Abstract
Background
Cisplatin (CDDP) is an efficacious anticancer agent used widely in chemotherapy despite its severe side effect related to neurotoxicity. Redox imbalance and inflammatory mechanism have been implicated in the pathophysiology of CDDP-induced neurotoxicity. Herein, we investigated whether Tiliacora triandra (TT) extract could inhibit CDDP-induced redox-mediated neurotoxicity and behavioural deficit in rats.
Materials and Methods
CDDP-induced redox-mediated neurotoxicity and behavioral deficit in rats. Rats were administered TT for five consecutive weeks (250 and 500 mg/kg bw), while weekly i.p. injection of CDDP commenced on the second week (2.5 mg/kg bw) of the TT administration.
Results
CCDDP caused significant body weight reduction and cognitive diminution as revealed by Morris water maze and Y maze tests. In the CDDP-induced cognitive impairment (CICI) rats, there were remarkable increases in the brain levels of TNF-α, IL-6 and IL-1β and malondialdehyde (MDA), whereas catalase (CAT), glutathione (GSH), glutathione peroxidase (GPx) and superoxide dismutase (SOD) activities considerably decreased compared to normal control. The brain acetylcholinesterase (AChE) activity in CDDP control rats was significantly increased compared to the normal control. The expression of caspase-3 and p53 proteins was upregulated by CDDP injection, whereas Bcl2 was downregulated coupled with histopathological alterations in the rat brain. Interestingly, treatment with TT significantly abated neurobehavioral deficits, MDA and cytokine levels and restored CAT, GPx, GSH, SOD, and AChE activities compared to the CDDP control rats. Caspase-3 level as well as Bcl2 and p53 expressions were modulated with alleviated changes in histopathology.
Conclusion
The findings highlight neuroprotective and cognitive function improvement efficacy of TT against CICI via redox-inflammatory balance and antiapoptotic mechanism in rats.
Keywords: Tiliacora triandra, cisplatin, neurotoxicity, oxidative stress, inflammation
Introduction
Cancer is one of the major causes of global mortality, accounting for more than nine million deaths in 2018. The gruesome burden including financial, physical, and emotional stress associated with cancer is growing at an exponential rate especially in low and middle-income countries with little or no adequate resources to manage the burden. As such, rigorous efforts have been invested into developing several therapies that can increase the survival and improve the quality of life of cancer patients.1 Chemotherapy is one widely used approach for treating cancers and tumors and several anticancer drugs have been developed and approved including cyclophosphamide, doxorubicin, cisplatin, vincristine among others.2–4
Cisplatin, a platinum-based anticancer drug is a major and widely used drug for treating several malignancies such as head and neck, testicular, and non-small-cell lung cancer.5,6 In spite of the relative success attained by cisplatin, its applicability has been extremely curtailed due to a number of serious side effects including ototoxicity, renal toxicity, testicular toxicity, and neuronal toxicity.7,8 Cisplatin-induced cognitive impairment (CICI) also known as “chemobrain” encompasses a wide range of features including reduction in processing speed, learning and memory dysfunction, and loss of locomotive activity.1,9 At present the pathophysiology of CICI is yet to be fully comprehended, thus making effective treatment for patients a daunting task. However, multiple pathophysiological mechanisms have been postulated to decipher CICI including oxidative stress, inflammation, mitochondrial dysfunction, DNA damage and apoptosis.10,11 Unfortunately, there are no effective therapies for mitigating CICI, and as such it is imperative to consider alternative treatment that can interfere with the proposed underlying mechanism associated with CICI.
Tiliacora triandra (TT) is an edible vegetable and herbal plant used for treating malaria, diabetes, fever, and as a detoxification agent. TT possesses multiple pharmacological properties such as antidiabetic, antimicrobial, antioxidant, antimalaria, and neuroprotective effects.12–14 The application of TT as a neuroprotective agent has been previously explored. The extracts from the leaves of the plant enhanced memory and learning as well as prevented neuronal death in models of cerebral ischemia/reperfusion. In addition, TT enhanced antioxidative enzymes and reduced acetylcholinesterase activity in ethanol dependence animals.13,15–17 However, the protective effects of TT against CICI is unknown and unexplored. In this work, the protective effects of TT extract against CDDP induced CICI in rats was explored.
Materials and Methods
Plant Specimen
The collection of T. triandra as well as the preparation of TT extract was according to a previous study.18 Briefly, the leaves were washed, oven dried, powdered and 200 g of the powder was macerated in 2 L of 70% ethanol on a shaker for 24 h. Thereafter, the extract was filtered and concentrated to one third of its original volume and kept at 4°C overnight. The solution was decanted, centrifuged, and lyophilized to obtain a light brown hydroscopic powder (TT), which was stored in an airtight container at 4°C until use.
UHPLC-DAD-ESI-QTOF-MS Profiling of TT Extract
The identification of bioactive metabolites in TT extract was performed using UPLC-ESI-Q-TOF-MS analysis. Briefly, 50 mg of TT was dissolved in 1 mL of 50% methanol, centrifuged and the supernatant obtained was filtered using 0.22 µm nylon membrane. The extract subjected to UHPLC-ESI-QTOF-MS analysis.
Animals
Adult specific pathogen-free male Wistar rats (120±40 g) were used in this study. The rats were kept in stainless steel cages with six rats per cage and housed in a well ventilated animal house facility. The rats were given continuous access to tap water and normal rat food for one week before the commencement of the study. The study was approved by the Ethics Committee of Anhui Medical College (ethic approval number: Anhuiyxgdzkxx-2020-07008) and followed, the guidelines of the US National Institute of Health for the Care and Use of Laboratory Animals (NIH, revised 1978).
Experimental Design
After the seven days of adaptation, the rats were randomized into four groups as follows:
Healthy control rats (HNC): administered with normal saline.
CDDP control rats (CDDP): administered with normal saline.
TT-250-treated rats: administered with 250 mg/kg of TT extract.
TT-500-treated rats: administered with 500 mg/kg of TT extract.
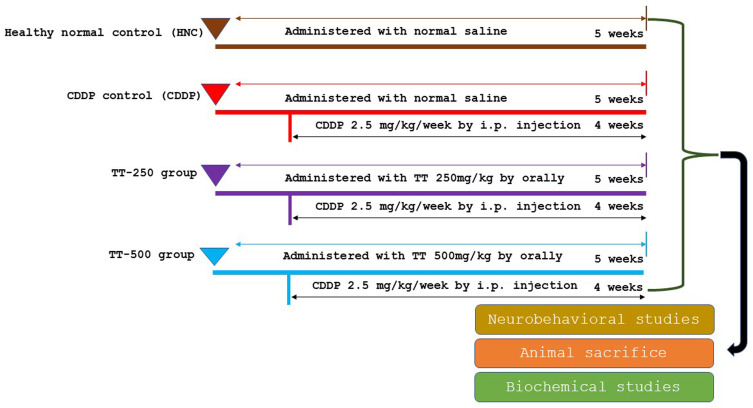
The rats in the TT-250 and TT-500 groups were given TT on a daily basis for 35 days and were simultaneously given a weekly i.p. injection of 2.5 mg/kg of CDDP for four weeks, beginning on the second week of TT administration. Likewise, the CDDP control rats received a weekly i.p. injection of CDDP (2.5 mg/kg) for four weeks from the second week of normal saline administration (Figure 1). The choice of TT dose was adopted from previous studies.12,13,18 The animals were subjected to behavioral studies upon completion of the treatment.
Figure 1.
Experimental design and animal grouping.
Behavioral Studies
Morris Water Maze Test
The method used for the Morris water maze (MWM) test was according to previous report.19 A circular pool-like container with the following dimensions was used for the MWM test: diameter of 150 cm and a height of 40 cm, with water depth of 30 cm. The water was made opaque with milk powder and a hidden platform was placed 1 cm below the surface of the water. The rats were trained individually on day 0 prior to the start of the experiment. The acquisition trail was conducted on days 1–4, while the fifth day was the exploration period. For the training and acquisition trail, the rats were gently placed into the pool along the walls of the four different arbitrary quadrants and gently released with their heads raised up to swim and locate the position of the hidden platform within 60 seconds. The time taken for the rats to locate the submerged platform before the expiration of the 60 seconds was recorded. The rats that were unable to locate the platform within 60 seconds were gently guided to the position of the platform and allowed to stay on the platform for 10 seconds. On the fifth day the platform was removed and the time that each rat stayed in the target quadrant where the platform was previously placed was recorded within 60 seconds.
Y Maze Test
The Y maze test was used to evaluate spontaneous alternation of recognition using a previously described method.20 The maze was made up of three identical arms X, Y, and Z with dimensions of 35 cm long, 30 cm high, and a width of 15 cm, stationed at equal angles. The rats were introduced into the maze from the end of one arm and allowed to freely navigate through the maze within five minutes. Spontaneous alternation was evaluated by visually recording the pattern of complete entry into each arm (the rat’s hind paws goes entirely into the arm). The frequency of alternation into the arms was recorded based on successive entries into the three arms on overlapping triplet sets (XYZ, YZX, ZXY).
Animal Sacrifice
Following the behavioral studies, the rats were euthanized with pentobarbital sodium and the brain tissues were carefully removed from the cranium, washed with normal saline to remove residual blood, and weighed. Some part of the brain tissues was kept in 10% buffered formalin solution for histopathologic analyses using routine hematoxylin and eosin (H&E) staining techniques. The remaining portion of the brain tissues was homogenized in ice-cold 0.1 M phosphate buffer (pH 7.4) and thereafter subjected to high speed centrifugation and the resulting brain tissue supernatant was used in the determination of biochemical analyses.
Evaluation of Biochemical Parameters in the Brain
Oxidative stress parameters including lipid peroxidation products malondialdehyde (MDA), superoxide dismutase (SOD), catalase (CAT), glutathione (GSH) and glutathione peroxidase (GPx) activities in the brain homogenates were assayed using assay kits from Nanjing Bioengineering Institute, China. Moreover, the levels of TNF-α, IL-6, IL-1β and caspase-3 were assayed with ELISA kits from Abcam (Cambridge, UK). Acetylcholinesterase (AChE) activity was determined using the previously reported method.21
Histology
Brain histological examination was performed on 4–5-μm tissue sections stained with hematoxylin and eosin using standard protocol.
Immunohistochemistry
Immunostaining of the brain tissues for the expression of Bcl2 and p53 proteins were performed following previously reported method.22 Quantitative analysis of the immunostained intensity of Bcl2 and p53 proteins was performed using image J software.
Statistical Evaluation
All data are shown as mean ±SD and analyzed using one-way ANOVA with Tukey’s HSD multiple range post hoc test using GraphPad Prism version 5. Data was considered statistically significant at p<0.05, p<0.01 or p<0.001.
Results
TT Ameliorated CDDP-triggered Weight Loss
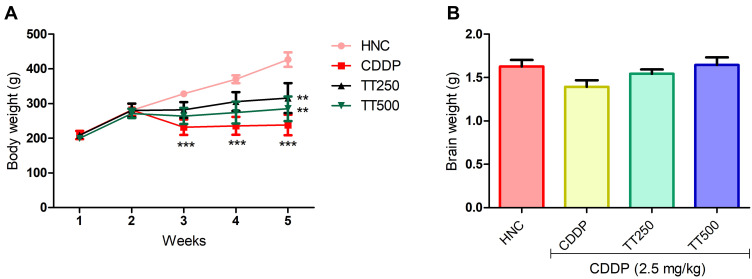
The body weight of the rats were not significantly different at the beginning of the experiment prior to CDDP injection. Whereas, the weight of the CDDP control rats was significantly reduced by the end of the experiment compared to the HNC rats (Figure 2). In contrast, TT significantly prevented body weight loss in the treated groups compared to the CDDP group. Moreover, the weight of the brain of the rats in the CDDP group was observed to be lower than the HNC and TT treated groups (Figure 2).
Figure 2.
Effect of TT on (A) body and (B) brain weight in CDDP induced neurotoxicity in rats. Results are expressed as mean ±SD (n=6) and analyzed using one way ANOVA followed by Tukey's post hoc test. *p<0.001 indicates significant difference compared to HNC group; **p<0.001 indicates significant difference compared to CDDP group.
TT Alleviated CDPP Induced Cognitive Dysfunction in Rats
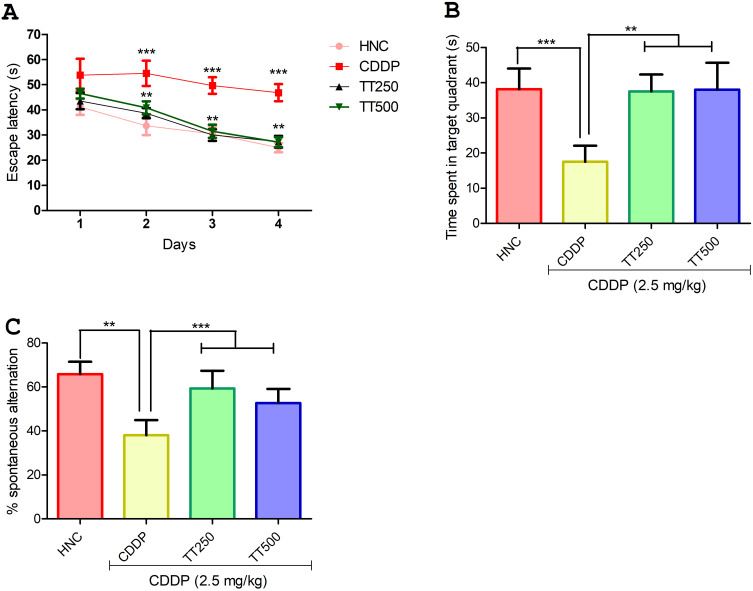
In the MWM test, the latency time of rats in the CDDP control group to locate the hidden platform was significantly increased compared to the HNC rats throughout the acquisition trial period (p<0.001; Figure 3A). In contrast, the time taken by the rats in the TT-treated group was observed to be significantly shortened from day two of the acquisition trial compared to the CDDP control animals (Figure 3A). In addition, in the exploration test, the CDDP control rats showed shorter retention memory as observed by significant reduction in the time spent in the target quadrant compared to the HNC and TT-treated groups, suggesting decreased spatial memory in CDDP control rats (p<0.001; Figure 3B). Furthermore, in the Y-maze test, the CDDP control rats showed significantly reduced percentage spontaneous alternation compared to HNC group (p<0.001; Figure 3C), but the rats in the TT-treated groups showed significant increase in the percentage spontaneous alternation when compared to the untreated CDDP control rats (p<0.001; Figure 3C).
Figure 3.
Effect of TT on neurobehavioral parameters in CDDP induced neurotoxicity in rats. (A and B) Morris water maze test (C) Y maze test. Results are expressed as mean ±SD (n=6) and analyzed using one-way ANOVA followed by Tukey's post hoc test. *p<0.001 indicates significant difference compared to HNC group; **p<0.001 indicates significant difference compared to CDDP group.
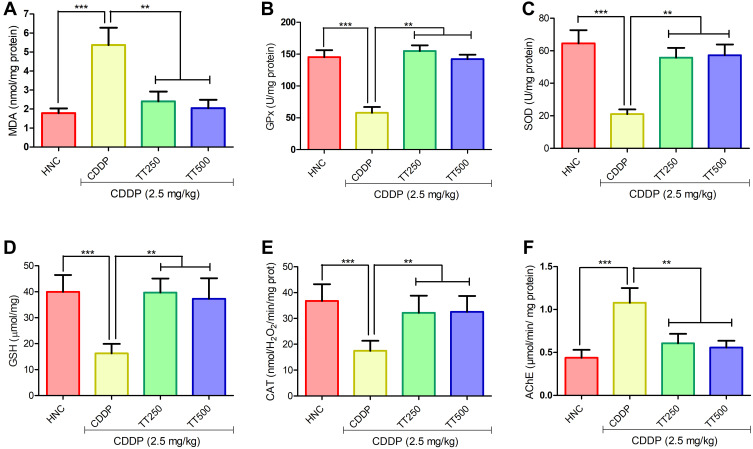
TT Attenuated CDDP-induced Oxidative Stress in the Brain of Rats
Figure 4 portrays the effects of TT on oxidative stress indices in the brain of the experimental rats. The administration of CDDP elicited significant increase in MDA levels in the CDDP control rats in comparison with HNC group (p<0.001; Figure 4A). However, treatment with TT markedly abated MDA levels compared to CDDP control rats (p<0.001; Figure 4A). In addition, Figure 3B–E depicts the effect of TT on antioxidant enzymes activities in the brain of experimental rats. The activities of brain GPx, SOD, GSH, and CAT in CDDP control animals were markedly reduced compared with the HNC group. Treatment with TT upregulated the activities of these antioxidative enzymes in the brain tissues in comparison with CDDP control group (p<0.001; Figure 4B–E).
Figure 4.
Effect of TT on brain oxidative stress biomarkers (A) MDA, (B) GPx, (C) SOD, (D) GSH, (E) CAT and (F) brain acetylcholinesterase activity in CDDP-induced neurotoxicity in rats. Results are expressed as mean ±SD (n=6) and analyzed using one-way ANOVA followed by Tukey's post hoc test. *p<0.001 indicates significant difference compared to HNC group; **p<0.001 indicates significant difference compared to CDDP group.
TT Improved AChE Activity in the Brain Tissue of Rats
The AChE activity in the brain tissue of CDDP control rats was significantly increased in comparison with HNC group. Conversely, TT administration elicited marked improvement by reducing the activity of AChE in the brain of the treated rats (p<0.001; Figure 4F).
TT Suppressed Biomarkers of Inflammation and Apoptosis
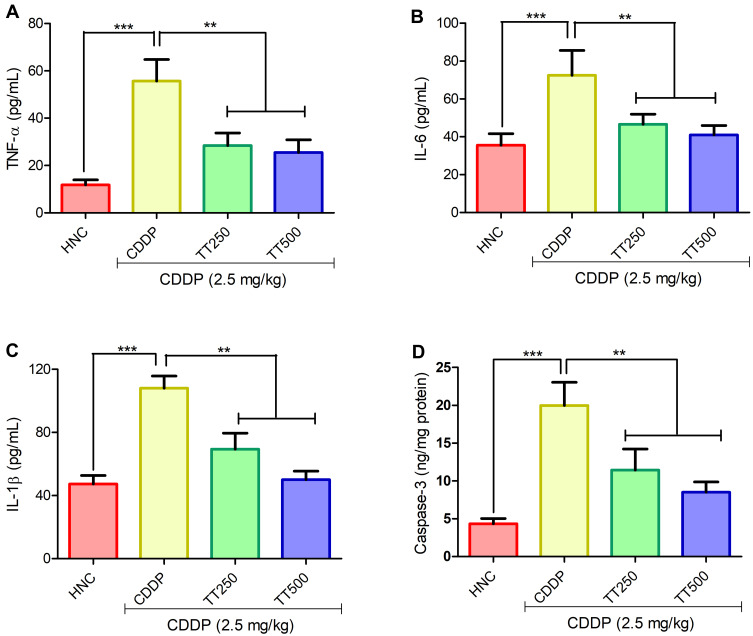
As depicted in Figure 5, TNF-α level was significantly increased in the brain tissues of CDDP animals when compared with the HNC counterpart. IL-6 and IL-1β levels were also observed to be markedly increased in the CDDP control group. The concentration of TNF-α, IL-6 and IL-1β were significantly reduced in comparison with CDDP control rats in the TT treated groups (p<0.001; Figure 5A–C). In addition, CDDP significantly upregulated the concentration of caspase-3 in the brain tissues of the CDDP control rats relative to the HNC group. Treatment with TT at 250 and 500 mg/kg significantly decreased the concentration of caspase-3 in the brain tissues of treated rats compared to CDDP alone group (Figure 5D).
Figure 5.
Effect of TT on brain proinflammatory cytokines (A) TNF-α, (B) IL-6, (C) IL-1β and (D) caspase-3 activity in CDDP induced neurotoxicity in rats. Results are expressed as mean ±SD (n=6) and analyzed using one-way ANOVA followed by Tukey post hoc test. *p<0.001 indicates significant difference compared to HNC group; **p<0.001 indicates significant difference compared to CDDP group.
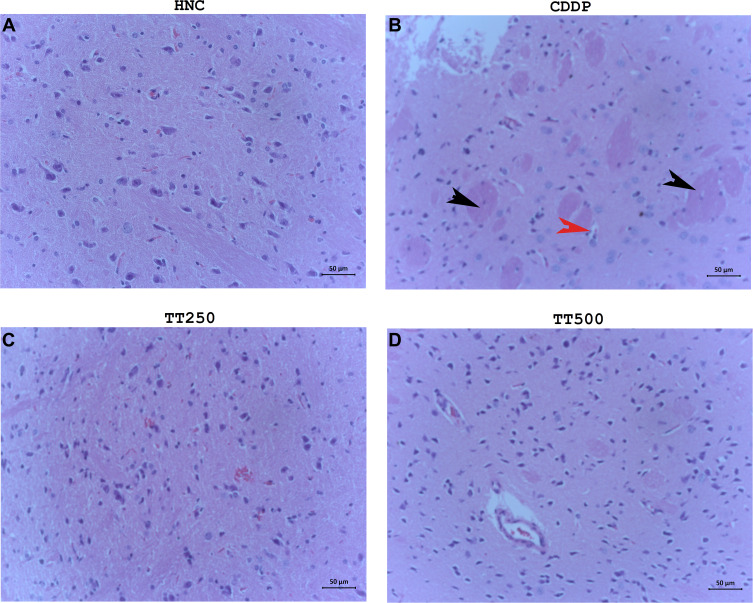
TT Alleviated Pathological Damages in the Brain Tissues of Rats
The histopathological results according to H&E staining revealed normal brain architecture including neurons of variable sizes without any obvious indications of apoptosis and glial proliferation of the neurons. In contrast, the CDDP control rats showed obvious signs of neuronal apoptosis, inflammation of neuronal cells, disordered pyramidal cell layer as well as glial proliferation. Treatment with TT produced significant alleviation to the pathological alterations in the brain tissues of the treated rats (Figure 6A–D).
Figure 6.
H&E photomicrographs showing histology of brain tissues of (A) HNC, (B) CDDP, (C) TT250 and (D) TT500 groups, respectively. H&E ×400.
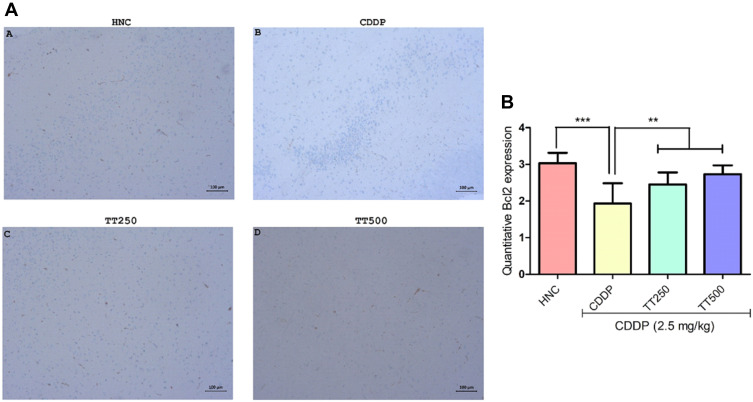
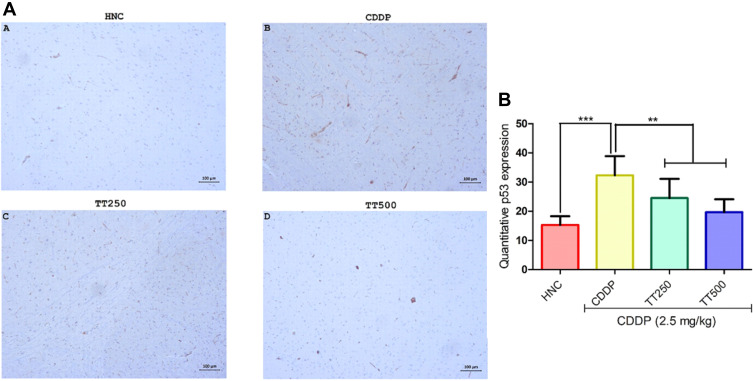
Effect of TT on Immunostaining Intensity of Bcl2 and p53 Proteins
The effect of TT on immunostaining intensity of Bcl2 and p53 proteins are presented in Figures 7 and 8. Treatment with CDDP only showed reduced immunostaining intensity of Bcl2, whereas p53 protein immunostaining intensity increased (Figures 6 and 7). However, combined treatment with TT indicated significantly increased intensity for Bcl2 and reduced intensity for p53 in relation to CDDP control (Figures 6 and 7).
Figure 7.
Effect of TT on immunostaining intensity for Bcl2 protein in CDDP induced neurotoxicity in rats. (A) Representative photomicrographs (×400) of Bcl2 immunohistochemistry of rat brain, (B) Quantitative expression of Bcl2. Results are expressed as mean ±SD (n=6) and analyzed using one-way ANOVA followed by Tukey's post hoc test. *p<0.001 indicates significant difference compared to HNC group; **p<0.001 indicates significant difference compared to CDDP group.
Figure 8.
Effect of TT on immunostaining intensity for p53 protein in CDDP induced neurotoxicity in rats. (A) Representative photomicrographs (×400) of p53 immunohistochemistry of rat brain, (B) Quantitative expression of p53. Results are expressed as mean ±SD (n=6) and analyzed using one-way ANOVA followed by Tukey's post hoc test. *p<0.001 indicates significant difference compared to HNC group; **p<0.001 indicates significant difference compared to CDDP group.
UHPLC-DAD-ESI-QTOF-MS Profiling of TT
The results of the phytochemical profiling of the bioactive compounds in TT extract using UHPLC-ESI-QTOF-MS/MS analysis (negative mode) is shown in Table 1. The data revealed the presence of various classes of compounds majorly flavonoid and phenolic glycosides, including kaempferol-7 O-glucoside, isovitexin, luteolin 3ʹ-methyl ether 7,4ʹ-dixyloside, luteolin 6-C-glucoside 8-C-arabinoside, okanin 4ʹ-O-(4″,6″-di-O-acetyl glucoside), cutellarein 7,4ʹ-dirhamnoside and vestitone 7-glucoside. Other compounds such as nonglycosylated flavones including amentoflavone, a para-benzoquinone (embelin), fatty acids and coumarin derivative (esculetin) were also identified.
Table 1.
Tentative Identification of Compounds in TT Extract Using LC-ESI-QTOF-MS Analysis
| No | Rt (Min) | Accurate Mass (m/z) | Calculated Mass (Da) | Base Peak (m/z) | Score (DB) | Predicted Formula | Compound Identity |
|---|---|---|---|---|---|---|---|
| 1 | 2.285 | 191.058 | 192.0653 | 191.0571 | C7H12O6 | Quinic acid | |
| 2 | 4.092 | 134.0475 | 135.0547 | 134.0475 | 98.56 | C5H5N5 | Adenine |
| 3 | 4.544 | 111.0090 | 112.0163 | 111.0088 | 87.48 | C5H4O3 | 2,3-Dihydroxy-2,4-cyclopentadien-1-one |
| 4 | 4.644 | 290.0884 | 291.0956 | 128.0351 | 98.38 | C11H17NO8 | Sarmentosin epoxide |
| 5 | 5.222 | 124.0406 | 125.0480 | 124.0401 | 96.95 | C6H7NO2 | 4-Aminocatechol |
| 6 | 5.799 | 359.0988 | 360.1060 | 197.0457 | 99.36 | C15H20O10 | 6ʹ-Methoxy-polygoacetophenoside |
| 7 | 5.900 | 167.0352 | 168.0425 | 167.035 | 86.56 | C8H8O4 | Dihydroxyphenylacetic acid |
| 8 | 6.251 | 341.0879 | 342.0951 | 341.0869 | 99.67 | C15H18O9 | Glucocaffeic acid |
| 9 | 6.402 | 241.0722 | 242.0797 | 211.0614 | 95.05 | C11H14O6 | Elenaic acid |
| 10 | 6.929 | 401.1453 | 402.1527 | 401.1445 | 97.5 | C18H26O10 | Benzyl O-[arabinofuranosyl-(1->6)-glucoside] |
| 11 | 7.180 | 137.0247 | 138.0319 | 137.0244 | 87.45 | C7H6O3 | 2,5-Dihydroxybenzaldehyde |
| 12 | 7.331 | 395.1565 | 396.1637 | 395.1556 | 97.78 | C16H28O11 | 1-(3-Methylbutanoyl)-6-apiosylglucose |
| 13 | 7.406 | 177.0199 | 178.0272 | 177.0197 | 97.53 | C9H6O4 | Esculetin |
| 14 | 7.431 | 415.1609 | 416.1681 | 415.1604 | 97.68 | C19H28O10 | Phenylethyl primeveroside |
| 15 | 7.507 | 577.1561 | 578.1632 | 577.1552 | 98.21 | C27H30O14 | Scutellarein 7,4ʹ-dirhamnoside |
| 16 | 7.531 | 533.1299 | 534.1370 | 533.128 | 98.82 | C25H26O13 | Okanin 4ʹ-O-(4″,6″-di-O-acetyl glucoside) |
| 17 | 7.556 | 563.1401 | 564.1474 | 563.1385 | 96.4 | C26H28O14 | Luteolin 3ʹ-methyl ether 7,4ʹ-dixyloside |
| 18 | 7.607 | 609.1458 | 610.1530 | 609.1451 | 98.49 | C27H30O16 | Luteolin 6-C-glucoside 8-C-arabinoside |
| 19 | 7.758 | 431.0988 | 432.1060 | 431.0988 | 98.65 | C21H20O10 | Isovitexin |
| 20 | 7.958 | 593.1519 | 594.1589 | 593.1518 | 98.41 | C27H30O15 | Saponarin |
| 21 | 8.209 | 547.1457 | 548.1529 | 547.1453 | 98.89 | C26H28O13 | Puerarin xyloside |
| 22 | 8.335 | 447.0931 | 448.1004 | 447.0933 | 97.83 | C21H20O11 | Kaempferol 7-O-glucoside |
| 23 | 8.560 | 401.0879 | 402.0953 | 401.0866 | 99.19 | C20H18O9 | 5-Hydroxy-3,3ʹ,7,8-tetramethoxy-4ʹ,5ʹ-methylenedioxyflavone |
| 24 | 9.062 | 433.1502 | 434.1573 | 433.1489 | 96.57 | C22H26O9 | Vestitone 7-glucoside |
| 25 | 10.393 | 327.2179 | 328.2253 | 327.2172 | 96.92 | C18H32O5 | (9R,10S,12Z)-9,10-Dihydroxy-8-oxo-12-octadecenoic acid |
| 26 | 10.845 | 329.2333 | 330.2405 | 329.2331 | 98.81 | C18H34O5 | 9S,10S,11R-trihydroxy-12Z-octadecenoic acid |
| 26 | 11.247 | 287.223 | 288.2303 | 287.2228 | 99.47 | C16H32O4 | 9,10-dihydroxy-hexadecanoic acid |
| 27 | 11.309 | 537.0825 | 538.0898 | 537.0822 | 99.57 | C30H18O10 | Amentoflavone |
| 28 | 11.749 | 285.2072 | 286.2145 | 285.207 | 97.78 | C16H30O4 | Hexadecanedioic acid |
| 29 | 13.180 | 293.1766 | 294.1839 | 293.1786 | 97.19 | C17H26O4 | Embelin |
Discussion
Cisplatin (CDDP) is one of the leading anticancer agents in cancer chemotherapy. The consistent worry of clinicians about cisplatin use is the variegated side effects chiefly linked with neurotoxicity, nephrotoxicity, and testicular damage.7,23,24 However, studies probing into the mechanisms of CDDP toxicity show that a network of oxidative stress, pro-inflammation and redox-mediated apoptosis play a pivotal role.25 Emerging research reports have revealed increasing interest for exploration of natural products to combat the integrated mechanism underlying the severe side effects of CDDP. We thus explored whether TT could mitigate CDDP neurotoxicity and to evaluate possible mechanism of action.
In this study, the intraperitoneal injection of CDDP at 2.5 mg/kg triggered significant loss of body weight in CDDP group compared to the normal control. Previous studies have also reported loss of body weight due to injection of CDDP.26,27 We observed that the intake of food by the rats reduced as well as the water intake. Conceivably, reduced food consumption occasioned by the CDDP may contribute to the loss of weight as energy expenditure may be greater than energy harvested during the reduced food intake. The study of Fulco et al suggests that CDDP causes metabolic dysfunction and inhibits citrate synthase contributing to depressed energy generation.28 The co-administration of TT was found to improve the food intake and consequently prevented loss of weight in TT+CDDP groups. Accumulating evidence depicts neurotoxicity and/or CICI as the overriding off-target tissue toxicity of CDDP.29,30 Cisplatin is a potent penetrator of the blood–brain barrier, and so it can debilitate mature neurons to induce cognitive impairment and behavioral deficit.29,31 Cisplatin is clinically known to cause structural CNS mutilations which correlate with the cognitive deficits observed in CDDP‐treated patient.23 Therefore, our study examined CICI and behavior of rats after CDDP injection via MWM and Y maze tests. Morris water-maze test is a very common behavioral model to test rodent spatial memory and learning.32 The learning and memory exercise demonstrates that the CDDP adversely affected the rat brain such that there were considerable alterations in latency time and retention memory of rats compared to normal control rats. Furthermore, in the Y-maze test, the CDDP control rats showed significantly reduced spontaneous alternation compared to healthy normal control. However, Y-maze test assesses short-term hippocampus-dependent spatial working memory.33 Taken together herein, the tests suggest that the neurotoxic effect of CDDP reduced learning and memory capacity of the rats. This finding is consistent with our observation of the effect of CDDP on AChE activity in this study. In the CDDP control rats, the brain AChE activity markedly increased compared to normal control rats.34,35 This implies increased hydrolysis of acetylcholine, a crucial neurotransmitter involved in regulating cognitive function and cholinergic integrity. These findings are in consonance with the earlier investigations which demonstrated that CDDP treatment induced learning and memory dysfunctions in rats.36–38 In contrast, the rats in TT-treated groups showed significant improvement in latency time, retention memory and increase in the percentage spontaneous alternation when compared to the untreated CDDP control rats. It is noteworthy that the TT-250 treated group showed higher percentage spontaneous alternation than the TT-500 treated group albeit not statistically different. TT inhibited the brain activity of AChE for restoration of cognitive integrity. Therefore, AChE inhibition may be pivotal to ameliorating CDDP side effect on the brain. The neuroprotective efficacy of TT in our study is consistent with reports in previous studies. In a systematic study of Phunchago et al, TT improves memory impairment, neurodegeneration and cholinergic disruption induced by ethanol.14 The study suggests that the HPLC-detected gallic acid, cyanidin, and quercetin may be responsible for the neuroprotective potential of TT. In a recent publication by Thong-asa and Bullangpoti, the neuroprotective potential of TT was observed against brain infarction and neuronal death in the cerebral cortex and hippocampus in a model of cerebral ischemia reperfusion.16
Publications have implicated oxidative stress as the leading contributor to the pathogenesis of CDDP-induced neurotoxicity. Redox imbalance orchestrates cellular machinery triggering DNA damage, inflammation and apoptosis.39,40 Cisplatin generates reactive oxygen species (ROS), including hydrogen peroxide and hydroxyl radicals which attack lipid membrane and inhibit antioxidant enzyme activity to promote lipid peroxidation and oxidative stress, respectively.29,41 In this study, the injected CDDP instigated preponderant elevations in the brain MDA levels in the CDDP model compared to the normal control. In addition, the brain activities of SOD, CAT, GSH, and GPx were also appreciably reduced compared to the normal control. In normal physiological interplay, cell antioxidant mechanisms modulate metabolic generation of ROS to maintain homeostasis. However, alterations to this milieu usually aggravate ROS generation. Herein, CDDP altered the milieu and increased ROS production leading to inhibition of SOD, GSH, CAT, and GPx activities and the consequent attack on brain cell membrane, hence, increased levels of MDA. Depressed activities of SOD and CAT may provoke accumulation of superoxide radical and hydrogen peroxide evolving further damage consistent with oxidative histopathological lesions found in the rat brain. Our findings regarding the aforementioned redox indices agreed with earlier reports on the oxidative effect of CDDP on the brain, kidney, and testis.7,23,39,42,43 It was interesting to observe that TT co-administration mitigated MDA release and significantly restored brain antioxidant defences dose dependently. Therefore, the beneficial health effect of TT in the current study demonstrates its antioxidant property and also support the reported action of TT in previous studies.14–16
The molecular mechanisms of CDDP neurotoxicity are multifactorial and complicated, consisting of oxidative stress, inflammation, apoptosis and autophagy leading to cell death.20,44 Accumulating evidence cleared that CDDP toxicity is a trigger of pro-inflammatory cascades through a significant decline in antioxidant mechanism. Meanwhile aberrant alterations in redox-inflammatory processes provoke apoptosis and autophagy by changes in signalling protein expression, including Bcl2 and p53.25,45,46 Our results depict that CDDP administration caused deteriorating pro-inflammation by considerable unregulation of pro-inflammatory cytokines, TNF-α, IL-6 and IL-1β, in CDDP rats. Supportive evidence confirms that CDDP-induced oxidative stress-mediated activation of NF-κB unlocks translation of nuclear TNF-α, IL-6 and IL-1β genes.43,47 Several studies have shown that injection of CDDP induces increased expression of NF-κB, TNF-α, IL-6 and IL-1β and other inflammatory mediators.24,29,43,47 The co-treatment with TT prevented overexpression of TNF-α, IL-6 and IL-1β compared to the CDDP rats. Tiliacora triandra leaves and a Thai polyherbal remedy containing TT have been shown to possess anti-inflammatory effects.48,49
Studies indicate Bcl-2 family and the caspase family play an extremely important regulatory role in the apoptotic pathway.25 The expression of the antiapoptotic marker, Bcl2, was markedly reduced, while caspase-3 and p53 protein levels increased in CDDP rats. Cisplatin has been reported to reduce Bcl2 expression.44 The modulation of p53 determines the fate of stressed cells either by a cycle arrest or by removal of these cells by inducing apoptosis.45 The significant alterations in Bcl2 and caspase-3 expressions in our study suggest CDDP-induced brain apoptosis. These results suggest that CDDP evokes apoptosis in the brain of experimental rats confirming data in published papers.25,29 Interestingly, TT alleviated these altered pathological expression.
Antioxidant compounds, particularly flavonoids and phenolics, are one of the most prevalent class of compounds identified in a vast number of plant species. These classes of compounds have been a source of extensive research over several decades due to their various pharmacological properties, especially in oxidative and inflammatory related disorders.50,51 Weerawatanakorn et al and Pasachan et al identified the presence of a high content of phenolic constituents in T. triandra leaves extract and these compounds were adjudged as the active principles showing antioxidant, anti-inflammatory and antidiabetic effects in their study.48,52 Our UHPLC-DAD-ESI-QTOF-MS analysis of TT extract indicated the presence of phenolic and flavonoids, which may have accounted for the excellent antioxidant and anti-inflammatory effects against CDDP induced neurotoxicity. For instance Liu et al reported that isovitexin protected against CDDP induced renal injury via anti-inflammatory and antioxidative properties.53 Amentoflavone, a bioflavonoid tentatively identified in TT was reported to show antioxidant and anti-inflammatory effects. It was also reported to display neuroprotective properties including antidepressant and anxiolytic effects, and ameliorated scopolamine-induced memory via inhibition of AChE and increasing antioxidant enzyme activities.54 In addition, kaempferol 7-O-glucoside and esculetin have also been reported as excellent antioxidant, anti-inflammatory, and neuroprotective agents.55–57
Conclusion
In conclusion, this study elucidated, for the first time, that TT can attenuate the neurotoxicity caused by CDDP via improving cognitive function and integrity associated with enhanced antioxidant defense mechanism, anti-inflammatory and antiapoptotic pathways. The protective effect of TT involves restoration of redox balance and suppression of AChE, TNF-α, IL-6, IL-1β levels and apoptotic Bcl2/caspase-3/p53.
Acknowledgment
This scientific work was supported by 2019 support program for outstanding young talents in colleges and universities of Anhui Province (gxyq2019190).
Disclosure
The authors report no conflicts of interest for this work.
References
- 1.Ongnok B, Chattipakorn N, Chattipakorn SC. Doxorubicin and cisplatin induced cognitive impairment: the possible mechanisms and interventions. Exp Neurol. 2020;324:113118. doi: 10.1016/j.expneurol.2019.113118 [DOI] [PubMed] [Google Scholar]
- 2.Oun R, Moussa YE, Wheate NJ. The side effects of platinum-based chemotherapy drugs: a review for chemists. Dalton Trans. 2018;47:6645–6653. doi: 10.1039/C8DT00838H [DOI] [PubMed] [Google Scholar]
- 3.Stankovic JSK, Selakovic D, Mihailovic V, Rosic G. Antioxidant supplementation in the treatment of neurotoxicity induced by platinum-based chemotherapeutics-A review. Int J Mol Sci. 2020;21:7753. doi: 10.3390/ijms21207753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Falzone L, Salomone S, Libra M. Evolution of cancer pharmacological treatments at the turn of the third millennium. Front Pharmacol. 2018;9:1300. doi: 10.3389/fphar.2018.01300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou W, Kavelaars A, Heijnen CJ. Metformin prevents cisplatin-induced cognitive impairment and brain damage in mice. PLoS One. 2016;11(3):e0151890. doi: 10.1371/journal.pone.0151890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim SJ, Park C, Lee JN, Park R. Protective roles of fenofibrate against cisplatin-induced ototoxicity by the rescue of peroxisomal and mitochondrial dysfunction. Toxicol Appl Pharmacol. 2018;353:43–54. doi: 10.1016/j.taap.2018.06.010 [DOI] [PubMed] [Google Scholar]
- 7.Wang L, He Y, Li Y, et al. Protective effects of nucleosides-rich extract from Cordyceps cicadae against cisplatin induced testicular damage. Chem Biodivers. 2020;11:e2000671. [DOI] [PubMed] [Google Scholar]
- 8.Yin M, Li N, Makinde EA, Olatunji OJ, Ni ZN. N6-2-hydroxyethyl-adenosine ameliorate cisplatin induced acute kidney injury in mice. All Life. 2020;13:244–251. doi: 10.1080/26895293.2020.1760149 [DOI] [Google Scholar]
- 9.Wang L, Apple AC, Schroeder MP, et al. Reduced prefrontal activation during working and long-term memory tasks and impaired patient-reported cognition among cancer survivors post chemotherapy compared with healthy controls. Cancer. 2016;122:258–268. doi: 10.1002/cncr.29737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chtourou Y, Gargouri B, Kebieche M, Fetoui H. Naringin abrogates cisplatin-induced cognitive deficits and cholinergic dysfunction through the down-regulation of AChE expression and iNOS signaling pathways in hippocampus of aged rats. J Mol Neurosci. 2015;56(2):349–362. doi: 10.1007/s12031-015-0547-0 [DOI] [PubMed] [Google Scholar]
- 11.Salih NA, Al-Baggou BK. Effect of memantine hydrochloride on cisplatin-induced neurobehavioral toxicity in mice. Acta Neurol Belg. 2020;120(1):71–82. doi: 10.1007/s13760-019-01161-z [DOI] [PubMed] [Google Scholar]
- 12.Makinde EA, Radenahmad N, Adekoya AE, Olatunji OJ. Tiliacora triandra extract possesses antidiabetic effects in high fat diet/streptozotocin-induced diabetes in rats. J Food Biochem. 2020;44(6):e13239. doi: 10.1111/jfbc.13239 [DOI] [PubMed] [Google Scholar]
- 13.Makinde EA, Ovatlarnporn C, Adekoya AE, Nwabor OF, Olatunji OJ. Antidiabetic, antioxidant and antimicrobial activity of the aerial part of Tiliacora triandra. S Afr J Bot. 2019;125:337–343. doi: 10.1016/j.sajb.2019.08.012 [DOI] [Google Scholar]
- 14.Phunchago N, Wattanathorn J, Chaisiwamongkol K. Tiliacora triandra, an anti-intoxication plant, improves memory impairment, neurodegeneration, cholinergic function, and oxidative stress in hippocampus of ethanol dependence rats. Oxid Med Cell Longev. 2015;2015:918426. doi: 10.1155/2015/918426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Song P, Sun C, Li J, et al. Tiliacora triandra extract and its major constituent attenuates diabetic kidney and testicular impairment by modulating redox imbalance and pro-inflammatory responses in rats. J Sci Food Agric. 2021;101:1598–1608. doi: 10.1002/jsfa.10779 [DOI] [PubMed] [Google Scholar]
- 16.Thong-Asa W, Bullangpoti V. Neuroprotective effects of Tiliacora triandra leaf extract in a mice model of cerebral ischemia reperfusion. Avicenna J Phytomed. 2020;10:202–212. [PMC free article] [PubMed] [Google Scholar]
- 17.Thong-Asa W, Tumkiratiwong P, Bullangpoti V, Kongnirundonsuk K, Tilokskulchai K. Tiliacora triandra (Colebr.) Diels leaf extract enhances spatial learning and learning flexibility and prevents dentate gyrus neuronal damage induced by cerebral ischemia/reperfusion injury in mice. Avicenna J Phytomed. 2017;7:389–400. [PMC free article] [PubMed] [Google Scholar]
- 18.Liu C, Ma M, Wen C, Uz Zaman R, Olatunji OJ. Antiallodynic and anti-hyperalgesia effects of Tiliacora triandra against cisplatin-induced peripheral neuropathy. All Life. 2021;14:441–449. doi: 10.1080/26895293.2021.1927204 [DOI] [Google Scholar]
- 19.Chen X, Famurewa AC, Tang J, Olatunde OO, Olatunji OJ. Hyperoside attenuates neuroinflammation, cognitive impairment and oxidative stress via suppressing TNF-α/NF-κB/caspase-3 signaling in type 2 diabetes rats. Nutr Neurosci. 2021;16:1–11. doi: 10.1080/1028415X.2021.1901047 [DOI] [PubMed] [Google Scholar]
- 20.Wall PM, Messier C. Infralimbic kappa opioid and muscarinic M1 receptor interactions in the concurrent modulation of anxiety and memory. Psychopharmacology. 2002;160:233–244. doi: 10.1007/s00213-001-0979-9 [DOI] [PubMed] [Google Scholar]
- 21.Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959;82:70–77. doi: 10.1016/0003-9861(59)90090-6 [DOI] [PubMed] [Google Scholar]
- 22.Ahmad ST, Arjumand W, Seth A, et al. Preclinical renal cancer chemopreventive efficacy of geraniol by modulation of multiple molecular pathways. Toxicol. 2011;290:69–81. doi: 10.1016/j.tox.2011.08.020 [DOI] [PubMed] [Google Scholar]
- 23.El‐Deeb OS, Soliman GM, Elesawy RO. Linagliptin, the dipeptidyl peptidase‐4 enzyme inhibitor, lessens CHOP and GRP78 biomarkers levels in cisplatin‐induced neurobehavioral deficits: a possible restorative gateway. J Biochem Mol Toxicol. 2020;34:e22541. doi: 10.1002/jbt.22541 [DOI] [PubMed] [Google Scholar]
- 24.Park YJ, Kim KS, Park JH, et al. Protective effects of dendropanoxide isolated from Dendropanax morbifera against cisplatin-induced acute kidney injury via the AMPK/mTOR signaling pathway. Food Chem Toxicol. 2020;145:111605. doi: 10.1016/j.fct.2020.111605 [DOI] [PubMed] [Google Scholar]
- 25.Chen X, Wei W, Li Y, Huang J, Ci X. Hesperetin relieves cisplatin-induced acute kidney injury by mitigating oxidative stress, inflammation and apoptosis. Chem Biol Interact. 2019;308:269–278. doi: 10.1016/j.cbi.2019.05.040 [DOI] [PubMed] [Google Scholar]
- 26.Potočnjak I, Marinić J, Batičić L, Šimić L, Broznić D, Domitrović R. Aucubin administered by either oral or parenteral route protects against cisplatin-induced acute kidney injury in mice. Food Chem Toxicol. 2020;142:111472. doi: 10.1016/j.fct.2020.111472 [DOI] [PubMed] [Google Scholar]
- 27.Famurewa AC, Ekeleme-Egedigwe CA, Onwe CS, et al. Ginger juice prevents cisplatin-induced oxidative stress, endocrine imbalance and NO/iNOS/NF-κB signalling via modulating testicular redox-inflammatory mechanism in rats. Andrologia. 2020;52:e13786. doi: 10.1111/and.13786 [DOI] [PubMed] [Google Scholar]
- 28.Fulco BC, Jung JT, Brum LO, Zborowski VA, Goulart TA, Nogueira CW. Similar hepatoprotective effectiveness of Diphenyl diselenide and Ebselen against cisplatin-induced disruption of metabolic homeostasis and redox balance in juvenile rats. Chem Biol Interact. 2020;330:109234. doi: 10.1016/j.cbi.2020.109234 [DOI] [PubMed] [Google Scholar]
- 29.Gomaa DH, Hozayen WG, Al-shafeey H, Elkelawy AM, Hashem KS. Ginkgo biloba alleviates cisplatin-mediated neurotoxicity in rats via modulating APP/A/P2X7R/P2Y12R and XIAP/BDNF-dependent caspase-3 apoptotic pathway. Appl Sci. 2020;10:4786–4801. doi: 10.3390/app10144786 [DOI] [Google Scholar]
- 30.Sałat K. Chemotherapy-induced peripheral neuropathy: part 1—current state of knowledge and perspectives for pharmacotherapy. Pharmacol Rep. 2020;72:486–507. doi: 10.1007/s43440-020-00109-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seigers R, Schagen S, Van Tellingen O, Dietrich J. Chemotherapy-related cognitive dysfunction: current animal studies and future directions. Brain Imaging Behav. 2013;7:453–459. doi: 10.1007/s11682-013-9250-3 [DOI] [PubMed] [Google Scholar]
- 32.Morris RG, Garrud P, Rawlins JA, O’Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297:681–683. doi: 10.1038/297681a0 [DOI] [PubMed] [Google Scholar]
- 33.Hao R, Song X, Li F, Tan X, Sun-Waterhouse D, Li D. Caffeic acid phenethyl ester reversed cadmium-induced cell death in hippocampus and cortex and subsequent cognitive disorders in mice: involvements of AMPK/SIRT1 pathway and amyloid-tau-neuroinflammation axis. Food Chem Toxicol. 2020;144:111636. doi: 10.1016/j.fct.2020.111636 [DOI] [PubMed] [Google Scholar]
- 34.Wachiryah T, Hathaipat L. Enhancing effect of Tiliacora triandra leaves extract on spatial learning, memory and learning flexibility as well as hippocampal choline acetyltransferase activity in mice. Avicenna J Phytomed. 2018;8:380–388. [PMC free article] [PubMed] [Google Scholar]
- 35.Ingkaninan K, Temkitthawon P, Chuenchom K, Yuyaem T, Thongnoi W. Screening for acetylcholinesterase inhibitory activity in plants used in Thai traditional rejuvenating and neurotonic remedies. J Ethnopharmacol. 2003;89:261–264. doi: 10.1016/j.jep.2003.08.008 [DOI] [PubMed] [Google Scholar]
- 36.Kandeil MA, Gomaa SB, Mahmoud MO. The effect of some natural antioxidants against cisplatin-induced neurotoxicity in rats: behavioral testing. Heliyon. 2020;6:e04708. doi: 10.1016/j.heliyon.2020.e04708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Abdelkader NF, Saad MA, Abdelsalam RM. Neuroprotective effect of nebivolol against cisplatin-associated depressive-like behavior in rats. J Neurochem. 2017;141:449–460. doi: 10.1111/jnc.13978 [DOI] [PubMed] [Google Scholar]
- 38.Oz M, Atalik KE, Yerlikaya FH, Demir EA. Curcumin alleviates cisplatin-induced learning and memory impairments. Neurobiol Learn Mem. 2015;123:43–49. doi: 10.1016/j.nlm.2015.05.001 [DOI] [PubMed] [Google Scholar]
- 39.Eren H, Mercantepe T, Tumkaya L, et al. Evaluation of the protective effects of amifostine and melatonin against cisplatin induced testis injury via oxidative stress and apoptosis in rats. Exp Mol Pathol. 2020;112:104324. doi: 10.1016/j.yexmp.2019.104324 [DOI] [PubMed] [Google Scholar]
- 40.Ueki M, Ueno M, Morishita J, Maekawa N. Curcumin ameliorates cisplatin-induced nephrotoxicity by inhibiting renal inflammation in mice. J Biosci Bioeng. 2013;115:547–551. doi: 10.1016/j.jbiosc.2012.11.007 [DOI] [PubMed] [Google Scholar]
- 41.Famurewa AC, Edeogu CO, Offor FI, Besong EE, Akunna GG, Maduagwuna EK. Downregulation of redox imbalance and iNOS/NF-ĸB/caspase-3 signalling with zinc supplementation prevents urotoxicity of cyclophosphamide-induced hemorrhagic cystitis in rats. Life Sci. 2021;266:118913. doi: 10.1016/j.lfs.2020.118913 [DOI] [PubMed] [Google Scholar]
- 42.Sohail N, Hira K, Kori JA, et al. Nephroprotective effect of ethanol extract and fractions of a sea lettuce, Ulva fasciata against cisplatin-induced kidney injury in rats. Environ Sci Pollut Res Int. 2021;28:9448–9461. doi: 10.1007/s11356-020-11321-x [DOI] [PubMed] [Google Scholar]
- 43.Yarijani ZM, Godini A, Madani SH, Najafi H. Reduction of cisplatin-induced renal and hepatic side effects in rat through antioxidative and anti-inflammatory properties of Malva sylvestris L. extract. Biomed Pharmacother. 2018;106:1767–1774. doi: 10.1016/j.biopha.2018.07.115 [DOI] [PubMed] [Google Scholar]
- 44.Abdel-Razek EA, Abo-Youssef AM, Azouz AA. Benzbromarone mitigates cisplatin nephrotoxicity involving enhanced peroxisome proliferator-activated receptor-alpha (PPAR-α) expression. Life Sci. 2020;243:117272. doi: 10.1016/j.lfs.2020.117272 [DOI] [PubMed] [Google Scholar]
- 45.Domitrovic R, Cvijanovic O, Šušnic V, Katalinic N. Renoprotective mechanisms of chlorogenic acid in cisplatin-induced kidney injury. Toxicology. 2014;324:98–107. doi: 10.1016/j.tox.2014.07.004 [DOI] [PubMed] [Google Scholar]
- 46.Kaygusuzoglu E, Caglayan C, Kandemir FM, et al. Zingerone ameliorates cisplatin‐induced ovarian and uterine toxicity via suppression of sex hormone imbalances, oxidative stress, inflammation and apoptosis in female Wistar rats. Biomed Pharmacother. 2018;102:517–530. doi: 10.1016/j.biopha.2018.03.119 [DOI] [PubMed] [Google Scholar]
- 47.Hu J, Yang J, Jiang S, et al. Panax quinquefolium saponins protect against cisplatin evoked intestinal injury via ROS-mediated multiple mechanisms. Phytomedicine. 2021;82:153446. doi: 10.1016/j.phymed.2020.153446 [DOI] [PubMed] [Google Scholar]
- 48.Weerawatanakorn M, Rojsuntornkitti K, Pan M, Wongwaiwech D. Some phytochemicals and anti-inflammation effect of juice from Tiliacora triandra leaves. J Food Nutr Res. 2018;6:32–38. doi: 10.12691/jfnr-6-1-6 [DOI] [Google Scholar]
- 49.Juckmeta T, Itharat A. Anti-inflammatory and antioxidant activities of Thai traditional remedy called ya-ha-rak. J Health Res. 2012;26:205–210. [Google Scholar]
- 50.Arfaoui L. Dietary plant polyphenols: effects of food processing on their content and bioavailability. Molecules. 2021;26(10):2959. doi: 10.3390/molecules26102959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hano C, Tungmunnithum D. Plant polyphenols, more than just simple natural antioxidants: oxidative stress, aging and age-related diseases. Medicines. 2020;7:26. doi: 10.3390/medicines7050026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pasachan T, Duangjai A, Ontawong A, et al. Tiliacora triandra (Colebr.) Diels leaf aqueous extract inhibits hepatic glucose production in HepG2 cells and type 2 diabetic rats. Molecules. 2021;26:1239. doi: 10.3390/molecules26051239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu S, Zhang X, Wang J. Isovitexin protects against cisplatin-induced kidney injury in mice through inhibiting inflammatory and oxidative responses. Int Immunopharmacol. 2020;83:106437. doi: 10.1016/j.intimp.2020.106437 [DOI] [PubMed] [Google Scholar]
- 54.Yu S, Yan H, Zhang L, et al. A review on the phytochemistry, pharmacology, and pharmacokinetics of amentoflavone, a naturally-occurring biflavonoid. Molecules. 2017;22:299. doi: 10.3390/molecules22020299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang J, Fang X, Ge L, et al. Antitumor, antioxidant and anti-inflammatory activities of kaempferol and its corresponding glycosides and the enzymatic preparation of kaempferol. PLoS One. 2018;13:e0197563. doi: 10.1371/journal.pone.0197563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pruccoli L, Morroni F, Sita G, Hrelia P, Tarozzi A. Esculetin as a bifunctional antioxidant prevents and counteracts the oxidative stress and neuronal death induced by amyloid protein in SH-SY5Y cells. Antioxidants. 2020;9:551. doi: 10.3390/antiox9060551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhu L, Nang C, Luo F, et al. Esculetin attenuates lipopolysaccharide (LPS)-induced neuroinflammatory processes and depressive-like behavior in mice. Physiol Behav. 2016;163:184–192. doi: 10.1016/j.physbeh.2016.04.051 [DOI] [PubMed] [Google Scholar]