Abstract
Glucocorticoids exert profound effects on the brain and behavior, but cortisol concentrations are rarely linked to subjectively reported emotional states in humans. This study examined whether the link between cortisol and subjective anxiety varied by childhood maltreatment history. To do this, 97 individuals (60.8% female) participated in a standardized stress task in the laboratory (Trier Social Stress Test, TSST) while providing serial ratings of their feelings of anxiety as well as cortisol samples in blood. These measurements were collected nine times across the laboratory visit, from immediately before the TSST to 65 minutes after stress initiation. We estimated the within-person association between cortisol concentrations and momentary feelings of anxiety for individuals with and without exposure to childhood maltreatment, measured via self-report on the Childhood Trauma Questionnaire (CTQ). Individuals exposed to maltreatment during childhood reported the greatest feelings of anxiety when cortisol concentrations were lowest. This pattern was exaggerated among female participants, those with posttraumatic stress disorder (PTSD), and those exposed to emotional neglect relative to other forms of maltreatment. Early life adversity, such as parental maltreatment, may alter the role of cortisol in affective experiences. This observation may provide preliminary, translational evidence of a novel pathway through which stress may lead to and maintain internalizing symptoms in humans. More studies accounting for the moderating role of childhood maltreatment in biobehavioral pathways are needed.
Keywords: Glucocorticoid, childhood maltreatment, stress, developmental psychopathology, cortisol, HPA axis, TSST, early life stress
Introduction
Almost half of all psychiatric disorders that begin in childhood, and nearly one third of those beginning in adulthood, can be attributed to childhood adversity (Green et al., 2010). One of the most pernicious forms of childhood adversity is maltreatment by a caregiver. Individuals exposed to childhood maltreatment are 50–110% more likely to have a mood disorder and 60–90% more likely to have an anxiety disorder (Green et al., 2010). Yet, how childhood maltreatment leads to internalizing psychiatric disorders remains largely unknown.
The aim of the present study was to determine whether the association between cortisol concentrations and subjectively reported affective experience differed by childhood maltreatment exposure. One of the putative neurobiological mechanisms involved in internalizing psychopathology is hypothalamic-pituitary-adrenal (HPA) axis dysregulation (Baumeister et al., 2014; Pariante & Lightman, 2008). Indeed, stress-induced endogenous as well as exogenously administered glucocorticoids (GCs), cortisol in humans, lead to striking changes in neurotransmission, neural functioning, and cognition (Erickson et al., 2003; Harrewijn et al., 2020; Joëls et al., 2008; Lupien et al., 2009), which can lead to changes in behavior and mood. Yet, cortisol and affective responses to acute psychosocial stress in the laboratory only correlate 25% of the time (Campbell & Ehlert, 2012). Understanding of the links between these constructs may be obscured by extensive, unsuccessful efforts to directly link cortisol concentrations to affect without taking into account individual differences in glucocorticoid receptor (GR) and mineralocorticoid receptor (MR) distribution, density, and sensitivity as shaped by earlier experience.
Childhood maltreatment may alter the effect GCs have on the rest of the body via differential expression of the receptors to which they bind. Anxiety-like behavior occurs as a function of cortisol binding to MRs while termination of the acute stress response occurs via GRs (Joëls et al., 2008). Chronic adversity in childhood, like maltreatment, could decrease the number, density, sensitivity of MRs in particular brain regions, and thus create a tendency to experience negative affect whenever GCs are released in response to a later stressor. Experimental studies with animals have indeed shown that chronic stress (e.g. repeated social defeat) does alter expression and function of both MRs and GRs (Buwalda et al., 1999), that reduced hippocampal MRs and GRs are lasting indicators of early life stress (ELS) exposure (Arabadzisz et al., 2010), and that ELS-related reduction in MR expression is linked to later potentiation of anxiety-like behavior (Bonapersona et al., 2019). Additionally, childhood abuse is associated with epigenetic regulation of hippocampal glucocorticoid receptors in postmortem studies with humans (McGowan et al., 2009). Despite this intriguing evidence, our understanding of whether chronic social stressors, such as childhood maltreatment, alter the impact of GCs on affective experience in adult humans remains limited.
One potential explanation for this gap in the literature is that affect is not consistently measured or reported in studies of biological responses to acute laboratory stress; one systematic review found that more than one third do not report affective responses (Campbell & Ehlert, 2012). Among those that do, biological responses to acute psychosocial stress in the laboratory only correspond with subjective experience about 25% of the time (Campbell & Ehlert, 2012). Of course, some exceptions have been observed, such that cortisol and affective ratings correlate during TSST administration among healthy men (Hellhammer & Schubert, 2012), and that cortisol may protect women from the effect of stress on negative mood (Het & Wolf, 2007). This is consistent with the broader literature on emotional experiences which finds little concordance between biological and subjective measures of emotional experience (Mauss et al., 2005). Importantly, concordance between HPA and affective responses to stress in the laboratory appear to be observed more often in samples exposed to chronic stress (Campbell & Ehlert, 2012), suggesting that environmental experience may contribute to whether and in what ways cortisol responses to stress shape subjective affective experience of that stress.
The present study used intensive, repeated measures of cortisol and affective ratings throughout participation in the Trier Social Stress Test (TSST). This protocol provided an ideal context in which to interrogate the association between GCs and affective experience because both cortisol and affect exhibit large within- and between-person variability across the session. The extant literature linking biological and psychological responses to stress has focused on between-person associations (i.e. is anxiety highest among the individuals with the highest concentrations of cortisol during the laboratory protocol?). However, between-person analytic approaches neglect to address the role of change within people (i.e. do individuals’ anxiety ratings increase when cortisol increases?). This approach also allowed us to analytically overcome the threat to group-to-individual generalizability crisis that currently plagues translational human subjects research (Fisher et al., 2018). Here, we focused on within-person variability, examining the role of maltreatment in explaining whether within-person changes in anxiety vary as a function of within-person changes in cortisol across the session.
Materials and methods
Participants
Participants were recruited from the community (using online advertisements and flyers in local businesses, including outpatient mental health service providers) as part of a larger study designed to disentangle the role of trauma timing (childhood vs adulthood) and comorbid PTSD in the HPA axis dysregulation observed in depression. For this reason, participants were recruited into five groups based upon current psychiatric status: major depressive disorder (MDD), comorbid MDD with posttraumatic stress disorder (PTSD) related to an adult trauma, comorbid MDD with PTSD related to childhood trauma, comorbid MDD and social anxiety disorder (SAD) with childhood onset, and sex- and age-matched healthy controls. For more information on the parent study, see Mayer and colleagues (2020).
Exclusion criteria included serious medical illness, history of psychosis, history of DSM-IV alcohol or drug dependence, drug or alcohol abuse in the past 2 years, regular alcohol use during the past 6 months of >7 cans of beer per week or equivalent, any use of street drugs during the past 2 years, positive urine drug screen at enrollment visit, daily consumption of >500 mg of caffeine, daily tobacco use, active suicidal ideation, or past month major chronobiological disruption or phase shift, or were completely sedentary or vigorously athletic, or recently taking medications that alter HPA axis function (including oral contraceptives or any past month use of topical or inhaled steroids or herbal antidepressants). Participants were free of psychotropic drugs including antidepressants for at least 2 weeks, with no use of fluoxetine for 6 weeks. Healthy controls were additionally excluded if they had any history of prescribed psychiatric medications.
Procedures
All procedures were approved by the University of Michigan Institutional Review Board. Participants provided informed consent prior to any research procedures. Participants reported to an outpatient neuroendocrine study suite first for a clinical diagnostic assessment, and again for the laboratory stress procedure.
Acute psychosocial stress was administered using the Trier Social Stress Test (TSST) (Birkett, 2011; Kirschbaum et al., 1993; Kudielka et al., 2007), a standard approach designed specifically to activate the HPA axis (Dickerson & Kemeny, 2004), but also activating the sympathetic nervous system (Nater et al., 2006) and the innate immune system (Steptoe et al., 2007). The TSST yields robust, short-term increases in cortisol and anxiety (Campbell & Ehlert, 2012; Kirschbaum et al., 1993; Kudielka et al., 2007). It involves public speaking and mental arithmetic tasks performed in front of judges (1 male, 1 female), trained to appear highly evaluative but studiously non-disclosing. First, participants were given 3 min to prepare for a mock job interview (to promote their candidacy for a job tailored to their interests), presented remarks for 5 min, with structured interrogation by the panel if they did not fill the time. This was followed by a 5-min mental arithmetic task, asking them to serially subtract 13 from 1022, while being told to stop and begin again after any error. To further intensity social-evaluative threat, the procedure was videotaped, and participants were told their performance would be evaluated by experts in non-verbal communication.
Measures
HPA axis reactivity
The session began at 2:30 PM or later to control for circadian influence on HPA axis function, and serial blood collection via intravenous catheter began only after 60 min of accommodation. Blood was collected at 0, 5, 10, 15, 25, 35, 45, 55, and 65 min after the start of the TSST. Samples were placed on ice immediately and then centrifuged for 10 minutes at 4 °C to separate plasma. Plasma was stored at −80 °C until assayed for ACTH and cortisol. A rapid, sensitive, and precise semi-automated chemiluminescent assay was conducted using Immulite, per manufacturer’s directions (Siemens Healthcare Diagnostics, Tarrytown, NY). Intra-assay variability was <5%, and inter-assay variability was 5–7% for both cortisol and ACTH.
Anxiety ratings
Participants rated subjective anxiety using a visual analog scale (VAS) nine times throughout the visit, responding to the question “How anxious do you feel right now?” on a 100-mm scale (“calm” to “anxious”). Assessments occurred at 0, 5, 10, 15, 25, 35, 45, 55, and 65 min after the start of the TSST and correspond to the serial blood collection. The VAS is a valid, reliable, and commonly used measure of state anxiety (Rossi & Pourtois, 2012).
Childhood maltreatment
Participants completed the Childhood Trauma Questionnaire-Short Form (CTQ) (Bernstein et al., 2003; Bernstein & Fink, 1998). The CTQ has 28 items assessing self-reported childhood exposure to abuse and neglect. Each item begins with “When I was growing up … ” and then lists various childhood experiences, such as: “I was punished with a belt/board/cord/other hard object,” “People in my family said hurtful or insulting things to me,” and “My parents were too drunk or high to take care of the family.” Participants recorded the frequency of each experience on a six-point Likert-type scale ranging from 0 “never true” to 5 “very often true”. Higher scores indicate more severe and frequent maltreatment experiences. Participants were categorized into two mutually exclusive groups (maltreatment exposed or no maltreatment) using established cut-points (Walker et al., 1999): emotional abuse ≥ 10, emotional neglect ≥ 15, physical abuse ≥ 8, physical neglect ≥ 8, or sexual abuse ≥ 8. These thresholds have ≥ .85 sensitivity and specificity compared to clinical interviews identifying maltreatment (Bernstein & Fink, 1998).
Psychiatric morbidity
Diagnoses of Major Depressive Disorder and Social Anxiety Disorder were determined using the Structured Clinical Interview for DSM-IV Disorders (SCID) (First & Gibbon, 2004). Presence of active depression was further verified by a score >14 on the Hamilton Depression Rating Scale (HAM-D). PTSD was diagnosed using the Clinician-Administered PTSD Scale for DSM-IV (CAPS) (Blake et al., 1995). Depressive and anxiety symptom severity were also assessed via the Brief Symptom Inventory (BSI) with separate subscales (Derogatis & Melisaratos, 1983).
Data analysis
All variables were examined for normality and heteroscedasticity. Cortisol was transformed using the natural log to adjust for skew and kurtosis. All continuous variables were mean-centered.
Analyses were conducted using mixed linear models predicting ratings of anxiety as a function of minutes since stress onset, between-person cortisol concentrations, within-person cortisol reactivity, childhood maltreatment, the interaction between within-person cortisol and maltreatment. All data were nested within time points within individuals, and all models included random slopes and intercepts with an unstructured covariance matrix.
Between-person cortisol concentrations reflect the degree to which an individual’s cortisol concentrations varied from the rest of the sample. Within-person cortisol reactivity was computed by calculating a mean cortisol concentration for each participant using all of their available samples, then subtracting that individual mean from each of their cortisol observations. This within-person variable reflects the degree to which an individual varied from their own average cortisol concentration across the visit. Thus, models including both of these terms can simultaneously estimate whether anxiety ratings vary as a function of how high or low cortisol concentrations are relative to other people or as a function of how high or low cortisol concentrations are relative to themselves at other measurement points.
All models were computed unadjusted and then adjusted for several covariates known to impact the HPA axis or affective ratings: BMI, age, female sex, depressive symptoms, anxiety symptoms, and ethnic minority status (non-Hispanic white vs other). Cortisol and ACTH responses to acute laboratory stress measured in blood do not vary by menstrual phase as they do when measured in saliva (Kirschbaum et al., 1999). Using day 14 of their cycle as a cut-point, 26.8% (n=15) of the women were in the follicular phase of their cycle on the day of the TSST and 73.2% (n=41) were in the luteal phase. There were no differences between women in these two phases of their cycle in the percent who showed an HPA axis response to the TSST, χ2=0.08, p = .78, nor did they differ in cortisol concentrations at baseline, F(1,52) = 0.03, p = .86, or their peak following the TSST, F(1,52) = 0.10, p = .76.
In models where the hypothesized interaction was significant, p < .05, we then computed the conditional effects of within-person cortisol on anxiety at each value of the moderator (i.e. childhood maltreatment). Finally, we conducted a series of theoretically-driven post-hoc analyses to determine the role of maltreatment type, sex, physiological state of the HPA axis, and psychiatric morbidity in our observations (see Kuhlman et al., 2017 for review).
Results
Childhood maltreatment was prevalent, with nearly half (42.3%) of this sample reporting considerable exposure to abuse or neglect (see Table 1 for demographics).
Table 1.
Participant demographics (n = 97).
| M (SD) | % (n) | |
|---|---|---|
|
| ||
| Age | 30.53 (12.33) | |
| Female | 60.8 (59) | |
| Race/ethnicity* | ||
| Asian | 6.2 (6) | |
| African American | 13.4 (13) | |
| Caucasian | 73.2 (71) | |
| Latino/Hispanic | 1 (1) | |
| Native American | 1 (1) | |
| Multi-racial | 6.2 (6) | |
| BMI | 25.02 (4.13) | |
| Normal (18–<25) | 58.8 (57) | |
| Overweight (25 – <30) | 30.9 (30) | |
| Obese (>30) | 10.3 (10) | |
| Childhood maltreatment | 42.3 (41) | |
| Physical neglect | 7.04 (2.87) | 30.9 (30) |
| Emotional neglect | 9.76 (4.59) | 52.6 (51) |
| Physical abuse | 6.42 (3.17) | 12.4 (12) |
| Emotional abuse | 9.11 (5.27) | 34 (33) |
| Sexual abuse | 5.88 (2.73) | 15.5 (15) |
| Diagnostic status* | ||
| Healthy controls | 42.2 (41) | |
| Major depressive disorder | 57.7 (56) | |
| Post-traumatic stress disorder | 30.9 (30) | |
| Social anxiety disorder (SAD) | 12.4 (12) | |
| Depressive symptoms (BSI) | 1.24 (1.26) | |
| Anxiety Symptoms (BSI) | 0.78 (0.93) | |
Groups not mutually exclusive.
Affective and HPA-axis responses to acute stress
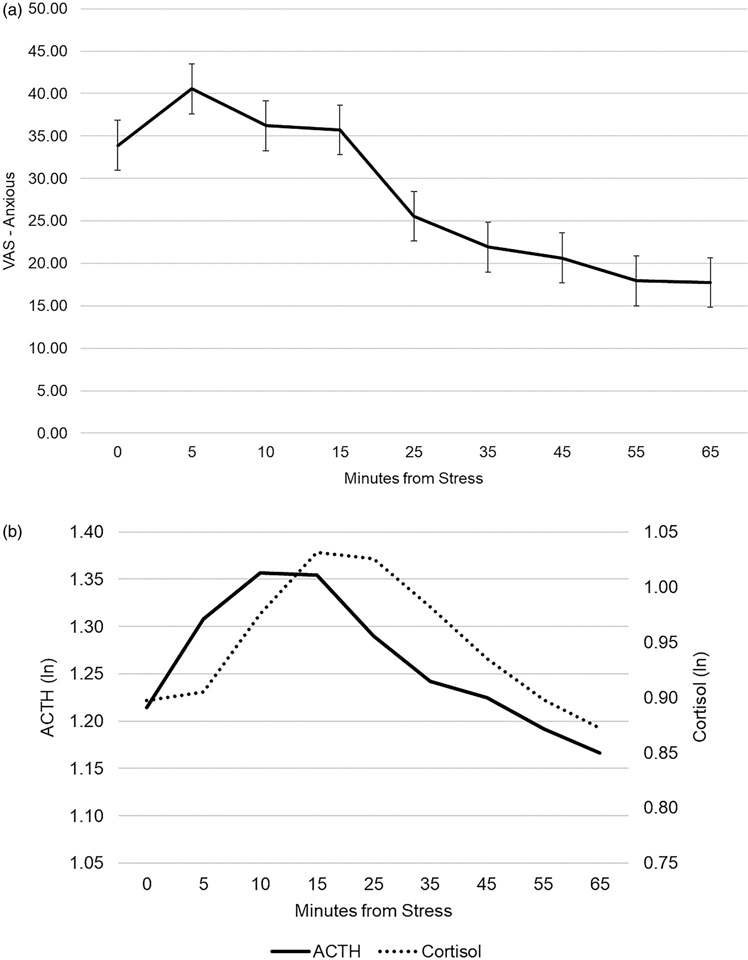
See Figure 1(a) for anxiety and Figure 1(b) for cortisol and ACTH responses to the TSST. Ratings of anxiety were highest during the TSST (at 5, 10, and 15 min) and returned to baseline by 25 minutes after stress-onset. A cubic model was the best fit to ratings of feeling anxious across the visit (AIC cubic = 6938.37, AIC quadratic = 6953.62, AIC linear = 6958.01). Feelings of anxiety were already elevated upon stress initiation and increased somewhat during the TSST, then recovered throughout the rest of the visit, minutes from stress b = .09, SE = .17, p = .60, minutes from stress2 b = −0.02, SE = .006, p < .001, minutes from stress3 b = .0003, SE=6.32E-5, p < .001. Participants exhibited a robust, nonlinear HPA axis response (both ACTH and cortisol). ACTH reached a post-stress peak at 10 min, that was sustained at 15 min, and concentrations returned to baseline by 55 min. ACTH increased significantly after stress initiation, b = .002, SE = .001, p < .001, and began to decline significantly at 15-min post-stress initiation, b = −6.97E-5, SE=7.91E-6, p < .001. Cortisol concentrations reached a post-stress peak at 15 min, that was sustained at 25 min, and concentrations returned to baseline by 55 min. Cortisol increased significantly after stress initiation, b = .007, SE = .001, p < .001, and began to decline at 25 min post-stress initiation, b = −0.0001, SE = −7.40E-6, p < .001.
Figure 1.
Anxiety and HPA axis response to TSST.
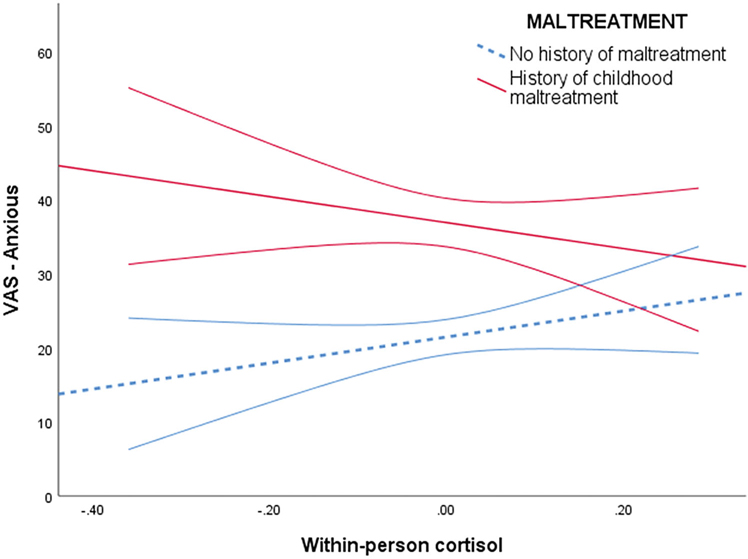
Variability in cortisol across the visit, maltreatment, and their interaction accounted for 2.0% of variance in ratings of anxiety (AIC = 6798.18). In this model, childhood maltreatment moderated the association between within-person variability in cortisol and feelings of anxiety, b = −27.89, SE−9.81, p = .005. This interaction remained after accounting for BMI, ethnic minority status, depressive symptoms, and anxiety symptoms, b = −22.04, SE=9.89, p = .026. See Table 2 for adjusted coefficient estimates. Specifically, within-person variability in cortisol was not associated with feelings of anxiety for participants without childhood maltreatment, b = −2.00, SE=5.22, p = .70 (see Figure 2). Meanwhile, there was a negative association between cortisol and anxiety ratings among participants with a history of childhood maltreatment reported, b = −21.84, SE=9.42, p = .021, such that their highest ratings of anxiety occurred when their cortisol was lowest relative to their other assessments.
Table 2.
Coefficient estimates of cortisol, maltreatment, and their interactions predicting feelings of anxiety across the laboratory visit.
| Unadjusted |
Adjusted |
|
| 6798.18 |
6347.96 |
|
| AIC | b(SE) | b(SE) |
|
| ||
| Intercept | 33.22 (3.70)*** | 40.93 (4.33)*** |
| Minutes from stress | −.37 (.04)*** | −0.36 (.04)*** |
| Cortisol (between-person) | −13.42 (14.71) | −7.40 (14.83) |
| Cortisol (within-person) | 10.66 (16.39) | 5.36 (16.31) |
| Childhood maltreatment | 12.79 (4.62)** | −3.46 (4.93) |
| Cortisol (within-person) × Childhood maltreatment | −27.89 (9.81)** | −22.04 (9.89)* |
| Sex (female) | 1.14 (4.44) | |
| Age | 0.08 (0.17) | |
| Depressive symptoms | 8.61 (3.42)* | |
| Anxiety symptoms | 4.21 (4.59) | |
| BMI | 0.14 (0.56) | |
| Ethnic minority status | −8.86 (5.01)+ | |
p < .001
p < .01
p < .05
p < .10.
Figure 2.
Association between within-person change in cortisol and feelings of anxiety by childhood maltreatment exposure.
Post hoc analyses
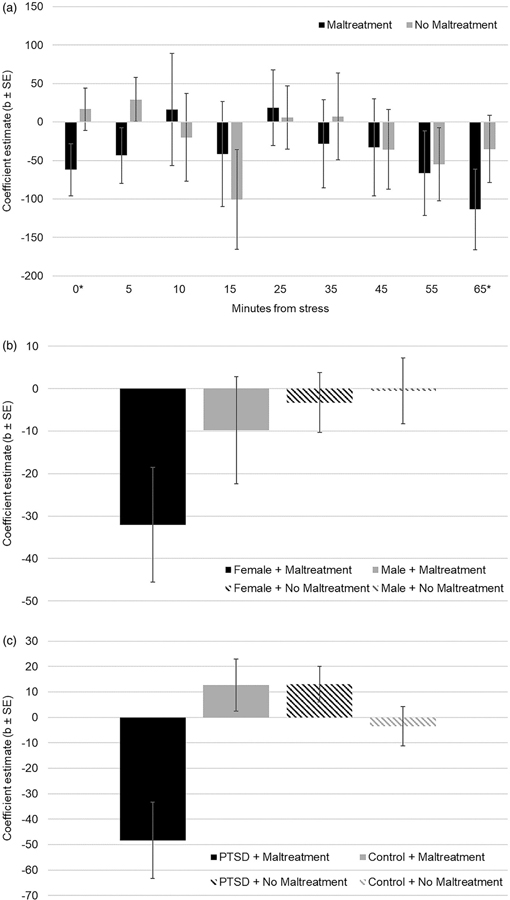
We unpacked this observation—that individuals with a history of childhood maltreatment reported the highest anxiety when their cortisol has shown the greatest decreases relative to other assessments—in post hoc analyses. First we examined whether moderation by maltreatment exposure occurred throughout the visit, or at specific time points, by computing the coefficient estimate of anxiety as a function of within-person cortisol for individuals with and without childhood maltreatment separately at each assessment. Maltreatment exposed individuals exhibited a negative association between cortisol and anxiety that was non-significant at baseline, b = −61.98, SE=33.81, p = .07, and significant at 65 min post-stress onset, b = −113.39, SE=52.32, p = .03. This association was non-significant at all other assessments for participants with maltreatment exposure, all ps > .22, and at all assessments among participants not exposed to maltreatment, all ps > 13. Qualitatively, the coefficient was negative for individuals exposed to maltreatment for 7 of the 9 assessments across the visit, increasing in magnitude from 35 to 65 min post-stress onset (Figure 3(a)).
Figure 3.
(a) Coefficient estimates of the effect of within-person cortisol variability on anxiety across the laboratory visit by maltreatment history. (b) Coefficient estimates of the effect of within-person cortisol variability across the laboratory visit on anxiety by sex and maltreatment history. (c) Coefficient estimates of the effect of within-person cortisol variability on anxiety by PTSD and maltreatment history.
To explore whether types of maltreatment differentially accounted for the observed moderation, we next conducted a single mixed model predicting feelings of anxiety as a function of within-person cortisol variability and exposure to physical abuse, physical neglect, emotional abuse, emotional neglect, or sexual abuse, and the interaction of each of these maltreatment subtypes with within-person cortisol variability. Emotional neglect moderated the association between within-person cortisol and feeling anxious, b = −3.96, SE=1.91, p = .038, with a significant negative link for participants exposed to emotional neglect, b = −25.41, SE=9.60, p = .009, and a non-significant link for participants exposed to little to no emotional neglect, b=1.77, SE=4.90, p = .72. No other maltreatment subtype moderated the association between cortisol variability and feeling anxious, physical abuse b=3.86 (SE=2.61), p = .14, physical neglect b = −1.46 (SE=3.06), p = .63, emotional abuse b = −2.41 (SE=1.97), p = .22, and sexual abuse b=3.44 (SE=3.06), p = .26.
We then examined the observed moderation by re-running the primary moderation analysis stratified by sex. There was a significant interaction between maltreatment and cortisol among female participants, b = −30.08, SE=13.79, p = .03, but not for males, b = −12.52, SE=13.80, p = .37. The association between within-person cortisol and anxiety was negative and significant for females with a maltreatment history, b = −32.05, SE=13.49, p = .019, but non-significant for males with a maltreatment history, b = −9.79, SE=12.57, p = .44, and females and males without maltreatment, b = −3.28, SE=7.06, p = .64 and b = −0.56, SE=7.76, p = .94 respectively (Figure 3(b)).
Current PTSD also moderated the observed association. The three-way interaction between within-person cortisol, childhood maltreatment, and PTSD was significant, b = −76.47, SE=24.52, p = .002. Specifically, the association between within-person cortisol variability and feelings of anxiety was negative and significant for individuals with PTSD and a childhood maltreatment history, b = −48.29, SE=14.97, p = .002, but was non-significant for individuals with PTSD without childhood maltreatment, b=13.08, SE=14.03, p = .36, as well as controls with and without childhood maltreatment, b=12.72, SE=10.23, p = .22 and b = −3.48, SE=5.62, p = .54 respectively (Figure 3(c)).
Discussion
The purpose of this study was to determine whether the role of cortisol in affective ratings of anxiety differed between individuals with and without a history of childhood maltreatment. Individuals with a history of childhood maltreatment exhibited exaggerated feelings of anxiety when cortisol concentrations were lowest relative to other assessments throughout the laboratory stress protocol, while anxiety and cortisol were unrelated in the absence of maltreatment. Sensitivity analyses indicated that this association was driven by females, individuals with PTSD, and individuals exposed to emotional neglect in childhood. Childhood maltreatment, and other forms of chronic stress during childhood, may alter the association between GCs and affective experiences, with potential implications for our theoretical and practical understanding of how childhood maltreatment may lead to internalizing symptoms, and importantly how we develop interventions for this high-risk population.
The majority of research on the role of childhood maltreatment in risk for psychiatric diseases has focused on direct pathways through which early life experiences alter functioning within physiological stress systems (i.e. HPA axis, inflammation) that then lead to psychiatric symptoms. This study provides additional, preliminary evidence of an alternate model in which the association between cortisol and affective experience is altered among individuals with a history of maltreatment. This is consistent with emerging evidence that cortisol may play a role in interfering with memory for negative-valenced information under acute stress for youth exposed to adversity (Kuhlman et al., 2021), and that the role of inflammation in affective and behavioral domains associated with depression also varies by early life adversity (Kuhlman et al., 2020). This alternate model may inform targeted interventions. For example, there is evidence that both behavioral and pharmacological treatment effectiveness differs for individuals exposed to childhood maltreatment (e.g. Lewis et al., 2010; Nanni et al., 2012; Nemeroff et al., 2003; Shamseddeen et al., 2011). It is plausible that underlying neurobiological processes that contribute to pathogenesis and maintenance of internalizing disorders differ for individuals exposed to maltreatment.
The HPA axis may be involved in the pathogenesis of internalizing psychiatric disorders via the effects of cortisol on MRs (Derijk et al., 2008; ter Heegde et al., 2015; Von Werne Baes et al., 2012). Indeed, the MR gene NR3C2 exerts pleiotropic effects on HPA axis stress-reactivity and on risk for stress-related disorders (Gerritsen et al., 2017). The MR plays a critical role in how the HPA axis enables an individual to appraise novel situations and select responses to deal with the immediate circumstances (Joëls et al., 2008). Notably, studies that have linked MR genotype and function to risk for psychiatric disorders have shown this association to be more robust in individuals exposed to maltreatment in childhood (Derijk et al., 2008; Gerritsen et al., 2017; Normann & Buttenschøn, 2020; ter Heegde et al., 2015; Von Werne Baes et al., 2012). And the interaction between childhood maltreatment and NR3C2 predicts negative valence memory bias (Vrijsen et al., 2015), which may be involved in both risk and maintenance of internalizing symptoms.
In this study, individuals with and without maltreatment reported similar levels of anxiety when their cortisol was highest, but individuals with a history of maltreatment reported increasing anxiety when their cortisol was lowest. In the context of a standardized laboratory stress protocol, this difference between individuals with and without maltreatment emerges when cortisol concentrations are lowest and is likely when the binding mechanism for cortisol changes from GRs to MRs or vice versa (See Figure 3(a)). If anxiety-like behavior occurs as a function of cortisol binding to MRs (Joëls et al., 2008), and ELS causes lasting alterations to MR expression and function (Arabadzisz et al., 2010; Bonapersona et al., 2019; Buwalda et al., 1999), one plausible explanation for the present findings is that childhood maltreatment has changed the point during TSST recovery at which the binding mechanism changes from GR to MR, thus leading to increasing reports of anxiety despite declining cortisol concentrations. Our data cannot speak to the specific role of MR or GR function or sensitivity. Environmental factors that affect the expression and functioning of MRs may indirectly decrease the threshold at which cortisol potentiates feelings of anxiety by altering the effect GCs have on the brain. Indeed, overexpression of MRs in mice is associated with less anxiety-like behavior while over-expression of GRs is associated with more (Rozeboom et al., 2007). Pharmacological-stimulation of MRs has shown some promise as a treatment for depression (Otte et al., 2010). Yet, to our knowledge, no study has explored whether MR stimulation improves treatment outcomes specifically among individuals with a history of childhood maltreatment. One important implication of alterations to glucocorticoid sensitivity in the brain may be impact on the time-course through which cortisol exerts opposite and complementary effects on attention to valenced information (Henckens et al., 2012). While these time-dependent effects are conserved to allow individuals to adapt to changing environmental demands, sustained alterations to this time-course may explain why the role of stress-induced cortisol on cognitive and affective processes appears to differ for individuals varying in childhood adversity exposure (Kuhlman et al., 2021).
It is important to note that the altered association between cortisol and anxiety was qualified by several factors, including female sex, diagnosis with PTSD, and exposure to emotional neglect. These observations reflect patterns that are consistent with previous research and warrant further investigation. For example, gonadal hormones provide a context for glucocorticoid signaling that render females more vulnerable to the effects of stress on the brain (Flügge, 1999; Karandrea et al., 2000; Kitraki et al., 2004), while cortisol has also been identified as protective in the effects of stress on negative mood in healthy, young women (Het & Wolf, 2007). The role of MRs in risk for depression appears to be either greater in or specific to females (Endedijk et al., 2020; Vinkers et al., 2015). Indeed, women with PTSD exhibit attenuated salivary cortisol responses but exaggerated subjective perceived stress responses to the TSST relative to women without PTSD (Metz et al., 2020). Our findings extend this observation by showing that this effect may be driven by altered affective sensitivity to cortisol within women and those with PTSD, rather than group differences in the magnitude of the HPA axis response to the TSST.
With respect to maltreatment type, relative to other forms of childhood maltreatment, emotional neglect has previously been identified in the link between psychobiological responses to stress and psychopathology. For example, exposure to emotional neglect is associated with exaggerated threat-related amygdala reactivity, particularly among those with the “more environmentally sensitive” Iso/Iso MR genotype (Bogdan et al., 2012). Nonetheless, emotional neglect was also far more frequent than any other form of maltreatment in this sample (52.6%), and a posthoc power analysis indicated that the present study was under-powered to detect this moderation for the least frequent maltreatment subtypes (e.g. 12.4% physical abuse and sexual abuse 15.5%). Replication of this finding in a sample recruited specifically for less frequent maltreatment experiences is warranted. Finally, low density or sensitivity of MRs has previously been linked to risk for PTSD (ter Heegde et al., 2015). Taken together, these findings may help to reconcile the mixed literature to date on the disproportionate risk for internalizing symptoms among those with a history of maltreatment.
These results should be considered in the context of the study’s strengths and limitations. One important strength of this study was the availability of intensive-repeated measures of cortisol which enabled us to model within-person variability in cortisol across the protocol in a clinical sample (Fisher et al., 2018). However, the parent study was designed to understand the role of trauma timing (childhood vs adulthood) and comorbid PTSD in HPA axis functioning among individuals with MDD (Mayer et al., 2020). This may limit generalizability since exposure to childhood maltreatment and the presence of psychopathology (i.e. MDD, PTSD, SAD) were over-represented relative to what would be observed in the general population. Additionally, the relatively strict exclusion criteria for patient groups with regard to medication may limit generalizability of our findings to individuals with untreated symptoms. Given the aims of the parent study, a placebo TSST or no stress control group were not included, so causal inferences cannot be made about the role of the stressor itself in the within- and between-person variability observed across the protocol. Attention in future research to whether and how stress alters individual differences in affective sensitivity to cortisol is needed and may further elucidate the potential role of GRs and MRs. Another limitation to the present study is that childhood maltreatment was measured using retrospective self-report, which is prone to measurement error as a result of age, memory and appraisal biases, and current distress (Epel et al., 2018; Monroe, 2008). Another important limitation is that childhood maltreatment was the only measure of early life adversity. There are many different types of early adversity that have shown differential effects on brain development (Sheridan & McLaughlin, 2014) and physiological stress response systems (Kuhlman et al., 2017, 2015). Whether this pattern of results generalizes to other forms of early adversity is unknown but warrants clarification, particularly if this discourse translates to the development of targeted interventions for this high-risk population.
One conclusion is clear – studies examining links between HPA axis activity and affective experience as well as psychiatric symptoms need to account for and examine the moderating role of childhood maltreatment in these relationships. Adversity in childhood not only presages the development of psychiatric diseases, but is also a significant contributor to years lived with disability, increased health care costs, and early mortality. Identification of specific and potentially modifiable biobehavioral pathways of risk and disease pathogenesis in this population are of great value to individuals and society.
Funding
Data collection for this project was funded by the National Institute of Mental Health via a grant awarded to Elizabeth A. Young, which was transferred to James L. Abelson after her passing [R01 MH078975]. Composition of this manuscript was supported by the National Institute of Mental Health [K08MH112773; PI: Kuhlman] and the National Institute of Aging [K99 AG062778; PI: Mayer].
Footnotes
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Arabadzisz D, Diaz-Heijtz R, Knuesel I, Weber E, Pilloud S, Dettling AC, Feldon J, Law AJ, Harrison PJ, & Pryce CR (2010). Primate early life stress leads to long-term mild hippocampal decreases in corticosteroid receptor expression. Biological Psychiatry, 67(11), 1106–1109. 10.1016/j.biopsych.2009.12.016 [DOI] [PubMed] [Google Scholar]
- Baumeister D, Lightman SL, & Pariante CM (2014). The interface of stress and the HPA axis in behavioural phenotypes of mental illness. In Behavioral neurobiology of stress-related disorders, current topics in behavioral neurosciences (pp. 13–24). Springer. 10.1007/7854_2014_304 [DOI] [PubMed] [Google Scholar]
- Bernstein DP, & Fink LA (1998). CTQ: Childhood trauma questionnaire: A retrospective self-report. The Psychological Corporation. [Google Scholar]
- Bernstein DP, Stein JA, Newcomb MD, Walker E, Pogge D, Ahluvalia T, Stokes J, Handelsman L, Medrano M, Desmond D, & Zule W (2003). Development and validation of a brief screening version of the Childhood Trauma Questionnaire. Child Abuse & Neglect, 27(2), 169–190. 10.1016/S0145-2134(02)00541-0 [DOI] [PubMed] [Google Scholar]
- Birkett MA (2011). The trier social stress test protocol for inducing psychological stress. Journal of Visualized Experiments, 56, 3238. 10.3791/3238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Gusman FD, Charney DS, & Keane TM (1995). The development of a Clinician-Administered PTSD Scale. Journal of Traumatic Stress, 8(1), 75–90. 10.1002/jts.2490080106 [DOI] [PubMed] [Google Scholar]
- Bogdan R, Williamson DE, & Hariri AR (2012). Mineralocorticoid receptor iso/Val (rs5522) Genotype Moderates the Association Between Previous Childhood emotional Neglect and Amygdala reactivity. The American Journal of Psychiatry, 169(5), 515–522. 10.1176/appi.ajp.2011.11060855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonapersona V, Damsteegt R, Adams ML, van Weert LTCM, Meijer OC, Joëls M, & Sarabdjitsingh RA (2019). Sex-dependent modulation of acute stress reactivity after early life stress in mice: Relevance of mineralocorticoid receptor expression. Frontiers in Behavioral Neuroscience, 13, 181. 10.3389/fnbeh.2019.00181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buwalda B, de Boer SF, Schmidt ED, Felszeghy K, Nyakas C, Sgoifo A, Van der Vegt BJ, Tilders FJ, Bohus B, & Koolhaas JM (1999). Long-lasting deficient dexamethasone suppression of hypothalamic-pituitary-adrenocortical activation following peripheral CRF challenge in socially defeated rats. Journal of Neuroendocrinology, 11(7), 513–520. 10.1046/j.1365-2826.1999.00350.x [DOI] [PubMed] [Google Scholar]
- Campbell J, & Ehlert U (2012). Acute psychosocial stress: Does the emotional stress response correspond with physiological responses? Psychoneuroendocrinology, 37(8), 1111–1134. 10.1016/j.psyneuen.2011.12.010 [DOI] [PubMed] [Google Scholar]
- Derijk RH, van Leeuwen N, Klok MD, & Zitman FG (2008). Corticosteroid receptor-gene variants: modulators of the stress-response and implications for mental health. European Journal of Pharmacology, 585(2–3), 492–501. 10.1016/j.ejphar.2008.03.012 [DOI] [PubMed] [Google Scholar]
- Derogatis LR, & Melisaratos N (1983). The Brief Symptom Inventory: An introductory report. Psychological Medicine, 13(3), 595–605. 10.1017/S0033291700048017 [DOI] [PubMed] [Google Scholar]
- Dickerson SS, & Kemeny ME (2004). Acute stressors and cortisol responses: A theoretical integration and synthesis of laboratory research. Psychological Bulletin, 130(3), 355–391. 10.1037/0033-2909.130.3.355 [DOI] [PubMed] [Google Scholar]
- Endedijk HM, Nelemans SA, Schür RR, Boks MPM, Lier P, van Meeus W, Branje S, & Vinkers CH (2020). The role of stress and mineralocorticoid receptor haplotypes in the development of symptoms of depression and anxiety during adolescence. Frontiers in Psychiatry, 11, 367. 10.3389/fpsyt.2020.00367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epel ES, Crosswell AD, Mayer SE, Prather AA, Slavich GM, Puterman E, & Mendes WB (2018). More than a feeling: A unified view of stress measurement for population science. Frontiers in Neuroendocrinology, 49, 146–169. 10.1016/j.yfrne.2018.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson K, Drevets W, & Schulkin J (2003). Glucocorticoid regulation of diverse cognitive functions in normal and pathological emotional states. Neuroscience and Biobehavioral Reviews, 27(3), 233–246. 10.1016/S0149-7634(03)00033-2 [DOI] [PubMed] [Google Scholar]
- First MB, & Gibbon M (2004). The Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I) and the Structured Clinical Interview for DSM-IV Axis II Disorders (SCID-II). In Hilsenroth MJ & Segal DL (Eds.), Comprehensive handbook of psychological assessment, Vol. 2. Personality assessment (pp. 134–143). John Wiley & Sons, Inc. [Google Scholar]
- Fisher AJ, Medaglia JD, & Jeronimus BF (2018). Lack of group-to-individual generalizability is a threat to human subjects research. Proceedings of the National Academy of Sciences of the United States of America, 115(27), E6106–E6115. 10.1073/pnas.1711978115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flügge G (1999). Regulation of monoamine receptors in the brain: Dynamic changes during stress. In Jeon KW (Ed.), International review of cytology (pp. 145–213). Academic Press. 10.1016/S0074-7696(08)62705-9 [DOI] [PubMed] [Google Scholar]
- Gerritsen L, Milaneschi Y, Vinkers CH, van Hemert AM, van Velzen L, Schmaal L, & Penninx BW (2017). HPA Axis genes, and their interaction with childhood maltreatment, are related to cortisol levels and stress-related phenotypes. Neuropsychopharmacology, 42(12), 2446–2455. 10.1038/npp.2017.118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green JG, McLaughlin KA, Berglund PA, Gruber MJ, Sampson NA, Zaslavsky AM, & Kessler RC (2010). Childhood adversities and adult psychiatric disorders in the National Comorbidity Survey Replication I: Associations with first onset of DSM-IV disorders. Archives of General Psychiatry, 67(2), 113–123. 10.1001/archgenpsychiatry.2009.186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrewijn A, Vidal-Ribas P, Clore-Gronenborn K, Jackson SM, Pisano S, Pine DS, & Stringaris A (2020). Associations between brain activity and endogenous and exogenous cortisol - a systematic review. Psychoneuroendocrinology, 120, 104775. 10.1016/j.psyneuen.2020.104775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellhammer J, & Schubert M (2012). The physiological response to Trier Social Stress Test relates to subjective measures of stress during but not before or after the test. Psychoneuroendocrinology, 37(1), 119–124. 10.1016/j.psyneuen.2011.05.012 [DOI] [PubMed] [Google Scholar]
- Henckens MJAG, van Wingen GA, Joëls M, & Fernández G (2012). Time-dependent effects of cortisol on selective attention and emotional interference: a functional MRI study. Frontiers in Integrative Neuroscience, 6, 66. 10.3389/fnint.2012.00066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Het S, & Wolf OT (2007). Mood changes in response to psychosocial stress in healthy young women: Effects of pretreatment with cortisol. Behavioral Neuroscience, 121(1), 11–20. 10.1037/0735-7044.121.1.11 [DOI] [PubMed] [Google Scholar]
- Joëls M, Karst H, DeRijk R, & de Kloet ER (2008). The coming out of the brain mineralocorticoid receptor. Trends in Neurosciences, 31(1), 1–7. 10.1016/j.tins.2007.10.005 [DOI] [PubMed] [Google Scholar]
- Karandrea D, Kittas C, & Kitraki E (2000). Contribution of sex and cellular context in the regulation of brain corticosteroid receptors following restraint stress. Neuroendocrinology, 71(6), 343–353. 10.1159/000054555 [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Kudielka BM, Gaab J, Schommer NC, & Hellhammer DH (1999). Impact of gender, menstrual cycle phase, and oral contraceptives on the activity of the hypothalamus-pituitary-adrenal axis. Psychosomatic Medicine, 61(2), 154–162. 10.1097/00006842-199903000-00006 [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Pirke KM, & Hellhammer DH (1993). The “Trier Social Stress Test” - A tool for investigating psychobiology stress responses in a laboratory setting. Neuropsychobiology, 28(1–2), 76–81. 10.1159/000119004 [DOI] [PubMed] [Google Scholar]
- Kitraki E, Kremmyda O, Youlatos D, Alexis MN, & Kittas C (2004). Gender-dependent alterations in corticosteroid receptor status and spatial performance following 21 days of restraint stress. Neuroscience, 125(1), 47–55. 10.1016/j.neuroscience.2003.12.024 [DOI] [PubMed] [Google Scholar]
- Kudielka BM, Hellhammer DH, & Kirschbaum C (2007). Ten years of research with the Trier Social Stress Test—revisited. In Harmon-Jones E & Winkielman P (Eds.), Social neuroscience: Integrating biological and psychological explanations of social behavior (pp. 56–83). Guilford Press. [Google Scholar]
- Kuhlman KR, Chiang JJ, Horn S, & Bower JE (2017). Developmental psychoneuroendocrine and psychoneuroimmune pathways from childhood adversity to disease. Neuroscience and Biobehavioral Reviews, 80, 166–184. 10.1016/j.neubiorev.2017.05.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhlman KR, Geiss EG, Vargas I, & Lopez-Duran NL (2015). Differential associations between childhood trauma subtypes and adolescent HPA-axis functioning. Psychoneuroendocrinology, 54, 103–114. 10.1016/j.psyneuen.2015.01.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhlman KR, Mayer SE, Vargas I, & Lopez-Duran NL (2021). Early life stress sensitizes adolescents to the influence of stress on affective memory. Developmental Psychobiology, 10.1111/DEV.22105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhlman KR, Robles TF, Haydon MD, Dooley LN, Boyle CC, & Bower JE (2020). Early life stress sensitizes individuals to the psychological correlates of mild fluctuations in inflammation. Developmental Psychobiology, 62(3), 400–408. 10.1002/dev.21908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis CC, Simons AD, Nguyen LJ, Murakami JL, Reid MW, Silva SG, & March JS (2010). Impact of childhood trauma on treatment outcome in the treatment for adolescents with depression study (TADS). Journal of the American Academy of Child & Adolescent Psychiatry, 49(2), 132–140. 10.1016/j.jaac.2009.10.007 [DOI] [PubMed] [Google Scholar]
- Lupien SJ, McEwen BS, Gunnar MR, & Heim C (2009). Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nature Reviews. Neuroscience, 10(6), 434–445. 10.1038/nrn2639 [DOI] [PubMed] [Google Scholar]
- Mauss IB, Levenson RW, McCarter L, Wilhelm FH, & Gross JJ (2005). The tie that binds? Coherence among emotion experience, behavior, and physiology. Emotion (Washington, D.C.), 5(2), 175–190. 10.1037/1528-3542.5.2.175 [DOI] [PubMed] [Google Scholar]
- Mayer SE, Peckins M, Kuhlman KR, Rajaram N, Lopez-Duran NL, Young EA, & Abelson JL (2020). The roles of comorbidity and trauma exposure and its timing in shaping HPA axis patterns in depression. Psychoneuroendocrinology, 120, 104776. 10.1016/j.psyneuen.2020.104776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGowan PO, Sasaki A, D’Alessio AC, Dymov S, Labonté B, Szyf M, Turecki G, & Meaney MJ (2009). Epigenetic regulation of the glucocorticoid receptor in human brain associates with childhood abuse. Nature Neuroscience, 12(3), 342–348. 10.1038/nn.2270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metz S, Duesenberg M, Hellmann-Regen J, Wolf OT, Roepke S, Otte C, & Wingenfeld K (2020). Blunted salivary cortisol response to psychosocial stress in women with posttraumatic stress disorder. Journal of Psychiatric Research, 130, 112–119. 10.1016/j.jpsychires.2020.07.014 [DOI] [PubMed] [Google Scholar]
- Monroe SM (2008). Modern approaches to conceptualizing and measuring human life stress. Annual Review of Clinical Psychology, 4, 33–52. 10.1146/annurev.clinpsy.4.022007.141207 [DOI] [PubMed] [Google Scholar]
- Nanni V, Uher R, & Danese A (2012). Childhood maltreatment predicts unfavorable course of illness and treatment outcome in depression: A meta-analysis. The American Journal of Psychiatry, 169(2), 141–151. 10.1176/appi.ajp.2011.11020335 [DOI] [PubMed] [Google Scholar]
- Nater UM, La Marca R, Florin L, Moses A, Langhans W, Koller MM, & Ehlert U (2006). Stress-induced changes in human salivary alpha-amylase activity - associations with adrenergic activity. Psychoneuroendocrinology, 31(1), 49–58. 10.1016/j.psyneuen.2005.05.010 [DOI] [PubMed] [Google Scholar]
- Nemeroff CB, Heim CM, Thase ME, Klein DN, Rush AJ, Schatzberg AF, Ninan PT, McCullough JP, Weiss PM, Dunner DL, Rothbaum BO, Kornstein S, Keitner G, & Keller MB, others. (2003). Differential responses to psychotherapy versus pharmacotherapy in patients with chronic forms of major depression and childhood trauma. Proceedings of the National Academy of Sciences of the United States of America, 100(24), 14293–14296. 10.1073/pnas.2336126100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Normann C, & Buttenschøn HN (2020). Gene–environment interactions between HPA-axis genes and childhood maltreatment in depression: A systematic review. Acta Neuropsychiatrica, 32(3), 111–121. 10.1017/neu.2020.1 [DOI] [PubMed] [Google Scholar]
- Otte C, Hinkelmann K, Moritz S, Yassouridis A, Jahn H, Wiedemann K, & Kellner M (2010). Modulation of the mineralocorticoid receptor as add-on treatment in depression: A randomized, double-blind, placebo-controlled proof-of-concept study. Journal of Psychiatric Research, 44(6), 339–346. 10.1016/j.jpsychires.2009.10.006 [DOI] [PubMed] [Google Scholar]
- Pariante CM, & Lightman SL (2008). The HPA axis in major depression: Classical theories and new developments. Trends in Neurosciences, 31(9), 464–468. 10.1016/j.tins.2008.06.006 [DOI] [PubMed] [Google Scholar]
- Rossi V, & Pourtois G (2012). Transient state-dependent fluctuations in anxiety measured using STAI, POMS, PANAS or VAS: A comparative review. Anxiety, Stress, and Coping, 25(6), 603–645. 10.1080/10615806.2011.582948 [DOI] [PubMed] [Google Scholar]
- Rozeboom AM, Akil H, & Seasholtz AF (2007). Mineralocorticoid receptor overexpression in forebrain decreases anxiety-like behavior and alters the stress response in mice. Proceedings of the National Academy of Sciences of the United States of America, 104(11), 4688–4693. 10.1073/pnas.0606067104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamseddeen W, Asarnow JR, Clarke G, Vitiello B, Wagner KD, Birmaher B, Keller MB, Emslie G, Iyengar S, Ryan ND, McCracken JT, Porta G, Mayes T, & Brent DA (2011). Impact of Physical and Sexual Abuse on Treatment Response in the Treatment of Resistant Depression in Adolescent Study (TORDIA). Journal of the American Academy of Child & Adolescent Psychiatry, 50(3), 293–301. 10.1016/j.jaac.2010.11.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheridan MA, & McLaughlin KA (2014). Dimensions of early experience and neural development: Deprivation and threat. Trends in Cognitive Sciences, 18(11), 580–585. 10.1016/j.tics.2014.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steptoe A, Hamer M, & Chida Y (2007). The effects of acute psychological stress on circulating inflammatory factors in humans: A review and meta-analysis. Brain, Behavior, and Immunity, 21(7), 901–912. 10.1016/j.bbi.2007.03.011 [DOI] [PubMed] [Google Scholar]
- ter Heegde F, De Rijk RH, & Vinkers CH (2015). The brain mineralocorticoid receptor and stress resilience. Psychoneuroendocrinology, 52, 92–110. 10.1016/j.psyneuen.2014.10.022 [DOI] [PubMed] [Google Scholar]
- Vinkers CH, Joëls M, Milaneschi Y, Gerritsen L, Kahn RS, Penninx BWJH, & Boks MPM (2015). Mineralocorticoid receptor haplotypes sex-dependently moderate depression susceptibility following childhood maltreatment. Psychoneuroendocrinology, 54, 90–102. 10.1016/j.psyneuen.2015.01.018 [DOI] [PubMed] [Google Scholar]
- Von Werne Baes C, de Carvalho Tofoli SM, Martins CMS, & Juruena MF (2012). Assessment of the hypothalamic-pituitary-adrenal axis activity: Glucocorticoid receptor and mineralocorticoid receptor function in depression with early life stress - a systematic review. Acta Neuropsychiatrica, 24(1), 4–15. 10.1111/j.1601-5215.2011.00610.x [DOI] [PubMed] [Google Scholar]
- Vrijsen JN, Vogel S, Arias-Vásquez A, Franke B, Fernández G, Becker ES, Speckens A, & van Oostrom I (2015). Depressed patients in remission show an interaction between variance in the mineralocorticoid receptor NR3C2 gene and childhood trauma on negative memory bias. Psychiatric Genetics, 25(3), 99–105. 10.1097/YPG.0000000000000081 [DOI] [PubMed] [Google Scholar]
- Walker EA, Unutzer J, Rutter C, Gelfand A, Saunders K, VonKorff M, Koss MP, & Katon W (1999). Costs of health care use by women HMO members with a history of childhood abuse and neglect. Archives of General Psychiatry, 56(7), 609–613. 10.1001/archpsyc.56.7.609 [DOI] [PubMed] [Google Scholar]