Abstract
Background
Paliperidone 3-monthly (PP3M) long-acting injection has proven efficacy and effectiveness in schizophrenia. Little is known of its effectiveness in other diagnoses.
Methods
All patients starting PP3M were followed up for 2 years. Main outcome measures were relapse and discontinuation from PP3M. Post hoc we examined outcomes in those switched back to one monthly paliperidone (PP1M) long-acting injection.
Results
Overall, 186 patients were followed-up. At the 2-year end point, 110 patients (59%) were still receiving PP3M, and 129 (70%) were receiving some form of paliperidone long-acting injection. Discontinuation from paliperidone long-acting injections (PPLAIs) was more likely with a nonschizophrenia diagnosis (hazard ratio [HR] for continuation 0.429 [95% confidence intervals (CI) – 0.21, 0.87 p = 0.018)), and prior clozapine use [in PP3M patients; HR for discontinuation 1.87 [95% CI – 1.05, 3.30 p = 0.032]). Relapse occurred in 20 (11%) of those receiving PP3M. Relapse on PP3M and PPLAIs was more likely in nonschizophrenia diagnosis (HR 0.17 for remaining relapse-free [95% CI – 0.06, 0.50; p = 0.001]; HR 0.21 [95% CI – 0.08, 0.58 p = 0.002], respectively), polypharmacy in PP3M patients (HR for relapse 7.91 [95% CI – 3.73, 22.9; p < 0.001]) and PPLAI patients (HR for relapse 6.45 [95% CI – 2.49, 16.5; p < 0.001]), and prior clozapine use in PP3M patients (HR for relapse 6.11 [95% CI – 1.82, 20.5; p = 0.003]) and PPLAI patients (HR for relapse 4.52 (95% CI – 1.51, 13.5; p = 0.007).
Conclusions
Outcomes with PP3M are excellent in practice, even when used outside its formal license. PP3M was relatively more effective in those with an F20 schizophrenia diagnosis and in those never before considered for or prescribed clozapine.
Keywords: discontinuation, paliperidone, psychotic disorders, relapse, three monthly
Introduction
Paliperidone long-acting injections (PPLAIs) are effective in maintenance treatment of adults with schizophrenia and licensed for this use in most of the world. There are relatively limited reports of one monthly paliperidone (PP1M) use in schizoaffective disorder [1–3] or bipolar disorder [3–5]. These studies do however support their value in reducing hospital admissions and the number of manic or mixed episodes, and the risk of relapse and hospitalization. To the best of our knowledge, no studies have examined Paliperidone 3-monthly (PP3M) use in conditions outside schizophrenia.
The main aim of this study was to investigate naturalistic outcomes—relapse and discontinuation—resulting from licensed and unlicensed prescribing of PP3M in clinical practice. A secondary aim was to determine factors relating to relapse and discontinuation.
Method
We followed up patients treated at the South London and Maudsley NHS foundation trust—a specialist mental health organization that serves 1.2 million people in south east London. The study was approved by the South London and Maudsley Drugs and Therapeutics Committee in 2016 (approval code SLaM/DTC/2016/3) as a service development not requiring individual patient consent. This was a prospective, noninterventional evaluation which did not alter patient treatment in any way. Patients were prescribed PP3M according to clinical need and did not undergo any additional investigation or monitoring. Patient data were collected using the Trust’s bespoke electronic patient record system—ePJS. Patient data were viewed only by pharmacy staff normally involved in patient care and data were anonymized at source and held on a secure database. No patient can be identified in this report.
Our a priori main outcomes were continuation with, and relapse on, PP3M in all patients prescribed PP3M regardless of indication. Our secondary aim was to find predictors for these outcomes based on a robust statistical model of the results. All patients starting on PP3M with South London and Maudsley were registered (by prescriber initiation form) and followed up for 2 years from the date of initiation. All included patients received at least one dose of PP3M. The prescriber initiation form included the following information: patient’s age, primary diagnosis (ICD-10), confirmation patient was neither pregnant nor breastfeeding, renal function, and PP1M maintenance doses. Each form was screened by a pharmacist for completeness and kept secure at the pharmacy. Information provided from initiation forms was checked against information stored on the patient electronic record system. Information collected and confirmed included: care setting on initiation, having ever been prescribed or considered for clozapine (a proxy measure for treatment responsiveness/resistance), any additional antipsychotic prescribed over follow-up (excluding PRN) and their PP1M initiation date and dose. Electronic patient medical records were accessed to gain information on outcome (continuation and relapse), reasons for discontinuation and medication prescribed after discontinuation. Additionally, information was collected on the average number of injections per year, time between injections and dose strengths. Adverse effects were reported anonymously to Medicines and Healthcare products Regulatory Agency and Janssen-Cilag Ltd. (Buckinghamshire, UK).
Discontinuation was defined as not receiving PP3M for 4 months (the 3-month dose interval + 1 month). The index date was taken as time of first prescription for PP3M and the date of discontinuation was counted as their last injection plus 3 months. Discontinuation from PP3M owing to prescriber decision relating to a worsening of condition was not, for the purposes of this study, defined as a relapse unless it resulted in a step-up in clinical care. Time to discontinuation was calculated from index to discontinuation or to attrition.
Relapse was defined as a step-up in clinical care and included referral to home-treatment team, attendance in Accident and Emergency (ER) services for reasons of psychiatric deterioration resulting in psychiatric admission, or direct admission to a psychiatric hospital. Time to relapse on PP was taken as time from index to the step-up in care. Discontinuation was treated as a censoring variable in continuous analysis of relapse.
Post hoc secondary outcomes were continuation and relapse on all PPLAI formulations (PP3M alone or PP3M followed immediately by PP1M) prescribed over the 2-year follow-up period. Discontinuation from PP1M (post PP3M) was dated as the last injection plus 1 month.
Statistical analysis
Baseline characteristics were summarized using measures of central tendency and variability. The association between diagnosis and time to relapse and time to discontinuation on PP3M and then PPLAI was examined with use of Cox proportional hazard models.
All regression models were adjusted for age, inpatient (Y/N), sex, polypharmacy, ethnicity, and dose, prior use of clozapine. Model assumptions were examined using Scholfeld’s residuals and plots for each variable in the model and overall. Adjusted cumulative hazard plots by F20 diagnosis were constructed at the mean of model covariates plots. Statistical analysis was conducted in Stata version 15.0 (StataCorp LLC, College Station, TX) and R version 4.0.2. (R Foundation for Statistical Computing, Vienna, Austria).
Results
In total, 186 patients were initiated on PP3M between 2016 and January 2019.
Continuation
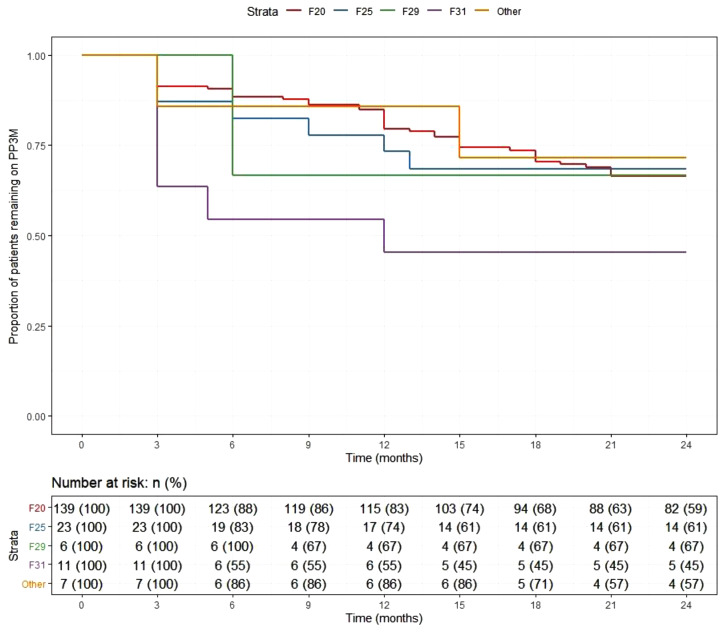
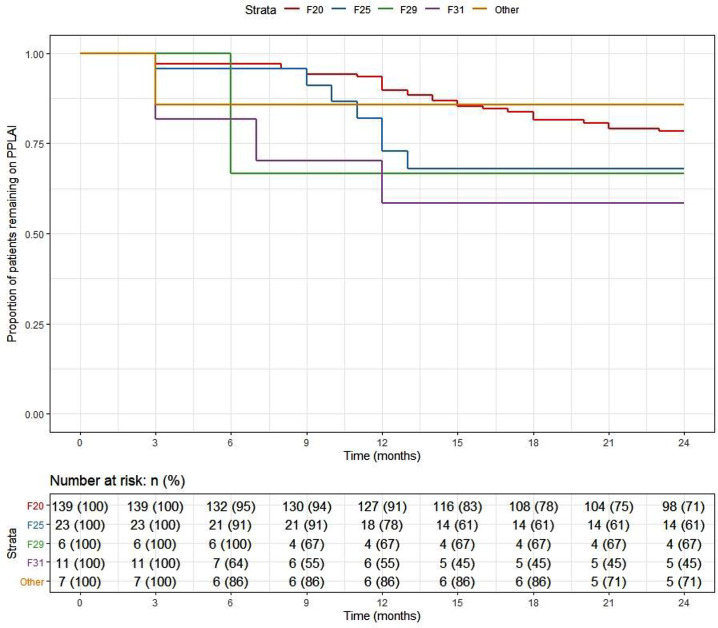
At the end of the 2-year follow-up, 110 patients (59%) had continuously received PP3M for the whole study period. Of those who discontinued, nearly half (n = 29) changed their medication to PP1M (10 later discontinued). The continuation rate for all PPLAI use from the PP3M initiation date was 70% (129/186). Figures 1 and 2 show these continuation rates within diagnosis groups. The most frequent reasons recorded for discontinuation were patient refusal (n = 18), perceived inefficacy (n = 15), and adverse effects (n = 11) (Table 1). Adverse effects cited as the reason for discontinuing were: hyperprolactinemia (n = 4), weight gain (n = 4), erectile dysfunction (n = 2), restlessness (n = 1), blurred vision (n = 1), anorgasmia (n = 1), extrapyramidal side effects (n = 1), gynaecomastia (n = 1), anhedonia (n = 1), lumps at injection sites (n = 1), and deranged liver function test (n = 1). Discontinuation statistics are displayed in Table 1. The baseline characteristics for each outcome group are shown in Table 2 and PP3M and PPLAI characteristics in Table 3.
Figure 1.
Kaplan–Meier plot showing the proportion of patients prescribed paliperidone 3-monthly (PP3M) since initiation in diagnosis groups.
Figure 2.
Kaplan–Meier plot showing the proportion of patients prescribed paliperidone long-acting injection (PPLAI) since initiation in diagnosis groups.
Table 1.
Discontinuation from PP3M.
| Clinical outcome after 2 years (PP3M) | ||
|---|---|---|
| n = 186 | ||
| Outcome 2 years after initiating PP3M [n (%)] | ||
| Continuation | 110 | (59.1) |
| Discontinuation | 62 | (33.3) |
| Attrition | 14 | (7.5) |
| n = 62 | ||
| Reasons for discontinuation [n (%)] | ||
| Patient refusal | 18 | (29.0) |
| Perceived inefficacy | 15 | (24.2) |
| Adverse effects | 11 | (17.7) |
| Patient request | 6 | (9.7) |
| Need more flexible dose adjustment | 4 | (6.5) |
| Independent health condition | 3 | (4.8) |
| Discharged from mental health services | 3 | (4.8) |
| Age concern (>65 years old) | 2 | (3.2) |
| Next medication (3 months after last depot) [n (%)] | ||
| PP1M | 29 | (46.8) |
| No medication | 21 | (33.9) |
| Risperidone (oral) | 5 | (8.1) |
| Aripiprazole (LAI) | 2 | (3.2) |
| Aripiprazole (oral) | 1 | (1.6) |
| Clozapine (oral) | 1 | (1.6) |
| Flupenthixol (LAI) | 1 | (1.6) |
| Haloperidol (LAI) | 1 | (1.6) |
| Haloperidol (oral) | 1 | (1.6) |
| n = 14 | ||
| Reasons for attrition [n (%)] | ||
| Lost to follow-up a | 8 | (57.1) |
| Died b | 6 | (42.9) |
Abbreviations: LAI, long-acting injection; PP3M, Paliperidone 3-monthly.
Lost to follow up: left country (n = 4), missing person (n = 2), changed trust (n = 1), and disengagement (n = 1).
Deaths classified using ePJS and not at a systems level: unknown (n = 2), heroin overdose (n = 1), natural causes (n = 1), nonadherence to diabetes medication (n = 1), and sepsis (n = 1).
Table 2.
Baseline characteristics for continuation outcomes on PP3M and PPLAI.
| Characteristic | Total | PP3M continuers | PP3M discontinuers a | PPLAI continuers | PPLAI discontinuers a | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| n = 186 | n = 110 | n = 76 | n = 129 | n = 57 | ||||||
| Age on initiation of PP3M (years) | ||||||||||
| Mean, SD | 45, 13.2 | 46, 11.7 | 44, 15.1 | 47, 12.3 | 42, 14.4 | |||||
| Min, max | 20, 77 | 22, 74 | 20, 77 | 22, 77 | 20, 74 | |||||
| Prior duration of PP1M (months) | ||||||||||
| Mean, SD | 34, 24.5 | 35, 25.1 | 34, 23.7 | 37, 24.9 | 28, 22.5 | |||||
| Min, max | 3, 89 | 3, 89 | 3, 81 | 3, 89 | 3, 81 | |||||
Abbreviations: PPLAI, paliperidone long-acting injection; PP1M, one monthly paliperidone; PP3M, Paliperidone 3-monthly; SD, standard deviation.
Discontinuers includes those who stopped due to death or being lost to follow-up.
Asian refers to Indian—subcontinent as per NHS ethnicity classification.
Table 3.
PP3M and PPLAI characteristics for continuation.
| PP3M | Total | PP3M continuers | PP3M discontinuers a | PPLAI continuers | PPLAI discontinuers a | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| n = 186 | n = 110 | n = 76 | n = 129 | n = 57 | ||||||
| Average PP3M injections/year = | ||||||||||
| Mean, SD | 4.0, 0.1 | 4.0, 0.1 | 4.0, 0.2 | 4.0, 0.1 | 4.0, 0.2 | |||||
| Min, max | 3.6, 4.7 | 3.6, 4.6 | 3.6, 4.7 | 3.6, 4.6 | 3.6, 4.7 | |||||
| Average no. days between PP3M injections | ||||||||||
| Mean, SD | 92, 3.5 | 92, 2.6 | 92, 5.0 | 93, 2.6 | 92, 5.5 | |||||
| Min, max | 70, 106 | 86, 105 | 70, 106 | 86, 105 | 70, 106 | |||||
Abbreviations: PPLAI, Paliperidone 3-monthly; PP3M, paliperidone long-acting injection; SD, standard deviation.
Discontinuers includes those who stopped due to death or being lost to follow-up.
Relapse
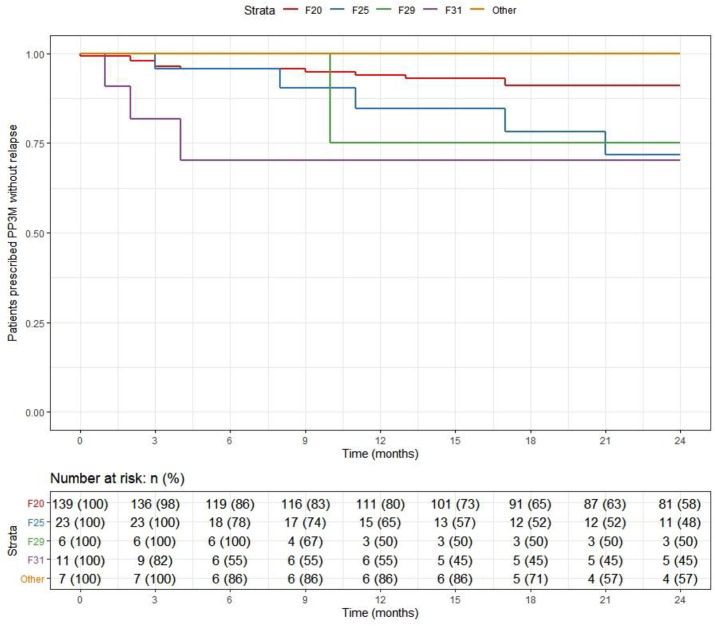
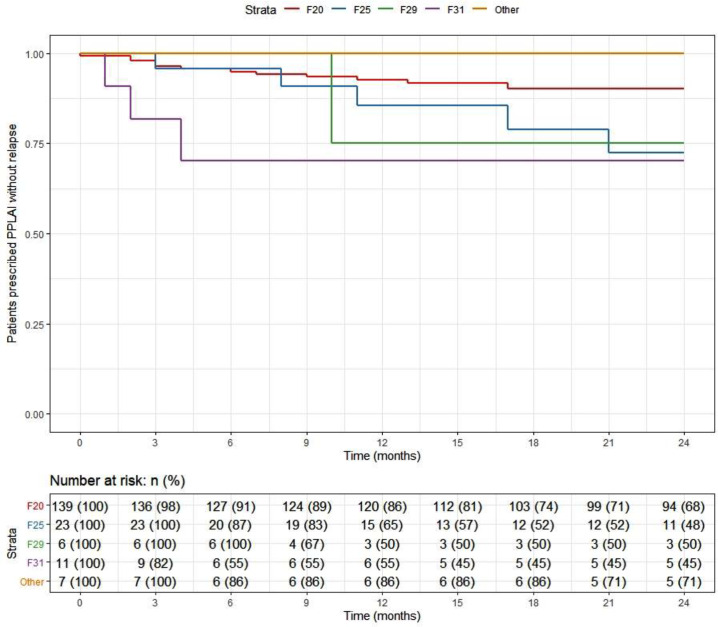
Overall, 166 patients (89%) who began treatment on PP3M remained relapse-free over the 2-year follow-up. The relapse-free rate was 88% (164/186) patients in the cohort on PPLAI. Figures 3 and 4 show these relapse rates within diagnosis groups. Tables 4 and 5 show the baseline and PP characteristics for those who relapsed and those who did not.
Figure 3.
Kaplan–Meier plot showing the proportion of patients that relapsed while being prescribed Paliperidone 3-monthly (PP3M) since initiation in diagnosis groups.
Figure 4.
Kaplan–Meier plot showing the proportion of patients that relapsed while being prescribed paliperidone long-acting injection (PPLAI) since initiation in diagnosis groups.
Table 4.
Baseline characteristics for relapse outcomes on PP3M and PPLAI.
| Characteristic | Total | PP3M no relapse | PP3M relapse | PPLAI no relapse | PPLAI relapse | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| n = 186 | n = 166 | n = 20 | n = 164 | n = 22 | ||||||
| Age on initiation of PP3M (years) | ||||||||||
| Mean, SD | 45, 13.2 | 46, 12.8 | 40, 15.9 | 46, 12.8 | 40, 15.3 | |||||
| Min, max | 20, 77 | 20, 77 | 22, 77 | 20, 77 | 22, 77 | |||||
| Prior duration of PP1M (months) | ||||||||||
| Mean, SD | 34, 24.5 | 34, 24.6 | 33, 24.1 | 34, 24.6 | 35, 23.8 | |||||
| Min, max | 3, 89 | 3, 89 | 4, 75 | 3, 89 | 4, 75 | |||||
Abbreviations: PPLAI, paliperidone long-acting injection; PP1M, one monthly paliperidone; PP3M, Paliperidone 3-monthly; SD, standard deviation.
Discontinuers includes those who stopped due to death or being lost to follow-up.
Table 5.
PP3M characteristics for relapse outcomes on PP3M and PPLAI.
| PP3M | Total | PP3M no relapse | PP3M relapse | PPLAI no relapse | PPLAI relapse | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| n = 186 | n = 166 | n = 20 | n = 164 | n = 22 | ||||||
| Average PP3M injections/year | ||||||||||
| Mean, SD | 4.0, 0.1 | 4.0, 0.1 | 4.0, 0.1 | 4.0, 0.1 | 4.0, 0.1 | |||||
| Min, max | 3.6, 4.7 | 3.6, 4.7 | 3.7, 4.2 | 3.6, 4.7 | 3.7, 4.2 | |||||
| Average no. days between PP3M injections | ||||||||||
| Mean, SD | 92, 3.5 | 92, 3.4 | 93, 4.6 | 92, 3.4 | 92, 4.4 | |||||
| Min, max | 70, 106 | 70, 106 | 84, 105 | 70, 106 | 84, 105 | |||||
Abbreviations: PPLAI, paliperidone long-acting injection; PP3M, Paliperidone 3-monthly; SD, standard deviation.
Regression analysis
The regression analysis for outcomes is displayed in Table 6. The hazard ratio for relapse while on PP3M and PPLAI was 0.17 and 0.21, respectively for those with an F20 diagnosis compared with those with another diagnosis. Prior clozapine use was associated with a markedly increased risk of relapse 6.11-fold (on PP3M and 4.52 on PPLAI) as was polypharmacy, which increased risk 7.91-fold (on PP3M and 6.45 on PPLAI). For every additional year of patient age the risk of relapse on PPLAI increased by 4%.
Table 6.
Regression modeling for time to relapse and discontinuation from PP3M and PPLAI.
| Model | Time to relapse (PP3M) | Time to discontinuation (PP3M) | Time to relapse (PPLAI) | Time to discontinuation (PPLAI) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Covariates | HR | 95% CI | p-value | HR | 95% CI | p-value | HR | 95% CI | p-value | HR | 95% CI | p-value |
| Age | 0.97 | (0.93, 1.00) | 0.056 | 0.97 | (0.95, 0.99) | 0.017 | 0.96 | (0.93, 0.99) | 0.045 | 0.95 | (0.93, 0.98) | <0.001 |
| Inpatient | 0.81 | (0.31, 2.10) | 0.662 | 1.54 | (0.89, 2.65) | 0.121 | 0.90 | (0.36,2.23) | 0.816 | 1.75 | (0.88, 3.47) | 0.109 |
| Male gender | 0.84 | (0.31, 2.31) | 0.741 | 0.52 | (0.30, 0.88) | 0.016 | 0.79 | (0.32,1.92) | 0.606 | 0.60 | (0.31, 1.17) | 0.132 |
| Polypharmacy | 7.91 | (2.73, 22.9) | <0.001 | 1.05 | (0.53, 2.04) | 0.897 | 6.45 | (2.49, 16.5) | <0.001 | 0.92 | (0.42, 1.99) | 0.831 |
| Ethnicity | 1.37 | (0.48, 4.21) | 0.586 | 1.18 | (0.65, 2.15) | 0.581 | 1.19 | (0.41,3.48) | 0.754 | 0.79 | (0.35,1.79) | 0.572 |
| Dose | 0.86 | (0.48, 1.51) | 0.594 | 0.97 | (0.73, 1.31) | 0.857 | 0.90 | (0.52,1.54) | 0.692 | 1.13 | (0.78, 1.63) | 0.524 |
| Prior clozapine | 6.11 | (1.82, 20.5) | 0.003 | 1.87 | (1.05, 3.30) | 0.032 | 4.52 | (1.51, 13.5) | 0.007 | 1.15 | (0.54, 2.41) | 0.721 |
| F20 | 0.17 | (0.06, 0.50) | 0.001 | 0.62 | (0.34, 1.13) | 0.119 | 0.21 | (0.08, 0.58) | 0.002 | 0.429 | (0.21, 0.87) | 0.018 |
Abbreviations: CI, confidence intervals; HR, hazard ratio; PPLAI, paliperidone long-acting injection; PP3M, Paliperidone 3-monthly; SD, standard deviation.
Older age was also associated with discontinuation, 3% increased risk per year on PP3M and 5% on PPLAI. Being male and prior clozapine use were associated with discontinuation from PP3M and a non-F20 diagnosis when on PPLAI.
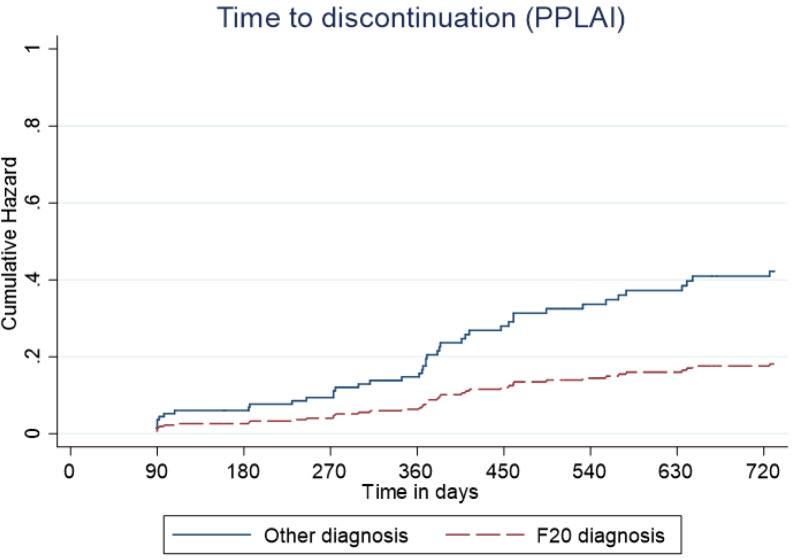
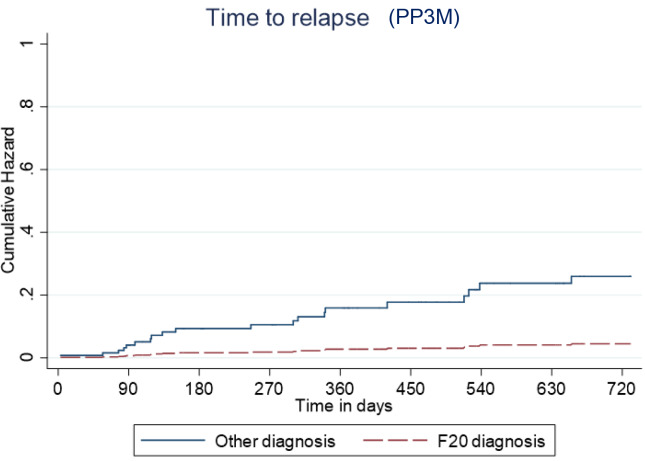
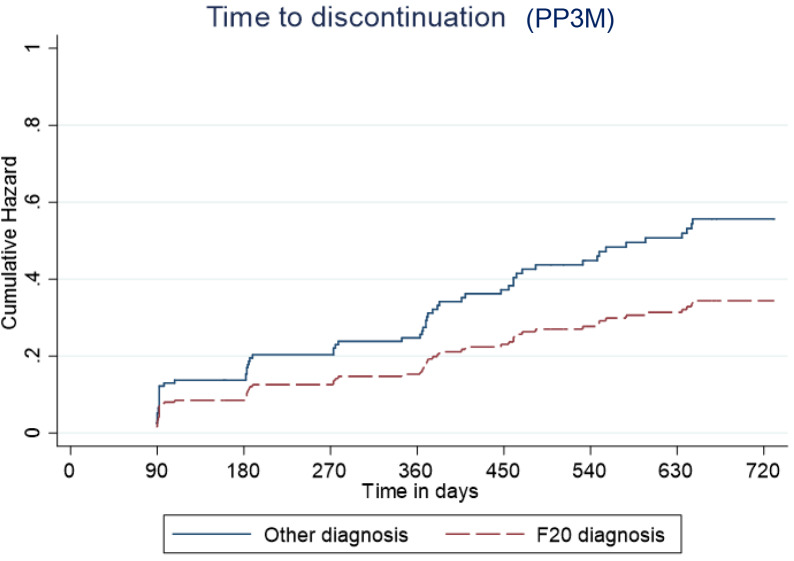
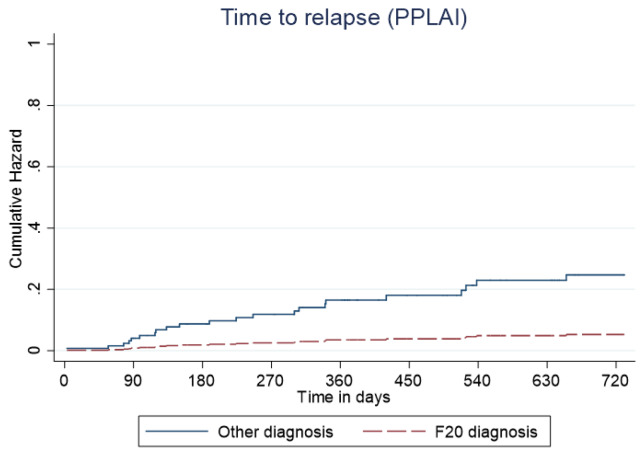
Predictive cumulative and adjusted hazards for the outcome events for those with and an F20 diagnosis and those with another diagnosis are shown in Figures 5–8.
Figure 6.
Kaplan–Meier plot showing the predictive cumulative hazard for discontinuation from paliperidone long-acting injection (PPLAI) in days using the regression model. F20 diagnosed patients are represented in red and non-F20 in blue.
Figure 7.
Kaplan–Meier plot showing the predictive cumulative hazard for relapse on Paliperidone 3-monthly (PP3M) in days using the regression model. F20 diagnosed patients are represented in red and non-F20 in blue.
Figure 5.
Kaplan–Meier plot showing the predictive cumulative hazard for discontinuation from Paliperidone 3-monthly (PP3M) in days using the regression model. F20 diagnosed patients are represented in red and non-F20 in blue.
Figure 8.
Kaplan–Meier plot showing the predictive cumulative hazard for relapse on paliperidone long-acting injection (PPLAI) in days using the regression model. F20 diagnosed patients are represented in red and non-F20 in blue.
Discussion
Overall, 70% of patients started on PP3M were still on PPLAI in some form 2 years later, and most were still on PP3M. Relapse over the 2 years after starting PP3M was 11% with PP3M and 12% with any paliperidone formulation. These very positive results were largely driven by the licensed use of PP3M in people with a diagnosis of schizophrenia (F20). We found that patients with an F20 diagnosis had an 83% reduced risk of relapse on PP3M compared with non-F20 patients. An F20 diagnosis was also associated with a lower likelihood of PPLAI discontinuation. In addition to this, prior clozapine use and concurrent polypharmacy were associated with a substantially greater risk of relapse while on PPLAIs. Increased age was also linked to a somewhat higher rate of discontinuation from PPLAIs.
Discontinuation
The association between age and discontinuation was statistically significant, but the effect was small and probably clinically insignificant. The strong link between polypharmacy and discontinuation is difficult to explain given the observational nature of this study. Polypharmacy regimes are more poorly tolerated than monotherapy and are associated with higher adverse effect burden [6]. Polypharmacy is also often an indication of more difficult-to-treat illness, although in our study nearly half of patients on polypharmacy were co-prescribed aripiprazole. Locally, it is our practice to use aripiprazole to treat or prevent hyperprolactinaemia rather than because of poor response. The association between prior clozapine therapy (our proxy for treatment resistance) and increased risk of discontinuation might have reflected a relatively poor response to paliperidone and subsequent discontinuation. Clozapine is, in our trust, only used as a treatment for refractory schizophrenia so prior use is assumed to indicate poor response to non-clozapine antipsychotics.
The association between non-F20 diagnoses (in this study, mainly schizoaffective and bipolar disorders) with higher discontinuation rates is on one level not surprising as in the UK/EU PPLAIs are indicated for the treatment only of schizophrenia [7, 8]. Better outcome might be expected in those with a diagnosis that reflects the bulk of research of a drug’s effects [9,10]. Continuation with treatment might also not be expected with all cases of some diagnoses, such as F29 and F31, which may not have a life-long course. It is also notable that other formulations of risperidone or paliperidone do have a formal license in the EU or USA for schizoaffective disorder (F25) [11,12] and bipolar disorder (F30/31) [13,14]. One recent observational study of LAIs looking at discontinuation rates over a year long period found no association between outcome and different diagnoses [15], but it is noteworthy that continuation rates in this study are much better than in similar observational studies of LAI treatment [16].
Relapse
Patients prescribed antipsychotic polypharmacy were almost eight times more likely to experience relapse while on PP3M than those on monotherapy. Antipsychotic polypharmacy is highly controversial, but one recent study suggested that co-prescribing two antipsychotics was associated with a slightly lower risk of relapse than was monotherapy [17]. However, our results seem to show that concomitant antipsychotic treatment predicts higher risk of relapse while on PPLAIs. In this context, as noted above, the need for polypharmacy may indicate poor overall response to treatment and so higher rates of relapse might thus be anticipated.
All patients starting PP3M in this study were previously clinically stable on PP1M. Nonetheless, patients who have been previously considered for, or given, clozapine had a higher risk of relapse while on PPLAIs. So, despite clinical stability on starting PP3M the prior use or consideration of clozapine might well indicate treatment resistance at some level and therefore a greater risk of relapse (or at least poor outcome) when receiving nonclozapine antipsychotics.
As with discontinuation, patients with an F20 diagnosis were less likely to relapse than those with other diagnoses. Again, this is perhaps to be expected given the relative paucity of data relating to efficacy in non F20 diagnoses.
Reasons for discontinuation
The most common reason for PP3M discontinuation was patient refusal. Almost half of patients switched back to PP1M after PP3M discontinuation. To some extent, this may indicate patients’ preference of PP1M over PP3M although our data did not indicate the stated reasons for the patients’ reasons for switching back to PP1M. Given that they were switched to the same drug, we can assume that the reason for the switch was not related to paliperidone itself, but rather the formulation and the frequency of its use. In conventional trials, PP1M and PP3M have near identical efficacy and tolerability [18].
Comparison with other studies
Risperidone and paliperidone have demonstrated efficacy in Randomised Controlled Trials (RCTs) in the treatment of schizoaffective disorder [19,20] and are associated with a significant reduction in the risk of relapse [20,21]. Their use in bipolar disorder is supported by RCTs in which they are reported to delay the recurrence of mood episodes [22–24] and manic relapse [25,26]. Our study suggests that PP3M/PPLAI may be relatively less effective in these conditions than in schizophrenia. We are unaware of any other data which suggest this difference in outcomes. To the best of our knowledge, there are currently no other published observational studies looking at PP3M use in non F20 patients.
Our findings are consistent with other naturalistic studies affirming PP3M is highly effective in relapse prevention and reducing hospital admissions [27,28]. One recent study reports better outcomes for younger patients and suggests that earlier implementation of PP3M in therapy is critical for better long term outcomes and symptomatic remission [29]. Our findings on the effect of age on outcome are consistent with this study.
Limitations
This was an open, naturalistic, and noninterventional analysis. As such there was no control group or blinding. By nature of being prescribed PP3M, the patients in this cohort were already stabilized on PP1M, so it might be expected that relapse and discontinuation rates would be low. Our dichotomous classification of ‘relapse’ may have been insensitive to some symptomatic worsening (although our definition was based on standard practice). The sample size was fairly small in respect to non-F20 diagnoses and participants were from one clinical environment—a socially deprived area of South East London—making it difficult to establish generalizability. Black people were over-represented in our sample which again may affect generalizability. We had no information on several factors that might influence outcome, such as substance or alcohol misuse. Lastly, diagnosis in practice is often less than certain and changes in diagnosis over time are far from unusual.
What the study means for practice
Continuation with treatment at 2 years was more likely for F20-diagnosed patients and those of younger age. PPLAIs were less successful in other diagnoses but the outcomes were generally good, especially in respect to relapse. Patients previously considered for or given clozapine had a much higher likelihood of relapse and discontinuation from PPLAIs. We have demonstrated this association in several previous studies [30–33]. Patients co-prescribed two antipsychotics were at a greater risk of relapse and subsequent discontinuation than patients on PPLAI monotherapy. The best outcomes were seen in patients diagnosed with schizophrenia on PPLAI monotherapy who had never been prescribed or considered for clozapine.
Acknowledgments
Statistical advice was provided by Dr. Kia-Chong Chua of King’s College, London.
Data Availability Statement
The data that support the findings of this study are available from david.taylor@slam.nhs.uk on request. Patient-identifying data will be removed to protect patient confidentiality.
Author Contributions
Conceptualization: D.T.; Formal analysis: I.C., P.W., and V.C.; Investigation: I.C.; Supervision: I.C. and D.T.; Methodology: P.W.; Software: V.C.; Validation: V.C.; Writing – original draft: I.C.; Writing – review & editing: I.C. and D.T.
Conflict of Interest
David Taylor has received research funding and consulting payments from Sunovion, Lundbeck, Janssen, Mylan HLS and Recordati. I.C., P.W. and V.C. declare none.
References
- 1.Fu DJ, Turkoz I, Simonson RB, Walling DP, Schooler NR, Lindenmayer JP, et al. Paliperidone palmitate once-monthly reduces risk of relapse of psychotic, depressive, and manic symptoms and maintains functioning in a double-blind, randomized study of schizoaffective disorder. J Clin Psychiatry. 2015;76(3):253–62. [DOI] [PubMed] [Google Scholar]
- 2.Alphs L, Fu D-J, Turkoz I. Paliperidone for the treatment of schizoaffective disorder. Expert Opin Pharmacother. 2016;17(6):871–83. [DOI] [PubMed] [Google Scholar]
- 3.Nikolic N, Page N, Akram A, Khan M. The impact of paliperidone palmitate long-acting injection on hospital admissions in a mental health setting. Int Clin Psychopharmacol. 2017;32(2):95–102. [DOI] [PubMed] [Google Scholar]
- 4.Yan T, Greene M, Chang E, Touya M, Broder MS. Impact of initiating long-acting injectable antipsychotics on hospitalization in patients with bipolar I disorder. J Comp Eff Res. 2018;7(11):1083–93. [DOI] [PubMed] [Google Scholar]
- 5.Li K, Liao Y, Yang Z, Yang C, Chen M, Wu X, et al. Case report: paliperidone palmitate in the management of bipolar I disorder with non-compliance. Front Psychiatry. 2020;11:529672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gallego JA, Nielsen J, De Hert M, Kane JM, Correll CU. Safety and tolerability of antipsychotic polypharmacy. Expert Opin Drug Saf. 2012;11(4):527–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Janssen-Cilag Ltd. Summary of product characteristics. Xeplion 25 mg, 50 mg, 75 mg, 100 mg, and 150 mg prolonged-release suspension for injection; 2018. https://www.medicines.org.uk/emc/medicine/31329.
- 8.Janssen-Cilag Limited. Summary of product characteristics. TREVICTA 175 mg, 263 mg, 350 mg, 525 mg prolonged release suspension for injection; 2019. https://www.medicines.org.uk/emc/medicine/32050.
- 9.Mihaljevic-Peles A, Sagud M, Filipcic IS, Grosic V, Pedisic I, Emsley R. Remission and employment status in schizophrenia and other psychoses: one-year prospective study in Croatian patients treated with risperidone long acting injection. Psychiatr Danub. 2016;28(3):263–72. [PubMed] [Google Scholar]
- 10.Vieta E, Nuamah IF, Lim P, Yuen EC, Palumbo JM, Hough DW, et al. A randomized, placebo- and active-controlled study of paliperidone extended release for the treatment of acute manic and mixed episodes of bipolar I disorder. Bipolar Disord. 2010;12(3):230–43. [DOI] [PubMed] [Google Scholar]
- 11.Janssen Pharmaceuticals. Highlights of prescribing information; 2009. https://www.janssenlabels.com/package-insert/product-monograph/prescribing-information/INVEGA+SUSTENNA-pi.pdf.
- 12.Janssen-Cilag Ltd. Summary of product characteristics. INVEGA 3 mg, 6 mg, 9 mg prolonged-release tablets; 2017. https://www.medicines.org.uk/emc/.
- 13.Janssen Pharmaceutical Companies. Highlights of prescribing information: RISPERDAL® (risperidone) tablets, RISPERDAL® (risperidone) oral solution, RISPERDAL® M-TAB® (risperidone) orally disintegrating tablets; 2019. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/020272s082,020588s070,021444s056lbl.pdf.
- 14.Accord Healthcare Limited. Risperidone 0.5/1/2/3/4/6 mg film coated tablets; 2020. https://www.medicines.org.uk/emc/PIL.25958.latest.pdf.
- 15.Bertolini F, Ostuzzi G, Pievani M, Aguglia A, Bartoli F, Bortolaso P, et al. Comparing long-acting antipsychotic discontinuation rates under ordinary clinical circumstances: a survival analysis from an observational, pragmatic study. CNS Drugs. 2021;35(6):655–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mahlich J, Olbrich K, Wilk A, Wimmer A, Wolff-Menzler C. Time to treatment discontinuation in German patients with schizophrenia: long-acting injectables versus oral antipsychotics. Clin Drug Investig. 2021;41(1):99–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tiihonen J, Taipale H, Mehtälä J, Vattulainen P, Correll CU, Tanskanen A. Association of antipsychotic polypharmacy vs monotherapy with psychiatric rehospitalization among adults with schizophrenia. JAMA Psychiatry. 2019;76(5):499–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nash AI, Turkoz I, Savitz AJ, Mathews M, Kim E. Predictors of achieving remission in schizophrenia patients treated with paliperidone palmitate 3-month formulation. Neuropsychiatr Dis Treat. 2019;15:731–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Conley RR, Mahmoud R. A randomized double-blind study of risperidone and olanzapine in the treatment of schizophrenia or schizoaffective disorder. Am J Psychiatry. 2001;158(5):765–74. [DOI] [PubMed] [Google Scholar]
- 20.Greenberg WM, Citrome L. Paliperidone palmitate for schizoaffective disorder: a review of the clinical evidence. Neurol Ther. 2015;4(2):81–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gaebel W, Schreiner A, Bergmans P, de Arce R, Rouillon F, Cordes J, et al. Relapse prevention in schizophrenia and schizoaffective disorder with risperidone long-acting injectable vs quetiapine: results of a long-term, open-label, randomized clinical trial. Neuropsychopharmacology. 2010;35(12):2367–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Quiroz JA, Yatham LN, Palumbo JM, Karcher K, Kushner S, Kusumakar V. Risperidone long-acting injectable monotherapy in the maintenance treatment of bipolar I disorder. BiolPsychiatry. 2010;68(2):156–62. [DOI] [PubMed] [Google Scholar]
- 23.Vieta E, Montgomery S, Sulaiman AH, Cordoba R, Huberlant B, Martinez L, et al. A randomized, double-blind, placebo-controlled trial to assess prevention of mood episodes with risperidone long-acting injectable in patients with bipolar I disorder. Eur Neuropsychopharmacol. 2012;22(11):825–35. [DOI] [PubMed] [Google Scholar]
- 24.Berwaerts J, Melkote R, Nuamah I, Lim P. A randomized, placebo- and active-controlled study of paliperidone extended-release as maintenance treatment in patients with bipolar I disorder after an acute manic or mixed episode. J Affect Disord. 2012;138(3):247–58. [DOI] [PubMed] [Google Scholar]
- 25.Kishi T, Oya K, Iwata N. Long-acting injectable antipsychotics for prevention of relapse in bipolar disorder: a systematic review and meta-analyses of randomized controlled trials. Int J Neuropsychopharmacol. 2016;19(9):pyw038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Macfadden W, Alphs L, Haskins JT, Turner N, Turkoz I, Bossie C, et al. A randomized, double-blind, placebo-controlled study of maintenance treatment with adjunctive risperidone long-acting therapy in patients with bipolar I disorder who relapse frequently. Bipolar disorders. 2009;11(8):827–39. [DOI] [PubMed] [Google Scholar]
- 27.Fernández-Miranda JJ, Díaz-Fernández S, De Berardis D, López-Muñoz F. Paliperidone palmitate every three months (PP3M) 2-year treatment compliance, effectiveness and satisfaction compared with paliperidone palmitate-monthly (PP1M) in people with severe schizophrenia. J Clin Med 2021;10(7):1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chung Y, Yang Y, Sulaiman A, Bergman P, Wea T. Asian subgroup analysis of the REMISSIO study: a long-term efficacy and safety study of paliperidone palmitate 3-month formulation in patients with stable schizophrenia in a naturalistic clinical setting. Clinical Psychopharmacology and Neuroscience: The Official Scientific Journal of the Korean College of Neuropsychopharmacology. 2021 https://www.cpn.or.kr/journal/view.html?uid=1255&vmd=Full#body01. [DOI] [PMC free article] [PubMed]
- 29.Pungor K, Bozikas V, Emsley R, Llorca PM, Gopal S, Mathews M, et al. Stable patients with schizophrenia switched to paliperidone palmitate 3-monthly formulation in a naturalistic setting: impact of patient age and disease duration on outcomes. Ther Adv Psychopharmacol. 2020;10:204512532098150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Taylor DM, Young C, Patel MX. Prospective 6-month follow-up of patients prescribed risperidone long-acting injection: factors predicting favourable outcome. Int J Neuropsychopharmacol. 2006;9:685–94. [DOI] [PubMed] [Google Scholar]
- 31.Wallman P, Clark I, Taylor D. Discontinuation and relapse with paliperidone palmitate three-monthly for maintenance of schizophrenia: two year follow-up of use in clinical practice. J. Psychopharmacol. 2021;35(9):1091–8. [DOI] [PubMed] [Google Scholar]
- 32.Taylor D, Cornelius V. Risperidone long-acting injection: factors associated with changes in bed stay and hospitalisation in a 3-year naturalistic follow-up. J. Psychopharmacol. 2010;24:995–1000. [DOI] [PubMed] [Google Scholar]
- 33.Osborne IJ, Mace S, Taylor D. A prospective year-long follow-up of lurasidone use in clinical practice: factors predicting treatment persistence. Ther Adv Psychopharmacol. 2018;8(4):117–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from david.taylor@slam.nhs.uk on request. Patient-identifying data will be removed to protect patient confidentiality.