130 | 12 | 157 | 15 | .17 | |||
| Mo 6 | 146 | 72 | 146 | 54 | 146 | 17 | 147 | 14 | .97 | |||
| Mo 9 | 140 | 61 | 141 | 43 | 137 | 16 | 143 | 12 | .75 | |||
| Mo 12 | 158 | 61 | 145 | 49 | 158 | 15 | 145 | 14 | .52 | |||
| Fasting C-peptide, ng/mL | .54 | .01 | .72 | |||||||||
| Baseline | 0.68 | 0.40 | 0.80 | 0.82 | 0.71 | 0.10 | 0.80 | 0.19 | .67 | |||
| Mo 3 | 0.65 | 0.38 | 0.59 | 0.40 | 0.69 | 0.09 | 0.71 | 0.12 | .90 | |||
| Mo 6 | 0.71 | 0.55 | 0.46 | 0.29 | 0.71 | 0.13 | 0.51 | 0.08 | .19 | |||
| Mo 9 | 0.44 | 0.24 | 0.42 | 0.24 | 0.49 | 0.07 | 0.46 | 0.06 | .80 | |||
| Mo 12 | 0.50 | 0.47 | 0.35 | 0.21 | 0.50 | 0.11 | 0.37 | 0.06 | .29 | |||
| Stimulated C-peptide, ng/mL | .08 | < .001 | .31 | |||||||||
| Baseline | 2.22 | 1.31 | 1.78 | 1.18 | 2.27 | 0.31 | 1.78 | 0.28 | .24 | |||
| Mo 3 | 1.74 | 0.94 | 1.28 | 0.78 | 1.76 | 0.21 | 1.56 | 0.25 | .55 | |||
| Mo 6 | 1.51 | 1.08 | 1.17 | 0.92 | 1.53 | 0.25 | 1.40 | 0.26 | .73 | |||
| Mo 9 | 1.35 | 1.34 | 1.05 | 0.84 | 1.36 | 0.31 | 1.25 | 0.24 | .79 | |||
| Mo 12 | 1.14 | 1.27 | 0.82 | 0.77 | 1.15 | 0.29 | 1.05 | 0.22 | .80 | |||
| HbA1c, % | .09 | < .001 | .04 | |||||||||
| Baseline | 7.47 | 1.69 | 7.62 | 1.35 | 7.47 | 0.39 | 7.62 | 0.32 | .76 | |||
| Mo 3 | 6.67 | 1.23 | 7.20 | 1.54 | 6.67 | 0.28 | 7.25 | 0.37 | .21 | |||
| Mo 6 | 7.12 | 1.51 | 7.26 | 1.18 | 7.15 | 0.34 | 7.28 | 0.27 | .77 | |||
| Mo 9 | 7.59 | 1.37 | 7.50 | 1.42 | 7.61 | 0.32 | 7.48 | 0.35 | .77 | |||
| Mo 12 | 8.01 | 1.70 | 7.64 | 2.14 | 8.03 | 0.41 | 7.65 | 0.57 | .59 | |||
| IDAA1c | .03 | < .001 | .02 | |||||||||
| Baseline | 9.4 | 2.4 | 9.7 | 1.8 | 9.4 | 0.6 | 9.9 | 0.4 | .51 | |||
| Mo 3 | 8.5 | 1.8 | 9.7 | 2.3 | 8.4 | 0.4 | 9.7 | 0.6 | .05 | |||
| Mo 6 | 9.0 | 2.4 | 9.8 | 1.9 | 9.0 | 0.5 | 10.0 | 0.5 | .17 | |||
| Mo 9 | 10.1 | 2.2 | 10.2 | 2.1 | 10.1 | 0.5 | 10.4 | 0.5 | .62 | |||
| Mo 12 | 10.7 | 2.5 | 10.3 | 2.3 | 10.7 | 0.6 | 10.6 | 0.6 | .89 |
P values were obtained from repeated-measures trend analysis using generalized linear model with dependent variable equal to clinical parameter, and independent variables equal to group, time, and their interactions. Generalized estimating equation was used for repeated measures.
Abbreviations: FBG, fasting blood glucose; HbA1c, glycated hemoglobin A1c; IDAA1c, insulin dose–adjusted A1c; LSE, least square estimate; TDDI, total daily dose of insulin.
Figure 3.
A, Trend analysis of the least square estimates (LSE) of the means for fasting C-peptide showing no statistically significant difference in the changes in fasting C-peptide concentration between the ergocalciferol- and placebo-treated patients with type 1 diabetes during the 12-month trial (P = .72). B, Trend analysis of the LSEs of the means for stimulated C-peptide showing no statistically significant difference in the change in stimulated C-peptide concentration at 90 minutes between the ergocalciferol- and placebo-treated patients with type 1 diabetes during the 12-month trial (P = .31). C, LSEs of the means for glycated hemoglobin A1c (HbA1c) showing the change in HbA1c between the ergocalciferol and placebo groups during the trial. Trend analysis shows an increase in HbA1c value for the combined groups (P < .0001). There was evidence of a faster rate of increase in HbA1c in the placebo compared to the vitamin group (P = .044). D, LSEs of the means for insulin dose–adjusted A1c (IDAA1c) showing the changes in IDAA1c between the ergocalciferol and placebo groups during the trial. Trend analysis shows an increase in IDAA1c value for the combined groups (P < .0001). Though IDAA1c was statistically significantly lower in the placebo group at 3 months, it subsequently rose sharply when compared to the vitamin group (P = .015), suggesting that individuals in the placebo group had greater residual β-cell function at the beginning of the study but lost this function at a faster rate than individuals in the ergocalciferol group.
Glycated Hemoglobin A1c, Fasting Blood Glucose, and Total Daily Dose of Insulin
Trend analysis showed an increase in HbA1c for the combined groups (P < .001; Fig. 3C). Although there was no statistically significant difference in the overall mean of HbA1c between the groups (P = .09) (see Table 5), there was a faster rate of increase in HbA1c in the placebo group, mean rate of change of 0.46% every 3 months, compared to the ergocalciferol group, mean rate of change of 0.14% every 3 months, (P = .04) (see Table 5, Fig. 3C). There were no significant differences in trend between the groups for FBG (P = .10) and TDDI (P = .10) (see Table 5).
Insulin Dose–adjusted A1c
IDAA1c increased over time in the combined placebo and ergocalciferol groups (P < .001; Fig. 3D). IDAA1c was lower in the placebo group at 3 months (P = .05), but subsequently rose sharply in the placebo group, at a mean rate of change of 0.77 every 3 months, whereas the increase was significantly blunted in the ergocalciferol group, at a mean rate of change of 0.30 every 3 months (P = .02) (see Fig. 3D).
Longitudinal Changes in Cytokines
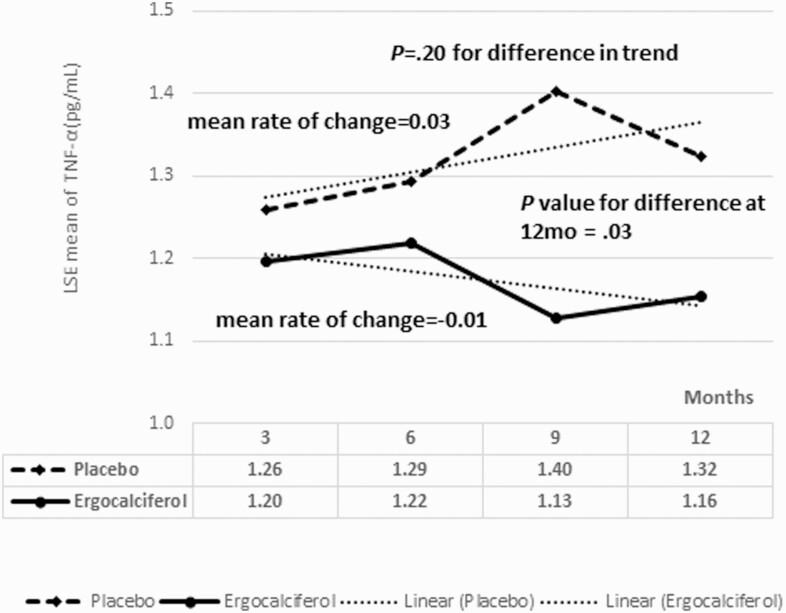
Fig. 4 shows the longitudinal changes in serum TNF-α concentration. The mean rate of change in TNF-α was 0.03 per 3 months for the placebo group, and –0.01 per 3 months for the ergocalciferol group (P = .20). At 12 months, the ergocalciferol group had a statistically significantly lower serum TNF-α concentration than the placebo group, 1.12 ± 0.1 pg/mL vs 1.32 ± 0.3 pg/mL (P = .03) (Table 6).
Figure 4.
Least square estimates of the means for serum tumor necrosis factor α (TNF-α) concentration showing the changes in TNF-α between the ergocalciferol and placebo groups during the trial. The mean of rates of change in TNF-α was 0.03 per 3 months for the placebo group, and 0.01 per 3 months for the ergocalciferol group. Serum TNF-α concentration was statistically significantly lower in the ergocalciferol group at 12 months.
Table 6.
Longitudinal changes in inflammatory cytokines during the trial
| Parameters | Time | Placebo (n = 18) | Ergocalciferol (n = 18) | P, from LSE estimates | ||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | |||
| IL-2, pg/mL | Baseline | 0.57 | 0.28 | 1.74 | 5.34 | .34 |
| Mo 3 | 0.48 | 0.25 | 1.55 | 4.12 | .28 | |
| Mo 6 | 0.51 | 0.23 | 1.68 | 4.10 | .26 | |
| Mo 9 | 0.50 | 0.24 | 1.67 | 4.31 | .26 | |
| Mo 12 | 0.47 | 0.24 | 1.84 | 4.55 | .23 | |
| IL-4, pg/mL | Baseline | 0.08 | 0.06 | 0.06 | 0.04 | .18 |
| Mo 3 | 0.08 | 0.07 | 0.07 | 0.07 | .84 | |
| Mo 6 | 0.10 | 0.13 | 0.05 | 0.03 | .41 | |
| Mo 9 | 0.16 | 0.41 | 0.06 | 0.03 | .39 | |
| Mo 12 | 0.14 | 0.34 | 0.06 | 0.04 | .39 | |
| IL-6, pg/mL | Baseline | 0.67 | 0.65 | 0.62 | 0.33 | .75 |
| Mo 3 | 0.51 | 0.25 | 0.61 | 0.41 | .35 | |
| Mo 6 | 0.71 | 0.51 | 0.80 | 0.38 | .56 | |
| Mo 9 | 1.12 | 1.54 | 0.61 | 0.33 | .19 | |
| Mo 12 | 0.73 | 0.60 | 0.62 | 0.36 | .57 | |
| IL-8, pg/mL | Baseline | 2.61 | 0.76 | 2.79 | 0.97 | .54 |
| Mo 3 | 2.74 | 0.86 | 3.33 | 2.12 | .26 | |
| Mo 6 | 2.61 | 0.58 | 2.98 | 1.07 | .12 | |
| Mo 9 | 2.83 | 1.08 | 2.88 | 1.00 | .85 | |
| Mo 12 | 3.20 | 1.49 | 2.99 | 1.27 | .73 | |
| IL-10, pg/mL | Baseline | 0.53 | 0.30 | 0.56 | 0.30 | .75 |
| Mo 3 | 0.51 | 0.20 | 0.54 | 0.28 | .78 | |
| Mo 6 | 0.64 | 0.68 | 0.79 | 0.94 | .61 | |
| Mo 9 | 0.66 | 0.61 | 0.55 | 0.39 | .53 | |
| Mo 12 | 0.68 | 0.85 | 0.48 | 0.08 | .36 | |
| IL-12p70, pg/mL | Baseline | 0.37 | 0.15 | 0.30 | 0.18 | .20 |
| Mo 3 | 0.34 | 0.14 | 0.35 | 0.18 | .89 | |
| Mo 6 | 0.35 | 0.17 | 0.51 | 0.78 | .43 | |
| Mo 9 | 0.41 | 0.42 | 0.37 | 0.16 | .70 | |
| Mo 12 | 0.32 | 0.14 | 0.32 | 0.17 | .98 | |
| IL-13, pg/mL | Baseline | 3.24 | 0.71 | 2.86 | 0.58 | .08 |
| Mo 3 | 3.01 | 0.58 | 3.13 | 0.64 | .72 | |
| Mo 6 | 3.12 | 0.51 | 3.20 | 0.71 | .83 | |
| Mo 9 | 3.05 | 0.60 | 3.00 | 0.70 | .81 | |
| Mo 12 | 2.79 | 0.49 | 3.25 | 0.84 | .09 | |
| IL-1β, pg/mL | Baseline | 0.14 | 0.11 | 0.13 | 0.10 | .84 |
| Mo 3 | 0.11 | 0.08 | 0.16 | 0.15 | .18 | |
| Mo 6 | 0.13 | 0.08 | 0.18 | 0.15 | .12 | |
| Mo 9 | 0.12 | 0.08 | 0.16 | 0.11 | .18 | |
| Mo 12 | 0.11 | 0.10 | 0.18 | 0.11 | .06 | |
| IFN-γ, pg/mL | Baseline | 5.87 | 7.32 | 5.73 | 5.68 | .95 |
| Mo 3 | 3.69 | 1.36 | 4.41 | 2.98 | .37 | |
| Mo 6 | 7.82 | 16.07 | 8.27 | 15.51 | .94 | |
| Mo 9 | 4.79 | 4.24 | 3.55 | 1.14 | .28 | |
| Mo 12 | 7.84 | 10.93 | 3.28 | 0.93 | .09 | |
| TNF-α, pg/mL | Baseline | 1.28 | 0.27 | 1.22 | 0.35 | .56 |
| Mo 3 | 1.26 | 0.29 | 1.19 | 0.39 | .58 | |
| Mo 6 | 1.30 | 0.26 | 1.21 | 0.36 | .50 | |
| Mo 9 | 1.41 | 0.76 | 1.13 | 0.33 | .17 | |
| Mo 12 | 1.32 | 0.26 | 1.12 | 0.13 | .03 |
Median test was also performed to ensure that results provided in this table were not affected by outliers. Statistically significant P value is shown in bold.
Abbreviations: IFN, interferon; IL, interleukin; LSE, least square estimate, based on generalized linear model with repeated measures; TNF, tumor necrosis factor.
Occurrence of Dysglycemia and Other Adverse Events
Adverse events are summarized in Table 7. One individual in the placebo group had an episode of diabetic ketoacidosis, while another participant in the placebo group had a confirmed COVID-19 infection. Biochemical monitoring showed no evidence of hypercalcemia, hypercalciuria, vitamin D toxicity, or pregnancy (data not shown).
Table 7.
Adverse events
| Adverse events | Vitamin D arm | Placebo arm |
|---|---|---|
| Upper respiratory tract complaints | 1 | 5 |
| Sinusitis | 1 | 1 |
| Otitis media | 0 | 1 |
| Hyperglycemia | 3 | 1 |
| Diabetic ketoacidosis | 0 | 1 |
| Mild hypoglycemia | 3 | 1 |
| Moderate hypoglycemia | 1 | 0 |
| Diarrhea | 1 | 0 |
| Stomachache | 0 | 1 |
| Vomiting | 1 | 0 |
| Transient weight loss | 0 | 1 |
| Skin rash | 1 | 0 |
| Mild transient hair loss | 1 | 1 |
| Confirmed COVID-19 infection | 0 | 1 |
Discussion
Our RCT showed that vitamin D’s actions are better demonstrated by a functional dynamic marker, IDAA1c, than an absolute, static test, SCP. Despite a statistically significant increase in serum 25(OH)D in the ergocalciferol group compared to the placebo group at 6 and 9 months, this 12-month RCT found no statistically significant differences between the groups for the duration of PR, magnitude of RBCF, IDAA1c, and glycemia. However, a statistically significantly faster rate of increase of HbA1c and IDAA1c values in the placebo group suggested a faster rate of loss of RBCF in that group, which indicates protection of RBCF by high-dose ergocalciferol supplementation in the experimental group. These results are supported by a recent report on the limitations of SCP concentration to denote PR [22]. In that study, 55% of children and adolescents with a SCP greater than 300 pmol/L (0.9 ng/mL) at 14.5 months after T1D diagnosis had low IS and thus failed to demonstrate the classic PR phenotype when assessed by IDAA1c [22]. The authors noted that differences in IS exist in PR and suggested that patients in PR with low IS could benefit from interventions to improve IS, and thus blunt increases in IDAA1c values [22]. Thus, IDAA1c is a superior marker for PR than SCP [22].
The statistically significantly lower serum TNF-α concentration, a proinflammatory agent [23], in the ergocalciferol group is mechanistically significant because it suggests that vitamin D could lower inflammation in early T1D by decreasing serum TNF-α concentrations. IL-2 promotes regulatory T cells [24]; however, the apparently higher IL-2 levels in the ergocalciferol group were due to one individual with extremely high levels throughout the study and was not a treatment effect.
Glycemic control was optimized in both groups by the application of TTIR [17, 19] so the overall average HbA1c levels for the ergocalciferol and placebo groups were 7.51% vs 7.61% (P = .79). This robust degree of glycemic control from TTIR could have prevented the detection of small differences in glycemia arising from ergocalciferol supplementation. Also, despite aggressive ergocalciferol dosing, the mean achieved serum 25(OH)D in the treatment group peaked at 30.6 ng/mL, possibly too low for maximal benefit.
It is unclear why the study participants did not attain much higher serum 25(OH)D concentrations than 30.6 ng/mL while receiving 50 000 IU of ergocalciferol weekly for 2 months, and then 25 000 IU weekly for 10 months. However, a similar peak 25(OH)D concentration was reported from India by Khadilkar et al [25] in an RCT of 50 girls aged 14 to 15 years who received 300 000 IU of ergocalciferol or placebo 4 times in one year while on 250-mg elemental calcium daily. Khadilkar and colleagues reported a peak serum 25(OH)D concentration of 30.2 ng/mL in the experimental arm, and 11.2 ng/mL in the placebo arm. The similarity of these peak 25(OH)D concentrations from studies conducted both in temperate and tropical climates argues against the effect of seasonality on peak 25(OH)D concentrations in this study.
A number of reasons have been advanced to explain failures of RCTs to demonstrate the expected health benefits of vitamin D supplementation [26]. These include study design effects that could affect the attainment and maintenance of elevated 25(OH)D concentrations; variations in 25(OH)D effect thresholds; nonsupplemental vitamin D intakes during RCTs and variations in the intestinal absorption of vitamin D; the effect of dietary factors that modulate vitamin D efficiency; and the variation of serum 25OHD values with genetic polymorphisms [11, 26, 27]. A possible study design effect on this study is the preponderance of male participants, who usually have robust PR [28], in the placebo arm compared to the ergocalciferol arm. This could explain the initial robust PR in the placebo arm that was then followed by an accelerated loss of RBCF; whereas individuals in the ergocalciferol arm, though made up mostly of female patients, who experience less robust PR, interestingly had a slower rate of decline in RBCF, suggesting that this mismatch of participants by sex could have skewed our results and reduced the observed effect of ergocalciferol on the study outcomes.
Our results are similar to reports in youth that found no statistically significant effect of ergocalciferol supplementation on PR [29] or glycemic control [6, 29] but differ from the study that reported a slower rate of decline of residual β-cell function in a combined population of youth and adults with T1D [6]. Mishra et al [29] conducted an RCT of vitamin D and calcium using cholecalciferol 2000 IU per day and placebo in 30 children aged 6 to 12 years and found no difference in glycemic control or PR at the end of the study. Gabbay et al [6] conducted an 18-month RCT of vitamin D and placebo in a mixed population of 38 children, adolescents, and adults aged 7 to 30 years. Using cholecalciferol 2000 IU per day or placebo and an inclusion criterion of a higher serum C-peptide level of 0.6 ng/mL, they found no difference in markers of glycemic control and cytokine levels, but a statistically significant increase in C-peptide in the first year and a slower C-peptide decay in the second year. Though these 2 studies used a similar dose of cholecalciferol, 2000 IU/day, Gabbay and colleagues [6] reported that 25(OH)D rose from a baseline level of 26.3 ng/mL to a peak level of 60.88 ng/mL at 6 months, whereas Mishra et al [29] reported a baseline level of 27.6 ng/mL that peaked in 6 months at 32.8 ng/mL. The intervention group in our study had a baseline 25(OH)D level of 22 ng/mL, which rose to 30.6 ng/mL at 3 months, and then to 29.2 ng/mL at 6 months and 26.5 ng/mL at 9 months. Thus, it is unclear whether the slower decline in C-peptide in Gabbay’s study was due to the robust rise in serum 25(OH)D or to some other factors such as patient selection or geographical location. Our study, however, is the first to demonstrate statistically significant functional and dynamic differences between ergocalciferol and placebo as depicted by statistically significant reductions in the rates of change both of HbA1c and IDAA1c in the ergocalciferol arm. Our study is also distinct as it is the longest of such studies in an exclusive pediatric T1D population using a standardized insulin regimen and high-dose ergocalciferol.
The study’s limitation is its single-center setting. Its strengths include an RCT design, long duration of follow-up, the use of a high dose of ergocalciferol, the standardization of insulin therapy in both groups, and an adequate sample size for statistical power. The RCT design and participant characteristics make the findings generalizable to youth with new-onset T1D.
Conclusions
Adjunctive ergocalciferol supplementation statistically significantly reduced serum TNF-α concentration and significantly blunted the rates of increase both in A1c and IDAA1c, suggesting a protection of RBCF and PR in youth with newly diagnosed T1D. This suggests that ergocalciferol slowed the rise in insulin requirements by improving IS in youth with newly diagnosed T1D. Larger studies are needed to quantify the effect of vitamin D on IS in youth with T1D.
Acknowledgment
We thank Prof Alan D. Rogol for his scientific review of the manuscript.
Funding: This work was supported by an investigator-initiated research grant from the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health (grant No. 1 R21 DK113353-03, to B.U.N.). The funder had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author Contributions: All the authors accept responsibility for the entire content of this submitted manuscript and approved submission. B.U.N. conceived the study and contributed to study design, data acquisition, and interpretation. L.C.A. and B.A.B. contributed to study conception and design. S.P., G.J., J.F., B.U.N., and R.B.S. contributed to study design and data acquisition. A.F.L., B.A.B., and B.U.N. interpreted and analyzed the data. B.U.N. generated the initial draft of the manuscript.
Clinical Trial Information: Clinical Trials.gov. registration number NCT03046927 (registered February 8, 2017).
Glossary
Abbreviations
- 25(OH)D
25-hydroxyvitamin D
- BMI
body mass index
- CGM
continuous glucose monitoring
- FBG
fasting blood glucose
- HbA1c
glycated hemoglobin A1c
- IDAA1c
insulin dose–adjusted A1c
- IDS
Investigational Drug Services
- IL
interleukin
- IS
insulin sensitivity
- LSE
least square estimate
- PR
partial clinical remission
- RBCF
residual β-cell function
- RCT
randomized controlled trial
- SCP
stimulated C-peptide
- T1D
type 1 diabetes
- TDDI
total daily dose of insulin
- TNF
tumor necrosis factor
- TTIR
treat-to-target insulin regimen
- UMMS
University of Massachusetts Medical School
Additional Information
Conflict of interest: The authors have no conflict of interest to disclose.
Data Availability
Some or all data generated or analyzed during this study are included in this published article or in the data repositories listed in “References.”
References
- 1. Barnett R. Type 1 diabetes. Lancet. 2018;391(10117):195. [DOI] [PubMed] [Google Scholar]
- 2. Sherry NA, Tsai EB, Herold KC. Natural history of beta-cell function in type 1 diabetes. Diabetes. 2005;54(Suppl 2):S32-S39. [DOI] [PubMed] [Google Scholar]
- 3. Oram RA, Jones AG, Besser RE, et al. The majority of patients with long-duration type 1 diabetes are insulin microsecretors and have functioning beta cells. Diabetologia. 2014;57(1):187-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yu MG, Keenan HA, Shah HS, et al. Residual β cell function and monogenic variants in long-duration type 1 diabetes patients. J Clin Invest. 2019;129(8):3252-3263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Arha D, Pandeti S, Mishra A, et al. Deoxyandrographolide promotes glucose uptake through glucose transporter-4 translocation to plasma membrane in L6 myotubes and exerts antihyperglycemic effect in vivo. Eur J Pharmacol. 2015;768:207-216. [DOI] [PubMed] [Google Scholar]
- 6. Gabbay MA, Sato MN, Finazzo C, Duarte AJ, Dib SA. Effect of cholecalciferol as adjunctive therapy with insulin on protective immunologic profile and decline of residual β-cell function in new-onset type 1 diabetes mellitus. Arch Pediatr Adolesc Med. 2012;166(7):601-607. [DOI] [PubMed] [Google Scholar]
- 7. Sherry N, Hagopian W, Ludvigsson J, et al. ; Protégé Trial Investigators . Teplizumab for treatment of type 1 diabetes (Protégé study): 1-year results from a randomised, placebo-controlled trial. Lancet. 2011;378(9790):487-497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rapini N, Schiaffini R, Fierabracci A. Immunotherapy strategies for the prevention and treatment of distinct stages of type 1 diabetes: an overview. Int J Mol Sci. 2020;21(6):2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rachid O, Osman A, Abdi R, Haik Y. CTLA4-Ig (abatacept): a promising investigational drug for use in type 1 diabetes. Expert Opin Investig Drugs. 2020;29(3):221-236. [DOI] [PubMed] [Google Scholar]
- 10. Ouyang H, Wen J, Song K. Decreased interleukin-35 levels and CD4+EBI3+ T cells in patients with type 1 diabetes and the effects of the antibody against CD20 (rituximab). Arch Med Sci. 2021;17(1):258-261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pozzilli P, Manfrini S, Crinò A, et al. ; IMDIAB group . Low levels of 25-hydroxyvitamin D3 and 1,25-dihydroxyvitamin D3 in patients with newly diagnosed type 1 diabetes. Horm Metab Res. 2005;37(11):680-683. [DOI] [PubMed] [Google Scholar]
- 12. Nwosu BU, Parajuli S, Jasmin G, et al. 2021 Investigational Study Protocol. Accessed November 22, 2021. https://escholarship.umassmed.edu/datasets/3/
- 13. American Diabetes Association. 2. Classification and diagnosis of diabetes: Standards of Medical Care in Diabetes—2020. Diabetes Care. 2020;43(Suppl 1):S14-S31. [DOI] [PubMed] [Google Scholar]
- 14. The Diabetes Control and Complications Trial Research Group. Effect of intensive therapy on residual beta-cell function in patients with type 1 diabetes in the diabetes control and complications trial. A randomized, controlled trial. Ann Intern Med. 1998;128(7):517-523. [DOI] [PubMed] [Google Scholar]
- 15. Sacks DB, John WG. Interpretation of hemoglobin A1c values. JAMA. 2014;311(22):2271-2272. [DOI] [PubMed] [Google Scholar]
- 16. Ucar F, Erden G, Ginis Z, et al. Estimation of biological variation and reference change value of glycated hemoglobin (HbA(1c)) when two analytical methods are used. Clin Biochem. 2013;46(15):1548-1553. [DOI] [PubMed] [Google Scholar]
- 17. Nwosu BU, Maranda L, Cullen K, et al. A randomized, double-blind, placebo-controlled trial of adjunctive metformin therapy in overweight/obese youth with type 1 diabetes. PLoS One. 2015;10(9):e0137525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Smith KE, Levine BA, Clement SC, Hu MJ, Alaoui A, Mun SK. Impact of MyCareTeam for poorly controlled diabetes mellitus. Diabetes Technol Ther. 2004;6(6):828-835. [DOI] [PubMed] [Google Scholar]
- 19. Blonde L, Merilainen M, Karwe V, Raskin P; TITRATE Study Group . Patient-directed titration for achieving glycaemic goals using a once-daily basal insulin analogue: an assessment of two different fasting plasma glucose targets—the TITRATE study. Diabetes Obes Metab. 2009;11(6):623-631. [DOI] [PubMed] [Google Scholar]
- 20. Institute of Medicine (US) Committee to Review Dietary Reference Intakes for Vitamin D and Calcium; Ross AC, Taylor CL, Yaktine AL, Del Valle HB, eds. Dietary Reference Intakes for Calcium and Vitamin D. National Academy Press; 2010. [PubMed] [Google Scholar]
- 21. Bizzarri C, Pitocco D, Napoli N, et al. ; IMDIAB Group . No protective effect of calcitriol on beta-cell function in recent-onset type 1 diabetes: the IMDIAB XIII trial. Diabetes Care. 2010;33(9):1962-1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mørk FCB, Madsen JOB, Jensen AK, et al. Differences in insulin sensitivity in the partial remission phase of childhood type 1 diabetes; a longitudinal cohort study. Diabet Med. Published online September 26, 2021. doi:10.1111/dme.14702 [DOI] [PubMed] [Google Scholar]
- 23. Martens PJ, Gysemans C, Verstuyf A, Mathieu AC. Vitamin D’s effect on immune function. Nutrients. 2020;12(5):1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dwyer CJ, Ward NC, Pugliese A, Malek TR. Promoting immune regulation in type 1 diabetes using low-dose interleukin-2. Curr Diab Rep. 2016;16(6):46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Khadilkar AV, Sayyad MG, Sanwalka NJ, et al. Vitamin D supplementation and bone mass accrual in underprivileged adolescent Indian girls. Asia Pac J Clin Nutr. 2010;19(4):465-472. [PubMed] [Google Scholar]
- 26. Boucher BJ. Why do so many trials of vitamin D supplementation fail? Endocr Connect. 2020;9(9):R195-R206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pani MA, Knapp M, Donner H, et al. Vitamin D receptor allele combinations influence genetic susceptibility to type 1 diabetes in Germans. Diabetes. 2000;49(3):504-507. [DOI] [PubMed] [Google Scholar]
- 28. Marino KR, Lundberg RL, Jasrotia A, et al. A predictive model for lack of partial clinical remission in new-onset pediatric type 1 diabetes. PLoS One. 2017;12(5):e0176860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mishra A, Dayal D, Sachdeva N, Attri SV. Effect of 6-months’ vitamin D supplementation on residual beta cell function in children with type 1 diabetes: a case control interventional study. J Pediatr Endocrinol Metab. 2016;29(4):395-400. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Some or all data generated or analyzed during this study are included in this published article or in the data repositories listed in “References.”