Abstract
Introduction
The objective of this study is to design a co-culture system of cancer cells and three-dimensional (3D) mesenchymal stem cells (MSC) aggregates for the in vitro evaluation of cancer invasion.
Methods
First, the MSC of an immunosuppressive phenotype (MSC2) were prepared by the MSC stimulation of polyriboinosinic polyribocytidylic acid. By simple mixing MSC2 and gelatin hydrogel microspheres (GM) in a U-bottomed well of 96 well plates which had been pre-coated with poly (vinyl alcohol), 3D MSC2 aggregates incorporating GM were obtained. The amount of chemokine (C–C motif) ligand 5 (CCL5) secreted from the MSC2 aggregates incorporating GM. Finally, an invasion assay was performed to evaluate the cancer invasion rate by co-cultured cancer cells and the 3D MSC2 incorporating GM.
Results
The amount of CCL5 secreted for the 3D MSC2 aggregates incorporating GM was significantly higher than that of two-dimensional (2D) MSC, 2D MSC2, and 3D MSC aggregates incorporating GM. When MDA-MB-231 human breast cancer cells were co-cultured with the 3D MSC2 aggregates incorporating GM, the invasion rate of cancer cells was significantly high compared with that of 2D MSC or 2D MSC2 and 3D MSC aggregates incorporating GM. In addition, high secretion of matrix metalloproteinase-2 was observed for the 3D MSC2 aggregates/cancer cells system.
Conclusions
It is concluded that the co-culture system of 3D MSC2 aggregates incorporating GM and cancer cells is promising to evaluate the invasion of cancer cells in vitro.
Keywords: Cancer invasion model, Anti-cancer drug screening, Three-dimensional cell culture, Mesenchymal stem cells, Gelatin hydrogel microspheres
Abbreviations: 2D, two-dimensional; 3D, three-dimensional; CAF, cancer-associated fibroblasts; (CCL)5, chemokine (C–C motif) ligand; DDW, double-distilled water; DMEM, Dulbecco's modified Eagle's medium; ELISA, enzyme-linked immunosolvent assay; FCS, fetal calf serum; GM, gelatin hydrogel microspheres; MEM, minimum essential medium; MMP, matrix metalloproteinase; MSC, mesenchymal stem cells; MSC2, MSC of an immunosuppressive phenotype; PBS, phosphate buffered-saline; PVA, poly (vinyl alcohol); TAM, tumor-associated macrophages
Highlights
-
•
This invasion model is an important tool for anti-cancer drug screening.
-
•
Mesenchymal stem cells of an immunosuppressive phenotype (MSC2) were obtained.
-
•
3D MSC2 aggregates incorporating gelatin hydrogel microspheres were prepared.
-
•
3D MSC2 aggregates promoted the invasion rate of cancer cells.
1. Introduction
The polarization of cells is one of the important properties to affect their biological functions. For example, macrophages are polarized to M1 (pro-inflammatory) and M2 (anti-inflammatory) phenotypes responding to the environment. The balance of the two phenotypes determines the inflammation induction or tissue regeneration [1,2]. M2 macrophages secrete anti-inflammatory cytokines of interleukin (IL)-10, leading to the repairing and regeneration of injured tissues [3,4]. On the other hand, M2 macrophages are involved in the cancer progression or metastasis and are recognized as tumor-associated macrophages (TAM) [5]. It has been reported that IL-17 and 23 secreted from TAM promote the cancer proliferation [6]. Tumor-necrosis factor-α (TNF-α), vascular endothelial growth factor (VEGF), and transforming growth factor-beta 1 (TGF-β1) secreted from TAM promote cancer metastasis [7]. In addition to macrophages, it has been demonstrated that mesenchymal stem cells (MSC) are also polarized to MSC1 or MSC2. MSC1 have a capacity for cancer cell diminishment or anti-cancer effect, while MSC2 are involved in the growth, invasion, and metastasis of cancer cells [[8], [9], [10]]. Therefore, MSC2 are one of the major component cells in the cancer environment to survive cancer cells or enhance their functions. As the cancer-related function of MSC2, the secretion of chemokine (C–C motif) ligand (CCL)5 or interleukin (IL)-1RA has been reported [9].
Drug discovery is one of the most important researches to treat diseases, such as cancer, infection, and autoimmune diseases. The therapeutic effects or the side effects of drugs have been so far evaluated by the cell culture system of a two-dimension (2D). However, the 2D cell culture conditions are quite different from those of in vivo environment. This difference often causes a big gap in the drug effect between the in vitro and in vivo systems, failing in drug development [11]. Therefore, to mimic the in vivo conditions, three-dimensional (3D) culture models, such as cell aggregates [12,13], spheroids [14,15], and organoids [[16], [17], [18]], have been recently investigated. For example, cells in cell aggregates well interacted with each other in a 3D manner, leading to an enhanced cell function of differentiation [19], metabolism [20], and extracellular matrix [21] or cytokine secretion [22]. Although such the 3D cell aggregates mimic the in vivo environment, cells present in the center of cell aggregates are not always proliferated well because oxygen or nutrient supplies to cells are poor [23,24]. As one trial to tackle the issue, hydrogel microspheres of a biodegradable natural polymer, gelatin (GM), have been explored to incorporate into cell aggregates for their enhanced functions and prolonged culture [13,25,26]. Because the water phase of GM matrices allows oxygen and nutrients to permeate into the aggregates [13]. Based on this feature, 3D cell aggregates incorporating GM would be a promising cell culture system for drug discovery.
This study is undertaken to design a co-culture system of cancer cells and 3D MSC2 aggregates incorporating GM, and evaluate the efficacy in the in vitro cancer cells invasion. First, 3D MSC2 aggregates incorporating GM were prepared to allow MSC2 to enhance biological function. The MSC2 function was assessed based on the secretion of a chemokine (C–C motif) ligand (CCL) 5 to compare with other control groups. Following the co-culture of cancer cells and 3D MSC2 aggregates incorporating GM, the invasion rate of cancer cells was evaluated in vitro. We examined the secretion of matrix metalloproteinase (MMP)-2 as a measure of cancer invasion promotion.
2. Materials and methods
2.1. Preparation of gelatin hydrogel microspheres
Gelatin hydrogel microspheres (GM) were prepared by the chemical crosslinking of gelatin in a water-in-oil emulsion state according to the method previously reported [13,25]. Briefly, an aqueous solution (20 ml) of 10 wt % gelatin (isoelectric point 5.0, weight-averaged molecular weight = 100,000, Nitta Gelatin Inc., Osaka, Japan) was preheated at 40 °C, followed by stirring at 300 rpm for 10 min to prepare a water-in-oil emulsion. The emulsion temperature was decreased at 4 °C for the natural gelation of gelatin solution to obtain non-crosslinked hydrogel microspheres. The resulting GM were washed three times with cold acetone in combination with centrifugation (5000 rpm, 4 °C, 5 min) to completely exclude the residual oil. Then, GM were fractionated by size using sieves with apertures of 32 and 53 μm (Iida Seisakusho Ltd, Osaka, Japan) and air dried at 4 °C. Then, non-crosslinked and dried GM (200 mg) were treated in a vacuum oven at 140 °C to allow them to dehydrothermally crosslink for 72 h. The picture of GM in the swollen state was taken with a microscope (BZ-X710, KEYENCE Ltd, Osaka, Japan). The size of 100 microspheres for each sample was measured using the computer program Image J (NIH Inc., Bethesda, USA) to calculate the average diameters.
2.2. Cell culture
Human bone marrow-derived mesenchymal stem cells (MSC) were kindly provided by professor Junya Toguchida (Institute for Frontier Life and Medical Sciences, Kyoto University). MSC were cultured in Modified Eagle's Medium alpha (MEM alpha). MDA-MB-231 of human breast cancer cells were cultured in Dulbecco's modified Eagle's medium (DMEM) (Thermo, Inc., Waltham, MA). Each culture medium was supplemented with 10 vol % fetal calf serum (FCS) (Thermo, Inc.) and 50 U/mL penicillin/streptomycin.
2.3. Induction of MSC polarization to MSC2
The polarization induction of MSC to MSC2 was performed according to the protocols previously established [9]. In brief, 1 μg/ml of polyriboinosinic polyribocytidylic acid (poly [I: C]) was added to the culture medium of MSC. After 1 h incubation, the fresh medium was changed to continue the culture for the following experiment.
2.4. Preparation of 3D MSC or MSC2 aggregates incorporating gelatin hydrogel microspheres
A poly (vinyl alcohol) (PVA) sample (the degree of polymerization = 1800 and the saponification = 88 mol%) kindly supplied from Unichika (Tokyo, Japan) was dissolved in phosphate-buffered saline (PBS) (pH 7.4, 1 wt %). To make the cell culture well non-adherent, the PVA solution was added to each well of round-bottomed (U-bottomed) 96-well culture plate (200 μl/well) and incubated at 37 °C for 15 min. Then, the solution was removed by aspiration, and the wells were washed twice with PBS (200 μl/well). GM and MSC or MSC2 were separately suspended in the standard medium. After the suspensions of GM (2 × 103 microspheres/mL, 100 μl) and MSC or MSC2 suspensions (2 × 104 cells/ml, 100 μl) were mixed, the mixture was added to the well pre-coated. After 2 days culture, cell aggregates were formed. The pictures of 3D MSC or MSC2 aggregates incorporating GM (3D MSC-GM or 3D MSC2-GM) were taken with a microscope (CKX41; Olympus Ltd., Tokyo, Japan). 3D MSC aggregates incorporating GM and 3D MSC2 aggregates incorporating GM were named 3D MSC-GM and 3D MSC2-GM, respectively.
2.5. Evaluation of CCL5 secretion
As a measure of MSC2 function, the amount of CCL5 secreted from 2D MSC, 2D MSC2, 3D MSC-GM, and 3D MSC2-GM was measured by Enzyme-Linked ImmunoSorbent Assay (ELISA) 2 and 4 days after incubation.
2.6. Evaluation of 3D MSC2 aggregate effect on invasion rate of cancer cells
Following the co-culture between various types of MSC (2D MSC, 2D MSC2, 3D MSC-GM, and 3D MSC2-GM) and cancer cells, a cancer invasion assay was performed by using Cytoselect 96 well invasion assay (Cell Biolabs, Inc., San Diego, USA) [27,28]. This kit is a transwell via a polycarbonate membrane with a pore size of 8 μm coated with the basement membrane components of collagen and laminin. Invasive cells migrate through the pore associated with the degradation of components coated by the MMP enzyme secreted. The assay is extensively performed to evaluate the invasion ability of cancer cells in vitro [[29], [30], [31], [32], [33], [34]].
In this experiment, 150 μl of MSC2 suspension (8.0 × 105 cells/well in medium containing FCS) or 3D MSC and MSC2 aggregates (2 or 4 days after incubation) were added (150 μl) into the tubes. After the membrane chamber was placed into the feeder tray, the cancer cell suspension (2.0 × 105 cells/well in FCS free medium) was added (100 μl) to the membrane chamber. The samples were incubated for 24 h. After completely dislodging the cancer cells from the underside of membrane, a lysis buffer dye solution was added. Then, the fluorescent intensity was measured in a fluorescence spectrometer (F-2000, HITACHI Ltd, Tokyo, Japan) at excitation and emission wavelengths of 480 and 520 nm, respectively. The invasion rate of cancer cells was calculated as follows: the number of cancer cells in the underside of membrane was divided by 2.0 × 105. In addition, the culture medium was collected, and the amount of MMP-2 secreted was evaluated using ELISA.
2.7. Statistical analysis
All the data were statistically analyzed and expressed as the mean ± the standard error of the mean. The data were analyzed by student t-test or Tukey's test to determine the statistically significant difference while the significance was accepted at p < 0.05. Experiments for each sample were performed three wells independently unless otherwise mentioned.
3. Results
3.1. Morphology of 3D MSC or MSC2 aggregates incorporating gelatin hydrogel microspheres
Fig. 1 shows the microscopic picture of GM. GM were spherical and had a smooth surface. The size in the swollen condition was 48.1 ± 6.47 μm. Fig. 2 shows the light microscopic pictures of 3D MSC-GM and 3D MSC2-GM 2 and 4 days after incubation. The size of 3D MSC-GM and 3D MSC2-GM was about 800 μm. The MSC phenotype did not affect the morphology of cell aggregates.
Fig. 1.
A light microscope photograph of GM dispersed in water. Scale bar; 50 μm.
Fig. 2.
Light microscope photographs of 3D MSC-GM, and 3D MSC2-GM 2 and 4 days after incubation. Scale bar; 200 μm.
3.2. CCL5 secretion of 3D MSC2-GM culture
Fig. 3 shows the amount of CCL5 secreted from 2D MSC, 2D MSC2, 3D MSC-GM, and 3D MSC2-GM 2 and 4 days after incubation. The secretion amount of 3D MSC2-GM 2 days after incubation was high compared with that of other groups (Fig. 3A). However, the similar tendency was not observed after 4 days incubation (Fig. 3B).
Fig. 3.
Relative CCL5 secretion amount for 2D MSC, 2D MSC2, 3D MSC-GM, and 3D MSC2-GM 2 (A) and 4 (B) days after incubation. ∗p < 0.05; significant against CCL5 secretion amount for other groups at the corresponding time.
3.3. Invasion of cancer cells in the co-culture system of 3D MSC2-GM and MMP2 secretion
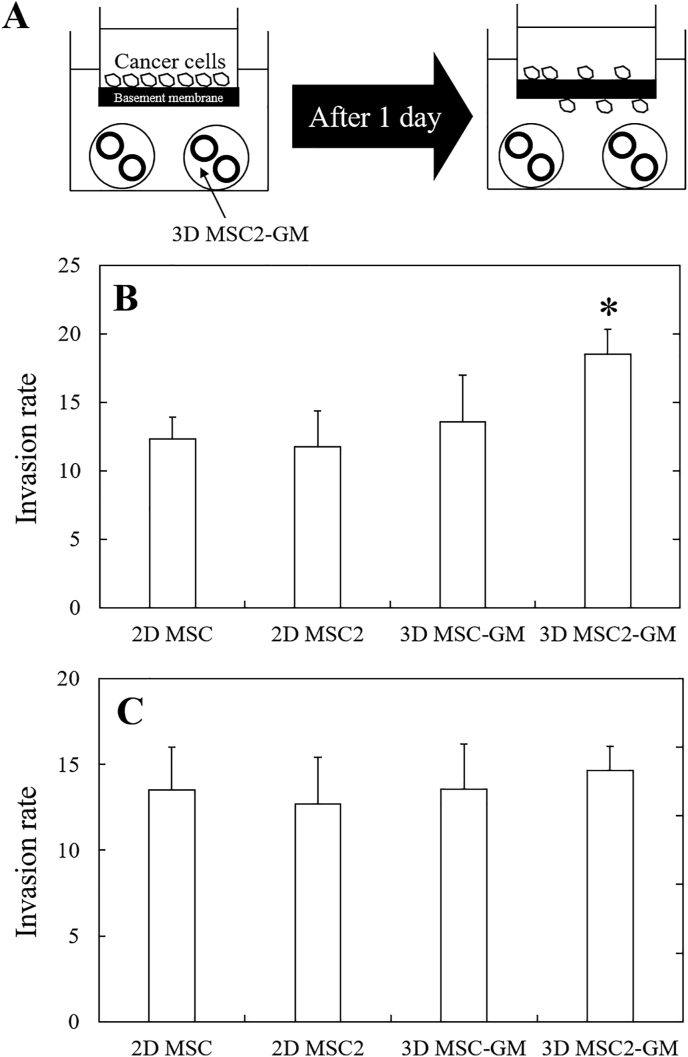
Fig. 4B shows that the invasion rate of cancer cells co-cultured with 2D MSC, 2D MSC2, 3D MSC-GM, and 3D MSC2-GM for 2 days. When the cancer cells were co-cultured with 3D MSC2-GM, the invasion rate of cancer cells was high compared with that of other groups. Such a tendency was not observed in the case of 3D MSC2-GM 4 days after incubation (Fig. 4C). For the co-culture with 3D MSC2-GM for 2 days, a significantly higher secretion level of MMP-2 was observed than that of other groups (Fig. 5).
Fig. 4.
(A) Illustration of cancer invasion assay based on the interaction between 3D MSC2 aggregates/cancer cells via model basement membrane. Invasion rate of cancer cells in co-culture system with 2D MSC, 2D MSC-2, 3D MSC-GM, and 3D MSC2-GM cultured 2 (B) and 4 (C) days after incubation. ∗p < 0.05; significant against invasion rate of cancer cells for other groups at the corresponding time.
Fig. 5.
Secretion amount of MMP-2 in co-culture system with 2D MSC, 2D MSC-2, 3D MSC-GM, and 3D MSC2-GM cultured 2 (A) and 4 (B) days after incubation. ∗p < 0.05; significant against secretion amount of MMP-2 for other groups at the corresponding time.
4. Discussion
An invasion of cancer cells is one of the most important issues to be solved in cancer therapy because the invasion leads to cancer metastasis, which is the main death cause of cancer diseases. In their connection, a culture system capable of evaluating the cancer invasion in vitro is highly needed to study the invasion of cancers for the development of anti-invasion drugs. Therefore, in this study, we focused on designing a cancer invasion model in vitro.
Three-dimensional (3D) cell culture with stromal cells composing of the tumor microenvironment, such as cancer-associated fibroblasts (CAF) [28,35], tumor-associated macrophages (TAM) [36], endothelial cells [37], and mesenchymal stem cells (MSC) [8,10], has been investigated for the drug discovery of cancer. In our previous studies, 3D CAF and TAM aggregates have been prepared to co-culture with cancer cells as an in vitro cancer invasion model [22,28,34]. Among the MSC, MSC2 play a major role in surviving cancer cells through their interaction. To evaluate the interaction, Waterman et al. report the co-culture of human bone marrow-derived MSC2 and human ovarian adenocarcinoma via the transwell. The invasion rate was high, but the MMP secretion did not change, although the effect of MMP2 and 9 on the cancer invasion has been proven [10]. To effectively enhance the MMP secretion level and increase the invasion rate of cancer cells, we hypothesized that it is important to enhance the MSC2 function by introducing 3D cell culture. However, little has been reported on the interaction of cancer cells with the 3D culture of MSC2. This is because cells readily weaken or die inside the 3D cell aggregates due to the poor nutrients and oxygen supply. To break through this issue, several trials with biomaterials technology have been reported [[38], [39], [40]]. For example, the culture on collagen scaffolds [35,41,42], the encapsulation by alginate microspheres [43], the usage of hyaluronic-based formulation [44,45], or polyethylene glycol-based matrices [[46], [47], [48]], have been demonstrated to survive cells of the 3D aggregates for a long time period. It is well known that biomaterials can assist a 3D culture system of cancer cells because they consist of extracellular matrix components [11,[49], [50], [51]]. In this study, as a biomaterial, gelatin is selected since it is a cell-friendly and biodegradable natural polymer that has been extensively for medical and pharmaceutical applications [[52], [53], [54]]. Microspheres of this gelatin have been used as the cell scaffolds and the carriers of drug release [28,55,56]. Their feasibility in interaction with cells has been proved. It is demonstrated that the incorporation of gelatin microspheres allows cells to enhance their survival and functions even in the 3D cell aggregates [13,25]. Based on these findings, in this study, 3D MSC2 aggregates incorporating the gelatin hydrogel microspheres (GM) were designed to improve the biological activity of MSC2. The diameter of GM used was 32–53 μm, and GM were hydrothermally crosslinked at 140°C for 72 h. This condition is confirmed by our previous studies to be an optimized property of GM in terms of GM incorporation into the cell aggregates [57]. The mixing ratio of GM/cells of 1:10 was selected since this ratio is suitable to form cell aggregates with a high biological function [13]. In addition, it is demonstrated that the biological functions of 3D MSC aggregates incorporating GM were much higher than those of 3D MSC aggregates without GM incorporation [25]. Therefore, 3D MSC2 aggregates without GM were not prepared as a control.
Fig. 2 shows that the microscopic pictures of 3D MSC-GM and 3D MSC2-GM. The size of cell aggregates did not change, irrespective of the MSC phenotypes. This indicates that the MSC phenotype did not affect the formation and morphology of cell aggregates. Next, the amount of CCL5 secreted from 3D MSC2-GM was significantly higher than that from other groups (Fig. 3A). This may be due to the activation of cells in 3D culture. The 3D cell–cell interaction enhances the cell activity [[58], [59], [60], [61]]. However, 4 days after incubation, the enhanced CCL5 secretion was no longer observed (Fig. 3B). It is highly conceivable that the MSC2 function in 3D MSC2 aggregates was reduced and lost for the longer time period of 4 days. The culture period is an important factor affecting the maintenance of MSC phenotype. For the invasion of cancer cells, the invasion rate of cancer cells was not significantly different between 2D MSC and 2D MSC2 groups (Fig. 4B). This is mainly because that there is a certain distance between cancer cells and MSC2 in this culture system. It would be difficult to maintain a sustained cell–cell interaction because of the distance. Next, the significantly strong invasion of cancer cells by co-culture with 3D MSC2-GM was observed than that of other groups. From this result, in the 3D MSC2-GM, the MSC2 activity is maintained even in the in vitro system, which effectively interacts with cells, resulting in an enhanced cancer invasion. However, when the 3D MSC2 aggregates incubated for 4 days were used, the enhanced phenomenon was not observed (Fig. 4C). This is again due to the loss of MSC2 activity 4 days after incubation. Another technology should be added to maintain the activity of MSC2 for a longer time period. For example, the controlled release of drugs that enable to activate or maintain the MSC2 function from GM would be effective. Therefore, when a longer time period is needed to investigate the function of 3D MSC2 aggregates, 3D MSC2 aggregates incorporating GM containing drugs should be prepared, although the drug release system is not performed in this study. In addition, the MMP-2 secretion amount upon co-culturing with 3D MSC2-GM 2 days after incubation was significantly higher than that of other groups (Fig. 5). Taken together, it is important to consider the culture period of 3D MSC2 aggregates to evaluate the in vitro invasion of cancer cells.
GM have been used for in vivo regenerative therapy [62]. It has been demonstrated that wound healing is accelerated only by injecting the GM incorporating basic fibroblast growth factor (b-FGF) to the target site. This is because bioactive bFGF is released from GM with the degradation to activate the cell activity of tissue regeneration therapy, while and then vascularization of an important factor for wound healing is induced [56,63]. GM are valuable to activate cell functions not only in regenerative therapy, but also in regenerative research of drug research [11,64]. It is reported that the incorporation of GM into the insulinoma cell aggregates enhances the insulin secretion. The insulinoma cell aggregates will be effective in drug discovery of type 1 diabetes [65]. Based on the findings, we can say with certainty that the GM incorporation into 3D cell aggregates is promising to design and prepare tissue-like models for drug research.
5. Conclusion
This study designed the drug screening model based on the interaction between cancer cells and MSC, especially the immunosuppressive MSC phenotype (MSC2). First, MSC2 aggregates incorporating gelatin hydrogel microspheres (GM) with about 50 μm were prepared. The aggregates were almost spherical, and the size was about 800 μm. The secretion amount of chemokine (C–C motif) ligand 5 was enhanced as the functions of MSC2. The MSC2 aggregates incorporating GM promoted the invasion ability of cancer cells by matrix metalloproteinase secretion. A 3D culture system of MSC2 incorporating GM is promising to evaluate the invasion behavior of cancer cells in vitro. A 3D culture system of MSC2 incorporating GM and cancer cells is promising to evaluate the invasion behavior of cancer cells in vitro.
Declaration of competing interest
The authors declare no conflict of interest.
Footnotes
Peer review under responsibility of the Japanese Society for Regenerative Medicine.
Contributor Information
Teruki Nii, Email: nii.teruki.204@m.kyushu-u.ac.jp.
Yasuhiko Tabata, Email: yasuhiko@infront.kyoto-u.ac.jp.
References
- 1.Gordon S., Taylor P.R. Monocyte and macrophage heterogeneity. Nat Rev Immunol. 2005;5:953–964. doi: 10.1038/nri1733. [DOI] [PubMed] [Google Scholar]
- 2.Yoshimoto Y., Jo J.I., Tabata Y. Preparation of antibody-immobilized gelatin nanospheres incorporating a molecular beacon to visualize the biological function of macrophages. Regen Ther. 2020;14:11–18. doi: 10.1016/j.reth.2019.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.da Silva M.D., Bobinski F., Sato K.L., Kolker S.J., Sluka K.A., Santos A.R.S. IL-10 cytokine released from M2 macrophages is crucial for analgesic and anti-inflammatory effects of acupuncture in a model of inflammatory muscle pain. Mol Neurobiol. 2015;51:19–31. doi: 10.1007/s12035-014-8790-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Momotori N., Jo J.I., Tabata Y. Preparation of polymer microspheres capable for pioglitazone release to modify macrophages function. Regen Ther. 2019;11:131–138. doi: 10.1016/j.reth.2019.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sica A., Schioppa T., Mantovani A., Allavena P. Tumour-associated macrophages are a distinct M2 polarised population promoting tumour progression: potential targets of anti-cancer therapy. Eur J Cancer. 2006;42:717–727. doi: 10.1016/j.ejca.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 6.Grivennikov S.I., Wang K., Mucida D., Stewart C.A., Schnabl B., Jauch D., et al. Adenoma-linked barrier defects and microbial products drive IL-23/IL-17-mediated tumour growth. Nature. 2012;491:254–258. doi: 10.1038/nature11465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tomita T., Sakurai Y., Ishibashi S., Maru Y. Imbalance of Clara cell-mediated homeostatic inflammation is involved in lung metastasis. Oncogene. 2011;30:3429–3439. doi: 10.1038/onc.2011.53. [DOI] [PubMed] [Google Scholar]
- 8.Barcellos-de-Souza P., Gori V., Bambi F., Chiarugi P. Tumor microenvironment: bone marrow-mesenchymal stem cells as key players. Biochim Biophys Acta Rev Cancer. 2013;1836:321–335. doi: 10.1016/j.bbcan.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 9.Waterman R.S., Tomchuck S.L., Henkle S.L., Betancourt A.M. A new mesenchymal stem cell (MSC) paradigm: polarization into a pro-inflammatory MSC1 or an immunosuppressive MSC2 phenotype. PLoS One. 2010;5 doi: 10.1371/journal.pone.0010088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Waterman R.S., Henkle S.L., Betancourt A.M. Mesenchymal stem cell 1 (MSC1)-Based therapy attenuates tumor growth whereas MSC2-treatment promotes tumor growth and metastasis. PLoS One. 2012;7 doi: 10.1371/journal.pone.0045590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nii T., Makino K., Tabata Y. Three-dimensional culture system of cancer cells combined with biomaterials for drug screening. Cancers (Basel) 2020;12:1–24. doi: 10.3390/cancers12102754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abecasis B., Aguiar T., Arnault É., Costa R., Gomes-Alves P., Aspegren A., et al. Expansion of 3D human induced pluripotent stem cell aggregates in bioreactors: bioprocess intensification and scaling-up approaches. J Biotechnol. 2017;246:81–93. doi: 10.1016/j.jbiotec.2017.01.004. [DOI] [PubMed] [Google Scholar]
- 13.Nii T., Makino K., Tabata Y. Influence of shaking culture on the biological functions of cell aggregates incorporating gelatin hydrogel microspheres. J Biosci Bioeng. 2019;128:606–612. doi: 10.1016/j.jbiosc.2019.04.013. [DOI] [PubMed] [Google Scholar]
- 14.Nunes A.S., Barros A.S., Costa E.C., Moreira A.F., Correia I.J. 3D tumor spheroids as in vitro models to mimic in vivo human solid tumors resistance to therapeutic drugs. Biotechnol Bioeng. 2019;116:206–226. doi: 10.1002/bit.26845. [DOI] [PubMed] [Google Scholar]
- 15.Brüningk S.C., Rivens I., Box C., Oelfke U., Ter Haar G. 3D tumour spheroids for the prediction of the effects of radiation and hyperthermia treatments. Sci Rep. 2020;10:1–13. doi: 10.1038/s41598-020-58569-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim J., Koo B.K., Knoblich J.A. Human organoids: model systems for human biology and medicine. Nat Rev Mol Cell Biol. 2020;21:571–584. doi: 10.1038/s41580-020-0259-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lancaster M.A., Huch M. Disease modelling in human organoids. DMM Dis Model Mech. 2019;12 doi: 10.1242/dmm.039347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Driehuis E., Kretzschmar K., Clevers H. Establishment of patient-derived cancer organoids for drug-screening applications. Nat Protoc. 2020;15:3380–3409. doi: 10.1038/s41596-020-0379-4. [DOI] [PubMed] [Google Scholar]
- 19.Fukuda J., Sakai Y., Nakazawa K. Novel hepatocyte culture system developed using microfabrication and collagen/polyethylene glycol microcontact printing. Biomaterials. 2006;27:1061–1070. doi: 10.1016/j.biomaterials.2005.07.031. [DOI] [PubMed] [Google Scholar]
- 20.Rodríguez-Enríquez S., Gallardo-Pérez J.C., Avilés-Salas A., Marín-Hernández A., Carreño-Fuentes L., Maldonado-Lagunas V., et al. Energy metabolism transition in multi-cellular human tumor spheroids. J Cell Physiol. 2008;216:189–197. doi: 10.1002/jcp.21392. [DOI] [PubMed] [Google Scholar]
- 21.Lin R.Z., Chang H.Y. Recent advances in three-dimensional multicellular spheroid culture for biomedical research. Biotechnol J. 2008;3:1172–1184. doi: 10.1002/biot.200700228. [DOI] [PubMed] [Google Scholar]
- 22.Nii T., Makino K., Tabata Y. A cancer invasion model of cancer-associated fibroblasts aggregates combined with TGF-β1 release system. Regen Ther. 2020;14:196–204. doi: 10.1016/j.reth.2020.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kellner K., Liebsch G., Klimant I., Wolfbeis O.S., Blunk T., Schulz M.B., et al. Determination of oxygen gradients in engineered tissue using a fluorescent sensor. Biotechnol Bioeng. 2002;80:73–83. doi: 10.1002/bit.10352. [DOI] [PubMed] [Google Scholar]
- 24.Compañ V., Guzmán J., Riande E. A potentiostatic study of oxygen transmissibility and permeability through hydrogel membranes. Biomaterials. 1998;19:2139–2145. doi: 10.1016/S0142-9612(98)00113-6. [DOI] [PubMed] [Google Scholar]
- 25.Hayashi K., Tabata Y. Preparation of stem cell aggregates with gelatin microspheres to enhance biological functions. Acta Biomater. 2011;7:2797–2803. doi: 10.1016/j.actbio.2011.04.013. [DOI] [PubMed] [Google Scholar]
- 26.Tajima S., Tabata Y. Preparation of epithelial cell aggregates incorporating matrigel microspheres to enhance proliferation and differentiation of epithelial cells. Regen Ther. 2017;7:34–44. doi: 10.1016/j.reth.2017.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Desai S.D., Reed R.E., Burks J., Wood L.M., Pullikuth A.K., Haas A.L., et al. ISG15 disrupts cytoskeletal architecture and promotes motility in human breast cancer cells. Exp Biol Med. 2012;237:38–49. doi: 10.1258/ebm.2011.011236. [DOI] [PubMed] [Google Scholar]
- 28.Nii T., Makino K., Tabata Y. A cancer invasion model combined with cancer-associated fibroblasts aggregates incorporating gelatin hydrogel microspheres containing a p53 inhibitor. Tissue Eng - Part C Methods. 2019;25:711–720. doi: 10.1089/ten.tec.2019.0189. [DOI] [PubMed] [Google Scholar]
- 29.Leung W.H., Vong Q.P., Lin W., Janke L., Chen T., Leung W. Modulation of NKG2D ligand expression and metastasis in tumors by spironolactone via RXRγ activation. J Exp Med. 2013;210:2675–2692. doi: 10.1084/jem.20122292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen Z., Zhang D., Yue F., Zheng M., Kovacevic Z., Richardson D.R. The iron chelators Dp44mT and DFO inhibit TGF-β-induced epithelial-mesenchymal transition via up-regulation of N-Myc downstream-regulated gene 1 (NDRG1) J Biol Chem. 2012;287:17016–17028. doi: 10.1074/jbc.M112.350470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nakayama K. CAMP-response element-binding protein (CREB) and NF-κB transcription factors are activated during prolonged hypoxia and cooperatively regulate the induction of matrix metalloproteinase MMP1. J Biol Chem. 2013;288:22584–22595. doi: 10.1074/jbc.M112.421636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eckstein N., Servan K., Hildebrandt B., Pölitz A., Von Jonquières G., Wolf-Kümmeth S., et al. Hyperactivation of the insulin-like growth factor receptor I signaling pathway Is an essential event for cisplatin resistance of ovarian cancer cells. Cancer Res. 2009;69:2996–3003. doi: 10.1158/0008-5472.CAN-08-3153. [DOI] [PubMed] [Google Scholar]
- 33.Neil J.R., Johnson K.M., Nemenoff R.A., Schiemann W.P. Cox-2 inactivates Smad signaling and enhances EMT stimulated by TGF-β through a PGE2-dependent mechanisms. Carcinogenesis. 2008;29:2227–2235. doi: 10.1093/carcin/bgn202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nii T., Kuwahara T., Makino K., Tabata Y. A co-culture system of three-dimensional tumor-associated macrophages and three-dimensional cancer-associated fibroblasts combined with biomolecule release for cancer cell migration. Tissue Eng - Part A. 2020;26:1272–1282. doi: 10.1089/ten.tea.2020.0095. [DOI] [PubMed] [Google Scholar]
- 35.Miyazaki K., Oyanagi J., Hoshino D., Togo S., Kumagai H., Miyagi Y. Cancer cell migration on elongate protrusions of fibroblasts in collagen matrix. Sci Rep. 2019;9:1–15. doi: 10.1038/s41598-018-36646-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu W., Song J., Du X., Zhou Y., Li Y., Li R., et al. AKR1B10 (Aldo-keto reductase family 1 B10) promotes brain metastasis of lung cancer cells in a multi-organ microfluidic chip model. Acta Biomater. 2019;91:195–208. doi: 10.1016/j.actbio.2019.04.053. [DOI] [PubMed] [Google Scholar]
- 37.Mazio C., Casale C., Imparato G., Urciuolo F., Netti P.A. Recapitulating spatiotemporal tumor heterogeneity in vitro through engineered breast cancer microtissues. Acta Biomater. 2018;73:236–249. doi: 10.1016/j.actbio.2018.04.028. [DOI] [PubMed] [Google Scholar]
- 38.Anada T., Fukuda J., Sai Y., Suzuki O. An oxygen-permeable spheroid culture system for the prevention of central hypoxia and necrosis of spheroids. Biomaterials. 2012;33:8430–8441. doi: 10.1016/j.biomaterials.2012.08.040. [DOI] [PubMed] [Google Scholar]
- 39.Pedraza E., Coronel M.M., Fraker C.A., Ricordi C., Stabler C.L. Preventing hypoxia-induced cell death in beta cells and islets via hydrolytically activated, oxygen-generating biomaterials. Proc Natl Acad Sci U S A. 2012;109:4245–4250. doi: 10.1073/pnas.1113560109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Patil P.S., Mansouri M., Leipzig N.D. Fluorinated chitosan microgels to overcome internal oxygen transport deficiencies in microtissue culture systems. Adv Biosyst. 2020;1900250:1–10. doi: 10.1002/adbi.201900250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lv D., Yu S.C., Ping Y.F., Wu H., Zhao X., Zhang H., et al. A three-dimensional collagen scaffold cell culture system for screening anti-glioma therapeutics. Oncotarget. 2016;7:56904–56914. doi: 10.18632/oncotarget.10885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reynolds D.S., Tevis K.M., Blessing W.A., Colson Y.L., Zaman M.H., Grinstaff M.W. Breast cancer spheroids reveal a differential cancer stem cell response to chemotherapeutic treatment. Sci Rep. 2017;7:1–12. doi: 10.1038/s41598-017-10863-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.DelNero P., Lane M., Verbridge S.S., Kwee B., Kermani P., Hempstead B., et al. 3D culture broadly regulates tumor cell hypoxia response and angiogenesis via pro-inflammatory pathways. Biomaterials. 2015;55:110–118. doi: 10.1016/j.biomaterials.2015.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Baker A.E.G., Tam R.Y., Shoichet M.S. Independently tuning the biochemical and mechanical properties of 3D hyaluronan-based hydrogels with oxime and diels-alder chemistry to culture breast cancer spheroids. Biomacromolecules. 2017;18:4373–4384. doi: 10.1021/acs.biomac.7b01422. [DOI] [PubMed] [Google Scholar]
- 45.Huang Y.J., Hsu S.H. Acquisition of epithelial-mesenchymal transition and cancer stem-like phenotypes within chitosan-hyaluronan membrane-derived 3D tumor spheroids. Biomaterials. 2014;35:10070–10079. doi: 10.1016/j.biomaterials.2014.09.010. [DOI] [PubMed] [Google Scholar]
- 46.Moriyama K., Naito S., Wakabayashi R., Goto M., Kamiya N. Enzymatically prepared redox-responsive hydrogels as potent matrices for hepatocellular carcinoma cell spheroid formation. Biotechnol J. 2016;11:1452–1460. doi: 10.1002/biot.201600087. [DOI] [PubMed] [Google Scholar]
- 47.Yang X., Sarvestani S.K., Moeinzadeh S., He X., Jabbari E. Three-dimensional-engineered matrix to study cancer stem cells and tumorsphere formation: effect of matrix modulus. Tissue Eng - Part A. 2013;19:669–684. doi: 10.1089/ten.tea.2012.0333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pradhan S., Clary J.M., Seliktar D., Lipke E.A. A three-dimensional spheroidal cancer model based on PEG-fibrinogen hydrogel microspheres. Biomaterials. 2017;115:141–154. doi: 10.1016/j.biomaterials.2016.10.052. [DOI] [PubMed] [Google Scholar]
- 49.Nii T., Takeuchi I., Kimura Y., Makino K. Effects of the conformation of PLGA molecules in the organic solvent on the aerodynamic diameter of spray dried microparticles. Colloids Surfaces A Physicochem Eng Asp. 2018;539:347–353. doi: 10.1016/j.colsurfa.2017.12.042. [DOI] [Google Scholar]
- 50.Hinderer S., Layland S.L., Schenke-Layland K. ECM and ECM-like materials - biomaterials for applications in regenerative medicine and cancer therapy. Adv Drug Deliv Rev. 2016;97:260–269. doi: 10.1016/j.addr.2015.11.019. [DOI] [PubMed] [Google Scholar]
- 51.Nii T., Katayama Y. Biomaterial-assisted regenerative medicine. Int J Mol Sci. 2021;22:1–18. doi: 10.3390/ijms22168657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Matsuo T., Masumoto H., Tajima S., Ikuno T., Katayama S., Minakata K., et al. Efficient long-term survival of cell grafts after myocardial infarction with thick viable cardiac tissue entirely from pluripotent stem cells. Sci Rep. 2015;5:1–14. doi: 10.1038/srep16842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bello A.B., Kim D., Kim D., Park H., Lee S.H. Engineering and functionalization of gelatin biomaterials: from cell culture to medical applications. Tissue Eng - Part B Rev. 2020;26:164–180. doi: 10.1089/ten.teb.2019.0256. [DOI] [PubMed] [Google Scholar]
- 54.Nakamura K., Saotome T., Shimada N., Matsuno K., Tabata Y. A gelatin hydrogel nonwoven fabric facilitates metabolic activity of multilayered cell sheets. Tissue Eng - Part C Methods. 2019;25:344–352. doi: 10.1089/ten.tec.2019.0061. [DOI] [PubMed] [Google Scholar]
- 55.Tabata Y., Ikada Y. Protein release from gelatin matrices. Adv Drug Deliv Rev. 1998;31:287–301. doi: 10.1016/S0169-409X(97)00125-7. [DOI] [PubMed] [Google Scholar]
- 56.Tabata Y., Ikada Y. Vascularization effect of basic fibroblast growth factor released from gelatin hydrogels with different biodegradabilities. Biomaterials. 1999;20:2169–2175. doi: 10.1016/S0142-9612(99)00121-0. [DOI] [PubMed] [Google Scholar]
- 57.Tajima S., Tabata Y. Preparation and functional evaluation of cell aggregates incorporating gelatin microspheres with different degradabilities. J Tissue Eng Regen Med. 2013;7:801–811. doi: 10.1002/term. [DOI] [PubMed] [Google Scholar]
- 58.Cave D.D., Rizzo R., Sainz B., Gigli G., Del Mercato L.L., Lonardo E. The revolutionary roads to study cell–cell interactions in 3d in vitro pancreatic cancer models. Cancers (Basel) 2021;13:1–19. doi: 10.3390/cancers13040930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Peng W.C., Logan C.Y., Fish M., Anbarchian T., Aguisanda F., Álvarez-Varela A., et al. Inflammatory cytokine TNFα promotes the long-term expansion of primary hepatocytes in 3D culture. Cell. 2018;175:1607–1619.e15. doi: 10.1016/j.cell.2018.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chaicharoenaudomrung N., Kunhorm P., Noisa P. Three-dimensional cell culture systems as an in vitro platform for cancer and stem cell modeling. World J Stem Cells. 2019;11:1065–1083. doi: 10.4252/wjsc.v11.i12.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Desai P.K., Tseng H., Souza G.R. Assembly of hepatocyte spheroids using magnetic 3D cell culture for CYP450 inhibition/induction. Int J Mol Sci. 2017;18 doi: 10.3390/ijms18051085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nii T. Strategies using gelatin microparticles for regenerative therapy and drug screening applications. Molecules. 2021;26:1–10. doi: 10.3390/molecules26226795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kawai K., Suzuki S., Tabata Y., Ikada Y., Nishimura Y. Accelerated tissue regeneration through incorporation of basic fibroblast growth factor-impregnated gelatin microspheres into artificial dermis. Biomaterials. 2000;21:489–499. doi: 10.1016/S0142-9612(99)00207-0. [DOI] [PubMed] [Google Scholar]
- 64.Mitsui R., Matsukawa M., Nakagawa K., Isomura E., Kuwahara T., Nii T., et al. Efficient cell transplantation combining injectable hydrogels with control release of growth factors. Regen Ther. 2021;18:372–383. doi: 10.1016/j.reth.2021.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Inoo K., Bando H., Tabata Y. Enhanced survival and insulin secretion of insulinoma cell aggregates by incorporating gelatin hydrogel microspheres. Regen Ther. 2018;8:29–37. doi: 10.1016/j.reth.2017.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]