Abstract
Purpose
Distant metastasis is the main pattern of treatment failure in nasopharyngeal carcinoma (NPC) in the era of intensity-modulated radiotherapy (IMRT). We aimed to establish and validate a prognostic nomogram to identify patients with a high risk of distant metastasis.
Patients and Methods
A total of 503 patients with nonmetastatic NPC were included in this retrospective study. We established a prognostic nomogram for distant metastasis-free survival (DMFS) based on the Cox proportional hazards model. The predictive discriminative ability and accuracy of the nomogram were assessed with the concordance index (C-index), receiver operating characteristic (ROC) curve, and calibration curve. The nomogram’s clinical utility was also evaluated using decision curve analysis (DCA) and Kaplan–Meier method. The predictive ability of the nomogram was validated in an independent cohort.
Results
The multivariate analysis showed that circulating CD4+ T lymphocytes, lactate dehydrogenase (LDH), serum ferritin (SF), and N stage were independent prognostic factors for DMFS. Then, we constructed the nomogram based on these factors. The C-indexes of the nomogram for distant metastasis were 0.763 (95% CI: 0.685–0.841) and 0.760 (95% CI: 0.643–0.877) in the training cohort and validation cohort, respectively, which was higher than the 8th TNM staging system (0.672 and 0.677). The calibration curve showed that the prediction results of the nomogram were in high agreement with the actual observation. The ROC curve indicated that the nomogram had a better predictive ability than TNM staging. The DCA also demonstrated that the nomogram was clinically beneficial. In addition, the patients were classified into two different risk groups (high-risk, low-risk) by the nomogram.
Conclusion
As a supplement to TNM staging, our nomogram could provide a more effective and accurate prognostic prediction of distant metastasis in NPC patients. It has the potential to guide the individualized treatment of patients to improve their survival.
Keywords: nasopharyngeal carcinoma, nomogram, distant metastasis, CD4+ T lymphocytes, lactate dehydrogenase, prognosis
Introduction
Nasopharyngeal carcinoma (NPC) is a malignant tumor that originates from the mucosal epithelium of the nasopharynx and has a high incidence in Southeast Asian countries.1 Approximately 130,000 new cases per year have been reported, with an incidence of 3.26/100,000 in endemic areas.2,3 Radiotherapy alone is the primary treatment modality for patients with early-stage NPC, and combined radiotherapy and chemotherapy are recommended for locally advanced disease.1 In the era of intensity-modulated radiotherapy (IMRT), locoregional control has improved considerably. But even after the combination of IMRT and induction or adjuvant chemotherapy, about 20% of NPC patients still develop distant metastasis.4 Nowadays distant metastasis is the main cause of treatment failure in NPC patients.4,5 Therefore, it is necessary to identify patients at high risk of distant metastasis by developing reliable prognostic models for early intervention that may prolong their survival.
At present, the tumor–node–metastasis (TNM) staging system is a mainstay for prognosis prediction and for guiding clinician treatment decisions in NPC. However, large differences in clinical outcomes have been observed in patients of the same stage after receiving similar regimens in the clinic.6,7 This demonstrates that the TNM staging system based on anatomical structure is insufficient to effectively predict which patients may develop distant metastasis. The traditional TNM staging system fails to consider the influence of non-anatomical factors which may account for the different survival outcomes of patients in the same stage.8 Although plasma EBV DNA is closely associated with the prognosis of NPC, and it is one of the ideal markers. Considering the lack of uniform standards for the detection of EBV DNA, there are large differences in the results of testing in different regions. Finding risk factors that reflect the biological heterogeneity of tumors is still urgently needed.
Extensive studies have shown that immune escape and inflammation are key in tumor progression and are associated with tumor prognosis.9–12 Combining immune and inflammation-related parameters with the TNM staging system has the potential to create more individualized prognostic models. CD4+ T lymphocytes are key players in the host immune response to tumors.13 Additionally, circulating CD4+ T lymphocytes and the CD4/CD8 ratio have previously been reported to be associated with distant metastasis in NPC, but few studies have further explored this.14 Lactate dehydrogenase (LDH) is an important enzyme in the human body that is widely distributed in the heart, liver, kidney, and skeletal muscle. When tissues are damaged due to inflammation or tumor growth, they release more LDH into the blood.15,16 Because of the rapid proliferation of tumor cells under hypoxic conditions, LDH catalyzes the conversion of pyruvate to lactate, providing energy for the tumor cells.17 Consequently, serum LDH can reflect the degree of tumor cell hypoxia to some extent. Some studies have shown that pretreatment LDH level can be used as one of the serum markers for distant metastasis of NPC.18,19 In addition, serum ferritin (SF) is a major iron-binding protein that reflects whether the body is in a state of iron deficiency or iron overload. It was reported that serum SF and albumin (ALB) were associated with distant metastasis in NPC.20 However, the relationship between SF, ALB levels, and distant metastasis in NPC remains unclear. These serum markers are potential biomarkers for predicting distant metastasis in NPC and are easily accessible in the clinic.
Compared with TNM stage, a nomogram can more accurately predict the probability of patients to develop clinical events by integrating relevant prognostic factors.21 In this study, we aimed to determine the factors that affect distant metastasis and to establish a prognostic nomogram that can identify patients at high risk of distant metastasis and help guide individualized treatment for NPC.
Materials and Methods
Patients
We retrospectively analyzed the data of patients with newly diagnosed, nonmetastatic NPC at Guangxi Medical University Cancer Hospital from 2011 to 2014. The inclusion criteria for our study were as follows: 1) pathologically confirmed, nonmetastatic NPC; 2) receiving IMRT; 3) KPS score ≥ 70 points; 4) adequate clinical data. Exclusion criteria were: 1) severe complications; 2) comorbid malignancies; 3) failure to receive complete radiotherapy; 4) lost to follow-up. We included a total of 503 NPC patients and randomized them at a ratio of 7:3 into a training cohort (n = 352) and an independent validation cohort (n = 151). We restaged all patients according to the 8th edition of the American Joint Committee on Cancer staging manual. The Ethics Committee of Guangxi Medical University Cancer Hospital approved this study and waived the requirement for informed consent. All data information has been anonymized.
Collection of Pretreatment Baseline Data
We collected data on serum parameters measured within 1 week before treatment and clinical characteristics of NPC patients, including gender, age, smoking history, family history, pathological type, clinical stage, radiotherapy dose, chemotherapy regimen, and pretreatment ALB, hemoglobin (HGB), SF, LDH, neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), percentage of CD4+ T lymphocytes, and CD4/CD8 ratio. NLR was calculated as the ratio of absolute counts between peripheral neutrophil and lymphocyte measurements, and PLR was calculated as the ratio of absolute counts between peripheral platelet and lymphocyte measurements.
Treatment Methods
All patients completed IMRT as planned. Patients with stage I disease received radiotherapy alone, patients with stage II disease received radiotherapy alone or concurrent chemoradiotherapy, and patients with stage III–IVa disease received concurrent chemoradiotherapy with or without induction and/or adjuvant chemotherapy. The delineation of target areas and organs at risk (OARs) was performed with reference to the International Commission on Radiation Units and Measurements (ICRU) Reports 50 and 62. Regarding the prescribed radiation doses, the primary gross tumor volume (GTVnx) was 68–75Gy/30–33f, cervical lymph node tumor volume (GTVnd) was 60–72Gy/30–33f, the clinical target volume with high risk (CTV1) was 60–64Gy/30–32f, and the low-risk clinical target volume (CTV2) was 54–58Gy/30–32f. All patients received radiotherapy once a day, 5 times per week. Concurrent chemotherapy was based on cisplatin (100 mg/m2 on days 1 to 3), and carboplatin was administered to some intolerant patients. Concurrent chemotherapy was administered every 3 weeks for 2–3 cycles. Induction chemotherapy or adjuvant chemotherapy was performed with TPF (docetaxel 60 mg/m2, day 1; cisplatin 60 mg/m2, day 1; 5-fluorouracil 600 mg/m2, continuous intravenous infusion for 120 hours), TP (docetaxel 75 mg/m2, day 1; cisplatin 75 mg/m2, day 1), or PF (cisplatin 80 mg/m2, days 1 to 3; 5-fluorouracil 750 mg/m2, continuous intravenous infusion for 120 hours) regimens. Induction chemotherapy or adjuvant chemotherapy was administered every 3 weeks for 2–3 cycles.
Follow-Up
All patients were assessed every 3 months during the first 1–2 years after completion of treatment, every 6 months during the next 3–5 years, and annually thereafter until death. Each evaluation was performed with relevant physical examination, laboratory testing, and imaging examination. The study endpoint was distant metastasis-free survival (DMFS), defined as the time from the date of the patient’s diagnosis to the date the patient developed distant metastasis or the last follow-up. Our follow-up ranged from 3–126 months, with a median follow-up of 77 months. The date of the last follow-up was November 2020.
Statistical Analysis
Statistical analyses were performed using SPSS25 (IBM Corp, Armonk, NY, USA) and R software (Version 4.0.3). First, we assessed the comparability between the training cohort and the validation cohort. Categorical variables were presented as frequencies (proportions), and the chi-square test was used to compare their differences. Continuous variables were presented as medians (first quartile, third quartile) to describe their characteristics, and the Mann–Whitney U-test was used for their comparison. The optimal cut-off values for continuous variables were determined by X-tile software (version 3.6.1, Yale University, USA), and then these continuous variables were transformed into categorical variables according to the cut-off values. In the training cohort, variables with P < 0.05 in the univariate Cox regression analysis were included in the multivariate analysis, and variables with P < 0.05 in the multivariate analysis were considered independent prognostic factors for distant metastasis of NPC. A prognostic nomogram was developed based on the independent prognostic factors screened. We evaluated the performance of the established nomogram in predicting distant metastasis using the concordance index (C-index), receiver operating characteristic (ROC) curve, and calibration curve. The clinical utility of the nomogram was assessed by decision curve analysis (DCA). After the risk score of the nomogram was determined, the optimal cut-off value for the score of patients in the training cohort was obtained by X-tile. Patients were divided into low-risk and high-risk groups according to the cut-off value of the risk score. The survival was calculated by the Kaplan Meier method, and log rank tests were used to compare survival differences between risk groups. In addition, the predictive ability of the nomogram was compared with the TNM staging system, and validated using an external validation cohort.
Results
Patient Characteristics
A total of 503 NPC patients were included in this study. There were 367 males and 136 females with a median age of 46 years (range 12–78 years). The distribution of clinical stages was as follows: 5 patients (1.0%) were in stage I, 117 (23.3%) were in stage II, 239 (47.5%) were in stage III, and 142 (28.2%) were in stage IVa. Distant metastasis occurred in 73 (14.5%) patients. The 5-year DMFS was 86.7% and 85.8% in the training and validation cohorts, respectively. There were no significant differences in baseline characteristics between the two cohorts (Table 1).
Table 1.
Baseline Characteristics of the Patients in Training Cohort and Validation Cohort
| Variables | Number of NPC Patients | P-value | |
|---|---|---|---|
| Training Cohort (n = 352) | Validation Cohort (n = 151) | ||
| Categorical variables | |||
| Gender | |||
| Female | 91(25.9%) | 45(29.8%) | 0.361 |
| Male | 261(74.1%) | 106(70.2%) | |
| Smoking | |||
| No | 228(64.8%) | 104(68.9%) | 0.373 |
| Yes | 124(35.2%) | 47(31.1%) | |
| Family history of NPC | |||
| No | 316(89.8%) | 137(90.7%) | 0.743 |
| Yes | 36(10.2%) | 14(9.3%) | |
| WHO pathological type | |||
| II | 33(9.4%) | 15(9.9%) | 0.845 |
| III | 319(90.6%) | 136(90.1%) | |
| T stage | |||
| T1–2 | 131(37.2%) | 55(36.4%) | 0.866 |
| T3–4 | 221(62.8%) | 96(63.6%) | |
| N stage | |||
| N0–1 | 192(54.5%) | 84(55.6%) | 0.823 |
| N2–3 | 160(45.5%) | 67(44.4%) | |
| Clinical stage | |||
| I–II | 85(24.1%) | 37(24.5%) | 0.932 |
| III–IVa | 267(75.9%) | 114(75.5%) | |
| Induction chemotherapy | |||
| No | 264(75.0%) | 118(78.1%) | 0.449 |
| Yes | 88(25.0%) | 33(21.9%) | |
| Concurrent chemotherapy | |||
| No | 18(5.1%) | 9(6.0%) | 0.699 |
| Yes | 334(94.9%) | 142(94.0%) | |
| Adjuvant chemotherapy | |||
| No | 256(72.7%) | 111(73.5%) | 0.856 |
| Yes | 96(27.3%) | 40(26.5%) | |
| Distant metastasis | |||
| No | 302(85.8%) | 128(84.8%) | 0.764 |
| Yes | 50(14.2%) | 23(15.2%) | |
| Continuous variables | |||
| Age, year | 46(39, 53) | 47(38, 55) | 0.584 |
| CD4+ T cells, % | 33.2(27.7, 40.0) | 34.1(27.6, 39.5) | 0.849 |
| CD4/CD8 ratio | 1.5(1.1, 2.1) | 1.4(1.0, 1.8) | 0.054 |
| NLR | 2.1 (1.6, 2.8) | 2.2(1.7, 2.9) | 0.264 |
| PLR | 138.8(109.4, 174.8) | 142.1(107.5, 187.6) | 0.668 |
| LDH, U/L | 176(156, 202) | 176(157, 203) | 0.945 |
| HGB, g/L | 139(127, 150) | 140(125, 151) | 0.890 |
| ALB, g/L | 44.8(42.1, 47.4) | 44.0(42.0, 46.6) | 0.081 |
| SF, μg/L | 271(154, 423) | 245(139, 361) | 0.128 |
Abbreviations: NLR, neutrophil-to-lymphocyte ratio; PLR, platelet-to-lymphocyte ratio; LDH, lactate dehydrogenase; HGB, hemoglobin; ALB, albumin; SF, serum ferritin.
Independent Prognostic Factors in the Training Cohort
The optimal cut-off values of the continuous variables were obtained by X-tile software. The cut-off values of CD4+ T lymphocytes, CD4/CD8 ratio, NLR, PLR, LDH, HGB, ALB, and SF before treatment were 37%, 1.3, 2.8, 168.8, 174 U/L, 117 g/L, 48.4 g/L, and 292 μg/L, respectively. The univariate analysis showed that T stage, N stage, clinical stage, CD4+ T lymphocytes, CD4/CD8 ratio, LDH, and SF were associated with distant metastasis. The significant factors (P < 0.05) were included in the multivariate Cox regression model. The multivariate analysis showed that N stage, CD4+ T lymphocytes, LDH, and SF were independent prognostic factors for DMFS (Table 2).
Table 2.
Univariate and Multivariable Cox Hazards Analysis of the Training Cohort
| Variables | Univariate | Multivariate | ||
|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | |
| Gender | ||||
| Female | Reference | |||
| Male | 1.410(0.705–2.819) | 0.331 | ||
| Age(years) | ||||
| ≤46 | Reference | |||
| >46 | 1.079(0.620–1.878) | 0.788 | ||
| Smoking | ||||
| No | Reference | |||
| Yes | 1.148(0.649–2.033) | 0.635 | ||
| Family history of NPC | ||||
| No | Reference | |||
| Yes | 0.772(0.278–2.145) | 0.620 | ||
| WHO pathological type | ||||
| II | Reference | |||
| III | 1.146(0.413–3.184) | 0.794 | ||
| T stage | ||||
| T1–2 | Reference | Reference | ||
| T3–4 | 2.990(1.453–6.154) | 0.003 | 1.741(0.716–4.236) | 0.222 |
| N stage | ||||
| N0–1 | Reference | Reference | ||
| N2–3 | 2.689(1.497–4.831) | 0.001 | 1.986(1.037–3.802) | 0.038 |
| Clinical stage | ||||
| I–II | Reference | Reference | ||
| III–IVa | 5.624(1.750–18.071) | 0.004 | 2.509(0.539–11.673) | 0.241 |
| Treatment | ||||
| RT alone | Reference | |||
| CCRT | 0.292(0.040–2.135) | 0.225 | ||
| CCRT+IC/AC | 0.596(0.331–1.073) | 0.085 | ||
| CD4+ T cells (%) | ||||
| ≤37 | Reference | Reference | ||
| >37 | 0.228(0.097–0.534) | 0.001 | 0.299(0.118–0.754) | 0.011 |
| CD4/CD8 ratio | ||||
| ≤1.3 | Reference | Reference | ||
| >1.3 | 0.444(0.253–0.778) | 0.005 | 0.673(0.365–1.240) | 0.204 |
| NLR | ||||
| ≤2.8 | Reference | |||
| >2.8 | 1.744(0.971–3.132) | 0.063 | ||
| PLR | ||||
| ≤168.8 | Reference | |||
| >168.8 | 1.549(0.869–2.761) | 0.138 | ||
| LDH (U/L) | ||||
| ≤174 | Reference | Reference | ||
| >174 | 2.811(1.515–5.215) | 0.001 | 2.407(1.282–4.518) | 0.006 |
| HGB (g/L) | ||||
| ≤117 | Reference | |||
| >117 | 0.997(0.425–2.339) | 0.994 | ||
| ALB (g/L) | ||||
| ≤48.4 | Reference | |||
| >48.4 | 0.609(0.259–1.428) | 0.254 | ||
| SF (μg/L) | ||||
| ≤292 | Reference | Reference | ||
| >292 | 2.260(1.268–4.026) | 0.006 | 2.271(1.270–4.062) | 0.006 |
Abbreviations: RT, radiotherapy; CCRT, concurrent chemotherapy; IC, induction chemotherapy; AC, adjuvant chemotherapy; NLR, neutrophil-to-lymphocyte ratio; PLR, platelet-to-lymphocyte ratio; LDH, lactate dehydrogenase; HGB, hemoglobin; ALB, albumin; SF, serum ferritin.
Establishing a Nomogram for DMFS
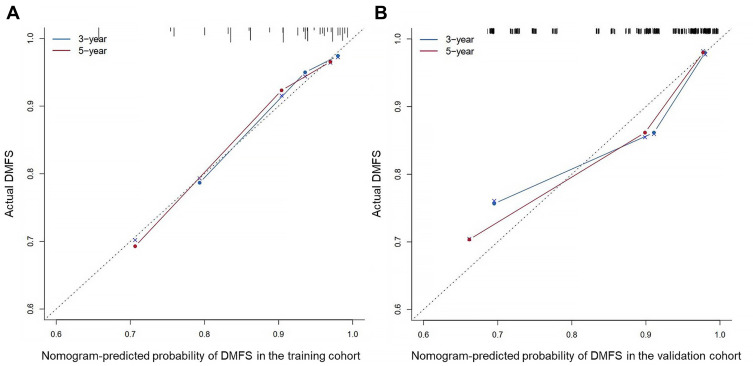
Based on the multivariate analysis of the training cohort, a nomogram for predicting DMFS was established (Figure 1). The value range of each variable was marked on the corresponding line segment. The length of the line segment reflected the contribution of the variable to the outcome event. Each variable had a corresponding score at different value ranges. Adding the scores of all variables obtained a total point. According to the calculated total point, we estimated the corresponding probability of 3-year and 5-year DMFS. The C-index of the nomogram was 0.763 (95% CI: 0.685–0.841) in the training cohort. The model exhibited good accuracy in predicting DMFS in NPC. The calibration curve showed good agreement between the predicted probabilities of the nomogram and the actual observed values of DMFS at 3 and 5 years (Figure 2A).
Figure 1.
Prognostic nomogram for DMFS in the training cohort.
Note: The scores of each variable were added to obtain the total score, and a vertical line was drawn on the total score to obtain the corresponding probability of DMFS at 3 and 5 years.
Abbreviations: LDH, lactate dehydrogenase; SF, serum ferritin; DMFS, distant metastasis-free survival.
Figure 2.
Calibration curves of the nomogram to predict 3- and 5-year DMFS in the training cohort (A) and validation cohort (B).
Validation of the Nomogram Based on the Validation Cohort
To verify the efficacy of the nomogram in predicting distant metastasis, we performed external validation. The nomogram C-index for DMFS was 0.760 (95% CI: 0.643–0.877) in the validation cohort. The calibration curve similarly showed good accuracy between the probability of the nomogram in predicting distant metastasis and the actual observation (Figure 2B).
Comparison of the Predictive Ability of DMFS Between the Nomogram and TNM Staging System
We evaluated the predictive ability of the model using the C-index, ROC curve, and assessed the clinical benefit of the model using DCA. First, the C-index of the nomogram was 0.763 (95% CI: 0.685–0.841), which was higher than the TNM stage (C-index: 0.672, 95% CI: 0.594–0.751) in the training cohort. In the validation cohort, the C-index of the nomogram was 0.760 (95% CI: 0.643–0.877), which was also higher than the TNM stage (C-index: 0.677, 95% CI: 0.560–0.795). A larger C-index indicated a more accurate prognostic value. Second, the ROC curve similarly showed that the nomogram had better predictive ability than TNM staging in the training and validation cohorts (Figure 3). Third, the DCA curve showed that the nomogram model (black dotted line) had a higher net benefit compared with the TNM staging system (red dotted line) (Figure 4). The DCA suggested that the nomogram had better clinical utility. The above results indicated that the nomogram had a higher predictive ability and clinical application value for DMFS.
Figure 3.
ROC curves for the nomogram of DMFS in the training cohort (A) and validation cohort (B).
Figure 4.
The Decision Curves Analysis curve for the nomogram of DMFS in the training cohort (A) and validation cohort (B).
Notes: In the decision curve analysis curve, the horizontal axis represents the threshold value, which is the reference probability of whether a patient receives treatment. The vertical axis represents the net benefit rate after disadvantages are subtracted from the advantages. Under the same threshold probability, a larger net benefit implies that patients can obtain the maximum benefit using the diagnosis model. A larger net benefit in the DCA graph corresponds to a higher value of the diagnosis model.
Performance of the Nomogram in Stratifying Risk of Patients
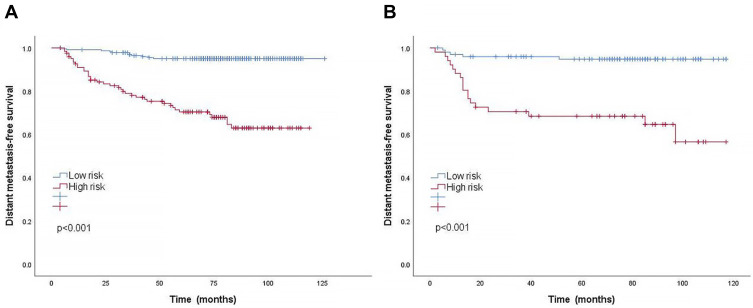
In the training cohort, the cut-off value for the total point calculated by the nomogram was determined by X-tile software. Patients were classified into two risk groups for the prediction of distant metastasis: low-risk group (< 189.9) and high-risk group (≥ 189.9), according to the cut-off values. The 5-year DMFS of the low-risk and high-risk groups in the training cohort were 95.0% and 70.4%, respectively (P < 0.001). Similarly, a significant DMFS difference was also observed in the validation cohort (94.8% vs 68.4%, P < 0.001). Kaplan–Meier curves in both the training and validation cohorts clearly indicated that NPC patients in the high-risk group were more likely to develop distant metastasis (Figure 5). This risk stratification can effectively distinguish NPC patients at a high risk of distant metastasis.
Figure 5.
Kaplan–Meier curves of DMFS according to low-risk or high-risk groups stratified by the nomogram in the training cohort (A) and validation cohort (B).
Discussion
In our study, we analyzed the effects of peripheral blood immune and inflammatory-related indicators on NPC distant metastasis. Univariate and multivariate Cox regression analyses indicated that N stage, CD4+ T lymphocytes, LDH, and SF were independent prognostic factors of DMFS, and we established a nomogram based on these factors. Our nomogram showed satisfactory prediction and calibration abilities based on C-index, ROC curve, and calibration curve, in both the training cohort and external validation cohort. Moreover, the DCA curve indicated that the nomogram had a high clinical benefit. Furthermore, significantly different DMFS rates were found when NPC patients were divided into high-risk and low-risk groups. This will help clinicians to identify NPC patients at high risk of distant metastasis more effectively and select an appropriate treatment.
Currently, radiotherapy is the main treatment for NPC. However, in the IMRT era, even with the improvement of locoregional control, about 20% of NPC patients will still develop distant metastasis after treatment.4 Distant metastasis becomes the major cause of treatment failure in NPC patients.4,5 An accurate estimation of prognosis before treatment, especially predicting the risk of distant metastasis, is essential to improve the clinical management of NPC. However, there are few relevant studies on prognostic models for distant metastasis in nasopharyngeal carcinoma, and effective prognostic models are lacking. Determining how to identify patients at high risk of distant metastasis to provide timely intervention and improve their survival has become an urgent clinical problem. Therefore, we developed a nomogram to predict DMFS in NPC patients. The nomogram we constructed showed better predictive ability than the 8th edition TNM staging system. Our nomogram has the potential to be a good complement to the TNM staging system for predicting prognosis. Numerous studies have shown that T stage is closely associated with locoregional recurrence, whereas N stage is associated with distant metastasis in NPC.4,22–24 A higher N stage is a risk factor for distant metastasis in NPC,23,25 which is consistent with the results of our study.
In addition to anatomical factors, we included indicators reflecting the patients’ immune and inflammatory conditions. To our knowledge, we combined immune and inflammation related indicators for the first time in a prognostic model of distant metastasis in NPC. It is well known that immune escape and inflammation are key in tumor progression. An increasing number of studies have shown that immune cells are involved in the whole process of tumor progression and have a dual function of promoting or inhibiting tumor growth. Inflammatory cells are involved in constituting the tumor microenvironment, which promote the proliferation and metastasis of tumors. The immune environment and inflammation are closely related to tumor prognosis.9–12 The introduction of immune parameters into the TNM staging system will perhaps improve the predictive power of tumor prognosis.26 We found that NPC patients with lower CD4+ T lymphocyte levels before treatment were more likely to develop distant metastasis. CD4+ T lymphocytes are major players in the body’s antitumor response and can exert potent tumoricidal effects by activating NK cells through the production of interleukin II and assisting B cells to produce antibodies.13 The decrease of CD4+ T lymphocytes may contribute to the development of tumor immune escape.10,27,28 A decrease in CD4+ T lymphocytes associated with poor prognosis has also been observed in other malignancies.29,30 A recent study showed that the peripheral T cell receptor β chain (TCRB) can be used to predict distant metastasis of NPC.31 Furthermore, our results showed that high pretreatment LDH levels were a risk factor for the development of distant metastasis in NPC patients. Recently, as an inflammation related serological marker, LDH has been identified as a prognostic factor in several malignancies, including renal cell carcinoma, osteosarcoma, and lung cancer.32–34 Several studies have shown that high LDH levels are associated with poor prognosis in NPC patients and are related to distant metastasis.18,19,35,36 Our study further confirmed that serum LDH could serve as one of the biomarkers for distant metastasis of NPC. Although the prognostic value of LDH has been extensively studied, the molecular mechanisms between LDH and distant metastasis remain unknown. First, it is generally believed to be associated with the glycolysis of cancer cells. There are different metabolic programs in cancer cells and normal cells. During the process of generating energy, cancer cells preferentially utilize the anaerobic pathway of glycolysis despite the existence of oxygen, which is histologically known as the Warburg effect.37 LDH catalyzes the conversion of pyruvate to lactate under hypoxic conditions and plays a key role in anaerobic glycolysis. With the production of large amounts of lactate, the upregulation of LDH levels ensures the efficiency of the activity. Second, lactic acidosis has a strong immunosuppressive effect and may mediate the occurrence of tumor immune escape.38 Moreover, tumor tissues often exhibit marked cellular damage, releasing more intracellular enzymes (including LDH) than normal tissues.16
SF is the main storage form of iron in the human body. Iron is an essential trace element required for cell growth, and dysregulated iron metabolism has been declared one of the metabolic hallmarks of cancer cells.39 Iron metabolism is thought to be involved in tumor progression and is associated with poor prognosis in several malignancies such as pancreatic, hepatocellular, and colorectal cancer.39–42 Studies have shown that cancer cells employ multiple mechanisms to increase the bioavailability of iron to promote tumor growth.43 Excess iron decreases T and B cell numbers and inhibits lymphocyte function as well as macrophage and monocyte tumoricidal effects.39,44,45 Disturbance of immune function may lead to an increased risk of distant metastasis in NPC patients. Our study found a high risk of distant metastasis in patients with high SF levels.
The TNM staging system is the decisive factor of prognosis prediction and risk stratification for treatment decisions in NPC, but its predictive ability for distant metastasis is insufficient.7,8 The individual condition of patients also affects the prognosis of tumors. Combining multiple factors to predict the risk of distant metastasis may be more valuable for the management of NPC patients. As a predictive tool, nomograms have been shown to provide higher predictive accuracy than conventional TNM staging.21,46 Tang et al reported a molecular signature consisting of 13 genes and constructed a nomogram prediction model incorporating gene expression profiles, N stage, gender, serum LDH, and C-reactive protein (CRP), concluding that the nomogram (C-index = 0.748) could well predict distant metastasis of NPC.25 However, the detection of gene expression profiles is expensive and not widely available in the clinic. Additionally, a nomogram (C-index 0.737) constructed by lymph node related features on MRI was reported to predict distant metastasis in NPC patients. But MRI parameters are complex to acquire and are similarly not easy to apply clinically.24 In our study, the nomogram constructed by combining CD4+ T lymphocytes, LDH, SF and N stage showed good predictive performance in both the training cohort (C-index = 0.763) and the validation cohort (C-index = 0.760). Moreover, circulating CD4+ T lymphocytes, serum LDH, and serum SF are readily detectable and inexpensive in the clinic with high reproducibility.
There are several limitations to our study. First, the study was a single center retrospective study and there was inevitably a selection bias. Large prospective cohort studies are needed to validate the independent predictive role of CD4+ T lymphocytes in distant metastasis, and more external cohort validation is needed for the utility of the nomogram. With that said, a retrospective study is still valuable as it has implications for some prospective studies. Second, considering that there is a lack of uniform criteria for the detection of EBV DNA in plasma, we did not include EBV DNA in our study despite its important influence on the prognosis of NPC.
Conclusion
In summary, our study showed that CD4+ T lymphocytes, LDH, SF and N stage were independent predictors of distant metastasis in NPC. The nomogram based on CD4+ T lymphocytes and lactate dehydrogenase was effective in predicting distant metastasis for NPC patients, which can be used as a good supplement to the TNM staging system. Risk stratified management may provide more prognostic information and guide individualized treatment for NPC patients, contributing to their prolonged survival.
Acknowledgments
We thank Guangxi Medical University Cancer Hospital for data support. We are grateful to the valuable comments provided by the reviewers and editors.
Funding Statement
This work was sponsored by Guangxi Key R&D Program (No. GuikeAB18221007), the National Natural Science Foundation Program (No. 81760544), and the Scientific Research & Technical Development Project of Wuming District, Nanning city (No. 20200214).
Ethics Approval
This study was approved by the Ethics Committee of Guangxi Medical University Cancer Hospital (LW2021072), in compliance with the Declaration of Helsinki.
Disclosure
The authors declare that they have no conflicts of interest in this work.
References
- 1.Chen YP, Chan ATC, Le QT, Blanchard P, Sun Y, Ma J. Nasopharyngeal carcinoma. Lancet. 2019;394(10192):64–80. doi: 10.1016/S0140-6736(19)30956-0 [DOI] [PubMed] [Google Scholar]
- 2.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 3.Fu ZT, Guo XL, Zhang SW, et al. [Incidence and mortality of nasopharyngeal carcinoma in China, 2014]. Zhonghua Zhong Liu Za Zhi. 2018;40(8):566–571. Chinese. doi: 10.3760/cma.j.issn.0253-3766.2018.08.002 [DOI] [PubMed] [Google Scholar]
- 4.Mao YP, Tang LL, Chen L, et al. Prognostic factors and failure patterns in non-metastatic nasopharyngeal carcinoma after intensity-modulated radiotherapy. Chin J Cancer. 2016;35(1):103. doi: 10.1186/s40880-016-0167-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tian YM, Liu MZ, Zeng L, et al. Long-term outcome and pattern of failure for patients with nasopharyngeal carcinoma treated with intensity-modulated radiotherapy. Head Neck. 2019;41(5):1246–1252. doi: 10.1002/hed.25545 [DOI] [PubMed] [Google Scholar]
- 6.Huang CL, Guo R, Li JY, et al. Nasopharyngeal carcinoma treated with intensity-modulated radiotherapy: clinical outcomes and patterns of failure among subsets of 8th AJCC stage IVa. Eur Radiol. 2020;30:816–822. doi: 10.1007/s00330-019-06500-5 [DOI] [PubMed] [Google Scholar]
- 7.Wu LR, Zhang XM, Xie XD, Lu Y, Wu JF, He X. Validation of the 8th edition of AJCC/UICC staging system for nasopharyngeal carcinoma: results from a non-endemic cohort with 10-year follow-up. Oral Oncol. 2019;98:141–146. doi: 10.1016/j.oraloncology.2019.09.029 [DOI] [PubMed] [Google Scholar]
- 8.Tang LL, Chen YP, Mao YP, et al. Validation of the 8th edition of the UICC/AJCC staging system for nasopharyngeal carcinoma from endemic areas in the intensity-modulated radiotherapy era. J Natl Compr Canc Netw. 2017;15(7):913–919. doi: 10.6004/jnccn.2017.0121 [DOI] [PubMed] [Google Scholar]
- 9.Strausberg RL. Tumor microenvironments, the immune system and cancer survival. Genome Biol. 2005;6(3):211. doi: 10.1186/gb-2005-6-3-211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Biswas SK. Metabolic reprogramming of immune cells in cancer progression. Immunity. 2015;43(3):435–449. doi: 10.1016/j.immuni.2015.09.001 [DOI] [PubMed] [Google Scholar]
- 11.Clancy T, Pedicini M, Castiglione F, et al. Immunological network signatures of cancer progression and survival. BMC Med Genomics. 2011;4:28. doi: 10.1186/1755-8794-4-28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Candido J, Hagemann T. Cancer-related inflammation. J Clin Immunol. 2013;33(Suppl 1):S79–84. doi: 10.1007/s10875-012-9847-0 [DOI] [PubMed] [Google Scholar]
- 13.Gerloni M, Zanetti M. CD4 T cells in tumor immunity. Springer Semin Immunopathol. 2005;27(1):37–48. doi: 10.1007/s00281-004-0193-z [DOI] [PubMed] [Google Scholar]
- 14.Tao CJ, Chen YY, Jiang F, et al. A prognostic model combining CD4/CD8 ratio and N stage predicts the risk of distant metastasis for patients with nasopharyngeal carcinoma treated by intensity modulated radiotherapy. Oncotarget. 2016;7(29):46653–46661. doi: 10.18632/oncotarget.9695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Drent M, Cobben NA, Henderson RF, Wouters EF, van Dieijen-visser M. Usefulness of lactate dehydrogenase and its isoenzymes as indicators of lung damage or inflammation. Eur Respir J. 1996;9(8):1736–1742. doi: 10.1183/09031936.96.09081736 [DOI] [PubMed] [Google Scholar]
- 16.Certo M, Tsai CH, Pucino V, Ho PC, Mauro C. Lactate modulation of immune responses in inflammatory versus tumour microenvironments. Nat Rev Immunol. 2021;21(3):151–161. doi: 10.1038/s41577-020-0406-2 [DOI] [PubMed] [Google Scholar]
- 17.Biswas S, Lunec J, Bartlett K. Non-glucose metabolism in cancer cells–is it all in the fat? Cancer Metastasis Rev. 2012;31(3–4):689–698. doi: 10.1007/s10555-012-9384-6 [DOI] [PubMed] [Google Scholar]
- 18.Long G, Tang W, Fu X, et al. Pre-treatment serum lactate dehydrogenase predicts distant metastasis and poor survival in nasopharyngeal carcinoma. J Cancer. 2019;10(16):3657–3664. doi: 10.7150/jca.32716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang M, Wei S, Su L, Lv W, Hong J. Prognostic significance of pretreated serum lactate dehydrogenase level in nasopharyngeal carcinoma among Chinese population: a meta-analysis. Medicine. 2016;95(35):e4494. doi: 10.1097/MD.0000000000004494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen X, Long X, Liang Z, et al. Higher N stage and serum ferritin, but lower serum albumin levels are associated with distant metastasis and poor survival in patients with nasopharyngeal carcinoma following intensity-modulated radiotherapy. Oncotarget. 2017;8(42):73177–73186. doi: 10.18632/oncotarget.17418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Balachandran VP, Gonen M, Smith JJ, DeMatteo RP. Nomograms in oncology: more than meets the eye. Lancet Oncol. 2015;16(4):e173–180. doi: 10.1016/S1470-2045(14)71116-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leung TW, Tung SY, Sze WK, et al. Treatment results of 1070 patients with nasopharyngeal carcinoma: an analysis of survival and failure patterns. Head Neck. 2005;27(7):555–565. doi: 10.1002/hed.20189 [DOI] [PubMed] [Google Scholar]
- 23.Sun X, Su S, Chen C, et al. Long-term outcomes of intensity-modulated radiotherapy for 868 patients with nasopharyngeal carcinoma: an analysis of survival and treatment toxicities. Radiother Oncol. 2014;110(3):398–403. doi: 10.1016/j.radonc.2013.10.020 [DOI] [PubMed] [Google Scholar]
- 24.Xie C, Li H, Yan Y, et al. A nomogram for predicting distant metastasis using nodal-related features among patients with nasopharyngeal carcinoma. Front Oncol. 2020;10:616. doi: 10.3389/fonc.2020.00616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tang XR, Li YQ, Liang SB, et al. Development and validation of a gene expression-based signature to predict distant metastasis in locoregionally advanced nasopharyngeal carcinoma: a retrospective, multicentre, cohort study. Lancet Oncol. 2018;19(3):382–393. doi: 10.1016/S1470-2045(18)30080-9 [DOI] [PubMed] [Google Scholar]
- 26.Bruni D, Angell HK, Galon J. The immune contexture and Immunoscore in cancer prognosis and therapeutic efficacy. Nat Rev Cancer. 2020;20(11):662–680. doi: 10.1038/s41568-020-0285-7 [DOI] [PubMed] [Google Scholar]
- 27.Fridman WH, Pagès F, Sautès-Fridman C, Galon J. The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer. 2012;12(4):298–306. doi: 10.1038/nrc3245 [DOI] [PubMed] [Google Scholar]
- 28.Fridman WH, Zitvogel L, Sautès-Fridman C, Kroemer G. The immune contexture in cancer prognosis and treatment. Nat Rev Clin Oncol. 2017;14(12):717–734. doi: 10.1038/nrclinonc.2017.101 [DOI] [PubMed] [Google Scholar]
- 29.Trédan O, Manuel M, Clapisson G, et al. Patients with metastatic breast cancer leading to CD4+ T cell lymphopaenia have poor outcome. Eur J Cancer. 2013;49(7):1673–1682. doi: 10.1016/j.ejca.2012.11.028 [DOI] [PubMed] [Google Scholar]
- 30.Péron J, Cropet C, Tredan O, et al. CD4 lymphopenia to identify end-of-life metastatic cancer patients. Eur J Cancer. 2013;49(5):1080–1089. doi: 10.1016/j.ejca.2012.11.003 [DOI] [PubMed] [Google Scholar]
- 31.Zhang Y, Zhu Y, Wang J, et al. A comprehensive model based on temporal dynamics of peripheral T cell repertoire for predicting post-treatment distant metastasis of nasopharyngeal carcinoma. Cancer Immunol Immunother. 2021:1–14. doi: 10.1007/s00262-021-03016-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fu Y, Lan T, Cai H, Lu A, Yu W. Meta-analysis of serum lactate dehydrogenase and prognosis for osteosarcoma. Medicine. 2018;97(19):e0741. doi: 10.1097/MD.0000000000010741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shen J, Chen Z, Zhuang Q, et al. Prognostic value of serum lactate dehydrogenase in renal cell carcinoma: a systematic review and meta-analysis. PLoS One. 2016;11(11):e0166482. doi: 10.1371/journal.pone.0166482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Deng T, Zhang J, Meng Y, Zhou Y, Li W. Higher pretreatment lactate dehydrogenase concentration predicts worse overall survival in patients with lung cancer. Medicine. 2018;97(38):e12524. doi: 10.1097/MD.0000000000012524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen Z, Guo Q, Lu T, et al. Pretreatment serum lactate dehydrogenase level as an independent prognostic factor of nasopharyngeal carcinoma in the intensity-modulated radiation therapy era. Med Sci Monit. 2017;23:437–445. doi: 10.12659/MSM.899531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhou GQ, Ren XY, Mao YP, et al. Prognostic implications of dynamic serum lactate dehydrogenase assessments in nasopharyngeal carcinoma patients treated with intensity-modulated radiotherapy. Sci Rep. 2016;6:22326. doi: 10.1038/srep22326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liberti MV, Locasale JW. The Warburg effect: how does it benefit cancer cells? Trends Biochem Sci. 2016;41(3):211–218. doi: 10.1016/j.tibs.2015.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Siska PJ, Singer K, Evert K, Renner K, Kreutz M. The immunological Warburg effect: can a metabolic-tumor-stroma score (MeTS) guide cancer immunotherapy? Immunol Rev. 2020;295(1):187–202. doi: 10.1111/imr.12846 [DOI] [PubMed] [Google Scholar]
- 39.Shen L, Zhou Y, He H, et al. Crosstalk between macrophages, T cells, and iron metabolism in tumor microenvironment. Oxid Med Cell Longev. 2021;2021:8865791. doi: 10.1155/2021/8865791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang SL, Cao S, Wu R, et al. Serum ferritin predicted prognosis in patients with locally advanced pancreatic cancer. Future Oncol. 2015;11(21):2905–2910. doi: 10.2217/fon.15.186 [DOI] [PubMed] [Google Scholar]
- 41.Song A, Eo W, Kim S, Shim B, Lee S. Significance of serum ferritin as a prognostic factor in advanced hepatobiliary cancer patients treated with Korean medicine: a retrospective cohort study. BMC Complement Altern Med. 2018;18(1):176. doi: 10.1186/s12906-018-2240-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee S, Song A, Eo W. Serum ferritin as a prognostic biomarker for survival in relapsed or refractory metastatic colorectal cancer. J Cancer. 2016;7(8):957–964. doi: 10.7150/jca.14797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hsu MY, Mina E, Roetto A, Porporato PE. Iron: an essential element of cancer metabolism. Cells. 2020;9:12. doi: 10.3390/cells9122591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pfeifhofer-Obermair C, Tymoszuk P, Petzer V, Weiss G, Nairz M. Iron in the tumor microenvironment-connecting the dots. Front Oncol. 2018;8:549. doi: 10.3389/fonc.2018.00549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sacco A, Battaglia AM, Botta C, et al. Iron metabolism in the tumor microenvironment-implications for anti-cancer immune response. Cells. 2021;10(2):303. doi: 10.3390/cells10020303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tang LQ, Li CF, Li J, et al. Establishment and validation of prognostic nomograms for endemic nasopharyngeal carcinoma. J Natl Cancer Inst. 2016;108(1):djv291. doi: 10.1093/jnci/djv291 [DOI] [PubMed] [Google Scholar]