Abstract
In the Brazilian Atlantic Rainforest (AF), amphibians (625 species) face habitat degradation leading to stressful thermal conditions that constrain animal activity (e.g., foraging and reproduction). Data on thermal ecology for these species are still scarce. We tested the hypothesis that environmental occupation affects the thermal tolerance of amphibian species more than their phylogenetic relationships. We evaluated patterns of thermal tolerance of 47 amphibian species by assessing critical thermal maxima and warming tolerances, relating these variables with ecological covariates (e.g., adult macro‐ and microhabitat and site of larval development). We used mean and maximum environmental temperature, ecological covariates, and morphological measurements in the phylogenetic generalized least squares model selection to evaluate which traits better predict thermal tolerance. We did not recover phylogenetic signal under a Brownian model; our results point to a strong association between critical thermal maxima and habitat and development site. Forest species were less tolerant to warm temperatures than open area or generalist species. Species with larvae that develop in lentic environment were more tolerant than those in lotic ones. Thus, species inhabiting forest microclimates are more vulnerable to the synergistic effect of habitat loss and climate change. We use radar charts as a quick evaluation tool for thermal risk diagnoses using aspects of natural history as axes.
Keywords: climate changes, CTMax , deforestation, future vulnerability, tropical amphibians, warming tolerance
This study describes a strong association between critical thermal maximums and the habitat and place of development for 47 species of amphibians from the Atlantic Forest. We show that ecological profiles and aspects of natural history (rather than phylogenetic relationship) are the main predictors of thermal sensitivity.

Research Highlights.
The thermal vulnerability among the Atlantic Rainforest amphibians is correlated to ecological pattern of habitat occupancy.
Forest species that reproduce in streams and whose adults are arboreal are the most susceptible to climate change.
1. INTRODUCTION
Among vertebrates, amphibians have particular characteristics (e.g., permeable skin) that make them extremely dependent on abiotic factors such as temperature and humidity (Duellman & Trueb, 1994). Amphibians are the most endangered group of vertebrates in the world (Alroy, 2015; Ceballos et al., 2017; Stuart et al., 2004). Recent studies estimate that approximately 200 amphibian species (2.4% of all global diversity) are already extinct, and direct and indirect factors related to human activities threaten 41% (Alroy, 2015; Hoffmann et al., 2010; IUCN, 2020). Even those classified as Least Concern (LC) by the IUCN (International Union for Conservation of Nature—an international effort to estimate the extinction risk of species) may be facing declines and/or loss of populations (Ceballos et al., 2017). With ca. 1,137 species, Brazil is the country with the largest amphibian richness (Frost, 2020; Segalla et al., 2019), but a significant number of these species are still classified as Data Deficient (approx. 281 spp.) (IUCN, 2021; Tapley et al., 2018).
The Brazilian Atlantic Forest is one of Earth's biodiversity hot spots, having been reduced to only 12.4% of its original area (SOS Atlantic Forest, 2017). It harbors more than 50% (ca. 650 spp.) of amphibian species recognized for Brazil (Myers et al., 2000; Rossa‐Feres et al., 2017; Segalla et al., 2019). In the south of Bahia state, the Atlantic Rainforest is particularly important for amphibian conservation given the high number of species, several endemic (e.g., Dias et al., 2014; Mira‐Mendes et al., 2018). The overall diversity of amphibians in this region remains understudied given the numerous recent additions to knowledge of regional diversity by descriptions of new species (Carnaval et al., 2009; Dias et al., 2017, 2020; Orrico et al., 2018). Therefore, southern Bahia can be a considerate priority area for amphibian conservation taking into account the high diversity of species, lineages, distinct life history guilds, and current pressures as climate change and fragmentation (Campos et al., 2017, 2020; Loyola et al., 2008; Vasconcelos et al., 2014).
Both on a local (southern Bahia) and on a biome (Brazilian Atlantic Forest) scale, habitat fragmentation and climate may act in synergism producing disconnection between the habitat from different amphibian ontogenetic stages (Becker et al., 2007). Therefore, amphibian conservation efforts can benefit from considering life history variation in habitat use, because microhabitat, activity windows, and sensibility may vary along with the ontogenetic development in amphibians (Enriquez‐Urzelai et al., 2019).
Thermal exposure is among the main threats to amphibian conservation in the Atlantic Forest (Hof et al., 2011; Silvano & Segalla, 2005). Therefore, it is paramount to investigate ecophysiological aspects linked to the thermal niche (e.g., CTMax) to understand the consequences of climate change for these animals (Li et al., 2013). In a warming Earth, ecophysiological studies provide data to assess climatic vulnerability of species, mainly in ectotherm animals such as amphibians (Tejedo et al., 2012). The critical thermal maximum (CTMax) is the thermal point where the animal loses its locomotor ability to respond to a stressful thermal environment (Cowles & Bogert, 1944; Taylor et al., 2020). The use of CTMax combined with the environmental thermal data allows to infer the species level of sensitivity (e.g., warming tolerance) to future global warming scenarios (Deutsch et al., 2008; Duarte et al., 2012; Gutiérrez‐Pesquera et al., 2016; Simon et al., 2015). Additionally, studies have recovered phylogenetic signals in thermal niche dimensions (e.g., CTMax and CTMin) for amphibians (Gutiérrez‐Pesquera et al., 2016; Hof et al., 2010). The presence of phylogenetic signal has been tested in tadpoles of anuran species from the Atlantic Rainforest of southern Bahia, but nothing is known about adults (Gutiérrez‐Pesquera et al., 2016). Since the larval and adult stages are exposed to different environmental conditions, it is paramount to understand the physiological dimensions of both development stages and elucidate any phylogenetic signatures involved, and the implications for the species fitness (i.e., population growth rate).
In Brazil, research on the impacts of global warming on amphibians is still scarce (Winter et al., 2016). Herein, we evaluate patterns of upper thermal tolerance by accessing the CTMax in adults of 47 amphibian species from the Atlantic Forest of southern Bahia. We expect that macro‐ and microhabitat play a role in buffering against the exposition to high temperatures. Furthermore, we investigate patterns of thermal tolerance among species groups organized by macro‐ and microhabitats of adults, and site of larval development. We hypothesize that forest‐associated species have lower heat tolerance than generalists and those who inhabit open areas. We also expect that arboreal species will reveal lower CTMax values if compared with terrestrial, fossorial, and cryptozoic species. We used a phylogenetic model to identify whether ecological, allometric, and microclimatic covariates can predict thermal tolerance of species. Therefore, we use model selection to evaluate whether the CTMax of the studied species (obtained from experiments with adults) results from an evolutionary process predicted by Brownian motion, as already predicted for CTMax of larval forms of species in the study region (Gutiérrez‐Pesquera et al., 2016). Last, we use radar charts as a rapid assessment tool for thermal risk diagnoses, using CTMax as a ranking factor to categorize ecological groups according to their thermal sensitivity.
2. MATERIALS AND METHODS
2.1. Specimen collection and study area
We captured specimens from Atlantic Forest fragments in southern Bahia state, Brazil. The climate of the region is tropical humid, Af (tropical wet), and Am (tropical monsoon) in the Köppen classification, with annual average temperature and precipitation around 25°C and 1200 mm, respectively. Sampling was performed at the following locations: Almadina municipality (14°42′0.51″S, 39°37′48″W), Serra Bonita Private Reserve of Natural Heritage (15º23′S, 39º33′W, Camacã municipality), Provisão Farm (14°39′19.09″S, 39°13′13.90″W, Ilhéus municipality), cabruca agroforestry system in Universidade Estadual de Santa Cruz campus (14°47′45″S, 39°10′20″W, Ilhéus municipality), Michelin Ecological Reserve (Igrapiúna municipality), Bonfim Farm (14°36′24.68″S, 39°21′17.99″W, Uruçuca municipality), village of Acuípe (Ilhéus–Una Road, Ilhéus municipality), and Camamú Bay Islands (Maraú municipality). We performed visual encounter surveys through habitat and collected adult individuals of different amphibian species (orders Anura, Gymnophiona) by hand.
2.2. Critical thermal maximum and warming tolerance
We subjected individuals to Hutchison's dynamic method (Lutterschmidt & Hutchison, 1997), through a constant and gradual increase in temperature until reaching the Critical thermal maximum (CTMax) (see Taylor et al., 2020 for a review on thermal tolerance methods). We acclimated individuals for 72 hr at a room temperature of 25°C prior to assessing CTMax, following a 12‐hr photoperiod of light and dark regimes. We conducted the experiments in a specimen‐size experimental chamber with a 5‐mm high dechlorinated water layer (controlling the risk of desiccation), covered with a net to prevent individuals from escaping. We placed the experimental chambers inside a water bath to create a homogeneous heating system for experimental trials.
We started the bath's temperature at 25°C and gradually increased it by 0.25°C min−1 (Gutiérrez‐Pesquera et al., 2016). We used the “belly‐up” condition (Brattstrom, 1968; Taylor et al., 2020) to diagnose CTMax for specimens by the lack of response to periodically employed stimuli (5 touches per min with a glass rod). Once the specimens were flipped over, exposing their venters, but remained immobile (not returning to normal position in 10 s), we considered the temperature of the chamber to have reached the CTMax and the gradual warming was stopped. Subsequently, the chamber bottom temperature was measured using a contact thermometer (Miller & Weber, Inc.; 0.1°C accuracy). The specimens were then placed in a 23–25°C water container for cooling. We monitored all individuals for 24 hr, to ensure the lethal temperature was not reached during experiments, and only data from those who survived this period were included in the analyses. The CTMax for each species was defined as the average of its sampled individuals. For each specimen, we measured snout–vent length (SVL) and head width (HW) with a 1‐mm precision caliper after the experimental protocol (including the observation time) in order to minimize the influence of handling in their survivorship. We also weigh (W) each individual just before experimental procedures using a 0.01‐g precision scale.
We obtained microclimate data (average temperature—T Mean; maximum temperature—T Max) by installing temperature data loggers (HOBO Pendant Temp/Light, UA‐002–64) in an area of regenerating Atlantic Forest remnants (secondary forest) during the three‐month (November—February) period with highest annual temperatures in the studied region. We installed a data logger in each microhabitat category used by amphibians (see section below). The warming tolerance (WT) was calculated from the difference between the CTMax of each species and the maximum temperature of the microhabitat they use (sensu Deutsch et al., 2008), and we projected the future vulnerability by using the most recent International Panel on Climate Change (IPCC) indices for more and less acute warming scenarios (RCP 8.5–4.8°C; RCP 4.5–2.6°C, respectively) available on WorldClim database (worldclim.org). CTMax and warming tolerance were used to access the thermal vulnerability of species for both scenarios. The Animal Research Ethics Committee of Santa Cruz State University authorized all experiments (Protocol No. 012/15), while collection license 13,708 was issued by SISBio/ICMBio.
2.3. Groupings and statistical analyses
We analyzed how ecological features of species covariate with CTMax through a perspective of macrohabitat use (forest—Fo; generalist—Ge; and open areas—Op) and according to the site of larval development (lentic; lotic; marsupial; and terrestrial). To analyze possible trends in WT, we divided the species into groups by microhabitat (i.e., arboreal, cryptozoic, fossorial, and terrestrial). We based the functional group allocation of each individual species (see Table S2), following the ecological aspects available in Haddad et al. (2013).
Within functional groups, CTMax was calculated from the average CTMax of all individuals of the species assigned to each group. We compared our data on CTMax of the adult's specimens graphically with those available in the literature for their respective conspecific tadpoles looking for ontogenetic tendencies on thermal tolerance (Supplementary Material in Gutiérrez‐Pesquera et al., 2016). We assessed differences in CTMax and warming tolerance between groups by the nonparametric Kruskal–Wallis and the Dunn post hoc tests, with a significance level of 0.05.
We constructed radar charts to evaluate the thermal vulnerability of species given ecological covariates (e.g., macrohabitat, site of larval development, and microhabitat). We used the mean values of CTMax obtained for each category (e.g., forest, generalist, and open areas) within the ecological covariates as a categorization parameter on a scale ranging from 1 to 3, where the value 1 was given to categories with the lowest average CTMax within each covariate and the value 3 was given to those with the highest average CTMax.
2.4. Phylogenetic comparative methods
Comparative methods that take phylogenetic information under consideration are broadly used when data on several species violate assumptions of statistical independence. Phylogenetic generalized least squares (PGLS) models use phylogenies to make estimates on expected covariance among species data (Garamszegi, 2014). We performed regression analysis by PGLS in a Brownian evolutionary motion model, using the CAPER package (Orme et al., 2013) in the statistical Program R (R Core Team, 2020) to test the effects of allometric (SVL, HL, and W), ecological (macrohabitat and microhabitat), and microclimatic (mean and maximum temperature—T Mean and T Max) variables on CTMax. To account for phylogeny in analyses, we used the topology of Pyron and Wiens (2011), restricting the phylogeny to 23 species common with our sampling (Figure 1). We admitted the position of the nearest taxon, similar to Gutiérrez‐Pesquera et al. (2016), for three taxa (Aplastodiscus gr. albosignatus; Scinax gr. ruber; and Scinax cf. x‐signatus) that were not included in the phylogeny of Pyron and Wiens (2011). We used the lambda value to verify whether the covariance between the variables used follows the evolutionary pattern predicted by a Brownian motion model (where λ = 1) (Garamszegi, 2014). We ranked and selected among competing models using Akaike's information criteria (AIC). The model AIC weight value (wi) was used to evaluate the relative likelihood of model support (Burnham & Anderson, 2002). Analysis was conducted in R (R Core Team, 2020).
FIGURE 1.
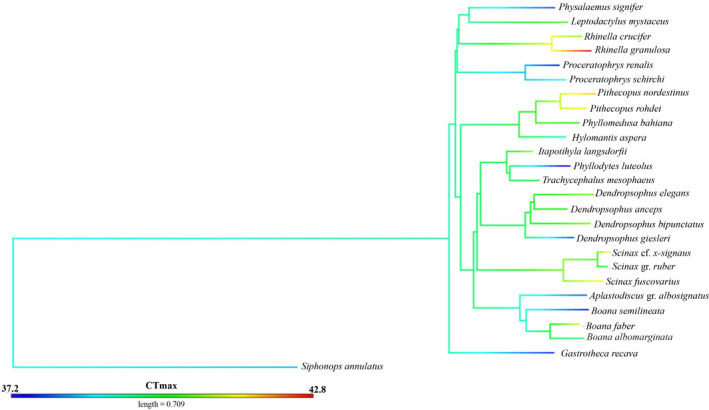
Phylogenetic tree including the 26 species studied (sensu Pyron & Wiens, 2011). Branch colors denote critical thermal maximum values for species. Color's gradient reaching from blue (lower CTMax) to red (higher CTMax)
3. RESULTS
3.1. Environment and CTMax
We evaluated the environmental influences of CTMax of 47 species (Table S1). CTMax differed among species’ macrohabitat groups (H = 26.77, df =2, p < 0.01, n = 308) (Tables S1 and S2). CTMax values of generalist species are intermediate between species that inhabit open environments (that reach higher values) and forest specialists (lower values) (Table 1). Pairwise statistics among groups of each covariate (macrohabitat, microhabitat, and site of larval development) are provided in Table 2.
TABLE 1.
Variation of critical thermal maximum (CTMax) and warming tolerance (WT) by ecological covariates. Means and their respective standard deviations (mean ± SD), Sample size (N), Lower and upper ranges of the 95% confidence intervals (lower 95 and upper 95, respectively), The Kruskal–Wallis chi‐squared index (H) and analyses p‐value (p)
| Critical thermal maximum | ||||||
|---|---|---|---|---|---|---|
| Mean ± SD | N | Lower 95 | Upper 95 | H | p | |
| Habitat | 26.8 | <0.01 | ||||
| Forest | 38.6 ± 1.7 | 192 | 38.3 | 38.8 | ||
| Generalist | 39.5 ± 2.8 | 80 | 38.7 | 40.0 | ||
| Open Habitats | 40.3 ± 1.5 | 36 | 39.8 | 41.0 | ||
| Larval development | 27.7 | <0.01 | ||||
| Lentic | 39.3 ± 2.1 | 259 | 39.1 | 39.7 | ||
| Lotic | 37.5 ± 0.9 | 31 | 36.9 | 37.7 | ||
| Marsupial | 37.9 ± 0.8 | 4 | 36.6 | 39.1 | ||
| Terrestrial | 38.6 ± 0.6 | 9 | 38.1 | 39.0 | ||
| Warming tolerance | ||||||
|---|---|---|---|---|---|---|
| Mean ± SD | N | Lower 95 | Upper 95 | H | p | |
| Microhabitat | 65.3 | <0.01 | ||||
| Arboreal | 7.0 ± 2.0 | 214 | 6.7 | 7.25 | ||
| Terrestrial | 6.0 ± 1.8 | 27 | 5.27 | 6.71 | ||
| Cryptozoic | 9.9 ± 1.3 | 34 | 9.5 | 10.4 | ||
| Fossorial | 13.7 ± 0.0 | 9 | 13.2 | 14.1 | ||
TABLE 2.
Pairwise differences (Dunn's post hoc test) between covariates groups. Bold p‐values denote significant differences in the CTMax of compared groups
| Pairwise groups | Z‐test statistic | p‐value | Covariate |
|---|---|---|---|
| Forest – generalist | −3.06 | <0.01 | Macrohabitat |
| Forest – open areas | −4.73 | <0.01 | Macrohabitat |
| Generalist – open areas | −2.26 | 0.01 | Macrohabitat |
| Lentic – lotic | 5.12 | <0.01 | Site of larval development |
| Lotic – marsupial | −0.52 | 0.3 | Site of larval development |
| Lentic – marsupial | 1.38 | 0.08 | Site of larval development |
| Marsupial – terrestrial | −0.74 | 0.22 | Site of larval development |
| Terrestrial – lotic | −1.91 | 0.03 | Site of larval development |
| Terrestrial – lentic | 0.73 | 0.23 | Site of larval development |
| Arboreal – cryptozoic | −6.86 | <0.01 | Microhabitat |
| Arboreal – fossorial | −5.42 | <0.01 | Microhabitat |
| Arboreal – terrestrial | 1.82 | 0.03 | Microhabitat |
| Cryptozoic – fossorial | −1.54 | 0.06 | Microhabitat |
| Cryptozoic – terrestrial | 6.35 | <0.01 | Microhabitat |
| Fossorial – terrestrial | 5.76 | <0.01 | Microhabitat |
CTMax values between groups with different larval development sites were also different (H = 27.7, df = 3, p = 0.00, n = 303) (Tables S1 and S2). Species with larval development in lotic habitats had lower CTMax values than species with larvae developing in lentic habitats (p < 0.01; Table 2). Our only marsupial frog species had lower CTMax than the other groups with exception of the lotic species (Table 1). We sampled a single species of Caecilian (Siphonops annulatus, the only species in the terrestrial group). Its CTMax was higher than that of marsupial anurans and species with lotic development and lower than that of lentic species. Our results regarding the terrestrial and marsupial group must be interpreted with caution, once in our sampling we were only able to collect individuals of only one species for each of these groups.
We rank‐scaled our ecological covariates according to thermal tolerance within radar graphs using each covariate as one axis and recovered ten general patterns for our sampled species. Eight patterns had at least one covariate limiting their tolerance and increasing the vulnerability (value 1 in chart scale) (Figure 2). The most vulnerable pattern is of terrestrial species, with lotic larval development, that inhabit forests. In contrast, species that combine fossorial adult microhabitat with lentic larval development, and are habitat generalists, presented the most tolerant profile.
FIGURE 2.
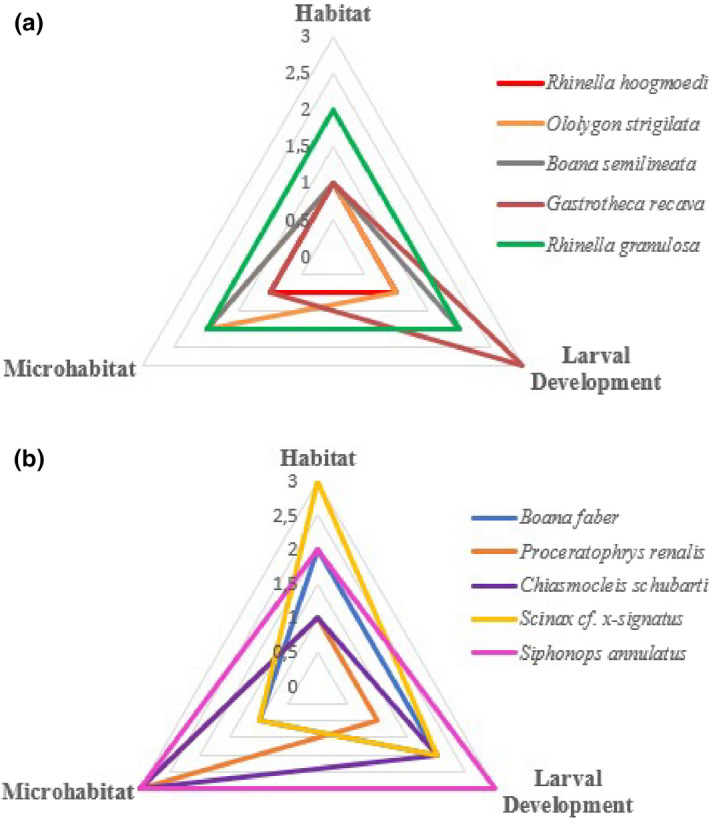
Radar charts showing ten patterns found for vulnerability according to ecological variables of macrohabitat, microhabitat, and larval development site. Each chart (a and b) is composed by five representative species for the general ecological patterns studied in the current study. Ecological categories were classified (1, 2, or 3) by CTMax (ºC) values (1—less tolerant species; 3—more tolerant species) according to the results of ecological covariates in the group analyses. Charts with smaller areas denote greater thermal vulnerability than those with larger areas
3.2. Warming tolerance
The WT of the 47 studied amphibian species differed according to their microhabitats (H = 65.322, df = 3, p = 0.001, n = 284). Fossorial and cryptozoic species had higher warming tolerances than arboreal and terrestrial species (Figure 3). All functional groups showed values of warming tolerance different from each other (p < 0.05, Table 2), with the exception of cryptozoic and fossorial (z = −1.54, p = 0.06) (Table 1). Predicted climate change scenario of 2.6°C (RCP4.5) will raise thermal conditions (microhabitat maximum temperate) above the CTMax of one species (Rhinella hoogmoedi) and near the thermal maximum of another one (Dendropsophus haddadi). Another predicted warming scenario (of 4.8°C) would increase the microhabitat maximum temperature above the CTMax of three species (both previously mentioned and Bokermannohyla capra). This same scenario would drive nine additional species to experience thermal conditions near to their CTMax (Table 3).
FIGURE 3.
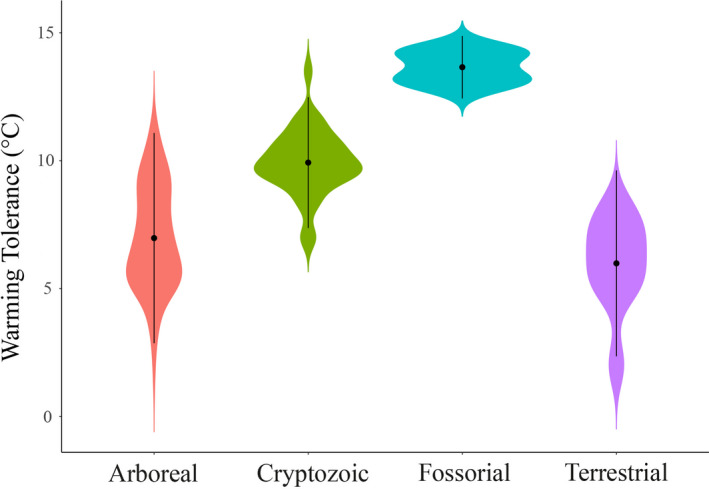
Effect of microhabitat on the warming tolerance of amphibian species from the Atlantic Forest of southern Bahia. Vertical bars denote 95% confidence intervals around the mean (point) of each functional group
TABLE 3.
Species most sensitive to temperature increase in view of more and less pessimistic scenarios proposed by the IPCC (2014) (RCP8.5 and RCP4.5, respectively). Values in the table refer to the maximum critical temperature (CTMax), species microhabitat, maximum critical temperature of microhabitats (T Max), current heating tolerance (WT), and those based on warming projections of IPCC (2014) (WTRCP4.5 and WTRCP8.5, respectively)
| CTMax (°C) | Microhabitat | T Max (°C) | WT | WTRCP4.5 | WTRCP8.5 | |
|---|---|---|---|---|---|---|
| Rhinella hoogmoedi | 37.5 | Ter | 35.4 | 2.1 | −0.5 | −2.7 |
| Dendropsophus haddadi | 35.8 | Arb | 31.9 | 3.9 | 1.3 | −0.9 |
| Bokermannohyla capra | 36.5 | Arb | 31.9 | 4.6 | 2.0 | −0.2 |
| Ololygon melanodactyla | 36.8 | Arb | 31.9 | 4.9 | 2.3 | 0.1 |
| Phyllodytes luteolus | 37.2 | Arb | 31.9 | 5.3 | 2.7 | 0.5 |
| Ololygon strigilata | 37.2 | Arb | 31.9 | 5.3 | 2.7 | 0.5 |
| Rhinella crucifer | 40.8 | Ter | 35.4 | 5.4 | 2.8 | 0.6 |
| Boana pombali | 37.3 | Arb | 31.9 | 5.4 | 2.8 | 0.6 |
| Boana semilineata | 37.5 | Arb | 31.9 | 5.6 | 3.0 | 0.8 |
| Aplastodiscus ibirapitanga | 37.6 | Arb | 31.9 | 5.7 | 3.1 | 0.9 |
| Gastrotheca recava | 37.8 | Arb | 31.9 | 5.9 | 3.3 | 1 |
| Dendropsophus giesleri | 37.9 | Arb | 31.9 | 6.0 | 3.4 | 1.2 |
3.3. PGLS model selection
The best‐supported model (AIC = 886, Wi = 0.63) indicated that amphibian CTMax is explained by adult habitat and larval development site (Table 4). We did not observe a phylogenetic signal on CTMax within the three best models.
TABLE 4.
PGLS models. Italicized models are the five best‐selected models discussed on our results. Covariance adjustment parameter to the Brownian evolutionary model (λ), Akaike's information criterion value (AIC), Akaike's weight (wi), Snout–vent length (SVL), Head width (HW), Site of larval development (LDS), Average microhabitat temperature (T Mean), Maximum microhabitat temperature (T Max) and Weight (W)
| Model | Formulation | λ | AIC | wi |
|---|---|---|---|---|
| m12 | CTMax ~ Habitat + LDS | 0 | 887 | 0.63 |
| m9 | CTMax ~ HW/SVL + Habitat + LDS | 0 | 923 | 0.10 |
| m10 | CTMax ~ W/SVL + Habitat + LDS | 0 | 924 | 0.10 |
| m5 | CTMax ~ Tmean + Tmax + Habitat + LDS | 0 | 926 | 0.01 |
| m7 | CTMax ~ HW/SVL + Tmean + Tmax + Habitat + LDS | 0 | 961 | 0.01 |
| m6 | CTMax ~ SVL * HW * W + T mean + T max + Habitat + LDS | 1 | 964 | 0.01 |
| m8 | CTMax ~ W/SVL + T mean + T max + Habitat + LDS | 0 | 964 | 0.01 |
| m11 | CTMax ~ SVL * HW * W + Habitat + LDS | 0.75 | 964 | 0.01 |
| m13 | CTMax ~ T mean + T max | 0 | 981 | 0.00 |
| m1 | CTMax ~ W/SVL + T max | 0 | 994 | 0.00 |
| m3 | CTMax ~ SVL * HW * W + T max | 0 | 10 | 0.00 |
| m4 | CTMax ~ SVL * HW * W + T mean | 0 | 101 | 0.00 |
| m2 | CTMax ~ W/SVL + T mean | 0 | 103 | 0.00 |
4. DISCUSSION
Literature suggests that available macro‐ and microhabitats play an important role in thermoregulation and ecophysiology of forest‐associated tropical amphibians (Nowakowski et al., 2018; Scheffers et al., 2013, 2014). Our results corroborate this notion because values of adult amphibian CTMax of forest species were significantly lower than those from generalist or open environment species. Canopy protection and the availability of thermal refuges seem to be crucial to avoid overheating of forest species due to their low heat tolerance (Scheffers et al., 2014). Thus, the species of amphibians that inhabit forests will depend on even less macro‐ and microhabitats available in a global context marked by habitat loss (Stuart et al., 2004) and the exposure of thermal refuges to rising temperatures (Ficetola et al., 2015).
Among the three best models, there was no association between the CTMax and the T Mean and T Max of the microhabitat. Literature suggests that the CTMax of aquatic ectothermic organisms (e.g., crustaceans, fish, and aquatic insects) is more influenced by average and maximum environment temperatures because terrestrial ectothermic organisms are able to use habitat heterogeneity to exploit different microenvironments in order to maintain their body temperatures independent of thermal averages from the air (Bogert, 1949; Gunderson & Stillman, 2015; Kearney et al., 2009; Stevenson, 1985; Sunday et al., 2011).
In a study conducted in Costa Rica, Frishkoff et al. (2015) found an association between the occurrences of lentic larval development species (i.e., puddles) with deforested areas, while those with lotic larval development (i.e., streams) or direct development seemed to prefer forests. In our study, species that reproduce in ponds tolerated higher temperatures than lotic species, suggesting that lentic species that can eventually use lotic water bodies for reproduction (e.g., some species from genus Boana, Aplastodiscus, and Rhinella) are better competitors than lotic breeders in warmer environments (Haddad et al., 2013). Due to the low sample size of marsupial and direct development species, we were unable to characterize variation in CTMax with confidence for these groups. However, species that do not depend on water bodies for their development depend on the humidity of the forest to prevent desiccation of eggs (Frishkoff et al., 2015; Scheffers et al., 2013).
The Atlantic Forest faces a historical crisis of deforestation and fragmentation (Moura et al., 2018; SOS Mata Atlântica, 2017; Wanger et al., 2020). As degradation usually implies increased edge effect on forest matrices (Kapos, 1989), and consequently a higher incidence of light, forest specialist taxa are exposed to microclimatic modifications of their thermal refuges (Nowakowski et al., 2018; Tuff et al., 2016). Once exposed to anomalous and stressful thermal conditions, forest specialist species amphibians in southern Bahia face the risk of isolation and population disturbance from forest fragmentation, which associated with possible thermal stress could lead to local extinction events (Becker et al., 2007; Tuff et al., 2016). In a region with high amphibian richness and endemism rates (Carnaval et al., 2009; Dias et al., 2014; Mira‐Mendes et al., 2018; Vasconcelos et al., 2014), such a scenario raises an alarm concerning the extinction risk of endemic species.
Amphibians are particularly sensitive to habitat loss and fragmentation (Becker et al., 2007), and regenerating environments (secondary forests) usually present lower species richness when compared to pristine environments (Thompson & Donelly, 2018). According to Schneider‐Maunoury et al. (2016), the consequences of edge effects (resulting from degradation of forest matrices) on amphibians are related to variables of the general biology of species such as body size and habitat specialization. Our results show a converging panorama when we recover that the CTMax, an ecophysiological variable, is strongly influenced by adult and larval habitat, as well as when we highlight considerable differences between the CTMax of forest species, generalists, and those inhabiting open environments.
In the present study, the most tolerant species were those with cryptozoic or fossorial microhabitat. Nevertheless, other categories (arboreal and terrestrial) maintained their WT within tolerable physiological limits, according to current thermal signatures. We found 2–12 species that will be at risk of extinction given likely climate change scenarios in the Atlantic Forest considering the projections of temperature increase between 2.6 and 4.8°C (RCP 4.5 and RCP 8.5, respectively—IPCC, 2014). Terrestrial and arboreal species were more sensitive (lower WT) than cryptozoic and fossorial ones. Given that microhabitat warming can also be a consequence of forest cover loss, cryptozoic and fossorial species are also endangered by the increase in temperature and risk of desiccation (Kapos, 1989; Nowakowski et al., 2018; Tuff et al., 2016).
Even though our results point to habitat as the variable that best explains CTMax, the influence of the environment is not restricted to the upper tolerance limits. Diversity within families of anuran amphibians is mainly explained by species microhabitat, and to a lesser extent by the thermal niche they occupy (Moen & Wiens, 2017). However, several studies have recovered phylogenetic signals in niche thermal dimensions for various groups, including amphibians (e.g., Gutiérrez‐Pesquera et al., 2016; Hof et al., 2010; Olalla‐Tárraga et al., 2011). Our results agree with Moen and Wiens (2017) because CTMax values do not result from an evolutionary process predicted by the Brownian motion model (best‐adjusted models, λ = 0). Instead, we recovered a strong influence of the adult and tadpole habitat for the adult CTMax within the studied species, thus indicating that the occupation of the environment is an important factor to explain the heating tolerance of amphibian species. Previous research also showed an ecological pattern for the thermal limits in some Neotropical anurans from the superfamily Brachycephaloidea where physiological traits were positively correlated with the altitudinal distribution of the evaluated taxa (von May et al., 2017).
Given that our analysis did not recover any phylogenetic signal, to what extent does the adaptive potential of the analyzed Amphibia groups influence survival in a warming landscape? According to Moritz et al. (2012), although no significant variation was found in CTMax between peripheral and central lineages of the same species, there are differences among upper thermal limits between edge species and those within forest. Hypothetically, populations at the periphery of fragments may present genotypes that give them some resilience to future warming scenarios (see Moritz et al., 2012). However, phenotypic plasticity seems to have little ability to buffer harmful effects in a progressive context of rising global temperatures (Bellard et al., 2012). Terrestrial ectotherms have low acclimation capacity when it comes to temperature rises, and therefore are less tolerant to temperature changes than aquatic ectotherms (Gunderson & Stillman, 2015). We observed that differences between the adult CTMax (this study) and tadpoles of 14 species (Gutiérrez‐Pesquera et al., 2016) confirm the previously presented pattern—tadpoles (aquatic environment) tend to present higher maximal CTMax than adults (environment specific) (Figure 4).
FIGURE 4.
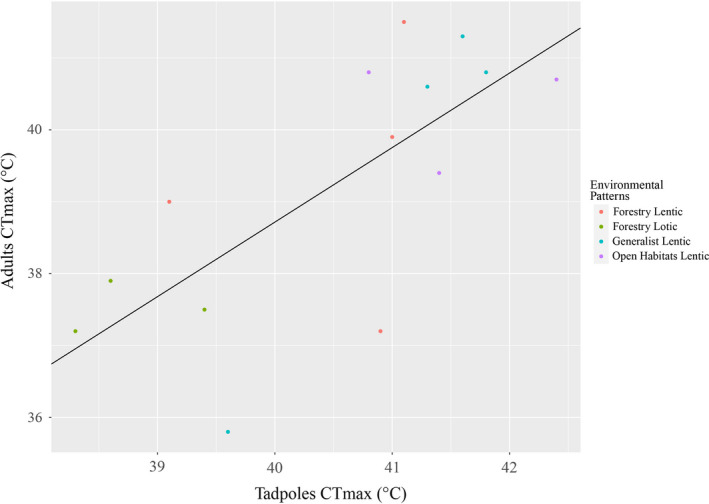
Distribution of CTMax data between adults and tadpoles of 14 species of anurans from southern Bahia. Data of tadpoles were obtained from Gutiérrez‐Pesquera et al. (2016)
For tadpoles, Tejedo et al. (2012) point out that the ability to adapt to temperature increase is linked to the thermal environments experienced by lineages. Thus, generalist species (tolerating more variable thermal environments) would be better adapted to cope with temperature rises than thermally specialized taxa (e.g., forest and open‐air species) (Tejedo et al., 2012). Although our data for adult amphibians also indicate such a pattern, it is still necessary to investigate whether larval forms exhibit similar behavior for thermal tolerance as a function of aquatic microhabitat. Although preliminary, we note that the tolerance patterns between larvae and adults signaled here point to a thermal separation between the adult and larval life stages (Becker et al., 2007).
By combining the data available from Gutiérrez‐Pesquera et al. (2016) for species with low plasticity in tolerance limits (Gunderson & Stillman, 2015; Tejedo et al., 2012), progressive reduction of forest patches (SOS Mata Atlântica), and the projections of temperature increase (IPCC, 2014), we point to threat scenarios close to those projected by previous studies (Ceballos et al., 2017). The consequences for such scenarios pass through population reductions (Becker et al., 2007) that can lead to loss of genetic diversity for populations (Ceballos et al., 2017; Moritz et al., 2012) and species (Carnaval et al., 2009), as well as extinction events (Alroy, 2015; Ceballos et al., 2017).
Overall, our results reinforce the general understanding that global warming is certainly a threat to biodiversity but produces different pressures according to the natural history of each species. Deforestation and homogenization of microhabitats can potentiate the effects of global warming and need to be evaluated synergistically. Interpretation of the results through the “Radar” charts (Figure 2) shows that even species classified as Least Concern or Data Deficient by IUCN are facing thermal risk. Overlapping graphical patterns (e.g., radar charts) from a scale established by the CTMax averages denoted the specific influence of macro‐ and microhabitat as a vulnerability predictor of southern Bahia amphibians. Even in radar patterns with the same area, different ecological aspects lead to classify species as vulnerable to thermal stress. The results obtained from the model selection confirmed the same trend. Therefore, the use of graphic components based on ecological variables, as well as the application of rigorously established scales, proved to be an efficient risk/vulnerability assessment strategy for the species studied here. The same approach may be used to estimate the susceptibility of other species, or even groups, to anthropogenic impacts.
The restrictions imposed by the thermal niches must be taken into account for conservation actions (Araújo et al., 2013; Damasceno et al., 2014). Thermal limit data are only available for about one tenth of Atlantic Forest amphibians—ca. 65 species. (Gutiérrez‐Pesquera et al., 2016; Simon et al., 2015; Tejedo et al., 2012; present study). In addition to CTMax, other ecophysiological variables (e.g., voluntary limits) could improve vulnerability projections for Atlantic Forest species if implemented in mechanistic niche models (Taylor et al., 2020). Our research shows that natural history plays a key role in thermal tolerance and thermal vulnerability in amphibians of the Brazilian Atlantic Forest and may be a proxy for thermal niche. Considering the low latitudinal variation of CTMax and conservationism in the upper thermal limits (Araújo et al., 2013), our results can be used for extrapolations within this biome.
CONFLICT OF INTEREST
None declared.
AUTHOR CONTRIBUTIONS
Leildo Machado Carilo Filho: Conceptualization (lead); Data curation (lead); Formal analysis (lead); Investigation (equal); Methodology (lead); Validation (lead); Visualization (lead); Writing – original draft (lead); Writing – review and editing (lead). Bruno Teixeira de Carvalho: Conceptualization (equal); Data curation (equal); Investigation (equal); Methodology (equal). Bruna Kelly Alves de Azevedo: Data curation (equal); Methodology (equal). Luis Miguel Gutiérrez‐Pesquera: Conceptualization (equal); Data curation (equal); Methodology (equal); Validation (equal); Writing – review and editing (equal). Caio Vinícius Mira‐Mendes: Conceptualization (equal); Methodology (equal); Writing – original draft (equal); Writing – review and editing (equal). Mirco Solé: Conceptualization (equal); Funding acquisition (equal); Methodology (equal); Resources (equal); Supervision (equal); Writing – original draft (equal); Writing – review and editing (equal). Victor Goyannes Dill Orrico: Conceptualization (equal); Formal analysis (equal); Funding acquisition (equal); Methodology (equal); Project administration (equal); Resources (equal); Supervision (equal); Writing – original draft (equal); Writing – review and editing (equal).
Supporting information
Tables S1 and S2
ACKNOWLEDGMENTS
LMCF thanks FAPESB for granting a scholarship and UESC for funding the research (#00220.1100.1727). MS and VGDO acknowledge CNPq for research grants (#304999/2015‐6 and #310467/2017‐9 respectively). MS also thanks Alexander von Humboldt‐Stiftung/Coordenação de Aperfeiçoamento de Pessoal de Nível Superior for a scholarship (BEX 0585/16‐5).
Carilo Filho, L. M. , de Carvalho, B. T. , Azevedo, B. K. A. , Gutiérrez‐Pesquera, L. M. , Mira‐Mendes, C. V. , Solé, M. , & Orrico, V. G. D. (2021). Natural history predicts patterns of thermal vulnerability in amphibians from the Atlantic Rainforest of Brazil. Ecology and Evolution, 11, 16462–16472. 10.1002/ece3.7961
DATA AVAILABILITY STATEMENT
Data sources used for statistical group analyses, species traits, and most sensitive taxa are provided in the Supporting Information. All files mentioned above are available online at https://doi.org/10.5061/dryad.ttdz08kzp.
REFERENCES
- Alroy, J. (2015). Current extinction rates of reptiles and amphibians. Proceedings of the National Academy of Sciences of the United States of America, 112(42), 13003–13008. 10.1073/pnas.1508681112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araújo, M. B. , Ferri‐Yáñez, F. , Bozinovic, F. , Marquet, P. A. , Valladares, F. , & Chown, S. L. (2013). Heat freezes niche evolution. Ecology Letters, 16(9), 1206–1219. 10.1111/ele.12155 [DOI] [PubMed] [Google Scholar]
- Becker, C. G. , Fonseca, C. R. , Haddad, C. F. B. , Batista, R. F. , & Prado, P. I. (2007). Habitat split and the global decline of amphibians. Science, 318(5857), 1775–1777. 10.1126/science.1149374 [DOI] [PubMed] [Google Scholar]
- Bellard, C. , Bertelsmeier, C. , Leadley, P. , Thuiller, W. , & Courchamp, F. (2012). Impacts of climate change on the future of biodiversity. Ecology Letters, 15(4), 365–377. 10.1111/j.1461-0248.2011.01736.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogert, C. M. (1949). Thermoregulation in reptiles, a factor in evolution. Evolution, 3(3), 195–211. 10.2307/2405558 [DOI] [PubMed] [Google Scholar]
- Brattstrom, B. H. (1968). Thermal acclimation in anuran amphibians as a function of latitude and altitude. Comparative Biochemistry and Physiology, 24(1), 93–111. 10.1016/0010-406X(68)90961-4 [DOI] [PubMed] [Google Scholar]
- Burnham, K. P. , & Anderson, D. R. (2002). Model selection and multi‐model inference: A practical information‐theoretic approach. Springer. [Google Scholar]
- Campos, F. S. , Lourenço‐de‐Moraes, R. , Llorente, G. A. , & Solé, M. (2017). Cost‐effective conservation of amphibian ecology and evolution. Science Advances, 3(6), e1602929. 10.1126/sciadv.1602929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos, F. S. , Lourenço‐de‐Moraes, R. , Ruas, D. S. , Mira‐Mendes, C. V. , Franch, M. , Llorente, G. A. , Solé, M. , & Cabral, P. (2020). Searching for networks: Ecological connectivity for amphibians under climate change. Environmental Management, 65(1), 46–61. 10.1007/s00267-019-01240-0 [DOI] [PubMed] [Google Scholar]
- Carnaval, A. C. , Hickerson, M. J. , Haddad, C. F. , Rodrigues, M. T. , & Moritz, C. (2009). Stability predicts genetic diversity in the Brazilian Atlantic forest hotspot. Science, 323(5915), 785–789. 10.1126/science.1166955 [DOI] [PubMed] [Google Scholar]
- Ceballos, G. , Ehrlich, P. R. , & Dirzo, R. (2017). Biological annihilation via the ongoing sixth mass extinction signalled by vertebrate population losses and declines. Proceedings of the National Academy of Sciences of the United States of America, 114(30), E6089–E6096. 10.1073/pnas.1704949114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowles, R. B. , & Bogert, C. M. (1944). A preliminary study of the thermal requirements of desert reptiles. Bulletin of the American Museum of Natural History, 83, 261–296. [Google Scholar]
- Damasceno, R. , Strangas, M. L. , Carnaval, A. C. , Rodrigues, M. T. , & Moritz, C. (2014). Revisiting the vanishing refuge model of diversification. Frontiers in Genetics, 5(353), 1–12. 10.3389/fgene.2014.00353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutsch, C. A. , Tewksbury, J. J. , Huey, R. B. , Sheldon, K. S. , Ghalambor, C. K. , Haak, D. C. , & Martin, P. R. (2008). Impacts of climate warming on terrestrial ectotherms across latitude. Proceedings of the National Academy of Sciences of the United States of America, 105(18), 6668–6672. 10.1073/pnas.0709472105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias, I. R. , Haddad, C. F. B. , Argôlo, A. J. S. , & Orrico, V. G. D. (2017). The 100th: An appealing new species of Dendropsophus (Amphibia: Anura: Hylidae) from northeastern Brazil. PLoS One, 12(3), e0171678. 10.1371/journal.pone.0171678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias, I. R. , Medeiros, T. T. , Nova, M. F. V. , & Solé, M. (2014). Amphibians of Serra Bonita, southern Bahia: A new hotpoint within Brazil’s Atlantic Forest hotspot. ZooKeys, 449, 105–130. 10.3897/zookeys.449.7494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias, I. R. , Novaes‐e‐Fagundes, G. , Mollo Neto, A. , Zina, J. , Garcia, C. , Recoder, R. S. , Dal Vechio, F. , Rodrigues, M. T. , & Solé, M. (2020). A new large canopy‐dwelling species of Phyllodytes Wagler, 1930 (Anura, Hylidae) from the Atlantic Forest of the state of Bahia, Northeastern Brazil. PeerJ, 8, 1–27. 10.7717/peerj.8642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duarte, H. , Tejedo, M. , Katzenberger, M. , Marangoni, F. , Baldo, D. , Beltrán, J. F. , Martí, D. A. , Richter‐Boix, A. , & Gonzalez‐Voyer, A. (2012). Can amphibians take the heat? Vulnerability to climate warming in subtropical and temperate larval amphibian communities. Global Change Biology, 18(2), 412–421. 10.1111/j.1365-2486.2011.02518.x [DOI] [Google Scholar]
- Duellman, W. E. , & Trueb, L. (1994). Biology of amphibians. Johns Hopkins University Press. [Google Scholar]
- Enriquez‐Urzelai, U. , Kearney, M. R. , Nicieza, A. G. , & Tingley, R. (2019). Integrating mechanistic and correlative niche models to unravel range‐limiting processes in a temperate amphibian. Global Change Biology, 25(8), 2633–2647. 10.1111/gcb.14673 [DOI] [PubMed] [Google Scholar]
- Ficetola, G. F. , Rondinini, C. , Bonardi, A. , Baisero, D. , & Padoa‐Schioppa, E. (2015). Habitat availability for amphibians and extinction threat: A global analysis. Diversity and Distributions, 21(3), 302–311. 10.1111/ddi.12296 [DOI] [Google Scholar]
- Frishkoff, L. O. , Hadly, E. A. , & Daily, G. C. (2015). Thermal niche predicts tolerance to habitat conversion in tropical amphibians and reptiles. Global Change Biology, 21(11), 3901–3916. 10.1111/gcb.13016 [DOI] [PubMed] [Google Scholar]
- Frost, D. R. (2020). Amphibian Species of the World: An online reference. American Museum of Natural History. [Google Scholar]
- Garamszegi, L. Z. (2014). Modern phylogenetic comparative methods and their application in evolutionary biology. Springer. [Google Scholar]
- Gunderson, A. R. , & Stillman, J. H. (2015). Plasticity in thermal tolerance has limited potential to buffer ectotherms from global warming. Proceedings of the Royal Society B: Biological Sciences, 282(1808), 20150401. 10.1098/rspb.2015.0401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutiérrez‐Pesquera, L. M. , Tejedo, M. , Olalla‐Tárraga, M. Á. , Duarte, H. , Nicieza, A. , & Solé, M. (2016). Testing the climate variability hypothesis in thermal tolerance limits of tropical and temperate tadpoles. Journal of Biogeography, 43(6), 1166–1178. 10.1111/jbi.12700 [DOI] [Google Scholar]
- Haddad, C. F. B. , Toledo, L. F. , Prado, C. P. A. , Loebmann, D. , Gasparini, J. L. , & Sazima, I. (2013). Guia dos Anfíbios da Mata Atlântica: Diversidade e Biologia. Anolis Books. [Google Scholar]
- Hof, C. , Araújo, M. B. , Jetz, W. , & Rahbek, C. (2011). Additive threats from pathogens, climate and land‐use change for global amphibian diversity. Nature, 480(7378), 516–519. 10.1038/nature10650 [DOI] [PubMed] [Google Scholar]
- Hof, C. , Rahbek, C. , & Araújo, M. B. (2010). Phylogenetic signals in the climatic niches of the world's amphibians. Ecography, 33(2), 242–250. 10.1111/j.1600-0587.2010.06309.x [DOI] [Google Scholar]
- Hoffmann, M. , Hilton‐Taylor, C. , Angulo, A. , Böhm, M. , Brooks, T. M. , Butchart, S. H. M. , Carpenter, K. E. , Chanson, J. , Collen, B. , Cox, N. A. , Darwall, W. R. T. , Dulvy, N. K. , Harrison, L. R. , Katariya, V. , Pollock, C. M. , Quader, S. , Richman, N. I. , Rodrigues, A. S. L. , Tognelli, M. F. , … Stuart, S. N. (2010). The impact of conservation on the status of the world’s vertebrates. Science, 330(6010), 1503–1509. 10.1126/science.1194442 [DOI] [PubMed] [Google Scholar]
- IPCC . (2014). Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change [Pachauri R., & Meyer L. (Eds.)]. IPCC, Geneva, Switzerland, 151 p. [Google Scholar]
- IUCN (2021). The IUCN red list of threatened species. Version 2020‐1. Retrieved from https://www.iucnredlist.org [Google Scholar]
- Kapos, V. (1989). Effects of isolation on the water status of forest patches in the Brazilian Amazon. Journal of Tropical Ecology, 5(2), 173–185. 10.1017/S0266467400003448 [DOI] [Google Scholar]
- Kearney, M. , Shine, R. , & Porter, W. P. (2009). The potential for behavioral thermoregulation to buffer “cold‐blooded” animals against climate warming. Proceedings of the National Academy of Sciences of the United States of America, 106(10), 3835–3840. 10.1073/pnas.0808913106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Y. , Cohen, J. M. , & Rohr, J. R. (2013). Review and synthesis of the effects of climate change on amphibians. Integrative Zoology, 8(2), 145–161. 10.1111/1749-4877.12001 [DOI] [PubMed] [Google Scholar]
- Loyola, R. D. , Becker, C. G. , Kubota, U. , Haddad, C. F. B. , Fonseca, C. R. , & Lewinsohn, T. M. (2008). Hung out to dry: Choice of priority ecoregions for conserving threatened Neotropical anurans depends on life‐history traits. PLoS One, 3(5), e2120. 10.1371/journal.pone.0002120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutterschmidt, W. I. , & Hutchison, V. H. (1997). The critical thermal maximum: History and critique. Canadian Journal of Zoology, 75(10), 1561–1574. 10.1139/z97-783 [DOI] [Google Scholar]
- Mira‐Mendes, C. B. , Ruas, D. S. , Oliveira, R. M. , Castro, I. M. , Dias, I. R. , Baumgarten, J. E. , Juncá, F. A. , & Solé, M. (2018). Amphibians of the Reserva Ecológica Michelin: A high diversity site in the lowland Atlantic Forest of southern Bahia, Brazil. ZooKeys, 753, 1–21. 10.3897/zookeys.753.21438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moen, D. S. , & Wiens, J. J. (2017). Microhabitat and climatic niche change explain patterns of diversification among frog families. The American Naturalist, 190(1), 29–44. 10.1086/692065 [DOI] [PubMed] [Google Scholar]
- Moritz, C. , Langham, G. , Kearney, M. , Krockenberger, A. , VanDerWal, J. , & Williams, S. (2012). Integrating phylogeography and physiology reveals divergence of thermal traits between central and peripheral lineages of tropical rainforest lizards. Philosophical Transactions of the Royal Society B: Biological Sciences, 367(1596), 1680–1687. 10.1098/rstb.2012.0018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moura, M. R. , Costa, H. C. , Peixoto, M. A. , Carvalho, A. L. , Santana, D. J. , & Vasconcelos, H. L. (2018). Geographical and socioeconomic determinants of species discovery trends in a biodiversity hotspot. Biological Conservation, 220, 237–244. 10.1016/j.biocon.2018.01.024 [DOI] [Google Scholar]
- Myers, N. , Mittermeier, R. A. , Mittermeier, C. G. , Da Fonseca, G. A. , & Kent, J. (2000). Biodiversity hotspots for conservation priorities. Nature, 403(6772), 853–858. 10.1038/35002501 [DOI] [PubMed] [Google Scholar]
- Nowakowski, A. J. , Watling, J. I. , Thompson, M. E. , Brusch, G. A. IV , Catenazzi, A. , Whitfield, S. M. , Kurz, D. J. , Suárez‐Mayorga, A. , Aponte‐Gutiérrez, A. , Donelly, M. A. , & Todd, B. D. (2018). Thermal biology mediates responses of amphibians and reptiles to habitat modification. Ecology Letters, 21(3), 345–355. 10.1111/ele.12901 [DOI] [PubMed] [Google Scholar]
- Olalla‐Tárraga, M. Á. , McInnes, L. , Bini, L. M. , Diniz‐Filho, J. A. F. , Fritz, S. A. , Hawkins, B. A. , Hortal, J. , Orme, D. , Rahbek, C. , Rodríguez, M. Á. , & Purvis, A. (2011). Climatic niche conservatism and the evolutionary dynamics in species range boundaries: Global congruence across mammals and amphibians. Journal of Biogeography, 38(12), 2237–2247. 10.1111/j.1365-2699.2011.02570.x [DOI] [Google Scholar]
- Orme, D. , Freckleton, R. , Thomas, G. , Petzoldt, T. , Fritz, S. , Isaac, N. , & Pearse, W. (2013). CAPER: Comparative analyses of phylogenetics and evolution in R. R package version 0.5.2. http://cran.r‐project.org/package=caper [Google Scholar]
- Orrico, V. G. , Dias, I. R. , & Marciano‐Jr, E. (2018). Another new species of Phyllodytes (Anura: Hylidae) from the Atlantic Forest of northeastern Brazil. Zootaxa, 4407(1), 101–110. 10.11646/zootaxa.4407.1.6 [DOI] [PubMed] [Google Scholar]
- Pyron, R. A. , & Wiens, J. J. (2011). A large‐scale phylogeny of Amphibia including over 2800 species, and a revised classification of extant frogs, salamanders, and caecilians. Molecular Phylogenetics and Evolution, 61(2), 543–583. 10.1016/j.ympev.2011.06.012 [DOI] [PubMed] [Google Scholar]
- R Core Team (2020). R: A language and environment for statistical computing. R Foundation for Statistical Computing. [Google Scholar]
- Rossa‐Feres, D. C. , Garey, M. V. , Caramaschi, U. , Napoli, M. F. , Nomura, F. , Bispo, A. A. , Brasileiro, C. A. , Thomé, M. T. C. , Sawaya, R. J. , Conte, C. E. , Cruz, C. A. G. , Nascimento, L. B. , Gasparini, J. L. , Almeida, A. P. , & Haddad, C. F. B. (2017). Anfíbios da Mata Atlântica: lista de espécies, histórico dos estudos, biologia e conservação. In Monteiro Filho E. L. A., & Conte C. E. (Eds.), Revisões em Zoologia: Mata Atlântica (1st ed., Vol. 1, pp. 237–314). Curitiba: Editora UFPR. [Google Scholar]
- Scheffers, B. R. , Brunner, R. M. , Ramirez, S. D. , Shoo, L. P. , Diesmos, A. , & Williams, S. E. (2013). Thermal buffering of microhabitats is a critical factor mediating warming vulnerability of frogs in the Philippine biodiversity hotspot. Biotropica, 45(5), 628–635. 10.1111/btp.12042 [DOI] [Google Scholar]
- Scheffers, B. R. , Edwards, D. P. , Diesmos, A. , Williams, S. E. , & Evans, T. A. (2014). Microhabitats reduce animal's exposure to climate extremes. Global Change Biology, 20(2), 495–503. 10.1111/gcb.12439 [DOI] [PubMed] [Google Scholar]
- Schneider‐Maunoury, L. , Lefebvre, V. , Ewers, R. M. , Medina‐Rangel, G. F. , Peres, C. A. , Somarriba, E. , Urbina‐Cardona, N. , & Pfeifer, M. (2016). Abundance signals of amphibians and reptiles indicate strong edge effects in Neotropical fragmented forest landscapes. Biological Conservation, 200, 207–215. 10.1016/j.biocon.2016.06.011 [DOI] [Google Scholar]
- Segalla, M. V. , Caramaschi, U. , Cruz, C. A. G. , Grant, T. , Haddad, C. F. B. , Garcia, P. C. A. , Berneck, B. V. M. , & Langone, J. A. (2019). Brazilian amphibians: List of species. Herpetologia Brasileira, 8(1), 65–96. [Google Scholar]
- Silvano, D. L. , & Segalla, M. V. (2005). Conservation of Brazilian amphibians. Conservation Biology, 19(3), 653–658. 10.1111/j.1523-1739.2005.00681.x [DOI] [Google Scholar]
- Simon, M. N. , Ribeiro, P. L. , & Navas, C. A. (2015). Upper thermal tolerance plasticity in tropical amphibian species from contrasting habitats: Implications for warming impact prediction. Journal of Thermal Biology, 48, 36–44. 10.1016/j.jtherbio.2014.12.008 [DOI] [PubMed] [Google Scholar]
- SOS Mata Atlântica‐Fundação SOS Mata Atlântica, INPE‐ Instituto Nacional de Pesquisas Espaciais (2017). Atlas dos Remanescentes Florestais da Mata Atlântica – Período 2015‐2016. Fundação SOS Mata Atlântica, São Paulo, p. 60. [Google Scholar]
- Stevenson, R. D. (1985). The relative importance of behavioral and physiological adjustments controlling body temperature in terrestrial ectotherms. The American Naturalist, 126(3), 362–386. 10.1086/284423 [DOI] [Google Scholar]
- Stuart, S. N. , Chanson, J. S. , Cox, N. A. , Young, B. E. , Rodrigues, A. S. , Fischman, D. L. , & Waller, R. W. (2004). Status and trends of amphibian declines and extinctions worldwide. Science, 306(5702), 1783–1786. 10.1126/science.1103538 [DOI] [PubMed] [Google Scholar]
- Sunday, J. M. , Bates, A. E. , & Dulvy, N. K. (2011). Global analysis of thermal tolerance and latitude in ectotherms. Proceedings of the Royal Society B: Biological Sciences, 278(1713), 1823–1830. 10.1098/rspb.2010.1295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapley, B. , Michaels, C. J. , Gumbs, R. , Böhm, M. , Luedtke, J. , Pearce‐Kelly, P. , & Rowley, J. J. (2018). The disparity between species description and conservation assessment: A case study in taxa with high rates of species discovery. Biological Conservation, 220, 209–214. 10.1016/j.biocon.2018.01.022 [DOI] [Google Scholar]
- Taylor, E. M. , Diele‐Viegas, L. M. , Gangloff, E. J. , Hall, J. M. , Halpern, B. , Massey, M. D. , Rödder, D. , Rollinson, N. , Spears, S. , Sun, B. , & Telemeco, R. S. (2020). The thermal ecology and physiology of reptiles and amphibians: A user's guide. Journal of Experimental Zoology Part A: Ecological and Integrative Physiology, jez.2396, 1–32. [DOI] [PubMed] [Google Scholar]
- Tejedo, M. , Duarte, H. , Gutiérrez‐Pesquera, L. M. , Beltran, J. F. , Katzenberger, M. , Marangoni, F. , Navas, C. A. , Nicieza, A. G. , Relyea, R. A. , Rezende, E. L. , Richter Boix, A. , Santos, M. , Simon, M. , & Solé, M. (2012). El estudio de las tolerancias térmicas para el examen de hipótesis biogeográficas y de la vulnerabilidad de los organismos ante el calentamiento global. Ejemplos en anfíbios. Boletín de la Asociación Herpetológica Española, 23(2), 2–27. [Google Scholar]
- Thompson, M. E. , & Donnelly, M. A. (2018). Effects of secondary forest succession on amphibians and reptiles: A review and meta‐analysis. Copeia, 106(1), 10–19. 10.1643/CH-17-654 [DOI] [Google Scholar]
- Tuff, K. T. , Tuff, T. , & Davies, K. F. (2016). A framework for integrating thermal biology into fragmentation research. Ecology Letters, 19(4), 361–374. 10.1111/ele.12579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasconcelos, T. S. , Prado, V. H. , da Silva, F. R. , & Haddad, C. F. (2014). Biogeographic distribution patterns and their correlates in the diverse frog fauna of the Atlantic Forest hotspot. PLoS One, 9(8), e104130. 10.1371/journal.pone.0104130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- von May, R. , Catenazzi, A. , Corl, A. , Santa‐Cruz, R. , Carnaval, A. C. , & Moritz, C. (2017). Divergence of thermal physiological traits in terrestrial breeding frogs along a tropical elevational gradient. Ecology and Evolution, 7(9), 3257–3267. 10.1002/ece3.2929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanger, T. C. , DeClerck, F. , Garibaldi, L. A. , Ghazoul, J. , Kleijn, D. , Klein, A.‐M. , Kremen, C. , Mooney, H. , Perfecto, I. , Powell, L. L. , Settele, J. , Solé, M. , Tscharntke, T. , & Weisser, W. (2020). Integrating agroecological production in a robust post‐2020 Global Biodiversity Framework. Nature Ecology & Evolution, 4(9), 1150–1152. 10.1038/s41559-020-1262-y [DOI] [PubMed] [Google Scholar]
- Winter, M. , Fiedler, W. , Hochachka, W. M. , Koehncke, A. , Meiri, S. , & De la Riva, I. (2016). Patterns and biases in climate change research on amphibians and reptiles: A systematic review. Royal Society Open Science, 3(9), 1–13. 10.1098/rsos.160158 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1 and S2
Data Availability Statement
Data sources used for statistical group analyses, species traits, and most sensitive taxa are provided in the Supporting Information. All files mentioned above are available online at https://doi.org/10.5061/dryad.ttdz08kzp.


