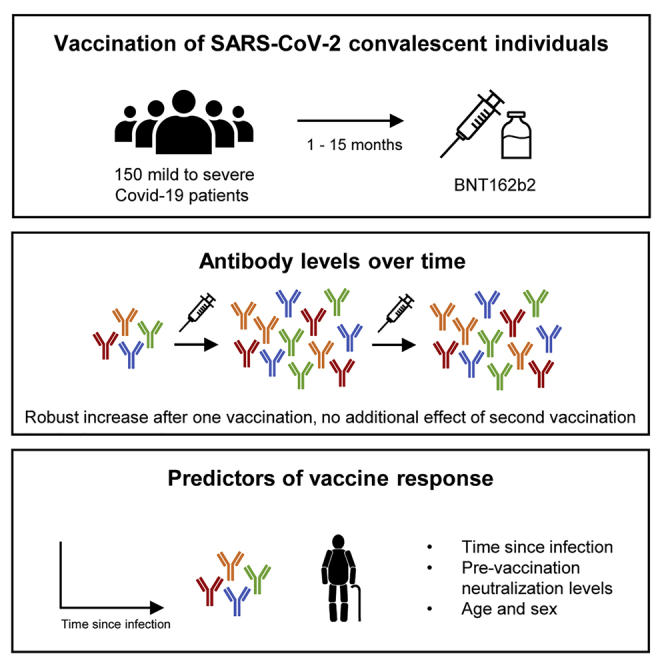
Summary
The urgent need for, but limited availability of, SARS-CoV-2 vaccines worldwide has led to widespread consideration of dose-sparing strategies. Here, we evaluate the SARS-CoV-2-specific antibody responses following BNT162b2 vaccination in 150 previously SARS-CoV-2-infected individuals from a population-based cohort. One week after first vaccine dose, spike protein antibody levels are 27-fold higher and neutralizing antibody titers 12-fold higher, exceeding titers of fully vaccinated SARS-CoV-2-naive controls, with minimal additional boosting after the second dose. Neutralizing antibody titers against four variants of concern increase after vaccination; however, overall neutralization breadth does not improve. Pre-vaccination neutralizing antibody titers and time since infection have the largest positive effect on titers following vaccination. COVID-19 severity and the presence of comorbidities have no discernible impact on vaccine response. In conclusion, a single dose of BNT162b2 vaccine up to 15 months after SARS-CoV-2 infection offers higher neutralizing antibody titers than 2 vaccine doses in SARS-CoV-2-naive individuals.
Keywords: COVID-19, SARS-CoV-2, previous infection, mRNA vaccine, BNT162b2, antibody response, neutralization, response predictors, variants
Graphical abstract
Highlights
-
•
150 SARS-CoV-2-infected individuals in a population-based prospective cohort study
-
•
The antibody titers increase within 1 week after a single dose of BNT162b2
-
•
Neutralization titers against the variants of concern show no increase in breadth
-
•
Pre-vaccination antibody titers have the largest effect on vaccine response
In a prospective cohort study, van Gils et al. find that a single dose of BNT162b2 mRNA vaccine up to 15 months after SARS-CoV-2 infection provides neutralizing titers exceeding 2 vaccine doses in SARS-CoV-2-naive individuals. This supports wide implementation of a single-dose mRNA vaccine strategy after prior SARS-CoV-2 infection.
Introduction
The unprecedented rapid development and emergency use authorization of several vaccines against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) allows for optimism in the global fight against the coronavirus disease 2019 (COVID-19) pandemic.1 However, in many regions, vaccination campaigns are hampered by limited supply or resources; hence, vaccine sparing strategies are desirable. Making use of immunological memory after prior natural SARS-CoV-2 infection,2, 3, 4 single dosing represents one such strategy for vaccines requiring two doses for optimal efficacy. A number of recent small studies in healthcare workers (HCWs) have shown similar or higher antibody responses and higher vaccine efficacy to a single-dose SARS-CoV-2 mRNA vaccine after prior infection, compared to two doses in SARS-CoV-2-naive individuals.5, 6, 7, 8, 9, 10, 11, 12, 13, 14 However, these studies were performed in relatively young and healthy individuals and provided limited information on the possible influence of COVID-19 severity and the duration since infection on vaccine responses. To inform potential wide implementation of a single-dose strategy following natural infection, we evaluated the titers and breadth of antibody responses after SARS-CoV-2 mRNA vaccination in an ongoing population-based prospective cohort study of COVID-19 patients, representing a range in age, the presence of comorbidities, COVID-19 severity, and time since infection. Antibody responses were compared to those observed after two doses in a cohort of SARS-CoV-2 naive HCWs and correlated with patient- and infection-related variables.
Results
Study population
A total of 150 participants of the RECoVERED cohort received 1 or 2 doses of the BNT162b2 mRNA vaccine after a median of 9 months following SARS-CoV-2 infection (interquartile range [IQR] 5–12 months; Figure S1). The median age of participants was 51 years (IQR 33–62), 35% were female, 44% had ≥1 comorbidities, and their SARS-CoV-2 infections were classified as mild, moderate, or severe/critical COVID-19 in 33%, 45%, and 22% of participants, respectively (Table 1). Overall, the vaccine was well tolerated, with only mild and self-limiting adverse events (Table S1). A total of 128 of the 150 participants (84%) reported ≥1 side effects within 48 h after the first vaccine dose, with pain at the injection site (84%) and fatigue (48%) reported most frequently. Similarly, 84 of the 101 participants (83%) who received the second dose of vaccine reported side effects within 48 h, with pain at the injection site (56%) and fatigue (57%) as the most common complaints. The control group consisted of 49 healthy HCWs (62% female, median age 44 years [IQR 33–53]) without evidence of previous SARS-CoV-2 infection who received 2 doses of the BNT162b2 mRNA vaccine.
Table 1.
Characteristics of the study participants
| First dose | Second dose | |
|---|---|---|
| Total participants | 150 | 101 |
| Age, y, median 51; range 22–80 (%) | ||
| ≤45 | 57 (38) | 39 (38.6) |
| 45–65 | 68 (45.3) | 52 (51.5) |
| >65 | 25 (16.7) | 10 (9.9) |
| Sex (%) | ||
| Male | 97 (64.7) | 65 (64.4) |
| Female | 53 (35.3) | 36 (35.6) |
| Disease severity (%) | ||
| Mild | 49 (32.7) | 25 (24.8) |
| Moderate | 68 (45.3) | 50 (49.5) |
| Severe | 15 (10) | 12 (11.9) |
| Critical | 18 (12) | 14 (13.9) |
| Time (months) between symptom onset and vaccination, median 9 months; range 1–15 months (%) | ||
| ≤6 | 57 (38) | 35 (34.7) |
| 7–12 | 58 (38.7) | 36 (35.6) |
| >12 | 35 (23.3) | 30 (29.7) |
| Comorbidities | ||
| Cardiovascular | 27 (18) | 19 (18.8) |
| Diabetes | 15 (10) | 9 (8.9) |
| Chronic respiratory | 20 (13.3) | 17 (16.8) |
| Cancer | 9 (6) | 4 (4) |
| Obesity | 30 (20) | 19 (18.8) |
SARS-CoV-2 IgG antibody responses
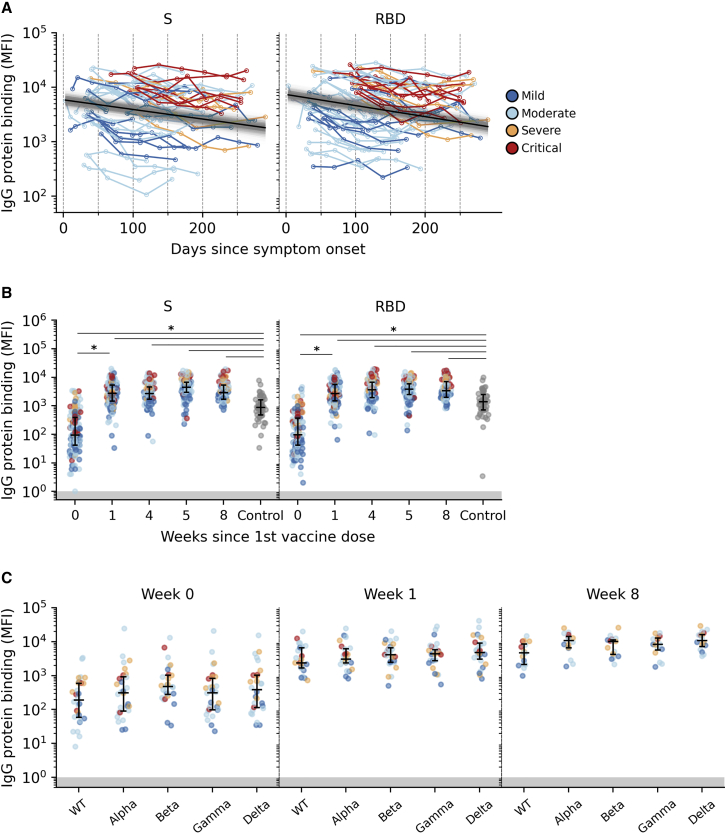
Following SARS-CoV-2 infection, levels of IgG antibodies binding to S, RBD, and N proteins exhibited a wide range, with overall higher levels observed in participants with previous severe/critical COVID-19 (Figures 1A and S2A). Using a constant decay model that best fit the data, immunoglobulin G (IgG) levels declined over time with estimated half-lives of 170 (95% credible interval [CrI]: 137–228), 148 (95% CrI: 124–180), and 99 days (95% CrI: 86–118) for S, RBD, and N proteins respectively, independent of peak IgG level or disease severity.
Figure 1.
Anti-SARS-CoV-2 IgG responses
(A) Post-infection serum IgG levels in SARS-CoV-2 S and RBD protein. The posterior median decay slope and its 95% credible interval are plotted as black and gray lines, respectively.
(B) Pre- (week 0 = before first dose, n = 150 participants) and post-vaccination (week 4 = before second dose, n = 101 participants) as well as HCW control (n = 49) distributions of serum IgG levels to SARS-CoV-2 S and RBD proteins.
(C) Pre- (week 0; n = 30 participants) and post-vaccination (week 1, n = 30 participants, and week 8, n = 21 participants) distributions of anti-S IgG levels to WT (Wuhan-Hu-1) and VOC lineages Alpha, Beta, Gamma, and Delta. Each point represents 1 participant colored by COVID-19 severity.
∗: distributions with non-overlapping 95% confidence interval (CI) of group mean effect size estimated using a Bayesian ANOVA model (Table S2). Median with interquartile range is depicted. Areas of binding values below detection limit are shaded in gray.
Sharp increases in anti-S and anti-RBD IgG were observed 1 week after the first vaccination (median fold increase 22.0 [IQR 7.7–53.1] and 22.55 [IQR 6.5–67.1], respectively) (Figures 1B and S2B). No further increases were observed 4 weeks after the first dose (before administration of the second dose) and 1 and 4 weeks after the second dose (weeks 5 and 8; Table S2). Using a Bayesian ANOVA model, we found substantial differences in anti-S (95% CrI of difference in effects: 1.22–1.49) and anti-RBD (95% CrI: 1.23–1.48) IgG levels between specimens collected before and after the first dose of vaccine. All subsequent changes after week 1 were found to be trivial (Figure S2B; Table S2). Achieved levels after week 1 were similar to or higher than those observed 4 weeks after 2 vaccinations in the SARS-CoV-2-naive HCW control group (Figure 1B). When looking at anti-S IgG to 4 variants of concern (VOCs; Alpha [B1.1.7], Beta [B.1.351], Gamma [P.1], and Delta [B.1.617.2]), levels were comparable to wild-type (WT) Wuhan-Hu-1 S protein both pre- and post-vaccination, with discernible increases for all VOCs S proteins after vaccination (Figure 1C; Table S2).
SARS-CoV-2 neutralizing antibody responses
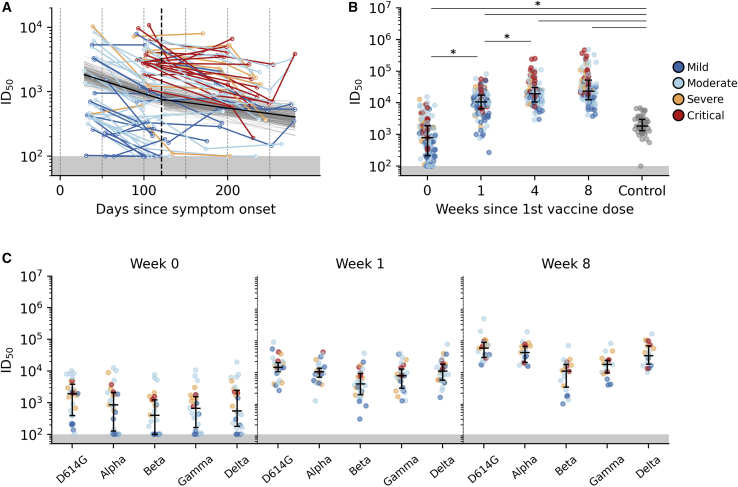
Over time after infection, SARS-CoV-2 neutralizing antibody responses developed, with higher titers observed for individuals with more severe COVID-19 disease outcomes (Figure 2A). A mixed-effects two-phase decay provided a better fit to the observed waning neutralization titers based on the Watanabe-Akaike information criterion (Table S3). Decay was faster during the first phase, with a median half-life of 74 days (95% CrI: 37–160) before transitioning into the second phase, with a slower decay at median half-life of 153 days (95% CrI: 94–809), independent of COVID-19 severity and peak neutralizing response. The median transition time point between the 2 phases was estimated to be on day 123 after onset of symptoms (95% CrI: 50–183).
Figure 2.
Serum neutralization of SARS-CoV-2 pseudovirus
(A) Post-infection serum neutralization levels to SARS-CoV-2. The posterior 2-phase median decay slope and its 95% credible interval are plotted as black and gray lines, respectively. The posterior median transition time point between the 2 phases is indicated as a dashed vertical line.
(B) Pre- (week 0 = before first dose, n = 150 participants), post-vaccination (week 4 = before second dose, n = 101 participants), and HCW control (n = 49) distributions of SARS-CoV-2 pseudovirus neutralization.
(C) Pre-vaccination (week 0, n = 30 participants) and post-vaccination (week 1, n = 30 participants, and week 8, n = 21 participants) serum neutralization distributions of WT D614G and VOC lineages Alpha, Beta, Gamma, and Delta. Each point represents 1 participant colored by COVID-19 severity.
∗: distributions with non-overlapping 95% CI of group mean effect size estimated using a Bayesian ANOVA model (Table S4). Median with interquartile range is depicted. Areas of neutralization titers below detection limit are shaded in gray.
Before vaccination, 132 of 150 (88%) participants still had detectable neutralization titers (median infectious dose [ID50] > 100). Similar to SARS-CoV-2-specific IgG, the neutralization titers increased sharply 1 week after vaccination (median fold increase 10.8 [IQR 4.1–26.0]). A further increase was observed 1 month after the first dose (additional median fold increase 0.8 [IQR 0.1–2.4], achieving higher titers than observed in the control group [Figures 2B, S2C, and S2D; Table S4]). However, further increases after the second dose were minimal (median fold increase between weeks 4 and 8, 0.4 [IQR −0.1 to 1.1]). Using the Bayesian ANOVA model, we estimated that the relative increase in mean neutralizing responses was non-trivial on weeks 1 (95% CrI: 0.66–0.81) and 4 (95% CrI: 0.3–0.23) but trivial on week 8 (95% CrI: −0.01 to 0.13).
The neutralization of VOCs was evaluated in a random selection of 30 participants with detectable WT D614G neutralization titers. While 11 of these 30 participants had undetectable neutralizing activity against ≥1 VOCs before vaccination, neutralization titers rose sharply after the first vaccine dose and were measurable at that time in all of the participants to all four VOCs. Further increases in neutralization titers were observed at 8 weeks post-vaccination (4 weeks post-second dose) to the Alpha and Delta variants, similar to the increase in WT D614G neutralization between weeks 1 and 8 (Figures 2C and S2E; Table S4). The differences between WT D614G and Alpha and Delta VOC neutralization titers were therefore trivial, but not for Beta (95% CrI: −0.24 to −0.07) and Gamma (95% CrI: −0.41 to −0.11), the neutralization titers of which lagged behind at week 8 post-vaccination (Figure S2E; Table S4).
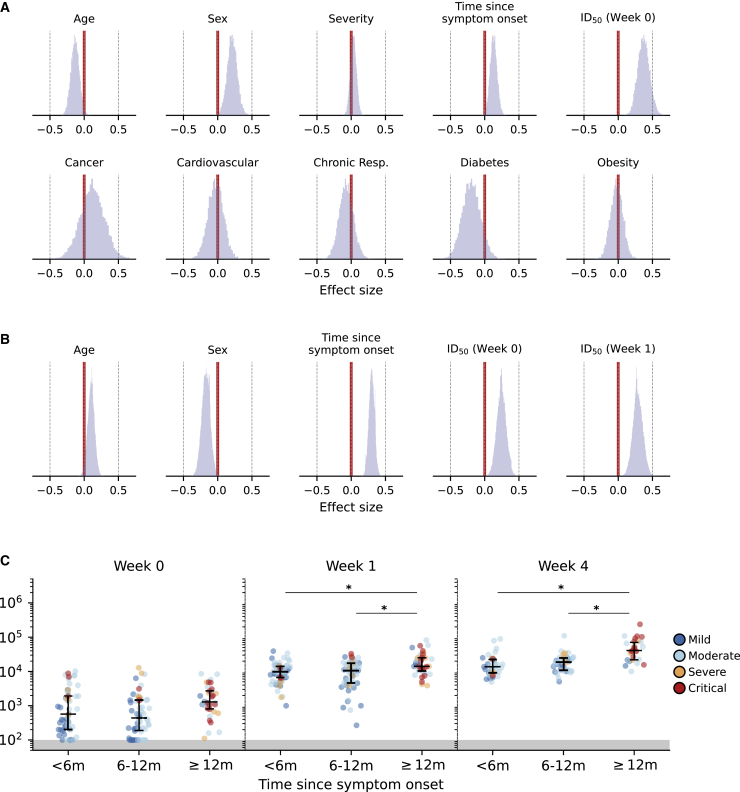
Predictors of vaccine response
We used a Bayesian multilevel regression model to estimate the effect size of variables potentially affecting neutralization levels after SARS-CoV-2 infection, as well as 1 and 4 weeks after the first dose of vaccine where non-trivial changes in neutralizing response were observed. Evidently, the time period since symptom onset has the strongest negative effect (95% CrI: −1.10 to −0.68) on post-infection neutralization titers as antibodies wane over time (Figure S3A). In addition, individuals who are older (95% CrI: 0.10–0.38) and have more severe disease outcomes (95% CrI: 0.11–0.28) are expected to have relatively higher neutralization titers. However, sex and the presence of comorbidities did not have any impact on post-infection neutralizing activity.
After administering the first dose of vaccine, pre-vaccination (week 0) neutralization levels showed the largest positive mean effect on neutralizing response 1 week after vaccination, with clear posterior support of non-trivial effects (95% CrI: 0.21–0.55) (Figure 3A). COVID-19 severity has trivial effect sizes (95% CrI: −0.05 to 0.12) on post-vaccination neutralizing activity, while comorbidities continue to have no impact on antibody responses after vaccination. However, age (95% CrI: −0.25 to −0.01), sex (95% CrI: 0.06–0.36), and the time since symptom onset (95% CrI: 0.03–0.23) exhibited modest non-trivial effects, indicating more pronounced responses in younger female individuals. We then investigated whether these factors continued to have an effect on the further increased neutralizing response 4 weeks after vaccination (Figure 3B). Positive effects were still observed for time since symptom onset (95% CrI: 0.21–0.38) and neutralization levels at previous time points (95% CrI, week 0 [0.11–0.38] and week 1 [0.15–0.44]). Interestingly, age (95% CrI: 0.00–0.20) and sex (95% CrI −0.30 to −0.05) now showed reverse non-trivial effects on neutralization titers, indicating higher responses in older male individuals 1 month after the first dose of vaccine. We repeated our analysis, including the second phase decay rate of post-infection neutralizing response as an additional variable to neutralization titers measured 4 weeks after vaccination (Figure S3B). In this reanalysis, using a smaller subset of participants (n = 66) for whom we were able to estimate the post-infection antibody decay rate, age (95% CrI: −0.09 to 0.19), week 0 neutralization titers (95% CrI: −0.01 to 0.47), and post-infection decay rate (95% CrI: −0.12 to 0.26) were found to have trivial effects. However, sex (95% CrI: −0.37 to −0.01), time since symptom onset (95% CrI: 0.05–0.48), and week 1 neutralization titers (95% CrI: 0.06–0.49) continued to have non-trivial effects.
Figure 3.
Predictors of vaccine response
(A and B) Joint contributions of participant and clinical factors on post-vaccination serum neutralization titers on weeks 1 (A) and 4 (B) after the first vaccine dose. The mean effects across study participants were estimated using a Bayesian multilevel model. All continuous predictors were mean centered and scaled such that effect sizes shown can be compared on a common scale (sex: male encoded as 0 and female encoded as 1).
(C) Distributions of pre- (week 0) and post-vaccination (weeks 1 and 4) in serum neutralization titers of study participants stratified according to time since symptom onset. Each point represents 1 participant colored by COVID-19 severity.
∗: distributions with non-overlapping 95% CI of group mean effect size estimated using a Bayesian ANOVA model (Table S4). Median with interquartile range is depicted. Areas of neutralization titers below detection limit are shaded in gray.
We further analyzed the differentiation in neutralization levels between participants grouped by age, sex, and time since symptom onset using the Bayesian ANOVA model. Before vaccination, neutralization levels could not be meaningfully distinguished between different groups of the aforementioned variables, as their estimated posterior distributions of mean neutralization titers overlapped with each other (Figure S4A). After vaccination, mean neutralization levels were discernible between different sexes (95% CrI difference, week 1 [0.02– 0.24], week 4 [−0.27 to −0.01]). However, post-vaccination neutralizing responses remained indiscernible between different age groups (Figures S4B and S4C). This indicates that while neutralization levels overlapped widely between different age groups, younger individuals were expected to achieve higher neutralizing responses 1 week after vaccination and older individuals were expected to undergo a larger increase in neutralization titers between weeks 1 and 4.
As for the length of time since symptom onset, individuals with illness onset dating >1 year before vaccination yielded discernibly higher mean neutralizing titers (Figures 3C, S4B, and S4C). While most of the recruited participants with severe or critical COVID-19 disease (n = 19/33; 58%) were diagnosed with COVID-19 >1 year before vaccination and these individuals were expected to have higher neutralizing responses post-infection, almost half of the participants with times since symptom onset >12 months (n = 16/35; 48%) had mild to moderate COVID-19 disease severity. Furthermore, the higher post-vaccination neutralizing response among participants with >12 months since illness onset was still observed when only those with mild or moderate COVID-19 disease were included in the analysis (Figure S4D). Pre-vaccination neutralization titers from these individuals were also not discernibly different from those with shorter times since symptom onset (Figures 3C and S4A), indicating that time since symptom onset was independently associated with higher vaccine responses.
Of note, 7 participants were infected with an Alpha lineage variant. While the week 1 binding and neutralizing antibodies responses for these individuals fell within the range of those observed in participants infected with non-VOCs (Figure S3C), the small number of Alpha-infected individuals prevented reliable assessment of any statistically meaningful differences.
Discussion
This study demonstrates that higher levels of neutralizing antibodies are achieved already within 1 week after a single dose of a SARS-CoV-2 mRNA vaccine in previously infected individuals, compared to those observed in fully vaccinated SARS-CoV-2 naive HCWs, irrespective of time since infection. This implies that a single dose in previously infected individuals administered up to >1 year after SARS-CoV-2 infection provides antibody responses associated with the vaccine efficacy observed in the Phase III study of the BNT162b2 mRNA vaccine.15 Furthermore, a second dose has no additional impact on antibody responses. Similar favorable vaccine responses after natural infection have been reported, but these studies were fairly small, restricted to relatively young and healthy HCWs with mostly mild disease who were vaccinated up to 9 months after infection.5, 6, 7, 8, 9, 10, 11, 12, 13, 14 Our prospective COVID-19 cohort allowed the extension of these findings to a broader population at risk and showed that these responses were not affected by the presence of underlying comorbidities, COVID-19 disease severity, or timing of vaccination since infection. Hence, our study supports wide implementation of single-dosing strategies for previously infected individuals.
The Bayesian multilevel regression model showed that pre-vaccination neutralization titers as well as time since infection were associated with higher neutralization titers after vaccination. This may suggest that preexisting antibodies potentially augment immune responses, perhaps through the formation of immune complexes by antibodies binding the vaccine antigen,16,17 and that over time, the memory B cells accumulate higher affinity, resulting in higher recall response after vaccination.2 In keeping with other studies,9,18, 19, 20, 21 we observed that age and male sex inversely correlated with vaccine responses early after vaccination; however, this leveled out again 4 weeks after vaccination, even though variation in the neutralization titers was large and effect sizes overlapped between the sex and age groups.
The emergence of SARS-CoV-2 variants may pose risks of infection despite immunity induced by natural infection and vaccination. Emerging observations indicate substantial reductions of vaccine-induced antibodies in binding and neutralization capacity against several VOCs, including Beta and Gamma.12,22, 23, 24, 25, 26, 27 After vaccination, we found sharp increases in IgG binding and neutralization levels for the 4 VOCs. However, neutralization titers for the Beta and Gamma variants lagged behind those for WT and the other VOCs after the second dose. Overall, these results suggest that neutralization breadth was not improved after vaccination, most likely because neutralization after vaccination is overwhelmingly dominated by RBD responses, which are shown to be more sensitive to the mutations in the VOC.28 Nevertheless, a degree of cross-neutralization of these four VOCs was observed in all of the participants already after a single dose in previously infected individuals, also against the highly prevalent Delta variant, with titers favorably compared to Beta and Gamma.
Longer follow-up will determine the longevity of the immune response and protective efficacy after single and double dosing in previously infected individuals. In addition, while similar antibody boost responses would be anticipated for other SARS-CoV-2 vaccines, this needs to be confirmed in future studies, especially for non-mRNA vaccines. In the meantime, the findings of this study support wide implementation of a single-dose mRNA vaccine strategy after prior SARS-CoV-2 infection to save vaccines and resources, hence expediting vaccination uptake at community levels worldwide.
Limitations of the study
There are several limitations of our study. As only symptomatic COVID-19 patients were enrolled in the RECoVERED cohort, we were unable to study vaccine responses after previous asymptomatic SARS-CoV-2 infection. However, an earlier study in HCWs observed no differences in antibody responses to a mRNA vaccine between individuals with prior asymptomatic and symptomatic SARS-CoV-2 infections.13 Furthermore, the SARS-CoV-2-naive HCW controls were not matched with the previously infected cohort participants for potentially relevant factors such as age, sex, or the presence of comorbidities. However, given that antibody responses in the healthier and younger HCW controls were lower, combined with our finding that age is inversely correlated with early antibody vaccine response, the observed difference in vaccine response may even have been more pronounced if controls were matched. Only serological responses have been studied, as these have been shown to strongly correlate with vaccine efficacy.29,30 However, other immune components such as T cells likely play important roles in illness protection as well. Finally, participants with severe COVID-19 were overrepresented in the subgroup with >12-month intervals between infection and vaccination, but fold increases in neutralization were very similar for all time interval subgroups, independent of disease severity.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Goat-anti-human IgG-PE | Southern Biotech | Cat# 2040-09; RRID: AB_2795648 |
| Biological samples | ||
| Sera COVID-19 patients | This paper | N/A |
| Chemicals, peptides, and recombinant proteins | ||
| Poly-L-Lysine Hydrobromide | Sigma-Aldrich | Cat# P1399 |
| Tetanus Toxoid | Creative Biolabs | Cat#: Vcar-Lsx003 |
| Respiratory syncytial virus fusion glycoprotein | McLellan et al. Science 201331 | N/A |
| Influenza A/H1N1pdm09 virus HA protein | Aartse et al. Vaccines 202132 | N/A |
| SARS-CoV-2 Nucleocapsid | Obtained from the lab of Gestur Vidarsson | N/A |
| SARS-CoV-2 Spike (VOCs) | Caniels et al. Science Advances 202127 | N/A |
| SARS-CoV-2 RBD | Brouwer et al. Science 202033 | N/A |
| Critical commercial assays | ||
| Nano-Glo Luciferase Assay System | Promega | Cat# N1130 |
| Luminex Magplex beads | Luminex | Cat#: MC10043-01 |
| Experimental models: cell lines | ||
| HEK293T/ACE2 cells | Obtained from the lab of Paul Bieniasz | N/A |
| HEK293F cells | Thermo Fisher | Cat# R79007 |
| HEK293T cells | ATCC | Cat# CRL-11268 |
| Recombinant DNA | ||
| pHIV-1NL43ΔENV-NanoLuc plasmid | Obtained from the lab of Paul Bieniasz | N/A |
| SARS-CoV-2-SΔ19 plasmid | Obtained from the lab of Paul Bieniasz | N/A |
| SARS-CoV-2 S pPPI4 plasmid | Brouwer et al. Science 202033 | N/A |
| SARS-CoV-2 RBD pPPI4 plasmid | Brouwer et al. Science 202033 | N/A |
| Software and algorithms | ||
| GraphPad Prism v8 | GraphPad | N/A |
| pymc3 | Python | N/A |
| Python/matplotlib | Python | N/A |
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Menno de Jong (m.d.dejong@amsterdamumc.nl).
Materials availability
This study did not generate new unique reagents.
Experimental model and subject details
The current vaccine study was embedded in the RECoVERED project, an ongoing prospective cohort study of individuals with laboratory-confirmed SARS-CoV-2 infection in Amsterdam, the Netherlands.34 The RECoVERED cohort was initiated in May 2020 and as of April 2021 enrolled 328 participants, including both home-cared patients with mild infections and hospitalized patients with moderate to severe or critical illness. RECoVERED participants are followed at 1-3-month intervals from illness onset whereby biological specimens and questionnaires are collected at each follow-up visit to address RECoVERED’s primary objectives relating to immunology and long-term sequelae of COVID-19. Clinical severity groups were defined in line with the WHO COVID-19 disease severity criteria35: Mild disease was defined as having a respiratory rate (RR) < 20/min and oxygen saturation (SpO2) on room air > 94% during acute illness; moderate disease as having a RR of 20-30/min, SpO2 90%–94% and/or receiving oxygen therapy; severe disease as having a RR > 30/min or SpO2 < 90%; and critical disease as requiring ICU admission.
Cohort participants, invited to receive vaccination according to the Dutch national vaccination campaign before 12 April 2021, were asked to participate in the present vaccine substudy. In addition, participants not yet prioritized for vaccination according to Dutch policy were asked to participate and receive the BNT162b2 (Pfizer-BioNTech) mRNA vaccine in April 2021, made available for our research aim by the Dutch Ministry of Health, Welfare and Sport. Participants with pregnancy, vaccine-related allergic reactions or laboratory-confirmed infections within 4 weeks of expected vaccination were excluded as per national guidelines. Serum samples for determination of antibody levels were collected over time after infection and vaccination (Figure S1) and participants completed questionnaires on the presence and severity of symptoms pre-vaccination and vaccine-related adverse effects within one week post-vaccination.
Vaccinated HCW without longitudinal serological evidence of prior SARS-CoV-2 infection, participating in a HCW cohort study at the Amsterdam University Medical Centers (S3 study), served as a control group.36 In this cohort, antibody responses were measured four weeks after the second dose of the BNT162b2 mRNA vaccine. The RECoVERED study, including the vaccine substudy, and the S3 study were approved by the medical ethical review board of the Amsterdam University Medical Centers (NL73759.018.20 and NL73478.029.20, respectively). All participants provided written informed consent.
Method details
SARS-CoV-2 binding IgG antibody levels
Levels of Immunoglobulin G (IgG) binding to SARS-CoV-2 receptor-binding domain (RBD), nucleocapsid (N) and spike (S) proteins of wild-type (WT) virus (Wuhan-Hu-1; GenBank: MN908947.3) and variants of concern (VOCs; Alpha (B1.1.7), Beta (B.1.351), Gamma (P.1) and Delta (B.1.617.2)), as well as to control proteins tetanus toxoid, respiratory syncytial virus fusion glycoprotein (RSV-F) and influenza A/H1N1pdm09 virus HA protein, were determined using a custom luminex assay as described previously.33 The VOCs S constructs contained the following mutations compared to the WT27; (ΔH69-V70, ΔY144, N501Y, A570D, D614G, P681H, T716I, S982A, D1118H in Alpha; L18F, D80A, D215G, L242H, R246I, K417N, E484K, N501Y, D614G, A701V in Beta; L18F, T20N, P26S, D138Y, R190S, K417T, E484K, N501Y, D614G, H655Y, T1027I in Gamma; T19R, K77T, G142D, L452R, T478K, D614G, D950N in Delta). In short, proteins were produced in HEK293F cells (Invitrogen) and purified from the cell culture supernatant using affinity chromatography with NiNTA agarose beads (QIAGEN). Proteins were covalently coupled to luminex magplex beads using a two-step carbodiimide reaction. Beads were incubated overnight with 1:100,000 diluted serum followed by detection with goat-anti-human IgG-PE (Southern Biotech) on a Magpix (Luminex) as the mean fluorescent intensity (MFI).
Pseudovirus neutralization assay
Pseudovirus neutralization assay was performed as previously described.33 Briefly, HEK293T/ACE2 cells37 were seeded in poly-L-lysine pre-coated 96-well plates. The next day, heat-inactivated 1:100 diluted sera were 3-fold serially diluted and mixed in a 1:1 ratio with pseudovirus Wuhan-Hu-1 D614G (WT D614G)37 or VOC27 (ΔH69-V70, ΔY144, N501Y, A570D, D614G, P681H, T716I, S982A, D1118H in Alpha; L18F, D80A, D215G, L242H, R246I, K417N, E484K, N501Y, D614G, A701V in Beta; L18F, T20N, P26S, D138Y, R190S, K417T, E484K, N501Y, D614G, H655Y, T1027I in Gamma; T19R, K77T, G142D, ΔE156-F157, R158G, L452R, T478K, D614G, D950N in Delta). After 1-hour incubation at 37°C the mixtures were added to the cells and incubated for 48 hours at 37°C. The luciferase activity in cell lysates was measured using the Nano-Glo Luciferase Assay System (Promega) and GloMax system (Turner BioSystems). The 50% inhibitory dilution (ID50) titers were determined as the serum dilution at which infectivity was inhibited by 50% using a non-linear regression curve fit (GraphPad Prism software version 8.3).
Neutralization decay model
We estimated the decay rates in antibody response after infection using data based on 66 participants for which serum samples were collected for at least two time points 4 weeks after their respective symptom onset date. We performed Bayesian hierarchical linear regression of the mean log response variable () against time since symptom onset (), partially pooling decay rates across participants . Following previous analyses by others,38 two models were considered, including a single-phase constant decay model:
and a two-phase decay model:
where
and are the participant-specific intercept and constant decay rate. In the two-phase decay model, is the difference in decay rates between the first and second phase. is the estimated transition time point between the two phases. Detailed prior formulations of , and are described in the supplemental information. The Watanabe-Akaike information criterion was computed to assess model fit.
Quantification and statistical analysis
A Bayesian hierarchical generalization of the one-way ANOVA model was used to compare control, pre- and post-vaccination neutralizing and IgG antibody binding titers. Differences between groups are reported as differences in effect sizes. A difference in effect size is non-trivial if it is non-zero, and substantial if greater/lower than 1/-1. This model was also used to estimate the individual effect size of age (i.e., ≤ 45 years, 46-65 years and > 65 years), sex (i.e., male and female) and time since symptom onset until vaccination (i.e., ≤ 6 months, 7-12 months and > 12 months) on the observed vaccine responses.
To identify and estimate the effect size of different predictor variables on the observed SARS-CoV-2 neutralization titers, we used a Bayesian hierarchical model that partially pooled effect size estimates across all study participants . We assumed a linear correlation between the mean-centered predicted log neutralization values and predictor variables :
where is the normalized effects of variable for participant and is the participant-specific intercept.
We assumed that the observed mean-centered and scaled neutralization values follow a Student-T distribution about the predicted with error-term standard deviation with degrees of freedom:
We assumed that is exponentially distributed with a mean of 30 such that high prior probability was allocated over parameter values that describe the range from normal to heavy-tailed data under the Student-T distribution39:
The intercepts were assumed to be normally distributed about a common mean intercept with standard deviation :
The participant-specific effect sizes of variable were assumed to be normally distributed about a common mean effect size with a predictor-specific standard deviation :
Weakly informative priors were placed on all standard deviation terms to constrain parameter inferences within biologically and mathematically plausible values40:
A weakly informative Gaussian prior was also placed for the mean intercept while a weakly informative Student-T prior was placed on the mean effect size for each predictor :
Furthermore, a Bayesian multilevel model that partially pooled effect size estimates across all study participants was used to estimate the effect size of the predictor variables individually and in combination on post-vaccination serum neutralization levels (weeks 1 and 4 after first dose of vaccine). We investigated if, and the degree to which, participants’ age, sex, presence of comorbidities (i.e., history of cancer, cardiovascular disease, chronic respiratory disease, diabetes mellitus and obesity, separately), COVID-19 severity, time since COVID-19 symptom onset, pre-vaccination neutralization titers, and post-infection decay rate of neutralizing response were correlated with vaccine response. Condition indices were computed to ensure that there was no collinearity among the predictor variables (i.e., condition index < 10). A distribution of normalized effect sizes (analogous to regression coefficients) was estimated for each predictor variable as a measure of their relative contributions to vaccine response. Similar to the Bayesian ANOVA model, an effect size is non-trivial if it is non-zero, and substantial if greater/lower than 1.
All models were fitted using Markov Chain Monte Carlo (MCMC) with pymc3,41 implementing a no-u-turn sampler. Four MCMC chains were run with at least 4000 burn-in steps and 2000 saved posterior samples. Convergence for all parameters were verified by checking trace plots, ensuring their values were < 1.05 with sufficient effective sample size (> 200).
Consortia
The members of the RECoVERED Study Group are as follows: Public Health Service of Amsterdam: Ivette Agard, Jane Ayal, Anders Boyd, Floor Cavdar, Marianne Craanen, Udi Davidovich, Annemarieke Deuring, Annelies van Dijk, Ertan Ersan, Laura del Grande, Joost Hartman, Nelleke Koedoot, Tjalling Leenstra, Dominique Loomans, Agata Makowska, Tom du Maine, Ilja de Man, Amy Matser, Lizenka van der Meij, Marleen van Polanen, Maria Oud, Clark Reid, Leeann Storey, Marije de Wit, and Marc van Wijk; Amsterdam University Medical Centers: Joyce van Assem, Joost van den Aardweg, Marijne van Beek, Thyra Blankert, Brigitte Boeser-Nunnink, Eric Moll van Charante, Karel van Dort, Orlane Figaroa, Leah Frenkel, Arginell Girigorie, Jelle van Haga, Agnes Harskamp-Holwerda, Mette Hazenberg, Soemeja Hidad, Nina de Jong, Marcel Jonges, Suzanne Jurriaans, Hans Knoop, Lara Kuijt, Anja Lok, Marga Mangas Ruiz, Irma Maurer, Pythia Nieuwkerk, Ad van Nuenen, Annelou van der Veen, Bas Verkaik, and Gerben-Rienk Visser.
Acknowledgments
The authors wish to thank all RECoVERED and S3 study participants; Mathieu A.F. Claireaux of Amsterdam UMC, Amsterdam, the Netherlands, for providing the control proteins; Dirk Eggink and Chantal Reusken of the National Institute for Public Health and the Environment, Bilthoven, the Netherlands, for providing the SARS-CoV-2 B.1.617.2 S protein; Gestur Vidarsson and Federica Linty of Sanquin, Amsterdam, the Netherlands, for providing the SARS-CoV-2 nucleocapsid protein; Dr. Paul Bieniasz and Theodora Hatziioannou of the Howard Hughes Medical Institute, Rockefeller University, New York, NY, USA, and Dr. Beatrice Hahn of the Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA, USA, for donating cells and reagents for pseudovirus neutralization assays; Mohamed Hoesein, Marco Hol, Melvin Angelovski, and their team at the Public Health Service of Amsterdam, Amsterdam, the Netherlands, for valuable logistical support of the vaccine study; and the Amsterdam UMC COVID-19 S3/HCW study group (D. van de Beek, M.C. Brouwer, D.T.P. Buis, N. Chekrouni, N. van Mourik, S.E. Olie, E.J.G. Peters, T.D.Y. Reijnders, M. Schinkel, M.A. Slim, and W.J. Wiersinga). This work was supported by the Netherlands Organization for Scientific Research (NWO) ZonMw (RECoVERED, grant agreement no. 10150062010002, to M.D.d.J., the S3 study, grant agreement no. 10430022010023, to M.K.B., and Vici grant no. 91818627, to R.W.S.), the Bill and Melinda Gates Foundation (grant nos. INV-002022 and INV008818, to R.W.S., and INV-024617, to M.J.v.G.), Amsterdam UMC through the AMC Fellowship (to M.J.v.G.) and the Corona Research Fund (to M.K.B.), the European Research Council (no. 818353, to C.A.R.), the European Union’s Horizon 2020 program (RECoVER, grant no. 101003589, to M.D.d.J.), and the Public Health Service of Amsterdam (Research & Development grant no. 21-14, to M.P.). The funders had no role in study design, data collection, data analysis, data interpretation, or data reporting.
Author contributions
Conceptualization, M.J.v.G., C.A.R., M. Prins, G.J.d.B., and M.D.d.J.; funding acquisition, M.J.v.G., A.X.H., J.J.S., R.W.S., C.A.R., M. Prins, and M.D.d.J.; investigation, H.D.G.v.W., E.W., K.v.d.S., J.A.B., M.O., K.T., M. Poniman, and J.H.B.; methodology, M.J.v.G., H.D.G.v.W., E.W., A.X.H., K.v.d.S., R.W.S., C.A.R., M. Prins, G.J.d.B., and M.D.d.J.; project administration, H.D.G.v.W., E.W., N.A.K., M. Prins, G.J.d.B., and M.D.d.J.; resources, H.D.G.v.W., E.W., A.V., R.L., M.D., J.A.B., M.O., K.T., M. Poniman, J.H.B., B.A., A.H.A.L., T.G.C., I.B., L.A.v.V., A.P.J.V., J.J.S., M.K.B., R.W.S., and the RECoVERED Study Group; data analysis, M.J.v.G., A.X.H., K.v.d.S., and M. Poniman; supervision, M.J.v.G., A.P.J.V., J.J.S., M.K.B., N.A.K., C.A.R., M. Prins., G.J.d.B., and M.D.d.J.; writing – original draft, M.J.v.G., A.X.H., C.A.R., and M.D.d.J.; writing – review & editing, all authors.
Declaration of interests
The authors declare no competing interests.
Inclusion and diversity
We worked to ensure gender balance in the recruitment of human subjects. We worked to ensure ethnic or other types of diversity in the recruitment of human subjects. We worked to ensure that the study questionnaires were prepared in an inclusive way. One or more of the authors of this article self-identifies as a member of the LGBTQ+ community. The author list of this article includes contributors from the location where the research was conducted who participated in the data collection, design, analysis, and/or interpretation of the work.
Published: December 14, 2021
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.xcrm.2021.100486.
Contributor Information
Godelieve J. de Bree, Email: g.j.debree@amsterdamumc.nl.
Menno D. de Jong, Email: m.d.dejong@amsterdamumc.nl.
RECoVERED Study Group:
Ivette Agard, Jane Ayal, Anders Boyd, Floor Cavdar, Marianne Craanen, Udi Davidovich, Annemarieke Deuring, Annelies van Dijk, Ertan Ersan, Laura del Grande, Joost Hartman, Nelleke Koedoot, Tjalling Leenstra, Dominique Loomans, Agata Makowska, Tom du Maine, Ilja de Man, Amy Matser, Lizenka van der Meij, Marleen van Polanen, Maria Oud, Clark Reid, Leeann Storey, Marije de Wit, Marc van Wijk, Joyce van Assem, Joost van den Aardweg, Marijne van Beek, Thyra Blankert, Brigitte Boeser-Nunnink, Eric Moll van Charante, Karel van Dort, Orlane Figaroa, Leah Frenkel, Arginell Girigorie, Jelle van Haga, Agnes Harskamp-Holwerda, Mette Hazenberg, Soemeja Hidad, Nina de Jong, Marcel Jonges, Suzanne Jurriaans, Hans Knoop, Lara Kuijt, Anja Lok, Marga Mangas Ruiz, Irma Maurer, Pythia Nieuwkerk, Ad van Nuenen, Annelou van der Veen, Bas Verkaik, and Gerben-Rienk Visser
Supplemental information
Data and code availability
Any additional information required to reanalyse the data reported in this paper is available from the lead contact upon request.
References
- 1.Omer S.B., Yildirim I., Forman H.P. Herd Immunity and Implications for SARS-CoV-2 Control. JAMA. 2020;324:2095–2096. doi: 10.1001/jama.2020.20892. [DOI] [PubMed] [Google Scholar]
- 2.Wang Z., Muecksch F., Schaefer-Babajew D., Finkin S., Viant C., Gaebler C., Hoffmann H.H., Barnes C.O., Cipolla M., Ramos V., et al. Naturally enhanced neutralizing breadth against SARS-CoV-2 one year after infection. Nature. 2021;595:426–431. doi: 10.1038/s41586-021-03696-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dan J.M., Mateus J., Kato Y., Hastie K.M., Yu E.D., Faliti C.E., Grifoni A., Ramirez S.I., Haupt S., Frazier A., et al. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science. 2021;371:eabf4063. doi: 10.1126/science.abf4063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wajnberg A., Amanat F., Firpo A., Altman D.R., Bailey M.J., Mansour M., McMahon M., Meade P., Mendu D.R., Muellers K., et al. Robust neutralizing antibodies to SARS-CoV-2 infection persist for months. Science. 2020;370:1227–1230. doi: 10.1126/science.abd7728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anichini G., Terrosi C., Gandolfo C., Gori Savellini G., Fabrizi S., Miceli G.B., Cusi M.G. SARS-CoV-2 Antibody Response in Persons with Past Natural Infection. N. Engl. J. Med. 2021;385:90–92. doi: 10.1056/NEJMc2103825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jalkanen P., Kolehmainen P., Häkkinen H.K., Huttunen M., Tähtinen P.A., Lundberg R., Maljanen S., Reinholm A., Tauriainen S., Pakkanen S.H., et al. COVID-19 mRNA vaccine induced antibody responses against three SARS-CoV-2 variants. Nat. Commun. 2021;12:3991. doi: 10.1038/s41467-021-24285-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bradley T., Grundberg E., Selvarangan R., LeMaster C., Fraley E., Banerjee D., Belden B., Louiselle D., Nolte N., Biswell R., et al. Antibody Responses after a Single Dose of SARS-CoV-2 mRNA Vaccine. N. Engl. J. Med. 2021;384:1959–1961. doi: 10.1056/NEJMc2102051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Manisty C., Otter A.D., Treibel T.A., McKnight Á., Altmann D.M., Brooks T., Noursadeghi M., Boyton R.J., Semper A., Moon J.C. Antibody response to first BNT162b2 dose in previously SARS-CoV-2-infected individuals. Lancet. 2021;397:1057–1058. doi: 10.1016/S0140-6736(21)00501-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Prendecki M., Clarke C., Brown J., Cox A., Gleeson S., Guckian M., Randell P., Pria A.D., Lightstone L., Xu X.N., et al. Effect of previous SARS-CoV-2 infection on humoral and T-cell responses to single-dose BNT162b2 vaccine. Lancet. 2021;397:1178–1181. doi: 10.1016/S0140-6736(21)00502-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krammer F., Srivastava K., Alshammary H., Amoako A.A., Awawda M.H., Beach K.F., Bermúdez-González M.C., Bielak D.A., Carreño J.M., Chernet R.L., et al. Antibody Responses in Seropositive Persons after a Single Dose of SARS-CoV-2 mRNA Vaccine. N. Engl. J. Med. 2021;384:1372–1374. doi: 10.1056/NEJMc2101667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ebinger J.E., Fert-Bober J., Printsev I., Wu M., Sun N., Prostko J.C., Frias E.C., Stewart J.L., Van Eyk J.E., Braun J.G., et al. Antibody responses to the BNT162b2 mRNA vaccine in individuals previously infected with SARS-CoV-2. Nat. Med. 2021;27:981–984. doi: 10.1038/s41591-021-01325-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stamatatos L., Czartoski J., Wan Y.H., Homad L.J., Rubin V., Glantz H., Neradilek M., Seydoux E., Jennewein M.F., MacCamy A.J., et al. mRNA vaccination boosts cross-variant neutralizing antibodies elicited by SARS-CoV-2 infection. Science. 2021;9175:eabg9175. doi: 10.1126/science.abg9175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saadat S., Rikhtegaran Tehrani Z., Logue J., Newman M., Frieman M.B., Harris A.D., Sajadi M.M. Binding and Neutralization Antibody Titers After a Single Vaccine Dose in Health Care Workers Previously Infected With SARS-CoV-2. JAMA. 2021;325:1467–1469. doi: 10.1001/jama.2021.3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hall V.J., Foulkes S., Saei A., Andrews N., Oguti B., Charlett A., Wellington E., Stowe J., Gillson N., Atti A., et al. SIREN Study Group COVID-19 vaccine coverage in health-care workers in England and effectiveness of BNT162b2 mRNA vaccine against infection (SIREN): a prospective, multicentre, cohort study. Lancet. 2021;397:1725–1735. doi: 10.1016/S0140-6736(21)00790-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., Perez J.L., Pérez Marc G., Moreira E.D., Zerbini C., et al. C4591001 Clinical Trial Group Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N. Engl. J. Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Michaud H.-A., Gomard T., Gros L., Thiolon K., Nasser R., Jacquet C., Hernandez J., Piechaczyk M., Pelegrin M. A crucial role for infected-cell/antibody immune complexes in the enhancement of endogenous antiviral immunity by short passive immunotherapy. PLoS Pathog. 2010;6:e1000948. doi: 10.1371/journal.ppat.1000948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wen Y.-M., Mu L., Shi Y. Immunoregulatory functions of immune complexes in vaccine and therapy. EMBO Mol. Med. 2016;8:1120–1133. doi: 10.15252/emmm.201606593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Müller L., Andrée M., Moskorz W., Drexler I., Walotka L., Grothmann R., Ptok J., Hillebrandt J., Ritchie A., Rabl D., et al. Age-dependent immune response to the Biontech/Pfizer BNT162b2 COVID-19 vaccination. Clin. Infect. Dis. 2021:ciab381. doi: 10.1093/cid/ciab381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zimmermann P., Curtis N. Factors That Influence the Immune Response to Vaccination. Clin. Microbiol. Rev. 2019;32:e00084-18. doi: 10.1128/CMR.00084-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klein S.L., Flanagan K.L. Sex differences in immune responses. Nat. Rev. Immunol. 2016;16:626–638. doi: 10.1038/nri.2016.90. [DOI] [PubMed] [Google Scholar]
- 21.Fischinger S., Boudreau C.M., Butler A.L., Streeck H., Alter G. Sex differences in vaccine-induced humoral immunity. Semin. Immunopathol. 2019;41:239–249. doi: 10.1007/s00281-018-0726-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barros-Martins J., Hammerschmidt S.I., Cossmann A., Odak I., Stankov M.V., Morillas Ramos G., Dopfer-Jablonka A., Heidemann A., Ritter C., Friedrichsen M., et al. Immune responses against SARS-CoV-2 variants after heterologous and homologous ChAdOx1 nCoV-19/BNT162b2 vaccination. Nat. Med. 2021;27:1525–1529. doi: 10.1038/s41591-021-01449-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tada T., Zhou H., Dcosta B.M., Samanovic M.I., Mulligan M.J., Landau N.R. SARS-CoV-2 Lambda Variant Remains Susceptible to Neutralization by mRNA Vaccine-elicited Antibodies and Convalescent Serum. bioRxiv. 2021 https://pesquisa.bvsalud.org/global-literature-on-novel-coronavirus-2019-ncov/resource/en/ppbiorxiv-450959 [Google Scholar]
- 24.Garcia-Beltran W.F., Lam E.C., St Denis K., Nitido A.D., Garcia Z.H., Hauser B.M., Feldman J., Pavlovic M.N., Gregory D.J., Poznansky M.C., et al. Multiple SARS-CoV-2 variants escape neutralization by vaccine-induced humoral immunity. Cell. 2021;184:2372–2383.e9. doi: 10.1016/j.cell.2021.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reynolds C.J., Pade C., Gibbons J.M., Butler D.K., Otter A.D., Menacho K., Fontana M., Smit A., Sackville-West J.E., Cutino-Moguel T., et al. UK COVIDsortium Immune Correlates Network. UK COVIDsortium Investigators Prior SARS-CoV-2 infection rescues B and T cell responses to variants after first vaccine dose. Science. 2021;1282:1–11. doi: 10.1126/science.abh1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goel R.R., Apostolidis S.A., Painter M.M., Mathew D., Pattekar A., Kuthuru O., Gouma S., Hicks P., Meng W., Rosenfeld A.M., et al. Distinct antibody and memory B cell responses in SARS-CoV-2 naïve and recovered individuals following mRNA vaccination. Sci. Immunol. 2021;6:eabi6950. doi: 10.1126/sciimmunol.abi6950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Caniels T.G., Bontjer I., van der Straten K., Poniman M., Burger J.A., Appelman B., Lavell H.A.A., Oomen M., Godeke G.J., Valle C., et al. Amsterdam UMC COVID-19 S3/HCW Study Group Emerging SARS-CoV-2 variants of concern evade humoral immune responses from infection and vaccination. Sci. Adv. 2021;7:eabj5365. doi: 10.1126/sciadv.abj5365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang P., Nair M.S., Liu L., Iketani S., Luo Y., Guo Y., Wang M., Yu J., Zhang B., Kwong P.D., et al. Antibody resistance of SARS-CoV-2 variants B.1.351 and B.1.1.7. Nature. 2021;593:130–135. doi: 10.1038/s41586-021-03398-2. [DOI] [PubMed] [Google Scholar]
- 29.Earle K.A., Ambrosino D.M., Fiore-Gartland A., Goldblatt D., Gilbert P.B., Siber G.R., Dull P., Plotkin S.A. Evidence for antibody as a protective correlate for COVID-19 vaccines. Vaccine. 2021;39:4423–4428. doi: 10.1016/j.vaccine.2021.05.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Khoury D.S., Cromer D., Reynaldi A., Schlub T.E., Wheatley A.K., Juno J.A., Subbarao K., Kent S.J., Triccas J.A., Davenport M.P. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat. Med. 2021;27:1205–1211. doi: 10.1038/s41591-021-01377-8. [DOI] [PubMed] [Google Scholar]
- 31.McLellan J.S., Chen M., Leung S., Graepel K.W., Du X., Yang Y., et al. Structure of RSV fusion glycoprotein trimer bound to a prefusion- specific neutralizing antibody. Science. 2013;340:1113–1117. doi: 10.1126/science.1234914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aartse A., Eggink D., Claireaux M., van Leeuwen S., Mooij P., Bogers W.M., et al. Influenza A Virus Hemagglutinin Trimer, Head and Stem Proteins Identify and Quantify Different Hemagglutinin-Specific B Cell Subsets in Humans. Vaccines (Basel). 2021;9:717. doi: 10.3390/vaccines9070717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brouwer P.J.M., Caniels T.G., van der Straten K., Snitselaar J.L., Aldon Y., Bangaru S., Torres J.L., Okba N.M.A., Claireaux M., Kerster G., et al. Potent neutralizing antibodies from COVID-19 patients define multiple targets of vulnerability. Science. 2020;369:643–650. doi: 10.1126/science.abc5902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wynberg E., van Willigen H.D.G., Dijkstra M., Boyd A., Kootstra N.A., van den Aardweg J.G., van Gils M.J., Matser A., de Wit M.R., Leenstra T., et al. Evolution of coronavirus disease 2019 (COVID-19) symptoms during the first 12 months after illness onset. Clin. Infect. Dis. 2021 doi: 10.1093/cid/ciab759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.World Health Organization . 2021. Global COVID-19 Clinical Platform Case Report Form (CRF) for Post COVID condition (Post COVID-19 CRF)https://www.who.int/publications/i/item/global-covid-19-clinical-platform-case-report-form-(crf)-for-post-covid-conditions-(post-covid-19-crf-) [Google Scholar]
- 36.Sikkens J.J., Buis D.T.P., Peters E.J.G., Dekker M., Schinkel M., Reijnders T.D.Y., Schuurman A.R., de Brabander J., Lavell A.H.A., Maas J.J., et al. Serologic Surveillance and Phylogenetic Analysis of SARS-CoV-2 Infection Among Hospital Health Care Workers. JAMA Netw. Open. 2021;4:e2118554. doi: 10.1001/jamanetworkopen.2021.18554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schmidt F., Weisblum Y., Muecksch F., Hoffmann H.H., Michailidis E., Lorenzi J.C.C., Mendoza P., Rutkowska M., Bednarski E., Gaebler C., et al. Measuring SARS-CoV-2 neutralizing antibody activity using pseudotyped and chimeric viruses. J. Exp. Med. 2020;217:e20201181. doi: 10.1084/jem.20201181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wheatley A.K., Juno J.A., Wang J.J., Selva K.J., Reynaldi A., Tan H.X., Lee W.S., Wragg K.M., Kelly H.G., Esterbauer R., et al. Evolution of immune responses to SARS-CoV-2 in mild-moderate COVID-19. Nat. Commun. 2021;12:1162. doi: 10.1038/s41467-021-21444-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kruschke J.K. Academic Press; 2011. Doing Bayesian Data Analysis : A Tutorial with R and BUGS. [Google Scholar]
- 40.Gelman A. Prior distributions for variance parameters in hierarchical models (comment on article by Browne and Draper) Bayesian Anal. 2006;1:515–534. [Google Scholar]
- 41.Salvatier J., Wiecki T.V., Fonnesbeck C. Probabilistic programming in Python using PyMC3. PeerJ Comput. Sci. 2016;2:e55. doi: 10.7717/peerj-cs.1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Any additional information required to reanalyse the data reported in this paper is available from the lead contact upon request.