Abstract
Background
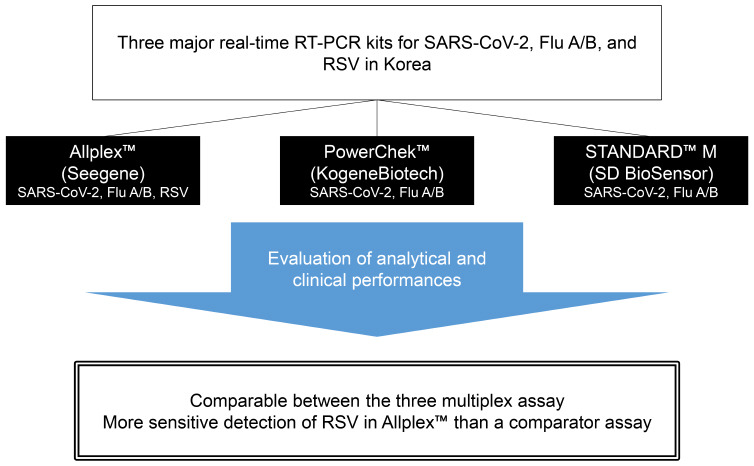
In the coronavirus disease 2019 (COVID-19) pandemic era, the simultaneous detection of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), influenza virus (Flu), and respiratory syncytial virus (RSV) is important in the rapid differential diagnosis in patients with respiratory symptoms. Three multiplex real-time reverse transcription polymerase chain reaction (rRT-PCR) assays have been recently developed commercially in Korea: PowerChek™ SARS-CoV-2, Influenza A&B Multiplex Real-time PCR Kit (PowerChek; KogeneBiotech); STANDARD™ M Flu/SARS-CoV-2 Real-time Detection Kit (STANDARD M; SD BioSensor); and Allplex™ SARS-CoV-2/FluA/FluB/RSV Assay (Allplex; Seegene). We evaluated the analytical and clinical performances of these kits.
Methods
A limit of detection tests were performed and cross-reactivity analysis was executed using clinical respiratory samples. Ninety-seven SARS-CoV-2-positive, 201 SARS-CoV-2-negative, 71 influenza A-positive, 50 influenza B-positive, 78 RSV-positive, and 207 other respiratory virus-positive nasopharyngeal swabs were tested using the three assays. The AdvanSure™ respiratory viruses rRT-PCR assay (AdvanSure; LG Life Sciences) was used as a comparator assay for RSV.
Results
Except in influenza B, in SARS-CoV-2 and influenza A, there were no significant differences in detecting specific genes of the viruses among the three assays. All three kits did not cross-react with common respiratory viruses. All three kits had greater than 92% positive percent agreement and negative percent agreement and ≥ 0.95 kappa value in the detection of SARS-CoV-2 and flu A/B. Allplex detected RSV more sensitively than AdvanSure.
Conclusion
The overall performance of three multiplex rRT-PCR assays for the concurrent detection of SARS-CoV-2, influenza A/B, and RSV was comparable. These kits will promote prompt differential diagnosis of COVID-19, influenza, and RSV infection in the COVID-19 pandemic era.
Keywords: SARS-CoV-2, Influenza A Virus, Influenza B Virus, Respiratory Syncytial Viruses, Multiplex Polymerase Chain Reaction
Graphical Abstract
INTRODUCTION
The coronavirus disease 2019 (COVID-19) pandemic is an ongoing global health crisis caused by a newly discovered coronavirus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).1 The COVID-19 pandemic began in December 2019 in Wuhan City, China, and has lasted for more than a year worldwide.2 Clinical presentation of COVID-19 ranges from asymptomatic cases and mild illness to critical conditions featuring acute respiratory distress syndrome.3 In Korea, patients less than 10 years and over 70 years are less frequently infected with COVID-19, and those aged between 20–59 years are more commonly infected.4 COVID-19 disease severity is associated with increased age.5 Influenza virus (Flu) and respiratory syncytial virus (RSV) are common respiratory pathogens that cause seasonal epidemics.6,7 Influenza infection tend to be less severe, typically resulting in uncomplicated upper respiratory tract illness, but, in rare cases, influenza infection can induce a complicated disease with severe viral pneumonia.8 Children are most likely to be infected with influenza, and people aged 65 years and older are least likely to become ill from influenza.9 RSV is recognized as the most common cause of bronchiolitis and pneumonia in children younger than 1 year. RSV infection can produce common cold-like symptoms but may be severe in infants and young children. In older adults, RSV usually causes no symptoms or upper respiratory infection but may lead to severe lower respiratory infection.10
SARS-CoV-2, influenza, and RSV infections should be managed in different ways. COVID-19 management requires strict quarantine and isolation strategies that are not applied to influenza and RSV infection management. Among these three infective diseases, influenza is the only effectively treatable condition; influenza can be treated with the drug, oseltamivir (Tamiflu).
Since the clinical presentations of SARS-CoV-2, influenza, and RSV infections have overlapping symptoms, differential diagnosis of these diseases is challenging. Specifically, rapid diagnosis is needed in elderly patients because SARS-CoV-2, influenza, and RSV infections result in substantial morbidity and mortality in these patients.6,11 In the COVID-19 pandemic era, clinicians may encounter a co-infection of SARS-CoV-2 with influenza or RSV.12,13,14 According to a Nature survey, many researchers expect SARS-CoV-2 to become endemic.15 In the future, SARS-CoV-2, influenza, and RSV are expected to be the most common respiratory viruses infected in humans. Therefore, multiplex detection of SARS-CoV-2, influenza, and RSV will be essential and commonly used.
Molecular testing is the gold standard for the detection of many viruses, and real-time reverse transcription polymerase chain reaction (rRT-PCR) is most widely used molecular method for qualitative diagnostic testing.16 Numerous rRT-PCR kits that solely detect SARS-CoV-2 have been developed and commercialized worldwide, including in Korea.17,18,19 Seegene has the highest market share in the SARS-CoV-2 single molecular diagnostic kit market in Korea, followed by KogeneBiotech and SD BioSensor. The United States Food and Drug Administration approved the use of SARS-CoV-2 molecular diagnostic kits produced by these three companies under Emergency Use Authorization in April 2020.20 Recently, these three manufacturers have developed molecular diagnostic kits for concurrent detection of SARS-CoV-2, influenza A/B, and RSV. The kits produced by Seegene and KogeneBiotech were approved for use by the Ministry of Food and Drug Safety in Korea in January 2021 and November 2020, respectively, although the kit produced by SD BioSensor has not yet been approved (last reviewed on October 20, 2021). In this study, we evaluated the analytical and clinical performance of these three multiplex rRT-PCR assays for simultaneous detection of SARS-CoV-2, influenza A/B, and RSV in nasopharyngeal swabs (NPS).
METHODS
Three molecular diagnostic kits for simultaneous detection of SARS-CoV-2 and influenza A/B and/or RSV
PowerChek™ SARS-CoV-2, Influenza A&B Multiplex Real-time PCR Kit (PowerChek; KogeneBiotech, Seoul, Korea) and STANDARD™ M Flu/SARS-CoV-2 Real-time Detection Kit (STANDARD M; SD BioSensor, Osong, Korea) are multiplex kits for the detection of SARS-CoV-2 and influenza A/B. Allplex™ SARS-CoV-2/FluA/FluB/RSV Assay (Allplex; Seegene, Seoul, Korea) is a multiplex kit for the detection of SARS-CoV-2, influenza A/B, and RSV.
To detect SARS-CoV-2, PowerChek™ SARS-CoV-2, Influenza A&B Multiplex Real-time PCR Kit (PowerChek; KogeneBiotech), and STANDARD™ M Flu/SARS-CoV-2 Real-time Detection Kit (STANDARD M; SD BioSensor) target the envelope (E) and open reading frame 1ab (ORF1ab) coding regions of the SARS-CoV-2 genome, whereas Allplex™ SARS-CoV-2/FluA/FluB/RSV Assay (Allplex; Seegene, Seoul, Korea) targets the nucleocapsid (N), RNA-dependent RNA polymerase (RdRp), and spike (S) coding regions. According to the World Health Organization guideline,21 rRT-PCR results are interpreted as positive when all target genes are detected. If no target genes are detected, the test is interpreted as negative. The cases where the target genes are partially detected are considered inconclusive. For the detection of influenza A, PowerChek, STANDARD M, and Allplex target the matrix (M) genes. For the detection of influenza B, PowerChek, STANDARD M, and Allplex target the nucleoprotein (NP), nonstructural (NS) 1, and NS genes, respectively. Allplex targets the matrix (M) gene for the detection of RSV. All kits are based on one-step rRT-PCR in one tube. Specifications of the three assays are described in Table 1.
Table 1. Specification of three commercial real-time RT-PCR kits for the multiplex detection of SARS-CoV-2, influenza virus A/B, and/or RSV.
| Variables | Kit name | ||
|---|---|---|---|
| PowerChek™ SARS-CoV-2, Influenza A&B Multiplex Real-time PCR Kit (KogeneBiotech) | STANDARD™ M Flu/SARS-CoV-2 Real-time Detection Kit (SD BioSensor) | Allplex™ SARS-CoV-2/FluA/FluB/RSV Assay (Seegene) | |
| Target genes (virus) | ORF1ab, E (SC2), M (influenza A), NP (influenza B) | ORF1ab, E (SC2), M (influenza A), NS1 (influenza B) | N, RdRp, S (SC2), M (influenza A), NS (influenza B), M (RSV) |
| Internal control | RNA process control, spiked into sample or 0.5 μL into PCR mixture | Recombinant RNA (Turnip yellow mosaic virus), 5 μL spiked into sample or 0.5 μL into PCR mixture | RNAse P (Endo IC) MS2 phage (Exo IC), spiked into sample |
| Applicable PCR analyzer | CFX96 Real-time PCR Detection System (Bio-Rad), Gentier 96S Real-time PCR System (Tianlong) | CFX96™ Dx System (Bio-Rad), Rotor-Gene Q (Qiagen) | CFX96™ Dx System (Bio-Rad) |
| Volume of nucleic acid extracts/PCR reaction mixtures per test, μL | 5/15 | 10/20 | 10/10 |
| No. of pre-amplification/amplification cycles | None/40 | 5/40 | 3/42 |
| PCR running time, min | 120 | 90 | 115 |
| Cut off for positive (Ct) | 38, 35 for IC | 36 | 38, 40 for RdRp |
| Sample types | Nasopharyngeal swab | Sputum, nasopharyngeal swab, oropharyngeal swab | Nasopharyngeal swab |
All kits are one-step RT-PCR kits. Pre-amplification was not counted for Ct.
RT-PCR = reverse transcription polymerase chain reaction, SARS-CoV-2 = severe acute respiratory syndrome-coronavirus-2, RSV = respiratory syncytial virus, SC2 = SARS-CoV-2, IC = internal control, exo IC = exogenous internal control, endo IC = endogenous internal control, Ct = cycle threshold.
Specimen collection
We selected 103 SARS-CoV-2 positive and 201 SARS-CoV-2 negative specimens that were collected from September 2020 to November 2020 and reported by STANDARD™ M new Coronavirus (nCoV) Real-Time Detection kit (STANDARD M nCoV; SD BioSensor). The specimens were nasopharyngeal and/or oropharyngeal swabs from patients. Aliquots of samples were stored at −80°C. We retrieved the aliquots and thawed them for testing using the three assays and STANDARD M nCoV. Re-testing using STANDARD M nCoV revealed that the results of six SARS-CoV-2 positive samples were changed from positive to inconclusive or negative, and the results of the remaining 97 SARS-CoV-2 positive samples were unchanged. We excluded the six specimens and finally included 97 SARS-CoV-2-positive and 201 SARS-CoV-2-negative specimens. All 97 SARS-CoV-2-positive samples were acquired from confirmed cases.
Four hundred and three respiratory virus-positive NPS samples tested by AdvanSure™ respiratory viruses (RV) real-time RT-PCR assay (AdvanSure; LG Life Sciences, Seoul, Korea) were collected from December 2017 to November 2020. A substantial portion of the respiratory virus-positive NPS samples tested by the AdvanSure diagnostic kit was acquired before the COVID-19 pandemic. Aliquots of samples were stored at −80°C. We retrieved the aliquots and thawed them for testing using the three assays. When the results of the three assays were different from the previously reported results of the AdvanSure kit, the corresponding aliquots were tested with the AdvanSure kit again. We used re-tested AdvanSure results if tested again; if not, the previously reported results were used for analysis. All specimens harbored one or more respiratory viruses including 71 influenza A-positive, 50 influenza B-positive, 78 RSV-positive, and 207 other respiratory virus-positive samples.
Sample processing and PCR
A 200 μL volume of the patient specimen was extracted using the EMAG system (bioMérieux, Marcy l'Etoile, France) according to the manufacturer’s instructions, with a nucleic acid elution volume of 50 μL. All real-time PCR analyses were performed using the CFX96 Real-Time Detection System (Bio-Rad, Hercules, CA, USA). PCR reactions were performed in a total volume of 20 μL (15 μL PCR reaction mixture and 5 μL template RNA), a total volume of 30 μL (20 μL PCR reaction mixture and 10 μL template RNA), and a total volume of 20 μL (9 μL PCR reaction mixture and 11 μL template RNA) using the PowerChek, STANDARD M, and Allplex kits, respectively.
Analytical performance: limit of detection (LoD) and cross-reactivity
The LoD was determined using the AccuPlex™ SARS-CoV-2, Flu A/B, and RSV reference material kits (SeraCare, Milford, MA, USA). AccuPlex virus products contained non-replicative recombinant viruses including full genomes of SARS-CoV-2 and influenza A/B and partial genome of RSV (NC_001803, 1..4380; 8460..15191). We obtained seven concentrations (5,000, 1,667, 500, 167, 50, 17, and 5 copies/mL) by serial dilution from reference non-replicative recombinant viruses. The first two concentrations (5,000 and 1,667 copies/mL) were tested with eight replicates each. The other concentrations were tested with 24 replicates each. LoD was defined as the input copy number per 1 mL with a 95% probability of a positive PCR and was calculated by probit regression analysis using International Business Machines (IBM) Statistical Package for the Social Sciences software package (version 25; IBM, Armonk, NY, USA) with 95% confidence intervals (CIs).
Cross-reactivity was verified using the 403 clinical samples containing typical respiratory viruses. Cross-reactivity was defined as the ability to generate a false-positive in similar viruses during RT-PCR analysis. Reference results were acquired using the AdvanSure diagnostic kits. Among the 403 respiratory virus-positive NPS samples, 47 samples tested positive for multiple respiratory pathogens. Since all three diagnostic kits were not regarded as cross-reactive to the 47 samples, each sample was regarded as multiple samples harboring different viruses.
Evaluation of the clinical performance of the three molecular diagnostic kits
Reference results for positive and negative agreements were defined as follows: With regards to SARS-CoV-2, positive samples were defined as the sum of positive samples for all three kits and confirmed cases with discrepant results between three kits. Negative samples were defined as samples that tested negative for all three kits. In terms of influenza A/B, positive or negative samples were defined when two or three kits showed positive or negative results, respectively. When one kit produced discrepant results with the other two kits, the result of this kit was regarded as false.
The RSV results of the Allplex diagnostic kit were compared with those of the AdvanSure comparator kit. The discordant results between the three kits and the AdvanSure comparator kit might be due to differences in sensitivity. Therefore, we estimated LoDs for influenza A/B and RSV in AdvanSure using the AccuPlex™ SARS-CoV-2, Flu A/B, and RSV reference material kits (SeraCare). RT-PCR and gene sequencing to identify RSV were performed on samples that produced discrepant results between Allplex and AdvanSure.
Positive percent agreements (PPAs) and negative percent agreements (NPAs) with 95% CIs were calculated using GraphPad Prism 7 (GraphPad, San Diego, CA, USA). Cohen’s kappa values with 95% CIs were calculated using GraphPad QuickCalcs (https://www.graphpad.com/quickcalcs/kappa1/) (GraphPad).
Ethics statement
This study was approved by the Institutional Review Board of the Seoul National University Boramae Medical Center and the requirement for informed consent was waived (IRB No. 20-2020-262).
RESULTS
Analytical performance
In comparison of the LoD for the ORF1ab or RdRp genes of SARS-CoV-2, the three kits showed no significant difference in detecting specific SARS-CoV-2 genes due to overlapping 95% CIs (Table 2). The LoDs for influenza A M gene were not significantly different between the three assays. In comparison of the LoD for the NP, NS1, or NS genes of influenza B, the PowerChek had a lower LoD than the STANDARD M and Allplex kits.
Table 2. Limit of detection test results of three commercial kits for the multiplex PCR detection of SARS-CoV-2 and influenza virus A/B detection with reference material (AccuPlex™ SARS-CoV-2 reference material kit).
| Target genes | No. detected/No. replicates at each concentration (copies/mL) | LoD in copies/mL (95% CI) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 5,000 | 1,667 | 500 | 167 | 50 | 17 | 5 | |||
| PowerChek | |||||||||
| SARS-CoV-2 E gene | 8/8 | 8/8 | 24/24 | 23/24 | 12/24 | 8/24 | 1/24 | 212.1 (126.7–507.5) | |
| SARS-CoV-2 ORF1ab gene | 8/8 | 8/8 | 24/24 | 13/24 | 2/24 | 0/24 | 0/24 | 402.3 (279.6–799.1) | |
| Influenza virus A M gene | 8/8 | 8/8 | 18/24 | 7/24 | 1/24 | 5/24 | 2/24 | 5,661.8 (875.7–62,746,173) | |
| Influenza virus B NP gene | 8/8 | 8/8 | 24/24 | 24/24 | 21/24 | 8/24 | 3/24 | 88.8 (55.8–203.0) | |
| STANDARD M | |||||||||
| SARS-CoV-2 E gene | 8/8 | 8/8 | 20/24 | 17/24 | 10/24 | 2/24 | 2/24 | 1,176.4 (571.2–3,932.9) | |
| SARS-CoV-2 ORF1ab gene | 8/8 | 8/8 | 24/24 | 21/24 | 18/24 | 4/24 | 4/24 | 259.7 (145.7–680.7) | |
| Influenza virus A M gene | 8/8 | 6/8 | 11/24 | 3/24 | 2/24 | 0/24 | 1/24 | 11,205 (1,744.8–624,142,878) | |
| Influenza virus B NS1 gene | 8/8 | 8/8 | 22/24 | 19/24 | 10/24 | 2/24 | 1/24 | 578.0 (325.5–1,470.9) | |
| Allplex | |||||||||
| SARS-CoV-2 N gene | 8/8 | 8/8 | 19/24 | 10/24 | 4/24 | 2/24 | 0/24 | 1,649.6 (838.4–5,316.7) | |
| SARS-CoV-2 RdRp gene | 8/8 | 8/8 | 24/24 | 21/24 | 14/24 | 5/24 | 2/24 | 283.5 (163.9–702.2) | |
| SARS-CoV-2 S gene | 8/8 | 8/8 | 24/24 | 20/24 | 14/24 | 7/24 | 3/24 | 361.0 (193.9–1,027.8) | |
| Influenza virus A M gene | 8/8 | 8/8 | 10/24 | 7/24 | 2/24 | 1/24 | 0/24 | 4,917.3 (2,070.1–24,922) | |
| Influenza virus B NS gene | 8/8 | 8/8 | 24/24 | 23/24 | 11/24 | 5/24 | 2/24 | 248.9 (147.1–596.1) | |
| RSV M gene | 8/8 | 8/8 | 24/24 | 23/24 | 12/24 | 3/24 | 4/24 | 282.48 (160.43–718.78) | |
PCR = polymerase chain reaction, SARS-CoV-2 = severe acute respiratory syndrome-coronavirus-2, LoD = limit of detection, CI = confidence interval, RSV = respiratory syncytial virus.
In the evaluation of cross-reactivity in the three kits, all kits were not cross-reactive with other common human respiratory viruses such as common cold viruses (coronavirus 2229E, OC43, and NL63), adenovirus, bocavirus, metapneumovirus, parainfluenza virus, and rhinovirus (Table 3).
Table 3. Cross-reactivity test results of three commercial real-time RT-PCR kits for the multiplex detection of SARS-CoV-2, influenza virus A/B, and/or RSV using clinical samples.
| Respiratory viruses | No. of samples | No. of positive samples | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| PowerChek | STANDARD M | Allplex | |||||||||
| SC2 | Flu A | Flu B | SC2 | Flu A | Flu B | SC2 | Flu A | Flu B | RSV | ||
| Adenovirus | 28 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Bocavirus | 34 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Coronavirus, 229E | 9 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Coronavirus, OC43 | 17 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Coronavirus, NL63 | 12 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Metapneumovirus | 50 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Parainfluenza virus 1 | 22 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Parainfluenza virus 2 | 19 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Parainfluenza virus 3 | 32 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Rhinovirus A/B/C | 41 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
RT-PCR = reverse transcription polymerase chain reaction, SARS-CoV-2 = severe acute respiratory syndrome-coronavirus-2, RSV = respiratory syncytial virus, Flu = influenza virus, SC2 = SARS-CoV-2.
Clinical performance for the detection of SARS-CoV-2, influenza A/B, and RSV
Compared with the reference results derived from the combinations of the three kits’ results, the three kits had greater than 92% PPA and NPA in the detection of SARS-CoV-2 and influenza A/B. Kappa values of the three kits indicated perfect agreements in the detection of SARS-CoV-2 and influenza A/B (Table 4). Furthermore, there were no significant differences in PPA, NPA, and kappa values among the three assays in both SARS-CoV-2 and influenza A/B detection. Discordant SARS-CoV-2 results were found in 10 specimens (Table 5). All the 10 specimens were acquired from confirmed COVID-19 patients. Additional SARS-CoV-2 data of the three kits were described in Supplementary Tables 1 and 2.
Table 4. Positive and negative agreement for the detection of SARS-CoV-2 and influenza A/B between the three commercial kits.
| Viruses | PowerChek | STANDARD M | Allplex | |
|---|---|---|---|---|
| SARS-CoV-2 | ||||
| PPA | 90/97 | 93/97 | 93/97 | |
| 92.8 (85.9–96.5) | 95.9 (89.9–98.4) | 95.9 (89.9–98.4) | ||
| NPA | 201/201 | 201/201 | 201/201 | |
| 100.0 (98.1–100.0) | 100.0 (98.1–100.0) | 100.0 (98.1–100.0) | ||
| Kappa | 0.95 (0.91–0.99) | 0.97 (0.94–1.00) | 0.97 (0.94–1.00) | |
| Influenza A | ||||
| PPA | 71/71 | 71/71 | 71/71 | |
| 100.0 (94.9–100.0) | 100.0 (94.9–100.0) | 100.0 (94.9–100.0) | ||
| NPA | 332/332 | 331/332 | 331/332 | |
| 100.0 (98.9–100.0) | 99.7 (98.3–100.0) | 99.7 (98.3–100.0) | ||
| Kappa | 0.97 (0.93–1.00) | 0.99 (0.98–1.00) | 0.99 (0.98–1.00) | |
| Influenza B | ||||
| PPA | 50/50 | 50/50 | 50/50 | |
| 100.0 (92.9–100.0) | 100.0 (92.9–100.0) | 100.0 (92.9–100.0) | ||
| NPA | 353/353 | 353/353 | 353/353 | |
| 100.0 (98.9–100.0) | 100.0 (98.9–100.0) | 100.0 (98.9–100.0) | ||
| Kappa | 1.00 (1.00–1.00) | 1.00 (1.00–1.00) | 1.00 (1.00–1.00) | |
PPA and NPA were presented as % with 95% confidence intervals and Kappa with 95% confidence intervals.
SARS-CoV-2 = severe acute respiratory syndrome-coronavirus-2, PPA = positive percent agreement, NPA = negative percent agreement.
Table 5. Discordant results of SARS-CoV-2 between the three commercial kits.
| Specimen No. | Interpretations and Ct values of target genes | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| PowerChek | STANDARD M | Allplex | ||||||||
| Interpr. | E | ORF1ab | Interpr. | E | ORF1ab | Interpr. | N | RdRp | S | |
| 6 | P | 33.8 | 37.5 | P | 34.4 | 30.5 | I | - | 34.7 | 32.8 |
| 36 | I | 35.8 | - | P | 34.0 | 33.1 | I | - | 35.3 | 34.0 |
| 65 | I | 36.7 | - | I | 36.4 | 35.1 | P | 35.2 | 35.7 | 36.0 |
| 66 | I | 38.3 | 35.1 | N | - | 36.4 | P | 34.9 | 31.7 | 32.3 |
| 67 | P | 36.0 | 37.6 | I | - | 33.5 | P | 35.7 | 34.8 | 34.9 |
| 72 | I | - | 37.2 | P | 35.3 | 33.4 | P | 34.7 | 36.8 | 36.0 |
| 75 | P | 36.8 | 36.7 | I | - | 33.1 | I | 35.2 | 36.2 | - |
| 80 | N | - | - | P | 33.9 | 31.0 | P | 34.1 | 32.5 | 31.4 |
| 94 | I | 35.1 | 38.1 | P | 33.3 | 32.6 | P | 33.4 | 33.6 | 32.5 |
| 97 | N | 38.7 | 38.3 | P | 34.8 | 34.7 | I | - | 35.4 | 36.1 |
All specimens were derived from coronavirus disease 2019 confirmed cases.
SARS-CoV-2 = severe acute respiratory syndrome-coronavirus-2, Ct = cycle threshold, Interpr. = interpretation, P = positive, I = inconclusive, N = negative.
There were two influenza A false-positive results; the STANDARD M and Allplex kits each produced a false-positive result in two different samples, although both assays had a NPA of 99.7% (Table 5, Supplementary Table 3). In the first sample, PowerChek and Allplex kits did not detect influenza A, but the STANDARD M did with a cycle threshold (Ct) value of 34.0. In the second sample, PowerChek and STANDARD M only detected influenza B, but the Allplex kit additionally detected influenza A with a Ct value of 34.0.
Five samples that tested negative for RSV using the AdvanSure comparator kit tested positive using the Allplex kit (Table 6, Supplementary Table 3). In the LoD test of the AdvanSure kit, the LoDs for influenza A, influenza B, and RSV were as follows: 250,172.6 copies/mL (95% CI, 23,630.6–4.74×1013; P = 0.988), 747.9 copies/mL (95% CI, 481.0–1,585.1; P = 0.807), and more than 5,000 copies/mL, respectively. A previous study reported that LoD of RSV in the AdvanSure kit was 2,920 copies/mL22 which was higher than that of the Allplex kit. RT-PCR and gene sequencing confirmed that among these five samples four were identified as RSV-positive, and their RSV Ct values in Allplex ranged from 34.4 to 35.9. However, one sample was not identified as RSV-positive, despite producing an RSV Ct value of 36.7 using the Allplex kit.
Table 6. Positive and negative agreement for detection of RSV between the Allplex and the comparator assay (AdvanSure™ respiratory viruses real-time RT-PCR assay) and analysis for samples with discrepant results.
| Allplex | |||
|---|---|---|---|
| + | − | ||
| AdvanSure | |||
| + | 78 | 0 | |
| − | 5 | 319 | |
| No. of positive samples of RSV sequencing in discrepant samples | 4 | NA | |
| PPA (%) (95% CI) | 94.0 (86.7–97.4) | ||
| NPA (%) (95% CI) | 100.0 (98.8–100.0) | ||
| Kappa (95% CI) | 0.96 (0.93–1.00) | ||
RSV = respiratory syncytial virus, RT-PCR = reverse transcription polymerase chain reaction, NA = not applicable, PPA = positive percent agreement, CI = confidence interval, NPA = negative percent agreement.
Finally, influenza- or RSV-positive cases did not occur in the SARS-CoV-2-positive samples, and SARS-CoV-2-positive cases did not in the influenza A/B-positive, RSV-positive, or other respiratory viruses-positive samples.
DISCUSSION
In the evaluation of analytical sensitivity, the LoD is dependent on reference material kits, extraction methods, PCR machines, and other experimental conditions. Therefore, the LoDs reported in our study may not correspond with practical LoDs in the real world. In the evaluation of cross-reactivity of the three assays, all three kits did not present cross-reactivity with common respiratory viruses. Especially, for SARS-CoV-2, three assays showed no reactive with coronavirus 229E, OC43, and NL63.
All three kits had 100% NPA in SARS-CoV-2 results, but PPAs in SARS-CoV-2 results ranged from 92.8% to 95.9%; among 97 SARS-CoV-2 positive samples, 10 (10.3%) produced discrepant results among the three assays. All three kits had 100% PPA in influenza A/B results. In contrast to 100% NPA in influenza B results in all three kits, NPAs in influenza A results ranged from 99.7% to 100.0% due to two false influenza A-positive reactions. According to the LoD testing of AdvanSure and Allplex kits and RSV sequencing results, Allplex detected RSV more sensitively than AdvanSure.
Molecular diagnostic kits for detecting SARS-CoV-2 have shifted from single SARS-CoV-2 detection to multi-detection of SARS-CoV-2 and other common respiratory pathogens. Nörz et al.23 adapted a laboratory-developed multiplex RT-PCR assay for the simultaneous detection of SARS-CoV-2 and influenza A/B on a fully automated high-throughput system and demonstrated analytical performance comparable with that of currently available commercial tests. Sensitivities of SARS-CoV-2, influenza A, and influenza B in the multiplex assay were 98.1%, 97.67%, and 100%, respectively.23 Xpert Xpress SARS-CoV-2/Flu-RSV (Xpert 4-in-1 assay) was launched with Emergency Use Authorization of the United States Food and Drug Administration in September 2020 and demonstrated a performance highly comparable with those of Xpert SARS-CoV-2 and Xpert Flu/RSV assays.24 PPAs and NPAs of SARS-CoV-2, influenza A, influenza B, and RSV in Xpert -in-1 assay were 98.48%/100%, 100%/100%, 100%/99.54%, and 100%/100%, respectively.24
A case of co-infection of SARS-CoV-2 and other respiratory viruses was absent in the present study. SARS-CoV-2-positive specimens were collected between September 2020 and November 2020. In Korea, RSV and influenza outbreak seasons classically begin in autumn and winter, respectively.25 Although the collection period overlapped with RSV outbreak season, due to COVID-19-related quarantine and improved hygiene, transmission rates of influenza and RSV were lower.26 A US research team reported that 20.7% of specimens positive for SARS-CoV-2 were positive for one or more additional pathogens, compared with 26.7% negative for SARS-CoV-2.27 The most commonly co-infected pathogens were rhinovirus/enterovirus followed by RSV and non-SARS-CoV-2 Coronaviridae.27 A single-centered retrospective study was performed in Wuhan, China, between January 12 and February 21, 2020 during the initial COVID-19 outbreak.28 Unexpectedly, among COVID-19 patients, only 42.7% (131/307) of patients were singly positive for SARS-CoV-2; 57.3% (176/307) of COVID-19 patients were also positive for influenza viruses including influenza A (49.8%) and influenza B (7.5%).28 Another single-centered retrospective study conducted in Lukou District, China showed that 14.1% (11/78) of COVID-19 patients were co-infected with other respiratory pathogens including RSV (3/11, 36.3%) and influenza B (1/11, 9.1%).14 In contrast, a Korean research team demonstrated co-infection cases of SARS-CoV-2 and influenza virus or RSV were absent.29 Using the Allplex RV-Essential Assay (Seegene) detecting seven respiratory viruses including adenovirus, influenza A virus, influenza B virus, metapneumovirus, parainfluenza virus, RSV, and human rhinovirus, the co-infection rate in COVID-19 patients was 2.2%.29 The most frequently detected virus was adenovirus, followed by human rhinovirus and metapneumovirus.29 However, comprehensive PCR testing to detect respiratory viruses other than influenza virus and RSV was not performed in SARS-CoV-2-positive specimens in the present study.
PowerChek, STANDARD M, and Allplex kits each specified different cycle conditions for rRT-PCR. PowerChek did not require any pre-amplification cycles during PCR. In the PCR protocol of STANDARD M, five pre-amplification cycles preceded the amplification cycles, and the time and temperature conditions of both cycles were the same. Allplex had three pre-amplification cycles with different time and temperature conditions from those of the amplification cycles. Volume of template RNA per test and the number of pre-amplification cycles and amplification cycles of the three multi-target kits were compared with corresponding single SARS-CoV-2 detection kits: PowerChek 2019-nCoV Real-time PCR (Kogene Biotech), STANDARD M nCoV Real-Time Detection (SD BioSensor), and Allplex 2019-nCoV (Seegene).17 KogeneBiotech and SD BioSensor had the same volume of template RNA per test and number of pre-amplification and amplification cycles in each pair of single- and multi-target assays. However, the multi-target assay of Allplex required 10 μL of template RNA compared with 8 μL in the single-target assay. Allplex single-target assay performed 45 amplification cycles without pre-amplification but the multi-target assay executed 3 pre-amplification and 42 amplification cycles. Allplex multi-target assay was thought to be designed to increase sensitivity than the single-target assay by increasing the volume of template RNA.
This study had some limitations. First, as previously mentioned, the evaluation of the LoD of the kits may not have reflected the practical analytical sensitivity because the measured LoD may have been affected by extraction method, type of PCR machine, etc. Second, for SARS-CoV-2, cross-reactivity test with other coronaviruses except 229E, OC43, and NL63 was not performed. Third, although the AdvanSure kit was treated as a comparator kit in this study, a true reference method was absent. Indeed, the AdvanSure kit was less sensitive for RSV compared with the Allplex kit. Finally, because multiplex PCR used to detect respiratory viruses other than influenza A/B and RSV was not performed in SARS-CoV-2-positive samples, co-infection rates of other respiratory viruses and SARS-CoV-2 could not be estimated.
In conclusion, the overall performance of these three multiplex rRT-PCR assays for concurrent detection of SARS-CoV-2, influenza A/B, and RSV was comparable. In the COVID-19 pandemic era, all three kits are suitable for the prompt differential diagnosis of COVID-19, influenza, and RSV infection in patients with respiratory symptoms.
ACKNOWLEDGMENTS
The authors would like to thank SD BioSensor for supplying the STANDARD™ M Flu/SARS-CoV-2 Real-time Detection Kit.
Footnotes
Funding: This work was supported by a grant from Seegene in Seoul, Korea (2020).
Disclosure: The authors have no potential conflicts of interest to disclose.
- Conceptualization: Park H, Kim N, Roh EY, Shin S, Yoon JH, Kim TS.
- Data curation: Park H.
- Formal analysis: Yun J, Park JH.
- Funding acquisition: Park H.
- Investigation: Park H, Yun J.
- Methodology: Park H.
- Writing - original draft: Yun J.
- Writing - review & editing: Park H, Park JH, Yun J.
SUPPLEMENTARY MATERIALS
SARS-CoV-2 results and Ct values of the three assays and STANDARD M nCoV
The target gene results in the detection of SARS-CoV-2 according to the three assays
Discordant samples among AdvanSure and three kits regarding influenza A/B and RSV
References
- 1.Hu B, Guo H, Zhou P, Shi ZL. Characteristics of SARS-CoV-2 and COVID-19. Nat Rev Microbiol. 2021;19(3):141–154. doi: 10.1038/s41579-020-00459-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu F, Zhao S, Yu B, Chen YM, Wang W, Song ZG, et al. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579(7798):265–269. doi: 10.1038/s41586-020-2008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Parasher A. COVID-19: Current understanding of its Pathophysiology, Clinical presentation and Treatment. Postgrad Med J. 2021;97(1147):312–320. doi: 10.1136/postgradmedj-2020-138577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.So W. Age distribution of coronavirus (COVID-19) cases in South Korea as of October 8, 2021. [Updated 2021]. [Accessed November 3, 2021]. http://www.statista.com/statistics/1102730/south-korea-coronavirus-cases-by-age/
- 5.Turke PW. Five reasons COVID-19 is less severe in younger age-groups. Evol Med Public Health. 2021;9(1):113–117. doi: 10.1093/emph/eoaa050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gaitonde DY, Moore FC, Morgan MK. Influenza: diagnosis and treatment. Am Fam Physician. 2019;100(12):751–758. [PubMed] [Google Scholar]
- 7.Borchers AT, Chang C, Gershwin ME, Gershwin LJ. Respiratory syncytial virus--a comprehensive review. Clin Rev Allergy Immunol. 2013;45(3):331–379. doi: 10.1007/s12016-013-8368-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peteranderl C, Herold S, Schmoldt C. Human influenza virus infections. Semin Respir Crit Care Med. 2016;37(4):487–500. doi: 10.1055/s-0036-1584801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention. Key facts about influenza (Flu) [Updated 2021]. [Accessed May 25, 2021]. http://www.cdc.gov/flu/about/keyfacts.htm .
- 10.Centers for Disease Control and Prevention. Respiratory syncytial virus infection (RSV) [Updated 2020]. [Accessed May 25, 2021]. http://www.cdc.gov/rsv/clinical/index.html .
- 11.Obando-Pacheco P, Justicia-Grande AJ, Rivero-Calle I, Rodríguez-Tenreiro C, Sly P, Ramilo O, et al. Respiratory syncytial virus seasonality: a global overview. J Infect Dis. 2018;217(9):1356–1364. doi: 10.1093/infdis/jiy056. [DOI] [PubMed] [Google Scholar]
- 12.Ozaras R, Cirpin R, Duran A, Duman H, Arslan O, Bakcan Y, et al. Influenza and COVID-19 coinfection: report of six cases and review of the literature. J Med Virol. 2020;92(11):2657–2665. doi: 10.1002/jmv.26125. [DOI] [PubMed] [Google Scholar]
- 13.Konala VM, Adapa S, Naramala S, Chenna A, Lamichhane S, Garlapati PR, et al. A case series of patients coinfected with influenza and COVID-19. J Investig Med High Impact Case Rep. 2020;8:2324709620934674. doi: 10.1177/2324709620934674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tang ML, Li YQ, Chen X, Lin H, Jiang ZC, Gu DL, et al. Co-Infection with common respiratory pathogens and SARS-CoV-2 in patients with COVID-19 pneumonia and laboratory biochemistry findings: a retrospective cross-sectional study of 78 patients from a single center in China. Med Sci Monit. 2021;27:e929783. doi: 10.12659/MSM.929783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nature. The coronavirus is here to stay — here’s what that means. [Updated 2021]. [Accessed June 2, 2021]. http://www.nature.com/articles/d41586-021-00396-2 .
- 16.Tahamtan A, Ardebili A. Real-time RT-PCR in COVID-19 detection: issues affecting the results. Expert Rev Mol Diagn. 2020;20(5):453–454. doi: 10.1080/14737159.2020.1757437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hur KH, Park K, Lim Y, Jeong YS, Sung H, Kim MN. Evaluation of four commercial kits for SARS-CoV-2 real-time reverse-transcription polymerase chain reaction approved by Emergency-Use-Authorization in Korea. Front Med (Lausanne) 2020;7:521. doi: 10.3389/fmed.2020.00521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhen W, Smith E, Manji R, Schron D, Berry GJ. Clinical evaluation of three sample-to-answer platforms for detection of SARS-CoV-2. J Clin Microbiol. 2020;58(8):e00783–e00720. doi: 10.1128/JCM.00783-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pfefferle S, Reucher S, Nörz D, Lütgehetmann M. Evaluation of a quantitative RT-PCR assay for the detection of the emerging coronavirus SARS-CoV-2 using a high throughput system. Euro Surveill. 2020;25(9):2000152. doi: 10.2807/1560-7917.ES.2020.25.9.2000152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.U.S. Food and Drug Administration. In vitro diagnostics EUAs - Molecular Diagnostic Tests for SARS-CoV-2. [Updated 2021]. [Accessed May 25, 2021]. http://www.fda.gov/medical-devices/coronavirus-disease-2019-covid-19-emergency-use-authorizations-medical-devices/in-vitro-diagnostics-euas-molecular-diagnostic-tests-sars-cov-2#individual-molecular .
- 21.World Health Organization. Laboratory testing for coronavirus disease (COVID-19) in suspected human cases: Interim guidance, 19 March 2020. [Updated 2020]. [Accessed May 25, 2021]. http://apps.who.int/iris/handle/10665/331501 .
- 22.Jung YJ, Kwon HJ, Huh HJ, Ki CS, Lee NY, Kim JW. Comparison of the AdvanSure™ real-time RT-PCR and Seeplex (®) RV12 ACE assay for the detection of respiratory viruses. J Virol Methods. 2015;224(2):42–46. doi: 10.1016/j.jviromet.2015.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nörz D, Hoffmann A, Aepfelbacher M, Pfefferle S, Lütgehetmann M. Clinical evaluation of a fully automated, laboratory-developed multiplex RT-PCR assay integrating dual-target SARS-CoV-2 and influenza A/B detection on a high-throughput platform. J Med Microbiol. 2021;70(2):001295. doi: 10.1099/jmm.0.001295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leung EC, Chow VC, Lee MK, Tang KP, Li DK, Lai RW. Evaluation of the Xpert Xpress SARS-CoV-2/Flu/RSV assay for simultaneous detection of SARS-CoV-2, influenza A/B and respiratory syncytial viruses in nasopharyngeal specimens. J Clin Microbiol. 2021;59(4):e02965–e02920. doi: 10.1128/JCM.02965-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Korea Disease Control and Prevention Agency. Pathogens & Vector Surveillance Weekly Report, PVSWR 2019. 10. 6.–10. 19. [Updated 2019]. [Accessed November 7, 2021]. http://www.kdca.go.kr/npt/biz/npp/portal/nppPblctDtaView.do?pblctDtaSeAt=3&pblctDtaSn=1988 .
- 26.Korea Disease Control and Prevention Agency. Pathogens & Vector Surveillance Weekly Report, PVSWR 2021. 8. 29.–2021. 9. 11. [Updated 2021]. [Accessed November 7, 2021]. http://www.kdca.go.kr/npt/biz/npp/portal/nppPblctDtaView.do?pblctDtaSeAt=3&pblctDtaSn=2443 .
- 27.Kim D, Quinn J, Pinsky B, Shah NH, Brown I. Rates of co-infection between SARS-CoV-2 and other respiratory pathogens. JAMA. 2020;323(20):2085–2086. doi: 10.1001/jama.2020.6266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yue H, Zhang M, Xing L, Wang K, Rao X, Liu H, et al. The epidemiology and clinical characteristics of co-infection of SARS-CoV-2 and influenza viruses in patients during COVID-19 outbreak. J Med Virol. 2020;92(11):2870–2873. doi: 10.1002/jmv.26163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim YG, Park H, Kim SY, Hong KH, Kim MJ, Lee JS, et al. Rates of coinfection between SARS-CoV-2 and other respiratory viruses in Korea. Ann Lab Med. 2022;42(1):110–112. doi: 10.3343/alm.2022.42.1.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
SARS-CoV-2 results and Ct values of the three assays and STANDARD M nCoV
The target gene results in the detection of SARS-CoV-2 according to the three assays
Discordant samples among AdvanSure and three kits regarding influenza A/B and RSV


