Abstract
Group B Streptococcus (GBS), also known as Streptococcus agalactiae is a Gram-positive bacterium commonly encountered as part of the microbiota within the human gastrointestinal tract. A common cause of infections during pregnancy, GBS is responsible for invasive diseases ranging from urinary tract infections to chorioamnionitis and neonatal sepsis. Diabetes mellitus (DM) is a chronic disease resulting from impaired regulation of blood glucose levels. The incidence of DM has steadily increased worldwide to affecting over 450 million people. Poorly controlled DM is associated with multiple health comorbidities including an increased risk for infection. Epidemiologic studies have clearly demonstrated that DM correlates with an increased risk for invasive GBS infections, including skin and soft tissue infections and sepsis in non-pregnant adults. However, the impact of DM on risk for invasive GBS urogenital infections, particularly during the already vulnerable time of pregnancy, is less clear. We review the evolving epidemiology, immunology, and pathophysiology of GBS urogenital infections including rectovaginal colonization during pregnancy, neonatal infections of infants exposed to DM in utero, and urinary tract infections in pregnant and non-pregnant adults in the context of DM and highlight in vitro studies examining why DM might increase risk for GBS urogenital infection.
Keywords: Streptococcus agalactiae, Group B Streptococcus, diabetes mellitus, hyperglycemia, vaginal colonization, neonatal sepsis, urinary tract infection
1. Introduction:
1.1. Group B Streptococcus
Streptococcus agalactiae, also known as Group B Streptococcus (GBS), is a Gram-positive bacterium that often asymptomatically colonizes the human gastrointestinal and female urogenital tracts.1 Although GBS is a common component of the gastrointestinal microbiota,2, 3 GBS can cause invasive infections in other niches.4 Worldwide, GBS is a leading cause of infections during pregnancy and early neonatal life.5 GBS is thought to cause perinatal infections by colonizing the vagina and ascending the reproductive tract to the gravid uterus.6 Recent meta-analyses estimate the prevalence of rectovaginal GBS colonization during pregnancy around 18% but varies by region, with the highest prevalence of colonization in the Caribbean (35%) and lowest regional prevalence in Southern (13%) and Eastern Asia (11%).7 GBS colonization of the vagina is a major risk factor for perinatal infections ranging from chorioamnionitis, or infection of the fetal membranes, to stillbirth, preterm birth, neonatal sepsis, and meningitis.1 These infections can result in lifelong health conditions for infants including neurodevelopmental impairments, chronic lung disease, and increased risk for cardiovascular and metabolic disorders.8, 9 The Centers for Disease Control and Prevention, American Academy of Pediatrics, and American College of Obstetricians and Gynecologists recommend routine screening for GBS rectovaginal colonization during the third trimester and antibiotic prophylaxis during labor for those that test positive.10 Following initiation of routine GBS screening and prophylaxis, maternal GBS colonization during pregnancy is now associated with lower odds of chorioamnionitis compared to prior to these interventions.11
Over the past two decades, the epidemiology of invasive GBS infections in non-pregnant adults has evolved. A recent population-based surveillance study suggests a rising incidence of invasive GBS infection in non-pregnant adults, especially with increasing age.4 Additionally, in nearly 95% of cases of GBS invasive disease, affected individuals had at least one underlying condition, most commonly obesity and diabetes.4 That study provided additional supporting evidence to prior observations suggesting that the incidence of GBS infections has been steadily increasing over the past 30 years, and that risk factors, such as diabetes, are driving the increasing numbers of GBS infections in non-pregnant adults.12, 13
1.2. Diabetes mellitus:
Diabetes mellitus (DM) is one of the world’s largest public health concerns and is a leading cause of mortality and reduced life expectancy.14 The International Diabetes Federation estimated that 451 million adults were living with DM worldwide in 2017 and projects an increase to 693 million by the year 2045 without adoption of effective prevention methods.14, 15 Incidences of both type 1 diabetes mellitus (autoimmune, T1DM)16 and type 2 diabetes mellitus (non-autoimmune, T2DM) are increasing.14 Gestational diabetes mellitus (GDM), or new-onset hyperglycemia during pregnancy, affects 7-10% of pregnancies worldwide17. GDM is thought to arise, in part, from placental hormone production leading to insulin resistance.17, 18 Increasing rates of GDM mirror those of T2DM, as patients with GDM are at increased risk of developing T2DM in the future.19
DM is associated with maternal and fetal morbidity and mortality during pregnancy.20 Recent population studies from the U.S. and Spain demonstrate that pregnant persons with DM are at risk for pregnancy complications including preeclampsia and hypertension and are more likely to require induction of labor, cesarean delivery, and to deliver prematurely.21, 22 Neonates born to persons with DM are at risk for short term and longer term complications.23 Compared to pregnant persons without DM, infants born to those with DM have an increased risk of several complications including macrosomia, birth trauma, shoulder dystocia, neonatal hypoglycemia, hyperbilirubinemia, polycythemia, and respiratory distress syndrome.22 Pregestational DM, particularly with poor glycemic control early in pregnancy, is associated with increased rates of congenital malformations including cardiac and neural tube defects, spontaneous abortions and stillbirth, preterm birth, and perinatal mortality.21, 24 Infants born to mothers with DM are also at increased risk for chronic diseases later in life including cardiovascular diseases, hypertension, obesity, and DM.24
Not only does DM increase risk for skin, bone, eye, ear, gastrointestinal, urinary tract, and respiratory infections, but these infections are associated with significantly increased hospitalization and mortality rates (reviewed in 25). The etiology of increased infection risk in DM is multifactorial and incompletely understood (reviewed in 26, 27); however, skin wounds can be more common in persons with DM suffering from autonomic and sensory neuropathy. Additionally, hyperglycemia may impair immune defenses, with leukocytes exhibiting multiple defects including impaired chemotactic, phagocytic, and microbicidal activities.28, 29
As DM becomes more common, it is imperative to understand the relationship between DM and infections to guide research and clinical care. We sought to define what is known from the literature regarding how DM might increase risk for GBS urogenital tract infections in pregnant and non-pregnant adults. Specifically, this review will examine GBS rectovaginal colonization during pregnancy, GBS neonatal infections of infants exposed to DM in utero, and urinary tract infections in pregnant and non-pregnant adults. While prior studies concerning the impact of hyperglycemia on immune function have been reviewed elsewhere, these studies generally ignore effects of hyperglycemia on invading microbes. Here, we also review evidence of how elevated glucose levels found in patients with DM (and/or GDM) might affect the pathophysiology of GBS infections and host reproductive immunology.
2. Methods:
We reviewed the existing literature regarding GBS clinical and basic pathophysiology of urogenital tract infections. The central research question was “What is known from the existing literature regarding how DM impacts risk for GBS urogenital tract infection including urinary tract infections (UTIs), rectovaginal colonization, and neonatal sepsis?” To address this question, we conducted a search using PubMed with search terms including ((diabetes) AND (Group B Streptococcus)) AND (conditions listed above). We reviewed papers with the following inclusion criteria: English language studies of human subjects that evaluated DM as a risk factor for the different conditions listed above. Our search strategy is presented as outlined by the PRISMA 2020 statement (Supplemental Figure 1).30 Due to very few studies specifically examining GBS neonatal sepsis, we expanded our search regarding studies investigating neonatal sepsis in general as shown in Supplemental Figure 1. To compare incidence rates across different studies, data regarding rates of GBS colonization in patients with and without DM were extracted and an unadjusted odds ratio with 95% confidence intervals was calculated using Prism 9 for macOS, Version 9.1.0. To further address questions regarding the impact of DM on reproductive immune responses, GBS virulence and pathophysiology, we also review in vitro and in vivo studies that evaluate reproductive immunology and GBS pathophysiology in the setting of DM or experimental conditions of elevated glucose. To investigate how DM changes reproductive immunology, we used the search terms: ((NOD mice) AND (pregnancy)) and ((type 1 diabetes) AND (pregnancy)), and for GBS pathophysiology, we used the terms: (group b streptococcus) AND (glucose) AND ((infection) OR (colonization)).
3. Epidemiology of GBS genitourinary infections in patients with DM:
3.1. Rectovaginal colonization during pregnancy:
Rectovaginal colonization during pregnancy is the primary risk factor for GBS perinatal infections.31 GBS ascends from the vagina to cause infection of the fetal membranes (chorioamnionitis) or via transmission to the infant during passage through the vaginal canal during labor.32 Due to the prevalence of invasive GBS disease, the Centers for Disease Control and Prevention, American Academy of Pediatrics, and American College of Obstetricians and Gynecologists first recommended routine screening for GBS rectovaginal colonization in 1996.33 With initiation of screening and antibiotic prophylaxis during labor, incidence of early onset neonatal sepsis (EONS), diagnosed within the first 7 days of life, decreased from 1.8 cases/1000 live births in 1990 to 0.23 cases/1000 live births in 2015.34 Current recommendations include routine screening for GBS colonization during the third trimester and antibiotic prophylaxis during labor.35 To understand how DM might impact rectovaginal colonization, we conducted a literature search for studies that compared rates of rectovaginal colonization during pregnancy in patients with and without DM. Studies that provide data allowing comparison of colonization rates are included in Table 1.
Table 1:
Studies that provided data regarding rates of GBS vaginal colonization in gestating parents with or without DM (preexisting or GDM).
| Study | Location | Study period |
Population | Mean age in years (SD) |
Rate of DM |
+GBS in DM |
+ GBS in non-DM |
Unadjusted OR [95% CI] |
|---|---|---|---|---|---|---|---|---|
| Ji et al. 37 | Inner Mongolian Maternal and Child Care Hospital, China | 01/2016-12/2016 | 9,770 pregnancies | NA | 947 (9.6%) with GDM | 39/947 (4.1%) | 228/8913 (2.6%) | 1.64 [1.15–2.32] |
| Edwards et al. 36 | Duke Health affiliated hospitals, North Carolina, USA | 01/2003-12/2015 | 60,029 pregnancies | GBS+: 28.0 (6.2) GBS −: 28.7 (6.2) |
1760 (2.9%) with preexisting DM | 466/1760 (26.4%) | 11915/56183 (21.2%) | 1.34 [1.20-1.49] |
| 2086 (3.5%) with GDM | 571/2086 (27.4%) | 1.40 [1.27-1.55] | ||||||
| Gopal Rao et al. 44 | North West Healthcare NHS Trust, London, UK | 03/2014-12/2015 | 6,309 pregnancies | 30.6 (5.6) | 217 (3.4%) with any type of DM | 76/217 (35%) | 1749/6034 (29.0%) | 1.32 [0.99-1.76] |
| Lukic et al. 42 | Rome, Italy | 11/2011-07-2013 | 473 pregnancies (346 controls) | DM: 34.9 (5.0) Non-DM: 32.8 (4.7) |
114 with any type of DM | 16/114 (14%) | 47/312 (15.1%) | 0.92 [0.50-1.71] |
| Obata-Yasuoka et al. 39 | Tsukuba, Japan | 01/2001-05/2010 | 5,379 pregnancies at 35-37 weeks | NA | 249 (4.6%) with any type of DM | 42/249 (16.8%) | 620/5130 (12.1%) | 1.48 [1.05-2.08] |
| Venkatesh et al. 49 | Consortium on Safe Labor Study – 12 sites in USA | 2002-2008 | 115,070 pregnancies delivering >37 weeks | GBS+: 27.1(6.0) GBS−: 27.2 (6.0) |
1799 (1.6%) with preexisting DM | 431/1799 (24.0%) | 23625/108418 (21.8%) | 1.13 [1.01-1.26] |
| 4853 (4.2%) with GDM | 1003/4853 (20.1%) | 0.94 [0.87-1.00] | ||||||
| Najmi et al. 105 | Karachi, Pakistan | 05/2007-08/2007 | 405 pregnancies at 35-37 week | 28.9 (4.6) | 9/405 (5.8%) with any type of DM | 3/9 (33%) | 66/396 (17%) | 2.5 [0.67-9.51] |
| Piper et al. 40 | San Antonio Texas, USA | 01/1992-06/1996 | 1492 pregnancies(1046 case controls) | DM: 28.5 (6.2) Non-DM: 23.6 (5.5) |
446 with GDM | 55/446 (12.3%) | 125/1046 (12.0%) | 1.03 [0.74-1.46] |
| Ramos et al. 106 | Jacksonville Florida, USA | 01/1995-03/1996 | 405 pregnancies (300 case controls) | DM: 27.0 (6.5) Non-DM: 24.6 (6.4) |
48 with preexisting DM and 57 with GDM | 46/105 (44%) | 69/300 (23%) | 2.61 [1.64-4.13] |
| Bey et al. 107 | New Orleans, Louisiana, USA | 04/1985-05/1988 | 201 pregnancies (100 case controls) | DM: 29.0 (6.0) Non-DM: 24.6 (4.8) |
101 with DM any type of DM | 32/101 (31.7%) | 19/100 (19%) | 1.98 [1.04-3.78] |
| Matorras et al. 108 | Madrid, Spain | 12/1981-02/1985 | 1050 pregnancies | NA | 37 (3.5%) with preexisting DM | 7/37 (8.9%) | 107/980 (10.9%) | 1.90 [0.78-4.38] |
| 33 (3.1%) with GDM | 7/33 (21.2%) | 2.20 [0.89-5.23] | ||||||
| Raimer and O’Sullivan 109 | Miami Florida, USA | 11 months | 199 pregnancies (101 case controls) | DM: 30.6 (6.2) Non-DM 28.5 (6.8) |
55 with preexisting DM | 8/98 (8.2%) | 3/101 (3.0%) | 2.87 [0.84-10.2] |
| 43 with GDM |
DM, diabetes mellitus; GDM, gestational diabetes mellitus; OR, odds ratio; CI confidence interval; NA, not available
Rates of GBS vaginal colonization and DM vary by population studied. A cohort study by Edwards et al. noted a GBS colonization rate of 21.6% in 60,029 deliveries at Duke Health affiliated hospitals.36 Adults with GBS vaginal colonization and invasive GBS disease were more likely to have preexisting DM compared to those testing negative for GBS (3.5% versus 2.8% GBS negative, P < 0.001). Multivariate modelling to evaluate predictors of GBS colonization showed that preexisting DM was independently associated with risk for colonization (adjusted relative risk (aRR) 1.12, 95% Confidence Interval (CI): 1.01-1.23). The authors also determined that the GBS colonized cohort had a higher risk of GDM (RR 1.21, CI: 1.11-1.32).36 Another prospective cohort study by Ji et al., focused on the Inner-Mongolian population in China and found lower rates of GBS colonization (2.7%) but noted GDM to be among the risk factors for GBS colonization (odds ratio (OR) 1.6, CI: 1.14–2.28).37 A study conducted by McCloskey et al. using the Barwon Infant Study Cohort from southeastern Australia, examined effects of gestating parent and infant microbiome on aortic intima-media thickness as a measure of risk for cardiovascular disease.38 As part of the Barwon Infant Study Cohort, they collected data including age of the gestating parent, GBS colonization, antibiotic use, and health comorbidities including DM. In their population, the baseline GBS colonization rate was 21% and GDM conferred a non-statistically significant risk for GBS colonization (OR 1.44, CI: 0.65-3.17). Obata-Yasuoka et al. examined GBS colonization in 5379 subjects in Japan and found that those with impaired glucose tolerance from GDM or preexisting DM had a higher GBS colonization rate (16.8%) compared to those with normal glucose tolerance (12.1%).39
Not all studies suggest an association between DM and GBS colonization. Piper et al. did not find a difference in GBS colonization between patients with GDM and those without DM.40 Additionally, a cross-sectional study of pregnant people in Brazil by Siqueira et al. demonstrated that this population had a 14.4% rate of GBS carriage, but rates did not differ significantly (OR 0.81) with or without GDM.41 Lukic et al., evaluated a cohort of pregnant people with or without DM in Rome, Italy for bacterial infections including GBS, Mycoplasma hominis, Ureaplasma urealyticum, Chlamydia trachomatis, and Gardnerella vaginalis. Compared to patients without DM, the authors found significantly higher rates of vaginal Mycoplasma/Ureaplasma but no differences in GBS vaginal colonization.42
Obesity is a well described risk factor for the development of DM including during pregnancy,43 and some studies have evaluated how obesity impacts GBS colonization during pregnancy. A single center, prospective, cross sectional study performed by Gopal Rao et al. in London described an increased frequency of GBS colonization in obese adults, regardless of DM, with an incremental increase in frequency of colonization from 23 to 35% with increasing body mass index (BMI) from <18.5 to >35 Kg/m2 in a multivariable analysis.44 There was also a trend towards increased risk of GBS colonization in those with DM compared to those without (35% vs 29%, P = 0.07).44 Melchor et al. conducted a historical cohort study (Cruces Perinatal Database) of 9,778 Spanish adults of normal weight and 2207 obese adults for GBS rectovaginal colonization and found that the obese cohort had higher rates of colonization (12.24% vs 15.86%, aOR 1.299, CI: 1.14-1.47).45 This population had similar rates of GDM, with 5.35% affected in the normal weight group and 5.02% affected in the obese group.45 Similarly, Kleweis et al. conducted a retrospective cohort study of 7,711 patients with singleton term pregnancies in St. Louis, Missouri comparing those with a BMI >30 Kg/m2 (obese) and <30 Kg/m2 (nonobese) for the outcome of GBS colonization.46 The rate of GBS colonization of pregnant persons in this study was 25.8%, but patients with a BMI >30 were more likely to be colonized (28.4% vs 22.2%) when adjusting for race, parity, smoking, and DM (aOR 1.35, CI: 1.21-1.5). The authors did not specifically examine the influence of DM on GBS colonization, but there was a significantly higher percentage of patients with DM in the obese group compared to the nonobese group (3% vs 0.47%). Additionally, the authors found a dose response relationship between increasing BMI and GBS colonization, with rates of 22.2% in those with BMI <30 Kg/m2, 27.3% with BMI 30-39.9, and 31.7% with BMI > 40 Kg/m2.46
In summary, the incidence of obesity and DM during pregnancy is increasing.47 These studies provide evidence that these metabolic syndromes impact risk for GBS rectovaginal colonization. As shown in Table 1, there are other variables that may interact with risk factors such as DM and/or obesity. While recent studies suggest that older maternal age does not increase risk for GBS colonization, age is a risk factor for invasive GBS disease in non-pregnant adults.12, 48, 49 Additionally, women with DM and/or obesity often have other obstetric complications including hypertension and preeclampsia that may also confer risk.23, 43 More studies are needed to understand the magnitude of risk independently conferred by DM and obesity. Additionally, rates of DM and GBS colonization vary widely based on factors including race, socioeconomic status, and region; therefore, more adequately powered studies are needed to understand the evolving nature of how factors such as DM may impact risk for bacterial infection during pregnancy. One important factor missing from many studies investigating the impact of DM on colonization and infection is maternal glycemic control. Future studies are needed to better examine if strict treatment adherence, which could improve blood glucose levels, might modify the colonization and potential infection risk seen in patients with DM.
3.2. Neonatal infections of infants exposed to DM in utero:
Few studies exist that specifically examine the risk of GBS infection in neonates exposed to DM during pregnancy, and those that have been completed provide mixed results. Piper et al. conducted a retrospective cohort of 446 pregnant persons with GDM and 1046 pregnant persons without DM.40 Despite that the diabetic group was older (28.5 ± 6.2 vs. 23.5 ± 5.5 years), more likely to undergo induction of labor, and less likely to deliver vaginally, the authors found no differences in intraamniotic infection between GBS-colonized pregnant persons with (16%) or without (13%) GDM.40 The authors noted that GBS colonization increased risk of neonatal sepsis (OR 3.71); however, this outcome was independent of parental DM status. Of note, patients in this study were screened for GBS between 1992 and 1994 because they had GDM. Universal GBS perinatal screening was not yet in practice, and patients without DM were screened based on provider preference, potentially biasing results. Additionally, GBS positive status did not routinely warrant intrapartum antibiotic treatment in this cohort. Another historical single center cohort study by Edwards et al. including over 50,000 pregnant patients between 2003-2015 in the United States describes higher risk of GDM in GBS colonized individuals (RR 1.21, CI: 1.11-1.32).36 However, GBS colonization in this cohort was associated with lower risk of chorioamnionitis (aRR 0.76, CI: 0.66-0.87) and preterm birth (<34 weeks aRR 0.30, CI: 0.26-0.35 and <37 weeks aRR 0.49, CI: 0.45-0.53). Sub-analysis of pregnancies complicated by invasive gestational GBS disease demonstrated increased risk of GDM, preterm labor, preterm birth, and chorioamnionitis.
More studies are available that examine risk of neonatal sepsis regardless of pathogen. In a multinational retrospective cohort study of high-income countries outside of the U.S., Persson et al. described that while pregnant persons with any type of DM had higher rates of hypertension, antenatal steroid treatment, and cesarean deliveries than those without DM, the authors found no increase in neonatal mortality or severe neonatal morbidity in infants exposed to DM in utero.50 While this study defined severe morbidity as including potentially infection-related necrotizing enterocolitis, they do not specifically mention sepsis nor any pathogen-specific infection.50 In contrast, Li et al. examined cohorts of 22 pregnant patients with GDM compared to 28 pregnant patients without DM to investigate differences in growth parameters, placental changes including weight and inflammation, and neonatal outcomes.51 The authors found that patients with a first-time diagnosis of GDM gave birth to neonates with higher rates of neonatal morbidity, including infection (18.1% in GDM vs 0% in those without DM, P < 0.05) and hyperbilirubinemia (27.2% in GDM vs 3.6% without DM, P < 0.05).51 When they examined differences in inflammation between pregnant persons with GDM or without, they found significantly higher levels of inflammatory cytokines IL-6 and IL-8 in the gestating parent and cord blood (P <0.05).51 However, limitations of their study included a small sample size (~50 pregnant patients), lack of consistency in defining neonatal infection, and exclusion of patients with chorioamnionitis and/or preterm rupture of membranes. While most studies focus on infection in full term neonates, Boghossian et al. studied 10,781 pregnancies complicated by extreme preterm birth (22-28 weeks) to examine risks of mortality, in-hospital morbidities, and neurodevelopmental outcomes at 18-22 months in preterm infants of pregnant parents with insulin use before pregnancy, with insulin initiation during pregnancy, and without DM.52 They describe higher risk of necrotizing enterocolitis (aRR 1.55, CI:1.17-2.05) and late-onset sepsis (aRR 1.26 CI: 1.07-1.48) in preterm deliveries complicated by insulin use by the gestating parent before pregnancy compared to infants born to those without DM.52 The authors, however, found no statistically significant differences in risk for early-onset neonatal sepsis (EONS) between the groups.52 Similar to the Boghossian study, Grandi et al. conducted a retrospective cohort study using data from the NEOCOSUR South American Network that accumulates data on all live-born very low birthweight infants in South American countries.53 This study evaluated neonatal outcomes of infants born to 304 pregnant persons with pre-existing DM. Compared to 10,563 pregnant persons without DM, the diabetic cohort tended to be older (32.6 ±6.5 vs. 27.4 ± 7.3, P = 0.008), had higher rates of hypertension (46.1% vs. 26.7%, P < 0.001), and was more likely to undergo a non-vaginal delivery (83.5% vs 68.8%, P <0.001).53 Very low birthweight infants born to patients with preexisting DM had lower rates of in-hospital death (18.9% vs. 25.3%, P = 0.007) and early onset sepsis (1.5% vs 3.6%, P = 0.035) but had higher rates of necrotizing enterocolitis (15.3% vs. 11.0%, P = 0.013).53 Knight et al. conducted a retrospective cohort study examining perinatal outcomes of patients with T2DM compared to non-diabetic patients that were matched by pre-pregnancy BMI.23 In their 213 matched pairs of pregnant patients, the authors found that the T2DM cohort was older (37.7 ± 6.1 vs. 28.3 ± 5.6, P < 0.001), had higher rates of preeclampsia (14.6% vs. 5.2%, P = 0.001), and cesarean delivery (56.8% vs 38.5%, P < 0.001) compared to non-diabetic controls.23 Neonates born to pregnant persons with T2DM had higher rates of preterm delivery (23.9% vs. 9.9%, P <0.001), hyperbilirubinemia (30% vs 13%, P = <0.001), and sepsis (4.2% vs 0, P = 0.002).23
As with vaginal colonization, increased weight and obesity in the absence of a diagnosis of DM may impact neonatal infectious outcomes. Rastogi et al., performed a secondary data analysis using maternal and neonatal outcomes collected in the Consortium on Safe Labor study database.43 Of the 109,488 women in this study sample, 17.7% had early pregnancy BMIs >30. The authors found that rates of DM and GDM, hypertension, and preeclampsia increased with increasing BMI.43 In terms of neonatal outcomes, the authors found that rates of neonatal sepsis, but not necrotizing enterocolitis, increased with increasing BMI. When the authors examined obesity associated morbidities, both preexisting DM and GDM increased risk of adverse neonatal outcomes 1.34-3.07 fold.43 In a nationwide, population-based retrospective cohort study of almost 2 million live births in the U.S., Villamor et al. found that as weight and obesity increased in the gestating parent, term infants had an increased risk of EONS from any bacteria.54 They describe 866 cases of GBS EONS and found that risk of GBS EONS increased with higher BMIs (adjusted hazard ratios for BMI 30-34.0: 1.71, CI: 1.35-2.16; BMI 35-39.9: 2.41, CI:1.70-3.41; and BMI >40: 2.56. CI: 1.49-4.38).54 They found similar trends of increasing BMI in EONS cases with other pathogens including Staphylococcus aureus (350 cases), Escherichia coli (114 cases), and other staphylococci (916 cases).54 Similarly, Hakansson and Kallen found an increased risk of GBS EONS in those with higher BMI.55 They conducted a retrospective cohort of Swedish patients to examine 136 cases GBS EONS and found an 80% increased risk for GBS EONS (OR 1.8, CI: 1.1-3.0) in infants born to obese subjects when controlling for maternal age, gestational age, and GDM.55
Few studies specifically examine rates of GBS neonatal infection in the context of DM exposure in utero. Evidence suggests an increased risk of neonatal GBS infection if DM is present in the gestating parent, but other factors that often intersect with DM such as obesity may also play a significant role in neonatal infection outcomes.54 Increased biomarkers including cytokines and altered innate immune cells as reported in Li et al., may support the fact that pregnant persons with DM or other metabolic syndromes including obesity could have underlying changes in inflammation that could predispose to adverse pregnancy outcomes.51, 56 The degree of risk for neonatal infection may be different in infants born preterm rather than at term.52, 53 More studies of neonatal outcomes from pregnancies complicated by DM are needed, especially now that universal screening and intrapartum antibiotics are routine in many high-income countries.
3.3. Urinary tract infection (UTI) in non-pregnant and pregnant adults:
In addition to infection of the reproductive tract, GBS also colonizes and infects the urinary tract in pregnant and non-pregnant adults. Disease manifestations range from asymptomatic bacteriuria to pyelonephritis, urethritis, and urosepsis.57 Incidence of urinary infection due to GBS continues to rise.58 GBS now accounts for up to 2% of UTIs, but 4.5% of UTIs in patients with T2DM.58 Data regarding rates of GBS urinary infection in non-pregnant adults is sparse, though some studies from as early as the 1970’s identified the relationship between DM and GBS urinary infection.59-61 Persson et al. examined GBS positive urine cultures from men and non-pregnant women in Sweden from 1982-1984.60 After reviewing over 67,000 urine specimens, the authors identified 240 GBS cultures from 163 patients, 19 males and 144 females. When they looked at 128 matched controls with patients that had a urine culture positive for E.coli, the authors found a non-statistically significant higher incidence of urinary tract abnormalities and DM (15 in GBS group vs 8 in E.coli group) in those patients with a urine culture positive for GBS.60 Girgitzova et al. took culture samples from urine in parallel with feces and ureteral or vaginal swabs.59 They found that GBS isolates from feces had similar antimicrobial sensitivity profiles to that of the urine and genital isolates, reinforcing that the GI tract is often the reservoir for GBS within the human host.59 A multicenter study by Vigliarolo et al. evaluated incidence of UTIs in adult patients from Argentina.58 They found GBS UTIs in non-pregnant adults were often associated with underlying diseases, of which DM was most common.58 It should be noted that enhanced risk of asymptomatic bacteriuria and urinary tract infections are not limited to GBS. Several groups have noted increased incidence of UTIs in diabetic patients with E. coli and other urogenital pathogens.62, 63
During pregnancy, bacteriuria and UTIs are particularly problematic as pregnant persons have a higher risk for progression to pyelonephritis; interestingly, antibiotic treatment of these infections decreases risk for preterm delivery and low birthweight infants.64 GBS bacteriuria with or without symptoms during pregnancy is thought to represent heavy genitourinary colonization.57 This concept relating GBS bacteriuria to genitourinary colonization was reinforced by Anderson et al., where the authors followed pregnant patients found to have GBS bacteriuria early in pregnancy (prior to 20 weeks of gestation).65 Those that were not treated for GBS bacteriuria had an adjusted odds ratio of 7.2 for development of chorioamnionitis.65 While this study does not present data from late pregnancy screening, they do note that the grade of histologic chorioamnionitis from this study correlated with colony count of GBS bacteriuria early in the pregnancy.65
DM increases risk for GBS bacteriuria during pregnancy. Wood and Dillon provided early evidence for increased risk of GBS UTIs during pregnancy. They evaluated 439 pregnant individuals and found that individuals affected by DM were more likely to have a UTI with GBS or another pathogen (13%) compared to their counterparts (8%).66 When they evaluated rates of positive GBS cultures (regardless of colony forming unit threshold), 47% of diabetic patients had a positive GBS culture compared to just 22% of nondiabetic patients. Alvarez et al. completed a retrospective case control study of 150 pregnant persons with pregestational DM and 294 nondiabetic controls.67 Rates of asymptomatic GBS bacteriuria in patients with DM was 24% compared to 10.9% in control patients (OR 2.59, CI: 1.53-4.37).67 Additionally, diabetic subjects with asymptomatic bacteriuria had higher hemoglobin A1c values compared to diabetic patients without bacteriuria, suggesting that the degree of glycemic control may influence risk of GBS bacteriuria.67 Further investigating these findings, Kessous et al. completed a retrospective cohort study investigating correlations between urinary GBS cultures and adverse pregnancy outcomes in patients with positive urine cultures, positive vaginal cultures, or negative GBS vaginal cultures.68 Of 218,402 deliveries, 2270 (1.04%) had a positive urine culture. Rates of preterm birth, low birth weight, intrapartum fever and chorioamnionitis were significantly higher in patients with a positive urine culture. Rates of DM were higher in those with a positive GBS urine culture (8.2%) than those with a positive vaginal culture (5.7%) or no positive GBS culture (6.3%).
4. Molecular mechanisms of GBS pathogenesis in the genitourinary tracts of diabetic patients.
To better understand mechanisms behind the increased risk of GBS colonization or urogenital infection in patients with DM, we reviewed studies investigating the role of DM or elevated glucose environments on immune responses within the genitourinary tract or those that examined changes in GBS pathogenesis.
4.1. DM alters immune cell function within the reproductive tract:
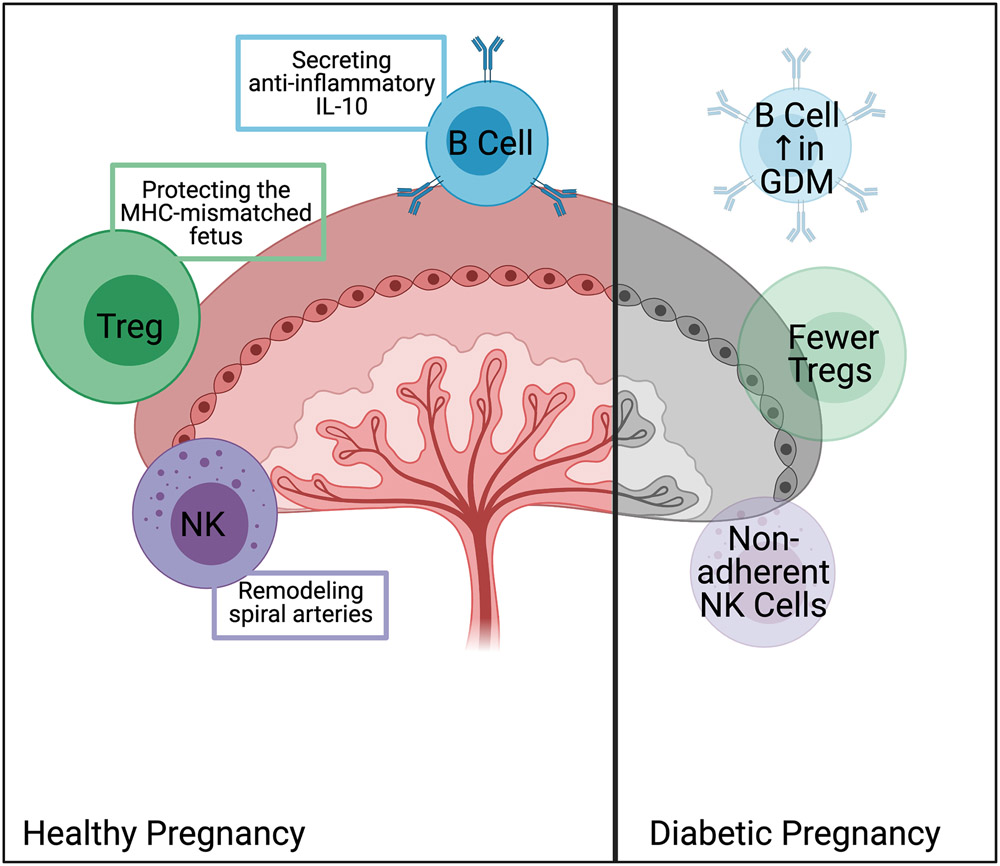
As outlined below, DM impacts adaptive and innate immunity, though the extent to which such changes alter perinatal GBS infection risk remains understudied. During gestation, the immune system must balance protecting the developing fetus from infection with suppressing immune responses to MHC-mismatched tissue.69, 70 Mouse and human studies show the necessity of regulatory T lymphocytes (Tregs), which dampen immune activity.70 In mice, increased numbers of Tregs (CD4+CD25+ cells) are found in blood, uterine-draining lymph nodes, spleen, and thymus two days after mating.70 Depletion of Tregs with anti-CD25 antibodies before mating leads to rejection of allogenic fetuses.71 In pregnant people, increased numbers of CD4+CD25+ Tregs are present in the decidua compared to peripheral blood;71 furthermore, a lower percentage of CD4+CD25+ cells are found in the decidua from people with recurrent miscarriages, indicating a similar role for Tregs in protection of the developing fetus.72 Immune cells contribute to initiation of labor.73 Cells isolated from decidua of patients who delivered via spontaneous vaginal delivery showed an increase in CD16+CD56low NK cells and CD19+ B cells, and a decrease in CD4+CD25+ Tregs compared to patients delivered via cesarean section.74 These same CD19+ B cells may also play a role in supporting fetal development, where IL-10 secreting B cells balance inflammatory cytokines like TNF-α.75 Induction of IL-10 in B cells may relate to changes in circulating factors reflective of pregnancy as B cells cultured with serum from pregnant people produce more IL-10 than those cultured with serum from non-pregnant individuals.76 Studies in mouse pregnancy show that healthy mice have an increase in IL-10 secreting B cells that is not seen in abortion-prone mice; transfer of these IL-10 secreting B cells increases Tregs and partially rescues fetal loss.77 B cells may also play a role in preventing fetal loss following inflammatory challenges such as infection.75 Treatment with LPS, an endotoxin found in the outer membrane of gram-negative bacteria, induces fetal loss in healthy mice, but IL-10 deficient mice experience similar levels of fetal loss when treated with a ten-fold lower dose of LPS.78 Transfer of IL-10 secreting B cells to mice treated with LPS increases Tregs and decreases fetal loss.75 These studies indicate a careful curation of the immune landscape to support developing fetuses, encourage parturition, and balance immune responses to infection by coordination across multiple immune cell types and functions.
This delicate balance is disrupted by the presence of an autoimmune disease like T1DM. In T1DM, mistakes in development of adaptive immune cells permit formation of auto-reactive T and B lymphocytes, leading to autoimmune attack of the pancreatic beta cells by autoreactive CD8+ lymphocytes.79 Additionally, circulating B lymphocytes produce autoantibodies reactive to islet proteins.80 Patients with T1DM can become pregnant and have healthy children but require a level of tolerance to foreign DNA that the patients do not have to their own organs. Perhaps unsurprisingly, patients with T1DM have increased CD4+CD25+ Tregs in cord blood compared to pregnant people without DM.74 Peripheral blood of pregnant people with T1DM show decreased NK cells, increased NK T cells, and increased CD3+ T cells.81, 82 CD56+ NK cells adhere to placental endothelium and aid in remodeling of spiral arteries; however, NK cells from pregnant people with both T1DM and T2DM were less able to adhere to the endothelium and traffic into the decidua, which could contribute to increased risk of preeclampsia in pregnant people with DM.83, 84 Additionally, healthy pregnancy is associated with an overall decrease in circulating lymphocytes, whereas pregnant people with T1DM have a stable number of lymphocytes, perhaps reflecting the relative increase of Tregs needed to prevent rejection of the developing fetus (Figure 1).85 Within pregnant patients with T1DM, patients who had a relative increase in number of Tregs also had an increase in C-peptide, perhaps alluding to broader tolerance induced by Treg expansion in pregnancy.86 Even in non-autoimmune DM (GDM or T2DM), alterations in immune cells impact disease outcome, as patients with GDM that were more insulin-resistant were found to have increased B cells; this increase was even predictive of which patients may have more difficult to treat DM.87
Figure 1.
Alterations in immune cells in diabetic pregnancies. All diabetic pregnancies are associated with changes in function and/or number of immune cells in the placenta, cord blood, and peripheral circulation. The disrupted actions of immune cells broadly affect the course of pregnancy, potentially including increasing susceptibility to infection. Image produced with BioRender.com.
Detailed studies to shape understanding of the immune repertoire and response to infectious and other inflammatory challenges during human pregnancy have not been undertaken. However, using the NOD mouse model of T1DM, Hu et al. showed that treatment of NOD pregnant mice with antibiotics decreased incidence of DM in offspring.88 Antibiotic treatment in utero increased the number of tolerogenic antigen-presenting cells in offspring, which tamped down the behavior of islet-reactive CD8+ T cells.88 When splenocytes from mice that received either antibiotics or received placebo in utero were transferred to an immune-deficient NOD mouse, CD8+ T cells from mice that received antibiotics were less diabetogenic. Antibiotic treatment appears to partially reset the immune landscape in NOD mice.88 In this regard, infection of the pregnant person with DM may present additional challenges that a healthy immune system would more readily overcome and makes it paramount to understand the role of infectious challenge in pregnancies with DM.
4.2. Glucose alters GBS growth and promotes biofilm production which may promote colonization.
To cause ascending infections during pregnancy, GBS must circumnavigate host immune responses and competition from commensal organisms to establish a niche in the vagina.89 Conditions that promote dysbiosis of the vaginal microbiota, including bacterial vaginosis, provide opportunities for GBS colonization.90 Recent studies examined the vaginal microbiome of adults with respect to DM. Wang et al. found that the microbiota of pregnant people and neonates were altered in the context of GDM.91 Zhang et al. found that individuals with GDM and dysbiosis of the vaginal flora had higher rates of premature rupture of membranes, premature delivery, and chorioamnionitis.92
Bacteria possess mechanisms, including two-component regulatory systems, to sense and react to environmental conditions.93 This interaction is particularly important for microorganisms like GBS, that live as a commensal in one host organ system but may cause infections in another. Available nutrients within the gastrointestinal tract can be very different from those found in the vagina.94 This difference likely stimulates changes in transcription and translation of genes involved in adhesion, nutrient acquisition, and survival against host immunity.93 The GBS two-component system, CovR/S, is thought to sense changes in available carbohydrates.93 Di Palo et al. evaluated transcriptomic changes of GBS cells cultured in media devoid of sugars or containing 55 mM glucose for 30 minutes. Exposure to high glucose conditions led to altered gene expression impacting carbohydrate metabolism, DNA replication, adhesion proteins, and toxin production.93 John et al. examined transcriptional changes in GBS grown in normal human urine or urine supplemented with 300 mg/dL glucose and observed higher mRNA transcript levels of the CovR/S two-component system, toxins cylE and CAMP factor B, and adhesion proteins.95 How host DM might alter glucose availability in the genitourinary microenvironment and thus promote changes in GBS metabolism and virulence is not well understood. More studies are needed to fully understand this intersection of host metabolic disease and bacterial pathogenesis.
GBS production of biofilm is thought to be important in maintaining colonization despite host immune responses.89 Biofilms are multicellular structures that enhance pathogen survival and provide protection against environmental challenges and host immune responses.96 Environmental factors alter bacterial biofilm production, and changes in vaginal pH, related to altered microbiota, may affect this process.97 In patients with DM, the concentration of glucose within vaginal secretions is not well defined. Rajan et al. used high performance liquid chromatography to investigate the carbohydrate content of vaginal fluid and found that glucose was the major carbohydrate (80-160 mM) component with smaller amounts of mannose (1-2 mM) and glucosamine.98 Whether individuals with DM have elevated levels of glucose in vaginal secretions has not been well studied, but variations in glucose content within the vagina may alter GBS pathogenesis. Several studies show that supplementing glucose into bacterial growth broth augments GBS biofilm production in vitro, but biofilm assembly can vary substantially between clinical isolates and laboratory strains.90, 99-101 Whether environmental glucose content alone stimulates biofilm production is not entirely clear. There are contrasting reports regarding whether increased biofilm effects are related to acidification of bacterial growth media as glucose is metabolized. For example, Ho et al. reported that GBS grown in nutrition-limited M9YE broth (with 0.4% glucose) produced enhanced biofilms at pH 4.5 relative to 7.0.96 Borges et al. examined GBS biofilm growth in simulated vaginal fluid102 that contained 5.0 g/L glucose at pH 4.2, 5.5 or 6.5 and reported increased biofilm production at pH 6.5 than 4.2.90 Differences in in vitro growth conditions, bacterial broth, and GBS strains likely contribute to conflicting reports, but also suggest more studies are needed to understand how environmental nutrients, such as glucose, influence GBS behavior and host colonization.
A couple of studies have examined the influence of DM on GBS urinary tract infection. John et al. utilized an in vitro bladder model, using human urine supplemented with glucose to 1000 mg/dL and bladder epithelial cells, to examine how changes in glucose affected GBS-epithelial interactions.95 GBS cells preincubated with glucose supplemented urine demonstrated significantly more adhesion to bladder cells compared to GBS cultured with urine without glucose. Exposure to 300 mg/dL glucose in urine also enhanced GBS cell resistance to the antimicrobial protein LL-37. The authors also examined a mouse UTI model, and GBS pre-exposed to high glucose urine resulted in higher bacterial burdens in kidneys compared to bacteria cultured in unsupplemented urine.95 Patras et al. examined the role of LL-37, a cathelicidin antimicrobial peptide expressed in the setting of UTIs, on GBS infections in a streptozotocin-induced diabetic mouse model of ascending UTI resulting in hyperglycemia and glucosuria.103 They found that streptozotocin-induced diabetic mice had higher GBS burdens in the bladder than mock-treated control mice. When the authors examined the immune cell populations responding to infection, they found similar numbers of neutrophils and monocytes, but higher populations of mast cells in diabetic mice, suggesting differences in immune responses may also contribute to the increased susceptibility of UTIs experienced by diabetic patients. While these studies begin to highlight some of the changes that may occur in the context of glucosuria, more studies are needed to understand the pathogenesis of GBS UTIs.
5. Conclusions and future directions:
We have provided data regarding evolving epidemiology, immunology, and pathophysiology of GBS urogenital infections including rectovaginal colonization during pregnancy, neonatal infections of infants exposed to DM in utero, and urinary tract infections. As DM becomes a more common condition affecting pregnancy, a better understanding of the influence of this condition on pregnancy and infection-related pregnancy outcomes are needed to guide research and clinical care. Regarding vaginal GBS colonization, studies over the past 3 decades have provided a mixed picture regarding the risk imparted by DM during pregnancy. Several studies suggest that pregnant persons with DM are at increased risk for GBS vaginal colonization and adverse pregnancy outcomes, but factors including baseline differences in colonization by race or regional populations, lack of data regarding the impact of glycemic control, interactions between obstetric comorbidities with DM, and evolving prevalence of DM over time, make it difficult to independently assess the risk for GBS vaginal colonization in the context of DM. Additionally, we found several studies that demonstrated that obesity, a metabolic health condition often closely linked to DM, also increases risk for GBS colonization.43, 49
Similarly, data specifically pertaining to the impact of DM on neonatal GBS infections is sparce. Larger number of studies exist that examine neonatal sepsis from any cause in pregnant parents with DM and/or obesity. Several of these studies found no increase in neonatal sepsis but found increased risk of other neonatal infectious outcomes including necrotizing enterocolitis.36, 52 These findings should be viewed in context of the success of perinatal GBS screening and antibiotic prophylaxis. With these interventions, recent studies have suggested that GBS colonization are associated with a lower odds of chorioamnionitis.11 Additionally, obstetric comorbidities are also associated with an increase in antibiotic use during pregnancy, which may in turn decrease rates of neonatal infections.104
While studies of invasive GBS infection in non-pregnant adults demonstrate increased incidence of infection, many of these studies do not independently investigate the incidence of GBS UTIs. Several studies have examined asymptomatic GBS bacteriuria during pregnancy, which is often considered to be an indicator of heavy rectovaginal colonization.65 Similar to GBS rectovaginal colonization, DM appears to increase risk for GBS bacteriuria and UTIs during pregnancy.67, 68 Alvarez et al. suggested that poor glycemic control was associated with a higher risk for GBS bacteriuria,67 but no other studies investigated the role of glycemic control to modify risk for bacteriuria or UTIs. Even though the rates of GBS UTIs in non-pregnant adults are rising, GBS still accounts for a small percentage of UTIs.58 As the prevalence of DM continues to rise, future studies should continue to monitor rates of GBS UTIs.
How DM increases risk for infections by pathogens such as GBS is not entirely clear. The presupposition is that DM results in altered immune function, increasing risk for infection.26, 27 Sustained hyperglycemia certainly leads to impairment in several aspects of immune function,28, 29 but it remains unclear if other aspects of sustained hyperglycemia such as microbiome alterations also confer risk.92 Additionally, the impact of elevated glucose concentrations on bacterial physiology and virulence at the host-bacterial interface is understudied. Emerging evidence suggests that as GBS moves from host niches such as the GI tract to the vagina, the bacteria encounter different available nutritional components.94 Recent studies have tried to model how changes in available glucose within patients with DM might alter the pathogenesis of GBS.95, 103 While more work is needed to fully understand how host metabolic disease might impact host-pathogen interactions, these studies suggest that both local immune responses103 and GBS virulence regulation95 are affected. These environmental changes may induce other changes in bacterial physiology, leading to upregulation of adhesion proteins and biofilm production, which may promote vaginal colonization.93, 95
In summary, as the incidence of DM and certain other risk factors for GBS invasive disease increase worldwide, we anticipate changing epidemiology with regards to urogenital colonization and infections with GBS. More work is needed to understand how poor glucose control might affect both sides of host-pathogen interactions, integrating alterations in immune cell function and signaling with changes in GBS virulence due to the available microenvironment nutrients. Evolving models of high glucose conditions including diabetic rodent models and advanced cell culture techniques such as organ-on-a-chip models are poised to provide new insights into the molecular mechanisms that underly the changing epidemiology of these infections.
Supplementary Material
Supplemental Figure 1: Flow diagram of search strategy for studies included in this review.
Acknowledgements:
This work has been funded by National Institutes of Health grants 1K08AI151100-01 (to R.S.D), 1F30DK121438-01A1 and T32GM007347 (to K.L.M.), and R01 HD090061 and R01 AI134036 (to D.M.A.).
Footnotes
Conflict of interest statement: The authors of this manuscript have no conflicts of interest to declare.
Ethical Statement: The authors confirm that the ethical policies of the journal, as noted on the journal’s author guidelines page, have been adhered to. No ethical approval was required as this is a review article with no original research data.’
References Cited
- 1.Armistead B, Oler E, Adams Waldorf K, Rajagopal L. The Double Life of Group B Streptococcus: Asymptomatic Colonizer and Potent Pathogen. J Mol Biol. July 26 2019;431(16):2914–2931. doi: 10.1016/j.jmb.2019.01.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dillon HC Jr., Gray E, Pass MA, Gray BM. Anorectal and vaginal carriage of group B streptococci during pregnancy. The Journal of infectious diseases. June 1982;145(6):794–9. doi: 10.1093/infdis/145.6.794 [DOI] [PubMed] [Google Scholar]
- 3.Shabayek S, Spellerberg B. Group B Streptococcal Colonization, Molecular Characteristics, and Epidemiology. Front Microbiol. 2018;9:437. doi: 10.3389/fmicb.2018.00437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Francois Watkins LK, McGee L, Schrag SJ, et al. Epidemiology of Invasive Group B Streptococcal Infections Among Nonpregnant Adults in the United States, 2008-2016. JAMA Intern Med. April 1 2019;179(4):479–488. doi: 10.1001/jamainternmed.2018.7269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Committee Opinion No. 797: Prevention of Group B Streptococcal Early-Onset Disease in Newborns: Correction. Obstetrics and gynecology. April 2020;135(4):978–979. doi: 10.1097/AOG.0000000000003824 [DOI] [PubMed] [Google Scholar]
- 6.Kolter J, Henneke P. Codevelopment of Microbiota and Innate Immunity and the Risk for Group B Streptococcal Disease. Frontiers in immunology. 2017;8:1497. doi: 10.3389/fimmu.2017.01497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Russell NJ, Seale AC, O'Driscoll M, et al. Maternal Colonization With Group B Streptococcus and Serotype Distribution Worldwide: Systematic Review and Meta-analyses. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. November 6 2017;65(suppl_2):S100–S111. doi: 10.1093/cid/cix658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kohli-Lynch M, Russell NJ, Seale AC, et al. Neurodevelopmental Impairment in Children After Group B Streptococcal Disease Worldwide: Systematic Review and Meta-analyses. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. November 6 2017;65(suppl_2):S190–S199. doi: 10.1093/cid/cix663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saigal S, Doyle LW. An overview of mortality and sequelae of preterm birth from infancy to adulthood. Lancet. January 19 2008;371(9608):261–9. doi: 10.1016/S0140-6736(08)60136-1 [DOI] [PubMed] [Google Scholar]
- 10.Prevention of Group B Streptococcal Early-Onset Disease in Newborns: ACOG Committee Opinion Summary, Number 797. Obstetrics and gynecology. February 2020;135(2):489–492. doi: 10.1097/AOG.0000000000003669 [DOI] [PubMed] [Google Scholar]
- 11.Venkatesh KK, Vladutiu CJ, Glover AV, et al. Is Group B Streptococcus Colonization Associated with Maternal Peripartum Infection in an Era of Routine Prophylaxis? Am J Perinatol. May 23 2020;doi: 10.1055/s-0040-1709666 [DOI] [PubMed] [Google Scholar]
- 12.Skoff TH, Farley MM, Petit S, et al. Increasing burden of invasive group B streptococcal disease in nonpregnant adults, 1990–2007. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. July 1 2009;49(1):85–92. doi: 10.1086/599369 [DOI] [PubMed] [Google Scholar]
- 13.Graux E, Hites M, Martiny D, et al. Invasive group B Streptococcus among non-pregnant adults in Brussels-Capital Region, 2005-2019. Eur J Clin Microbiol Infect Dis. March 2021;40(3):515–523. doi: 10.1007/s10096-020-04041-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lin X, Xu Y, Pan X, et al. Global, regional, and national burden and trend of diabetes in 195 countries and territories: an analysis from 1990 to 2025. Sci Rep. September 8 2020;10(1):14790. doi: 10.1038/s41598-020-71908-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cho NH, Shaw JE, Karuranga S, et al. IDF Diabetes Atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract. April 2018;138:271–281. doi: 10.1016/j.diabres.2018.02.023 [DOI] [PubMed] [Google Scholar]
- 16.Mobasseri M, Shirmohammadi M, Amiri T, Vahed N, Hosseini Fard H, Ghojazadeh M. Prevalence and incidence of type 1 diabetes in the world: a systematic review and meta-analysis. Health Promot Perspect. 2020;10(2):98–115. doi: 10.34172/hpp.2020.18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Behboudi-Gandevani S, Amiri M, Bidhendi Yarandi R, Ramezani Tehrani F. The impact of diagnostic criteria for gestational diabetes on its prevalence: a systematic review and meta-analysis. Diabetol Metab Syndr. 2019;11:11. doi: 10.1186/s13098-019-0406-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McElwain CJ, McCarthy FP, McCarthy CM. Gestational Diabetes Mellitus and Maternal Immune Dysregulation: What We Know So Far. Int J Mol Sci. April 20 2021;22(8)doi: 10.3390/ijms22084261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ferrara A Increasing prevalence of gestational diabetes mellitus: a public health perspective. Diabetes Care. July 2007;30 Suppl 2:S141–6. doi: 10.2337/dc07-s206 [DOI] [PubMed] [Google Scholar]
- 20.Jenner ZB, O'Neil Dudley AE, Mendez-Figueroa H, Ellis VS, Chen HY, Chauhan SP. Morbidity Associated with Fetal Macrosomia among Women with Diabetes Mellitus. Am J Perinatol. April 2018;35(5):515–520. doi: 10.1055/s-0037-1608811 [DOI] [PubMed] [Google Scholar]
- 21.Lopez-de-Andres A, Perez-Farinos N, Hernandez-Barrera V, et al. A Population-Based Study of Diabetes During Pregnancy in Spain (2009-2015): Trends in Incidence, Obstetric Interventions, and Pregnancy Outcomes. J Clin Med. February 21 2020;9(2)doi: 10.3390/jcm9020582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Battarbee AN, Venkatesh KK, Aliaga S, Boggess KA. The association of pregestational and gestational diabetes with severe neonatal morbidity and mortality. Journal of perinatology : official journal of the California Perinatal Association. February 2020;40(2):232–239. doi: 10.1038/s41372-019-0516-5 [DOI] [PubMed] [Google Scholar]
- 23.Knight KM, Pressman EK, Hackney DN, Thornburg LL. Perinatal outcomes in type 2 diabetic patients compared with non-diabetic patients matched by body mass index. The journal of maternal-fetal & neonatal medicine : the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet. June 2012;25(6):611–5. doi: 10.3109/14767058.2011.587059 [DOI] [PubMed] [Google Scholar]
- 24.Ornoy A, Becker M, Weinstein-Fudim L, Ergaz Z. Diabetes during Pregnancy: A Maternal Disease Complicating the Course of Pregnancy with Long-Term Deleterious Effects on the Offspring. A Clinical Review. Int J Mol Sci. March 15 2021;22(6)doi: 10.3390/ijms22062965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Erener S. Diabetes, infection risk and COVID-19. Mol Metab. September 2020;39:101044. doi: 10.1016/j.molmet.2020.101044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chastain CA, Klopfenstein N, Serezani CH, Aronoff DM. A Clinical Review of Diabetic Foot Infections. Clin Podiatr Med Surg. July 2019;36(3):381–395. doi: 10.1016/j.cpm.2019.02.004 [DOI] [PubMed] [Google Scholar]
- 27.Berbudi A, Rahmadika N, Tjahjadi AI, Ruslami R. Type 2 Diabetes and its Impact on the Immune System. Curr Diabetes Rev. 2020;16(5):442–449. doi: 10.2174/1573399815666191024085838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Delamaire M, Maugendre D, Moreno M, Le Goff MC, Allannic H, Genetet B. Impaired leucocyte functions in diabetic patients. Diabet Med. January 1997;14(1):29–34. doi: [DOI] [PubMed] [Google Scholar]
- 29.Turina M, Fry DE, Polk HC Jr. Acute hyperglycemia and the innate immune system: clinical, cellular, and molecular aspects. Crit Care Med. July 2005;33(7):1624–33. doi: 10.1097/01.ccm.0000170106.61978.d8 [DOI] [PubMed] [Google Scholar]
- 30.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. March 29 2021;372:n71. doi: 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Benitz WE, Gould JB, Druzin ML. Risk factors for early-onset group B streptococcal sepsis: estimation of odds ratios by critical literature review. Pediatrics. June 1999;103(6):e77. [DOI] [PubMed] [Google Scholar]
- 32.ACOG. Prevention of Group B Streptococcal Early-Onset Disease in Newborns: ACOG Committee Opinion, Number 782. Obstetrics and gynecology. July 2019;134(1):e19–e40. doi: 10.1097/AOG.0000000000003334 [DOI] [PubMed] [Google Scholar]
- 33.ACOG committee opinion. Prevention of early-onset group B streptococcal disease in newborns. Number 173--June 1996. Committee on Obstetric Practice. American College of Obstetrics and Gynecologists. Int J Gynaecol Obstet. August 1996;54(2):197–205. [PubMed] [Google Scholar]
- 34.Nanduri SA, Petit S, Smelser C, et al. Epidemiology of Invasive Early-Onset and Late-Onset Group B Streptococcal Disease in the United States, 2006 to 2015: Multistate Laboratory and Population-Based Surveillance. JAMA Pediatr. March 1 2019;173(3):224–233. doi: 10.1001/jamapediatrics.2018.4826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.ACOG Committee Opinion No. 485: Prevention of early-onset group B streptococcal disease in newborns. Obstetrics and gynecology. April 2011;117(4):1019–1027. doi: 10.1097/AOG.0b013e318219229b [DOI] [PubMed] [Google Scholar]
- 36.Edwards JM, Watson N, Focht C, et al. Group B Streptococcus (GBS) Colonization and Disease among Pregnant Women: A Historical Cohort Study. Infect Dis Obstet Gynecol. 2019;2019:5430493. doi: 10.1155/2019/5430493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ji Y, Zhao C, Ma XX, Peppelenbosch MP, Ma Z, Pan Q. Outcome of a screening program for the prevention of neonatal early-onset group B Streptococcus infection: a population-based cohort study in Inner Mongolia, China. J Med Microbiol. May 2019;68(5):803–811. doi: 10.1099/jmm.0.000976 [DOI] [PubMed] [Google Scholar]
- 38.McCloskey K, Vuillermin P, Carlin JB, et al. Perinatal microbial exposure may influence aortic intima-media thickness in early infancy. Int J Epidemiol. February 1 2017;46(1):209–218. doi: 10.1093/ije/dyw042 [DOI] [PubMed] [Google Scholar]
- 39.Obata-Yasuoka M, Hamada H, Yoshikawa H. Impaired glucose tolerance during pregnancy: possible risk factor for vaginal/anorectal colonization by Group B Streptococcus. J Obstet Gynaecol Res. September 2012;38(9):1233. doi: 10.1111/j.1447-0756.2012.01885.x [DOI] [PubMed] [Google Scholar]
- 40.Piper JM, Georgiou S, Xenakis EM, Langer O. Group B streptococcus infection rate unchanged by gestational diabetes. Obstetrics and gynecology. February 1999;93(2):292–6. doi: 10.1016/s0029-7844(98)00405-0 [DOI] [PubMed] [Google Scholar]
- 41.Siqueira F, Ferreira EM, de Matos Calderon I, Dias A. Prevalence of colonisation by group B streptococcus in pregnant patients in Taguatinga, Federal District, Brazil: a cross-sectional study. Arch Gynecol Obstet. March 2019;299(3):703–711. doi: 10.1007/s00404-019-05040-z [DOI] [PubMed] [Google Scholar]
- 42.Lukic A, Napoli A, Santino I, et al. Cervicovaginal bacteria and fungi in pregnant diabetic and non-diabetic women: a multicenter observational cohort study. Eur Rev Med Pharmacol Sci. May 2017;21(10):2303–2315. [PubMed] [Google Scholar]
- 43.Rastogi S, Rojas M, Rastogi D, Haberman S. Neonatal morbidities among full-term infants born to obese mothers. The journal of maternal-fetal & neonatal medicine : the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet. May 2015;28(7):829–35. doi: 10.3109/14767058.2014.935324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gopal Rao G, Hiles S, Bassett P, Lamagni T. Differential rates of group B streptococcus (GBS) colonisation in pregnant women in a racially diverse area of London, UK: a cross-sectional study. BJOG. October 2019;126(11):1347–1353. doi: 10.1111/1471-0528.15648 [DOI] [PubMed] [Google Scholar]
- 45.Melchor I, Burgos J, Del Campo A, Aiartzaguena A, Gutierrez J, Melchor JC. Effect of maternal obesity on pregnancy outcomes in women delivering singleton babies: a historical cohort study. J Perinat Med. August 27 2019;47(6):625–630. doi: 10.1515/jpm-2019-0103 [DOI] [PubMed] [Google Scholar]
- 46.Kleweis SM, Cahill AG, Odibo AO, Tuuli MG. Maternal Obesity and Rectovaginal Group B Streptococcus Colonization at Term. Infect Dis Obstet Gynecol. 2015;2015:586767. doi: 10.1155/2015/586767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hunt KJ, Schuller KL. The increasing prevalence of diabetes in pregnancy. Obstet Gynecol Clin North Am. June 2007;34(2):173–99, vii. doi: 10.1016/j.ogc.2007.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Haimbodi EL, Mukesi M, Moyo SR. Prevalence and molecular characterization of group B streptococcus in pregnant women from hospitals in Ohangwena and Oshikoto regions of Namibia. BMC Microbiol. August 5 2021;21(1):224. doi: 10.1186/s12866-021-02283-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Venkatesh KK, Vladutiu CJ, Strauss RA, et al. Association Between Maternal Obesity and Group B Streptococcus Colonization in a National U.S. Cohort. J Womens Health (Larchmt). December 2020;29(12):1507–1512. doi: 10.1089/jwh.2019.8139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Persson M, Shah PS, Rusconi F, et al. Association of Maternal Diabetes With Neonatal Outcomes of Very Preterm and Very Low-Birth-Weight Infants: An International Cohort Study. JAMA Pediatr. September 1 2018;172(9):867–875. doi: 10.1001/jamapediatrics.2018.1811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li YX, Long DL, Liu J, et al. Gestational diabetes mellitus in women increased the risk of neonatal infection via inflammation and autophagy in the placenta. Medicine (Baltimore). October 2 2020;99(40):e22152. doi: 10.1097/MD.0000000000022152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Boghossian NS, Hansen NI, Bell EF, et al. Outcomes of Extremely Preterm Infants Born to Insulin-Dependent Diabetic Mothers. Pediatrics. June 2016;137(6)doi: 10.1542/peds.2015-3424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Grandi C, Tapia JL, Cardoso VC. Impact of maternal diabetes mellitus on mortality and morbidity of very low birth weight infants: a multicenter Latin America study. J Pediatr (Rio J). May-Jun 2015;91(3):234–41. doi: 10.1016/j.jped.2014.08.007 [DOI] [PubMed] [Google Scholar]
- 54.Villamor E, Norman M, Johansson S, Cnattingius S. Maternal Obesity and Risk of Early-onset Neonatal Bacterial Sepsis: Nationwide Cohort and Sibling-controlled Studies. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. June 17 2020;doi: 10.1093/cid/ciaa783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hakansson S, Kallen K. High maternal body mass index increases the risk of neonatal early onset group B streptococcal disease. Acta Paediatr. October 2008;97(10):1386–9. doi: 10.1111/j.1651-2227.2008.00940.x [DOI] [PubMed] [Google Scholar]
- 56.Catalano PM, Shankar K. Obesity and pregnancy: mechanisms of short term and long term adverse consequences for mother and child. BMJ. February 8 2017;356:j1. doi: 10.1136/bmj.j1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ulett KB, Benjamin WH Jr., Zhuo F, et al. Diversity of group B streptococcus serotypes causing urinary tract infection in adults. Journal of clinical microbiology. July 2009;47(7):2055–60. doi: 10.1128/JCM.00154-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vigliarolo L, Arias B, Suarez M, et al. Argentinian multicenter study on urinary tract infections due to Streptococcus agalactiae in adult patients. J Infect Dev Ctries. January 31 2019;13(1):77–82. doi: 10.3855/jidc.10503 [DOI] [PubMed] [Google Scholar]
- 59.Girgitzova B, Minkov N, Zozikov B. Streptococcus agalactiae as a urinary tract pathogen in males and non-pregnant females. Int Urol Nephrol. 1991;23(4):365–9. doi: 10.1007/BF02549609 [DOI] [PubMed] [Google Scholar]
- 60.Persson KM, Grabe M, Kristiansen P, Forsgren A. Significance of group B streptococci in urine cultures from males and non-pregnant females. Scand J Infect Dis. 1988;20(1):47–53. doi: 10.3109/00365548809117216 [DOI] [PubMed] [Google Scholar]
- 61.Mhalu FS. Streptococcus agalactiae in urinary tract infections. Postgrad Med J. April 1977;53(618):216–8. doi: 10.1136/pgmj.53.618.216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Turan H, Serefhanoglu K, Torun AN, et al. Frequency, risk factors, and responsible pathogenic microorganisms of asymptomatic bacteriuria in patients with type 2 diabetes mellitus. Jpn J Infect Dis. May 2008;61(3):236–8. [PubMed] [Google Scholar]
- 63.Joshi N, Caputo GM, Weitekamp MR, Karchmer AW. Infections in patients with diabetes mellitus. The New England journal of medicine. December 16 1999;341(25):1906–12. doi: 10.1056/NEJM199912163412507 [DOI] [PubMed] [Google Scholar]
- 64.Smaill FM, Vazquez JC. Antibiotics for asymptomatic bacteriuria in pregnancy. Cochrane Database Syst Rev. November 25 2019;2019(11)doi: 10.1002/14651858.CD000490.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Anderson BL, Simhan HN, Simons KM, Wiesenfeld HC. Untreated asymptomatic group B streptococcal bacteriuria early in pregnancy and chorioamnionitis at delivery. American journal of obstetrics and gynecology. June 2007;196(6):524 e1–5. doi: 10.1016/j.ajog.2007.01.006 [DOI] [PubMed] [Google Scholar]
- 66.Wood EG, Dillon HC Jr. A prospective study of group B streptococcal bacteriuria in pregnancy. American journal of obstetrics and gynecology. July 1 1981;140(5):515–20. doi: 10.1016/0002-9378(81)90226-x [DOI] [PubMed] [Google Scholar]
- 67.Alvarez JR, Fechner AJ, Williams SF, Ganesh VL, Apuzzio JJ. Asymptomatic bacteriuria in pregestational diabetic pregnancies and the role of group B streptococcus. Am J Perinatol. March 2010;27(3):231–4. doi: 10.1055/s-0029-1239485 [DOI] [PubMed] [Google Scholar]
- 68.Kessous R, Weintraub AY, Sergienko R, et al. Bacteruria with group-B streptococcus: is it a risk factor for adverse pregnancy outcomes? The journal of maternal-fetal & neonatal medicine : the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet. October 2012;25(10):1983–6. doi: 10.3109/14767058.2012.671872 [DOI] [PubMed] [Google Scholar]
- 69.Ben Amara A, Gorvel L, Baulan K, et al. Placental macrophages are impaired in chorioamnionitis, an infectious pathology of the placenta. Journal of immunology. December 1 2013;191(11):5501–14. doi: 10.4049/jimmunol.1300988 [DOI] [PubMed] [Google Scholar]
- 70.Thuere C, Zenclussen ML, Schumacher A, et al. Kinetics of regulatory T cells during murine pregnancy. American journal of reproductive immunology. December 2007;58(6):514–23. doi: 10.1111/j.1600-0897.2007.00538.x [DOI] [PubMed] [Google Scholar]
- 71.Darrasse-Jèze G, Klatzmann D, Charlotte F, Salomon BL, Cohen JL. CD4+CD25+ regulatory/suppressor T cells prevent allogeneic fetus rejection in mice. Immunol Lett. January 15 2006;102(1):106–9. doi: 10.1016/j.imlet.2005.07.002 [DOI] [PubMed] [Google Scholar]
- 72.Yang H, Qiu L, Chen G, Ye Z, Lü C, Lin Q. Proportional change of CD4+CD25+ regulatory T cells in decidua and peripheral blood in unexplained recurrent spontaneous abortion patients. Fertil Steril. March 2008;89(3):656–61. doi: 10.1016/j.fertnstert.2007.03.037 [DOI] [PubMed] [Google Scholar]
- 73.Jongh RD, Jorens P, Student I, Heylen R. The contribution of the immune system to parturition. Mediators Inflamm. 1996;5(3):173–82. doi: 10.1155/S0962935196000233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sindram-Trujillo AP, Scherjon SA, van Hulst-van Miert PP, Kanhai HH, Roelen DL, Claas FH. Comparison of decidual leukocytes following spontaneous vaginal delivery and elective cesarean section in uncomplicated human term pregnancy. J Reprod Immunol. June 2004;62(1-2):125–37. doi: 10.1016/j.jri.2003.11.007 [DOI] [PubMed] [Google Scholar]
- 75.Busse M, Campe KJ, Nowak D, et al. IL-10 producing B cells rescue mouse fetuses from inflammation-driven fetal death and are able to modulate T cell immune responses. Sci Rep. June 27 2019;9(1):9335. doi: 10.1038/s41598-019-45860-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rolle L, Memarzadeh Tehran M, Morell-García A, et al. Cutting edge: IL-10-producing regulatory B cells in early human pregnancy. American journal of reproductive immunology. December 2013;70(6):448–53. doi: 10.1111/aji.12157 [DOI] [PubMed] [Google Scholar]
- 77.Jensen F, Muzzio D, Soldati R, Fest S, Zenclussen AC. Regulatory B10 cells restore pregnancy tolerance in a mouse model. Biol Reprod. October 2013;89(4):90. doi: 10.1095/biolreprod.113.110791 [DOI] [PubMed] [Google Scholar]
- 78.Robertson SA, Skinner RJ, Care AS. Essential role for IL-10 in resistance to lipopolysaccharide-induced preterm labor in mice. Journal of immunology. October 1 2006;177(7):4888–96. doi: 10.4049/jimmunol.177.7.4888 [DOI] [PubMed] [Google Scholar]
- 79.Santamaria P, Nakhleh RE, Sutherland DE, Barbosa JJ. Characterization of T lymphocytes infiltrating human pancreas allograft affected by isletitis and recurrent diabetes. Diabetes. January 1992;41(1):53–61. doi: 10.2337/diab.41.1.53 [DOI] [PubMed] [Google Scholar]
- 80.Taplin CE, Barker JM. Autoantibodies in type 1 diabetes. Autoimmunity. February 2008;41(1):11–8. doi: 10.1080/08916930701619169 [DOI] [PubMed] [Google Scholar]
- 81.Friebe-Hoffmann U, Antony L, Kruessel JS, Pawlowski B, Hoffmann TK. Peripheral Immunological Cells in Pregnant Women and their Change during Diabetes. Exp Clin Endocrinol Diabetes. November 2017;125(10):677–683. doi: 10.1055/s-0043-104935 [DOI] [PubMed] [Google Scholar]
- 82.Burke SD, Seaward AV, Ramshaw H, et al. Homing receptor expression is deviated on CD56+ blood lymphocytes during pregnancy in Type 1 diabetic women. PloS one. 2015;10(3):e0119526. doi: 10.1371/journal.pone.0119526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Groen B, Uuldriks GA, de Vos P, Visser JT, Links TP, Faas MM. Impaired trophoblast invasion and increased numbers of immune cells at day 18 of pregnancy in the mesometrial triangle of type 1 diabetic rats. Placenta. February 2015;36(2):142–9. doi: 10.1016/j.placenta.2014.12.004 [DOI] [PubMed] [Google Scholar]
- 84.Seaward AV, Burke SD, Ramshaw H, Smith GN, Croy BA. Circulating CD56+ cells of diabetic women show deviated homing potential for specific tissues during and following pregnancy. Hum Reprod. July 2011;26(7):1675–84. doi: 10.1093/humrep/der114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Groen B, van der Wijk AE, van den Berg PP, et al. Immunological Adaptations to Pregnancy in Women with Type 1 Diabetes. Sci Rep. September 22 2015;5:13618. doi: 10.1038/srep13618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Amouyal C, Klatzmann D, Tibi E, et al. Pregnant type 1 diabetes women with rises in C-peptide display higher levels of regulatory T cells: A pilot study. Diabetes Metab. September 3 2020:101188. doi: 10.1016/j.diabet.2020.04.005 [DOI] [PubMed] [Google Scholar]
- 87.Zhuang Y, Zhang J, Li Y, et al. B Lymphocytes Are Predictors of Insulin Resistance in Women with Gestational Diabetes Mellitus. Endocr Metab Immune Disord Drug Targets. 2019;19(3):358–366. doi: 10.2174/1871530319666190101130300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hu Y, Peng J, Tai N, et al. Maternal Antibiotic Treatment Protects Offspring from Diabetes Development in Nonobese Diabetic Mice by Generation of Tolerogenic APCs. Journal of immunology. November 1 2015;195(9):4176–84. doi: 10.4049/jimmunol.1500884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Patras KA, Nizet V. Group B Streptococcal Maternal Colonization and Neonatal Disease: Molecular Mechanisms and Preventative Approaches. Front Pediatr. 2018;6:27. doi: 10.3389/fped.2018.00027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Borges S, Silva J, Teixeira P. Survival and biofilm formation by Group B streptococci in simulated vaginal fluid at different pHs. Antonie Van Leeuwenhoek. March 2012;101(3):677–82. doi: 10.1007/s10482-011-9666-y [DOI] [PubMed] [Google Scholar]
- 91.Wang J, Zheng J, Shi W, et al. Dysbiosis of maternal and neonatal microbiota associated with gestational diabetes mellitus. Gut. September 2018;67(9):1614–1625. doi: 10.1136/gutjnl-2018-315988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhang X, Liao Q, Wang F, Li D. Association of gestational diabetes mellitus and abnormal vaginal flora with adverse pregnancy outcomes. Medicine (Baltimore). August 2018;97(34):e11891. doi: 10.1097/MD.0000000000011891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Di Palo B, Rippa V, Santi I, et al. Adaptive response of Group B streptococcus to high glucose conditions: new insights on the CovRS regulation network. PloS one. 2013;8(4):e61294. doi: 10.1371/journal.pone.0061294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rose C, Parker A, Jefferson B, Cartmell E. The Characterization of Feces and Urine: A Review of the Literature to Inform Advanced Treatment Technology. Crit Rev Environ Sci Technol. September 02 2015;45(17):1827–1879. doi: 10.1080/10643389.2014.1000761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.John PP, Baker BC, Paudel S, Nassour L, Cagle H, Kulkarni R. Exposure to Moderate Glycosuria Induces Virulence of Group B Streptococcus. The Journal of infectious diseases. March 3 2021;223(5):843–847. doi: 10.1093/infdis/jiaa443 [DOI] [PubMed] [Google Scholar]
- 96.Ho YR, Li CM, Yu CH, et al. The enhancement of biofilm formation in Group B streptococcal isolates at vaginal pH. Med Microbiol Immunol. April 2013;202(2):105–15. doi: 10.1007/s00430-012-0255-0 [DOI] [PubMed] [Google Scholar]
- 97.Rosini R, Margarit I. Biofilm formation by Streptococcus agalactiae: influence of environmental conditions and implicated virulence factors. Frontiers in cellular and infection microbiology. 2015;5:6. doi: 10.3389/fcimb.2015.00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Rajan N, Cao Q, Anderson BE, et al. Roles of glycoproteins and oligosaccharides found in human vaginal fluid in bacterial adherence. Infection and immunity. October 1999;67(10):5027–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ackerman DL, Doster RS, Weitkamp JH, Aronoff D, Gaddy JA, Townsend SD. Human Milk Oligosaccharides Exhibit Antimicrobial and Anti-Biofilm Properties Against Group B Streptococcus. ACS Infect Dis. June 01 2017;doi: 10.1021/acsinfecdis.7b00064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.D'Urzo N, Martinelli M, Pezzicoli A, et al. Acidic pH strongly enhances in vitro biofilm formation by a subset of hypervirulent ST-17 Streptococcus agalactiae strains. Applied and environmental microbiology. April 2014;80(7):2176–85. doi: 10.1128/AEM.03627-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Rinaudo CD, Rosini R, Galeotti CL, et al. Specific involvement of pilus type 2a in biofilm formation in group B Streptococcus. PloS one. February 15 2010;5(2):e9216. doi: 10.1371/journal.pone.0009216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Owen DH, Katz DF. A vaginal fluid simulant. Contraception. February 1999;59(2):91–5. [DOI] [PubMed] [Google Scholar]
- 103.Patras KA, Coady A, Babu P, et al. Host Cathelicidin Exacerbates Group B Streptococcus Urinary Tract Infection. mSphere. April 22 2020;5(2)doi: 10.1128/mSphere.00932-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Jess T, Morgen CS, Harpsoe MC, et al. Antibiotic use during pregnancy and childhood overweight: A population-based nationwide cohort study. Sci Rep. August 8 2019;9(1):11528. doi: 10.1038/s41598-019-48065-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Najmi N, Jehan I, Sikandar R, Zuberi NF. Maternal genital tract colonisation by group-B streptococcus: a hospital based study. J Pak Med Assoc. September 2013;63(9):1103–7. [PubMed] [Google Scholar]
- 106.Ramos E, Gaudier FL, Hearing LR, Del Valle GO, Jenkins S, Briones D. Group B streptococcus colonization in pregnant diabetic women. Obstetrics and gynecology. February 1997;89(2):257–60. doi: 10.1016/s0029-7844(96)00489-9 [DOI] [PubMed] [Google Scholar]
- 107.Bey M, Pastorek JG 2nd, Miller JM Jr. Group B streptococcal colonization in the diabetic gravida patient. Am J Perinatol. Sep-Nov 1992;9(5-6):425–7. doi: 10.1055/s-2007-999280 [DOI] [PubMed] [Google Scholar]
- 108.Matorras R, Garcia-Perea A, Usandizaga JA, Omenaca F. Recto-vaginal colonization and urinary tract infection by group B Streptococcus in the pregnant diabetic patient. Acta Obstet Gynecol Scand. 1988;67(7):617–20. doi: 10.3109/00016348809004274 [DOI] [PubMed] [Google Scholar]
- 109.Raimer K, O'Sullivan MJ. Influence of diabetes on group B Streptococcus colonization in the pregnant patient. J Matern Fetal Med. Mar-Apr 1997;6(2):120–3. doi: [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1: Flow diagram of search strategy for studies included in this review.