Abstract
Kisspeptin neurons in the arcuate nucleus (ARC), which co-express neurokinin B (NKB) and dynorphin A, are termed KNDy neurons. These neurons are candidates for the intrinsic gonadotropin-releasing hormone (GnRH) pulse generator. The central and peripheral administration of NKB or its receptor (NK3R) agonist evokes GnRH pulse generator activity and the subsequent pulsatile GnRH/luteinizing hormone (LH) secretion. However, the mechanism responsible for neural activation of the GnRH pulse generator in goats is unclear. We conducted electrophysiological and histochemical experiments to test the hypothesis that KNDy neurons receive NKB and that the signal is transmitted bilaterally to a population of KNDy neurons. Bilateral electrodes aimed at a cluster of KNDy neurons were inserted into the ovariectomized goat ARC. We observed the GnRH pulse generator activity, represented by characteristic increases in multiple-unit activity (MUA volleys). The unilateral administration of NKB or vehicle in the close vicinity of KNDy neurons under simultaneous MUA recording from both sides revealed that only NKB evoked MUA volley(s) immediately after administration. The timing of the MUA volley(s) evoked on the ipsilateral side was synchronized to that on the contralateral side. The double-labeled ISH for KISS1 and TACR3, which encode kisspeptin and NK3R, respectively, revealed that most KNDy neurons co-expressed TACR3. Therefore, NKB could directly stimulate KNDy neurons, following which the stimulatory signal is immediately transmitted to the entire population of KNDy neurons via connection with their fibers. This mechanism helps synchronize burst activity among KNDy neurons, thereby generating neural signals that govern pulsatile GnRH secretion.
Keywords: Arcuate nucleus, GnRH pulse generator, Kisspeptin, Neurokinin B
Reproductive function in mammals is controlled by the hypothalamic gonadotropin-releasing hormone (GnRH) pulse generator, which governs intermittent GnRH secretion into the portal vessels and regulates the release of pulsatile luteinizing hormone (LH) into the peripheral circulation. The activity of the GnRH pulse generator can be monitored by recording multiple-unit activity (MUA) in the mediobasal hypothalamus and has been represented as episodic bursts of MUA (MUA volleys) in monkeys [1], rats [2], and goats [3, 4]. Thus, neural substrates firing a high-frequency volley of action potentials command pulsatile GnRH secretion.
Kisspeptin plays an indispensable role in the control of GnRH release [5, 6]. Kisspeptin-expressing neurons (kisspeptin neurons) are exclusively distributed in the medial preoptic area (the anteroventral periventricular nucleus in rodents) and arcuate nucleus (ARC) [7, 8]. In several species, kisspeptin neurons in the ARC co-express neuropeptides implicated in the control of reproduction, including neurokinin B (NKB) and dynorphin A (Dyn) [9,10,11]. Therefore, kisspeptin neurons in the ARC are termed kisspeptin/NKB/Dyn (KNDy) neurons.
Our previous study on goats reported the GnRH pulse generator proximity of KNDy neurons, suggesting that the population of KNDy neurons is a candidate for the intrinsic GnRH pulse generator [10, 12, 13]. Several lines of evidence support this proposition. 1) KNDy neurons are located in the ARC, supported by a deafferentation study in rats that reported on the location of the GnRH pulse generator in the rat ARC [14]. 2) Genetic studies have suggested that mutations in several genes, including KISS1R, which encodes the kisspeptin receptor (GPR54), TAC3, and TACR3, which encode NKB and its receptor (NK3R), respectively, result in reproductive dysfunction in mice [15] and humans [16]. 3) The administration of GPR54 and NK3R antagonists suppresses pulsatile LH secretion and MUA volley following pulsatile LH secretion, respectively, in mice [17] and goats [18]. 4) The GnRH pulse generator mediates the negative feedback action of gonadal steroids. This can be attributed to the KNDy neuron-mediated expression of both estrogen (E2) receptor alpha and progesterone receptors in females or androgen receptors in male rats [19] and sheep [20]. Furthermore, E2 treatment reduces the frequency of MUA volleys in goats [10], reflecting the negative feedback action of gonadal steroids.
Most KNDy neurons drive synchronized bursts of neural activity to generate neural signals that control distinct GnRH pulses. Several morphological studies have reported the existence of a bilateral interconnected neural circuit that serves to synchronize neural activity among KNDy neurons through axon collaterals and/or dendrites. NKB/Dyn neurons are found in close proximity with fibers containing NKB/Dyn in rats [21]. Kisspeptin/NKB neurons in goats are surrounded by a dense network of fibers containing kisspeptin/NKB [10]. According to a neural tracing study that used anterograde and/or retrograde tracer, NKB neurons in the ARC (the likely candidate for KNDy neurons) send both ipsilateral and contralateral projections to other NKB neurons in rats [21] and goats [13]. Despite the presence of anatomical connections between the population of KNDy neurons in the ipsilateral and contralateral sides of the ARC, bilateral functional connections have not yet been proven.
Several lines of evidence suggest that NKB or its receptor agonist directly stimulate neural activity in KNDy neurons. The majority of KNDy neurons in mice [9], rats [19], and sheep [22] possess NK3R. However, this has not yet been reported in goats. Intracerebroventricular (icv) administration of an NK3R agonist induces c-Fos in KNDy neurons in rats [23] and sheep [24]. In a preparation that used mouse brain slices, NKB was found to elicit trains of action potentials in KNDy neurons via an NK3R-mediated mechanism [25]. The icv administration of NKB or the peripheral administration of NK3R agonist immediately accelerates the neural activity of KNDy neurons, as reflected by the MUA volleys in goats [10, 13, 26]. These findings imply that centrally or peripherally administered NK3R agonists directly stimulate KNDy neurons. NK3R-expressing neurons are distributed throughout the brain [22]. Hence, the possibility that KNDy neurons merely receive excitatory signals via NK3R-expressing neurons located in other NKB-activated brain areas cannot be ruled out.
We aimed to examine the precise mechanism for the generation of synchronized neural burst among bilateral subsets of KNDy neurons by NKB as a trigger of neural activity in goats. To verify this hypothesis, we established a method for unilateral NKB administration in the vicinity of KNDy neurons under bilateral MUA recordings from KNDy neurons.
Materials and Methods
Animals
We used 12 adult (four to eight years) female Shiba goats, weighing 20.0–28.0 kg. The goats were ovariectomized (OVX) for several months prior to the experiment. The animals were maintained with a standard pelleted diet and dry hay and had free access to water and supplemental minerals. We followed all experimental procedures for the Care and Use of Experimental Animals at the Institute of Livestock and Grassland Science, National Agriculture and Food Research Organization.
Recording electrodes with a guide cannula
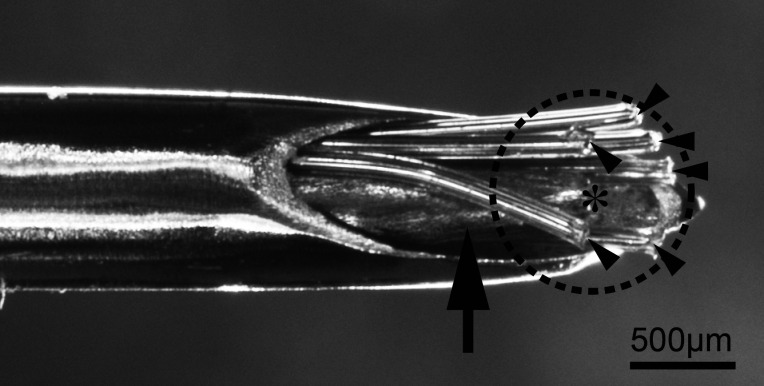
The MUA recording electrodes consisted of six Teflon-insulated platinum-iridium wires (75 µm bare and 139.7 µm coated in diameter, Cat #77000; A-M systems, WA, USA) in an 18-gauge stainless steel guide tube. We placed a 23-gauge stainless steel guide cannula and recording electrodes in a 16-gauge stainless steel guide tube for the left side of the ARC (Fig. 1). This helped us locally administer NKB or vehicle around the cluster of KNDy neurons under MUA recording from the administered area. The distance between the tip of the guide cannula and each electrode was < 500 µm (Fig. 1, dotted circle).
Fig. 1.
Photograph of the recording electrodes (arrowheads) with a guide cannula for the injection (arrow) in the stainless-steel guide tube. The asterisk indicates the tip of the 23-gauge guide cannula for the local injection. The dotted circle indicates 500 µm from the tip of the injector. Six wires for the MUA recording (arrowheads) have been set within 500 µm from the local injection site (asterisk). MUA, multiple-unit activity.
Surgery
OVX goats (n = 9) were stereotaxically implanted with an array of recording electrodes, and electrodes with the guide cannula were aimed at the caudal region of the ARC that densely contained KNDy neurons [10, 12]. The tip of the electrode was placed in close vicinity to or among the clusters of KNDy neurons, which was later confirmed by histological observation. Recording electrodes were implanted with a guide cannula for local injection on the left side of the ARC in each animal. In contrast, we implanted only the recording electrodes on the right side. The left and right sides of the ARC were defined as the ipsilateral and contralateral sides, respectively.
MUA recording and the intervolley interval in each animal
Following a recovery period of approximately four weeks, the goats (n = 9) were loosely held in individual stanchions in a condition-controlled room (12 L: 12 D, 23°C, and 50% relative humidity). We measured the MUA from the left and right side electrodes, as previously described [13]. When the recording electrodes were implanted in the vicinity of KNDy neurons, MUA volleys were recorded [12]. In five of nine goats, an MUA volley was not observed in either the ipsilateral or contralateral sides of the ARC. Therefore, these individuals could not be used for further experiments. Both sides of the electrodes were simultaneously connected to two independent input boxes to facilitate separate analysis of the bilateral MUA signals. We concurrently recorded MUA signals from the bilateral electrodes and stored them as counts per 20 sec on a personal computer.
To determine the timing of the local injection, we recorded the MUA for 2 h on the day of the experiment. We detected the spontaneous onset of MUA volleys. For the analysis of spontaneously occurring MUA volleys, the mean value and standard deviation (SD) of MUA counts/20 sec during control periods were set. We designated the start of the “volley” when the count exceeded twice the SD of the mean value at a time point. The MUA signals were analyzed as counts/20 sec. Moreover, the mean intervolley interval was calculated during the control period for each goat.
Local administration of NKB or vehicle under MUA recording
Four goats were used in the experiment. Prior to the experiment, we recorded the MUA for several hours. We inserted a 30-gauge stainless steel injector, similar in length to the 23-gauge guide cannula inserted at the left side of the ARC. Moreover, we used a microinjection pump (model ESP-32; Eicom, Kyoto, Japan) to administer NKB (100 pmol/µl) or vehicle (saline containing 0.01 N NaOH) as a control, at a rate of 100 nl/min for 1 min. The injection was performed at the midpoint between the MUA volleys. Following the injection, the injection cannula was retained at the site for an additional 1 min.
Statistical analysis
The effects of the injected NKB or vehicle on the intervolley interval were analyzed by a paired t-test using GraphPad Prism 9 software (GraphPad Software, CA, USA). Statistical significance was set at a confidence level of P < 0.05. Data are shown as the mean ± SEM in the three goats (#1–3).
Verifying the local injection area in the hypothalamus
We sacrificed three goats (#1, #3, and #4) after the local injection experiment and dissected their brain sections using a cryostat. We could not obtain the tissue from goat #2 to verify the position of the injection site because it died before being sacrificed. This helped us confirm the region of local administration in the hypothalamus. Goats #1 and #4 received 100 nL/min of 5% fluorescein isothiocyanate (FITC)-conjugated dextran (FITC-dextran, molecular weight; 4,000, Molecular probes, Eugene, OR, USA) for 1 min, before being sacrificed to visualize the region where the administrated NKB dispersed in the hypothalamus. The goats were sacrificed using an overdose of sodium pentobarbital, and their heads were bilaterally perfused with 4,000 ml of 10 mM phosphate buffer (PB) (pH 7.4) containing 0.9% sodium chloride, 13,000 U heparin/l, and 4% paraformaldehyde in 0.1 M phosphate buffer. We performed immunohistochemistry for kisspeptin using an anti-goat kisspeptin antibody (C2, 1:4,000, Okamura et al., 2017) [27] and alkaline phosphatase (AP) conjugated-anti-FITC polyclonal antibody (1:250, Roche, Indianapolis, IN, USA). Prior to any antibody reaction, we treated the sections with a solution containing 50% formamide, 10 mM Tris-HCl (pH 7.6), 600 mM NaCl, 0.25% sodium dodecyl sulfate at 60°C for 2 h, and 3% H2O2 in methanol for 30 min. This enabled quenching of the endogenous AP and peroxidase. The antibodies were then applied to the sections and allowed to react at 4°C for 18 h. We reacted AP-conjugated sheep anti-FITC Fab fragments (1:250. Roche). To detect the FITC-conjugated dextran, 4-nitro blue tetrazolium chloride and 5-bromo-4-chloro-3-indolyl-phosphatase (Roche) were used as chromogens. Biotinylated anti-rabbit IgG was used to visualize kisspeptin. After washing with PBS containing 0.1% Tween-20, the sections were reacted with ABC Elite kit (Vector Laboratories, Burlingame, CA, USA) and diaminobenzidine (DAB) for 5 min. We used DAB solution containing 0.2% nickel sulfate and 0.003% cobalt (II) chloride as the chromogen for brain sections derived from goat #3.
Double labeling of KISS1 and TACR3 in the ARC
We used three OVX goats to examine KISS1, which encodes kisspeptin and TACR3 expression overlap in the ARC. The goat TACR3 gene fragment was amplified by PCR using goat cDNA derived from the hypothalamus as a template. Nucleotide sequences for the PCR primers were based on bovine TACR3 in GeneBank and the following nucleotide sequences: sense, AACCAGTTCGTGCAGCCGTCCTG; anti-sense, CAGTAGATGATGGGGTTGTACAT. We inserted the NK3R (844 bp) into the pTA2 vector (Toyobo, Osaka, Japan) and used it as a template for digoxigenin (DIG)-labeled riboprobe. Brain blocks containing ARC were sectioned to 14 µm thickness using a cryostat. The sections were mounted on MAS-coated glass slides (Matsunami, Osaka, Japan). For double-labeled in situ hybridization (ISH), we labeled riboprobes for KISS1 and TACR3 with DIG and FITC, respectively. We used T3 and T7 RNA polymerase (Stratagene, La Jolla, CA, USA) and a labeling mixture (Roche). The method for double-labeled ISH has been described previously [10]. A Fast Red substrate kit (Sigma Aldrich, St. Louis, MO, USA) was used to visualize the signal. In addition, peroxidase-conjugated anti-FITC antibody (1:200; Roche) and Tyramide-FITC (1:150; PerkinElmer Life Sciences, Boston, MA, USA) were used as chromogens to detect the FITC-labeled probe. Two sections of the middle part of the ARC collected from each individual goat (six sections) were stained by double-labeled ISH as described above and used to count the number of KISS1-positive neurons, TACR3-positive neurons, and both KISS1- and TACR3-positive neurons, respectively.
Results
Effects of the local administration of NKB on multiple-unit activity
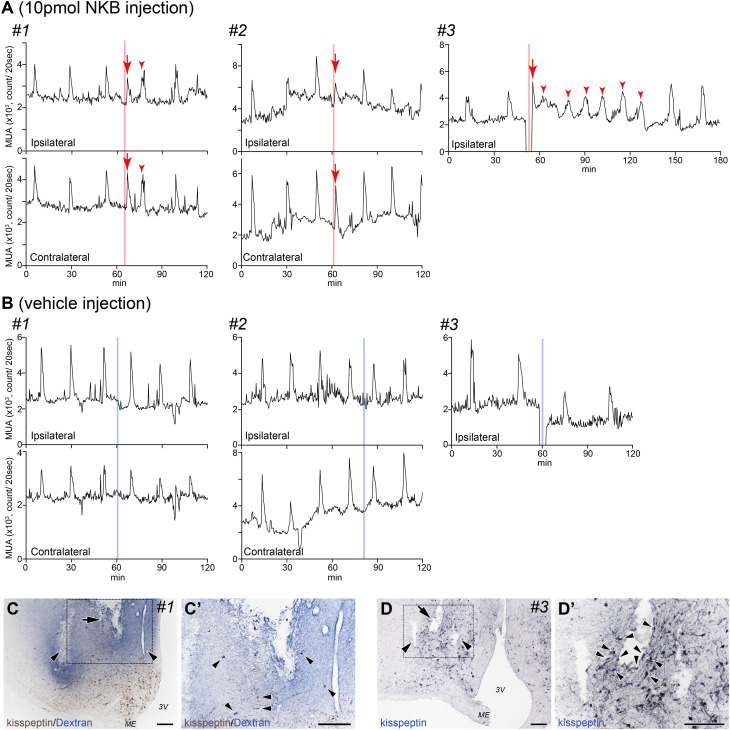
MUA volleys were observed in three goats on the ipsilateral side of the inserted cannula/recording electrodes (Fig. 2, goats #1, 2, and 3). In goats #1 and #2, fine MUA volleys derived from the ipsilateral (Fig. 2A, upper panels) and contralateral sides (Fig. 2A, lower panels) of the ARC were observed. The timing of recording the intrinsic MUA volleys from the ipsilateral and contralateral sides of the ARC were synchronized as previously reported [13]. The MUA volley was recorded only from the ipsilateral side in goat #3 (Fig. 2A, upper right panel). Therefore, we performed local administration of NKB and vehicle under MUA recording using the aforementioned three goats. There was no electric noise on the MUA when injecting goats #1 and #2. However, we encountered severe noise when the injector was inserted into the guide cannula in goat #3. We paused the MUA recording during the injection, following which the cannula was inserted. NKB administration immediately evoked fine MUA volley(s) in all three animals (Fig. 2A, arrows). We observed MUA volleys within 3 min of NKB injection, resulting in a significant reduction in the intervolley interval compared to the control period (23.83 ± 2.17 vs. 13.33 ± 1.76 min, P = 0.0023). Vehicle (saline containing 0.01 N NaOH) injection did not affect the intervolley intervals between pre- and post-vehicle injection (24.67 ± 3.71 vs. 22.0 ± 4.00 min). Following NKB injection, we observed MUA volleys with short intervolley intervals in goats #1 and #3 (Fig. 2A, arrowheads). MUA volleys from both the contralateral and ipsilateral sides were simultaneously observed in goats #1 and #2 (Fig. 2A, upper and lower panels). We could only record MUA volleys from the contralateral side of the ARC in goat #4. Furthermore, NKB administration to the ipsilateral side did not evoke any change in the MUA recording from the contralateral side (Fig. 3A). The injection of vehicle did not affect the MUA recordings (Fig. 3B).
Fig. 2.
A: Profiles of MUA in both the ipsilateral (upper panel) and contralateral (lower panel) sides of the ARC during the local injection of NKB. MUA signals (counts/20 sec) were simultaneously recorded from the left (upper panel) and right (lower panel) sides of the ARC in goats #1 (left) and #2 (middle). The MUA has only been recorded from the ipsilateral sides in goat #3 (right, upper panel). The MUA volley has been immediately evoked (arrows) after NKB administration (red lines). In goat #3, there are several NKB-induced MUA volleys (arrowheads in the right panel). B: Profiles of the MUA on the ipsilateral (upper panel) and contralateral (lower panel) sides of the ARC during local injection of vehicle. The blue lines indicate the local administration of vehicle to the ipsilateral side. Vehicle injection did not evoke any MUA volley. C: Photomicrograph of the control section displaying the placement of the MUA electrode (arrowheads) and guide cannula for local administration (arrow) in the ARC, with the distribution of kisspeptin immunoreactivity and injected FITC-conjugated dextran, before sacrifice in goat #1. The injected FITC-conjugated dextran was dispersed in the area containing kisspeptin neurons. C’: Higher magnification image from the dotted square in C. The arrowheads indicate kisspeptin neurons. D: Photomicrograph of the control section displaying the placement of the MUA electrodes (arrowheads) and guide cannula for local administration (arrow) in the ARC, with the distribution of kisspeptin immunoreactivity in goat #3. D’: Higher magnification image from the dotted square in D. The guide cannula was located close to the kisspeptin neurons (arrowheads) and fibers. MUA recording electrodes were also harbored close to kisspeptin immuno-positive cell bodies and fibers. MUA, multiple-unit activity; ARC, arcuate nucleus; FITC, fluorescein isothiocyanate; NKB, neurokinin B; 3V, third ventricle; ME, median eminence. Scale bars = 50 µm.
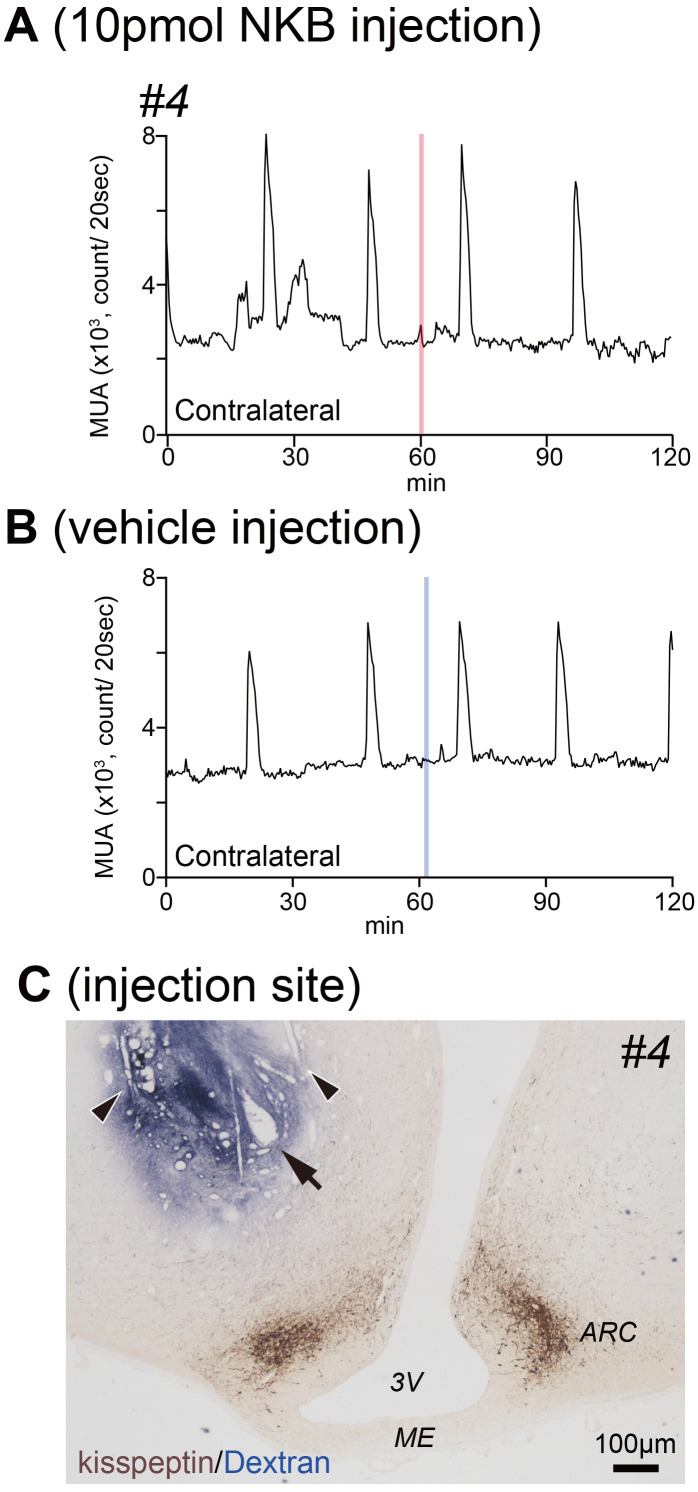
Fig. 3.
A: Profiles of MUA on the contralateral side of the ARC during the local injection of NKB in goat #4. The MUA has not been recorded from the ipsilateral side in goat #4. The NKB injection (red line) has not evoked any MUA volley. B: Profiles of MUA on the contralateral side of the ARC during local injection of vehicle control in goat #4. The blue lines indicate the local administration of vehicle to the ipsilateral side and are not affected on MUA. Vehicle injection did not evoke any MUA volley. C: The placement of electrodes and guide cannula in goat #4 that did not detect MUA volleys on the ipsilateral side. The FITC-dextran dispersed area is far from the kisspeptin neurons and their fibers in the ARC. MUA, multiple-unit activity; ARC, arcuate nucleus; FITC, fluorescein isothiocyanate; NKB, neurokinin B; 3V, third ventricle; ME, median eminence.
Determining the injection area in the goat hypothalamus
The injection/MUA recording sites were aimed at the caudal portion of the ARC, where dense clusters of KNDy neurons were located. We confirmed the electrode trace and terminal location of the bundle in two animals in which the MUA volleys were successfully recorded (goats #1 and #3). We could not verify the position of the injection site in goat #2 because it died before being sacrificed. We visualized the tips of the electrodes and guide in the ARC in both goats #1 and #3. The somata of KNDy neurons and dendrites were distributed around their tips. Goat #1 received FITC dextran injection via a guide cannula before being sacrificed. FITC-dextran was widely distributed around the area localizing KNDy neurons (Fig. 2C). There was an overlap between the stained area and distribution of kisspeptin-positive somata and dendrites (Fig. 2C’). We could clearly identify the placement of the recording electrodes and guide cannula within the area containing KNDy neurons in goat #3 (Fig. 2D and D’). Despite no signals of MUA volley on the ipsilateral side of goat #4, we injected FITC-dextran to confirm the position of the electrodes and guide cannula. The area stained with FITC-dextran did not overlap with the somata and dendrites of KNDy neurons (Fig. 3C).
TACR3 expression in KNDy neurons
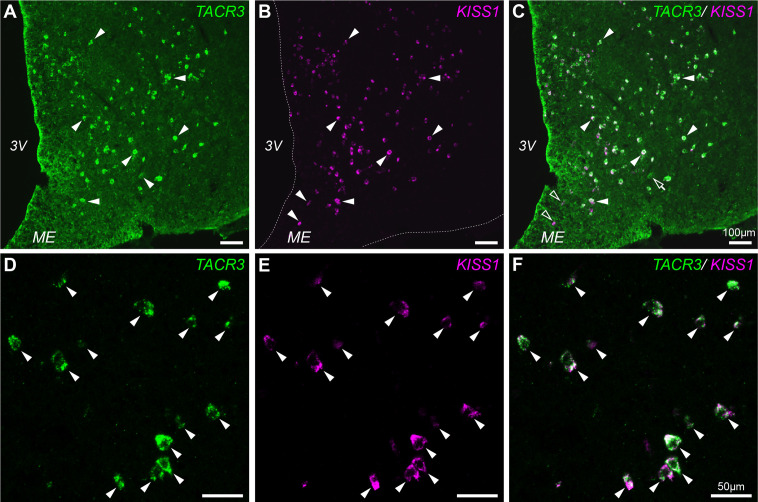
We determined the pattern of TACR3 expression in the ARC to evaluate NKB administration in the vicinity of KNDy neurons. We subsequently performed double labeling for TACR3 and KISS1 by ISH (Fig. 4A–C). Altogether, 1233 (93.5 ± 1.6%) of 1316 KISS1 positive cell bodies in the ARC contained TACR3 mRNA and 1233 (96.2 ± 0.2%) of 1280 TACR3 positive cell bodies in the ARC co-expressed KISS mRNA (Fig. 4). Some cell bodies containing exclusively TACR3 (Fig. 4A-C, open arrows) or KISS1-positive (Fig. 4A-C, open arrowheads), respectively, were observed in the ARC. TACR3 expression in the ARC is highly limited in KNDy neurons.
Fig. 4.
Distribution of TACR3 and KISS1-expressing neurons in goat ARC. A: Representative photograph of TACR3-expressing neurons in goat ARC. TACR3 mRNA-positive neurons are indicated by arrowheads. B: Representative photograph of KISS1-expressing neurons in the goat ARC. KISS1 mRNA-positive neurons are indicated by arrowheads. The dotted lines indicate the outline of brain tissue. C: Merged image of A and B. The open arrow and open arrowheads indicate TACR3-positive/ KISS1-negative neurons and KISS1-positive/ TACR3-negative neurons, respectively. D: A higher magnification photograph of TACR3-expressing neurons in the goat ARC. TACR3 mRNA-positive neurons are indicated by arrowheads. E: A higher magnification photograph of KISS1 mRNA-expressing neurons in the ARC. KISS1 mRNA-positive neurons are indicated by arrowheads. F: A merged image of D and E. All TACR3-positive signals overlapped with KISS1-positive neurons (arrowheads). Scale bars in A–C: 100 µm, D–F: 50 µm. ARC, arcuate nucleus; 3V, third ventricle; ME, median eminence.
Discussion
MUA recordings from the vicinity of KNDy neurons were previously used to simply monitor the activity of those neurons [12]. Here, we examined the neural response to the unilateral administration of NKB by taking simultaneous MUA recordings from the same brain region during administration. NKB application immediately evoked the MUA volleys derived from the ipsilateral side. Vehicle did not affect the MUA close to the recording area (Fig. 2). In the case of MUA volleys evoked by NKB injection, the guide cannula was located in the vicinity of KNDy neurons and their fibers (Fig. 2C, C’, D, and D’). Moreover, the recording electrodes and tip of the guide cannula were in close proximity (Fig 1, Fig 2C and D; arrows). Therefore, we successfully established a method for the local administration of some substances around KNDy neurons while recording the MUA from the region. The success rate of bilaterally obtaining MUA volleys was low (two of nine goats), thus necessitating a methodological improvement for future research.
Double labeling of KISS1 and TACR3 mRNA in the ARC revealed that most KNDy neurons co-express TACR3 in goats (Fig. 4), as previously reported in other species. Moreover, TACR3 signals were limited to KNDy neurons in the ARC, indicating that the TACR3 expression pattern in the ARC was highly limited to KNDy neurons (96.2 ± 0.2%). The majority of NK3R-immunopositive neurons are distributed in the ARC, ventral premammillary nucleus, periventricular nucleus, and preoptic area in sheep [23]. FITC-dextran, administered through a guide cannula, was dispersed in a limited area around the ARC. Furthermore, the administered NKB dispersed in the ARC might directly stimulate some KNDy neurons that co-express TACR3. Our results were supported by a previous study that used brain slices from male mice, including the ARC. The application of NKB resulted in depolarization and induced action potentials in KNDy neurons [25], indicating that NKB stimulates KNDy neurons in mice. Although the population of neurons containing TACR3 but not KISS1 (TACR3 (+)/KISS1 (–)) was small in the goat ARC, we could not rule out that part of the NKB-dependent stimulatory signal was transmitted to KNDy neurons via TACR3 (+)/KISS1 (–) neurons. Since the neural connections between TACR3 (+)/ KISS1 (–) neurons and KNDy neurons in the ARC have not yet been investigated, further analysis of this topic is required.
In this study, we were successful at simultaneously recording MUA volleys on the ipsilateral and contralateral sides in two goats (#1 and #2). MUA volleys were immediately evoked by NKB administration on the contralateral and ipsilateral sides of the ARC (Fig. 2A) and were completely synchronized between both sides. Unilaterally administered NKB could not directly affect the contralateral side of the ARC. Our results suggest that unilateral NKB administration stimulated a subset of KNDy neurons in the vicinity of the injection site. The stimulatory signal was then transmitted among another population of KNDy neurons on the contralateral side of the ARC. We previously reported that axons projecting from NKB-positive neurons in the ARC were directly connected to other NKB-positive neurons located bilaterally in the ARC [13]. In addition, bilateral communication of KNDy neurons is achieved via the ventral part of the caudal ARC under the third ventricle [13] and the internal layer of the median eminence (ME) [21]. The aforementioned findings suggest that these functional collaterals govern the synchronization between the bilateral clusters of KNDy neurons. The simultaneous bilateral burst of the entire population of KNDy neurons might be necessary for generating the MUA volley following transient GnRH secretion in the ME.
We observed a difference in the number of MUA volleys evoked by the local administration of NKB between animals (Fig. 2, arrowheads). One and two MUA volleys were evoked in goats #1 and #2, respectively. However, several MUA volleys with short intervals were evoked for approximately 2 h following NKB injection in goat #3 (Fig. 2). Considering the similar amount of administrated NKB, the positional relationship between the injection area and distribution of KNDy neurons was supposedly important. We observed a larger number of KNDy neurons and their fibers in goat #3 than in goat #1 around the injection guide cannula (Fig. 2C’ and D’). Considering that FITC-dextran did not reach the ARC in goat #4 (Fig. 3C), NKB could not stimulate KNDy neurons in the ARC. Therefore, the MUA derived from the contralateral side may not reveal any substantial changes.
In conclusion, the unilateral administration of NKB in the vicinity of KNDy neurons immediately evoked bilateral MUA volleys, reflecting the neural bursts of the majority of KNDy neurons. Most KNDy neurons in goats possess NK3R. Our findings, together with the existence of a previously reported bilateral neural connection between the population of KNDy neurons, suggest that NKB directly stimulates KNDy neurons. Stimulatory signals are transmitted to the majority of KNDy neurons on the ipsilateral and contralateral sides of the ARC using their neural networks. This is followed by immediate bursts in the majority of KNDy neurons. This necessitates a synchronized burst of the majority of KNDy neurons on the ipsilateral and contralateral sides of the ARC to control a distinct pulsatile GnRH/LH secretion.
Conflict of Interests
The authors have declared that no conflicts of interest exist.
Acknowledgments
This study was partially supported by a Grant-in-Aid from the Japan Society for the Promotion of Science KAKENHI (Grant Number 25871109 to YW) and the Research Program on Innovative Technologies for Animal Breeding, Reproduction, and Vaccine Development (REP2001) from the MAFF in Japan. We would like to thank Ms Y Sakairi for her technical assistance.
References
- 1.Cardenas H, Ordög T, O’Byrne KT, Knobil E. Single unit components of the hypothalamic multiunit electrical activity associated with the central signal generator that directs the pulsatile secretion of gonadotropic hormones. Proc Natl Acad Sci USA 1993; 90: 9630–9634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nishihara M, Hiruma H, Kimura F. Interactions between the noradrenergic and opioid peptidergic systems in controlling the electrical activity of luteinizing hormone-releasing hormone pulse generator in ovariectomized rats. Neuroendocrinology 1991; 54: 321–326. [DOI] [PubMed] [Google Scholar]
- 3.Mori Y, Nishihara M, Tanaka T, Shimizu T, Yamaguchi M, Takeuchi Y, Hoshino K. Chronic recording of electrophysiological manifestation of the hypothalamic gonadotropin-releasing hormone pulse generator activity in the goat. Neuroendocrinology 1991; 53: 392–395. [DOI] [PubMed] [Google Scholar]
- 4.Tanaka T, Mori Y, Hoshino K. Long-term recording of hypothalamic GnRH pulse generator activity during programmed administration of progesterone and estradiol in the ovaryectomized goat. J Reprod Dev 1994; 40: 183–188. [Google Scholar]
- 5.Maeda K, Adachi S, Inoue K, Ohkura S, Tsukamura H. Metastin/kisspeptin and control of estrous cycle in rats. Rev Endocr Metab Disord 2007; 8: 21–29. [DOI] [PubMed] [Google Scholar]
- 6.Oakley AE, Clifton DK, Steiner RA. Kisspeptin signaling in the brain. Endocr Rev 2009; 30: 713–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Adachi S, Yamada S, Takatsu Y, Matsui H, Kinoshita M, Takase K, Sugiura H, Ohtaki T, Matsumoto H, Uenoyama Y, Tsukamura H, Inoue K, Maeda K. Involvement of anteroventral periventricular metastin/kisspeptin neurons in estrogen positive feedback action on luteinizing hormone release in female rats. J Reprod Dev 2007; 53: 367–378. [DOI] [PubMed] [Google Scholar]
- 8.Clarkson J, Herbison AE. Postnatal development of kisspeptin neurons in mouse hypothalamus; sexual dimorphism and projections to gonadotropin-releasing hormone neurons. Endocrinology 2006; 147: 5817–5825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Navarro VM, Gottsch ML, Chavkin C, Okamura H, Clifton DK, Steiner RA. Regulation of gonadotropin-releasing hormone secretion by kisspeptin/dynorphin/neurokinin B neurons in the arcuate nucleus of the mouse. J Neurosci 2009; 29: 11859–11866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wakabayashi Y, Nakada T, Murata K, Ohkura S, Mogi K, Navarro VM, Clifton DK, Mori Y, Tsukamura H, Maeda K, Steiner RA, Okamura H. Neurokinin B and dynorphin A in kisspeptin neurons of the arcuate nucleus participate in generation of periodic oscillation of neural activity driving pulsatile gonadotropin-releasing hormone secretion in the goat. J Neurosci 2010; 30: 3124–3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ramaswamy S, Seminara SB, Ali B, Ciofi P, Amin NA, Plant TM. Neurokinin B stimulates GnRH release in the male monkey (Macaca mulatta) and is colocalized with kisspeptin in the arcuate nucleus. Endocrinology 2010; 151: 4494–4503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ohkura S, Takase K, Matsuyama S, Mogi K, Ichimaru T, Wakabayashi Y, Uenoyama Y, Mori Y, Steiner RA, Tsukamura H, Maeda K-I, Okamura H. Gonadotrophin-releasing hormone pulse generator activity in the hypothalamus of the goat. J Neuroendocrinol 2009; 21: 813–821. [DOI] [PubMed] [Google Scholar]
- 13.Wakabayashi Y, Yamamura T, Sakamoto K, Mori Y, Okamura H. Electrophysiological and morphological evidence for synchronized GnRH pulse generator activity among Kisspeptin/neurokinin B/dynorphin A (KNDy) neurons in goats. J Reprod Dev 2013; 59: 40–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ohkura S, Tsukamura H, Maeda K. Effects of various types of hypothalamic deafferentation on luteinizing hormone pulses in ovariectomized rats. J Neuroendocrinol 1991; 3: 503–508. [DOI] [PubMed] [Google Scholar]
- 15.Lapatto R, Pallais JC, Zhang D, Chan YM, Mahan A, Cerrato F, Le WW, Hoffman GE, Seminara SB. Kiss1-/- mice exhibit more variable hypogonadism than Gpr54-/- mice. Endocrinology 2007; 148: 4927–4936. [DOI] [PubMed] [Google Scholar]
- 16.Topaloglu AK, Reimann F, Guclu M, Yalin AS, Kotan LD, Porter KM, Serin A, Mungan NO, Cook JR, Imamoglu S, Akalin NS, Yuksel B, O’Rahilly S, Semple RK. TAC3 and TACR3 mutations in familial hypogonadotropic hypogonadism reveal a key role for Neurokinin B in the central control of reproduction. Nat Genet 2009; 41: 354–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roseweir AK, Kauffman AS, Smith JT, Guerriero KA, Morgan K, Pielecka-Fortuna J, Pineda R, Gottsch ML, Tena-Sempere M, Moenter SM, Terasawa E, Clarke IJ, Steiner RA, Millar RP. Discovery of potent kisspeptin antagonists delineate physiological mechanisms of gonadotropin regulation. J Neurosci 2009; 29: 3920–3929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sakamoto K, Wakabayashi Y, Yamamura T, Tanaka T, Takeuchi Y, Mori Y, Okamura H. A population of kisspeptin/neurokinin B neurons in the arcuate nucleus may be the central target of the male effect phenomenon in goats. PLoS One 2013; 8: e81017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burke MC, Letts PA, Krajewski SJ, Rance NE. Coexpression of dynorphin and neurokinin B immunoreactivity in the rat hypothalamus: Morphologic evidence of interrelated function within the arcuate nucleus. J Comp Neurol 2006; 498: 712–726. [DOI] [PubMed] [Google Scholar]
- 20.Foradori CD, Coolen LM, Fitzgerald ME, Skinner DC, Goodman RL, Lehman MN. Colocalization of progesterone receptors in parvicellular dynorphin neurons of the ovine preoptic area and hypothalamus. Endocrinology 2002; 143: 4366–4374. [DOI] [PubMed] [Google Scholar]
- 21.Krajewski SJ, Burke MC, Anderson MJ, McMullen NT, Rance NE. Forebrain projections of arcuate neurokinin B neurons demonstrated by anterograde tract-tracing and monosodium glutamate lesions in the rat. Neuroscience 2010; 166: 680–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Amstalden M, Coolen LM, Hemmerle AM, Billings HJ, Connors JM, Goodman RL, Lehman MN. Neurokinin 3 receptor immunoreactivity in the septal region, preoptic area and hypothalamus of the female sheep: colocalisation in neurokinin B cells of the arcuate nucleus but not in gonadotrophin-releasing hormone neurones. J Neuroendocrinol 2010; 22: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Navarro VM, Castellano JM, McConkey SM, Pineda R, Ruiz-Pino F, Pinilla L, Clifton DK, Tena-Sempere M, Steiner RA. Interactions between kisspeptin and neurokinin B in the control of GnRH secretion in the female rat. Am J Physiol Endocrinol Metab 2011; 300: E202–E210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sakamoto K, Murata K, Wakabayashi Y, Yayou K, Ohkura S, Takeuchi Y, Mori Y, Okamura H. Central administration of neurokinin B activates kisspeptin/NKB neurons in the arcuate nucleus and stimulates luteinizing hormone secretion in ewes during the non-breeding season. J Reprod Dev 2012; 58: 700–706. [DOI] [PubMed] [Google Scholar]
- 25.Navarro VM, Gottsch ML, Wu M, García-Galiano D, Hobbs SJ, Bosch MA, Pinilla L, Clifton DK, Dearth A, Ronnekleiv OK, Braun RE, Palmiter RD, Tena-Sempere M, Alreja M, Steiner RA. Regulation of NKB pathways and their roles in the control of Kiss1 neurons in the arcuate nucleus of the male mouse. Endocrinology 2011; 152: 4265–4275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yamamura T, Wakabayashi Y, Ohkura S, Navarro VM, Okamura H. Effects of intravenous administration of neurokinin receptor subtype-selective agonists on gonadotropin-releasing hormone pulse generator activity and luteinizing hormone secretion in goats. J Reprod Dev 2015; 61: 20–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Okamura H, Yamamura T, Wakabayashi Y. Mapping of KNDy neurons and immunohistochemical analysis of the interaction between KNDy and substance P neural systems in goat. J Reprod Dev 2017; 63: 571–580. [DOI] [PMC free article] [PubMed] [Google Scholar]